Abstract
The synthesis, structural characterization, and optical properties of the binary Zintl phases of α-EuP3, β-EuP3, EuP2, and α-K4P6 are reported in this study. These crystal structures demonstrate the versatility of P fragments with dimensionality varying from 0D (P6 rings in α-K4P6) to 1D chains (EuP2) to 2D layers (both EuP3). EuP2 is isostructural to previously reported SrP2 and BaP2 compounds. The thermal stabilities of the EuP2 and both EuP3 phases were determined using differential scanning calorimetry (DSC), with melting temperatures of 1086 K for the diphosphide and 1143 K for the triphosphides. Diffuse reflectance spectroscopy indicated that EuP2 is an indirect semiconductor with a direct bandgap of 1.12(5) eV and a smaller indirect one, less than 1 eV. Both EuP3 compounds had bandgaps smaller than 1 eV.
1. Introduction
An impressive variety of polyphosphides exist in the solid state [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33]. In these systems, P atoms form various coordination environments and multidimensional P fragments with a broad array of s, p-, and f-elements. On the basis of a 1988 review on polyphosphides, nearly a dozen binary phases exist in the K-P system, and only slightly fewer Eu–P binary phases exist [1,2]. Due to the presence of a partially filled f-electron shell on Eu atoms, some Eu phosphides show interesting magnetic properties [34,35].
The P fragments in these systems range from 0D to 3D, involving isolated atoms, dumbbells, rings, clusters, chains, layers, and frameworks of P atoms. A4P6 (A = K, Rb, Cs) compounds have a planar cyclic P structural unit, hexaphosphabenzene (P64–), of the point group D6h (6/mmm). A similar As64− anion was also reported [5,36,37,38].
All of these compounds belong to a broad family of Zintl phases [39,40]. The electropositive cations donate their valence electrons to the P anions, realizing a stable electron configuration of the noble gas. Phosphorus polyanions accept electrons and achieve an electron octet by forming P–P bonds and/or by forming electron lone pairs. In such a formalism, isolated P atoms bear a −3 charge, P atoms forming P–P dumbbells each have a −2 charge, while P atoms forming two and three homoatomic P–P bonds have −1 and 0 charges, correspondingly.
Most of alkali- or alkaline-earth phosphides are expected to be semiconductors on the basis of their charge-balanced nature. The bandgaps of these systems vary, leading to a range of colors from yellow to red and gray or black [1,6,7]. Recent discoveries in metal polyphosphide systems expand upon this library, introducing unique polyphosphide fragments which may be different from any previously reported [6,7]. Furthermore, an evaluation of the inorganic crystal structure database reveals that our understanding of many of the published binary phosphides is incomplete. In the current work, we present crystal structure information for some reported but only partially characterized or uncharacterized binary phosphides: α-EuP3, β-EuP3, EuP2, and α-K4P6.
2. Materials and Methods
2.1. Synthesis
All manipulations with the initial materials and sample handling were performed inside an Ar-filled glove box (p(O2) < 1 ppm). The starting materials of metallic europium (U.S. DOE Ames Laboratory, 99.9%), metallic potassium (Alfa Aesar, 99.95%), and red phosphorus (Alfa Aesar, 99%) were used as received.
Single crystals of β-EuP3 were originally synthesized as a side-product of the synthetic endeavors in the Eu-Ni-P system. The reactants (total amounts 500 mg) were placed in a glassy carbon crucible and sealed in a silica tube under vacuum; the residual pressure was 0.04 mbar. The annealing profile involved the ramping of reactants up to 1073 K over 17 h and holding at the maximum temperature for 140 h. The resulting products were gray-metallic binary Ni-phosphides and darker, blackish crystals of β-EuP3. Likewise, the original single crystals of α-EuP3 were found as a minor side product of a reaction of elemental Eu and P in a 1:3 stoichiometry sealed in a carbonized silica tube under vacuum. The sample was ramped up to 1173 K over 17 h and annealed for 48 h. Subsequent syntheses to form α-EuP3 samples were similar, but the samples were held at 1123 K for 217 h. β-EuP3 was formed by quenching from the melt at 1123 K. Alternatively, α-EuP3 was formed by controlled cooling of the melt over the course of 5 h.
EuP2 crystals were originally obtained by the low temperature attempt to make EuP3 from elements at 973 K. Eu and P were combined in a 1:3 stoichiometry in a carbonized silica ampoule and sealed under vacuum. The sample temperature was gradually increased to 973 K over 17 h and held there for 48 h. The reaction was incomplete after a single heating, where EuP2 formed on the surface of the partially reacted Eu. Therefore, to improve the yield of EuP2, the sample required additional grinding and re-annealing under the same conditions.
A single crystal of α-K4P6 was originally synthesized as a product of the reaction of elemental K with P in a 1:1.1 stoichiometry in an attempt to make the stoichiometric phase KP. Elemental P was placed at the bottom of a silica tube, and K was scooped into a small alumina crucible. The crucible was subsequently placed gently into the silica tube on top of the P, and the tube was sealed under vacuum. The sample was ramped up to 773 K over 17 h and annealed for 24 h. The resulting blackish product was found outside the crucible, in the bottom of the silica tube.
2.2. Single-Crystal X-ray Diffraction
A part of sample was placed in a Petri dish and covered with Paratone oil in the Ar-filled glove box (Hampton research corp, USA). Single crystals were selected using an optical microscope and placed under a stream of cold dry nitrogen on the single-crystal diffractometer. Datasets were collected on either a Bruker Apex II diffractometer or a Bruker D8 Venture diffractometer, both using Mo-Kα radiation. Data for α-EuP3, β-EuP3, and α-K4P6 were collected at 0.5° step width and 1 s per frame exposure time, while data for EuP2 were collected at 0.5° step width with a collection time of 10 s per frame. Data collection and integration and space group determination were performed using a Bruker APEX3 program suite (Bruker AXS USA). Multiscan absorption correction was applied to all crystals. Important single-crystal refinement parameters of all phases can be found in Table 1. Several crystals of EuP2 were measured, all exhibiting similar not-very-high qualities, presumably due to partial decomposition. The solutions and refinements of crystal structures were carried out using the SHELX suite of programs [41]. Further details of the crystal structure determinations may be found through Cambridge Crystallographic Data Centre by using CCDC #1885037-1885040 or in the Supplementary Materials.

Table 1.
Data collection and structure refinement parameters.
2.3. X-ray Powder Diffraction
X-ray powder diffraction patterns were measured using a Rigaku Miniflex 600 with Cu-Kα radiation and a Ni-Kβ filter. The experimental patterns were compared with the calculated ones based on the crystal structure models from single-crystal X-ray diffraction experiments. EuP3 phases were stable in air for a short period of time, which was sufficient for the purpose of X-ray powder diffraction (<10 min exposure to air). However, the quick decomposition of EuP2 and K4P6 in air at ambient temperature was evident by abrupt changes in color. Thus, homemade Kapton air-free holders were used for X-ray powder diffraction of EuP2 (Figure 1a).
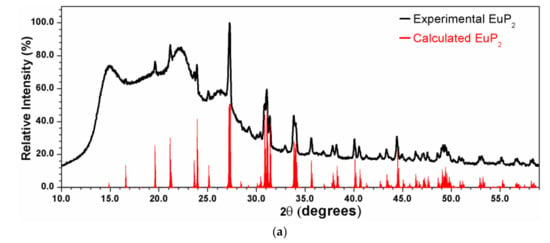
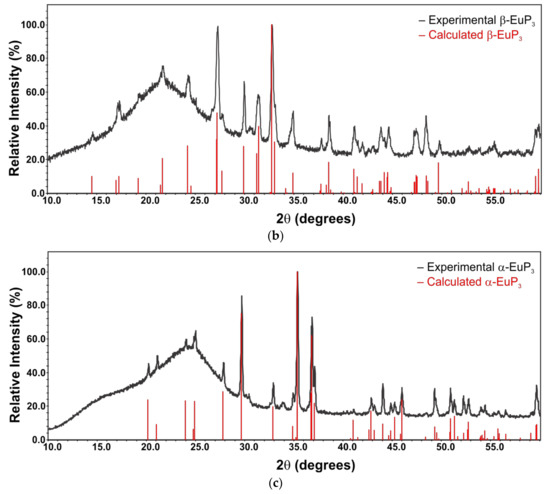
Figure 1.
X-ray powder diffraction patterns for EuP2 (a) and EuP3 (b and c) samples along with their calculated patterns from single-crystal data. The high background in the two bottom patterns may be due to the unidentified amorphous admixtures occurring as a result of partial sample decomposition in air. While the target phosphide is the main phase, low-intensity diffraction peaks of unidentified admixtures are present.
2.4. Differential Scanning Calorimetry
Two Netzsch Differential Scanning Calorimeters (DSC), either Netzsch STA 449 F3 Jupiter or Netzsch DSC 404 F3 Pegasus, were used to characterize the thermal behavior of synthesized phases. In order to maintain similar conditions to those of actual syntheses, DSC measurements were run using small evacuated and sealed silica ampoules with enough sample to cover the base of the ampoule (approximately 30–50 mg). The samples were initially heated to 673 K at a rate of 10 K/min, then the heating was slowed to 5 K/min over the 673–1273 K range. A similar cooling scheme was employed. Errors in the melting and crystallization temperatures were estimated not to exceed +/−3 K.
2.5. Solid-State Diffuse Reflectance Spectroscopy
Solid-state UV–Vis spectroscopy (Thermo Scientific Evolution 220 Spectrometer or BLACK-Comet C-SR-100 spectrometer, manufacturer, city, country) was employed for experimental bandgap determinations. Kubelka–Munk diffuse reflectance was used to help characterize the bandgaps of the three Eu-containing phases: α-EuP3, β-EuP3, and EuP2. For UV–Vis diffuse reflectance measurements, solid samples were ground into powders and heat-sealed in transparent polyethylene bags inside an Ar-filled glove box to prevent any phase degradation or color changes. A blank measurement of an empty polyethylene bag was used as a reference.
3. Results and Discussion
3.1. Synthesis and Thermal Stability
All synthesized polyphosphide phases decomposed in air over time. K4P6 was much more air-sensitive than the Eu phases. Even in paratone oil, the rich black and gold-tinted blocks of K4P6 fizzled and bubbled, turning a light red-orange color. The reactivity of K with silica and its high vapor pressure made it very difficult to control the amount of K in the reaction, thus K4P6 was formed in an excess of K. Furthermore, a strong reaction of K with the silica ampoule was observed by the discoloration of the ampoule’s inner surface. Thus, only single crystals of K4P6 were obtained, but no pure-phase synthesis was managed. The synthesis and thermal decomposition of α-K4P6 were previously explored by von Schnering et al. [5]. Their synthesis involved a stoichiometric reaction of elemental K with P up to a maximum temperature of 870 K. On the basis of their results, the selective synthesis of the α-phase requires the slow cooling of the K4P6 melt. Additionally, they showed the α-K4P6 phase decomposes into a K-deficient phase of K3P7 starting at 650 K (7K4P6 → 6K3P7 + 10K), which then begins to sublime at ≈ 830 K [5]. Because of the reactivity of α-K4P6 in air and lack of phase purity, the thermal stability of this phase was not further explored.
EuP2 was also air-sensitive, though less than K4P6. While the latter decomposed readily in oil, EuP2 did not. However, the phase decomposed when left in air, changing color to a light orange within minutes. EuP2 was synthesized from a 1:3 ratio of Eu/P at 973 K. Powder X-ray diffraction on initial annealed samples revealed little EuP2 formation; however, grinding and re-annealing this sample a second time drastically improved the yield of EuP2. The phosphorus excess sublimed to the top of the ampoule.
The syntheses of EuP3 phases were more straightforward. Oxidized portions of Eu were removed from the elemental metal, and the reaction of Eu with P at a low temperature (1073 K) formed a high-yield sample of β-EuP3. Tiny admixtures of α-EuP3 were discovered as a minor product. In addition to the formation of EuP2, work by von Schnering et al. in the early 1980s showed the possibility of forming β-EuP3 via the decomposition of EuP7 [3]. Subsequent syntheses to form EuP3 samples were conducted at a higher temperature, 1123 K. β-EuP3 was formed by quenching the sample at 1123 K. Alternatively, α-EuP3 was formed by controlled cooling of the sample over the course of 5 h. Typical X-ray powder diffraction patterns are shown in Figure 1.
Differential scanning calorimetry (DSC) was used to characterize the thermal stability of the Eu-containing compounds (Figure 2). Based on these results, both EuP3 phases had similar thermal stability melting and recrystallizing at around 1143 K and 1055 K, respectively. Previous results have shown α and β phases of binary phosphides may have very similar melting/decomposition temperatures. For example, in the case of dimorphic BaP3 phases, the melting temperatures were within error of one another at 1105(3) K and 1109(3) K for mP-BaP3 and mS-BaP3, respectively [6]. α-EuP3 has a 35 K higher melting point than the isostructural analogue mS-BaP3. EuP2 was found to melt at approximately 1086 K and recrystallize at 991 K. Powder diffraction on the DSC sample of EuP2 confirmed the recrystallization to be that of EuP2. This melting point is between those reported for BaP2 (1053 K) and SrP2 (1123 K) [7].
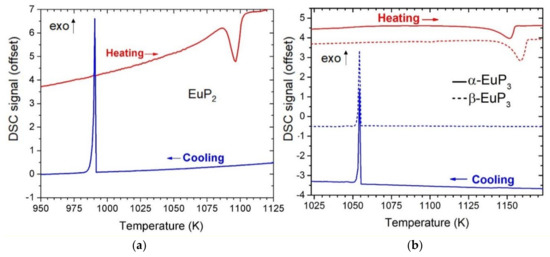
Figure 2.
DSC curves for EuP2 (a) and EuP3 (b) phases. Heating is shown in red, and cooling is shown in blue, exothermic direction is shown with exo arrow. For EuP3, the solid lines represent the α-phase, and the dashed lines represent the β-phase.
3.2. Crystal Structures
The crystal structures of α-EuP3 [1], β-EuP3 [1,34,35], EuP2 [1,3], and α-K4P6 [1,5] were mentioned in earlier works, although their complete crystal structure information is not available. For α-K4P6 and β-EuP3 the crystal structures determined by us agree well with previous model based on X-ray powder and single-crystal neutron diffraction data [5,35]. For α-EuP3 and EuP2, the structural models were proposed based on similarities of the unit cell parameters and powder diffraction patterns [1,3]. We confirmed the proposed models. The crystal structures of α- and β-EuP3 belong to well-known structure types in the realm of electropositive metal polyphosphides. The former is isostructural to mS-BaP3, and the latter is isostructural to SrP3. Both phases are made up of layers of infinite P sheets with Eu atoms sitting between the layers. Both P layers can be related to the layers of black P (Figure 3). Though both phases look nearly identical down the [010] direction, the ordering of their layers is different.
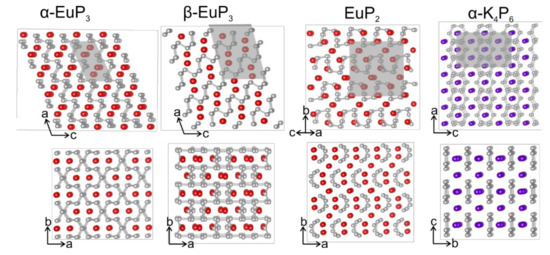
Figure 3.
The crystal structures of two EuP3 phases, EuP2, and α-K4P6 are shown along two different directions. The unit cells are highlighted with gray boxes. P: gray, Eu: red; K: purple.
In the case of α-EuP3, 14-membered P rings are present in the infinite P layers. These rings can be formed directly from black P by the removal of alternating P–P dumbbells in two directions. Interestingly, the infinite chair-like puckering in these P layers is maintained upon the evolution of P32- layers from black P (Figure 4). Similarly, two pairs of neighboring P–P dumbbells from the black P framework can be removed to form the 22-membered P rings in β-EuP3. These large rings are separated by leftover 6-membered P rings from the black P framework. Similar to α-EuP3, the black P puckering of the layers is maintained in β-EuP3 (Figure 4). In both EuP3 crystal structures, there are two-bonded (2b-) and three-bonded (3b-) P atoms in a 2:1 ratio. 3b-P atoms covalently connected to three P atoms have a formal oxidation state of 0, similar to P atoms in black phosphorus. 2b-P atoms, which are connected to only two P atoms, have a formal oxidation state of −1, thus making the compounds electron-balanced: (Eu2+)(2b-P1−)2(3b-P0)1.
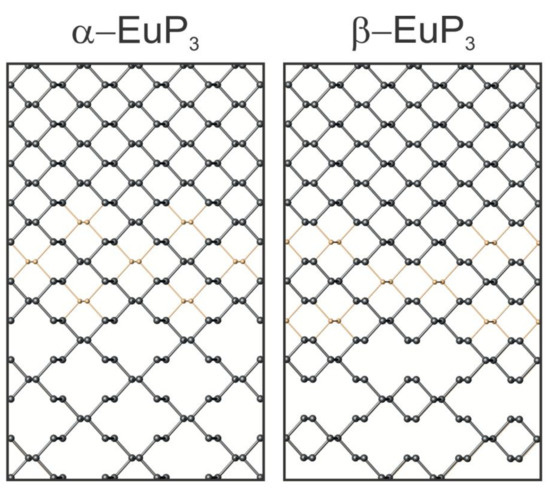
Figure 4.
The evolution of phosphorus frameworks from black phosphorus (top) to the two phases of EuP3 (bottom) are shown. The gold atoms depicted (middle) indicate the P atoms that are absent in the EuP3 frameworks.
EuP2 is isostructural to SrP2 and BaP2 [7]. As opposed to two-dimensional layers in the EuP3 phases, the P fragments of EuP2 consist of one-dimensional twisted chains of P (Figure 3). The unit cell parameters for EuP2 are slightly smaller than those of SrP2 [7]. A similar decrease in the unit cell parameters was observed in the crystal structures of A2SiP4 compounds, A = Sr, Eu [19].
α-K4P6 is very different from the other three phases, which all show some type of puckered infinite P fragments. The P in K4P6 is present as isolated 6-membered P64- rings, hexaphosphabenzenes, which can be derived from planar graphene-like P-layers by an ordered removal of a ¼ of P atoms [5]. The rings line up, but are not bound to each other, forming nearly flat layers of isolated hexagons, and the K atoms sit between the layers. The rings are planar, with D6h symmetry (6/mmm), likely due to their coordination with K atoms that coordinate to the faces of the rings. Previous works have explored these unique P rings in both the solid state and in solution, and As64− analogues are also known to exist [36,37,38].
The coordination spheres of Eu and K in the four phases are shown in Figure 5. α-EuP3 has one unique Eu site, while the other three phases have two unique Eu or K sites. Of the three Eu phases, the shortest Eu–Eu distance is present in β-EuP3 at 3.83 Å. The shortest K–K distance in α-K4P6 is 4.14 Å. Between the Eu and K samples, the P–P distances vary significantly. The shortest P–P distance for α-K4P6 is 2.152(1) Å, while the shortest P–P in Eu-containing compounds is 2.183(5) Å in EuP2. The former short length is close to the expected bond lengths in the P64− rings based on other A4P6 systems where A = Rb, Cs. This is due to the partial increase of the bond order over one by the formation of a quasi-aromatic π-system. Alternatively, the shortest length in EuP2, 2.183(5) Å, is close to the distance of a single P–P bond in black P at 2.23 Å at 300 K [1]. Furthermore, the closest distances between the P “layers” of each phase are 3.32 Å, 3.21 Å, 4.21 Å, and 4.68 Å for α-EuP3, β-EuP3, EuP2, and α-K4P6, respectively. The increased distances between the “layers” of P chains in EuP2 with respect to EuP3 phases can be explained by the higher ratio of Eu atoms to P atoms and the fact that P chains are present instead of layers.
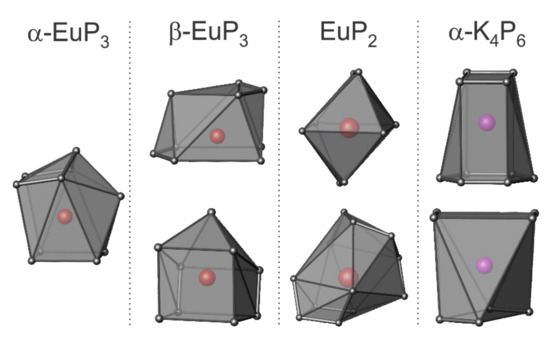
Figure 5.
The cations’ coordination in EuP3 phases, EuP2, and α-K4P6. P atoms are shown in gray, Eu atoms are red, and K atoms are purple.
3.3. Solid-State Diffuse Reflectance Spectroscopy
Previously published experimental resistivity measurements have shown semiconducting behavior for β-EuP3, and, with the structural similarities between the two phases, the α-phase may act electronically similar [34,35]. Kubelka-Munk diffuse reflectance was used to determine the bandgaps of the Eu-containing phases. For α-EuP3 and β-EuP3, no peaks in the Kubelka-Munk curve were present, indicating the likelihood of small bandgaps of less than 1 eV. This aligns with the observed black colors of the phases. Unfortunately, Tauc plots, and thus bandgap estimations, for the phases would likely be unreliable due to the small size of the bandgaps and the limitations of the instrument.
In turn, EuP2 showed an abrupt increase in absorption (Figure 6). Estimation of the direct bandgap using Tauc plots resulted in the values of 1.12(5) eV. The indirect bandgap appeared to be smaller and cannot be reliably determined because of the limitations of the instrument. The bandgap of EuP2 was smaller than the bandgap determined for isostructural SrP2, with indirect and direct bandgaps of 1.3(1) eV and 1.5(1) eV, respectively [7].
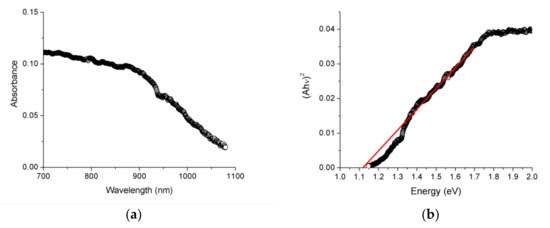
Figure 6.
Absorbance spectra (a) and solid-state UV-Vis direct bandgap Tauc plot (b) for EuP2.
4. Conclusions
Though numerous polyphosphides are known, many have not been fully characterized. It is likely that there may still be other binary phosphides that await discovery. Showing a broad variety of structure types and P fragments, the applications for these materials may be just as broad. Furthermore, a combination of phosphides could lead to interesting intermediates with controlled structures and properties. The four crystal structures of binary phosphide phases introduced here (α-EuP3, β-EuP3, EuP2, and α-K4P6) are no exception, and they broaden the library of binary polyphosphides. However, the synthesis of these phases is challenging due to the high vapor pressure of phosphorus at the reaction temperatures and the high reactivity and vapor pressure of K metal.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1996-1944/12/2/251/s1, details of the crystal structure determinations.
Author Contributions
Investigation, J.A.-D., J.M., S.L., N.T.; writing—original draft preparation, J.A.-D.; writing—review and editing, J.M., S.L., K.K.; supervision, K.K.
Funding
This research was funded by the U.S. Department of Energy, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering under Award DE-SC0008931. We thank the National Science Foundation MRI program, Grant 1531193, for the funding for the Bruker D8 Venture single-crystal X-ray diffractometer.
Acknowledgments
We thank Frank Osterloh (UC Davis) and Javier Vela (ISU) for access to the solid-state UV–Vis spectrometers.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hönle, W.; von Schnering, H.G. Chemistry and Structural Chemistry of Phosphides and Polyphosphides. 48. Bridging Chasms with Polyphosphides. Chem. Rev. 1988, 88, 243–273. [Google Scholar]
- Pöttgen, R.; Hönle, W.; von Schnering, H.G. Phosphides: Solid State Chemistry. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Von Schnering, H.G.; Wittman, M. Europium (II) Heptaphosphide EuP7. Zeitschrift für Naturforschung B 1980, 35, 824–831. [Google Scholar] [CrossRef]
- Schmettow, W.; Mensing, C.; von Schnering, H.-G. Zur Chemie und Strukturchemie der Phosphide und Polyphosphide. 34 [1] Dampfdruckuntersuchungen am System Europium–Phosphor. Z. Anorg. Allg. Chem. 1984, 510, 51–60. [Google Scholar] [CrossRef]
- Abicht, H.-P.; Hönle, W.; von Schnering, H.G. Zur Chemie und Strukturchemie von Phosphiden und Polyphosphiden. 36. Tetrakaliumhexaphosphid: Darstellung, Struktur und Eigenschaften von α-K4P6 und β-K4P6. Z. Anorg. Allg. Chem. 1984, 519, 7–23. [Google Scholar] [CrossRef]
- Dolyniuk, J.; Kaseman, D.C.; Sen, S.; Zhou, J.; Kovnir, K. mP-BaP3: A New Phase from an Old Binary System. Chem. Eur. J. 2014, 20, 10829–10837. [Google Scholar] [CrossRef] [PubMed]
- Dolyniuk, J.; He, H.; Ivanov, A.; Boldyrev, A.; Bobev, S.; Kovnir, K. Ba and Sr Binary Phosphides: Synthesis, Crystal Structures, and Bonding Analysis. Inorg. Chem. 2015, 54, 8608–8616. [Google Scholar] [CrossRef]
- Fulmer, J.; Kaseman, D.; Dolyniuk, J.; Lee, K.; Sen, S.; Kovnir, K. BaAu2P4: Layered Zintl Polyphosphide with Infinite ∞1(P–) Chains. Inorg. Chem. 2013, 52, 7061–7067. [Google Scholar] [CrossRef]
- Shatruk, M.M.; Kovnir, K.A.; Shevelkov, A.V.; Popovkin, B.A. Ag3SnP7: A Polyphosphide with a Unique (P7) Chain and a Novel Ag3Sn Heterocluster. Angew. Chem. Int. Ed. 2000, 39, 2508–2509. [Google Scholar] [CrossRef]
- Fässler, T.F. Relationships between Soluble Zintl Anions, Ligand-Stabilized Cage Compounds, and Intermetalloid Clusters of Tetrel (Si-Pb) and Pentel (P-Bi) Elements. In Zintl Ions: Principles and Recent Developments; Fässler, T.F., Ed.; Springer: Berlin, Germany, 2011; Volume 140, pp. 91–131. [Google Scholar]
- Scharfe, S.; Kraus, F.; Stegmaier, S.; Schier, A.; Fässler, T.F. Zintl Ions, Cage Compounds, and Intermetalloid Clusters of Group 14 and Group 15 Elements. Angew. Chem. Int. Ed. 2011, 50, 3630–3670. [Google Scholar] [CrossRef]
- Lange, S.; Sebastian, C.P.; Zhang, L.; Eckert, H.; Nilges, T. Ag3SnCuP10: Ag3Sn Tetrahedra Embedded Between Adamantane-Type P-10 Cages. Inorg. Chem. 2006, 45, 5878–5885. [Google Scholar] [CrossRef]
- Lange, S.; Sebastian, C.P.; Nilges, T. A New Preparative Approach To HgPbP14 Structure Type Materials: Crystal Structure of Cu0.73(1)Sn1.27(1)P14 And Characterization of M1-XSn1+XP14 (M = Cu, Ag) and AgSbP14. Z. Anorg. Allg. Chem. 2006, 632, 195–203. [Google Scholar] [CrossRef]
- Dolyniuk, J.; Zaikina, J.V.; Kaseman, D.C.; Sen, S.; Kovnir, K. Breaking the Tetra-Coordinated Framework Rule: New Clathrate Ba8M24P28+δ (M = Cu/Zn). Angew. Chem. Int. Ed. 2017, 56, 2418–2422. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.; Bawohl, M.; Weihrich, R.; Nilges, T. Mineralization Routes to Polyphosphides: Cu2P20 and Cu5InP16. Angew. Chem. Int. Ed. 2008, 47, 5654–5657. [Google Scholar] [CrossRef] [PubMed]
- Lincke, H.; Nilges, T.; Johrendt, D.; Pöttgen, R. Crystal and Electronic Structure of La3Zn2-xP4—New Phosphide with Isolated P3− species. Solid State Sci. 2008, 10, 1006–1011. [Google Scholar] [CrossRef]
- Wang, J.; Yox, P.; Voyles, J.; Kovnir, K. Synthesis, Crystal Structure, and Properties of Three La-Zn-P Compounds with Different Dimensionality of Zn-P Framework. Cryst. Growth Des. 2018, 18, 4076–4083. [Google Scholar] [CrossRef]
- Kovnir, K.; Zaikina, J.V.; Reshetova, L.N.; Olenev, A.V.; Dikarev, E.V.; Shevelkov, A.V. Unusually High Chemical Compressibility of Normally Rigid Type-I Clathrate Framework: Synthesis and Structural Study of Sn24P19.3BrxI8-x Solid Solution, the Prospective Thermoelectric Material. Inorg. Chem. 2004, 43, 3230–3236. [Google Scholar] [CrossRef] [PubMed]
- Mark, J.; Dolyniuk, J.; Tran, N.; Kovnir, K. Crystal and Electronic Structure and Optical Properties of AE2SiP4 (AE = Sr, Eu, Ba) and Ba4Si3P8. Z. Anorg. Allg. Chem. 2018. [Google Scholar] [CrossRef]
- Bawohl, M.; Schmidt, P.; Nilges, T. Temperature Initiated P-Polymerization in Solid [Cd3Cu]CuP10. Inorg. Chem. 2013, 52, 11895–11901. [Google Scholar] [CrossRef]
- Eckstein, N.; Hohmann, A.; Weihrich, R.; Nilges, T.; Schmidt, P. Synthesis and Phase Relations of Single-Phase Fibrous Phosphorus. Z. Anorg. Allg. Chem. 2013, 639, 2741–2743. [Google Scholar] [CrossRef]
- Dewalsky, M.V.; Jeitschko, W.; Wortmann, U. The Metallic Polyphosphide Ti2NiP5. Chem. Mater. 1991, 3, 316–319. [Google Scholar] [CrossRef]
- Eschen, M.; Wallinda, J.; Jeitschko, W. Preparation and Crystal Structure of Au1-xSn1+yP14 and Other Polyphosphides with HgPbP14-Type Structure. Z. Anorg. Allg. Chem. 2002, 628, 2764–2771. [Google Scholar] [CrossRef]
- Wang, J.; He, Y.; Mordvinova, N.E.; Lebedev, O.I.; Kovnir, K. The smaller the better: Hosting trivalent rare-earth guests in Cu-P clathrate cages. Chem 2018, 4, 1465–1475. [Google Scholar] [CrossRef]
- Eschen, M.; Jeitschko, W. Au2PbP2, Au2TlP2, and Au2HgP2: Ternary Gold Polyphosphides with Lead, Thallium, and Mercury in the Oxidation State Zero. J. Solid State Chem. 2002, 165, 238–246. [Google Scholar] [CrossRef]
- Bartsch, T.; Wiegand, T.; Ren, J.J.; Eckert, H.; Johrendt, D.; Niehaus, O.; Eul, M.; Pöttgen, R. Phosphide Oxides RE2AuP2O (RE = La, Ce, Pr, Nd): Synthesis, Structure, Chemical Bonding, Magnetism, and 31P and 139La Solid State NMR. Inorg. Chem. 2013, 52, 2094–2102. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dolyniuk, J.; Kovnir, K. Unconventional Clathrates with Transition Metal-Phosphorus Frameworks. Acc. Chem. Res. 2018, 51, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Pfannenschmidt, U.; Behrends, F.; Lincke, H.; Eul, M.; Schäfer, K.; Eckert, H.; Pöttgen, R. CaBe2Ge2 Type Phosphides REIr2P2 (RE = La-Nd, Sm) and Arsenides REIr2As2 (RE = La-Nd): Synthesis, Structure, and Solid State NMR Spectroscopy. Dalton Trans. 2012, 41, 14188–14196. [Google Scholar] [CrossRef] [PubMed]
- Eisenmann, B.; Röβler, U. A Polyphosphide Of Unusual Composition: The Crystal Structure of Ba5P9. Z. Anorg. Allg. Chem. 2003, 629, 459–462. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, L.P.; Yamanaka, S. High-Pressure Synthesis and Structural Characterization of Three New Polyphosphides, α-SrP3, BaP8, and LaP5. J. Solid State Chem. 2003, 173, 449–455. [Google Scholar] [CrossRef]
- Ivanov, A.S.; Morris, A.J.; Bozhenko, K.V.; Pickard, C.J.; Boldyrev, A.I. Inorganic Double-Helix Structures of Unusually Simple Lithium-Phosphorus Species. Angew. Chem. Int. Ed. 2012, 51, 8330–8333. [Google Scholar] [CrossRef]
- Wang, J.; Lebedev, O.I.; Lee, K.; Dolyniuk, J.; Klavins, P.; Bux, S.; Kovnir, K. A high-efficiency thermoelectric Ba8Cu14Ge6P26: Bridging the gap between tetrel-based and tetrel-free clathrates. Chem. Sci. 2017, 8, 8030–8038. [Google Scholar] [CrossRef]
- Dahlmann, W.; von Schnering, H.G. CaP3, A New Calcium Phosphide. Naturwissenschaften 1973, 60, 518. [Google Scholar] [CrossRef]
- Bauhofer, W.; Gmelin, E.; Mollendorf, M.; Nesper, R.; von Schnering, H.G. Electrical and Magnetic Properties of Single Crystalline EuAs3, β-EuP3 and Their Mixed Crystals. J. Phys. C Solid State Phys. 1985, 18, 3017–3035. [Google Scholar] [CrossRef]
- Chattopadhyay, T.; Voiron, J.; Bartholin, H. Magnetization investigations of the magnetic (H, T) phase diagrams of EuAs3, Eu(As1−xPx)3 and β-EuP3: Effects of hydrostatic pressure up to 15 kbar. J. Magn. Magn. Mater. 1988, 72, 35–44. [Google Scholar] [CrossRef]
- Kraus, F.; Hanauer, T.; Korber, N. Chemical Bond in the Cyclic Anions P64− and As64−: Synthesis, Crystal Structure, and Electron Localization Function of {Rb[18]crown-6)}2-Rb2As6-6NH3. Angew. Chem. Int. Ed. 2005, 44, 7200–7204. [Google Scholar] [CrossRef]
- Ystenes, M. Quantum Chemical Vibrational Analysis of Clusters Providing an Explanation for the Planarity of the P64− Ring. J. Mol. Struct. THEOCHEM 1996, 369, 23–28. [Google Scholar] [CrossRef]
- Scherer, O.J.; Sitzmann, H.; Wolmershauser, G. Hexaphosphabenzene as Complex Ligand. Angew. Chem. Int. Ed. 1985, 24, 351–353. [Google Scholar] [CrossRef]
- Kauzlarich, S.M. (Ed.) Chemistry, Structure and Bonding of Zintl Phases and Ions; VCH: New York, NY, USA, 1996. [Google Scholar]
- Miller, G.J.; Schmidt, M.W.; Wang, F.; You, T.-S. Struct. Bonding; Springer: Berlin, Germany, 2011; Volume 139, pp. 1–55. [Google Scholar]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. A 2008, A64, 112–122. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).