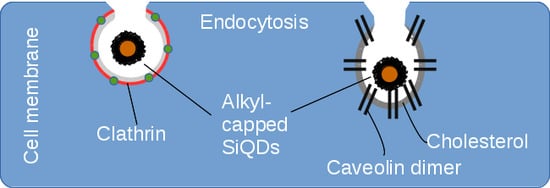
Endocytosis and Lack of Cytotoxicity of Alkyl-Capped Silicon Quantum Dots Prepared from Porous Silicon
Abstract
1. Introduction
2. Results and Discussion
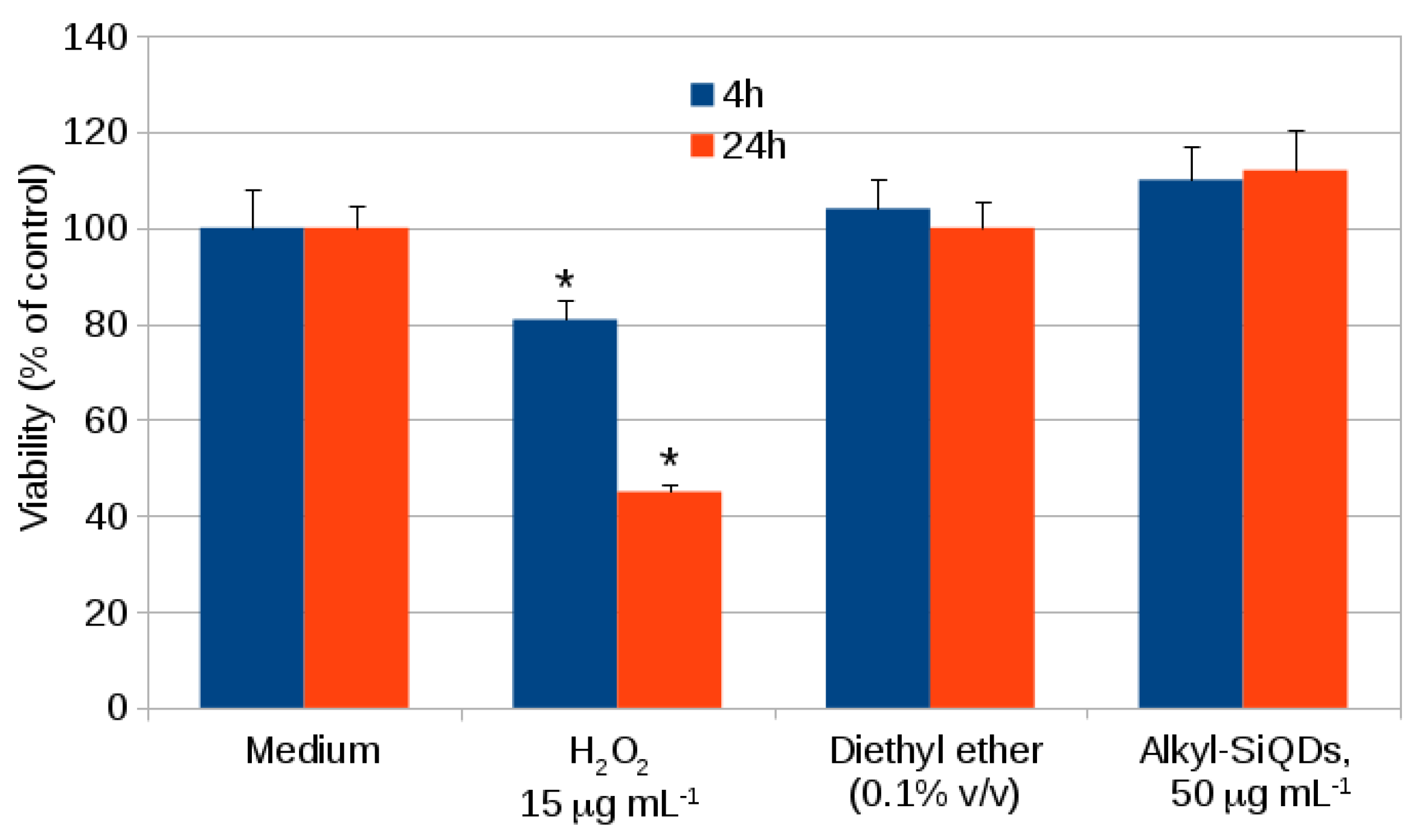
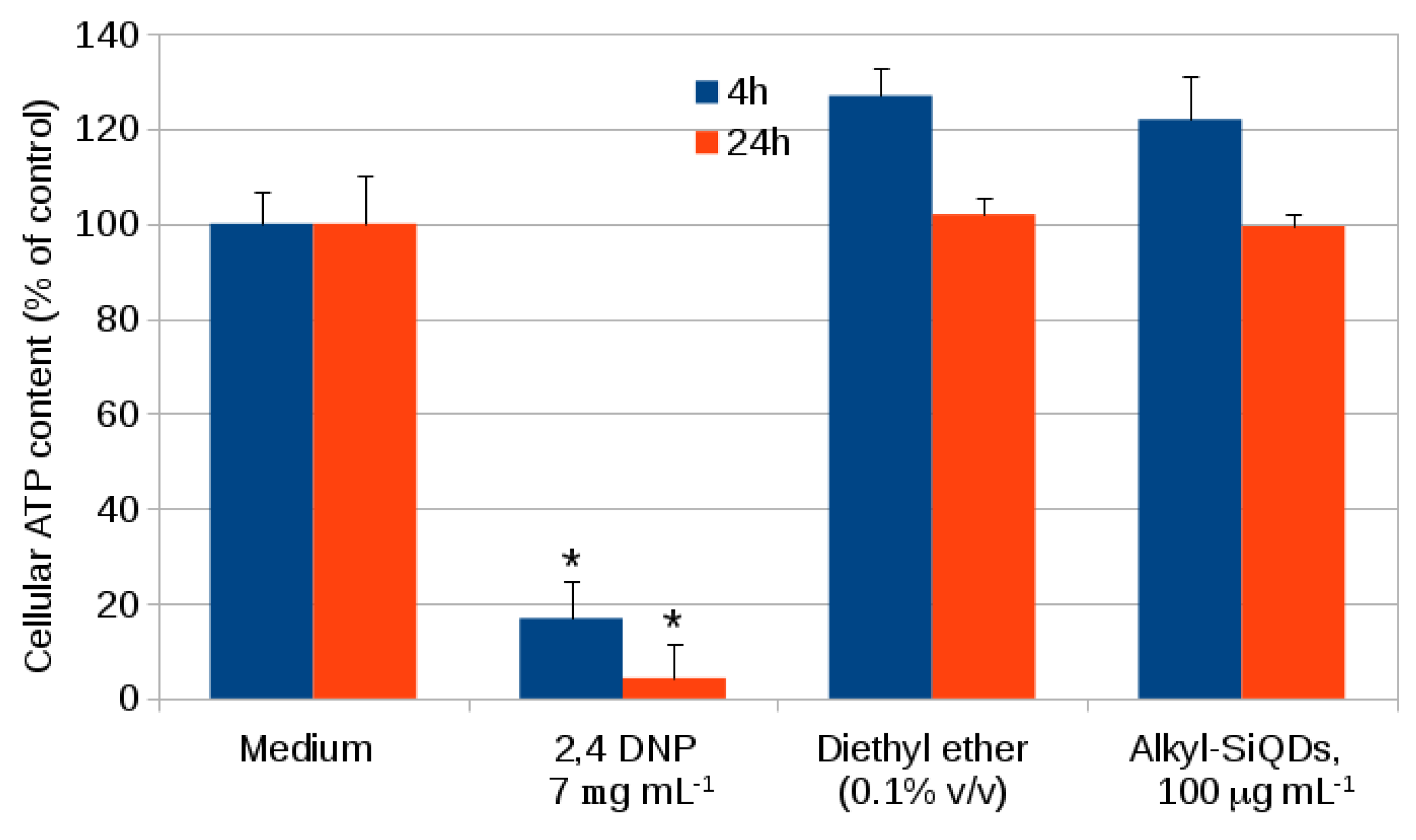
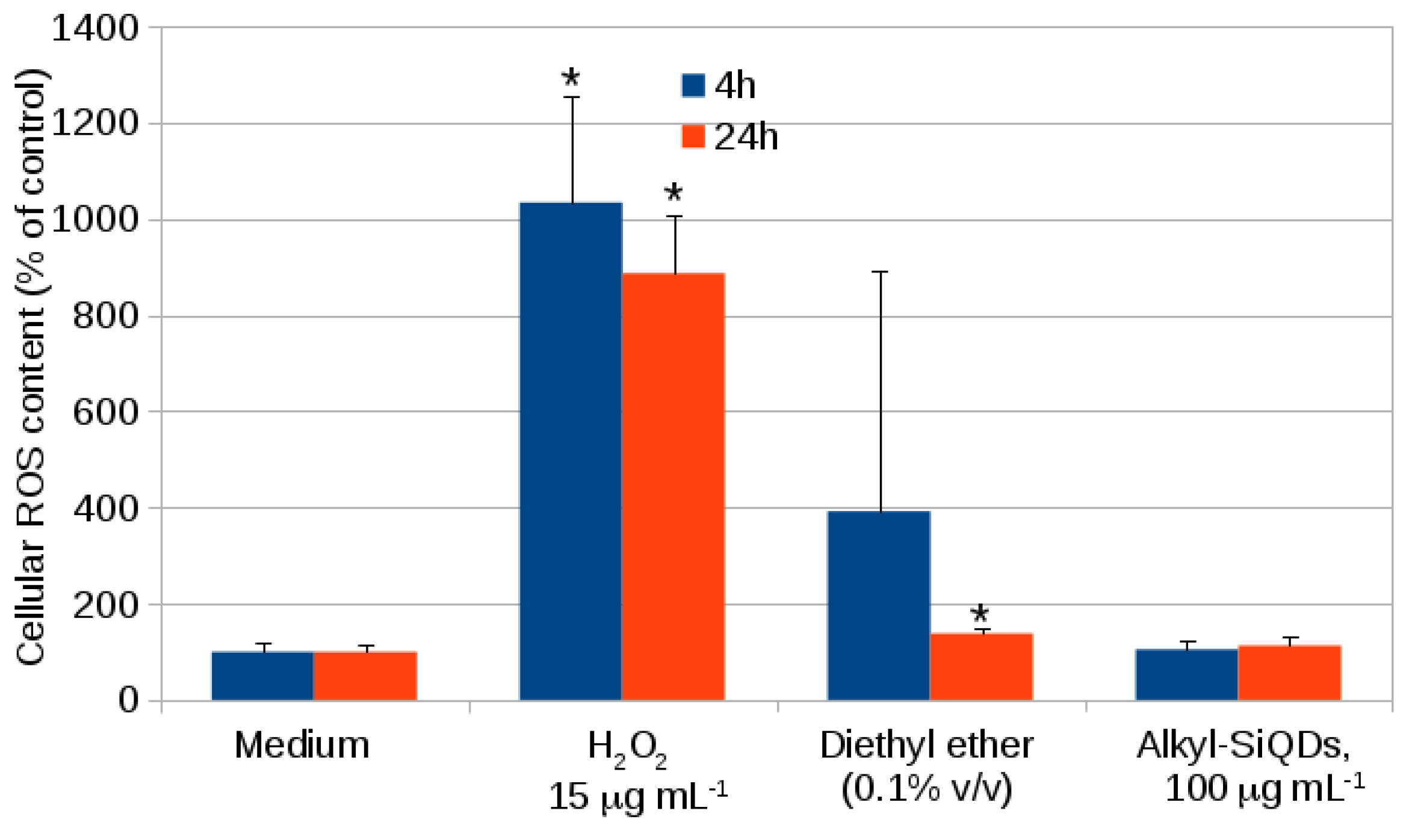
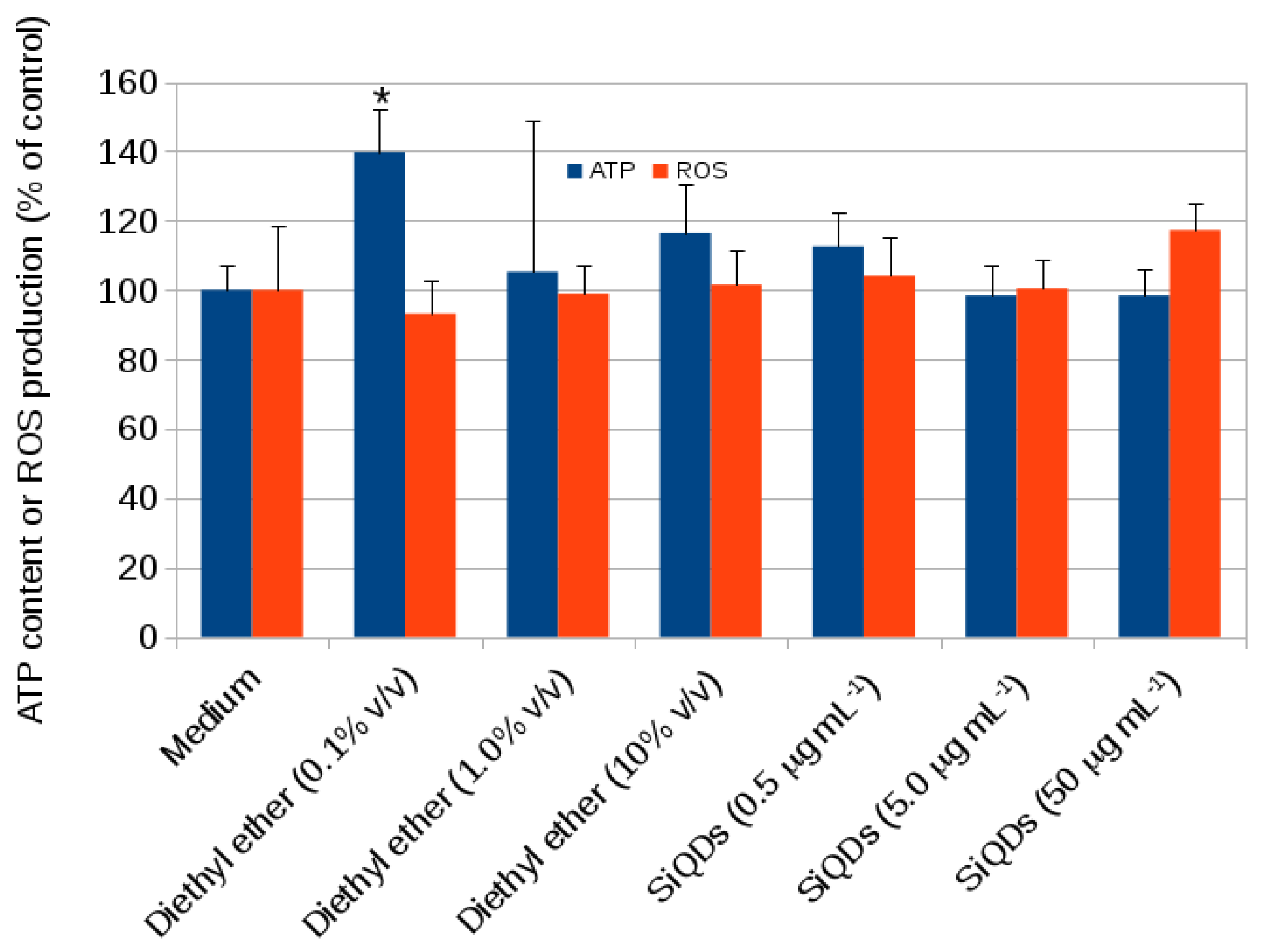
2.1. Cytotoxicity
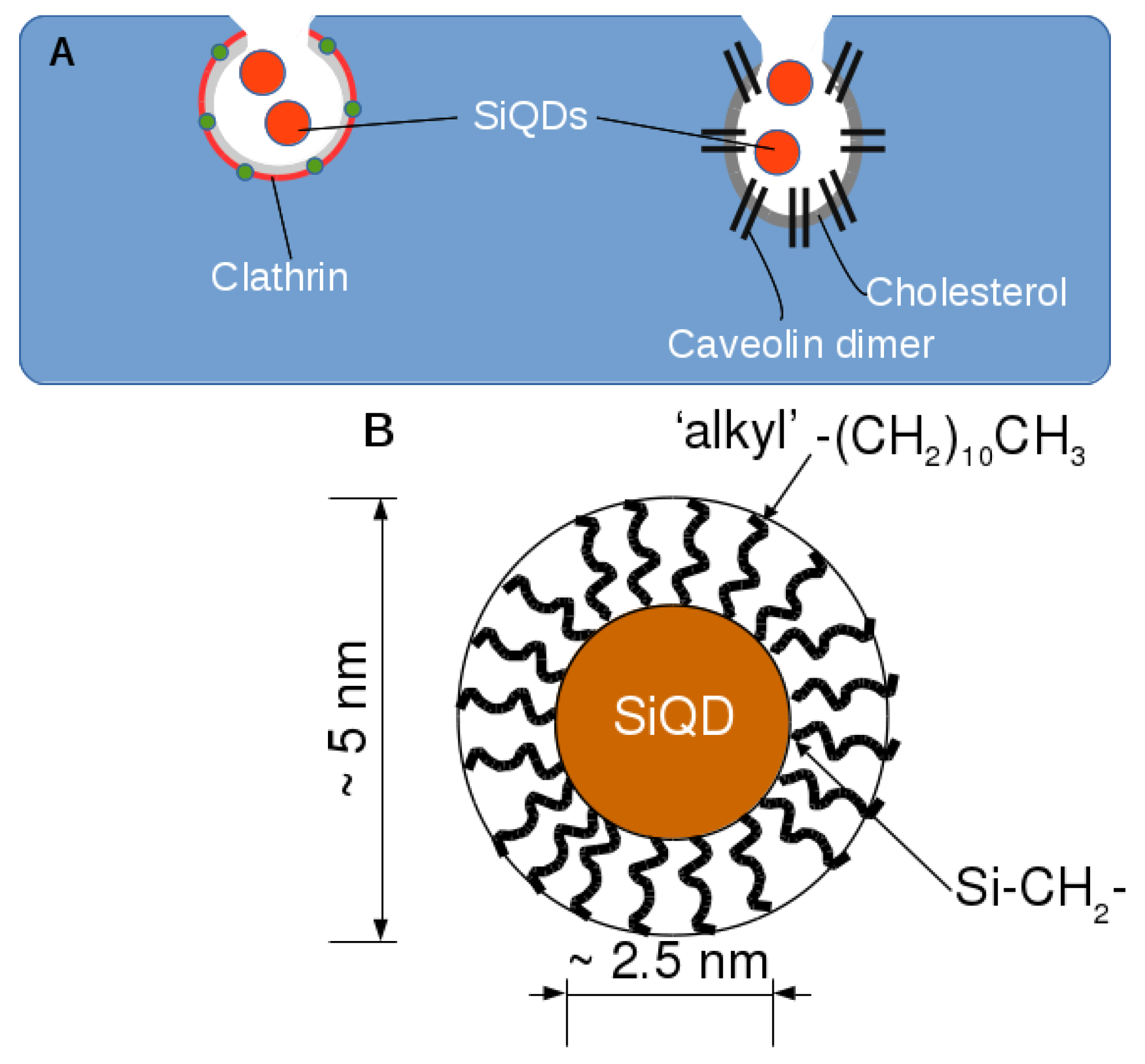
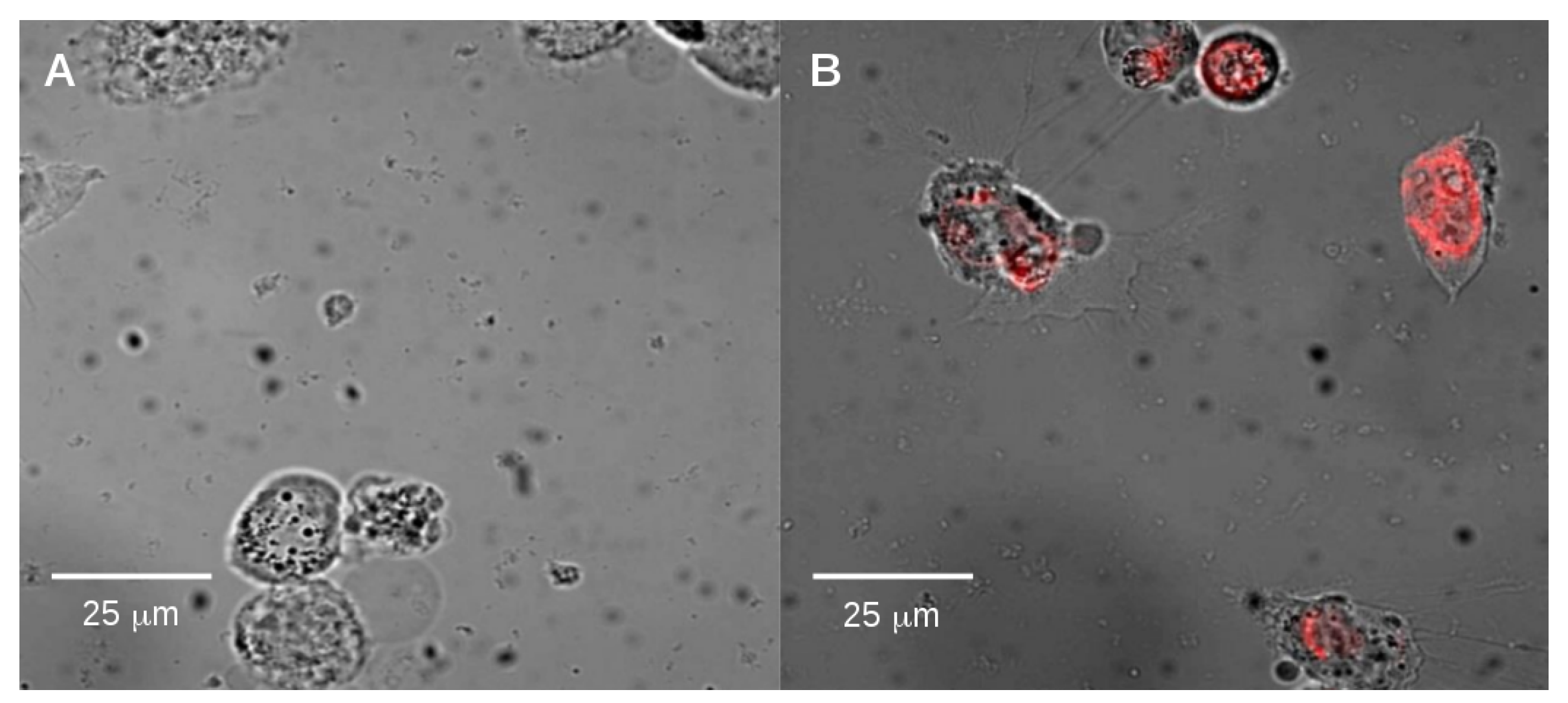
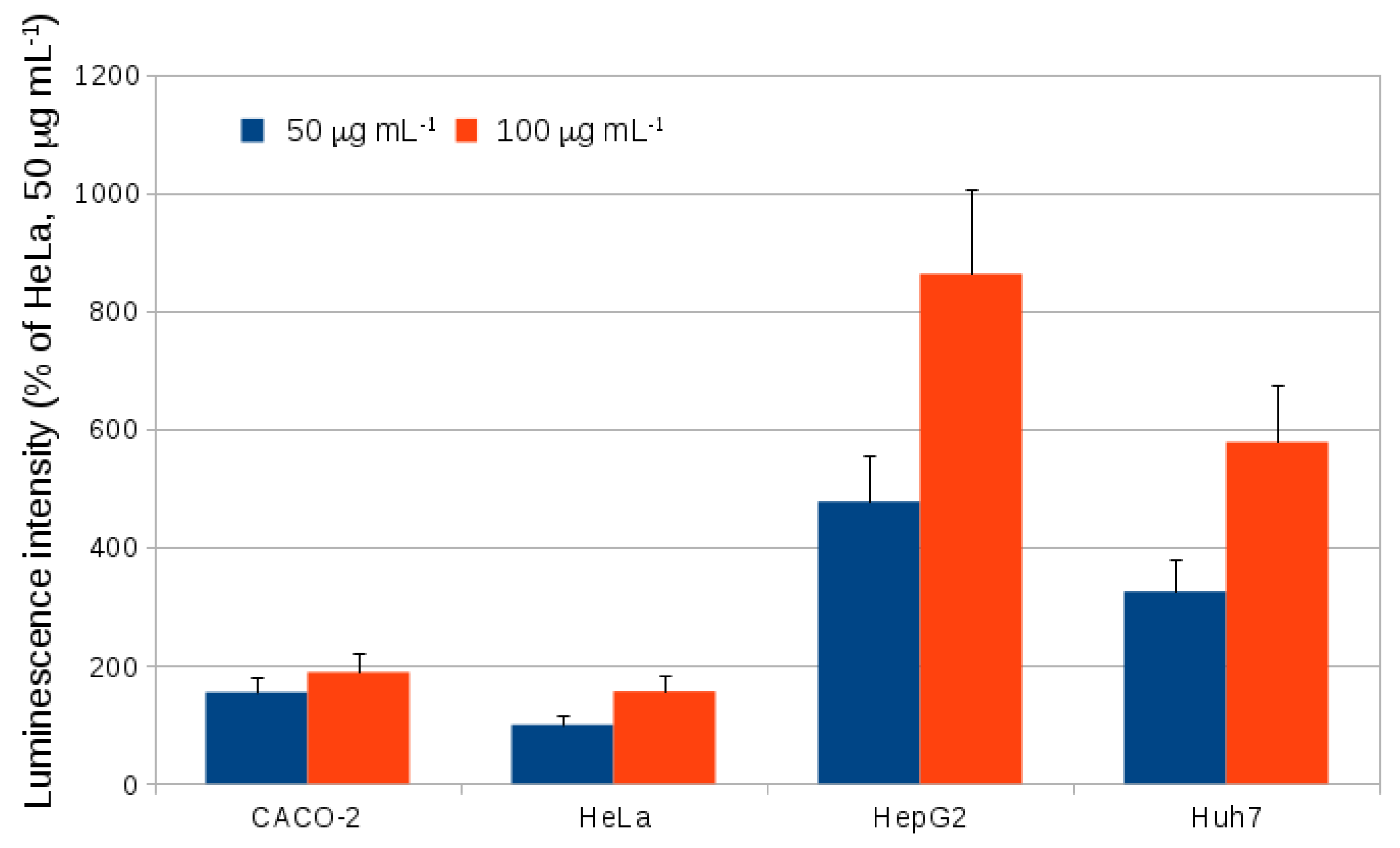
2.2. Endocytosis of Alkyl SiQDs
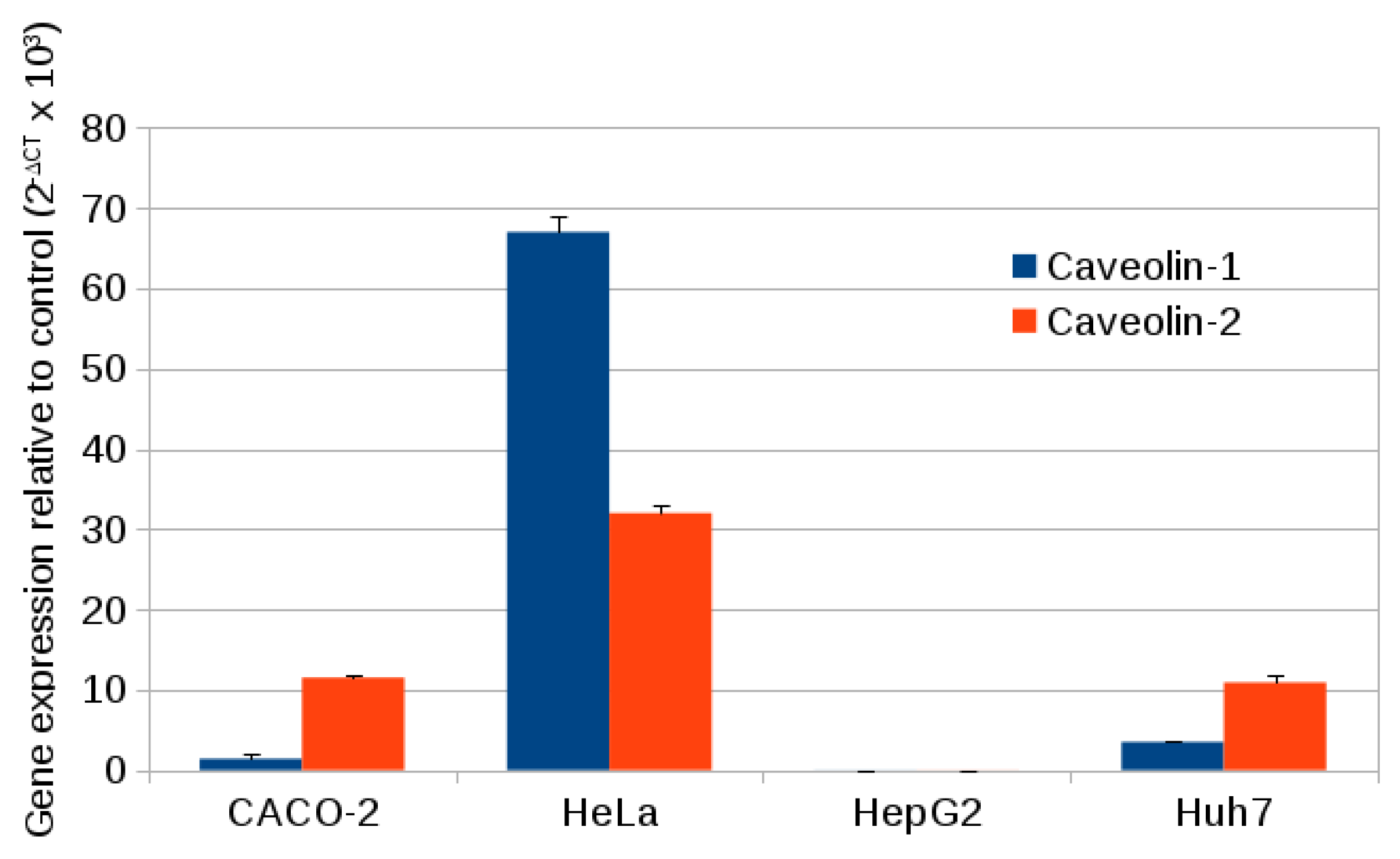
2.3. Caveolin Gene Expression
2.4. Chlorpromazine Inhibition
3. Materials and Methods
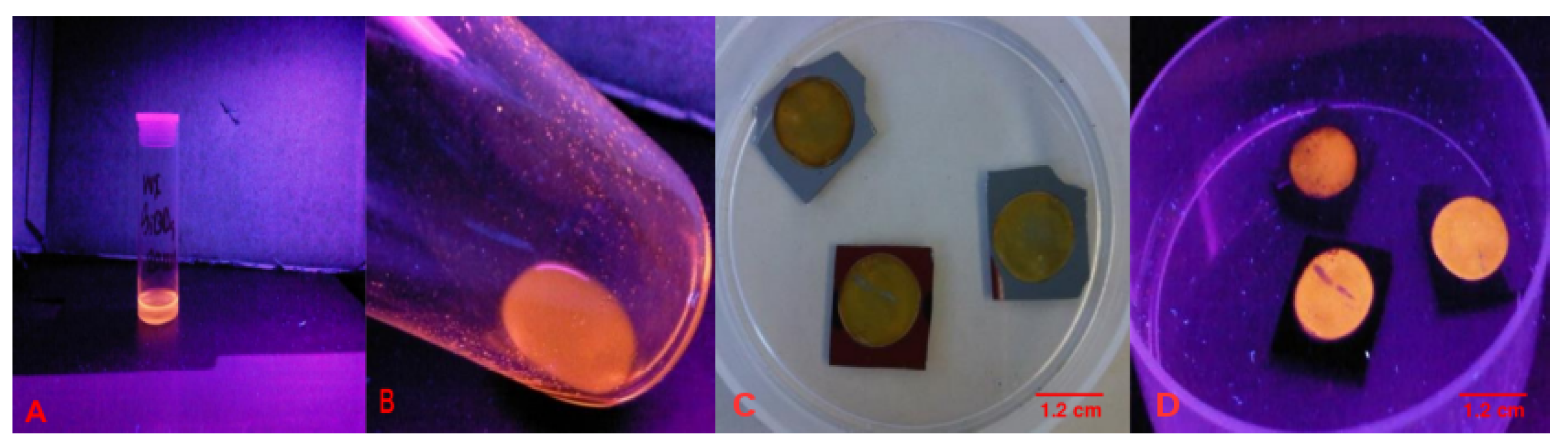
3.1. Preparation of Alkyl-SiQDs and Dispersions in Aqueous Media
3.2. CACO-2 Cell Culture
3.3. Optical and Epifluorescence Microscopy
3.4. Observation of Internalization of SiQDs by Epifluorescence Microscopy
3.5. CACO-2 Cell Viability (MTT Assay)
3.6. CACO-2 Intracellular ATP Content (FLASC Assay)
3.7. CACO-2 Oxidative Stress (H2DCFDA Assay)
3.8. CACO-2 DNA Damage (Comet Assay)
3.9. CACO-2 Chronic Exposure to Alkyl SiQDs
3.10. Internalization of SiQDs in Different Cell Lines
3.11. Specific Gene Expression of Caveolin 1 and Caveolin 2
3.11.1. RNA Preparation
3.11.2. cDNA Synthesis
3.11.3. Quantitative Reverse Transcriptase Real Time PCR Analysis
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Smith, A.M.; Gao, X.H.; Nie, S.M. Quantum dot nanocrystals for in vivo molecular and cellular imaging. Photochem. Photobiol. 2004, 80, 377–385. [Google Scholar] [CrossRef]
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum Dots for Live Cells, in Vivo Imaging, and Diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.C.; Maxwell, D.J.; Gao, X.; Bailey, R.E.; Han, M.; Nie, S. Luminescent quantum dots for multiplexed biological detection and imaging. Curr. Opin. Biotechnol. 2002, 13, 40–46. [Google Scholar] [CrossRef]
- Derfus, A.M.; Chan, W.C.W.; Bhatia, S.N. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 2004, 4, 11–18. [Google Scholar] [CrossRef]
- Kirchner, C.; Liedl, T.; Kudera, S.; Pellegrino, T.; Muñoz Javier, A.; Gaub, H.E.; Stölzle, S.; Fertig, N.; Parak, W.J. Cytotoxicity of colloidal CdSe and CdSe/ZnS nanoparticles. Nano Lett. 2005, 5, 331–338. [Google Scholar] [CrossRef]
- O’Farrell, N.; Houlton, A.; Horrocks, B.R. Silicon nanoparticles: Applications in cell biology and medicine. Int. J. Nanomed. 2006, 1, 451–472. [Google Scholar] [CrossRef]
- Das, A.; Snee, P.T. Synthetic Developments of Nontoxic Quantum Dots. ChemPhysChem 2016, 17, 598–617. [Google Scholar] [CrossRef]
- Park, J.-H.; Gu, L.; von Maltzahn, G.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat. Mat. 2009, 8, 331–336. [Google Scholar] [CrossRef]
- Coffer, J.L.; Whitehead, M.A.; Nagesha, D.K.; Mukherjee, P.; Akkaraju, G.; Totolici, M.; Saffie, R.S.; Canham, L.T. Porous silicon-based scaffolds for tissue engineering and other biomedical applications. Phys. Status Solidi A 2005, 202, 1451–1455. [Google Scholar] [CrossRef]
- Buriak, J.M. Organometallic chemistry on silicon and germanium surfaces. Chem. Rev. 2002, 102, 1271–1308. [Google Scholar] [CrossRef]
- Yu, Y.; Fan, G.; Fermi, A.; Mazzaro, R.; Morandi, V.; Smilgies, D.-M.; Korgel, B.A. Size-Dependent Photoluminescence Efficiency of Silicon Nanocrystal Quantum Dots. J. Phys. Chem. C 2017, 121, 23240–23248. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Swihart, M.T. Surface Functionalization of Silicon Nanoparticles Produced by Laser-Driven Pyrolysis of Silane followed by HF-HNO3 Etching. Langmuir 2004, 20, 4720–4727. [Google Scholar] [CrossRef]
- Clark, R.J.; Aghajamali, M.; Gonzalez, C.M.; Hadidi, L.; Islam, M.A.; Javadi, M.; Hosnay Mobarak, M.; Purkait, T.K.; Robidillo, C.J.T.; Sinelnikov, R.; et al. From Hydrogen Silsesquioxane to Functionalized Silicon Nanocrystals. Chem. Mater. 2017, 29, 80–89. [Google Scholar] [CrossRef]
- Lie, L.H.; Duerdin, M.; Tuite, E.M.; Houlton, A.; Horrocks, B.R. Preparation and characterisation of luminescent alkylated-silicon quantum dots. J. Electroanal. Chem. 2002, 538/539, 183–190. [Google Scholar] [CrossRef]
- Chao, Y.; Siller, L.; Krishnamurthy, S.; Coxon, P.R.; Bangert, U.; Gass, M.; Kjeldgaard, L.; Patole, S.N.; Lie, L.H.; O’Farrell, N.; et al. Evaporation and deposition of alkyl-capped silicon nanocrystals in ultrahigh vacuum. Nat. Nanotechnol. 2007, 2, 486–489. [Google Scholar] [CrossRef]
- Dickinson, F.M.; Alsop, T.A.; Al-Sharif, N.; Berger, C.E.M.; Datta, H.K.; Siller, L.; Chao, Y.; Tuite, E.M.; Houlton, A.; Horrocks, B.R. Dispersions of alkyl-capped silicon nanocrystals in aqueous media: Photoluminescence and ageing. Analyst 2008, 133, 1573–1580. [Google Scholar] [CrossRef]
- Zidek, K.; Pelant, I.; Trojanek, F.; Maly, P.; Gilliot, P.; Honerlage, B.; Oberle, J.; Siller, L.; Little, R.; Horrocks, B.R. Ultrafast stimulated emission due to quasidirect transitions in silicon nanocrystals. Phys. Rev. B 2011, 84, 085321. [Google Scholar] [CrossRef]
- Rostron, R.J.; Horrocks, B.R.; Roberts, G. Distributed luminescence from alkyl-capped silicon quantum dots. J. Appl. Phys. 2009, 105, 094302. [Google Scholar] [CrossRef]
- Siller, L.; Krishnamurthy, S.; Kjeldgaard, L.; Horrocks, B.R.; Chao, Y.; Houlton, A.; Chakraborty, A.K.; Hunt, M.R.C. Core and valence exciton formation in X-ray absorption, X-ray emission and X-ray excited optical luminescence from passivated Si nanocrystals at the Si L2,3 edge. J. Phys. Condens. Matter 2009, 21, 095005. [Google Scholar] [CrossRef]
- Al-Sharif, N.H.; Berger, C.E.M.; Varanasi, S.S.; Chao, Y.; Horrocks, B.R.; Datta, H.K. Alkyl–Capped Silicon Nanocrystals Lack Cytotoxicity and have Enhanced Intracellular Accumulation in Malignant Cells via Cholesterol-Dependent Endocytosis. Small 2009, 5, 221–228. [Google Scholar] [CrossRef]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; de Haan, L.H.J.; Evers, N.M.; Jiang, X.; Marcelis, A.T.M.; Zuilhof, H.; Rietjens, I.M.C.M.; Alink, G.M. Role of surface charge and oxidative stress in cytotoxicity of organic monolayer-coated silicon nanoparticles towards macrophage NR8383 cells. Part. Fibre Toxicol. 2010, 7, 1–12. [Google Scholar] [CrossRef]
- Shiohara, A.; Hanada, S.; Prabakar, S.; Fujioka, K.; Lim, T.H.; Yamamoto, K.; Northcote, P.T.; Tilley, R.D. Chemical reactions on surface molecules attached to silicon quantum dots. J. Am. Chem. Soc. 2010, 132, 248–253. [Google Scholar] [CrossRef]
- Wang, Q.; Bao, Y.; Zhang, X.; Coxon, P.R.; Jayasooriya, U.A.; Chao, Y. Uptake and Toxicity Studies of Poly-Acrylic Acid Functionalized Silicon Nanoparticles in Cultured Mammalian Cells. Adv. Healthc. Mater. 2012, 1, 189–198. [Google Scholar] [CrossRef]
- Zhi, B.; Mishra, S.; Hudson-Smith, N.V.; Kortshagen, U.R.; Haynes, C.L. Toxicity Evaluation of Boron- and Phosphorus-Doped Silicon Nanocrystals toward Shewanella oneidensis MR-1. ACS Appl. Nano Mater. 2018, 1, 4884–4893. [Google Scholar] [CrossRef]
- Singh, N.; Manshian, B.; Jenkins, G.J.S.; Griffiths, S.M.; Williams, P.M.; Maffeis, T.G.G.; Wright, C.J.; Doak, S.H. NanoGenotoxicology: The DNA damaging potential of engineered nanomaterials. Biomaterials 2009, 30, 3891–3914. [Google Scholar] [CrossRef]
- Choi, J.; Zhang, Q.; Reipa, V.; Wang, N.S.; Stratmeyer, M.E.; Hitchins, V.M.; Goering, P.L. Comparison of cytotoxic and inflammatory responses of photoluminescent silicon nanoparticles with silicon micron-sized particles in RAW 264.7 macrophages. J. Appl. Toxicol. 2009, 29, 52–60. [Google Scholar] [CrossRef]
- Fischer, H.C.; Chan, W.C.W. Nanotoxicity: The growing need for in vivo study. Curr. Opin. Biotechnol. 2007, 18, 565–571. [Google Scholar] [CrossRef]
- Santos, H.A.; Bimbo, L.M.; Herranz, B.; Shahbazi, M.-A.; Hirvonen, J.; Salonen, J. Nanostructured porous silicon in preclinical imaging: Moving from bench to bedside. J. Mater. Res. 2013, 28, 152–164. [Google Scholar] [CrossRef]
- Doherty, G.J.; McMahon, H.T. Mechanisms of Endocytosis. Annu. Rev. Biochem. 2009, 78, 857–902. [Google Scholar] [CrossRef]
- Pinto, M.; Robine-Leon, S.; Appay, M.D.; Kedinger, M.; Triadou, N.; Dussaulx, E.; Lacroix, B.; Simon-Assmann, P.; Haffen, K.; Fogh, J.; et al. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol. Cell 1983, 47, 323–330. [Google Scholar]
- Hidalgo, I.J.; Raub, T.J.; Borchardt, R.T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 1989, 96, 736–749. [Google Scholar] [CrossRef]
- Ahire, J.H.; Wang, Q.; Coxon, P.R.; Malhotra, G.; Brydson, R.; Chen, R.; Chao, Y. Highly Luminescent and Nontoxic Amine-Capped Nanoparticles from Porous Silicon: Synthesis and Their Use in Biomedical Imaging. ACS Appl. Mater. Interfaces 2012, 4, 3285–3292. [Google Scholar] [CrossRef]
- Gongalsky, M.B.; Osminkina, L.A.; Pereira, A.; Manankov, A.A.; Fedorenko, A.A.; Vasiliev, A.N.; Solovyen, V.V.; Kudryavtsev, A.A.; Sentis, M.; Kabashin, A.V.; et al. Laser-synthesized oxide-passivated bright Si quantum dots for bioimaging. Sci. Rep. 2016, 6, 24372. [Google Scholar] [CrossRef]
- Singh, S.; Bhatta, U.M.; Satyam, P.V.; Dhawan, A.; Sastry, M.; Prasad, B.L.V. Bacterial synthesis of silicon/silica nanocomposites. J. Mater. Chem. 2008, 18, 2601–2606. [Google Scholar] [CrossRef]
- Geng, X.; Li, Z.; Hu, Y.; Liu, H.; Sun, Y.; Meng, H.; Wang, Y.; Qu, L.; Lin, Y. One-Pot Green Synthesis of Ultrabright N-Doped Fluorescent Silicon Nanoparticles for Cellular Imaging by Using Ethylenediaminetetraacetic Acid Disodium Salt as an Effective Reductant. ACS Appl. Mater. Interfaces 2018, 10, 27979–27986. [Google Scholar] [CrossRef]
- Jensen, S.E.; Hubrechts, P.; Klein, B.M.; Haslov, K.R. Development and validation of an ATP method for rapid estimation of viable units in lyophilised BCG Danish 1331 vaccine. Biologicals 2008, 36, 308–314. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Liong, M.; Maedler, L.; Gilbert, B.; Shi, H.; Yeh, J.I.; Zink, J.I.; Nel, A.E. Comparison of the Mechanism of Toxicity of Zinc Oxide and Cerium Oxide Nanoparticles Based on Dissolution and Oxidative Stress Properties. ACS Nano 2008, 2, 2121–2134. [Google Scholar] [CrossRef]
- Sayes, C.M.; Reed, K.L.; Warheit, D.B. Assessing toxicity of fine and nanoparticles: Comparing in vitro measurements to in vivo pulmonary toxicity profiles. Toxicol. Sci. 2007, 97, 163–180. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Brant, J.; Hotze, M.; Sempf, J.; Oberley, T.; Sioutas, C.; Yeh, J.I.; Wiesner, M.R.; Nel, A.E. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006, 6, 1794–1807. [Google Scholar] [CrossRef]
- Nel, A.E.; Xia, T.; Madler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Scott, B.L.; Sochacki, K.A.; Low-Nam, S.T.; Bailey, E.M.; Luu, Q.; Hor, A.; Dickey, A.M.; Smith, S.; Kerkvliet, J.G.; Taraska, J.W.; et al. Membrane bending occurs at all stages of clathrin-coat assembly and defines endocytic dynamics. Nat. Commun. 2018, 9, 419. [Google Scholar] [CrossRef]
- Sigismund, S.; Confalonieri, S.; Ciliberto, A.; Polo, S.; Scita, G.; Di Fiore, P.P. Endocytosis and signaling: Cell logistics shape the eukaryotic cell plan. Physiol. Rev. 2012, 92, 273–366. [Google Scholar] [CrossRef]
- Conner, S.D.; Schmid, S.L. Regulated portals of entry into the cell. Nature 2003, 422, 37–44. [Google Scholar] [CrossRef]
- McMahon, H.T.; Boucrot, E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2011, 12, 517–533. [Google Scholar] [CrossRef]
- Cohen, A.W.; Hnasko, R.; Schubert, W.; Lisanti, M.P. Role of caveolae and caveolins in health and disease. Physiol. Rev. 2004, 84, 1341–1379. [Google Scholar] [CrossRef]
- Nabi, I.R.; Le, P.U. Caveolae/raft-dependent endocytosis. J. Cell Biol. 2003, 161, 673–677. [Google Scholar] [CrossRef]
- Le, P.U.; Guay, G.; Altschuler, Y.; Nabi, I.R. Caveolin-1 is a negative regulator of caveolae-mediated endocytosis to the endoplasmic reticulum. J. Biol. Chem. 2002, 277, 3371–3379. [Google Scholar] [CrossRef]
- Tachikawa, M.; Morone, N.; Senju, Y.; Sugiura, T.; Hanawa-Suetsugu, K.; Mochizuki, A.; Suetsugu, S. Measurement of caveolin-1 densities in the cell membrane for quantification of caveolar deformation after exposure to hypotonic membrane tension. Sci. Rep. 2017, 7, 7794. [Google Scholar] [CrossRef]
- Rejman, J.; Oberle, V.; Zuhorn, I.S.; Hoekstra, D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem. J. 2004, 377, 159–169. [Google Scholar] [CrossRef]
- Vercauteren, D.; Vandenbroucke, R.E.; Jones, A.T.; Rejman, J.; Demeester, J.; De Smedt, S.C.; Sanders, N.N.; Braeckmans, K. The Use of Inhibitors to Study Endocytic Pathways of Gene Carriers: Optimization and Pitfalls. Mol. Ther. 2010, 18, 561–569. [Google Scholar] [CrossRef]
- Palozza, P.; Sestito, R.; Picci, N.; Lanza, P.; Monego, G.; Ranelletti, F.O. The sensitivity to beta-carotene growth-inhibitory and proapoptotic effects is regulated by caveolin-1 expression in human colon and prostate cancer cells. Carcinogenesis 2008, 29, 2153–2161. [Google Scholar] [CrossRef]
- Gai, X.; Lu, Z.; Tu, K.; Liang, Z.; Zheng, X. Caveolin-1 is up-regulated by GLI1 and contributes to GLI1-driven EMT in hepatocellular carcinoma. PLoS ONE 2014, 9, e84551. [Google Scholar] [CrossRef]
- Truong, T.Q.; Aubin, D.; Falstrault, L.; Brodeur, M.R.; Brissette, L. SR-BI, CD36, and caveolin-1 contribute positively to cholesterol efflux in hepatic cells. Cell Biochem. Funct. 2010, 28, 480–489. [Google Scholar] [CrossRef]
- Nagasawa, S.; Ogura, K.; Tsutsuki, H.; Saitoh, H.; Moss, J.; Iwase, H.; Noda, M.; Yahiro, K. Uptake of Shiga-toxigenic Escherichia coli SubAB by HeLa cells requires an actin- and lipid raft-dependent pathway. Cell Microbiol. 2014, 16, 1582–1601. [Google Scholar] [CrossRef][Green Version]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C-T method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
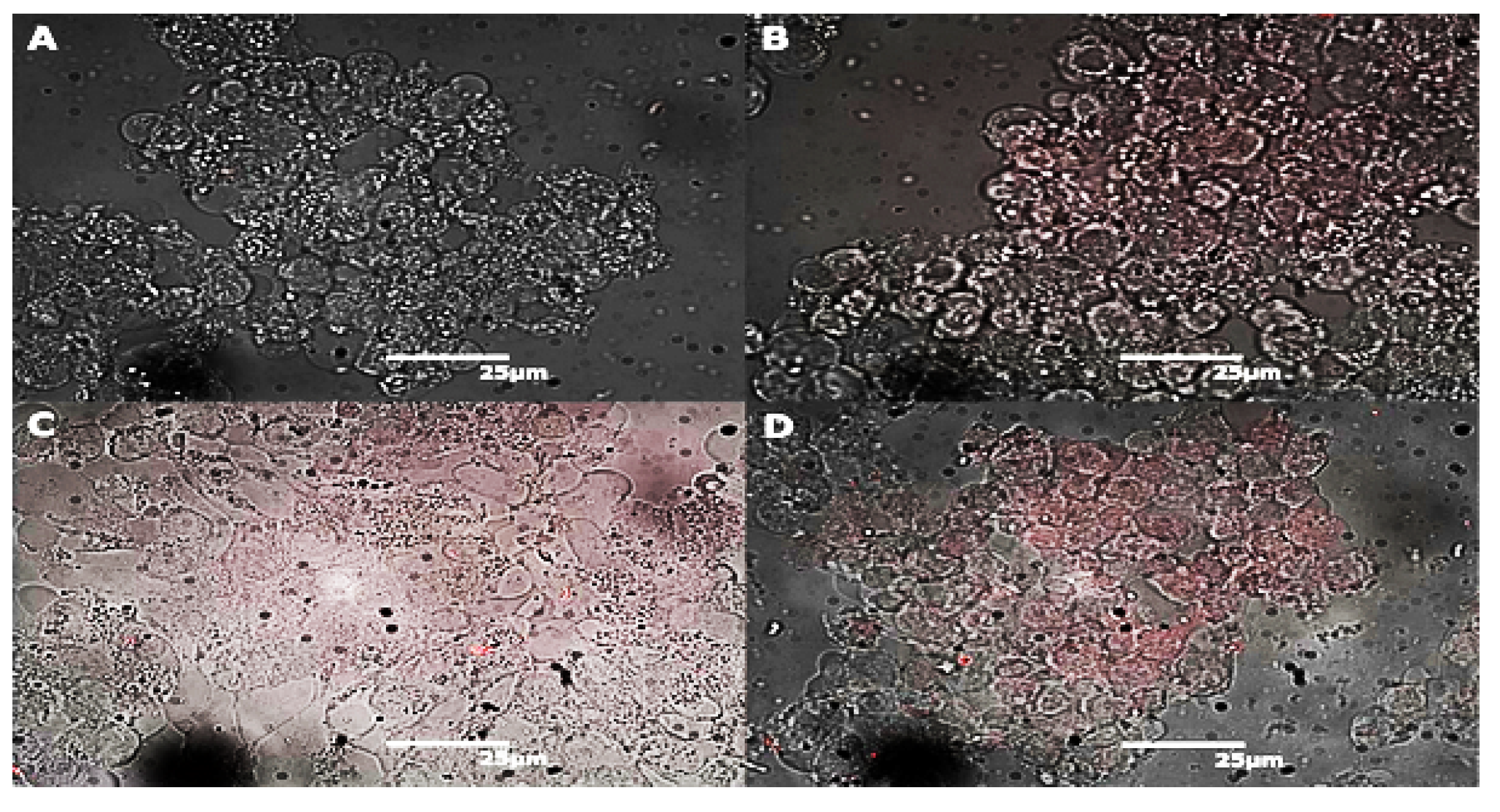
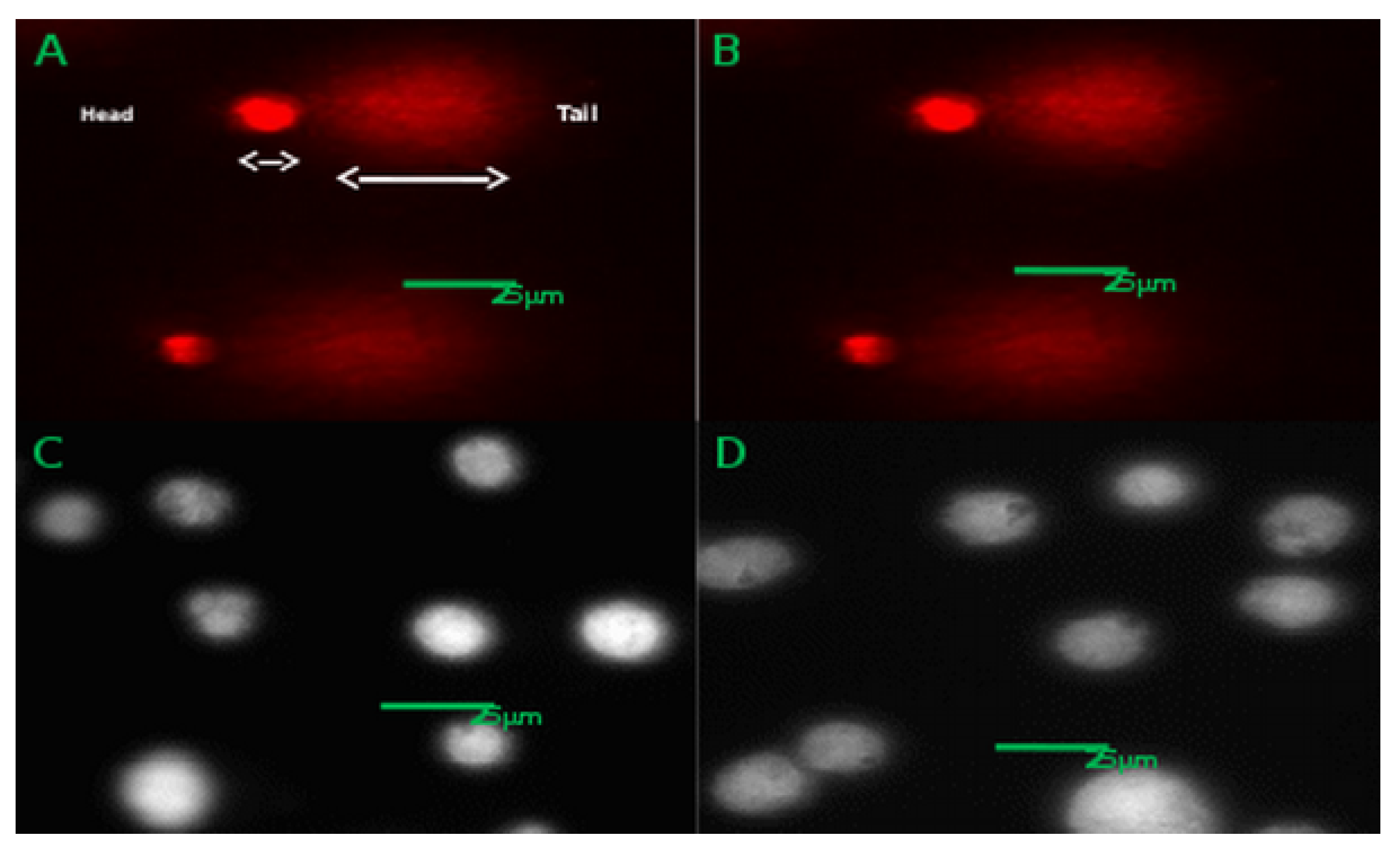
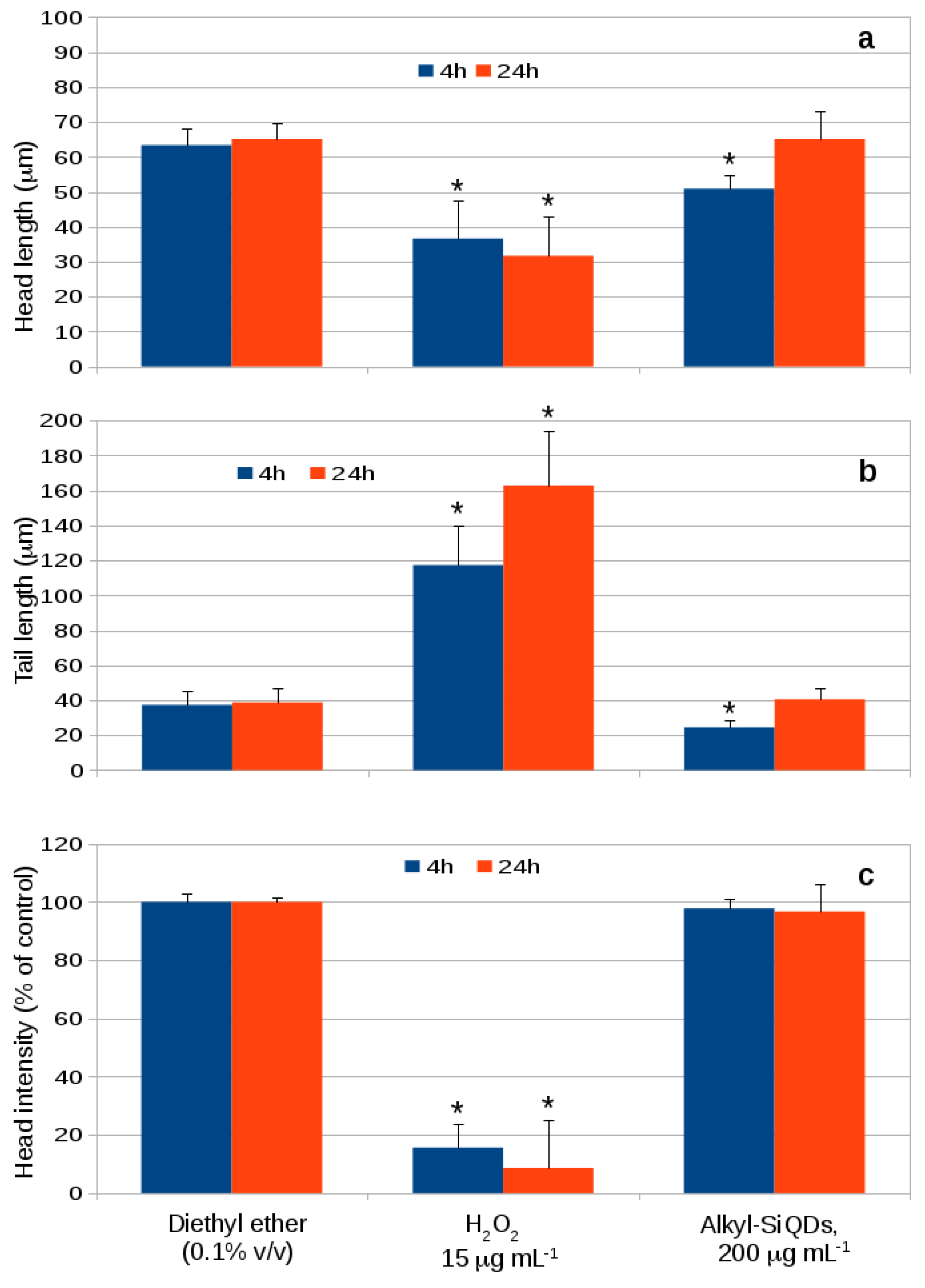
| Cell Line | Caveolin-1 | GAPDH | Caveolin-2 | GAPDH |
|---|---|---|---|---|
| CACO-2 | 27.03 ± 0.06 | 17.51 ± 0.02 | 23.99 ± 0.07 | 17.55 ± 0.03 |
| HeLa | 21.15 ± 0.08 | 17.24 ± 0.04 | 2.32 ± 0.06 | 17.33 ± 0.02 |
| HepG2 | 35.69 ± 0.31 | 17.65 ± 0.02 | 34.89 ± 0.43 | 17.66 ± 0.03 |
| Huh7 | 25.92 ± 0.05 | 17.77 ± 0.02 | 24.33 ± 0.17 | 17.81 ± 0.02 |
| Cell Line | Intensity as % of Control |
|---|---|
| HeLa | 94 ± 6.9 |
| Huh7 | 170 ± 26 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phatvej, W.; Datta, H.K.; Wilkinson, S.C.; Mutch, E.; Daly, A.K.; Horrocks, B.R. Endocytosis and Lack of Cytotoxicity of Alkyl-Capped Silicon Quantum Dots Prepared from Porous Silicon. Materials 2019, 12, 1702. https://doi.org/10.3390/ma12101702
Phatvej W, Datta HK, Wilkinson SC, Mutch E, Daly AK, Horrocks BR. Endocytosis and Lack of Cytotoxicity of Alkyl-Capped Silicon Quantum Dots Prepared from Porous Silicon. Materials. 2019; 12(10):1702. https://doi.org/10.3390/ma12101702
Chicago/Turabian StylePhatvej, Wipaporn, Harish K. Datta, Simon C. Wilkinson, Elaine Mutch, Ann K. Daly, and Benjamin R. Horrocks. 2019. "Endocytosis and Lack of Cytotoxicity of Alkyl-Capped Silicon Quantum Dots Prepared from Porous Silicon" Materials 12, no. 10: 1702. https://doi.org/10.3390/ma12101702
APA StylePhatvej, W., Datta, H. K., Wilkinson, S. C., Mutch, E., Daly, A. K., & Horrocks, B. R. (2019). Endocytosis and Lack of Cytotoxicity of Alkyl-Capped Silicon Quantum Dots Prepared from Porous Silicon. Materials, 12(10), 1702. https://doi.org/10.3390/ma12101702