Preclinical Studies of the Biosafety and Efficacy of Human Bone Marrow Mesenchymal Stem Cells Pre-Seeded into β-TCP Scaffolds after Transplantation
Abstract
1. Introduction
2. Material and Methods
2.1. Isolation and Culture of Bone Marrow-Derived hBMMSCs
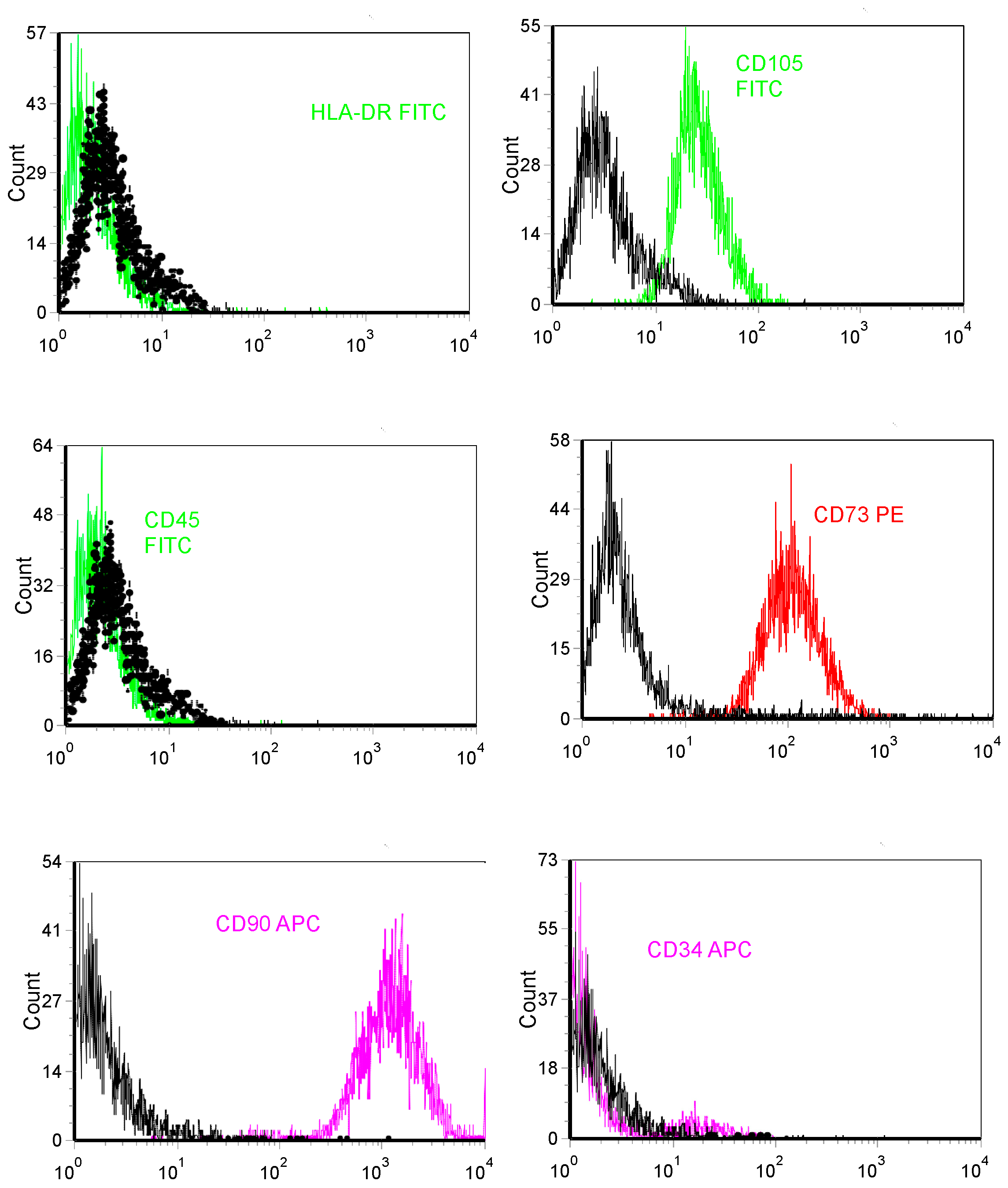
2.2. Immunophenotypic Profiles of hBMMSC Cultures
2.3. Human Bone Marrow-Derived Mesenchymal Stem Cells (hBMMSCs) Seeded into Scaffold (hBMMSCs/ β-TCP) Constructs Preparation
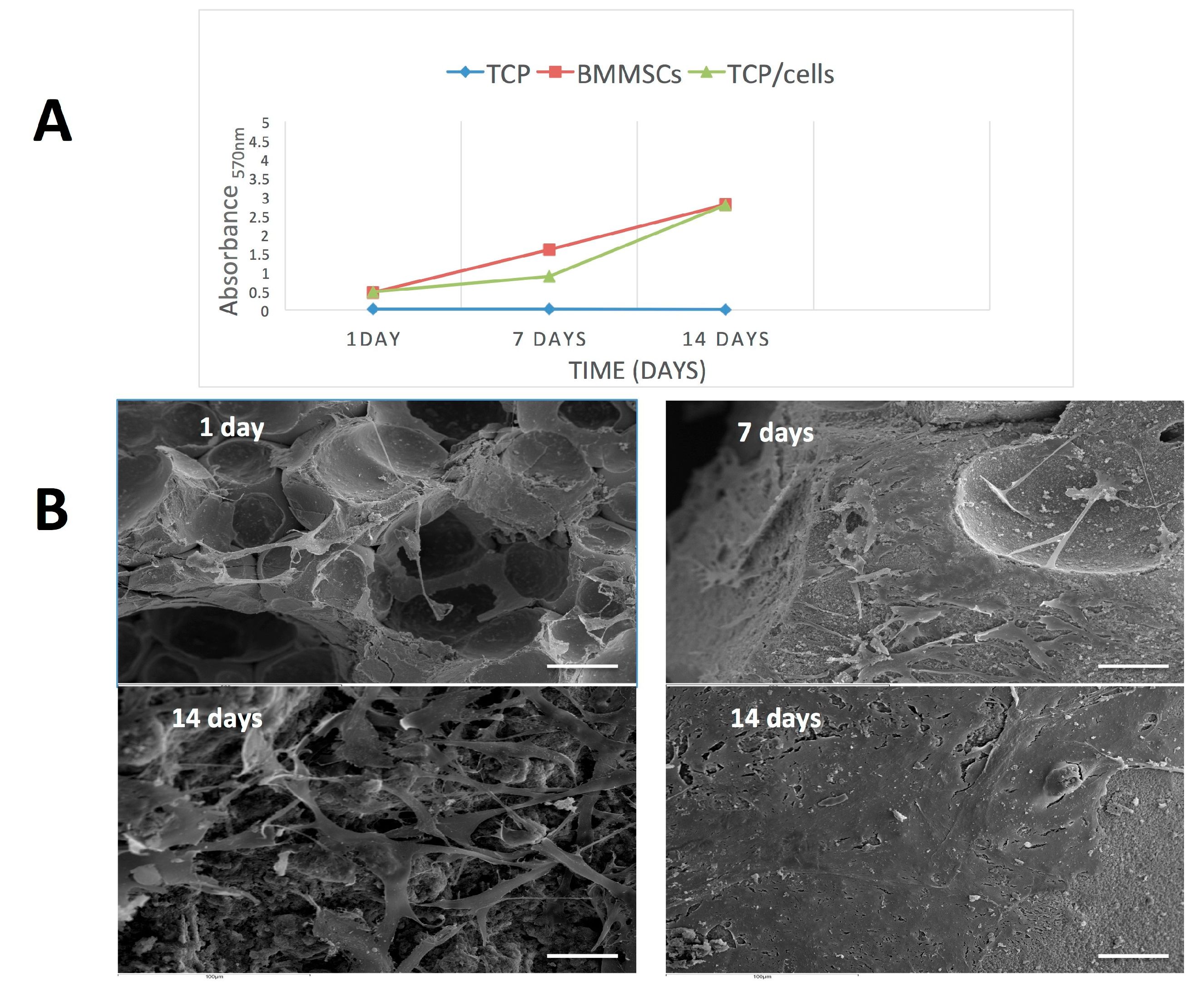
2.4. Cell Viability Assay
2.5. Scanning Electron Microscopy (SEM) Study of hBMMSCs Seeded on β-TCP
2.6. In vivo hBMMSCs/β-TCP Constructs Transplantation
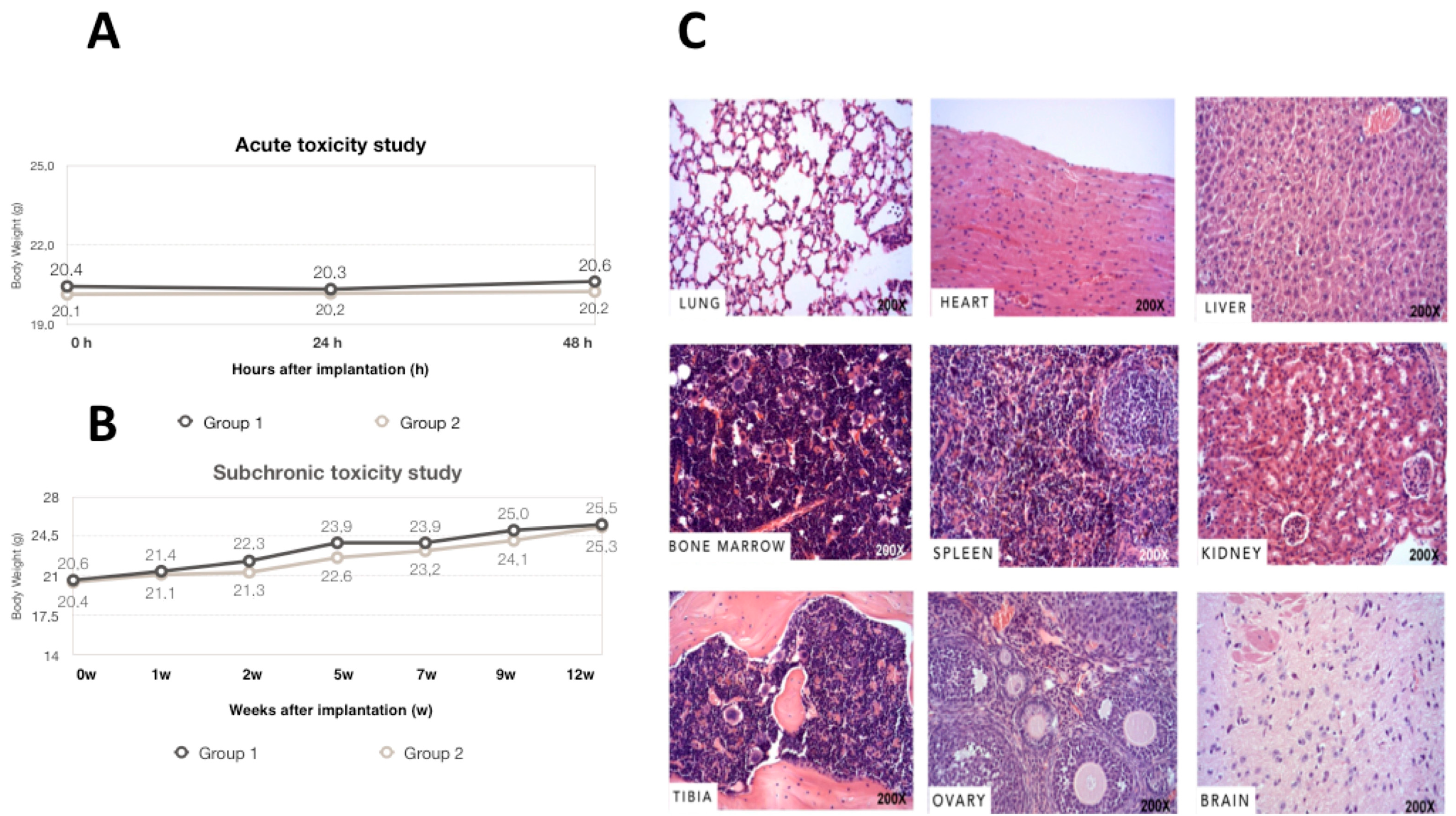
2.7. Acute and Subchronic Toxicity Study
2.8. Biodistribution
2.9. Anatomic Pathology Examination
2.10. Statistics
3. Results
3.1. Characterization of hBMMSCs In Vitro Experiments
3.2. Cell Proliferation and Attachment
3.3. Acute, Subchronic Toxicity Study
3.4. Biodistribution
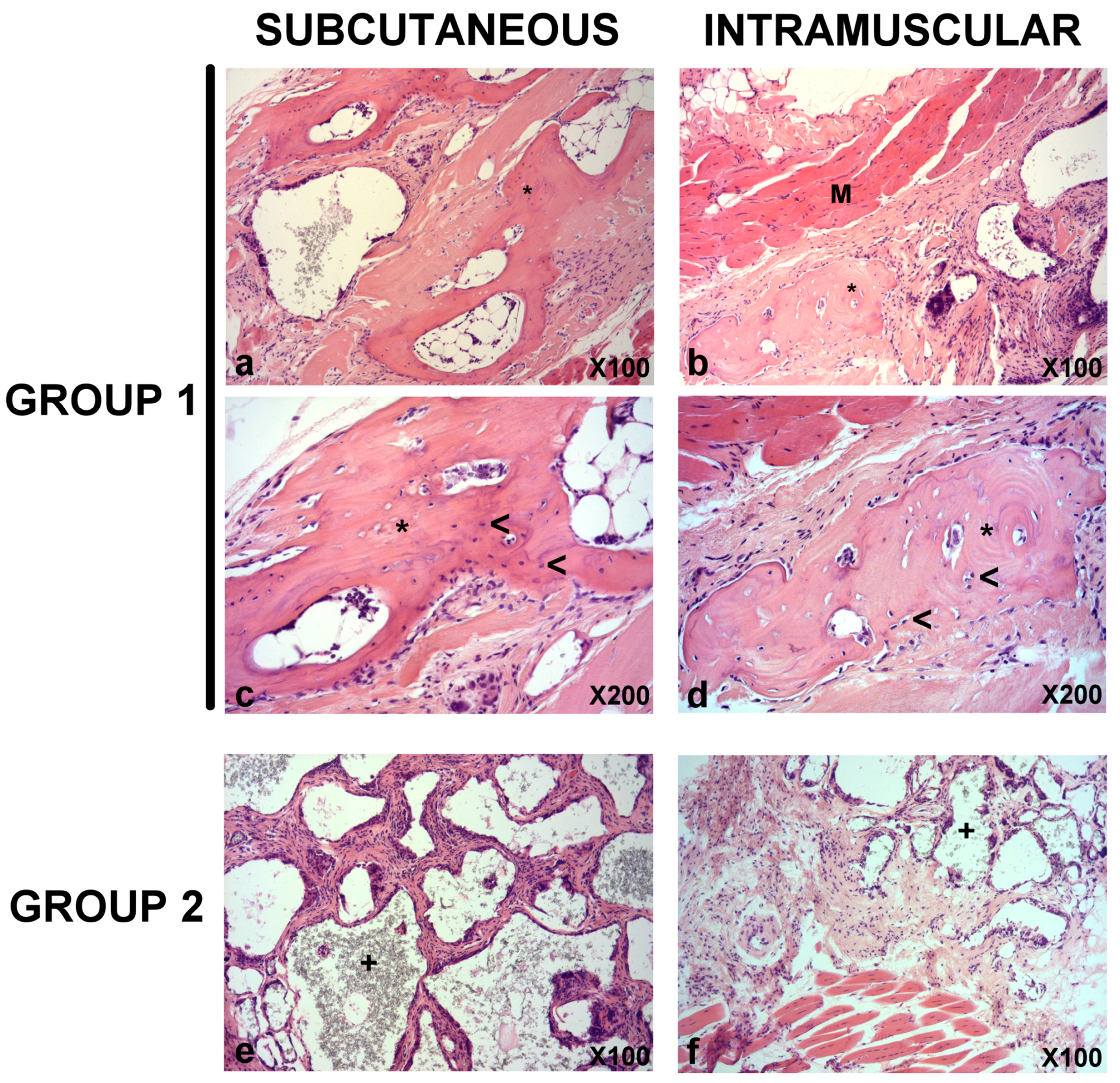
3.5. In Vivo Bone Formation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Delorme, B.; Chateauvieux, S.; Charbord, P. The concept of mesenchymal stem cells. Regen. Med. 2006, 1, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Moraleda, J.M.; Blanquer, M.; Bleda, P.; Iniesta, P.; Ruiz, F.; Bonilla, S.; Cabanes, C.; Tabares, L.; Martinez, S. Adult stem cell therapy: Dream or reality? Transpl. Immunol. 2006, 17, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Gonzálvez-García, M.; Rodríguez-Lozano, F.J.; Villanueva, V.; Segarra-Fenoll, D.; Rodríguez-González, M.A.; Oñate-Sánchez, R.; Blanquer, M.; Moraleda, J.M. Cell therapy in bisphosphonate-related osteonecrosis of the jaw. J. Craniofac. Surg. 2013, 24, 226–228. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Niu, L.; Zhao, T.; Shi, Z.; Di, T.; Feng, G.; Li, J.; Huang, Z. Combining mesenchymal stem cell sheets with platelet-rich plasma gel/calcium phosphate particles: A novel strategy to promote bone regeneration. Stem Cell Res. Ther. 2015, 6, 256. [Google Scholar] [CrossRef] [PubMed]
- Cella, L.; Oppici, A.; Arbasi, M.; Moretto, M.; Piepoli, M.; Vallisa, D.; Zangrandi, A.; Di Nunzio, C.; Cavanna, L. Autologous bone marrow stem cell intralesional transplantation repairing bisphosphonate related osteonecrosis of the jaw. Head Face Med. 2011, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Suenaga, H.; Furukawa, K.S.; Suzuki, Y.; Takato, T.; Ushida, T. Bone regeneration in calvarial defects in a rat model by implantation of human bone marrow-derived mesenchymal stromal cell spheroids. J. Mater. Sci. Mater. Med. 2015, 26, 254. [Google Scholar] [CrossRef] [PubMed]
- Shamsul, B.S.; Tan, K.K.; Chen, H.C.; Aminuddin, B.S.; Ruszymah, B.H. Posterolateral spinal fusion with ostegenesis induced BMSC seeded TCP/HA in a sheep model. Tissue Cell 2014, 46, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Jimi, E.; Hirata, S.; Osawa, K.; Terashita, M.; Kitamura, C.; Fukushima, H. The current and future therapies of bone regeneration to repair bone defects. Int. J. Dent. 2012, 2012, 148261. [Google Scholar] [CrossRef] [PubMed]
- Sunil, P.; Manikandhan, R.; Muthu, M.; Abraham, S. Stem cell therapy in oral and maxillofacial region: An overview. J. Oral Maxillofac. Pathol. 2012, 16, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Basu, J.; Assaf, B.T.; Bertram, T.A.; Rao, M. Preclinical biosafety evaluation of cell-based therapies: Emerging global paradigms. Toxicol. Pathol. 2015, 43, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Patrício, T.; Domingos, M.; Gloria, A.; D’Amora, U.; Coelho, J.F.; Bártolo, P.J. Fabrication and characterisation of PCL and PCL/PLA scaffolds for tissue engineering. Rapid Prototyping J. 2014, 20, 145–156. [Google Scholar] [CrossRef]
- Guarino, V.; Gloria, A.; Raucci, M.G.; De Santis, R.; Ambrosio, L. Bio-inspired composite and cell instructive platforms for bone regeneration. Int. Mater. Rev. 2013, 57, 256–275. [Google Scholar] [CrossRef]
- Frey-Vasconcells, J.; Whittlesey, K.J.; Baum, E.; Feigal, E.G. Translation of stem cell research: Points to consider in designing preclinical animal studies. Stem Cells Transl. Med. 2012, 1, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Phinney, D.G.; Bonnet, D.; Dominici, M.; Krampera, M. Mesenchymal stem cell biodistribution, migration, and homing in vivo. Stem Cells Int. 2014, 2014, 292109. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, C.F.; Maciulaitis, R.; Sladowski, D.; Narayanan, G. Cell and Gene Therapies: European View on Challenges in Translation and How to Address Them. Front. Med. (Lausanne) 2018, 5, 158. [Google Scholar] [CrossRef] [PubMed]
- De Aza, P.N.; Garcia-Bernal, D.; Cragnolini, F.; Velasquez, P.; Meseguer-Olmo, L. The effects of Ca2SiO4-Ca3(PO4)2 ceramics on adult human mesenchymal stem cell viability, adhesion, proliferation, differentiation and function. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 4009–4020. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A.; Therapy, I.S.f.C. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lozano, F.J.; Garcia-Bernal, D.; Onate-Sanchez, R.E.; Ortolani-Seltenerich, P.S.; Forner, L.; Moraleda, J.M. Evaluation of cytocompatibility of calcium silicate-based endodontic sealers and their effects on the biological responses of mesenchymal dental stem cells. Int. Endod. J. 2017, 50, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Llena, C.; Collado-Gonzalez, M.; Tomas-Catala, C.J.; Garcia-Bernal, D.; Onate-Sanchez, R.E.; Rodriguez-Lozano, F.J.; Forner, L. Human Dental Pulp Stem Cells Exhibit Different Biological Behaviours in Response to Commercial Bleaching Products. Materials (Basel) 2018, 11, 1098. [Google Scholar] [CrossRef] [PubMed]
- Eslaminejad, M.B.; Mirzadeh, H.; Nickmahzar, A.; Mohamadi, Y.; Mivehchi, H. Type I collagen gel in seeding medium improves murine mesencymal stem cell loading onto the scaffold, increases their subsequent proliferation, and enhances culture mineralization. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, M.H.; Foden, B.W.; Wolfensohn, S.E. Refinement: Promoting the three Rs in practice. Lab. Anim. 2008, 42, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Francois, S.; Bensidhoum, M.; Mouiseddine, M.; Mazurier, C.; Allenet, B.; Semont, A.; Frick, J.; Sache, A.; Bouchet, S.; Thierry, D.; et al. Local irradiation not only induces homing of human mesenchymal stem cells at exposed sites but promotes their widespread engraftment to multiple organs: A study of their quantitative distribution after irradiation damage. Stem Cells 2006, 24, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Francois, S.; Mouiseddine, M.; Allenet-Lepage, B.; Voswinkel, J.; Douay, L.; Benderitter, M.; Chapel, A. Human mesenchymal stem cells provide protection against radiation-induced liver injury by antioxidative process, vasculature protection, hepatocyte differentiation, and trophic effects. Biomed. Res. Int. 2013, 2013, 151679. [Google Scholar] [CrossRef] [PubMed]
- Creane, M.; Howard, L.; O’Brien, T.; Coleman, C.M. Biodistribution and retention of locally administered human mesenchymal stromal cells: Quantitative polymerase chain reaction-based detection of human DNA in murine organs. Cytotherapy 2017, 19, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.M.; Mendicino, M.; Au, P. An FDA perspective on preclinical development of cell-based regenerative medicine products. Nat. Biotechnol. 2014, 32, 721–723. [Google Scholar] [CrossRef] [PubMed]
- Zscharnack, M.; Krause, C.; Aust, G.; Thummler, C.; Peinemann, F.; Keller, T.; Smink, J.J.; Holland, H.; Somerson, J.S.; Knauer, J.; et al. Preclinical good laboratory practice-compliant safety study to evaluate biodistribution and tumorigenicity of a cartilage advanced therapy medicinal product (ATMP). J. Transl. Med. 2015, 13, 160. [Google Scholar] [CrossRef] [PubMed]
- Stratton, S.; Shelke, N.B.; Hoshino, K.; Rudraiah, S.; Kumbar, S.G. Bioactive polymeric scaffolds for tissue engineering. Bioact. Mater. 2016, 1, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Seebach, C.; Schultheiss, J.; Wilhelm, K.; Frank, J.; Henrich, D. Comparison of six bone-graft substitutes regarding to cell seeding efficiency, metabolism and growth behaviour of human mesenchymal stem cells (MSC) in vitro. Injury 2010, 41, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Lv, K.; Zhang, W.; Zhang, X.; Jiang, X.; Zhang, F. The healing of critical-size calvarial bone defects in rat with rhPDGF-BB, BMSCs, and beta-TCP scaffolds. J. Mater. Sci. Mater. Med. 2012, 23, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, W.; Lv, K.; Yu, W.; Jiang, X.; Zhang, F. Peri-Implant Bone Regeneration Using rhPDGF-BB, BMSCs, and beta-TCP in a Canine Model. Clin. Implant Dent. Relat. Res. 2016, 18, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Kang, N.; Wang, Q.; Dong, P.; Lv, X.; Cao, Y.; Xiao, R. The Dose-Effect Relationship Between the Seeding Quantity of Human Marrow Mesenchymal Stem Cells and In Vivo Tissue-Engineered Bone Yield. Cell Transplant 2015, 24, 1957–1968. [Google Scholar] [CrossRef] [PubMed]
- Arpornmaeklong, P.; Pressler, M.J. Effects of ss-TCP Scaffolds on neurogenic and osteogenic differentiation of Human Embryonic Stem Cells. Ann. Anat. 2017, 215, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Vina-Almunia, J.; Mas-Bargues, C.; Borras, C.; Gambini, J.; El Alami, M.; Sanz-Ros, J.; Penarrocha, M.; Vina, J. Influence of Partial O(2) Pressure on the Adhesion, Proliferation, and Osteogenic Differentiation of Human Dental Pulp Stem Cells on beta-Tricalcium Phosphate Scaffold. Int. J. Oral Maxillofac. Implants 2017, 32, 1251–1256. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Ruan, G.P.; Yao, X.; Liu, J.F.; Zhu, X.Q.; Zhao, J.; Pang, R.Q.; Li, Z.A.; Pan, X.H. Chronic Toxicity Test in Cynomolgus Monkeys For 98 Days with Repeated Intravenous Infusion of Cynomolgus Umbilical Cord Mesenchymal Stem Cells. Cell Physiol. Biochem. 2017, 43, 891–904. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.W.; Ahn, J.H.; Kwon, E.; Kim, S.H.; Kim, H.; Jang, J.J.; Kim, W.H.; Kim, J.H.; Han, S.Y.; Kim, J.T.; et al. Human umbilical cord-derived mesenchymal stem cells in acute liver injury: Hepatoprotective efficacy, subchronic toxicity, tumorigenicity, and biodistribution. Regul. Toxicol. Pharmacol. 2016, 81, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Reyes, B.; Coca, M.I.; Codinach, M.; Lopez-Lucas, M.D.; Del Mazo-Barbara, A.; Caminal, M.; Oliver-Vila, I.; Cabanas, V.; Lope-Piedrafita, S.; Garcia-Lopez, J.; et al. Assessment of biodistribution using mesenchymal stromal cells: Algorithm for study design and challenges in detection methodologies. Cytotherapy 2017, 19, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.A.; Yun, J.W.; Joo, K.M.; Lee, J.Y.; Kwak, P.A.; Lee, Y.E.; You, J.R.; Kwon, E.; Kim, W.H.; Wang, K.C.; et al. Preclinical Biosafety Evaluation of Genetically Modified Human Adipose Tissue-Derived Mesenchymal Stem Cells for Clinical Applications to Brainstem Glioma. Stem Cells Dev. 2016, 25, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Toupet, K.; Maumus, M.; Peyrafitte, J.A.; Bourin, P.; van Lent, P.L.; Ferreira, R.; Orsetti, B.; Pirot, N.; Casteilla, L.; Jorgensen, C.; et al. Long-term detection of human adipose-derived mesenchymal stem cells after intraarticular injection in SCID mice. Arthritis Rheum. 2013, 65, 1786–1794. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Nagata, K.; Yokota, T.; Honda, M.; Aizawa, M. Histological evaluations of apatite-fiber scaffold cultured with mesenchymal stem cells by implantation at rat subcutaneous tissue. Biomed. Mater. Eng. 2017, 28, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Ismail, T.; Osinga, R.; Todorov, A., Jr.; Haumer, A.; Tchang, L.A.; Epple, C.; Allafi, N.; Menzi, N.; Largo, R.D.; Kaempfen, A.; et al. Engineered, axially-vascularized osteogenic grafts from human adipose-derived cells to treat avascular necrosis of bone in a rat model. Acta Biomater. 2017, 63, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Bouvet-Gerbettaz, S.; Boukhechba, F.; Balaguer, T.; Schmid-Antomarchi, H.; Michiels, J.F.; Scimeca, J.C.; Rochet, N. Adaptive immune response inhibits ectopic mature bone formation induced by BMSCs/BCP/plasma composite in immune-competent mice. Tissue Eng. Part A 2014, 20, 2950–2962. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Gan, Y.; Zhuang, Y.; Wang, X.; Zhao, J.; Tang, T.; Dai, K. Mesenchymal stem cells and porous beta-tricalcium phosphate composites prepared through stem cell screen-enrich-combine(-biomaterials) circulating system for the repair of critical size bone defects in goat tibia. Stem Cell Res. Ther. 2018, 9, 157. [Google Scholar] [CrossRef] [PubMed]
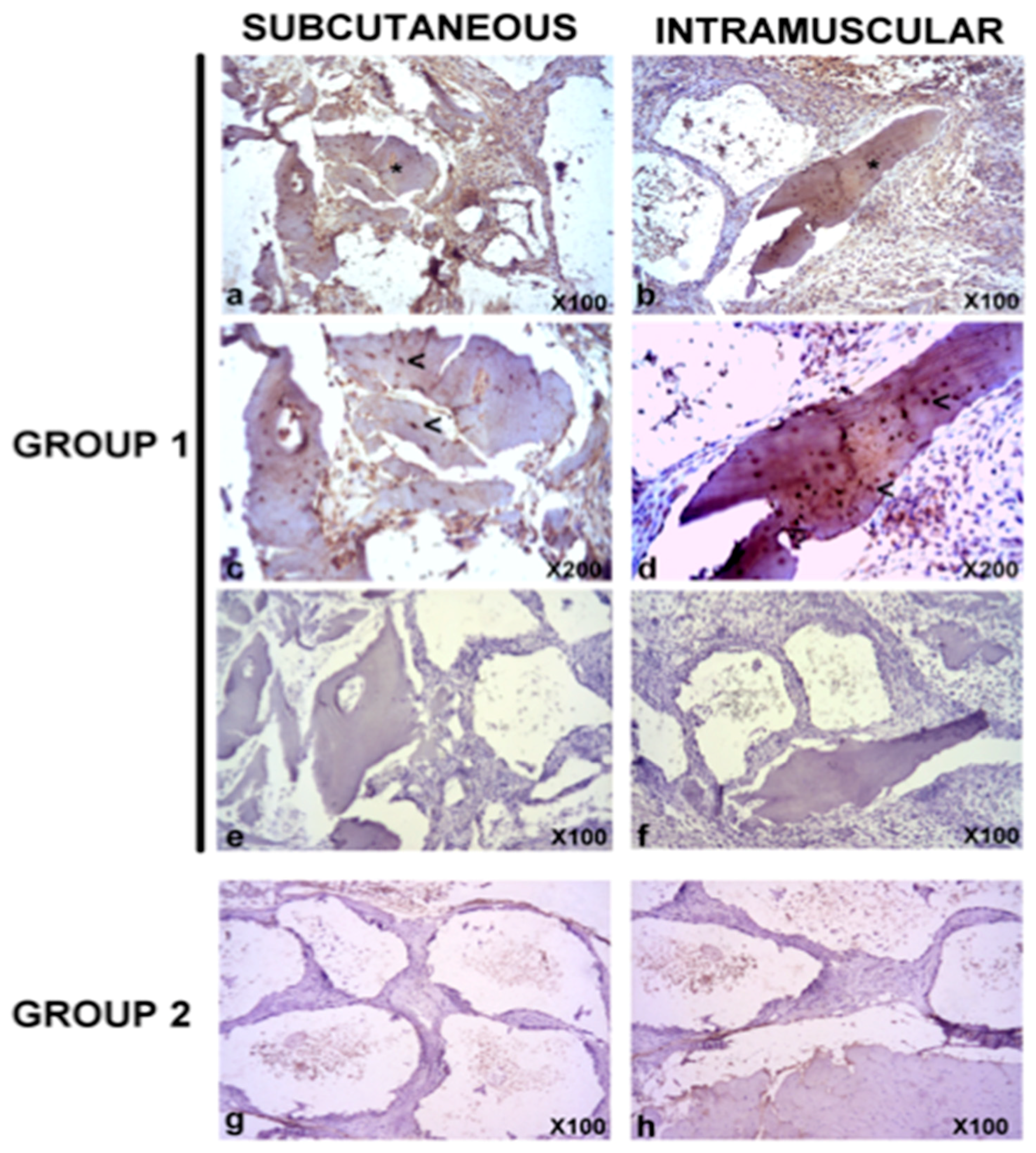
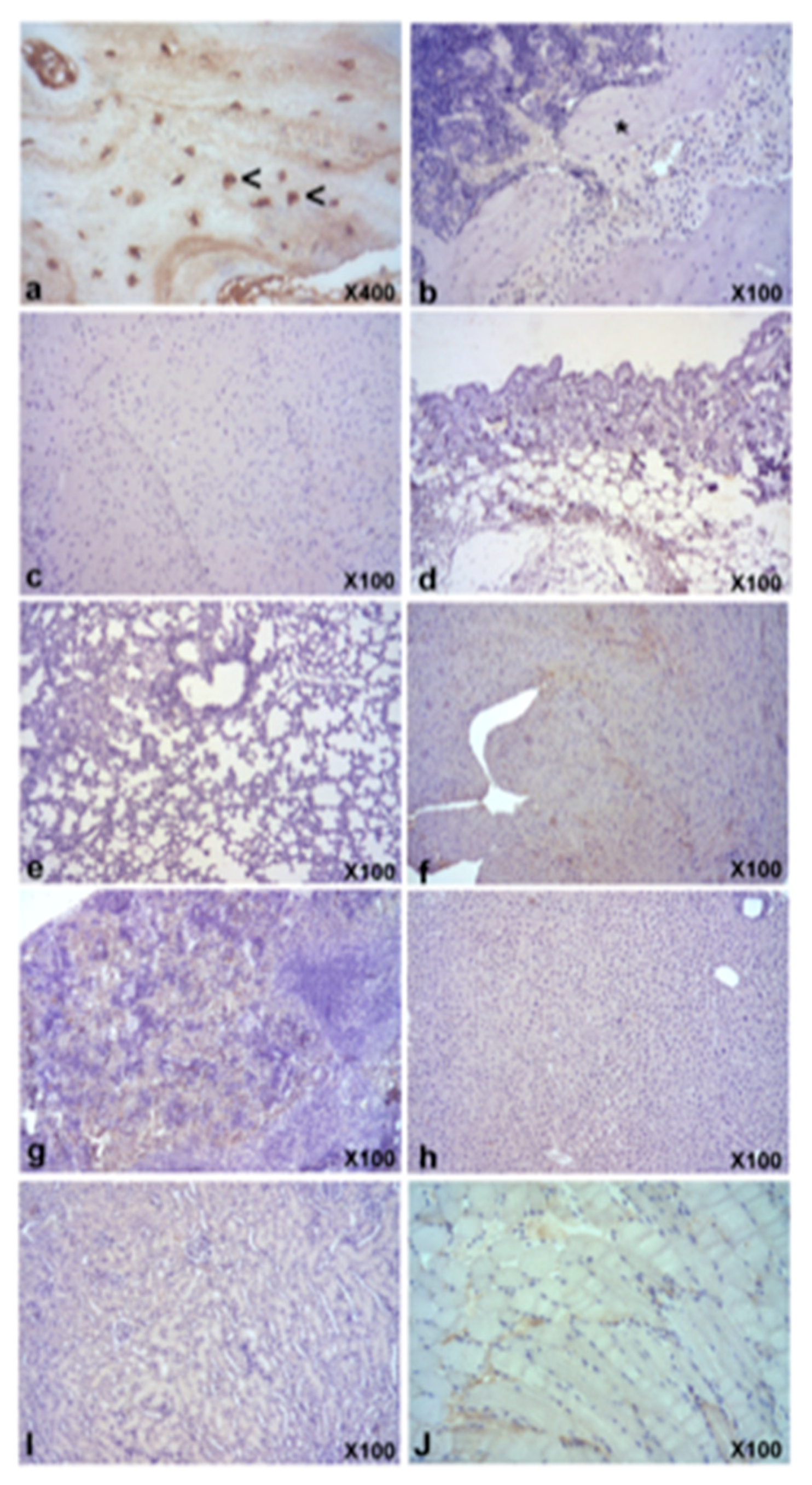
| Tissues/Organs | 24 h | 1 w | 2 w | 5 w | 7 w | 9 w | 12 w | Controls |
|---|---|---|---|---|---|---|---|---|
| Scaffold | + | + | + | + | + | + | + | − |
| Lung | − | − | − | − | − | − | − | − |
| heart | − | − | − | − | − | − | − | − |
| kidney | − | − | − | − | − | − | − | − |
| spleen | − | − | − | − | − | − | − | − |
| tibialis anterior muscle | − | − | − | − | − | − | − | − |
| brain | − | − | − | − | − | − | − | − |
| inguinal fat pad | − | − | − | − | − | − | − | − |
| bone marrow | − | − | − | − | − | − | − | − |
| stomach | − | − | − | − | − | − | − | − |
| intestine | − | − | − | − | − | − | − | − |
| liver | − | − | − | − | − | − | − | − |
| ovary | − | − | − | − | − | − | − | − |
| blood | − | − | − | − | − | − | − | − |
| skin | − | − | − | − | − | − | − | − |
| knee joint | − | − | − | − | − | − | − | − |
| Intramuscular Implant | Subcutaneous Implant | ||||
|---|---|---|---|---|---|
| Yes | No | Yes | No | ||
| Group I (hBMMSCs/TCP) | n = 18 ** | n = 7 | Group I (hBMMSCs/TCP) | n = 17 ** | n = 8 |
| Group II (Cell free TCP) | 0 | n = 5 | Group II (Cell free TCP) | 0 | n = 5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzálvez-García, M.; Martinez, C.M.; Villanueva, V.; García-Hernández, A.; Blanquer, M.; Meseguer-Olmo, L.; Oñate Sánchez, R.E.; Moraleda, J.M.; Rodríguez-Lozano, F.J. Preclinical Studies of the Biosafety and Efficacy of Human Bone Marrow Mesenchymal Stem Cells Pre-Seeded into β-TCP Scaffolds after Transplantation. Materials 2018, 11, 1349. https://doi.org/10.3390/ma11081349
Gonzálvez-García M, Martinez CM, Villanueva V, García-Hernández A, Blanquer M, Meseguer-Olmo L, Oñate Sánchez RE, Moraleda JM, Rodríguez-Lozano FJ. Preclinical Studies of the Biosafety and Efficacy of Human Bone Marrow Mesenchymal Stem Cells Pre-Seeded into β-TCP Scaffolds after Transplantation. Materials. 2018; 11(8):1349. https://doi.org/10.3390/ma11081349
Chicago/Turabian StyleGonzálvez-García, Mar, Carlos M. Martinez, Victor Villanueva, Ana García-Hernández, Miguel Blanquer, Luis Meseguer-Olmo, Ricardo E. Oñate Sánchez, José M. Moraleda, and Francisco Javier Rodríguez-Lozano. 2018. "Preclinical Studies of the Biosafety and Efficacy of Human Bone Marrow Mesenchymal Stem Cells Pre-Seeded into β-TCP Scaffolds after Transplantation" Materials 11, no. 8: 1349. https://doi.org/10.3390/ma11081349
APA StyleGonzálvez-García, M., Martinez, C. M., Villanueva, V., García-Hernández, A., Blanquer, M., Meseguer-Olmo, L., Oñate Sánchez, R. E., Moraleda, J. M., & Rodríguez-Lozano, F. J. (2018). Preclinical Studies of the Biosafety and Efficacy of Human Bone Marrow Mesenchymal Stem Cells Pre-Seeded into β-TCP Scaffolds after Transplantation. Materials, 11(8), 1349. https://doi.org/10.3390/ma11081349







