Droplet-Scale Combustion Analysis of Third-Generation Biodiesel–Diesel Blends
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
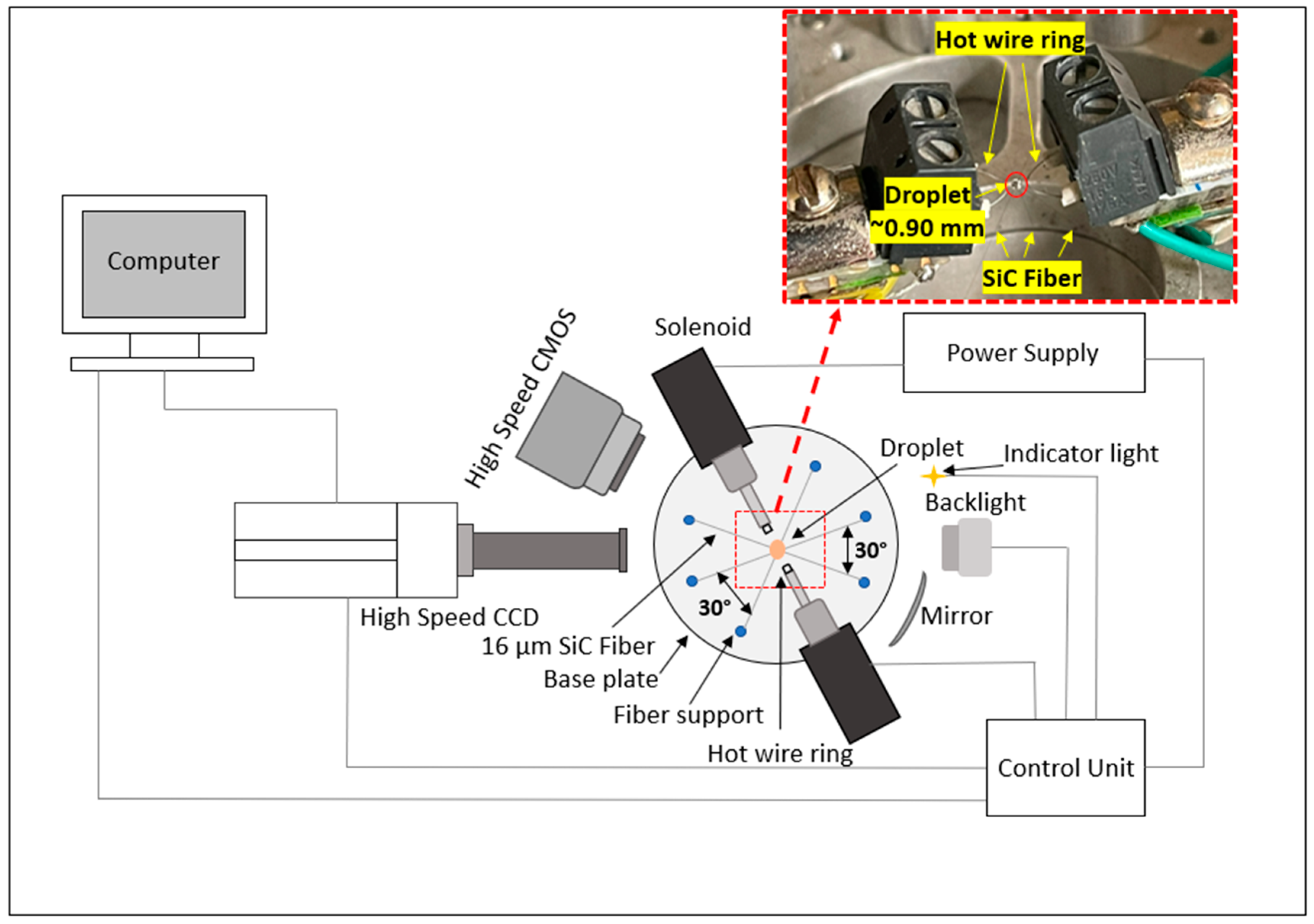
2.2. Experimental Setup
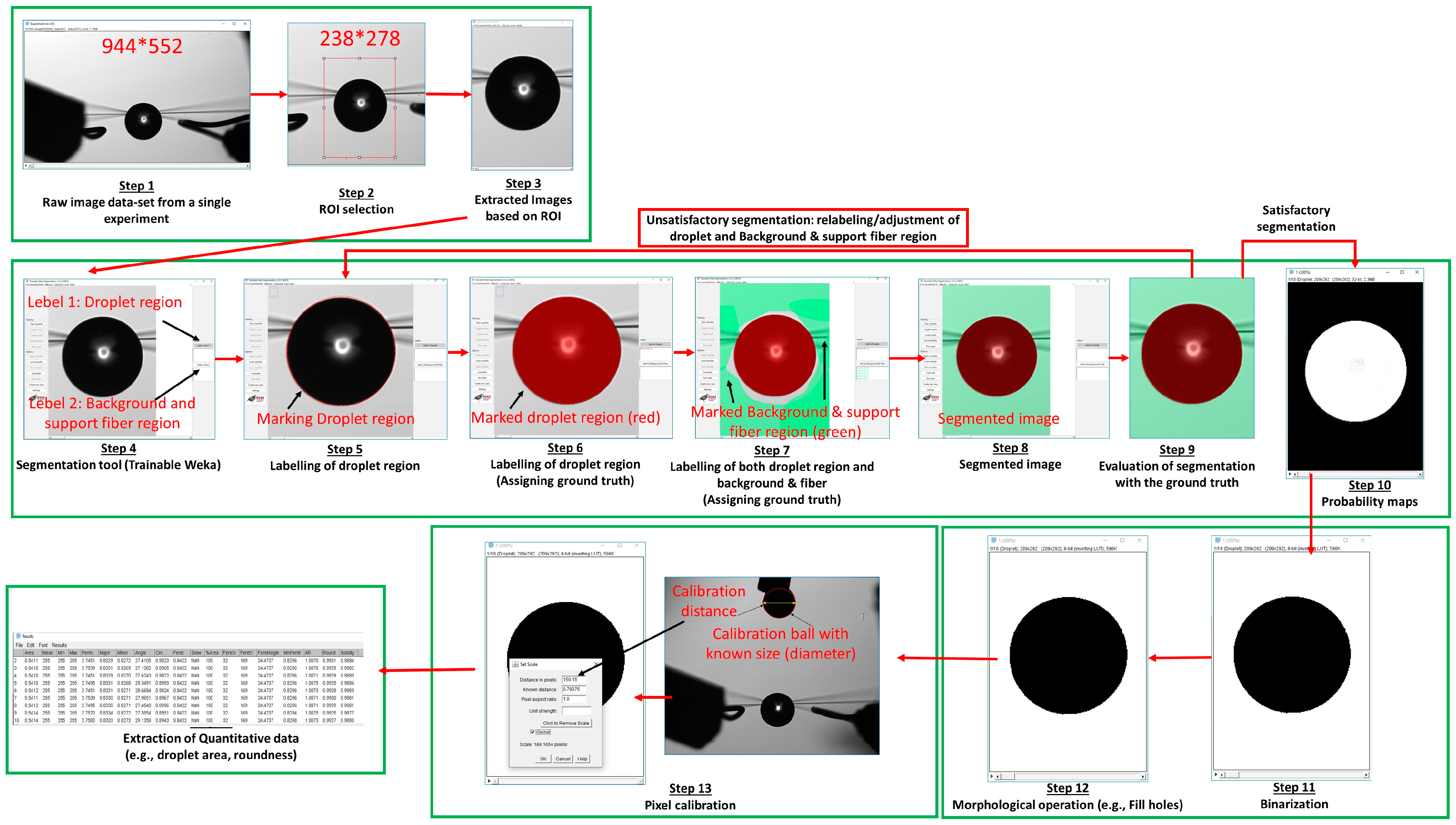
2.3. Image Post Processing
2.4. Uncertainty Analysis
3. Results and Discussion
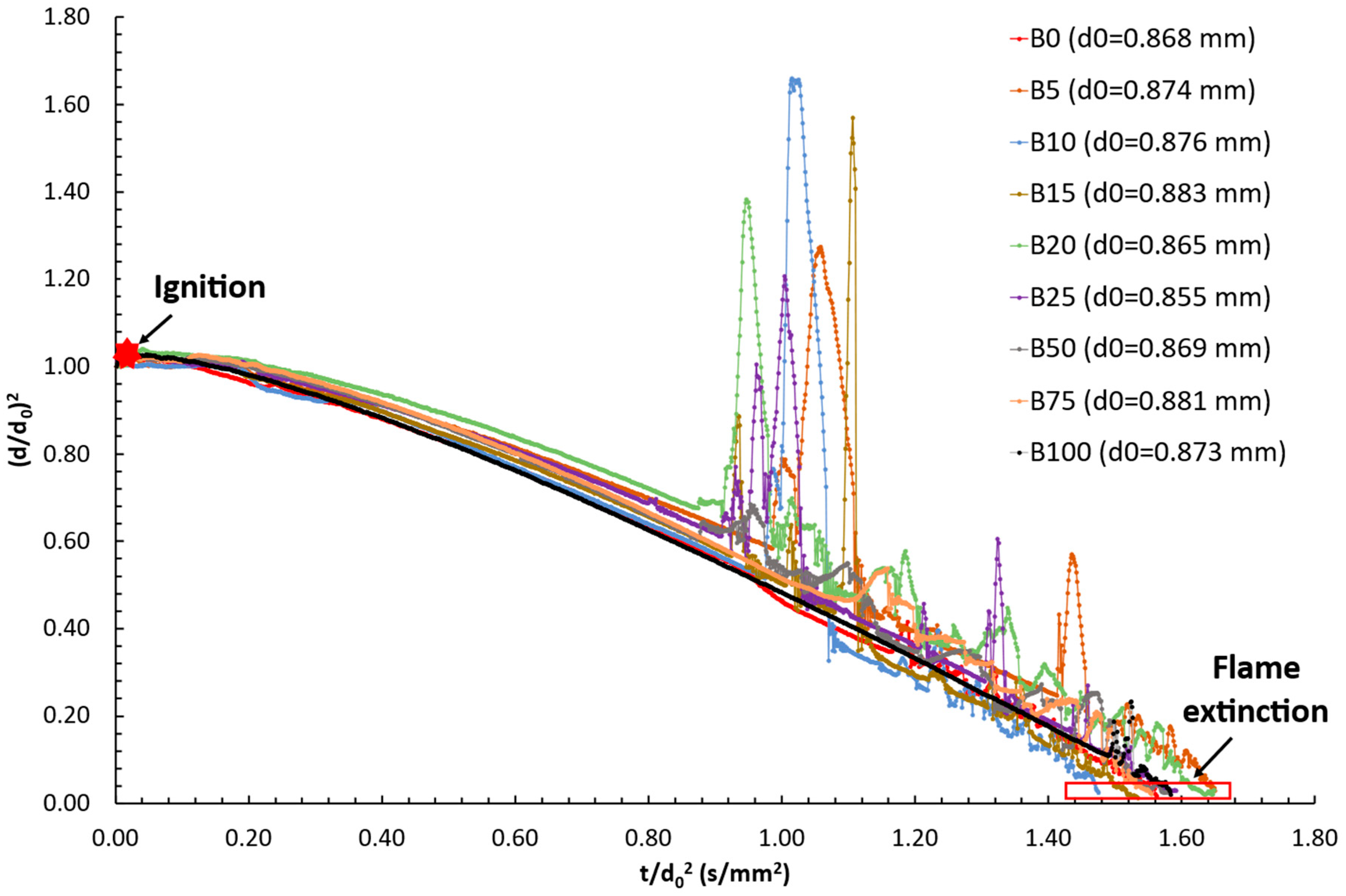
3.1. Burning Droplet Evolution over Time
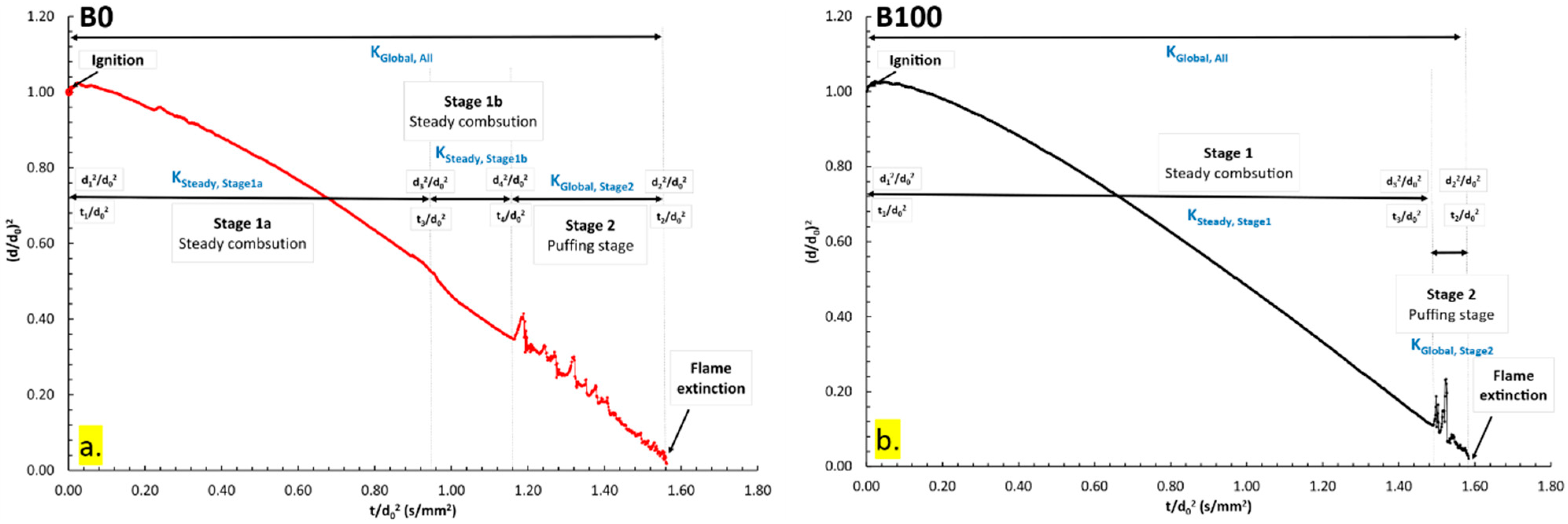
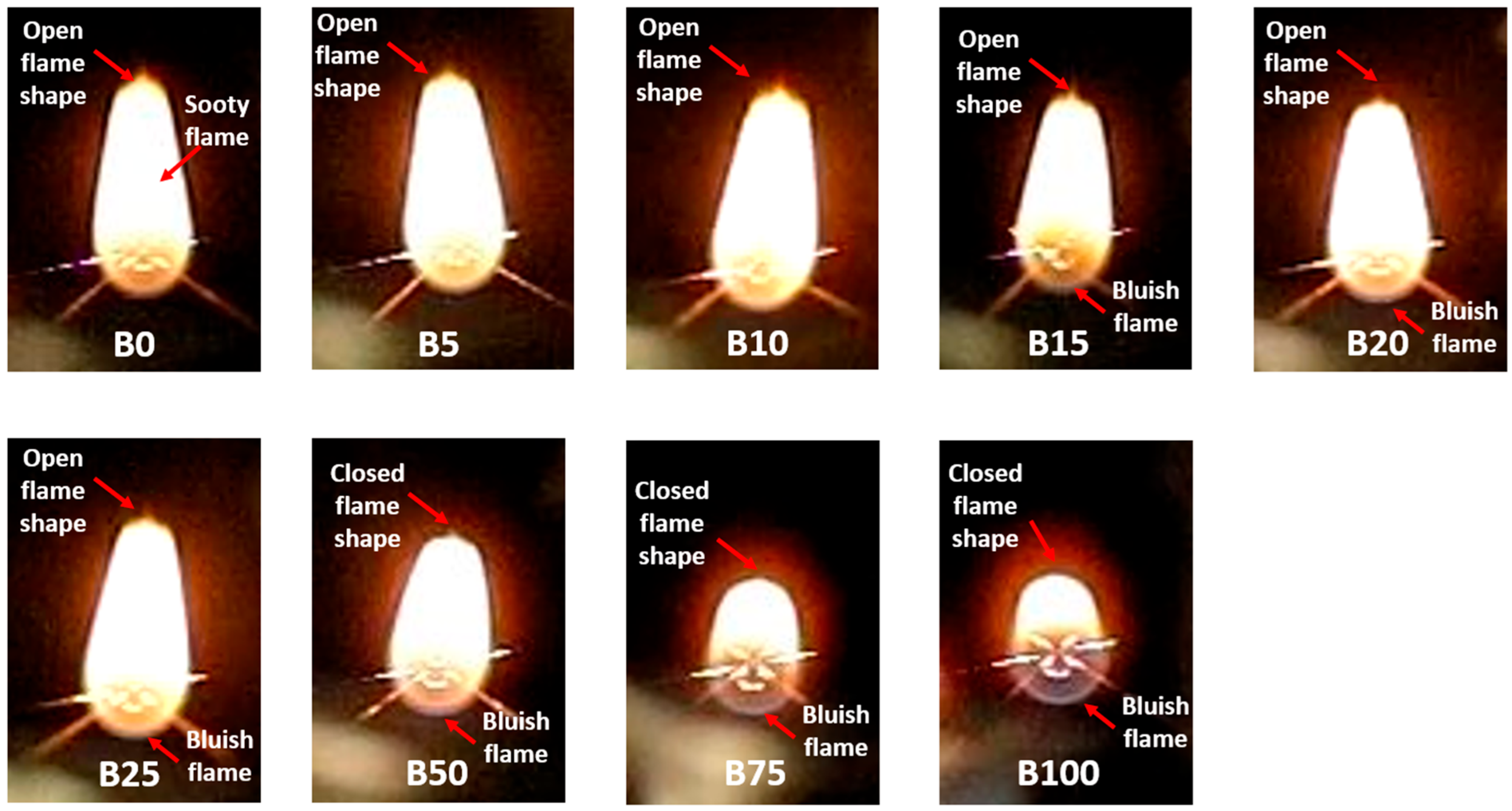
3.1.1. Combustion Characteristics of B0 and B100
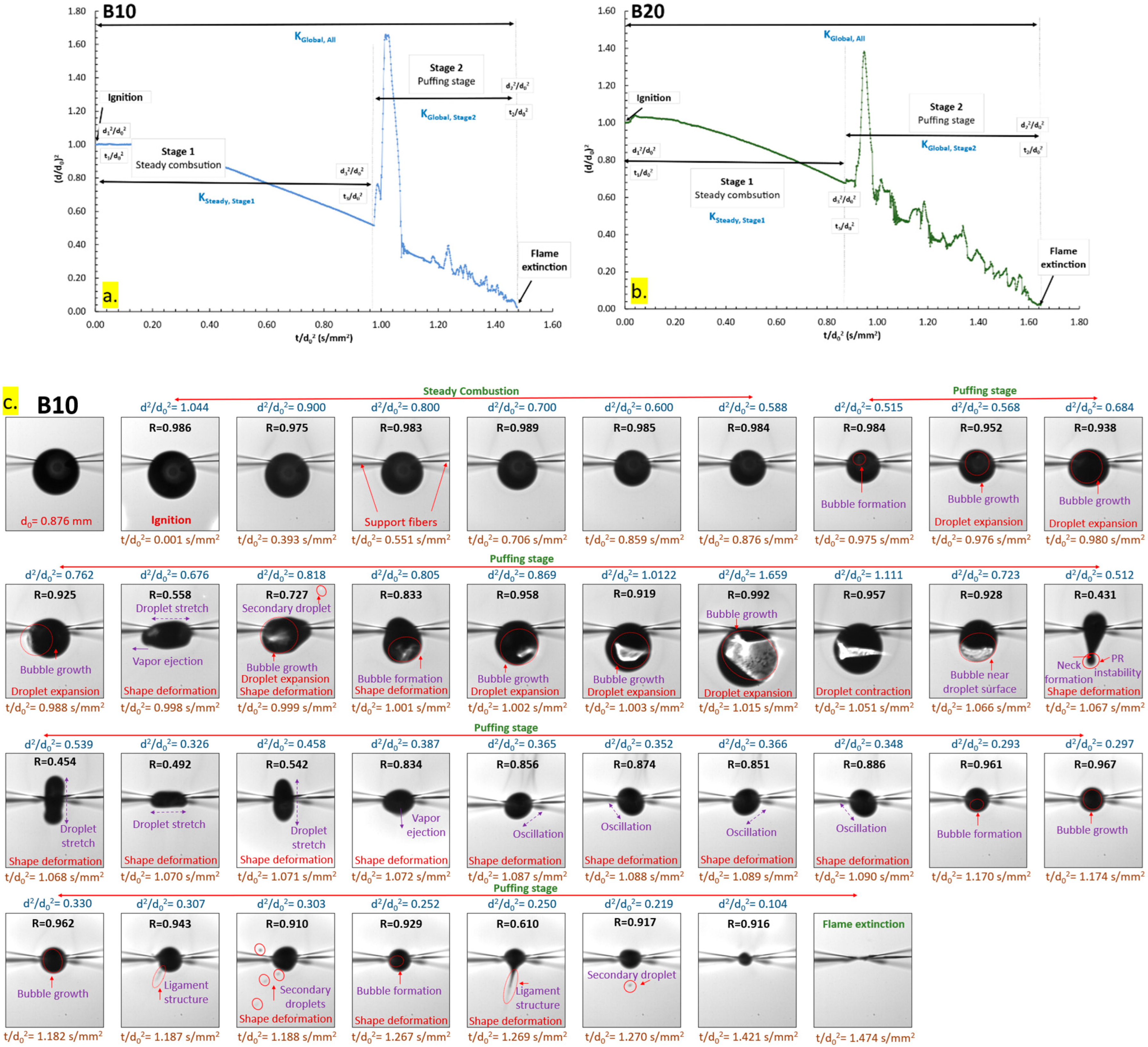
3.1.2. Combustion Characteristics of B5, B10, B15, and B20
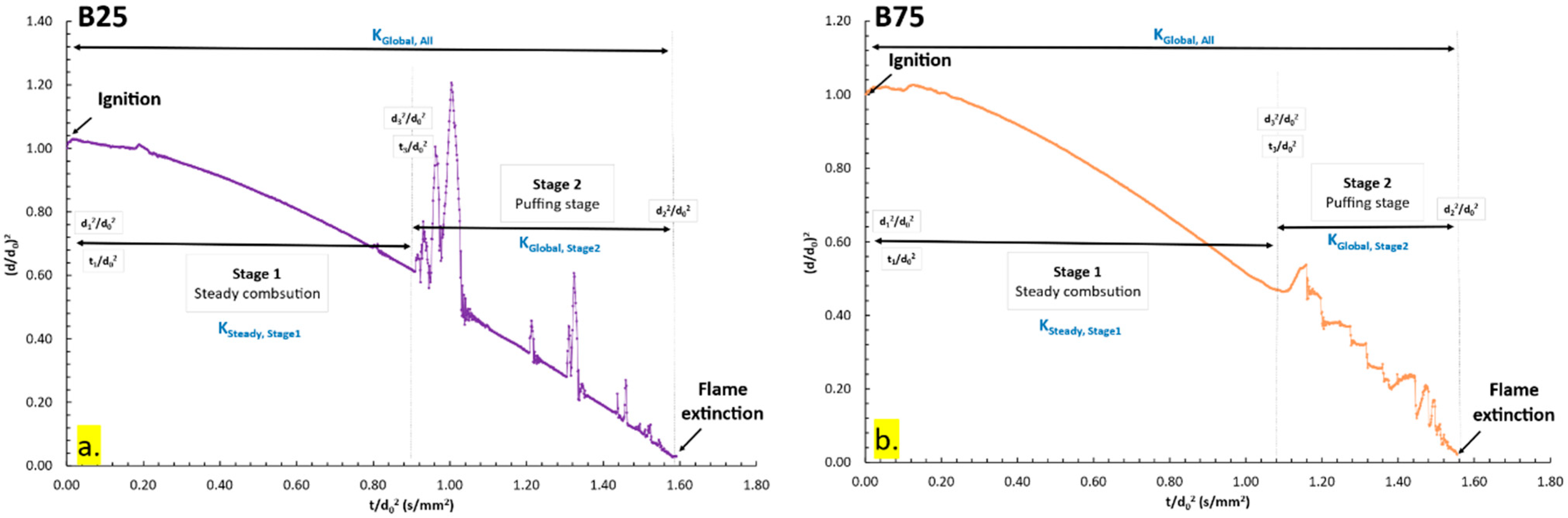
3.1.3. Combustion Characteristics of B25, B50, B75
3.2. Combustion Rate, Pre-Ignition Time and Burning Time
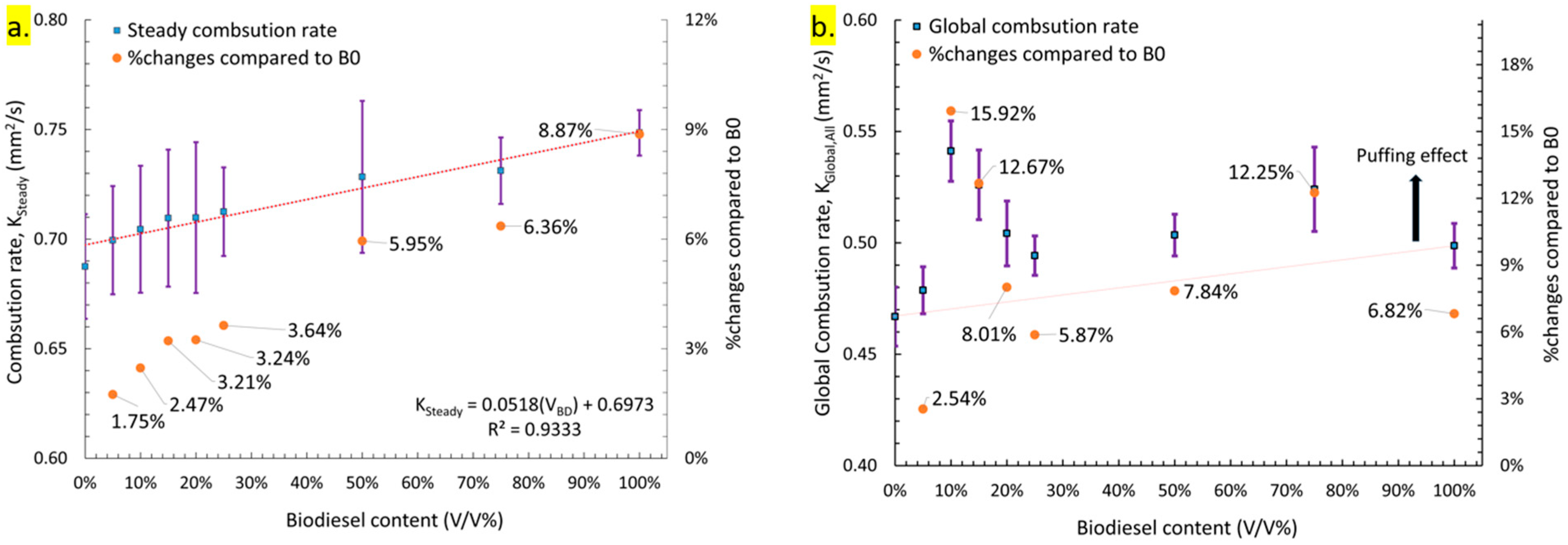
3.2.1. Combustion Rate Analysis
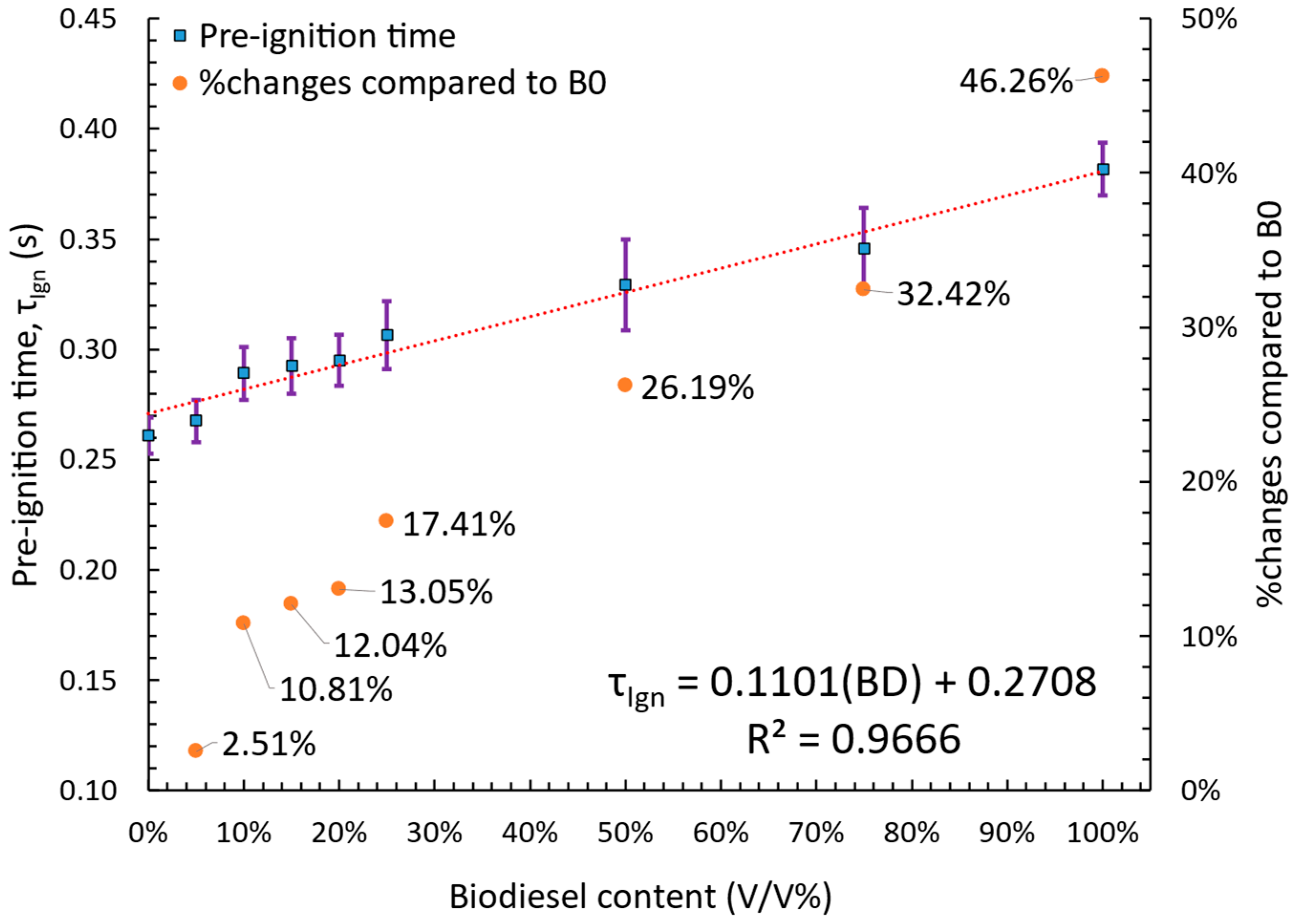
3.2.2. Pre-Ignition Time Analysis
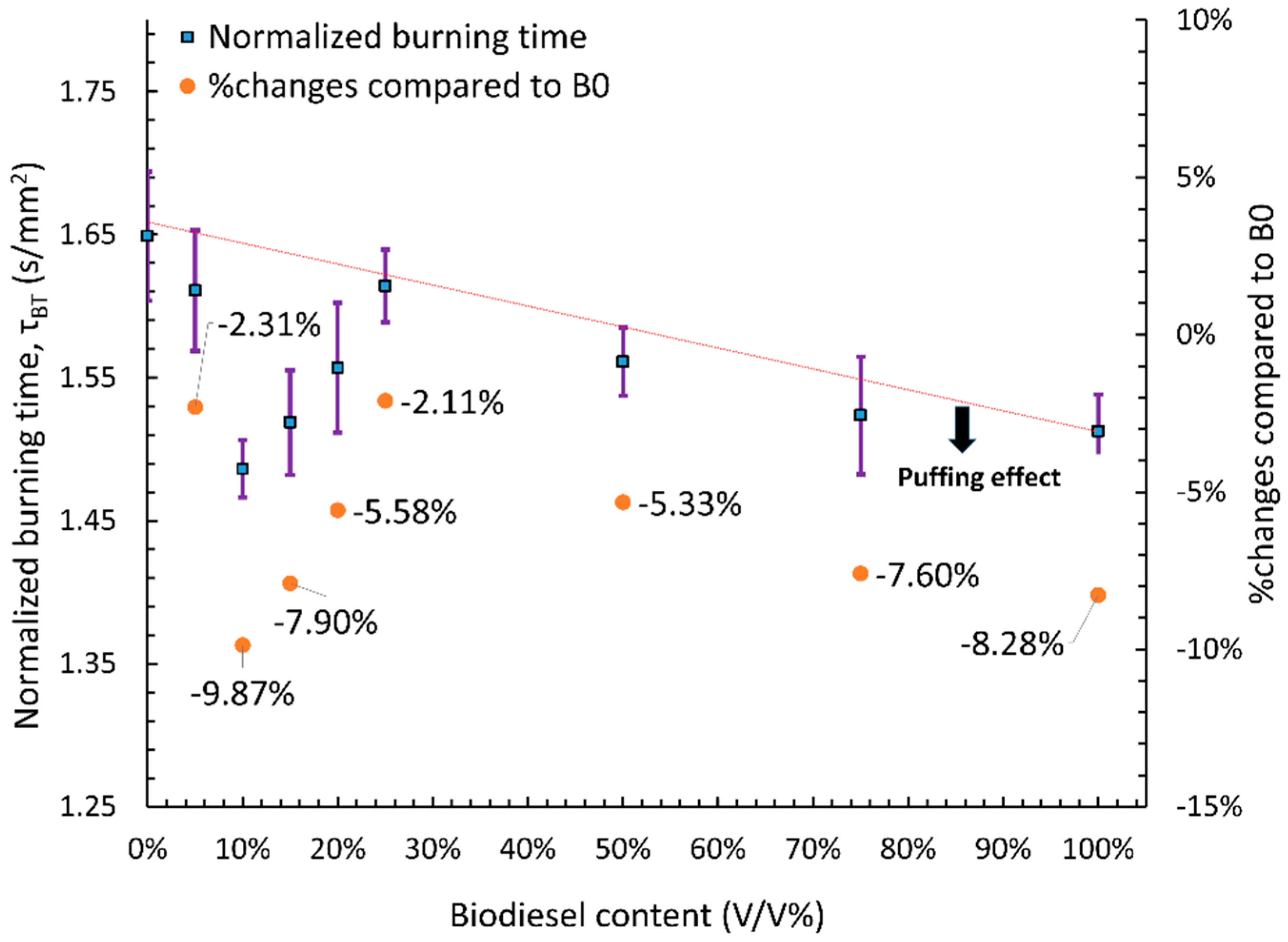
3.2.3. Burning Time Analysis
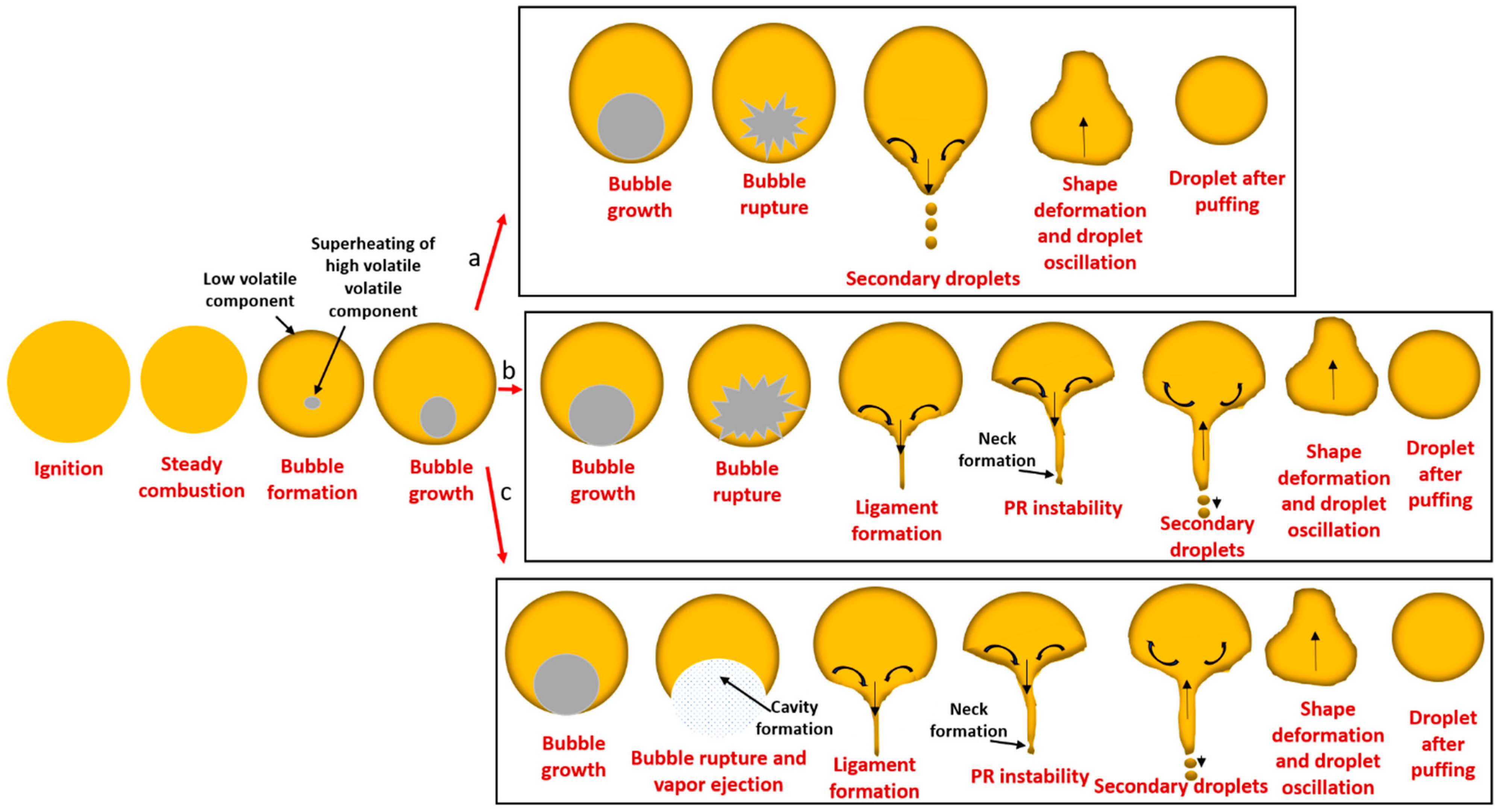
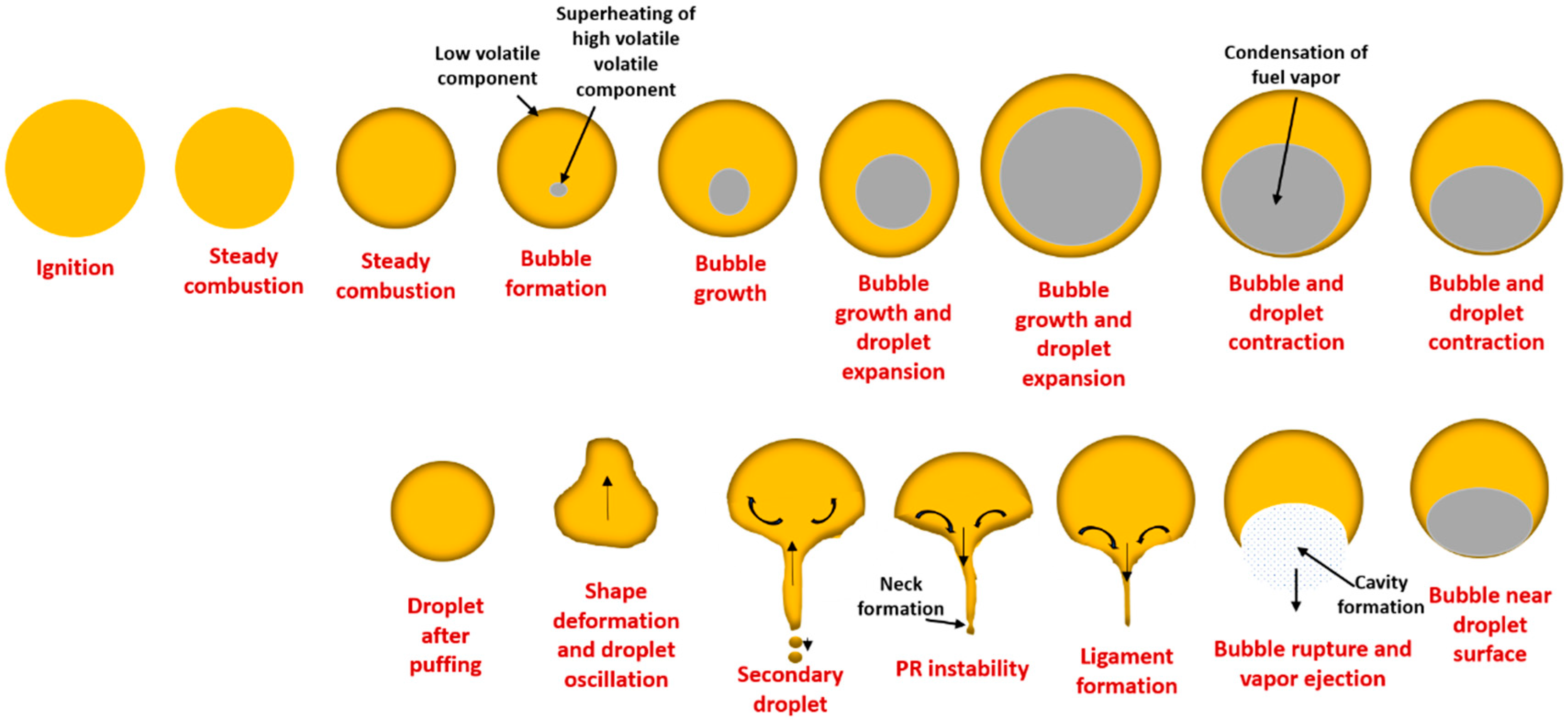
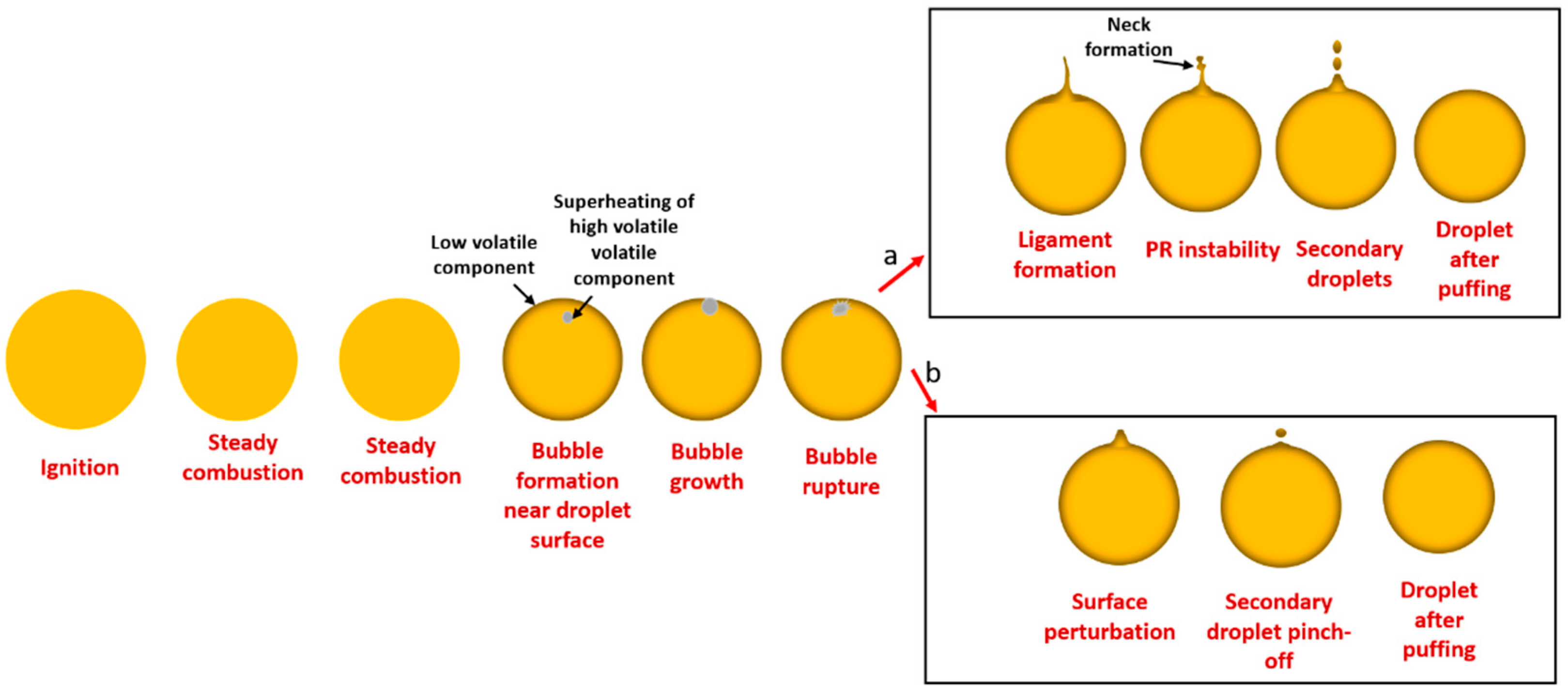
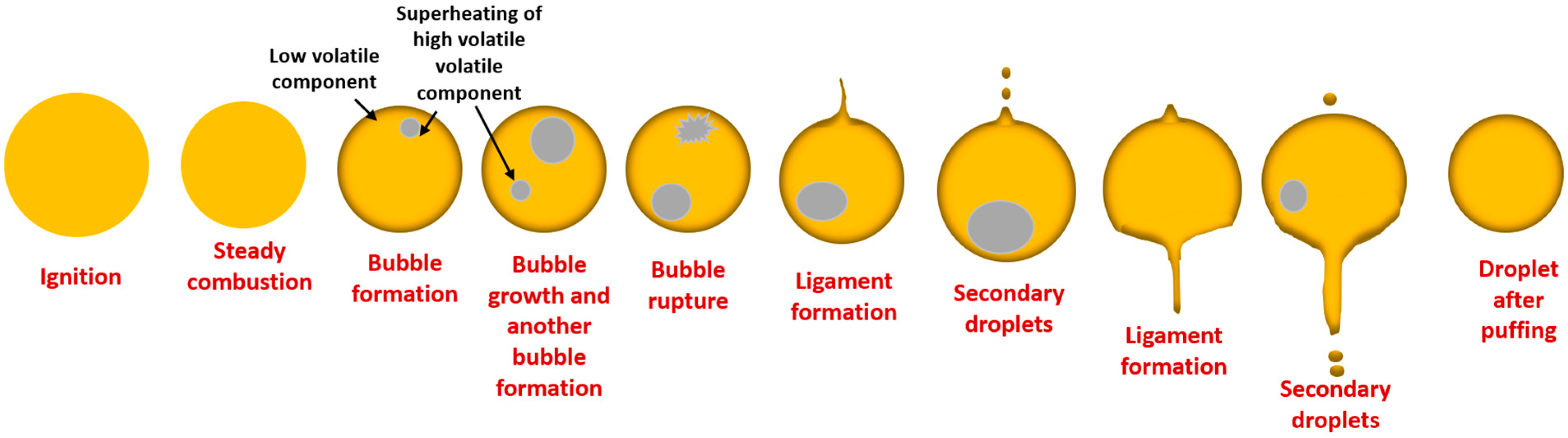
3.3. Puffing Modes and Characteristics
3.3.1. Puffing Mechanisms in Diesel–Biodiesel Blends
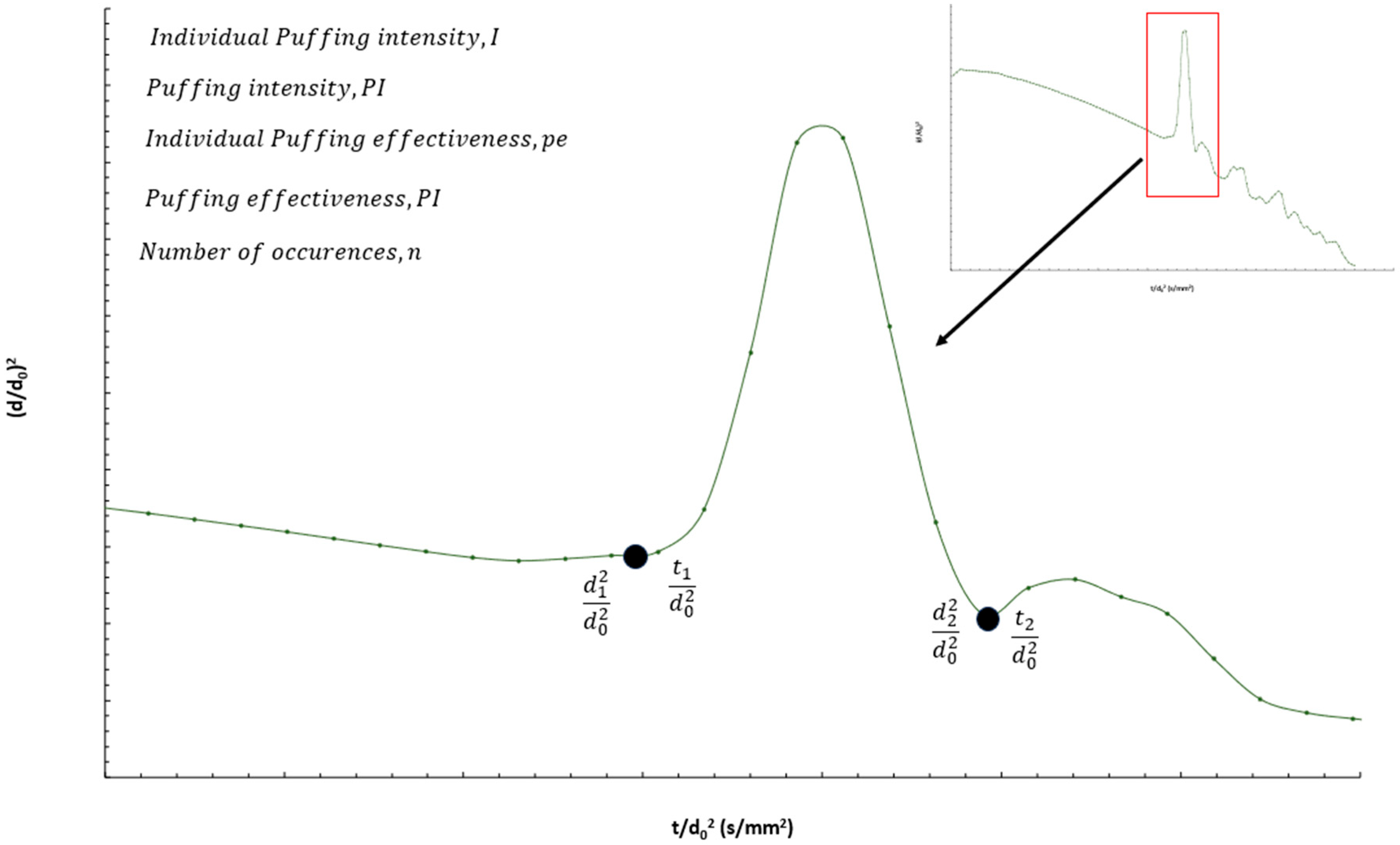
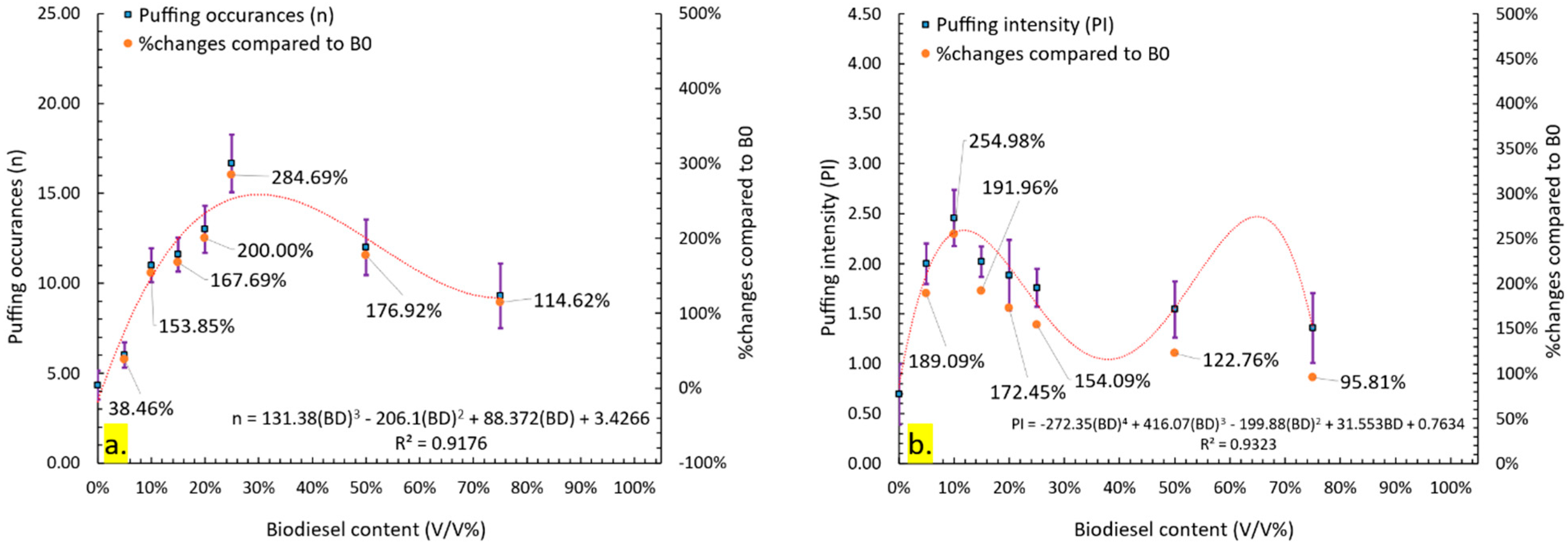
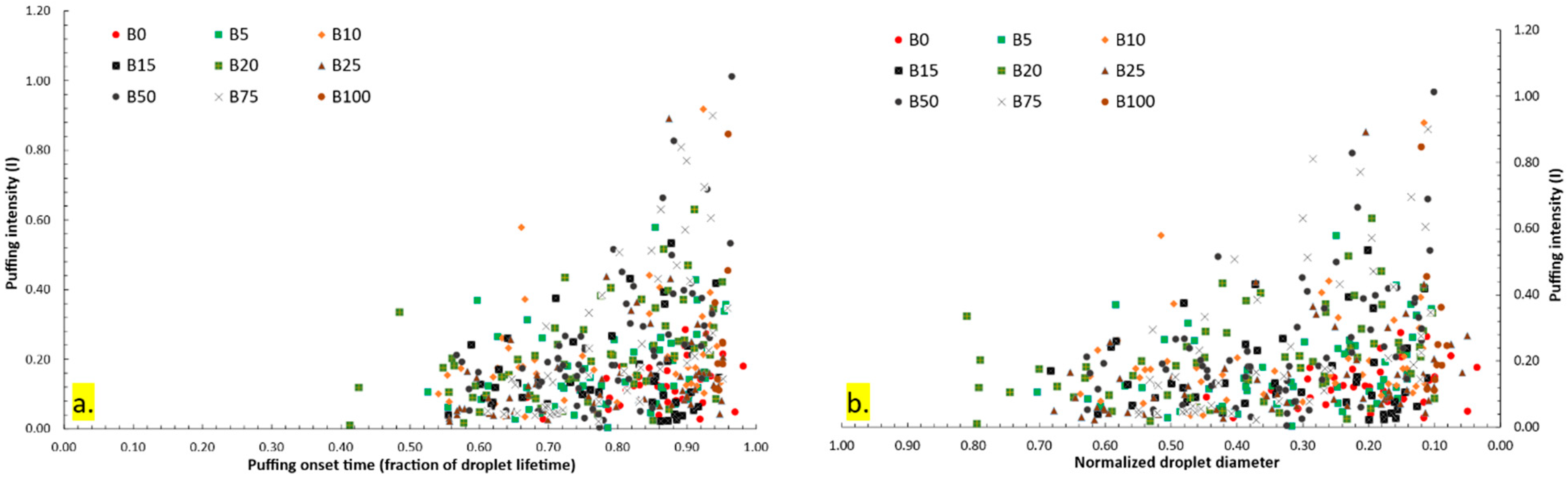
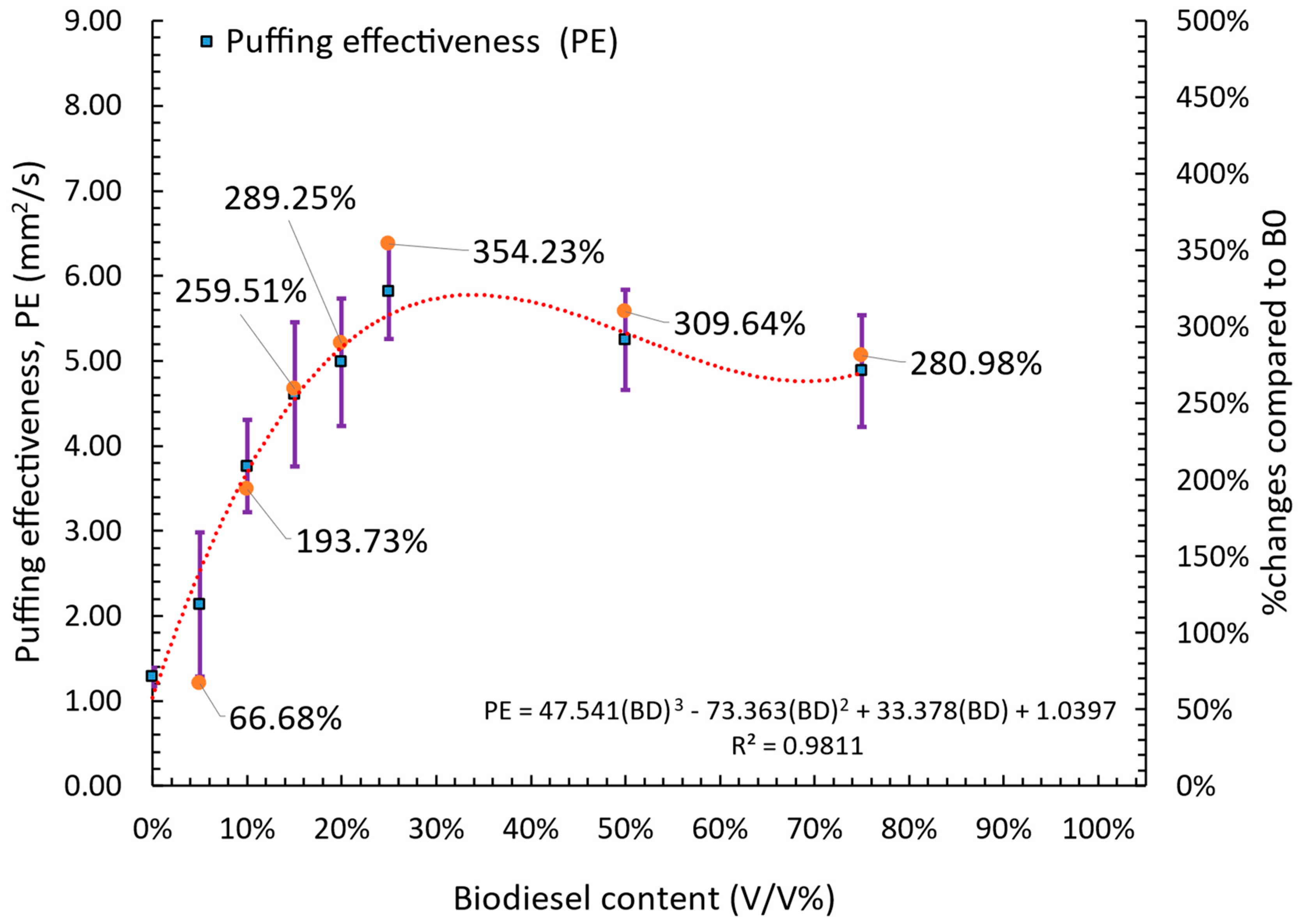
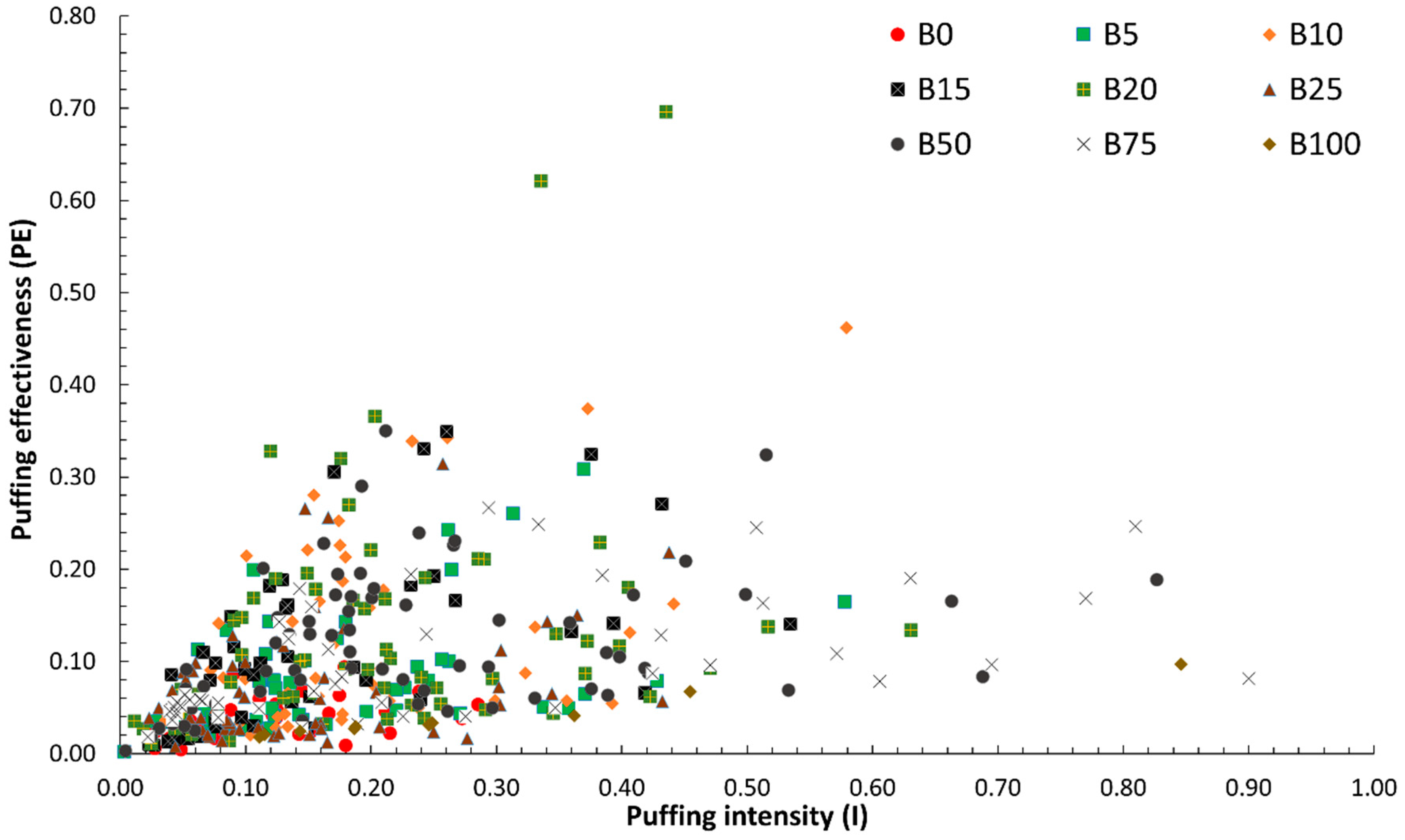
3.3.2. Quantitative Analysis of Puffing Occurrences, Intensity, and Effectiveness
4. Conclusions
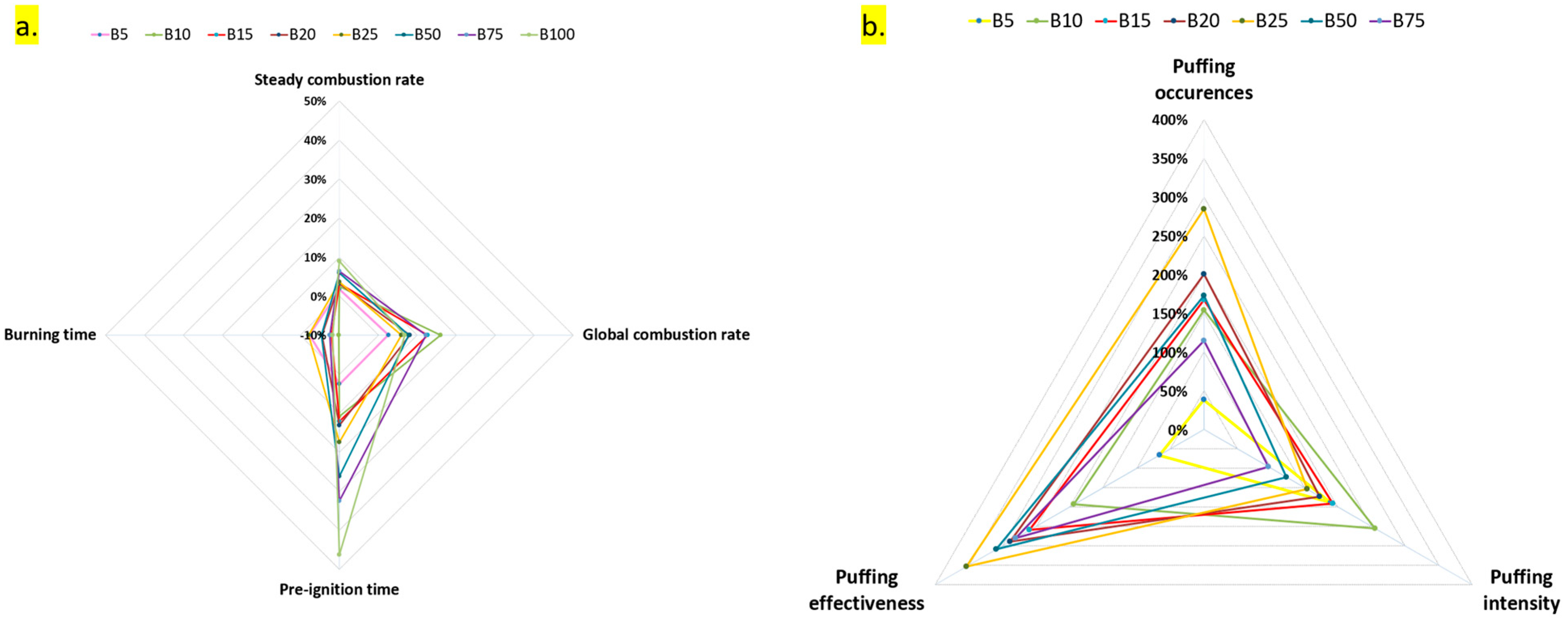
- Both neat fuels (B0 and B100) and blended fuels exhibited two distinct combustion stages: a steady combustion stage and a puffing stage. However, the characteristics and duration of these stages varied notably among different blends, particularly in puffing behavior between lower (B5–B25) and higher (B50, B75) biodiesel concentrations. The evolution of droplet morphology also showed distinct differences between these two blend categories.
- Four puffing mechanisms were identified. Mechanisms 1, 3, and 4 were observed in all fuels except B100, while mechanism 2 was exclusive to lower blends (B5–B25). Puffing characteristics, including the number of occurrences, intensity, and effectiveness, exhibited a non-linear trend with increasing biodiesel content. Puffing occurrences peaked at B25 before declining at higher blends (B50 and B75). Puffing intensity was highest at B10 and decreased beyond this concentration. Puffing effectiveness followed a similar trend, peaking at B10 and decreasing up to B25, but with a secondary enhancement at B50 and B75, indicating that puffing dynamics vary with blend composition. A comparison of individual puffing events revealed that high-intensity puffing did not necessarily correlate with increased effectiveness.
- The overall impact of droplet dynamics and puffing was quantified based on combustion rate, pre-ignition time, and burning time. Diesel (B0) exhibited the lowest steady combustion rate, while biodiesel (B100) had the highest. The steady combustion rate increased linearly with biodiesel concentration due to the additional oxygen content, which promoted more complete and cleaner combustion, as evident from flame appearance and color.
- The global combustion rate followed a non-linear trend with increasing biodiesel content. It initially increased, reaching a maximum at B10, then decreased up to B25. However, at B50 and B75, the global combustion rate increased again, suggesting that both high-intensity slow puffing and low-intensity rapid puffing can influence combustion rate. These results indicate that high-biodiesel blends can benefit from puffing, enhancing their viability for commercial applications.
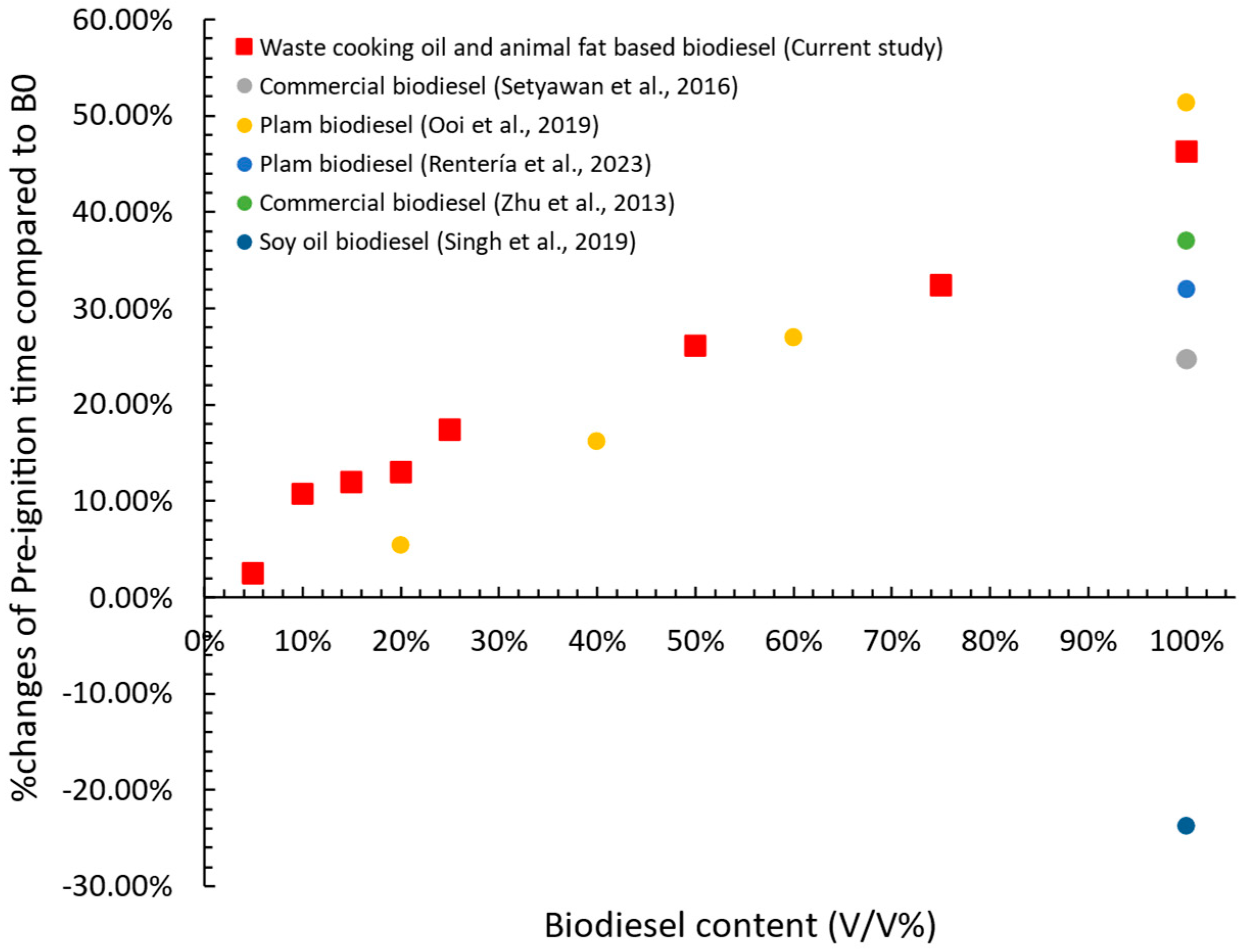
- Pre-ignition time was primarily influenced by the thermo-physical properties of the fuel, such as boiling point, flash point, and surface tension. A linear increase in pre-ignition time was observed with rising biodiesel content, with the longest delay recorded for B100 and the shortest for B0, while blended fuels exhibited intermediate values. Burning time followed the opposite trend of global combustion rate, with fuels exhibiting higher global combustion rates having shorter burning times.
- While these findings provide fundamental insights into droplet combustion, real-world systems operate under elevated pressures and temperatures, which can influence evaporation, ignition delay, and combustion efficiency. The results highlight how puffing enhances atomization, improving combustion rates in B10–B25, while B50–B75 benefit from distinct puffing behaviors that counteract atomization challenges. Future studies should explore high-pressure, high-temperature conditions to validate these trends in practical applications.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- DeCarolis, J.; LaRose, A. Annual Energy Outlook 2023; US Energy Information Administration: Washington, DC, USA, 2023.
- 2024 ExxonMobil Global Outlook Executive Summary. Available online: https://corporate.exxonmobil.com/-/media/global/files/global-outlook/2024/global-outlook-executive-summary.pdf (accessed on 21 February 2025).
- Suhara, A.; Karyadi Herawan, S.G.; Tirta, A.; Idris, M.; Roslan, M.F.; Putra, N.R.; Hananto, A.L.; Veza, I. Biodiesel sustainability: Review of progress and challenges of biodiesel as sustainable biofuel. Clean Technol. 2024, 6, 886–906. [Google Scholar] [CrossRef]
- Kaushik, V.; Swagatika Priyadarshini, L.A.; Kataria, R. Renewable Energy, Alternative Fuels, and Technological Innovations for Sustainable Development. In The Intersection of Global Energy Politics and Climate Change: A Comprehensive Analysis of Energy Markets and Economics; Springer Nature: Singapore, 2025; pp. 321–352. [Google Scholar]
- Manikandan, S.; Vickram, S.; Devarajan, Y. Cutting-Edge Technologies: Biofuel Innovations in Marine Propulsion Systems to lower black carbon emissions. Results Eng. 2025, 25, 104095. [Google Scholar] [CrossRef]
- Abdulwahab, M.R.; Al-attab, K.A.; Badruddin, I.A.; Bashir, M.N.; Lee, J.S. Biofuels spray and combustion characteristics in a new micro gas turbine combustion chamber design with internal exhaust recycling. Case Stud. Therm. Eng. 2025, 65, 105595. [Google Scholar] [CrossRef]
- Ennetta, R.; Soyhan, H.S.; Koyunoğlu, C.; Demir, V.G. Current technologies and future trends for biodiesel production: A review. Arab. J. Sci. Eng. 2022, 47, 15133–15151. [Google Scholar] [CrossRef]
- Pranta, M.H.; Cho, H.M. A comprehensive review of the evolution of biodiesel production technologies. Energy Convers. Manag. 2025, 328, 119623. [Google Scholar] [CrossRef]
- Yang, P.; Chen, Q.; Xu, W.; Jin, Y.; Sun, Y.; Xu, J. Strategic economic and energy analysis of integrated biodiesel production from waste cooking oil. Energy Convers. Manag. 2025, 325, 119354. [Google Scholar] [CrossRef]
- Alternaative Fuels Data Center. Available online: https://afdc.energy.gov/fuels/biodiesel-blends (accessed on 21 February 2025).
- Ghazali WN, M.W.; Mamat, R.; Masjuki, H.H.; Najafi, G. Effects of biodiesel from different feedstocks on engine performance and emissions: A review. Renew. Sustain. Energy Rev. 2015, 51, 585–602. [Google Scholar] [CrossRef]
- Yaqoob, H.; Teoh, Y.H.; Sher, F.; Farooq, M.U.; Jamil, M.A.; Kausar, Z.; Sabah, N.U.; Shah, M.F.; Rehman, H.Z.U.; Rehman, A.U. Potential of waste cooking oil biodiesel as renewable fuel in combustion engines: A review. Energies 2021, 14, 2565. [Google Scholar] [CrossRef]
- Loizides, M.I.; Loizidou, X.I.; Orthodoxou, D.L.; Petsa, D. Circular bioeconomy in action: Collection and recycling of domestic used cooking oil through a social, reverse logistics system. Recycling 2019, 4, 16. [Google Scholar] [CrossRef]
- Singh-Ackbarali, D.; Maharaj, R.; Mohamed, N.; Ramjattan-Harry, V. Potential of used frying oil in paving material: Solution to environmental pollution problem. Environ. Sci. Pollut. Res. 2017, 24, 12220–12226. [Google Scholar] [CrossRef]
- Xue, J. Combustion characteristics, engine performances and emissions of waste edible oil biodiesel in diesel engine. Renew. Sustain. Energy Rev. 2013, 23, 350–365. [Google Scholar]
- Zhang, Y.; Zhong, Y.; Lu, S.; Zhang, Z.; Tan, D. A comprehensive review of the properties, performance, combustion, and emissions of the diesel engine fueled with different generations of biodiesel. Processes 2022, 10, 1178. [Google Scholar] [CrossRef]
- Eremeeva, A.M.; Ilyushin, Y.V. Temperature Control During Storage of Raw Materials in the Process of Biodiesel Fuel Production. Inventions 2025, 10, 7. [Google Scholar] [CrossRef]
- Esmaeili, H.; Foroutan, R. Optimization of biodiesel production from goat tallow using alkaline catalysts and combining them with diesel. Chem. Chem. Technol. 2018, 12, 120–126. [Google Scholar]
- Daryono, E.D.; Jimmy, H.S. Production of Biodiesel Without Catalyst Separation with Palm Oil Interesterification Process Using Essential Oil Biocatalyst. Chemistry 2024, 18, 356–362. [Google Scholar] [CrossRef]
- Parveg, A.S.; Ratner, A. A comprehensive review of liquid fuel droplet evaporation and combustion behavior with carbon-based nanoparticles. Prog. Energy Combust. Sci. 2025, 106, 101198. [Google Scholar]
- Pham, P.X.; Nguyen, K.T.; Pham, T.V.; Nguyen, V.H. Biodiesels manufactured from different feedstock: From fuel properties to fuel atomization and evaporation. ACS Omega 2020, 5, 20842–20853. [Google Scholar]
- Ibadurrohman, I.A.; Hamidi, N.; Yuliati, L. The role of the unsaturation degree on the droplet combustion characteristics of fatty acid methyl ester. Alex. Eng. J. 2022, 61, 2046–2060. [Google Scholar]
- Zhou, W.; Wang, Z.; Liang, Y.; Zhang, X.; Yu, L.; Lu, X. The effect of the unsaturation degree on the gas-phase autoignition of methyl oleate and methyl linoleate: Experimental and modeling study. Combust. Flame 2024, 263, 113381. [Google Scholar]
- Benjumea, P.; Agudelo, J.R.; Agudelo, A.F. Effect of the degree of unsaturation of biodiesel fuels on engine performance, combustion characteristics, and emissions. Energy Fuels 2011, 25, 77–85. [Google Scholar]
- Muelas, Á.; Remacha, P.; Ballester, J. Droplet combustion and sooting characteristics of UCO biodiesel, heating oil and their mixtures under realistic conditions. Combust. Flame 2019, 203, 190–203. [Google Scholar] [CrossRef]
- Raghavan, V.; Mehta, P.S. Vaporization characteristics of suspended droplets of biodiesel fuels of Indian origin and their diesel blends–An experimental study. Int. J. Heat Mass Transf. 2015, 88, 28–41. [Google Scholar]
- Ooi, J.B.; Yap, J.H.; Tran, M.V.; Leong, J.C.K. Experimental investigation on the droplet burning behavior of diesel–palm biodiesel blends. Energy Fuels 2019, 33, 11804–11811. [Google Scholar] [CrossRef]
- Faik, A.M.D.; Zhang, Y. Multicomponent fuel droplet combustion investigation using magnified high speed backlighting and shadowgraph imaging. Fuel 2018, 221, 89–109. [Google Scholar]
- Li, T.X.; Zhu, D.L.; Akafuah, N.K.; Saito, K.; Law, C.K. Synthesis, droplet combustion, and sooting characteristics of biodiesel produced from waste vegetable oils. Proc. Combust. Inst. 2011, 33, 2039–2046. [Google Scholar]
- Faik, A.M.E.D. Quantitative Investigation of the Multicomponent Fuel Droplet Combustion Using High Speed Imaging and Digital Image Processing. Ph.D. Thesis, University of Sheffield, Sheffield, UK, 2017. [Google Scholar]
- Pan, K.L.; Li, J.W.; Chen, C.P.; Wang, C.H. On droplet combustion of biodiesel fuel mixed with diesel/alkanes in microgravity condition. Combust. Flame 2009, 156, 1926–1936. [Google Scholar]
- Singh, G.; Esmaeilpour, M.; Ratner, A. The effect of acetylene black on droplet combustion and flame regime of petrodiesel and soy biodiesel. Fuel 2019, 246, 108–116. [Google Scholar]
- ASTM D4052; Standard Test Method for Density, Relative Density, and API Gravity of Liquids by Digital Density Meter. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM D1298; Standard Test Method for Density, Relative Density (Specific Gravity), or API Gravity of Crude Petroleum and Liquid Petroleum Products by Hydrometer Method. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM D445; Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity). ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM D7094; Standard Test Method for Flash Point by Modified Continuously Closed Cup (MCCCFP) Tester. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM D93C; Standard Test Methods for Flash Point by Pensky-Martens Closed Cup Tester, Procedure C. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM D240; Standard Test Method for Heat of Combustion of Liquid Hydrocarbon Fuels by Bomb Calorimeter. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM D86; Standard Test Method for Distillation of Petroleum Products and Liquid Fuels at Atmospheric Pressure. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM D1160; Standard Test Method for Distillation of Petroleum Products at Reduced Pressure. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM D524; Standard Test Method for Ramsbottom Carbon Residue of Petroleum Products. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM D4530; Standard Test Method for Determination of Carbon Residue (Micro Method). ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM D613; Standard Test Method for Cetane Number of Diesel Fuel Oil. ASTM International: West Conshohocken, PA, USA, 2022.
- Parveg, A.S.; Zhou, Y.; Leblanc, R.M.; Ratner, A. Effects of gel-like carbon dots (G-CDs) and surfactant (Span80) on the droplet combustion dynamics of liquid fuels. Fuel 2025, 381, 133385. [Google Scholar]
- Wang, J.; Zhang, Q.; Wang, X.; Xu, J. Micro-explosion characteristics and mechanism of multi-component fuel droplet with high volatility differential. Fuel 2023, 333, 126370. [Google Scholar]
- Rao, D.C.K.; Karmakar, S.; Basu, S. Atomization characteristics and instabilities in the combustion of multi-component fuel droplets with high volatility differential. Sci. Rep. 2017, 7, 8925. [Google Scholar]
- Setyawan, H.Y.; Zhu, M.; Zhang, Z.; Zhang, D. Ignition and combustion characteristics of single droplets of a crude glycerol in comparison with pure glycerol, petroleum diesel, biodiesel and ethanol. Energy 2016, 113, 153–159. [Google Scholar]
- Zhu, M.; Ma, Y.; Zhang, D. Effect of a homogeneous combustion catalyst on combustion characteristics of single droplets of diesel and biodiesel. Proc. Combust. Inst. 2013, 34, 1537–1544. [Google Scholar] [CrossRef]
- Botero, M.L.; Huang, Y.; Zhu, D.L.; Molina, A.; Law, C.K. Synergistic combustion of droplets of ethanol, diesel and biodiesel mixtures. Fuel 2012, 94, 342–347. [Google Scholar] [CrossRef]
- Rentería, J.; Gallego, A.; Gamboa, D.; Cacua, K.; Herrera, B. Effect of amide-functionalized carbon nanotubes as commercial diesel and palm-oil biodiesel additives on the ignition delay: A study on droplet scale. Fuel 2023, 338, 127202. [Google Scholar]
- Huang, X.; Wang, J.; Wang, Y.; Qiao, X.; Ju, D.; Sun, C.; Zhang, Q. Experimental study on evaporation and micro-explosion characteristics of biodiesel/n-propanol blended droplet. Energy 2020, 205, 118031. [Google Scholar]
- Bennewitz, J.W.; Badakhshan, A.; Talley, D.G. Systematic measurement of hydrocarbon fuel droplet burning rate constants and ignition delays. J. Propuls. Power 2019, 35, 690–703. [Google Scholar]




| Properties | Test Method | B0 | Test Method | B100 |
|---|---|---|---|---|
| Density (298.15 K, kg/m3) | ASTM D4052 [33] | 849.56 | ASTM D1298 [34] | 879.00 |
| Kinematic viscosity @313.00K (mm2/s) | ASTM D445 [35] | 2.60 | ASTM D445 [35] | 4.42 |
| Flash point (K) | ASTM D7094 [36] | 336.48 | ASTM D93 C [37] | 393.50 |
| Heating value (MJ/kg) | ASTM D240 [38] | 44.75 | ASTM D240 [38] | 40.03 |
| Boiling point (K) | ASTM D86 [39] | 450.15 | ASTM D1160 [40] | 603.12 |
| Initial boiling point (K) | ASTM D86 [39] | 450.15 | ASTM D1160 [40] | 603.12 |
| Distillation 90 vol.% (°K) | ASTM D86 [39] | 610.15 | ASTM D1160 [40] | 626.00 |
| Final boiling point (K) | ASTM D86 [39] | 629.15 | ASTM D1160 [40] | 630.17 |
| Boiling point range (K) | ASTM D86 [39] | 450.15–629.15 | ASTM D1160 [40] | 603.12–630.17 |
| Carbon residue (% mass) | ASTM D524 [41] | 0.08 | ASTM D4530 [42] | 0.01 |
| Cetane number | ASTM D613 [43] | 49.60 | ASTM D613 [43] | 56.80 |
| Parameter | Coefficient of Variation | ||||||||
| B0 | B5 | B10 | B15 | B20 | B25 | B50 | B75 | B100 | |
| Steady combustion rate (mm2/s) | 3.47% | 3.53% | 4.11% | 4.41% | 4.84% | 2.83% | 4.76% | 2.07% | 1.39% |
| Global combustion rate (mm2/s) | 2.84% | 2.21% | 2.51% | 2.97% | 2.89% | 1.79% | 1.86% | 3.62% | 2.01% |
| Pre-ignition time (s) | 3.14% | 3.58% | 4.15% | 4.28% | 3.89% | 5.12% | 6.25% | 5.39% | 3.16% |
| Normalized burning time (s/mm2) | 2.73% | 2.61% | 1.35% | 2.41% | 2.92% | 1.57% | 1.53% | 2.70% | 1.69% |
| Puffing occurences | 20.09% | 12.44% | 8.71% | 8.44% | 10.54% | 9.54% | 12.97% | 19.39% | |
| Puffing intensity | 43.85% | 10.49% | 11.37% | 7.60% | 18.04% | 11.02% | 18.56% | 26.27% | |
| Puffing effectiveness (mm2/s) | 8.24% | 39.81% | 14.39% | 18.49% | 15.07% | 9.71% | 11.24% | 13.38% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parveg, A.S.M.S.; Ratner, A. Droplet-Scale Combustion Analysis of Third-Generation Biodiesel–Diesel Blends. Energies 2025, 18, 1692. https://doi.org/10.3390/en18071692
Parveg ASMS, Ratner A. Droplet-Scale Combustion Analysis of Third-Generation Biodiesel–Diesel Blends. Energies. 2025; 18(7):1692. https://doi.org/10.3390/en18071692
Chicago/Turabian StyleParveg, A. S. M. Sazzad, and Albert Ratner. 2025. "Droplet-Scale Combustion Analysis of Third-Generation Biodiesel–Diesel Blends" Energies 18, no. 7: 1692. https://doi.org/10.3390/en18071692
APA StyleParveg, A. S. M. S., & Ratner, A. (2025). Droplet-Scale Combustion Analysis of Third-Generation Biodiesel–Diesel Blends. Energies, 18(7), 1692. https://doi.org/10.3390/en18071692








