Abstract
This study evaluated the production potential of M. sacchariflorus and M. giganteus depending on plantation age and soil type. The analyses showed that the leaf area index was dependent on the Miscanthus genotype, soil type, and plantation age. The mean leaf angle, on the other hand, was mainly affected by plantation age. Significant differences in plant height were found, resulting from genotype, soil type, and plantation age. The biomass yield obtained from Miscanthus plantations was also dependent on soil type, plantation age, and genotype. The biomass moisture content was to a lesser extent affected by the interactions between genotype and soil type, and between soil type and plantation age, but it was dependent on the interaction between genotype and plantation age. The calorific value of the tested biomass was mainly influenced by the Miscanthus genotype and, to a lesser extent, by plantation age and soil type. The highest calorific value was found in the biomass of M. sacchariflorus, regardless of soil type and plantation age, while the lowest was recorded for M. giganteus biomass, irrespective of soil type.
1. Introduction
In the EU, biofuels are the most significant among renewable energy sources [1,2]. In Poland, the majority of renewable energy production still comes from biomass, and this trend is likely to continue in the coming years [3,4,5]. Various plant species can be used for biomass production, such as Salix sp., Sida hermaphrodita, Spartina pectinata, Panicum virgatum, and Phalaris arundinacea. Their common feature is that they accumulate sufficient amounts of lignocellulose and are capable of adapting to different habitats [6,7,8,9,10,11,12,13,14,15,16,17,18].
In addition to these species, grasses of the genus Miscanthus spp. are also used. Within this genus, three main species can be distinguished: two occurring naturally, i.e., sugar miscanthus (M. sacchariflorus) and Chinese miscanthus (M. sinensis), and one interspecific hybrid, i.e., giant miscanthus (M. giganteus). The latter is a sterile species under European conditions, developed by crossing diploid M. sinensis with tetraploid M. sacchariflorus [19,20,21,22].
These species, due to their high yield potential, large biomass production, and ability to adapt to various habitats, can be successfully used for biomass production for energy purposes [23,24,25,26]. The previously mentioned adaptability to diverse habitats and the production potential of Miscanthus giganteus and Miscanthus sacchariflorus may play a significant role in the context of cultivation within specific micro- or macro-regions of a given country. This is because the course of growth and development may strongly depend on habitat conditions, which will, in turn, influence the subsequent yield performance of these two Miscanthus genotypes. Therefore, it is crucial to conduct studies under various environmental conditions, referring to specific regions of a country or even different parts of the world. Moreover, they are characterized by high dry matter productivity because they belong to the C4 photosynthetic pathway plants, which allows for more efficient CO2 fixation and increased tolerance to xerophytic environments. Additionally, M. giganteus plants host specific endophytic diazotrophic bacteria (Azospirillum amazonense and Herbaspirillum frisingense), which enable the fixation of atmospheric nitrogen through nodulation [27].
In recent years, numerous studies worldwide have evaluated the productivity and energy potential of Miscanthus species under different environmental and management conditions. Numerous studies worldwide have shown that soil fertility, water availability, and temperature conditions have a significant impact on the biomass yield and quality of Miscanthus giganteus and M. sacchariflorus [28]. Studies conducted in Asia, particularly in China and Japan, also indicate that M. sacchariflorus exhibits greater adaptability to variable moisture conditions and higher calorific values than M. giganteus [29,30]. The factors that significantly affect biomass production include mineral fertilization, protection against weeds, diseases and pests, moisture and thermal conditions during the growing season, habitat (soil type and its nutrient content), as well as the age of the plantation [15,31,32,33,34,35,36,37,38,39]. The aim of this study was to evaluate the yield performance of giant miscanthus (M. giganteus) and sugar miscanthus (M. sacchariflorus) grown for energy purposes, depending on habitat conditions (soil type) and plantation age. However, despite numerous reports, most research has focused on single-factor effects-either plantation age or soil type-without considering their combined influence on yield and energetic parameters. Moreover, comparative studies between these two Miscanthus species under Central European climatic conditions remain limited. Therefore, this study aims to fill this research gap by assessing how plantation age and soil type jointly influence yield, physiological indicators, and energetic properties of M. giganteus and M. sacchariflorus.
2. Materials and Methods
2.1. Experiment Design
The field experiment was established in 2013 on two production fields located 10 km apart in a straight line, differing in habitat conditions. The fields belonged to the Agricultural and Horticultural Farm owned by Tadeusz Turczuk. Each of the two fields was divided into two parts, each with an area of 1 ha, where M. giganteus and M. sacchariflorus were planted.
This study covered the years 2013–2018, during which the following parameters were assessed: biomass moisture content, biomass yield, calorific value, miscanthus plant height, leaf area index (LAI), and mean leaf angle (MTA), depending on habitat conditions, miscanthus genotypes, and plantation age.
The M. giganteus and M. sacchariflorus seedlings were supplied by Tadeusz Turczuk (owner of the Agricultural and Horticultural Farm), who specialized in their production and sale. The miscanthus plants were planted on both plantations in the second decade of May 2013 (Wilczyce—16 May 2013; Krzyków—17 May 2013). The seedlings had developed 1 to 3 leaves, and were planted at a spacing of 1 × 1 m (plant density of 10,000 plants·ha−1).
In the first location (Wilczyce), the preceding crop was permanent grassland, which was plowed in the first decade of October 2012. In the second location (Krzyków), the preceding crop was maize, harvested in the second decade of October 2012. After maize harvest, post-harvest tillage and winter plowing were carried out.
Subsequently, in 2013, in the third decade of April (i.e., three weeks before planting M. giganteus and M. sacchariflorus), the soil in both locations (Wilczyce and Krzyków) was cultivated using a disk-cultivator.
Due to the objective of the experiment, which focused on the natural soil fertility, no mineral fertilization was applied on either field. A major problem, however, was the presence of mono- and dicotyledonous weeds. Therefore, in order to limit weed infestation on the newly established miscanthus plantations, an herbicide treatment was carried out.
A tank mixture of two herbicides was applied: Callisto 100 SC (a.i. mesotrione) at a dose of 1.5 l·ha−1 and Fernando 225 EC (a.i. clopyralid + fluroxypyr + triclopyr) at a dose of 3.0 l·ha−1. The aim of the treatment was to control annual monocotyledonous weeds (e.g., Echinochloa crus-galli) and annual dicotyledonous weeds (e.g., Chenopodium album, Amaranthus retroflexus, Viola arvensis, Galinsoga parviflora), as well as perennial dicotyledonous weeds (e.g., Taraxacum officinale, Rumex crispus, Urtica dioica, Arctium lappa). The herbicide treatment was carried out when the miscanthus plants were at the 5–7 leaf stage (BBCH 15–17) (Figure 1).

Figure 1.
Weedy miscanthus plantation (herbicide treatment with a tank mixture of Callisto 100 SC + Fernando 225 EC).
2.2. Study Site and Soil Properties
The experiments were located in the southwestern part of Poland, in the Lower Silesian Voivodeship, on fields belonging to the Agricultural and Horticultural Farm of Tadeusz Turczuk. The first field was situated in the village of Wilczyce (51°19′16.29″ N, 17°14′39.70″ E), while the second field was located near the village of Krzyków (51°09′45.21″ N, 17°22′31.70″ E).
Before establishing the experiments, soil samples were collected from each plantation to determine physico-chemical properties. The first experiment (Wilczyce) was established on medium-heavy alluvial soil, whereas the second experiment (Krzyków) was established on brown soil [40].
The characteristics of the habitats are presented in Table 1. Soil pH (pHKCl) was determined by the potentiometric method according to Polish Standard PN-ISO 10390/1997 [41]. Available phosphorus and potassium in the soils were determined according to the Egner Riehm method using applicable Polish Standards or Industry Procedures. The phosphorus level was measured using the spectrophotometric method according to Polish Standard PN-R-04023/1996 [42], while potassium was determined by atomic emission spectrometry according to Polish Standard PN-R-04022/1996+Az1/2002 [43]. Magnesium content was determined using the atomic spectrometry technique based on Polish Standard PN-R-04020/1994/Az1/2004 [44]. The particle size distribution was determined by the laser method and the organic matter content with the Tyurin method [45].

Table 1.
Habitat characteristics of giant miscanthus (M. giganteus) and sugar miscanthus (M. sacchariflorus) plantations.
2.3. Climatic Conditions
Meteorological data from 2013 to 2018 (Table 2 and Table 3) indicate differences in precipitation and air temperature. The warmest year was 2015, with a mean daily air temperature of 10.5 °C. In that year, positive temperatures were recorded in January and February (2.2 °C and 1.9 °C, respectively). The warmest months of 2015, in terms of mean daily temperature, were July (20.2 °C) and August (22.4 °C). In contrast, the coldest year was 2013, with a mean annual daily temperature of 9.1 °C. During this year, negative air temperatures were recorded in January (−1.7 °C) and March (−0.8 °C). The coldest month of the six-year study period was February 2018, when the mean daily air temperature was −2.7 °C.

Table 2.
Mean daily air temperature (°C).

Table 3.
Sums of atmospheric precipitation (mm).
During the growing season, the wettest year was 2017, when the highest average total precipitation was recorded, amounting to 544.2 mm. The heaviest rainfall occurred in June (130.0 mm) and September (97.7 mm) of 2013, as well as in July 2017 (106.1 mm). Furthermore, 2013 and 2014 had very similar total precipitation levels, amounting to 460.5 mm and 484.9 mm, respectively. The driest year was 2015, with the lowest annual total precipitation of 357.3 mm. In that year, from May to September—the period of intensive miscanthus growth—only 175.7 mm of rainfall was recorded.
2.4. Determination of the Leaf Area Index (LAI) and Mean Leaf Tip Angle (MTA)
Due to various measurement techniques, it is possible to quickly and non-invasively estimate yield during the growing season [46,47,48,49]. For this purpose, the LAI-2000 measuring device (LI-COR, Inc., Lincoln, NE, USA) can be used, which makes it possible to assess leaf area index (LAI) and mean leaf angle (MTA). These indices effectively describe changes occurring in the canopy and allow yield prediction in advance. This applies not only to typical agricultural crops but also to plants used for energy purposes [50,51,52,53].
The miscanthus canopy was assessed by determining the leaf area index (LAI) and the mean leaf angle (MTA) using the LAI-2000 device (LI-COR, Inc., USA). LAI and MTA measurements were conducted from 2013 to 2017 during the peak growing season, i.e., in the first decade of August, separately for each miscanthus genotype (Figure 2A,B) and location. Measurements included one assessment at the edge of the stand and four assessments within the canopy at 10 randomly selected points on each plantation.

Figure 2.
(A) Miscanthus × giganteus; (B) Miscanthus sacchariflorus.
2.5. Assessment of the Height, Yield, Total Moisture and Calorific Value of Miscanthus Biomass
The height of miscanthus plants was measured after the end of the growing season from 2014 to 2018, i.e., during the winter, approximately one week before biomass harvest (Figure 3A,B). Plant height was determined using a GR 500 Professional Bosch telescopic measuring rod (Robert Bosch Power Tools GmbH, Max-Lang-Strasse 40-46, 70771 Leinfelden-Echterdingen, Germany) on 10 randomly selected miscanthus plants at seven randomly chosen points on each plantation.

Figure 3.
(A) Plant height measurement (M. giganteus); (B) Plant height measurement (M. sacchariflorus).
Miscanthus biomass was harvested in a single-stage process during the winter using a Claas Jaguar 860 self-propelled chopper (Claas KGaA mbH Muhlenwinkel 1, 33428 Harsenwinkel, Nordrhein-Westfalen, Germany), which shredded the miscanthus stems into chips approximately 4 cm in length (Figure 4).

Figure 4.
Claas Jaguar 860 self-propelled chopper used for harvesting miscanthus biomass.
The first harvest of miscanthus biomass was carried out in the winter (second decade of March) in 2014 (one-year-old plantation). In the following years, harvesting was also performed in winter, but in the third decade of February (2015—two-year-old plantation; 2016—three-year-old plantation; 2017—four-year-old plantation). Only in 2018 was the harvest conducted in the third decade of March (five-year-old plantation) (Figure 5A,B).

Figure 5.
(A) Winter harvest M. giganteus; (B) Winter harvest M. sacchariflorus.
From 2014 to 2018, after cutting the miscanthus, biomass samples (chips) were collected from nine sampling points. From these, primary samples of 5.0 kg each were taken, followed by three subsamples of 1.0 kg each for further laboratory analyses to determine total moisture content [54] and calorific value in working condition [55]. These analyses were conducted at the coal laboratory of BOT Elektrownia Opole S.A. (Opole, Poland).
2.6. Statistical Analysis
The results concerning the yield, moisture, height, calorific value and LAI and MTA value were statistically processed using the classic analysis of variance at a significance level of p = 0.05. To determine significant differences in the results for the subsequent years of this study, the Tukey test was used at a significance level of p = 0.05. The analyses were carried out using the ARM (Agriculture Research Management) program version 2024.1.
3. Results and Discussion
The main criteria to be considered when selecting a plant for biomass production for energy purposes are high biomass yield, appropriate calorific value, and proper selection of the species according to habitat conditions [15,18,26,56].
International studies consistently show that Miscanthus yields rise during establishment and reach a plateau after approximately three to five years, with the steepest increases between years 1 and 3 [28,57]. In temperate Europe, mature stands typically achieve about 10–20 t·ha−1 dry matter depending on site and management [58]. Across regions, soil water availability and nutrient supply are key drivers of yield variation [35,58]. For East Asian environments, studies on M. sacchariflorus also report strong site effects on productivity across contrasting locations [59].
These findings confirm the universal nature of the interactions observed in our study and demonstrate that soil fertility and plantation maturity are critical drivers of the energetic performance of Miscanthus crops, irrespective of region or genotype.
In our study, conducted from 2013 to 2018, the production potential of M. giganteus and M. sacchariflorus for energy purposes was evaluated depending on variable habitat conditions (mainly soil type) and plantation age. These plantations were established on two soil types (Wilczyce—medium alluvial soil; Krzyków—brown soil) that differed in terms of moisture conditions, particle size distribution, soil pH, organic matter content, macronutrients such as phosphorus, potassium, and magnesium, as well as the preceding crop (Table 1).
This article discusses the results regarding LAI, MTA, miscanthus plant height, biomass yield and moisture content, as well as calorific value obtained during the 2013/2014 (one-year-old plantation), 2014/2015 (two-year-old plantation), 2015/2016 (three-year-old plantation), 2016/2017 (four-year-old plantation), and 2017/2018 (five-year-old plantation) seasons (Figure 6, Figure 7, Figure 8, Figure 9, Figure 10 and Figure 11).
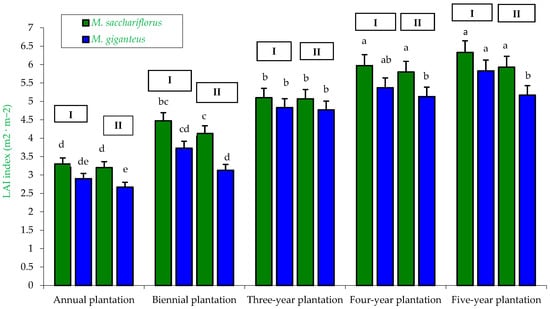
Figure 6.
Average Leaf Area Index (LAI) of M. sacchariflorus and M. giganteus depending on the plantation age and habitat conditions. I—first location (Wilczyce—medium-heavy alluvial soil); II—second location (Krzyków—brown soil); Means followed by same letter do not significantly differ (p = 0.05, Tukey’s HSD).
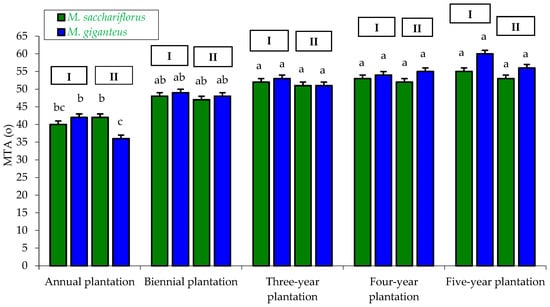
Figure 7.
Average Mean Leaf Tip Angle (MTA) of M. sacchariflorus and M. giganteus depending on the plantation age and habitat conditions. I—first location (Wilczyce—medium-heavy alluvial soil); II—second location (Krzyków—brown soil); Means followed by same letter do not significantly differ (p = 0.05, Tukey’s HSD).
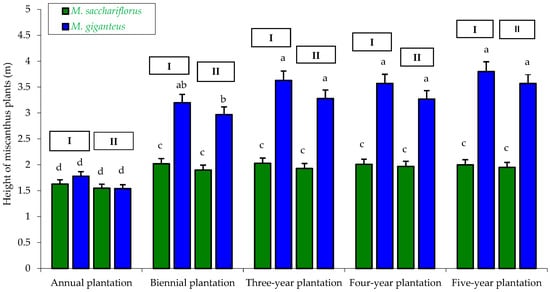
Figure 8.
Height of M. sacchariflorus and M. giganteus depending on the plantation age and habitat conditions. I—first location (Wilczyce—medium-heavy alluvial soil); II—second location (Krzyków—brown soil); Means followed by same letter do not significantly differ (p = 0.05, Tukey’s HSD).
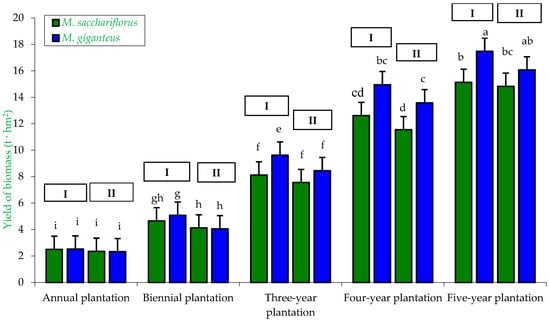
Figure 9.
Yield of M. sacchariflorus and M. giganteus depending on the plantation age and habitat conditions. I—first location (Wilczyce—medium-heavy alluvial soil); II—second location (Krzyków—brown soil); Means followed by same letter do not significantly differ (p = 0.05, Tukey’s HSD).
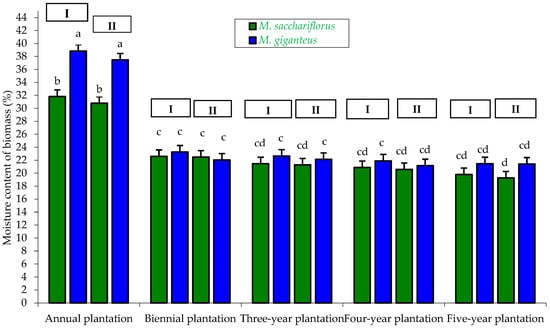
Figure 10.
Moisture content of biomass M. sacchariflorus and M. giganteus depending on the plantation age and habitat conditions. I—first location (Wilczyce—medium-heavy alluvial soil); II—second location (Krzyków—brown soil); Means followed by same letter do not significantly differ (p = 0.05, Tukey’s HSD).
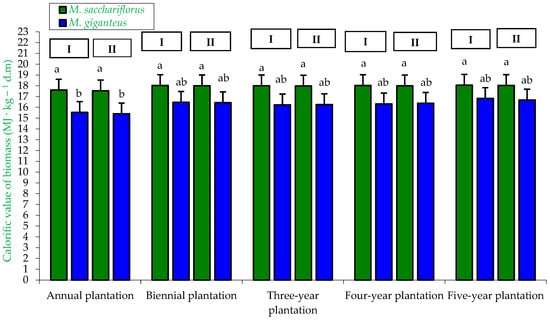
Figure 11.
Calorific value of biomass M. sacchariflorus and M. giganteus depending on the plantation age and habitat conditions. I—first location (Wilczyce—medium-heavy alluvial soil); II—second location (Krzyków—brown soil); Means followed by same letter do not significantly differ (p = 0.05, Tukey’s HSD).
3.1. Leaf Area Index and MTA
According to Brandić et al. [46], Parker [47], Cui et al. [48], Kross et al. [49] and Biskupski et al. [51], it is possible to estimate crop yield during the growing season using non-invasive methods, such as assessing the leaf area index (LAI) and the mean leaf angle (MTA). The Leaf Area Index (LAI) is a parameter that allows for a preliminary and rapid assessment of canopy structure and yield potential, as it provides an estimate of the plants’ ability to carry out photosynthesis and transpiration processes, which in turn determine biomass accumulation. The Mean Tilt Angle (MTA), on the other hand, is a parameter that enables the evaluation of canopy structure and the proper functioning of the photosynthetic process in plants. This is because the effective interception of solar radiation by leaves depends on their inclination angle, which is indirectly related to the overall nutritional status and growth performance of the entire plant.
In our study, the LAI and MTA of M. giganteus and M. sacchariflorus plants were also evaluated depending on variable habitat conditions and plantation age.
The highest average LAI, amounting to 6.33 (medium alluvial soil—Wilczyce) and 5.93 (brown soil—Krzyków), was observed for M. sacchariflorus plants in the last year of this study (five-year-old plantation). In contrast, the lowest LAI, 2.67 (brown soil—Krzyków), was recorded for M. giganteus plants in the first year of this study (one-year-old plantation) (Figure 6). Furthermore, it was found that the LAI was dependent on the miscanthus genotype, habitat, and plantation age (Table 4, Table 5 and Table 6).

Table 4.
The influence of genotype and habitat on the average yield, moisture content and calorific value of biomass as well as on height, leaf area index (LAI) and mean leaf tip angle (MTA).

Table 5.
The influence of genotype and years on the average yield, moisture content and calorific value of biomass as well as on height, leaf area index (LAI) and mean leaf tip angle (MTA).

Table 6.
The influence of habitat and years on the average yield, moisture content and calorific value of biomass as well as on height, leaf area index (LAI) and mean leaf tip angle (MTA).
The highest LAI for the genotype × habitat interaction was observed for M. sacchariflorus plants (LAI = 5.0) compared to M. giganteus plants (LAI = 4.2), which was confirmed by statistical analysis (Table 4).
In the genotype × year interaction, very pronounced differences in LAI were observed. The highest LAI values (5.5–6.1) were recorded in the last year of this study (five-year-old plantation), regardless of the miscanthus genotype. In contrast, the lowest LAI values were observed in the first year of this study (one-year-old plantation). In this case, significant differences between genotypes were also noted, with LAI for M. sacchariflorus plants being 3.3 and for M. giganteus plants 2.8 (Table 5).
Similarly, for the habitat × plantation age interaction, significant differences in LAI were observed. The highest LAI values were recorded for the four- and five-year-old plantations, amounting to 5.5–5.7 and 5.6–6.1, respectively, while the lowest LAI was obtained for the one-year-old plantation (LAI = 2.9–3.1) (Table 6).
The results for the mean leaf angle (MTA) differed. The highest MTA values (ranging from 51 to 60) were recorded for the three-, four-, and five-year-old plantations, regardless of genotype and habitat. In contrast, the lowest MTA values (ranging from 36 to 42) were observed for the one-year-old plantation. The differences in MTA between the one-year-old plantation and the three-, four-, and five-year-old plantations were statistically significant (Figure 7).
In the genotype × habitat interaction, despite observed differences in MTA values (46–52), statistical analysis did not confirm their significance (Table 4).
The genotype × plantation age interaction confirmed significant differences in MTA values between miscanthus from the four-year-old (MTA = 52–54) and five-year-old plantations (MTA = 54–58) and miscanthus from the one-year-old plantation (MTA = 39–41) (Table 5).
The habitat × plantation age interaction showed differences in MTA values between the one-year-old (39–41), two-year-old (48–49), and three-year-old plantations (44–51) compared to the four-year-old (53–54) and five-year-old plantations (55–57), which were confirmed statistically (Table 6).
Similar results regarding the influence of soil type, plantation age, and miscanthus genotype on LAI have also been reported by other researchers [43,53]. According to Kuś et al. [60], LAI was lowest in the two-year-old plantation, both on heavy soil (LAI = 4.2–5.4) and medium soil (LAI = 4.4–5.6). In the following years of cultivation (four-year-old plantation), LAI increased and was dependent on miscanthus genotype and soil type: M. sacchariflorus—heavy soil LAI = 7.5; medium soil LAI = 4.7; M. giganteus—heavy soil LAI = 5.9; medium soil LAI = 4.1.
In the study conducted by Feledyn-Szewczyk et al. [52], significant effects of plantation age and soil type were also observed on a seven- and eight-year-old plantation. On the seven-year-old plantation, LAI was 3.70 on light soil and 5.30 on heavy soil. On the eight-year-old plantation, LAI was 5.35 on light soil and 7.25 on heavy soil.
3.2. Height of Miscanthus Plants
Plant height measurements showed that over the four years of the experiment (two-, three-, four-, and five-year-old plantations), M. giganteus plants were the tallest compared to M. sacchariflorus, which was confirmed statistically. The height of M. giganteus plants ranged from 2.97 m to 3.80 m, whereas M. sacchariflorus plants ranged from 1.90 m to 2.03 m. The shortest plants, regardless of miscanthus genotype and habitat, were recorded in the first year of the experiment (one-year-old plantation), with heights ranging from 1.54 m to 1.78 m (Figure 8).
Miscanthus plant height was also dependent on the genotype × habitat interaction, which was statistically confirmed. The tallest plants were M. giganteus (3.20 m) from the plantation located on medium alluvial soil (Wilczyce), while the shortest were M. sacchariflorus plants, regardless of habitat type (Krzyków—1.86 m and Wilczyce—1.94 m) (Table 4).
Similarly, miscanthus height was influenced by the genotype × plantation age interaction. The tallest plants were M. giganteus from the three-year-old (3.46 m), four-year-old (3.42 m), and five-year-old plantations (3.58 m). In contrast, M. sacchariflorus plants were significantly shorter compared to M. giganteus, regardless of plantation age, which was statistically confirmed. Only in the first year of cultivation (one-year-old plantation) were plant heights similar between genotypes (M. sacchariflorus—1.59 m; M. giganteus—1.66 m), and these differences were statistically confirmed (Table 5).
Miscanthus plant height was also dependent on the habitat × plantation age interaction. The shortest plants were recorded in the one-year-old plantations (1.55–1.71 m), regardless of habitat. In contrast, plants from the two-year-old (2.43–2.61 m), three-year-old (2.61–2.83 m), four-year-old (2.62–2.79 m), and five-year-old plantations (2.66–2.90 m) were the tallest, showing significant differences compared to the one-year-old plantation (Table 6).
According to the study by Matyka and Kuś [61], the height of M. giganteus plants was significantly influenced by plantation age and soil type. The tallest plants (average over six years) were recorded on brown soil (2.5 m), while the shortest were on black soil (2.0 m). Plantation age also affected M. giganteus height, with the shortest plants observed in the two-year-old plantation (average 2.1 m) and the tallest in the six-year-old plantation (average 2.7 m).
In the study conducted by Feledyn-Szewczyk et al. [52], plantation age and soil type were also found to have a significant effect on the height of M. sacchariflorus × M. sinensis—clone M-115. On the seven-year-old plantation, miscanthus plant height was 2.21 m on medium soil and 2.56 m on heavy soil. On the eight-year-old plantation, the average plant height was 2.13 m on light soil and 3.76 m on heavy soil.
The results of Jeżowski [33] confirm our findings regarding the influence of miscanthus genotype and plantation age. According to his study, the height of different miscanthus genotypes was lowest in the first year of cultivation and highest in the third year. The tallest plants were M. giganteus (MG/1—2.87 m and MG/2—2.95 m), while the shortest were M. sinensis × M. sacchariflorus (MS/4—1.87 m) and M. sinensis (MS/5—1.73 m).
3.3. Yield of Biomass
Five-year observations showed that biomass yield was dependent on plantation age, habitat, and miscanthus genotype. The lowest biomass yields were obtained in the first year of the experiment (one-year-old plantation), ranging from 2.35 to 2.52 t·hm2. In the following years, miscanthus biomass yield increased progressively. The highest biomass yield (statistically confirmed), 17.48 t·hm2, was recorded in the last year of this study (five-year-old plantation) for M. giganteus on medium alluvial soil in Wilczyce, compared to M. sacchariflorus (regardless of habitat type). Significant differences in biomass yield were also observed due to genotype (biomass yield was always higher for M. giganteus regardless of plantation age) and habitat (yield was always higher on the medium alluvial soil in Wilczyce, regardless of age) (Figure 9).
Biomass yield from miscanthus was also dependent on the genotype × habitat interaction, which was statistically confirmed. The highest biomass yield, statistically significant, was obtained from M. giganteus plants (9.93 t·hm2) from the plantation located on medium alluvial soil (Wilczyce), while the lowest yield was recorded for M. sacchariflorus plants from the plantation on brown soil in Wilczyce (8.08 t·hm2) (Table 4).
Similarly, miscanthus biomass yield was influenced by the genotype × plantation age interaction. The highest biomass yield, statistically confirmed, was obtained from the five-year-old plantation, with M. giganteus yielding 16.78 t·hm2 and M. sacchariflorus 14.98 t·hm2. The lowest biomass yields were recorded from the one-year-old plantation (M. giganteus—2.42 t·hm2; M. sacchariflorus—2.43 t·hm2) and the two-year-old plantation (M. sacchariflorus—4.38 t·hm2; M. giganteus—4.57 t·hm2), which was statistically confirmed (Table 5).
Furthermore, miscanthus biomass yield was also dependent on the habitat × plantation age interaction. The lowest biomass yields were recorded for plants from the one-year-old plantation (2.33–2.51 t·hm2), regardless of habitat. The highest biomass yield, statistically confirmed, was obtained from the five-year-old plantation located on medium alluvial soil in Wilczyce (16.31 t·hm2). For the two-, three-, four-, and five-year-old plantations, differences in biomass yield were statistically confirmed and resulted from plantation location (habitat type) and plantation age (Table 6).
Similar results regarding biomass yield depending on genotype and plantation age were obtained by Jeżowski et al. [62], Jeżowski [33], and Dubis et al. [36].
The results of Jeżowski [33] also confirm our findings, as his study showed that biomass yield of different miscanthus genotypes was lowest in the first year of cultivation and highest in the third year. The highest biomass was produced by M. giganteus (MG/1—1.93 kg·m−1 and MG/2—2.17 kg·m−1), while the lowest was recorded for M. sinensis (MS/5—0.99 kg·m−1 and MS/6—1.06 kg·m−1).
Similar conclusions were drawn by Dubis et al. [36], who found that over 11 years of research, the lowest biomass yields were obtained in the first year, while the highest were recorded in the last years of the study. Average biomass yields were also dependent on miscanthus genotype. The authors reported that, averaged over 11 years, the productivity of M. giganteus (15.5 t·ha−1) was higher compared to M. sacchariflorus (9.3 t·ha−1).
The results of Borkowska and Molas [17] also indicate a significant influence of genotype and plantation age on miscanthus biomass yield. The lowest biomass yields were obtained in the first year of cultivation (M. giganteus—2.40 t·ha−1; M. sacchariflorus—0.44 t·ha−1). In contrast, the highest biomass yields were recorded in the last year (four-year-old plantation), with M. giganteus producing 29.43 t·ha−1 and M. sacchariflorus 10.12 t·ha−1.
According to Kuś and Matyka [15] and Matyka and Kuś [63], miscanthus biomass yield may depend on habitat (soil type) and genotype. Their studies showed that the highest dry matter yields were obtained for M. giganteus on heavy soil (25.4 t·ha−1), while the lowest dry matter yield was recorded for M. sinensis Silver Feather M-40 (14.4 t·ha−1) on medium soil. Different results were obtained in a five-year experiment conducted on heavy and light soils. The highest dry matter yields were obtained for M. giganteus (19.0–19.1 t·ha−1) and M. sacchariflorus Robustus × M. sinensis (M-115) (19.3–20.0 t·ha−1), regardless of soil type. The lowest dry matter yields were recorded for M. sinensis (M-40) on heavy soil (16.6 t·ha−1) and light soil (15.1 t·ha−1), and for M. sinensis (M-105)—heavy soil (15.1 t·ha−1), light soil (16.5 t·ha−1).
3.4. Moisture Content in Biomass
One of the most important parameters determining calorific value is the moisture content of the biomass. Air-dried biomass of good quality, intended for direct combustion, should contain up to 20% moisture [13]. In our study, the moisture content of miscanthus biomass was mainly dependent on plantation age and, to a lesser extent, on genotype and habitat. The highest moisture content was recorded for biomass obtained from M. giganteus in the one-year-old plantation, regardless of habitat (37.47–38.83%). The lowest moisture content, statistically confirmed, was found in biomass from M. sacchariflorus in the five-year-old plantation located on brown soil in Krzyków (19.27%) (Figure 10).
Miscanthus biomass moisture content was, to a lesser extent, dependent on the genotype × habitat interaction, which was statistically confirmed. The highest moisture content, statistically confirmed, was observed in biomass from M. giganteus (24.84–25.62%), regardless of habitat type. The lowest moisture content was recorded for biomass from M. sacchariflorus (22.88–23.32%), also independent of plantation location (habitat) (Table 4).
Miscanthus biomass moisture content was also dependent on the genotype × plantation age interaction. The highest moisture content, statistically confirmed, was recorded in biomass from the one-year-old plantation, with M. giganteus at 38.15% and M. sacchariflorus at 31.32%. The lowest biomass moisture content was obtained from M. sacchariflorus in the five-year-old plantation (19.53%), which was also statistically confirmed (Table 5).
Furthermore, miscanthus biomass moisture content was only slightly dependent on the habitat × plantation age interaction. The highest moisture content, statistically confirmed, was recorded for biomass from the one-year-old plantation (34.13–35.33%), regardless of habitat type. The lowest moisture content was observed in biomass from the five-year-old plantation located on brown soil in Krzyków (20.34%). For the two-, three-, four-, and five-year-old plantations, differences in biomass moisture content with respect to habitat (soil type) were not significant, which was statistically confirmed (Table 6).
According to Kuś and Matyka [15], harvesting miscanthus plants in late autumn results in biomass with increased moisture content (35–45%), whereas shifting the harvest to late winter or early spring allows for obtaining biomass with significantly lower moisture content (20–25%). A similar view was expressed by Borkowska and Molas [17], who demonstrated in their study that during autumn harvest (October), the moisture content of M. giganteus biomass can reach up to 49.78%, and M. sacchariflorus 27.0%. In contrast, during spring harvest (end of March), biomass moisture content decreases significantly, reaching 22.31% for M. giganteus and 13.9% for M. sacchariflorus.
3.5. Calorific Value
The most important thermophysical parameter of biomass intended for energy purposes is its calorific value. In our study, it was found that calorific value was mainly dependent on the miscanthus genotype. The highest calorific value was observed in biomass from M. sacchariflorus (17.53–18.05 MJ·kg−1 d.m.), regardless of habitat type and plantation age, which was statistically confirmed. In contrast, the lowest statistically confirmed calorific values were recorded for biomass from M. giganteus obtained from the one-year-old plantation (15.4–15.53 MJ·kg−1 d.m.), regardless of habitat type (Figure 11).
The highest calorific value of miscanthus biomass for the genotype × habitat interaction was observed only within the M. sacchariflorus genotype (17.91–17.94 MJ·kg−1 d.m.). Similarly, within the genotype, the lowest calorific value was obtained for biomass from M. giganteus (16.23–16.27 MJ·kg−1 d.m.), which was confirmed by statistical analysis (Table 4).
For the genotype × plantation age interaction, small but significant differences in calorific value were observed. The highest calorific value was recorded for biomass from M. sacchariflorus grown on two-year-old (18.01 MJ·kg−1 d.m.), three-year-old (17.99 MJ·kg−1 d.m.), four-year-old (18.00 MJ·kg−1 d.m.), and five-year-old plantations (18.04 MJ·kg−1 d.m.). In contrast, the lowest calorific value was observed for biomass from the one-year-old M. giganteus plantation (15.47 MJ·kg−1 d.m.), which was statistically confirmed (Table 5).
Moreover, the calorific value of miscanthus biomass was only slightly influenced by the habitat × plantation age interaction. The highest calorific value was recorded for biomass from two-, three-, four-, and five-year-old plantations (ranging from 17.12 to 17.43 MJ·kg−1 d.m.), regardless of habitat type, which was also statistically confirmed. In contrast, the lowest calorific value, ranging from 16.47 to 16.57 MJ·kg−1 d.m., was observed for biomass from the one-year-old plantations, regardless of location (soil type). These differences were statistically confirmed (Table 6).
According to Dłużniewska and Jaworska [64] and Stolarski et al. [65], the moisture content of biomass at harvest significantly affects its calorific value. These authors demonstrated that the harvest time (spring or autumn) can lead to an approximately twofold increase in the calorific value of giant miscanthus. At a moisture content of 55.0% (autumn harvest), the calorific value of miscanthus was 12.5 MJ·kg−1 d.m., whereas at a moisture content of 25.0% (spring harvest), the calorific value increased to 7.5 MJ·kg−1 d.m.
The calorific values of miscanthus biomass obtained in the present study are comparable to those reported by Lu et al. [29] and Kowalczyk-Juśko et al. [25], but are lower than the values reported by Lisowski and Borusiewicz [66] and Lisowski et al. [67].
The energetic assessment of biomass from M. giganteus and M. sacchariflorus demonstrated that both species exhibit high calorific values comparable to other lignocellulosic feedstocks, such as Sida hermaphrodita (16.5–17.2 MJ·kg−1 d.m.) [68], Phalaris arundinacea (17.5–18.2 MJ·kg−1 d.m.) [69] and Miscanthus (17–18 MJ·kg−1 d.m.) [70].
Considering the average calorific value of approximately 17.40 MJ·kg−1 d.m. and dry matter yields exceeding 15.88 t·ha−1 in mature Miscanthus plantations, the estimated gross energy yield reaches about 276,312 GJ·ha−1, confirming the high bioenergy potential of these species under Central European conditions. Similar energy outputs were reported in studies across Europe, where Miscanthus × giganteus achieved calorific values between 17 and 18 MJ·kg−1 d.m. and yields typically ranged from 12 to 20 t·ha−1 d.m., depending on site and management [70,71].
4. Conclusions
The non-invasive method used in the experiment, which enabled the assessment of the leaf area index (LAI) and mean leaf tip angle (MTA), demonstrated that it is possible to estimate biomass yield already during the growing season. These indices allow, with some advance, the identification of changes occurring within the Miscanthus canopy.
The analyses showed that the LAI was dependent on the Miscanthus genotype, soil type, and plantation age. Plant height, on the other hand, was influenced by plantation age and genotype. The biomass yield obtained from Miscanthus plantations depended on soil type, plantation age, and genotype. Biomass moisture content was to a lesser extent affected by the interaction between genotype and habitat, and between habitat and plantation age, but it was influenced by the interaction between genotype and plantation age.
The calorific value of the analyzed biomass was mainly determined by the Miscanthus genotype and, to a lesser extent, by plantation age and soil type. The highest calorific value was observed for the biomass obtained from M. sacchariflorus, regardless of soil type and plantation age, whereas the lowest was found for the biomass of M. giganteus, also irrespective of soil type.
The results of this study confirm that plantation age and soil type are key factors determining the yield and energetic value of Miscanthus biomass. Among the studied species, M. giganteus achieved higher biomass yields, while M. sacchariflorus exhibited slightly higher calorific values and lower moisture content. The findings provide a scientific basis for selecting suitable species–soil combinations in commercial bioenergy production systems. It is recommended that M. giganteus be cultivated on fertile alluvial soils to maximize yield, whereas M. sacchariflorus is more suitable for moderately fertile or brown soils where energy density and moisture reduction are priorities.
Future research should address long-term productivity under fertilized and unfertilized conditions, evaluate greenhouse gas balances, and integrate life-cycle energy assessments to support the sustainable use of Miscanthus as a renewable energy feedstock.
Author Contributions
Conceptualization, T.R.S.; methodology, T.R.S. and J.G.; validation, T.R.S. and M.Z.; formal analysis, T.R.S. and J.B.; investigation, T.R.S.; resources, T.R.S.; writing—original draft preparation, T.R.S. and J.G.; writing—review and editing, T.R.S., M.Z. and J.B.; visualization, T.R.S.; supervision, J.G., M.Z. and T.R.S.; funding acquisition, J.G. and M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
The authors wish to thank the Agricultural and Horticultural Farm of Tadeusz Turczuk for providing miscanthus seedlings, for valuable guidance on their cultivation and harvest, and for allowing the experiments to be conducted on the farm’s fields.
Conflicts of Interest
Author Justyna Belcar was employed by the company Farming Cooperative SAN. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- European Environment Agency. Share of Energy Consumption from Renewable Sources in Europe (8th EAP). Available online: https://www.eea.europa.eu/ims/share-of-energy-consumption-from (accessed on 25 August 2025).
- Bouter, A.; Hurtig, O.; Besseau, R.; Buffi, M.; Kulisic, B.; Scarlat, N. Updating the greenhouse gas emissions of liquid biofuels from Annex V of the Renewable Energy Directive II (RED II): An overview. Biomass Bioenergy 2025, 199, 107886. [Google Scholar] [CrossRef]
- Malec, M. Energy use of biomass—Polish contribution to renewable energy sources. Anal. IPE 2023, 2, 1–8. (In Polish) [Google Scholar]
- Statistics Poland. Energy from Renewable Sources in 2021; Statistics Poland: Warsaw, Poland, 2022; 96p.
- Syp, A.; Faber, A.; Borzęcka-Walker, M. An assessment of biomass production potential in Poland and impacts on food security. J. Food Agric. Environ. 2013, 11, 1721–1725. [Google Scholar]
- Marks-Bielska, R.; Bielski, S.; Pik, K.; Kurowska, K. The importance of renewable energy sources in Poland’s energy mix. Energies 2020, 13, 4624. [Google Scholar] [CrossRef]
- Pietrzak, M.B.; Igliński, B.; Kujawski, W.; Iwański, P. Energy transition in Poland—Assessment of the renewable energy sector. Energies 2021, 14, 2046. [Google Scholar] [CrossRef]
- Bełdycka-Bórawska, A.; Bórawski, P.; Borychowski, M.; Wyszomierski, R.; Bórawski, M.B.; Rokicki, T.; Ochnio, L.; Jankowski, K.; Mickiewicz, B.; Dunn, J.W. Development of solid biomass production in Poland, especially pellet, in the context of the world’s and the European Union’s climate and energy policies. Energies 2021, 14, 3587. [Google Scholar] [CrossRef]
- Borkowska, H.; Wardzińska, K. Some effects of Sida hermaphrodita R. cultivation on sewage sludge. Pol. J. Environ. Stud. 2003, 12, 119–122. [Google Scholar]
- Sekutowski, T.R.; Zardzewiały, M.; Belcar, J.; Gorzelany, J. The effect of harvest time and plantation age on the yield, chemical composition and calorific value of reed canary grass (Phalaris arundinacea L.) intended for energy purposes. Appl. Sci. 2024, 14, 11194. [Google Scholar] [CrossRef]
- Kieloch, R.; Gołębowska, H.; Sienkiewicz-Cholewa, U. Impact of habitat conditions on the biological traits of the reed canary grass (Phalaris arundinacea L.). Acta Agrobot. 2015, 68, 205–210. [Google Scholar] [CrossRef][Green Version]
- Majtkowski, W. Biodiversity of energetic crops as a basis for sustainable development. Probl. Inż. Rol. 2006, 2, 25–36. (In Polish) [Google Scholar][Green Version]
- Podlaski, S.; Chołuj, D.; Wiśniewski, G. Production of biomass from energy crops. Post. Nauk Rol. 2010, 62, 163–174. (In Polish) [Google Scholar][Green Version]
- Szczukowski, S.; Tworkowski, J.; Stolarski, M.; Przyborowski, J. Biomass yield of willow coppice grown on arable land in annual cutting cycle. Fragm. Agron. 2004, 21, 5–18. (In Polish) [Google Scholar][Green Version]
- Kuś, J.; Matyka, M. Productivity of selected crops planted for energy purposes depending on soil quality. Fragm. Agron. 2009, 26, 103–110. (In Polish) [Google Scholar][Green Version]
- Muylle, H.; van Hulle, V.; de Vliegher, A.; Baert, J.; van Bockstaele, E.; Roldán-Ruiz, I. Yield and energy balance of annual and perennial lignocellulosic crops for biorefinery use: A 4-year field experiment in Belgium. Eur. J. Agron. 2015, 63, 62–70. [Google Scholar] [CrossRef]
- Borkowska, H.; Molas, R. Yield comparison of four lignocellulosic perennial energy crop species. Biomass Bioenergy 2013, 51, 145–153. [Google Scholar] [CrossRef]
- Lewandowski, I. Biomass production from lignocellulosic energy crops. In Encyclopedia of Applied Plant Sciences, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 3, pp. 159–163. [Google Scholar] [CrossRef]
- Greef, J.M.; Deuter, M. Syntaxonomy of Miscanthus × giganteus. Angew. Bot. 1993, 67, 87–90. [Google Scholar]
- Anderson, E.; Arundale, R.; Maughan, M.; Oladelnde, A.; Wycislo, A.; Volgt, T. Growth and agronomy of Miscanthus × giganteus for biomass production. Biofuels 2011, 2, 71–87. [Google Scholar] [CrossRef]
- Scally, L.; Hodkinson, T.R.; Jones, M.B. Origins and taxonomy of Miscanthus. In Miscanthus for Energy and Fibre; Jones, M.B., Walsh, M., Eds.; James & James: London, UK, 2013; pp. 1–9. [Google Scholar]
- Cichorz, S.; Gośka, M.; Litwiniec, A. Perennial grasses from the Miscanthus genus—Potential source of renewable energy. Biul. IHAR 2014, 274, 133–151. (In Polish) [Google Scholar] [CrossRef]
- Strašil, Z. Evaluation of Miscanthus grown for energy use. Res. Agric. Eng. 2016, 62, 92–97. [Google Scholar] [CrossRef]
- Fowdar, H.; Payne, E.; Schang, C.; Zhang, K.; Deletic, A.; McCarthy, D. How well do stormwater green infrastructure respond to changing climatic conditions? J. Hydrol. 2021, 603, 126887. [Google Scholar] [CrossRef]
- Kowalczyk-Juśko, A.; Mazur, A.; Pochwatka, P.; Janczak, D.; Dach, J. Evaluation of the effects of using the giant miscanthus (Miscanthus × giganteus) biomass in various energy conversion processes. Energies 2022, 15, 3486. [Google Scholar] [CrossRef]
- Bilandzija, N.; Voca, N.; Jelcic, B.; Jurisic, V.; Matin, A.; Grubor, M.; Kricka, T. Evaluation of Croatian agricultural solid biomass energy potential. Renew. Sustain. Energy Rev. 2018, 93, 225–230. [Google Scholar] [CrossRef]
- Klama, J. Coexistence of bacterial endophytes and plants (review). Acta Sci. Pol. Agric. 2004, 3, 19–28. (In Polish) [Google Scholar]
- Clifton-Brown, J.; Hastings, A.; Mos, M.; McCalmont, J.P.; Ashman, C.; Awty-Carroll, D.; Cerazy, J.; Chiang, Y.-C.; Cosentino, S.L.; Cracroft-Eley, W.; et al. Progress in Upscaling Miscanthus Biomass Production for the European Bio-Economy with Seed-Based Hybrids. GCB Bioenergy 2017, 9, 6–17. [Google Scholar] [CrossRef]
- Lu, H.; Li, L.; Chen, J.; Nkoh, J.N.; Hao, D.; Li, J.; Wang, J.; Li, D.; Liu, J.; Guo, H.; et al. Effect of salt-induced stress on the calorific value of two Miscanthus sacchariflorus (Amur silvergrass) varieties. Agronomy 2024, 14, 1259. [Google Scholar] [CrossRef]
- Pignon, C.; Spitz, I.; Jørgensen, U.; Kørup, K.; Long, S.P.; Jørgensen, U. Siberian Miscanthus sacchariflorus Accessions Surpass the Exceptional Chilling Tolerance of the Most Widely Cultivated Clone of Miscanthus × giganteus. GCB Bioenergy 2019, 11, 883–897. [Google Scholar] [CrossRef]
- Chen, H.; Dai, Z.; Jager, H.I.; Wullschleger, S.D.; Xu, J.; Schadt, C.W. Influences of nitrogen fertilization and climate regime on the above-ground biomass yields of Miscanthus and switchgrass: A meta-analysis. Renew. Sustain. Energy Rev. 2019, 108, 303–311. [Google Scholar] [CrossRef]
- Haines, S.A.; Gehl, R.J.; Havlin, J.L.; Ranney, T.G. Nitrogen and phosphorus fertilizer effects on establishment of giant miscanthus. BioEnergy Res. 2015, 8, 17–27. [Google Scholar] [CrossRef]
- Jeżowski, S. Yield traits of six clones of Miscanthus in the first 3 years following planting in Poland. Ind. Crops Prod. 2008, 27, 65–68. [Google Scholar] [CrossRef]
- He, K.; Xu, Y.; He, G.; Zhao, X.; Wang, C.; Li, S.; Zhou, G.; Hu, R. Combined application of acidic biochar and fertilizer synergistically enhances Miscanthus productivity in coastal saline-alkaline soil. Sci. Total Environ. 2023, 893, 164811. [Google Scholar] [CrossRef]
- Cadoux, S.; Riche, A.B.; Yates, N.E.; Machet, J.M. Nutrient requirements of Miscanthus × giganteus: Conclusions from a review of published studies. Biomass Bioenergy 2012, 38, 14–22. [Google Scholar] [CrossRef]
- Dubis, B.; Jankowski, K.J.; Załuski, D.; Bórawski, P.; Szempliński, W. Biomass production and energy balance of Miscanthus over a period of 11 years: A case study in a large-scale farm in Poland. GCB Bioenergy 2019, 11, 1187–1201. [Google Scholar] [CrossRef]
- Gazoulis, I.; Kanatas, P.; Papastylianou, P.; Tataridas, A.; Alexopoulou, E.; Travlos, I. Weed management practices to improve establishment of selected lignocellulosic crops. Energies 2021, 14, 2478. [Google Scholar] [CrossRef]
- Covarelli, L.; Beccari, G.; Tosi, L. Miscanthus rhizome rot: A potential threat for the establishment and the development of biomass cultivations. Biomass Bioenergy 2012, 46, 263–269. [Google Scholar] [CrossRef]
- Pointeau, S.; Jaguenet, E.; Couty, A.; Dubois, F.; Rambaud, C.; Ameline, A. Differential performance and behavior of the corn leaf aphid, Rhopalosiphum maidis, on three species of the biomass crop Miscanthus. Ind. Crops Prod. 2014, 54, 135–141. [Google Scholar] [CrossRef]
- Polskie Towarzystwo Gleboznawcze. Particle size distribution and textural classes of soils and mineral materials—Classification of Polish Society of Soil Sciences 2008. Rocz. Gleboz. 2009, 60, 5–16. (In Polish) [Google Scholar]
- PN-ISO 10390/1997; Soil Quality—Determination of pH. Polish Committee for Standardization: Warszawa, Poland, 1997. (In Polish)
- PN-R-04023/1996; Agrochemical Soil Analysis—Determination of Assimilated Phosphorus Contents. Polish Committee for Standardization: Warszawa, Poland, 1996. (In Polish)
- PN-R-04022/1996+Az1/2002; Agrochemical Soil Analysis—Determination of Assimilated Potassium Contents. Polish Committee for Standardization: Warszawa, Poland, 2002. (In Polish)
- PN-R-04020/1994/Az1/2004; Agrochemical Soil Analysis—Determination of Assimilated Magnesium Contents. Polish Committee for Standardization: Warszawa, Poland, 2004. (In Polish)
- PN-ISO 14235:2003; Soil Quality: Determination of Organic Carbon in Soil by Sulfochromic Oxidation. Polish Committee for Standardization: Warsaw, Poland, 2003. (In Polish)
- Brandić, I.; Voća, N.; Leto, J.; Bilandžija, N. Modelling the yield and estimating the energy properties of Miscanthus × giganteus in different harvest periods. AgriEngineering 2024, 6, 423–437. [Google Scholar] [CrossRef]
- Parker, G.G. Tamm review: Leaf area index (LAI) is both a determinant and a consequence of important processes in vegetation canopies. For. Ecol. Manag. 2020, 477, 118496. [Google Scholar] [CrossRef]
- Cui, Y.; Ma, C.; Li, C.; Pei, H. Unmanned aerial vehicle digital image and hyperspectral data for estimating the comparison of leaf area index and biomass of potato at different growth stages. Appl. Math. Nonlinear Sci. 2024, 9, 1–16. [Google Scholar] [CrossRef]
- Kross, A.; McNairn, H.; Lapen, D.; Sunohara, M.; Champagne, C. Assessment of RapidEye vegetation indices for estimation of leaf area index and biomass in corn and soybean crops. Int. J. Appl. Earth Obs. Geoinf. 2015, 34, 235–248. [Google Scholar] [CrossRef]
- Aydoğdu, M.; Yıldız, H. Use of hyperspectral data for chlorophyll estimation based on leaf area index (LAI) in wheat. Turk. J. Remote Sens. 2024, 6, 97–111. [Google Scholar] [CrossRef]
- Biskupski, A.; Włodek, S.; Pabin, J. The influence of differentiated tillage on selected indices of canopy architecture and yielding of crops. Fragm. Agron. 2009, 26, 7–13. (In Polish) [Google Scholar]
- Feledyn-Szewczyk, B.; Matyka, M.; Staniak, M. Diversity of weed flora, selected biometric characteristics and yielding of Miscanthus spp. cultivated on light and heavy soil. Acta Agrobot. 2014, 67, 67–76. [Google Scholar] [CrossRef]
- Namoi, N.; Jang, C.; Behnke, G.D.; Woo Lee, J.; Yang, W.; Lee, D.K. Nitrogen fertilization effects on aged Miscanthus × giganteus stands: Exploring biomass yield, yield components, and biomass prediction using in-season morphological traits. GCB Bioenergy 2024, 16, e13139. [Google Scholar] [CrossRef]
- PN-80/G-04511; Solid Mineral Fuels–Coke—Determination of Moisture in the General Analysis Test Sample. Polish Committee for Standardization: Warszawa, Poland, 1980. (In Polish)
- PN-81/G-04513; Solid Mineral Fuels—Determination of Gross Calorific Value by the Bomb Calorimetric Method, and Calculation of Net Calorific Value. Polish Committee for Standardization: Warszawa, Poland, 1980. (In Polish)
- Lewandowski, W.M. Pro-Ecological Renewable Sources; WNT: Warsaw, Poland, 2012; p. 432. (In Polish) [Google Scholar]
- Hastings, A.; Clifton-Brown, J.; Wattenbach, M.; Mitchell, C.P.; Smith, P. The development of MISCANFOR: A new Miscanthus crop growth model. GCB Bioenergy 2009, 1, 320–333. [Google Scholar] [CrossRef]
- McCalmont, J.P.; Hastings, A.; McNamara, N.P.; Richter, G.M.; Robson, P.; Donnison, I.S.; Clifton-Brown, J. Environmental costs and benefits of growing Miscanthus for bioenergy in the UK. GCB Bioenergy 2017, 9, 489–507. [Google Scholar] [CrossRef]
- Hou, W.; Yi, Z. Adaptability Comparison and Application Assessment of Various Bioenergy Grasses on Different Marginal Lands in China. Energy 2023, 285, 129483. [Google Scholar] [CrossRef]
- Kuś, J.; Faber, A.; Stasiak, M.; Kawalec, A. Productivity of selected plant species grown for energy purposes in various habitats. Stud. I Rap. IUNG-PIB 2008, 11, 67–79. (In Polish) [Google Scholar]
- Matyka, M.; Kuś, J. Influence of soil quality for yielding and biometric features of Miscanthus × giganteus. Pol. J. Environ. Stud. 2016, 25, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Jeżowski, S.; Głowacka, K.; Bocianowski, J. Variation in selected clones of giant grasses from genus Miscanthus in terms of yielding at first years of cultivation. Zesz. Probl. Post. Nauk Rol. 2007, 517, 339–348. (In Polish) [Google Scholar]
- Matyka, M.; Kuś, J. Yielding and biometric characteristics of selected Miscanthus genotypes. Probl. Inż. Rol. 2011, 2, 157–163. (In Polish) [Google Scholar]
- Dłużniewska, J.; Jaworska, M. Biomass for energy potential and limitations. Ecol. Chem. Eng. 2007, 14, 919–925. [Google Scholar]
- Stolarski, M.; Szczurowski, S.; Tworkowski, J. Biofuels obtained from energetic perennials biomass. Energetyka I Ekol. 2008, 1, 77–80. (In Polish) [Google Scholar]
- Lisowski, J.; Borusiewicz, A. Comparison of yielding and energy values of the pennsylvanian mallow with the Miscanthus giganteus in three following years of cultivation. Fragm. Agron. 2019, 36, 1–7. (In Polish) [Google Scholar] [CrossRef]
- Lisowski, J.; Borusiewicz, A.; Porwisiak, H. Comparison of yield, combustion heat and calorific value of virginia mallow (Sida hermaphrodita L.) and Miscanthus giganteus (Miscanthus × giganteus) growth in the Podlaskie Voivodeship. Fragm. Agron. 2018, 35, 53–61. (In Polish) [Google Scholar] [CrossRef]
- Jablonowski, N.D.; Kollmann, T.; Nabel, M.; Damm, T.; Klose, T.; Müller, M.; Bläsing, M.; Seebold, S.; Krafft, S.; Kuperjans, I.; et al. Valorization of Sida (Sida hermaphrodita) Biomass for Multiple Applications—A Review. GCB Bioenergy 2017, 9, 231–245. [Google Scholar] [CrossRef]
- Lord, R.A. Reed Canarygrass (Phalaris arundinacea) Outperforms Miscanthus or Willow on Marginal Soils, Brownfield and Non-Agricultural Sites for Local Sustainable Energy Crop Production. Renew. Energy 2015, 83, 740–747. [Google Scholar] [CrossRef]
- Voća, N.; Leto, J.; Karažija, T.; Bilandžija, N.; Peter, A.; Kutnjak, H.; Šurić, J.; Poljak, M. Energy Properties and Biomass Yield of Miscanthus × giganteus Fertilized by Municipal Sewage Sludge. Molecules 2021, 26, 4371. [Google Scholar] [CrossRef] [PubMed]
- Winkler, B.; Mangold, A.; Lewandowski, I.; Iqbal, Y.; Kiesel, A. Implementing Miscanthus into Farming Systems: A Review of Agronomic Practices, Environmental Impacts and Adoption. Renew. Sustain. Energy Rev. 2020, 132, 110053. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).