Recent Advances of the Electrochemical Hydrogenation of Biofuels and Chemicals from Furfural
Abstract
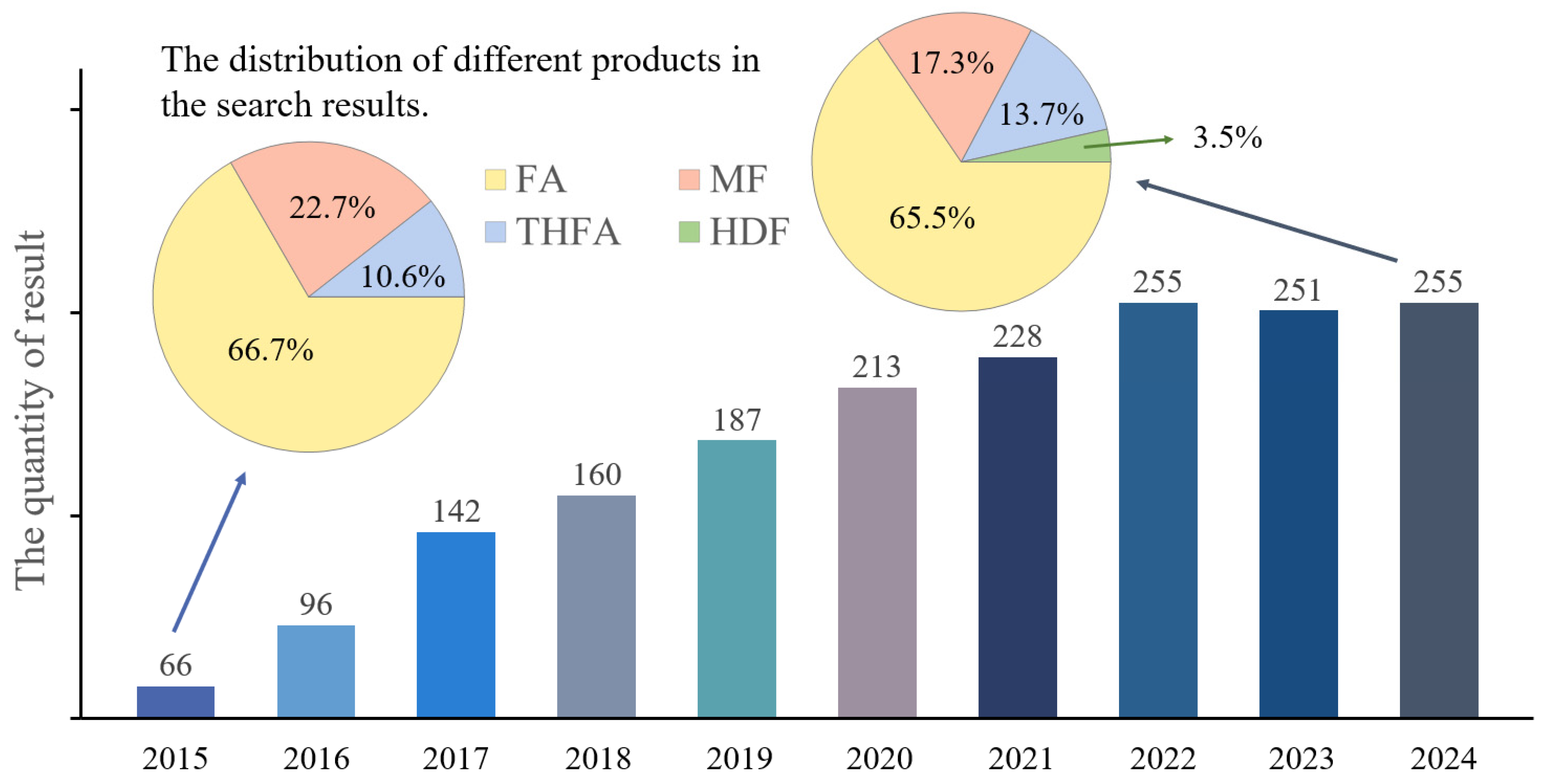
1. Introduction
- (1)
- Current research primarily focuses on the selective control of furfuryl alcohol and 2-methylfuran, lacking systematic understanding of the HDF formation pathways and competitive reaction mechanisms;
- (2)
- The dynamic interaction mechanisms between catalyst active sites and reaction intermediates are unclear and result in the selectivity toward HDF being below 40%;
- (3)
- Quantitative models have not yet been established for the influence patterns of operating conditions such as electrolyte pH and applied potential on product distribution, which constrains process optimization.
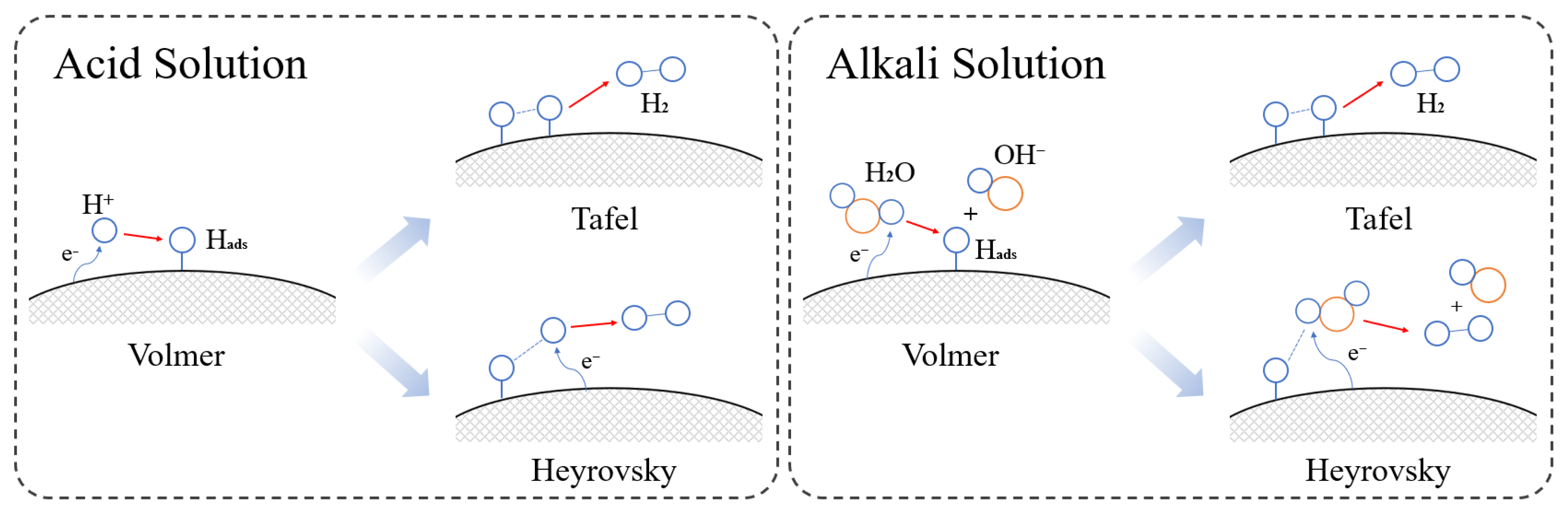
2. Overview of Electrocatalysis
3. Research Progress in Electroreduction of Furfural
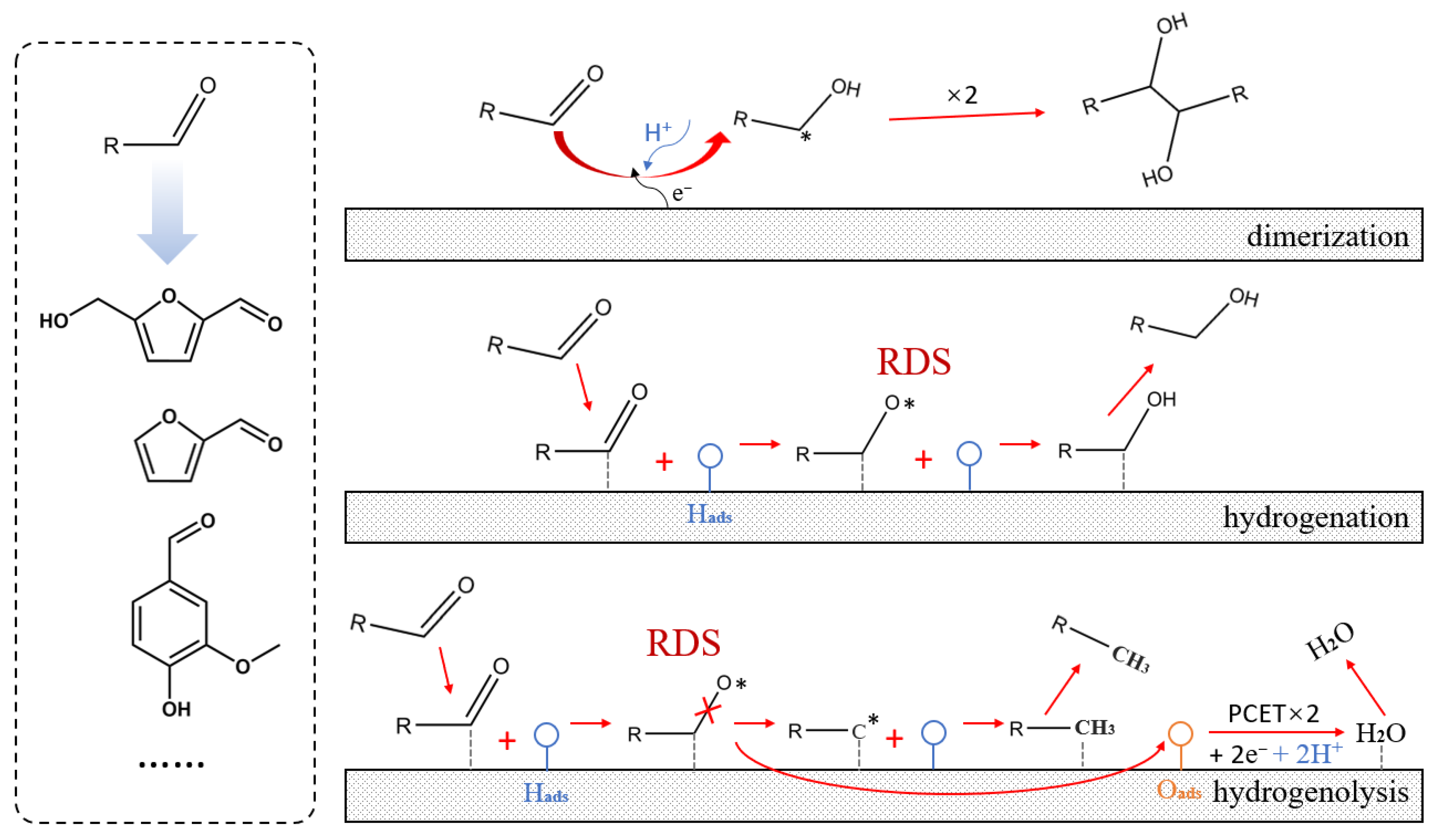
3.1. Overview of Furfural
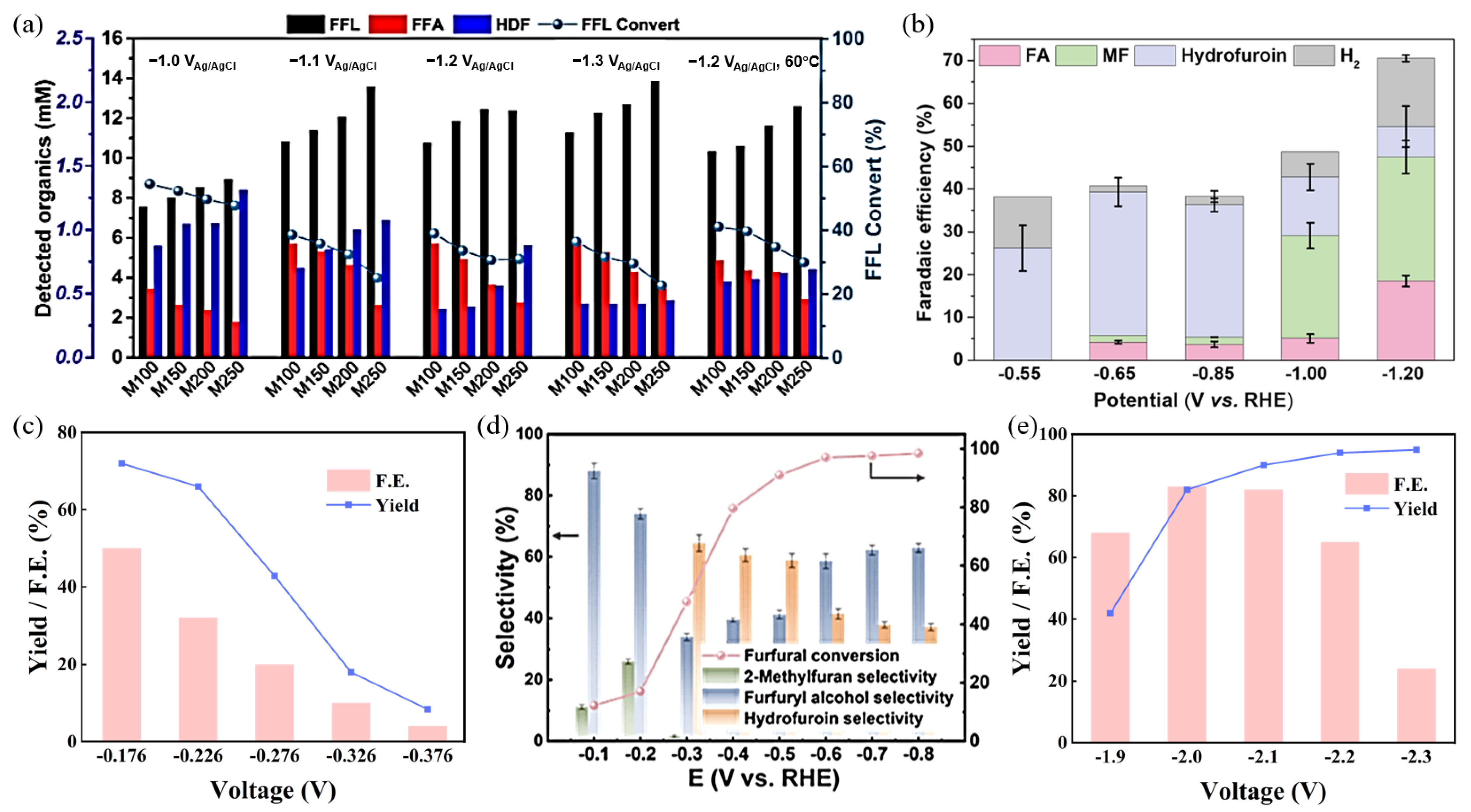
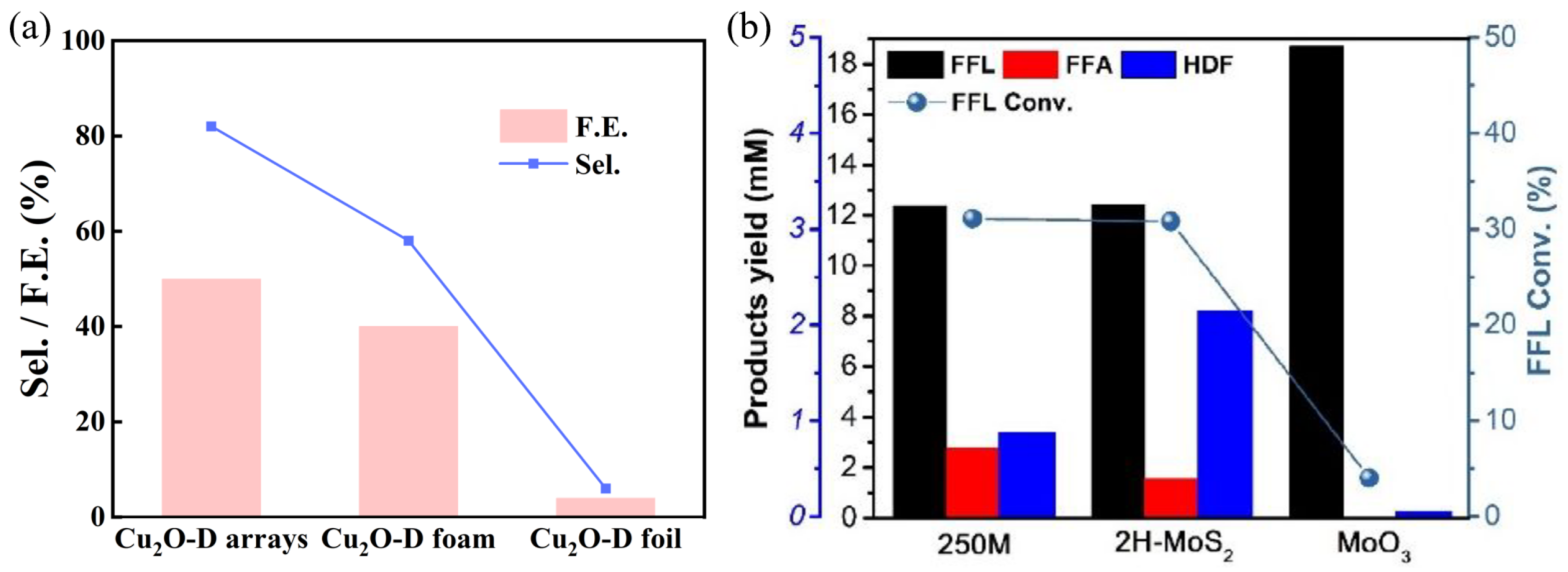
3.2. Research Progress on the Conversion of Furfural to Hydrofuroin
- (1)
- Mechanistic Studies
- (2)
- Catalyst Design
- (3)
- Influence of Reaction Environment
3.3. Research Progress on Furfural Conversion to Other Products
3.3.1. Furfuryl Alcohol
3.3.2. 2-Methylfuran
3.3.3. Tetrahydrofurfuryl Alcohol
4. Future Perspectives and Challenges
- (1)
- Deeply understand the competitive mechanisms and explore how to achieve targeted product selectivity by modulating reaction conditions.
- (2)
- Address Electrode Corrosion and Improving Recycling Efficiency in Electrocatalytic Processes.
- (3)
- Research Potential of Unknown Macromolecules in Electrochemical Hydrogenation.
- (4)
- Develop modular electrolyzer stack design to advance engineering applications.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, W.; Yu, C.; Chen, J.; Liu, Z. Electrochemical Hydrogenation of Biomass-Based Furfural in Aqueous Media by Cu Catalyst Supported on N-Doped Hierarchically Porous Carbon. Appl. Catal. B Environ. 2022, 305, 121062. [Google Scholar] [CrossRef]
- Temnikova, M.; Medvedev, J.; Medvedeva, X.; Delva, N.H.; Khairullina, E.; Krivoshapkina, E.; Klinkova, A. Electrochemical Hydrodimerization of Furfural in Organic Media as an Efficient Route to Jet Fuel Precursor. ChemElectroChem 2023, 10, e202200865. [Google Scholar] [CrossRef]
- International Energy Agency. World Energy Outlook 2023—Analysis. Available online: https://www.iea.org/reports/world-energy-outlook-2023 (accessed on 14 January 2025).
- Kunkel, R.; Schmidt, V.M. Electrochemical Hydrodimerization of Lignocellulose-Derived Carbonyls in Aqueous Electrolytes for Biobased Polymer and Long-Chained Synfuel Production: A Review. ChemSusChem 2025, 18, e202400638. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Gong, B.; Jin, Y.; Sit, P.H.-L.; Lam, J.C.-H. The Structural Phase Effect of MoS2 in Controlling the Reaction Selectivity between Electrocatalytic Hydrogenation and Dimerization of Furfural. ACS Catal. 2022, 12, 11340–11354. [Google Scholar] [CrossRef]
- Chen, J.; Wu, S.; Pan, X.; Zhou, X.; Zhang, X. Electrochemical Reduction Hydrogenation, Hydrogenolysis and Dimerization of Bio-Derived Aldehydes: A Review. Renew. Sustain. Energy Rev. 2025, 207, 114900. [Google Scholar] [CrossRef]
- Mukadam, Z.; Liu, S.; Pedersen, A.; Barrio, J.; Fearn, S.; Sarma, S.C.; Titirici, M.-M.; Scott, S.B.; Stephens, I.E.L.; Chan, K.; et al. Furfural Electrovalorisation Using Single-Atom Molecular Catalysts. Energy Environ. Sci. 2023, 16, 2934–2944. [Google Scholar] [CrossRef]
- Shang, X.; Yang, Y.; Sun, Y. Electrohydrodimerization of Biomass-Derived Furfural Generates a Jet Fuel Precursor. Green Chem. 2020, 22, 5395–5401. [Google Scholar] [CrossRef]
- Hu, Z.; Shen, J.; Tan, P.; Lou, D. Life Cycle Carbon Footprint of Biodiesel Production from Waste Cooking Oil Based on Survey Data in Shanghai, China. Energy 2025, 320, 135318. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Liu, H.; Li, W. Life Cycle Water Footprint Analysis and Future Prediction Based on LSTM for Arundo Donax in Sustainable Aviation Fuel Production. Water Resour. Manag. 2025, 39, 3109–3128. [Google Scholar] [CrossRef]
- Velvizhi, G.; Goswami, C.; Shetti, N.P.; Ahmad, E.; Kishore Pant, K.; Aminabhavi, T.M. Valorisation of Lignocellulosic Biomass to Value-Added Products: Paving the Pathway Towards Low-Carbon Footprint. Fuel 2022, 313, 122678. [Google Scholar] [CrossRef]
- Jeswani, H.K.; Chilvers, A.; Azapagic, A. Environmental Sustainability of Biofuels: A Review. Proc. R. Soc. A Math. Phys. Eng. Sci. 2020, 476, 20200351. [Google Scholar] [CrossRef] [PubMed]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Cao, Y.; Knijff, J.; Delparish, A.; d’Angelo, M.F.N.; Noël, T. A Divergent Paired Electrochemical Process for the Conversion of Furfural Using a Divided-Cell Flow Microreactor. ChemSusChem 2021, 14, 590–594. [Google Scholar] [CrossRef]
- Han, G.; Li, G.; Sun, Y. Electrocatalytic Dual Hydrogenation of Organic Substrates with a Faradaic Efficiency Approaching 200%. Nat. Catal. 2023, 6, 224–233. [Google Scholar] [CrossRef]
- Jiang, Z.; Zeng, Y.; Hu, D.; Guo, R.; Yan, K.; Luque, R. Chemical Transformations of 5-Hydroxymethylfurfural into Highly Added Value Products: Present and Future. Green Chem. 2023, 25, 871–892. [Google Scholar] [CrossRef]
- Yang, M.; Yuan, Z.; Peng, R.; Wang, S.; Zou, Y. Recent Progress on Electrocatalytic Valorization of Biomass-Derived Organics. Energy Environ. Mater. 2022, 5, 1117–1138. [Google Scholar] [CrossRef]
- Garedew, M.; Lam, C.H.; Petitjean, L.; Huang, S.; Song, B.; Lin, F.; Jackson, J.E.; Saffron, C.M.; Anastas, P.T. Electrochemical Upgrading of Depolymerized Lignin: A Review of Model Compound Studies. Green Chem. 2021, 23, 2868–2899. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Y.; Qian, L.; Al-Enizi, A.M.; Zhang, L.; Zheng, G. Heterogeneous Electrocatalysts for CO2 Reduction. ACS Appl. Energy Mater. 2021, 4, 1034–1044. [Google Scholar] [CrossRef]
- Creus, J.; Miola, M.; Pescarmona, P.P. Unravelling and Overcoming the Challenges in the Electrocatalytic Reduction of Fructose to Sorbitol. Green Chem. 2023, 25, 1658–1671. [Google Scholar] [CrossRef]
- Dang, K.; Dong, H.; Wang, L.; Jiang, M.; Jiang, S.; Sun, W.; Wang, D.; Tian, Y. Boosting Electrochemical Styrene Transformation via Tandem Water Oxidation over a Single-Atom Cr1/CoSe2 Catalyst. Adv. Mater. 2022, 34, 2200302. [Google Scholar] [CrossRef]
- Zhang, C.; Qi, Q.; Mei, Y.; Hu, J.; Sun, M.; Zhang, Y.; Huang, B.; Zhang, L.; Yang, S. Rationally Reconstructed Metal–Organic Frameworks as Robust Oxygen Evolution Electrocatalysts. Adv. Mater. 2023, 35, 2208904. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Luo, S.; Sun, M.; Wu, X.; Zhou, Y.; Liao, Y.; Tang, M.; Fan, X.; Huang, B.; Quan, Z. High-Entropy Intermetallic PtRhBiSnSb Nanoplates for Highly Efficient Alcohol Oxidation Electrocatalysis. Adv. Mater. 2022, 34, 2206276. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, S.L.; Lu, X.F.; Wu, Z.-P.; Luan, D.; Lou, X.W. Trimetallic Spinel NiCo2−xFexO4 Nanoboxes for Highly Efficient Electrocatalytic Oxygen Evolution. Angew. Chem. Int. Ed. 2021, 60, 11841–11846. [Google Scholar] [CrossRef]
- Rivadeneira-Mendoza, B.F.; Estrela Filho, O.A.; Fernández-Andrade, K.J.; Curbelo, F.; Fred Da Silva, F.; Luque, R.; Rodríguez-Díaz, J.M. MOF@biomass Hybrids: Trends on Advanced Functional Materials for Adsorption. Environ. Res. 2023, 216, 114424. [Google Scholar] [CrossRef]
- Wang, K.; Li, Z.; Guo, Z.; Huang, J.; Liu, T.; Zhou, M.; Hu, J.; Li, H. Electroreductive Upgradation of Biomass into High-Value Chemicals and Energy-Intensive Biofuels. Green Chem. 2024, 26, 2454–2475. [Google Scholar] [CrossRef]
- Munirathinam, B.; Schlüter, N.; Schröder, D. Recent Advances in Metallic Catalyst Materials for Electrochemical Upgrading of Furfurals to Drop-in Biofuels: A Mini Review. Energy Technol. 2023, 11, 2300307. [Google Scholar] [CrossRef]
- Wang, L.; Dou, Y.; Gan, R.; Zhao, Q.; Ma, Q.; Liao, Y.; Cheng, G.; Zhang, Y.; Wang, D. The Single Atom Anchoring Strategy: Rational Design of MXene-Based Single-Atom Catalysts for Electrocatalysis. Small 2025, 21, 2410772. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, F.; Xu, H.; Sun, M.; Wang, X.; Xiong, Y.; Zhou, J.; Liu, F.; Hu, Y.; Ma, Y.; et al. Interfacial Water Structure Modulation on Unconventional Phase Non-Precious Metal Alloy Nanostructures for Efficient Nitrate Electroreduction to Ammonia in Neutral Media. Angew. Chem. Int. Ed. 2025, e202508617. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, Y.; Zhu, J.; Tian, X.; Liu, G.; Feng, Z.; Pan, L.; Liu, X.; Han, N.; Tan, R. Material Engineering Strategies for Efficient Hydrogen Evolution Reaction Catalysts. Small Methods 2024, 8, 2400158. [Google Scholar] [CrossRef]
- Xiong, R.-Z.; Xu, H.-M.; Zhu, H.-R.; Zhang, Z.-J.; Li, G.-R. Recent Progress in Cu-Based Electrocatalysts for CO2 Reduction. Chem. Eng. J. 2025, 505, 159210. [Google Scholar] [CrossRef]
- Chadderdon, X.H.; Chadderdon, D.J.; Matthiesen, J.E.; Qiu, Y.; Carraher, J.M.; Tessonnier, J.-P.; Li, W. Mechanisms of Furfural Reduction on Metal Electrodes: Distinguishing Pathways for Selective Hydrogenation of Bioderived Oxygenates. J. Am. Chem. Soc. 2017, 139, 14120–14128. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Patel, D.M.; Chen, Y.; Lee, J.; Lee, T.-H.; Cady, S.D.; Cochran, E.W.; Roling, L.T.; Li, W. Unraveling Electroreductive Mechanisms of Biomass-Derived Aldehydes via Tailoring Interfacial Environments. ACS Catal. 2022, 12, 14072–14085. [Google Scholar] [CrossRef]
- Geng, W.; Zhang, D.; Zhen, N.; Du, J.; Dong, J.; Liu, C.; Chen, S.-L.; Chi, Y.; Hu, C. Electroreductive C–C Coupling of Furfural to Jet Fuel Precursors in Neutral Media via Synergistic Catalysis of the Polyoxotungstate and Cu Complex. ACS Catal. 2024, 14, 10040–10052. [Google Scholar] [CrossRef]
- Rabet, S.; Tobaschus, W.; Chung, G.; Gimpel, T.; Raabe, G.; Schroeder, D.; Munirathinam, B. Exploring Impurity Effects and Catalyst Surface Features in Furfural Electroreduction for Jet Fuel Precursor Production: Experimental and Molecular Dynamics Insights. ChemElectroChem 2024, 11, e202400336. [Google Scholar] [CrossRef]
- Liu, X.; Sun, Y.; Ren, H.; Lin, Y.; Wu, M.; Li, Z. Cu–Sn Bimetallic Activated Carbon–Carbon Coupling for Efficient Furfural Electroreduction. ACS Catal. 2024, 14, 5817–5826. [Google Scholar] [CrossRef]
- Ma, L.; Liu, H.; Wang, C. Switchable Selectivity to Electrocatalytic Reduction of Furfural over Cu2O-Derived Nanowire Arrays. Dalton Trans. 2024, 53, 10338–10346. [Google Scholar] [CrossRef]
- Liu, L.; Liu, H.; Huang, W.; He, Y.; Zhang, W.; Wang, C.; Lin, H. Mechanism and Kinetics of the Electrocatalytic Hydrogenation of Furfural to Furfuryl Alcohol. J. Electroanal. Chem. 2017, 804, 248–253. [Google Scholar] [CrossRef]
- Yang, Z.; Chou, X.; Kan, H.; Xiao, Z.; Ding, Y. Nanoporous Copper Catalysts for the Fluidized Electrocatalytic Hydrogenation of Furfural to Furfuryl Alcohol. ACS Sustain. Chem. Eng. 2022, 10, 7418–7425. [Google Scholar] [CrossRef]
- Dixit, R.J.; Bhattacharyya, K.; Ramani, V.K.; Basu, S. Electrocatalytic Hydrogenation of Furfural Using Non-Noble-Metal Electrocatalysts in Alkaline Medium. Green Chem. 2021, 23, 4201–4212. [Google Scholar] [CrossRef]
- Zhang, X.; Han, M.; Liu, G.; Wang, G.; Zhang, Y.; Zhang, H.; Zhao, H. Simultaneously High-Rate Furfural Hydrogenation and Oxidation Upgrading on Nanostructured Transition Metal Phosphides Through Electrocatalytic Conversion at Ambient Conditions. Appl. Catal. B Environ. 2019, 244, 899–908. [Google Scholar] [CrossRef]
- Qin, M.; Fan, S.; Li, X.; Duan, J.; Chen, G. Electrocatalytic Reduction of Furfural to Furfuryl Alcohol Using Carbon Nanofibers Supported Zinc Cobalt Bimetallic Oxide with Surface-Derived Zinc Vacancies in Alkaline Medium. J. Colloid Interface Sci. 2024, 660, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Biddinger, E.J. Controlling Competitive Side Reactions in the Electrochemical Upgrading of Furfural to Biofuel. Energy Technol. 2018, 6, 1370–1379. [Google Scholar] [CrossRef]
- Bharath, G.; Banat, F. High-Grade Biofuel Synthesis from Paired Electrohydrogenation and Electrooxidation of Furfural Using Symmetric Ru/Reduced Graphene Oxide Electrodes. ACS Appl. Mater. Interfaces 2021, 13, 24643–24653. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Tan, J.; Chen, Y.; Zhang, W.; Chen, P.; Tang, Y.; Gao, Q. Promoted Electrocatalytic Hydrogenation of Furfural in a Bi-Phasic System. Chem. Commun. 2023, 59, 3103–3106. [Google Scholar] [CrossRef]
- Lenk, T.; Rueß, V.; Gresch, J.; Schröder, U. Exploring the Electrochemical Ring Hydrogenation of Furanic Compounds. Green Chem. 2023, 25, 3077–3085. [Google Scholar] [CrossRef]
- Delima, R.S.; Stankovic, M.D.; MacLeod, B.P.; Fink, A.G.; Rooney, M.B.; Huang, A.; Jansonius, R.P.; Dvorak, D.J.; Berlinguette, C.P. Selective Hydrogenation of Furfural Using a Membrane Reactor. Energy Environ. Sci. 2022, 15, 215–224. [Google Scholar] [CrossRef]
- Orella, M.J.; Román-Leshkov, Y.; Brushett, F.R. Emerging Opportunities for Electrochemical Processing to Enable Sustainable Chemical Manufacturing. Curr. Opin. Chem. Eng. 2018, 20, 159–167. [Google Scholar] [CrossRef]
- Lee, D.K.; Kubota, S.R.; Janes, A.N.; Bender, M.T.; Woo, J.; Schmidt, J.R.; Choi, K.-S. The Impact of 5-Hydroxymethylfurfural (HMF)-Metal Interactions on the Electrochemical Reduction Pathways of HMF on Various Metal Electrodes. ChemSusChem 2021, 14, 4563–4572. [Google Scholar] [CrossRef]
- Anibal, J.; Xu, B. Electroreductive C–C Coupling of Furfural and Benzaldehyde on Cu and Pb Surfaces. ACS Catal. 2020, 10, 11643–11653. [Google Scholar] [CrossRef]
- de Luna, G.S.; Sacco, A.; Hernandez, S.; Ospitali, F.; Albonetti, S.; Fornasari, G.; Benito, P. Insights into the Electrochemical Reduction of 5-Hydroxymethylfurfural at High Current Densities. ChemSusChem 2022, 15, e202102504. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, Z.; Sun, C.; Ren, X.; Li, Q. High-Efficiency Electrochemical Hydrogenation of Biomass-Derived Benzaldehyde Compounds via a Durable and Versatile Dendritic-like Pd/Cu-CF Electrocatalyst. Fuel Process. Technol. 2022, 237, 107436. [Google Scholar] [CrossRef]


| Electrolyte | Catalysts | F.E/% | Conv./% | Yield/% | Sel./% | Potential | Refs. | |
|---|---|---|---|---|---|---|---|---|
| 1 | / | Pb | / | / | / | 28 | −0.985 R | [35] |
| 2 | 0.1 M KOH | C | 89 | 66 | 66 | 99 | −1.4 R | [8] |
| 3 | 0.1 M KOH | Cu | / | 100 | 60 | / | −1.4 R | [8] |
| 4 | phosphate buffer | Cu-Sn | 80 | 97.3 | / | 67 | −0.5 R | [36] |
| 5 | 0.1 M carbonate buffer | Cu | 60 | 17 | / | 80 | −0.6 R | [7] |
| 6 | 0.4 M phosphate buffer + wood spirit | Cu | / | 99 | 90 | 92.1 | −0.76 R | [34] |
| 7 | 1 M KOH | N-Cu | / | 93 | 52 | 56 | −0.45 R | [1] |
| 8 | 1 M KOH | Cu2O | 50 | 90 | / | 83.5 | −0.176 R | [37] |
| 9 | 0.4 M borate buffer + wood spirit | MoS2 | 15.4 | 98 | 7 | 42.7 | −1.0 A | [5] |
| Electrolyte | Catalyst | Product | F.E/% | Conv./% | Yield/% | Sel./% | Potential | Refs. | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.1 M carbonate buffer | Cu | FA | / | 62% | 45% | 87% | −1.4 A | [38] |
| 2 | PBS + methanol | Cu + Al | FA | 95 | 77 | / | 96 | −1.5 A | [39] |
| 3 | 0.5 M NaOH | Ni + Cu | FA | / | 100 | / | / | −0.45 R | [40] |
| 4 | 1.0 M KOH | Cu + P | FA | 98 | 100 | 99 | 100 | −0.35 R | [41] |
| 5 | 0.5 M NaOH | Zn + Mn | FA | 96 | / | / | 85 | −0.5 R | [42] |
| 6 | 0.5 M H2SO4 | Cu | MF | 15.2 | 70% | / | 71% | −0.8 R | [43] |
| 7 | 2.0 M H2SO4 | Ru | MF | 95 | 97 | 91 | / | −1.25 A | [44] |
| 8 | 0.5 M K2SO4 + cyclohexane | Cu | MF | 40 | 75 | 20 | / | −0.73 R | [45] |
| 9 | 0.5 M H2SO4 | Pd + Pt | THFA | / | 34 | / | 15.3 | −300 M | [46] |
| 10 | 1.0 M H2SO4 | Pt | THFA | / | 90 | 88 | 98 | −225 M | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, H.; Liu, K.; Zhang, X.; Zhang, Q.; Chen, L.; Chen, Y.; Zhuang, X.; Ma, L. Recent Advances of the Electrochemical Hydrogenation of Biofuels and Chemicals from Furfural. Energies 2025, 18, 3075. https://doi.org/10.3390/en18123075
Liang H, Liu K, Zhang X, Zhang Q, Chen L, Chen Y, Zhuang X, Ma L. Recent Advances of the Electrochemical Hydrogenation of Biofuels and Chemicals from Furfural. Energies. 2025; 18(12):3075. https://doi.org/10.3390/en18123075
Chicago/Turabian StyleLiang, Huiyi, Ke Liu, Xinghua Zhang, Qi Zhang, Lungang Chen, Yubao Chen, Xiuzheng Zhuang, and Longlong Ma. 2025. "Recent Advances of the Electrochemical Hydrogenation of Biofuels and Chemicals from Furfural" Energies 18, no. 12: 3075. https://doi.org/10.3390/en18123075
APA StyleLiang, H., Liu, K., Zhang, X., Zhang, Q., Chen, L., Chen, Y., Zhuang, X., & Ma, L. (2025). Recent Advances of the Electrochemical Hydrogenation of Biofuels and Chemicals from Furfural. Energies, 18(12), 3075. https://doi.org/10.3390/en18123075






