Abstract
This paper presents a sustainable recycling process for the separation and recovery of tempered glass from end-of-life photovoltaic (PV) modules. As glass accounts for 75% of the weight of a panel, its recovery is an important step in the recycling process. Current methods, such as mechanical, chemical and thermal processes, often lead to contamination of the glass and pose significant environmental risks. In response to these challenges, a thermal–mechanical delamination approach is proposed in this study. The method utilizes controlled heat application (hot air gun) to weaken the adhesive bond between the glass and encapsulant, allowing for separation with a thin stainless steel wire. Various analytical methods, including X-ray diffraction analysis (XRD), X-ray fluorescence (XRF) and scanning electron microscopy with energy-dispersive X-ray spectroscopy (SEM-EDS), were used to verify the effectiveness of the proposed method. The results show that the proposed method is effective. In less than a minute, the glass layer was separated and recovered with a success rate of over 99%, with no degradation of the material or release of gasses. The significance of this process lies in its ability to recover high-purity glass while minimizing the impact on the environment. This opens up the possibility of reusing the recovered tempered glass in new PV panels or other applications, reducing the need for virgin materials and lowering the overall environmental footprint of the solar energy industry.
1. Introduction
Photovoltaic (PV) panels, praised for their environmentally friendly energy production, pose a growing problem for the environment. The rapid expansion of the PV industry has led to an increase in the production and use of these panels []. When they reach the end of their approximately 25- to 30-year lifespan, they are expected to generate huge amounts of waste. In the worst-case scenario, this waste could exceed an estimated 160 million tons by 2050 [], as shown in Figure 1. The improper disposal of this waste is of major concern due to the presence of toxic heavy metals such as lead and tin. These substances pose serious environmental and health risks. This underlines the high need for sustainable methods for recycling PV waste to reduce the environmental impact. Poly- and monocrystalline PV panels, which currently dominate the market with a share of around 90%, are expected to contribute the most to end-of-life waste []. Figure 2 shows an illustration of a common poly-monocrystalline panel.
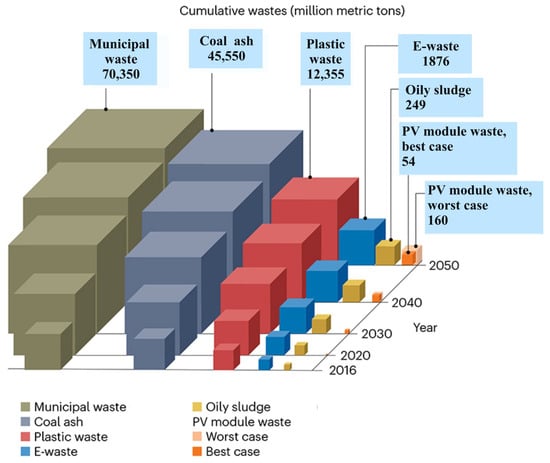
Figure 1.
Best-case and worst-case scenario PV module waste projections from 2016 to 2050 with other waste streams (in million metric tons) [,,,,,,].
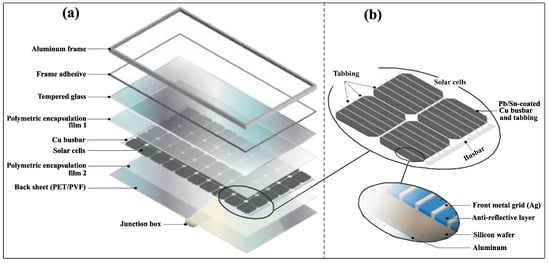
Figure 2.
Structure of silicon-based photovoltaic (PV) panels: (a) main layers of a PV panel; (b) detailed view of solar cell components, reprinted with permission from [].
Solar panels consist of several main components: monocrystalline or polycrystalline silicon solar cells that convert sunlight into electricity, tempered glass for protection, an aluminum frame for structural support, ethylene vinyl acetate (EVA) or polyolefin encapsulants, a polymer back sheet and a junction box for electrical connections. When examining the mass distribution, tempered glass makes up the largest proportion and accounts for more than 75% of the total mass of photovoltaic modules [] (Figure 3a). Despite its dominant mass, glass is often considered a low-value component, as it accounts for only about 8% of the total value of the modules []. Among the metals used in PV modules, silver is the most expensive material, followed by aluminum and silicon (Figure 3b).
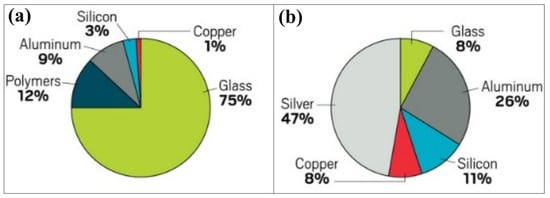
Figure 3.
Distribution of materials in a typical silicon photovoltaic panel: (a) by mass and (b) by value [].
Although glass may seem less valuable, its proper separation and recovery is important for two main reasons. First, efficient glass separation prevents the contamination of valuable metals. Secondly, due to the energy-intensive process of glass production from raw materials, glass reuse promotes sustainability through energy savings and waste reduction []. The main methods of glass separation proposed in the literature include mechanical processes, thermal treatment and chemical dissolution. Mechanical separation methods such as crushing, shredding and sieving are commonly used to crush PV modules and release their components. Ying Sim et al. (2023) demonstrated a crushing and sieving method that effectively separates polymers and metals into different particle size fractions []. Similarly, Li et al. (2023) utilized a combination of crushing and attrition scrubbing to delaminate the glass substrate and concentrate critical metals such as indium and gallium into fine fractions []. Bogust and Smith (2020) used a shredding and sieving process followed by cryogenic treatment to liberate glass and silicon wafers. With their method, they achieved a recovery rate of 86% and 88% for silicon wafers []. Tokoro et al. (2021) introduced selective crushing with an eccentric stirring mill, which significantly improved the separation of glass []. During thermal treatment, the PV modules are heated to decompose the encapsulation materials, which enables the recovery of glass and other components. Pagnanelli et al. (2019) compared solvent and thermal treatments and found that thermal treatment at 500 °C effectively removes the EVA and results in high-quality glass recovery []. Wahman and Surowiak (2022) followed a similar approach by placing panels in an oven at 500 °C for 60 min. The goal of complete removal of the polymer was achieved []. Wahman et al. (2023, 2024) proposed two methods to separate the backsheet of waste photovoltaic (PV) panels: a selective mechanical peeling process and a hot knife technique. They found that heating the panels without the backsheet to 400 °C for 5 min was sufficient to completely decompose the residual polymer and release the materials [,]. Belançon et al. (2022) used mechanical delamination in combination with heating to 85 °C and achieved a weight recovery of 71.5% of the high-quality glass []. Doni and Dughiero (2012) developed an electrothermal RF heating process that facilitated the removal of glass from PV laminates, significantly reduced the adhesive strength of EVA and enabled easy separation []. Chemical processes use solvents to dissolve the encapsulation materials and facilitate the recovery of glass and other components. Min et al. (2024) utilized limonene-induced swelling of EVA under ultrasonic conditions and achieved complete separation of the glass and backsheet from EVA without damaging the silicon cells []. Vanek et al. (2023) investigated different solvents for chemical delamination and found that toluene dissolved the EVA most effectively, although it caused significant cracking in the silicon wafer due to severe swelling []. Some studies have combined multiple methods to improve the efficiency of recovery and the purity of the material. Kamano et al. (2019) combined microwave heating with mechanical separation, which reduced the force required for glass removal by about 58% []. Królikowski et al. (2024) developed a comprehensive recycling process that combines thermal–mechanical treatment with chemical delamination. Their method successfully recovered glass, EVA, PET and PVDF polymers [].
Despite extensive research on the recycling of photovoltaic (PV) modules, the efficient recovery of high-purity tempered glass remains a challenge. Mechanical shredding of solar modules is effective, but often results in a mixture of glass, polymers, metals and silicon, which requires complicated separation processes. The chemical methods used are generally associated with environmental problems. Although they are promising and have a low temperature, they are not industrially feasible due to the long time period and large amounts of chemicals needed. On the other hand, thermal treatments require high temperatures and the associated release of toxic gasses [,,]. These problems underline the need for more sustainable processes.
This study addresses the critical need for a sustainable and effective method to recover tempered glass without degrading its quality or releasing harmful substances. The innovation lies in the use of a controlled thermal–mechanical delamination process that achieves a high recovery rate while preserving the integrity of the glass, a significant improvement over existing techniques.
2. Materials and Methods
2.1. Materials
Discarded monocrystalline silicon photovoltaic panels with broken tempered glass were used for the experiments (Figure 4a). The glass layer is the largest component of a PV panel and accounts for around 75% of its weight. It is bonded to the panel on the front side with an encapsulation of ethylene vinyl acetate (EVA) [] (Figure 4b). This type of glass, known as soda lime or soda-lime silicate glass, is a popular substrate for photovoltaic windows due to its advantageous properties [,]. It usually has an average thickness of 3 mm and a well-defined chemical composition specified in the German DIN standard EN572-1: 69–74% SiO2 (silicon dioxide), 10–16% Na2O (sodium oxide), 5–14% CaO (calcium oxide), 0–6% MgO (magnesium oxide), 0–3% Al2O3 (aluminum oxide) and 0–5% Fe2O3 (iron(III) oxide) and K2O (potassium oxide) [,,].
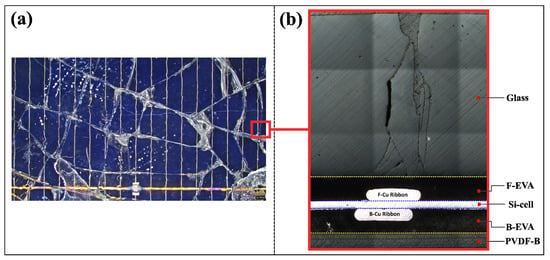
Figure 4.
(a) Discarded photovoltaic (PV) panel and (b) cross-section showing key components. F-EVA: front ethylene vinyl acetate encapsulant, B-EVA: back ethylene vinyl acetate encapsulant, PVDF-B: polyvinylidene fluoride back sheet, F-Cu ribbon: front copper collector ribbon, B-Cu ribbon: back copper collector ribbon.
2.2. Methods
In the first phase, the aluminum frame and the junction box were separated manually using standard tools. The unframed PV module was then attached to a workbench with the glass layer facing upwards. A transparent adhesive film was applied to the entire glass surface. This step was important to stabilize the glass fragments and prevent dust or glass particles from being released during separation. Figure 5 shows a setup for the separation process. The glass surface was heated to a temperature of 200 °C using a heat gun. This temperature was determined to be optimal for softening the EVA adhesives based on our previous research [,]. While the material is heated, a thin wire is carefully inserted along the perimeter of the glass layer. The wire is first oiled to reduce friction during separation. Two types of steel wires with different sizes and types were selected: a single solid stainless steel wire and a twisted stainless steel wire. The wire thicknesses tested are 0.15 mm, 0.2 mm, 0.3 mm, 0.5 mm, 0.7 mm and 1 mm. The separation process was monitored and timed to evaluate the effectiveness of each wire type and thickness. After successful glass separation, the glass fraction and the remaining layers were subjected to thermal treatment in an oven. They were heated at 400 °C for 5 min to decompose the remaining polymer residues. The surfaces and elemental composition of the recovered glass substrates were analyzed using scanning electron microscopy (SEM) with energy-dispersive spectroscopy (EDS). X-ray diffraction (XRD) was used to identify the amorphous or crystalline phases. The elemental composition of the obtained glasses and the remaining layers was also confirmed by X-ray fluorescence (XRF).
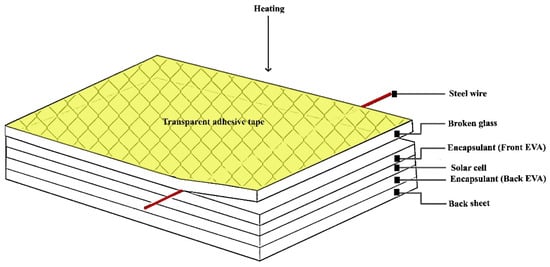
Figure 5.
Evaluation of sample subjected to the proposed process.
XRD analysis was performed using a Malvern PANalytical X’Pert Pro with a Johansson monochromator Ge(111), a Soller slit, a divergence slit of 1/2 degree and an antistreus slit of 1 degree.
The surface and cross-sectional were performed using the FEI Versa 3D scanning electron microscope (SEM) equipped with an Oxford Instruments Ultim Max energy-dispersive X-ray spectrometer (EDX). The SEM/EDS measurements were performed in low vacuum (LV) mode with an accelerating voltage of 15 kV.
X-ray fluorescence (XRF) analysis was performed using Malvern PANalytical Epsilon 3XLE spectrometer equipped with a rhodium (Rh) anode X-ray tube. The system operates with a voltage range of 4–50 kV and a current range of 1–1000 µA. For this analysis, the instrument was configured to use a primary beam filter suitable for the elements of interest to optimize detection and quantification.
3. Results and Discussion
3.1. Results
Figure 6a–c show the successful detachment and recovery of the glass layer using the proposed method. By heating the glass surface to 200 °C with a heat gun and simultaneously inserting a 0.5 mm thin solid stainless steel wire between the glass and the EVA layer, complete detachment of the glass was achieved in less than a minute. The recovered glass fragments appear clear with no visible dust or fine particles (Figure 6a), and the silicon cell lack significant residue (Figure 6b,c). The thinner wires that were tested (0.15 mm and 0.2 mm) broke easily during the process. The 0.3 mm wire provided a balance between strength and ease of insertion, but was not as reliable as the 0.5 mm wire. Wires thicker than 0.5 mm were difficult to insert and increased the risk of breaking the glass and underlying layers. The other type of wire (twisted stainless steel wires) was less efficient at all thicknesses tested. These wires generated more dust due to the increased friction during cutting and had a higher tendency to break, leading to possible damage to the remaining layers. The amount of glass recovered was calculated to be 99%. This was determined by weighing the glass before and after cutting and comparing the weight of the recovered glass with the initial weight of the glass. The glass fragments and the remaining layers were heat treated at 400 °C for 5 min to remove the polymer residues. The separating glass and the Si cell are shown in Figure 6d and Figure 6e, respectively.
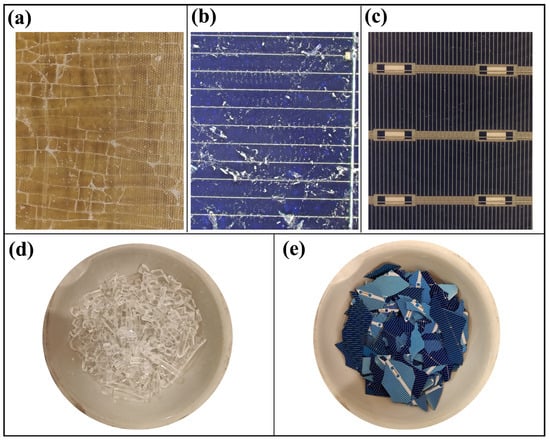
Figure 6.
(a) Separated glass. (b) Front surface of the treated silicon cells. (c) Backside of the silicon cells. (d) Glass after thermal treatment. (e) Si cell after thermal treatment.
3.1.1. SEM-EDS Analysis
The morphology and elemental composition of the obtained glass material were analyzed using SEM and EDS, as shown in Figure 7. The SEM image shows a uniform granular texture, indicating that the treated surface of the obtained glass has no visible impurities. The EDS analysis confirms that the elemental composition of the obtained glass is consistent with the typical soda-lime glass used in PV modules. The high concentrations of oxygen and silicon, as well as the significant amounts of sodium and calcium, indicate that the recovered glass retains its essential structural and functional properties. These results are consistent with previous studies [,,,,].
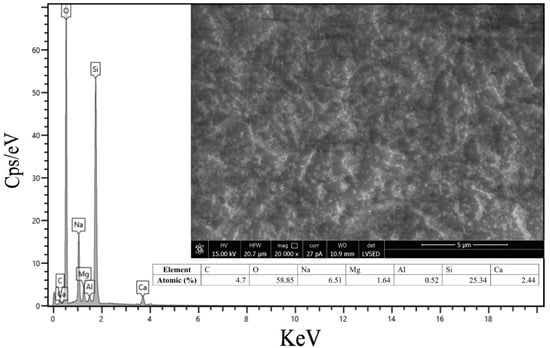
Figure 7.
Surface elemental analysis and morphology of the recovered glass material by EDS and SEM imaging.
3.1.2. XRD Analysis
The crystalline structure of the glass was analyzed using X-ray diffraction (XRD) (Figure 8). The X-ray diffractogram showed a broad hump at 2θ values of 20–30°, confirming the amorphous nature of the glass, which is consistent with standard soda-lime glass used in photovoltaic modules. Soda-lime glass is known for its amorphous structure, which contributes to its transparency and durability [,,].
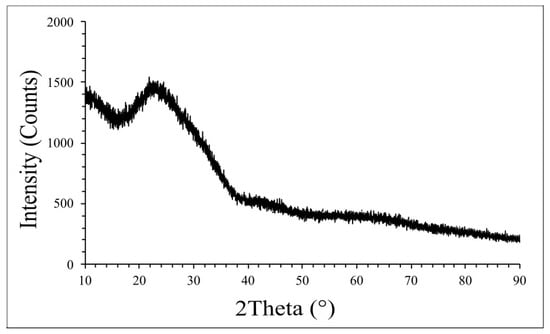
Figure 8.
XRD of the recovered glass.
3.1.3. XRF Analysis
The chemical composition of the recovered glass was determined using X-ray fluorescence spectroscopy (XRF). The main components and their compositions are listed in Table 1. XRF analysis revealed that the main constituents of the recovered glass are silicon dioxide (SiO2) at 66.355%, sodium oxide (Na2O) at 20.795% and calcium oxide (CaO) at 6.721%. These components are typical of soda-lime glass, indicating that the glass retains its basic properties after separation. There are slight deviations in the composition of SiO2 and Na2O, as they are outside the standard range []. These deviations may be due to the separation process or the original glass composition. It is possible that the glass was originally processed with a different waste material, resulting in a different proportion of Na2O alkali ions and a lower SiO2 content than typical soda-lime glass.

Table 1.
Composition of recovered glass vs. standard soda-lime glass.
The metal fraction was also subjected to XRF analysis to investigate possible impurities or changes in composition caused by the glass separation process. This approach is compared to the conventional crushing and sieving method described in the literature for glass separation. Table 2 shows the composition of the metal fraction obtained using the two methods. The proposed method yielded a metal fraction rich in silicon (Si) (83.00% vs. 41.67%, ratio of 1.99) and silver (Ag) (0.61% vs. 0.22%, ratio of 2.77). By contrast, glass-associated elements such as calcium (Ca) (0.02% vs. 4.24%, ratio of 0.005), magnesium (Mg) (0.01% vs. 0.49%, ratio of 0.02) and sodium (Na) (undetectable vs. 2.32%) were significantly reduced. The percentage of unidentified elements (UE), which are probably impurities due to polymers or glass residues (elements with atomic masses below 12, e.g., H, O and C), was also significantly lower with the proposed method (13.97%) than with crushing and sieving (47.93%), showing a ratio of 0.29. These results show that the proposed method separates the glass without contaminating the metal fraction with the glass particles.

Table 2.
Composition of metal fraction by method.
3.2. Discussion
The results show the effectiveness of the proposed thermal–mechanical method for separating and recovering glass from photovoltaic (PV) modules. Using a hot air gun to heat the glass surface to 200 °C and a 0.5 mm thick stainless steel wire, the glass was successfully separated with minimal impact on the underlying layers. Within one minute, more than 99% of the glass was recovered, and analytical methods confirmed its high purity. SEM-EDS analysis identified the composition as typical soda-lime glass (Si, O, Na and Ca) with a uniform texture, while XRD analysis confirmed its amorphous structure. XRF analysis showed that the glass maintained its primary chemical properties with only minor variations, making it suitable for reuse in solar module manufacturing or other applications. The process also effectively enriched the metal fraction, particularly valuable elements such as Si and Ag, while the presence of glass-related impurities was significantly reduced. This shows that the process efficiently separates the glass without contaminating the metal fraction. Reusing glass instead of producing it from raw materials offers significant environmental and economic benefits. Glass production is energy intensive and requires 2–3 kWh per kg due to the high melting temperatures. Greenhouse gasses are released during this process: it is estimated that 0.74 tons of CO2 are released per ton of glass [,]. Although the recovered glass has to be further processed, it still consumes less energy than the production of new glass, resulting in overall energy savings and less waste. Compared to conventional recycling methods (mechanical, high-temperature thermal treatment and chemical dissolution), the proposed method is more efficient and environmentally friendly. It operates at a lower temperature, is faster and avoids harmful gas emissions or the use of solvents. A key advantage of this method is its simplicity and scalability, making it highly suitable for widespread application in the PV recycling industry. With industrial-grade heating systems, automated wire separation mechanisms and batch processing, this method can efficiently handle large quantities of photovoltaic waste. According to Rio et al. (2022), around 686 kg of glass can be recycled from every ton of PV waste []. With a projected generation of 160 million tons of PV waste by 2050, this method could enable the recovery of at least 108 million tons of glass and thus make an important contribution to sustainable practices in the solar energy sector.
4. Conclusions
This study presents a novel thermal–mechanical method for the efficient separation and recovery of tempered glass from end-of-life photovoltaic (PV) modules. The main objective was to develop a method that not only achieves a high recovery rate and purity of the glass component but also overcomes the limitations of existing mechanical, chemical and thermal recycling processes. Using a controlled heat source, specifically a heat gun set at 200 °C, the adhesive bonds within the PV modules were effectively weakened. This allowed a 0.5 mm thin stainless steel wire to cleanly delaminate the glass layer without damaging the material or releasing harmful gasses. The results demonstrate a remarkable glass recovery rate of over 99% within just one minute. The recovered glass, which was characterized using SEM-EDS, XRD and XRF analyses, exhibited the composition and properties of typical soda-lime glass, proving its suitability for reuse in the manufacture of PV modules or other applications. The proposed process offers significant environmental and economic benefits compared to conventional recycling methods. By reusing glass instead of manufacturing it from raw materials, energy consumption and greenhouse gas emissions could be significantly reduced. The potential to recover over 108 million tons of glass from the projected 160 million tons of PV waste by 2050 underscores the method’s significant impact on reducing the solar industry’s environmental footprint. The simplicity, efficiency and scalability of the thermal–mechanical delamination process make it a promising solution to the growing challenge of PV waste management.
Author Contributions
Conceptualization, A.S.; data curation, M.W.; formal analysis, A.S. and M.W.; investigation, M.W.; methodology, M.W.; supervision, A.S.; writing—original draft, M.W.; writing—review and editing, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was carried out at the AGH University of Krakow, Doctoral School (no. 10.16.100.7999).
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- IRENA and IEA-PVPS. End-of-Life Management: Solar Photovoltaic Panels. 2016. Available online: https://www.irena.org/publications/2016/Jun/End-of-life-management-Solar-Photovoltaic-Panels (accessed on 4 June 2024).
- Mirletz, H.; Hieslmair, H.; Ovaitt, S.; Curtis, T.L.; Barnes, T.M. Unfounded concerns about photovoltaic module toxicity and waste are slowing decarbonization. Nat. Phys. 2023, 19, 1376–1378. [Google Scholar] [CrossRef]
- Xu, C.; Li, B.; Yuan, X.; Liu, C.; Shen, C.Y.; Dai, G.C. Separation of backsheets from waste photovoltaic (PV) modules by ultrasonic irradiation. IOP Conf. Ser. Earth Environ. Sci. 2019, 242, 032046. [Google Scholar] [CrossRef]
- Solid Waste Management. World Bank, 2022. Available online: https://go.nature.com/3PuZ9pL (accessed on 10 June 2024).
- Brown, M.A. Solid Waste from the Operation and Decommissioning of Power Plants. ORNL, 2017. Available online: https://go.nature.com/3LdqlYG (accessed on 3 June 2024).
- Agrawala, S. Global Plastics Outlook: Policy Scenarios to 2060—Policy Highlights. OECD, 2022. Available online: https://go.nature.com/48v2ayZ (accessed on 5 June 2024).
- Forti, V.; Baldé, C.P.; Kuehr, R.; Bel, G. Global e-Waste Monitor 2020: Quantities, Flows and the Circular Economy Potential. UNU/UNITAR, ITU, ISWA. 2020. Available online: https://go.nature.com/3RAfnAP (accessed on 3 June 2024).
- Dal Mas, F.; Zeng, X.; Huang, Q.; Li, J. Quantifying material flow of oily sludge in China and its implications. J. Environ. Manag. 2021, 287, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Hieslmair, H. Contextualizing PV waste to 2050 and the role of module reliability and degradation. In PV Reliability Workshop; NREL: Golden, CO, USA, 2023. [Google Scholar]
- Heath, G.A.; Silverman, T.J.; Kempe, M.; Deceglie, M.; Ravikumar, D.; Remo, T.; Cui, H.; Sinha, P.; Libby, C.; Shaw, S.; et al. Research and development priorities for silicon photovoltaic module recycling to support a circular economy. Nat. Energy 2020, 5, 502–510. [Google Scholar] [CrossRef]
- Rodríguez, K.T.; Vázquez, A.I.S.; Valdés, J.J.R.; Rodríguez, J.I.; Figueroa, M.G.P.; Porcar, S.; Méndez, A.Á. Photovoltaic glass waste recycling in the development of glass substrates for photovoltaic applications. Materials 2023, 16, 2848. [Google Scholar] [CrossRef]
- Peplow, M. Solar panels face recycling challenge. ACS Cent. Sci. 2022, 8, 299–302. [Google Scholar] [CrossRef]
- Sim, Y.; Tay, Y.B.; Pham, H.K.; Mathews, N. A facile crush-and-sieve treatment for recycling end-of-life photovoltaics. Waste Manag. 2023, 156, 97–106. [Google Scholar] [CrossRef]
- Li, M.; Widijatmoko, S.D.; Wang, Z.; Hall, P. A methodology to liberate critical metals in waste solar panel. Appl. Energy 2023, 337, 120900. [Google Scholar] [CrossRef]
- Bogust, P.; Smith, Y.R. Physical separation and beneficiation of end-of-life photovoltaic panel materials: Utilizing temperature swings and particle shape. JOM 2020, 72, 2615–2623. [Google Scholar] [CrossRef]
- Tokoro, C.; Nishi, M.; Tsunazawa, Y. Selective grinding of glass to remove resin for silicon-based photovoltaic panel recycling. Adv. Powder Technol. 2021, 32, 841–849. [Google Scholar] [CrossRef]
- Pagnanelli, F.; Moscardini, E.; Altimari, P.; Padoan, F.C.; Atia, T.A.; Beolchini, F.; Toro, L. Solvent versus thermal treatment for glass recovery from end of life photovoltaic panels: Environmental and economic assessment. J. Environ. Manag. 2019, 248, 109313. [Google Scholar] [CrossRef] [PubMed]
- Wahman, M.; Surowiak, A. Recycling of discarded photovoltaic modules using mechanical and thermal methods. Inz. Miner. 2022, 1, 107–116. [Google Scholar]
- Wahman, M.; Surowiak, A.; Berent, K.; Szymczak, P. Eco-efficient removal of polymer back sheet fraction and material separation from solar cell waste. Sol. Energy 2023, 264, 112085. [Google Scholar] [CrossRef]
- Wahman, M.; Surowiak, A.; Ebin, B.; Berent, K. PV back sheet recovery from c-Si modules using hot knife technique. Sol. Energy Mater. Sol. Cells 2024, 276, 113067. [Google Scholar] [CrossRef]
- Belançon, M.P.; Sandrini, M.; Tonholi, F.; Herculano, L.S.; Dias, G.S. Towards long term sustainability of c-si solar panels: The environmental benefits of glass sheet recovery. Renew. Energy Focus 2022, 42, 206–210. [Google Scholar] [CrossRef]
- Doni, A.; Dughiero, F. Electrothermal heating process applied to c-Si PV recycling. In Proceedings of the 38th IEEE Photovoltaic Specialists Conference, Austin, TX, USA, 3–8 June 2012; pp. 757–762. [Google Scholar]
- Min, R.; Deng, W.; Wang, Z.; Qi, T.; Zhang, Z.; Xiao, W.; Wang, D. Effective decapsulation method for photovoltaic modules: Limonene-induced eva controlled swelling under sonication and debonding mechanism analysis. J. Clean. Prod. 2024, 450, 141917. [Google Scholar] [CrossRef]
- Vaněk, J.; Jandova, K.; Vanýsek, P.; Maule, P. Chemical delamination applicable to a low-energy recycling process of photovoltaic modules. Processes 2023, 11, 3078. [Google Scholar] [CrossRef]
- Kamano, K.; Jaroenkhasemmeesuk, C.; Chaisartra, C.; Thoopkaew, T.; Tippayawong, N. Glass separation process for recycling of solar photovoltaic panels by microwave heating. AIP Conf. Proc. 2022, 2681, 020002. [Google Scholar]
- Królikowski, M.; Fotek, M.; Żach, P.; Michałowski, M. Development of a recycling process and characterization of eva, pvdf, and pet polymers from end-of-life pv modules. Materials 2024, 17, 821. [Google Scholar] [CrossRef]
- Danz, P.; Aryan, V.; Möhle, E.; Nowara, N. Experimental Study on Fluorine Release from Photovoltaic Backsheet Materials Containing PVF and PVDF during Pyrolysis and Incineration in a Technical Lab-Scale Reactor at Various Temperatures. Toxics 2019, 7, 47. [Google Scholar] [CrossRef]
- Tammaro, T.; Rimauro, J.; Fiandra, V.; Salluzzo, A. Thermal treatment of waste photovoltaic module for recovery and recycling: Experimental assessment of the presence of metals in the gas emissions and in the ashes. Renew. Energy 2015, 81, 103–112. [Google Scholar] [CrossRef]
- Sander, K. Study on the Development of a Take Back and Recovery System for Photovoltaic Products; Institut für Ökologie und Politik: Hamburg, Germany, 2007. [Google Scholar]
- Powalla, M.; Paetel, S.; Ahlswede, E.; Wuerz, R.; Wessendorf, C.D.; Friedlmeier, T.M. Thin-Film Solar Cells Exceeding 22% Solar Cell Efficiency: An Overview on CdTe-, Cu(In,Ga)Se2-, and Perovskite-Based Materials. Appl. Phys. Rev. 2018, 5, 041602. [Google Scholar] [CrossRef]
- DIN EN 572-1:2016-06; Glass in Building—Basic Soda-Lime Silicate Glass Products—Part 1: Definitions and General Physical and Mechanical Properties. German Version EN 572-1:2012+A1:2016; Beuth Verlag GmbH: Berlin, Germany, 2016.
- Blieske, U.; Stollwerck, G. Glass and other encapsulation materials. Semicond. Semimet. 2013, 89, 199–258. [Google Scholar]
- Riyatun; Bintari, P.L.; Purwanto, H.; Marzuki, A. Characterization of soda-lime glass with aluminium doping as a planar wave guide using electric-field-assisted solid-state ion exchange method. J. Phys. Conf. Ser. 2019, 1153, 012084. [Google Scholar] [CrossRef]
- Galvão, Á.C.P.; Farias, A.C.M.; Mendes, J.U.L. Characterization of waste of soda-lime glass generated from lapping process to reuse as filler in composite materials as thermal insulation. Cerâmica 2015, 61, 367–373. [Google Scholar] [CrossRef]
- Chakraborty, R.; Dey, A.; Mukhopadhyay, A.K. Loading rate effect on nanohardness of soda-lime-silica glass. Metall. Mater. Trans. A 2010, 41, 1301–1312. [Google Scholar] [CrossRef]
- Schmitz, A.; Kamiński, J.; Scalet, B.M.; Soria, A. Energy consumption and CO2 emissions of the European glass industry. Energy Policy 2011, 39, 142. [Google Scholar] [CrossRef]
- Rio, D.D.F.D.; Sovacool, B.K.; Foley, A.; Griffiths, S.; Bazilian, M.; Kim, J.; Rooney, D. Decarbonizing the glass industry: A critical and systematic review of developments, sociotechnical systems and policy options. Renew. Sustain. Energy Rev. 2022, 155, 111885. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).