Abstract
Unsymmetrical dimethylhydrazine (UDMH) is a common liquid propellant widely used in rocket engines and other applications. The safety of UDMH in service is affected by its slow oxidation during long-term storage to form impurities such as dimethylamine (DMA) and formaldehyde dimethylhydrazone (FDH). How these impurities affect combustion performance is not known, and in order to assess these effects, the present experiments investigated the combustion characteristics of self-igniting fuels and carried out ignition delay time measurements and flame propagation velocity measurements of pure UDMH and its denatured mixtures in a nitrogen tetroxide (NTO) atmosphere. This experiment was carried out to measure the delay time of hedge ignition of pure UDMH and qualitative analysis of its flame propagation properties under vacuum conditions at room temperature (T = 293 K). Ignition delay time measurements and flame propagation characterization were performed under the same experimental conditions for UDMH mixed with 1%, 5% and 10% FDH, UDMH mixed with 1%, 5% and 10% H2O, UDMH mixed with 1%, 5% and 10% DMA, as well as for UDMH mixed with the same proportions of the three substances (1%, 5% and 10%). The flame propagation characteristics were analyzed. The results showed that the incorporation of DMA, H2O and FDH in different proportions could inhibit the combustion of UDMH to varying degrees and prolong its ignition delay time. It is worth noting that the introduction of FDH had the least effect on it, and the least effect was observed at a concentration of 1%. In contrast, the effect of DMA on UDMH is more obvious, and the addition of H2O has the largest increase in the ignition delay time of UDMH. In the flame propagation experiment, the flame of the experimental group adding H2O can no longer fill the whole experimental window, while the other experimental groups can still make the window full of flame. Combined with the measurements of the ignition delay time, it can be seen that the moisture content has the greatest effect on the combustion characteristics.
1. Introduction
Hydrazine propellants are commonly used liquid propellants for launch vehicles. With the vigorous development of aerospace and military capabilities, their consumption has increased significantly. Unsymmetrical dimethylhydrazine (UDMH) has the advantages of high specific thrust and easy storage and is widely used as a propellant in military and aerospace fields [1,2]. Therefore, it is of great importance to study the ignition and flame propagation characteristics of UDMH.
At present, most of the domestic and international research on liquid propellant UDMH is focused on the treatment of UDMH wastewater and exhaust gas, and there are photocatalytic degradation, ozone micro and nano-bubbles, low-temperature plasma, and other methods and electrocatalytic oxidation to deal with the UDMH wastewater [3,4,5,6,7,8,9,10]. In the study of combustion characteristics of UDMH, Zhan Xiang et al. [11] used numerical simulation to simulate the reaction process of UDMH and NTO, during which a very small amount of UDMH and NTO react to form other methyl derivatives, and at the same time, the nitrogen dioxide reacts with the components in the air to form additional nitrous acid. Determination of UDMH by gas chromatography-mass spectrometry reveals that a small amount of FDH, DMA, H2O and other impurities are produced after its metamorphosis, all of these substances reduce the energy of UDMH, which affects its combustion characteristics, and it was found that there are about twelve metamorphic products of UDMH [12], and that the content of oxygen affects the rate of formation of the products after metamorphosis, as well as the quantity of products formed. Vikas K et al. [13] complexed UDMH by boron cyanide and cyanoborohydride, the physical and chemical properties of UDMH were completely changed, the combined UDMH boranes and UDMH diboranes were chemically stable, non-volatile, non-toxic, and harmless, and the ignition delay time of the complexes was significantly shortened compared to the ignition delay time of the pure UDMH, which is of great significance for the study of spontaneous combustion-type fuels. He Bo et al. [14] investigated the effect of temperature and pressure on the ignition delay and combustion time of spontaneous ignition of small droplets of UDMH organogel when NTO was used as an oxidizing agent. It was found that low temperature conditions lead to longer ignition delay time, the same droplet diameter, and with increasing pressure, the UDMH gel droplets first darken and gradually extinguish under high pressure conditions. The reason for this is that under high pressure, although the flame is very close to the droplet, most of the heat generated by combustion is transferred to the droplet, and for diffusion combustion to occur in the droplet under high pressure it needs to consume enough time to generate enough vapor to do so, but in the process the vapor on the surface of the UDMH droplet has already been consumed, resulting in the flame extinguishing. Songjiang Feng et al. [15] investigated the combustion characteristics of gelatinized UDMH. The effect of oxidant flow rate and temperature on the combustion behavior was studied in the presence of oxidant convection. It was found that the combustion process of gelled UDMH can be divided into four phases, heating and expansion phase, initial combustion phase, intense combustion and stabilization phase, and quenched combustion phase. The micro-explosion period in the droplet combustion process lasts for a long time, sometimes more than 70% of the entire combustion lifetime, and the burst vapor produced by the micro-explosion causes the phenomenon of gas jet combustion, and the transition of the combustion flame from a laminar enveloping flame structure to an escaping flame structure was also observed with the increase in convective intensity. The microburst intensity and burning rate increased with increasing convection temperature. Cesar A. V et al. [16] compared the combustion performance of hydrazine alone and mixed hydrazine with NTO and demonstrated that the vaporization length of monohydrazine decreases with increasing pressure. The effect of combustion chamber pressure, equivalence ratio, initial gas temperature, droplet size on the droplet evaporation length was analyzed. Umakant Swami et al. [17] investigated the ignition delay time of non-spontaneous high energy ionic liquid hydroxyethylhydrazine nitrate configured mixture mixed with meta-di-methylhydrazine. In the study of combustion characteristics of liquid propellant meta-di-methylhydrazine, the main method used is the preparation of meta-di-methylhydrazine gel droplets to investigate the ignition delay time as well as the flame propagation characteristics under different conditions such as temperature, flow rate and pressure of the oxidizer. Gelatinized UDMH combines the fluidity of liquid propellants with the stability of solid propellants that are easy to store and has been widely used in UDMH combustion characterization studies [18,19,20]. In the studies related to self-igniting fuels, most of the experiments are carried out in a heated oxidizer environment, and the main experimental methods include droplet experiments, droplet-to-droplet experiments, droplet airflow hedge experiments, and jet hedge experiments [21,22]. Others have done some research on hydrazine propellants, summarized in Table 1 below:

Table 1.
Hydrazine propellants have been studied by a number of scholars.
In addition, most of the current studies focus on the gas-liquid two-phase, and there are relatively few studies on the hedge combustion of two gases at room temperature, and relatively few studies on the effect of UDMH and its deteriorated products on its combustion characteristics at room temperature. It was difficult to realize the hedge ignition conditions required in this experiment with the previous equipment, so the laboratory built a set of experimental benches for the study of hedge ignition of self-igniting fuels by itself.
Based on this, this paper uses a self-constructed comprehensive test system for hedge flame combustion experiments to carry out a study on the ignition delay time measurement and flame propagation characteristics under the same experimental conditions of UDMH mixed with 1%, 5% and 10% of FDH; UDMH mixed with 1%, 5% and 10% of H2O; and UDMH mixed with 1%, 5% and 10% of DMA, as well as the mixture conditions of the three substances at the same ratios of 1%, 5% and 10%, respectively. Under the same conditions of 1%, 5%, and 10% mixture, we conducted measurements for ignition delay time and flame propagation.
2. Experimental Section
2.1. Experimental Setup
The experiment was conducted within a comprehensive test system for hedge flame combustion experiments self-developed and built in the laboratory, the schematic diagram of the experimental system is shown in Figure 1 and the physical diagram of the experimental system is shown in Figure 2.
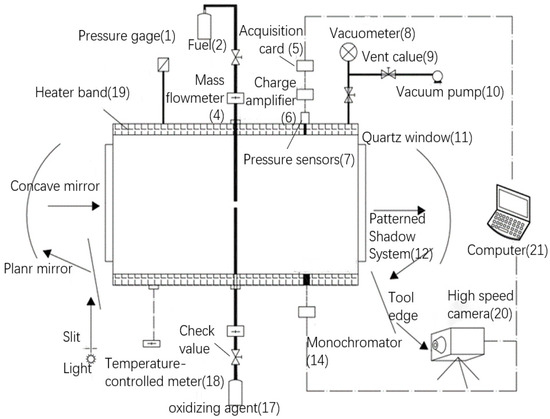
Figure 1.
Schematic diagram of the experimental system for the study of ignition and combustion characteristics of self-igniting fuels.
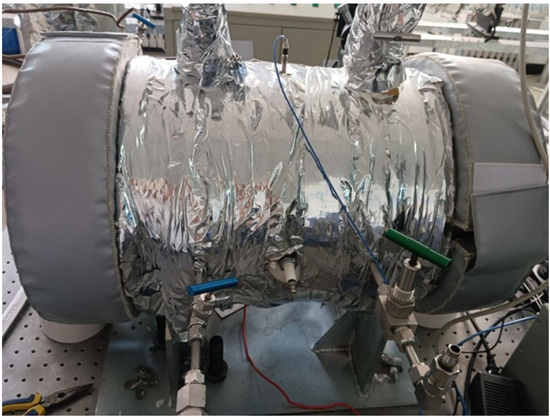
Figure 2.
Physical diagram of the experimental system.
This comprehensive experimental system is mainly composed of seven parts: combustion chamber, inlet system, exhaust system, temperature control system, data acquisition and processing system, pressure acquisition system and spectral acquisition system.
(1) The combustion chamber is mainly a hollow cylinder structure with openings at both ends, with an outer diameter of 300 mm and an inner diameter of 200 mm. The side walls of the combustion chamber are provided with holes of different diameters for connecting pressure sensors, temperature sensors, intake and exhaust piping and optical fibers. The openings at both ends are fitted with quartz glass forming a circular visualization window with a diameter of 110 mm. The optical system can be used for data acquisition during the experiment. The reaction process was visualized using high-speed cameras.
(2) The feed system consists of a fuel supply unit and an oxidizer supply unit, which includes a series of components such as fuel tank, oxidizer tank, solenoid valve, stainless steel inlet pipe, synchronous trigger device, mass flow meter and mechanical valves.
(3) The exhaust system is mainly composed of a vacuum table, mechanical valves, vacuum pumps, exhaust piping several parts. On the one hand, it can pump the experimental system to vacuum, on the other hand, it can discharge the exhaust gas after the experiment.
(4) The temperature control system is mainly composed of a heating tape wrapped around the outside of the combustion chamber, and a thermostat to control the temperature of the heating tape. The entire combustion reaction zone is heated to a suitable temperature for the experimental requirements prior to the start of the experiment.
(5) The data acquisition and processing system is mainly composed of a high-speed camera that facilitates the recording of the experimental process, a computer, and a data acquisition card, which allows the subsequent processing of the captured experimental images and the extraction of valid experimental data from them.
(6) The pressure acquisition system, mainly consisting of pressure sensors, charge amplifiers and data acquisition cards, which can record the pressure changes in the combustion chamber during the experiment.
(7) The spectral acquisition system mainly consists of optical fiber and monochromator, ignition will generate free radicals (OH, CH, etc.), which can produce a characteristic spectrum. It can be collected by the optical fiber, after the monochromator will convert the optical signal into an electrical signal, to provide a basis for the occurrence of the ignition phenomenon to provide a basis for discrimination.
The main equipment used during the construction of this lab bench includes the following: High-speed camera (Photron AX-200, IMAGICA GROUP Inc., Tokyo, Japan); reflective stroboscope ϕ200; INFICON vacuum pressure gauge (1000 torr), INFICON VGC-503 controller (INFICON, Bad Lagatz, Switzerland); YF-38 synchronous actuator (Shenzhen Yueyu Electronic Technology Co., Ltd., Dongguan, China); solenoid valve, computer data acquisition system and stainless-steel constant volume chamber.
In order to study the combustion characteristics of self-igniting fuel, the present experimental setup was designed and fabricated, and the whole experiment was conducted in a confined space, which is easy to operate and has better safety, and can ensure the smooth operation of the experiment.
2.2. Fuels
UDMH and NTO are stored in 304 stainless steel bottles with a pickled and passivated surface at a storage pressure of 2 MPa. Tokyo Kasei Industrial’s FDH, (purity > 98%), distilled H2O, and DMA gas (purity > 99.9%) were used.
2.3. Commissioning of Equipment
Given that this experiment took place within a custom-made hedge flame combustion experimental setup, a substantial amount of debugging work was undertaken to validate the viability of the apparatus. This pre-experimental phase revealed distinctive characteristics in the reaction between UDMH and NTO. Specifically, achieving hedge ignition required meticulous adjustments, necessitating the identification of the optimal distance between the outlet ends of the two substances. Furthermore, it was imperative to determine the optimal intake for both UDMH and NTO, ensuring that the ignition experiment could take place with precision within the visualization window of the experimental setup. It was found that when the fuel and oxidant inlet piping was 3 mm, the amount of UDMH and NTO entering the combustion chamber per unit of time was too small to allow ignition to occur, as shown in Figure 3 below.
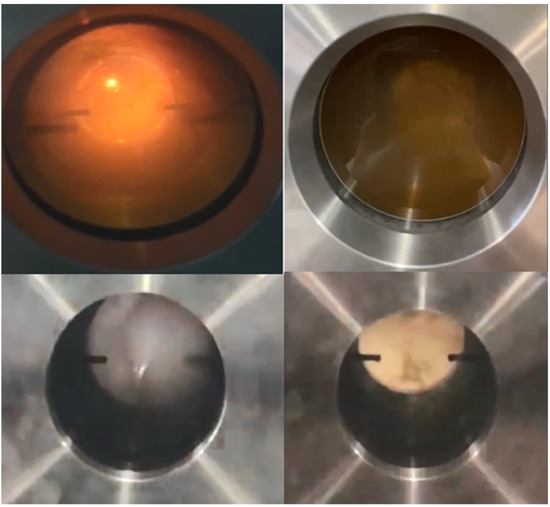
Figure 3.
Experimental results of UDMH/NTO hedge ignition under 3 mm inlet duct conditions (Pure UDMH and NTO).
Through a large number of experiments, it was found that when using a 3 mm inlet pipe, changing the inlet order, charging UDMH and then NTO, or changing the distance between the outlet ends of UDMH and NTO, when the two gases entered the combustion chamber, the flame did not appear as expected.
Two phenomena were observed during the experiment: (1) when NTO was in excess, no ignition phenomenon was observed, but the whole experiment was accompanied by the occurrence of chemical processes, and a large amount of water vapor was produced on the whole quartz glass after the reaction was completed; (2) when UDMH was in excess, it was visible to the naked eye, and a large amount of white smoke was generated, and some water vapor was produced, and ignition was not observed during this process either. The experimental phenomenon of using a ripple-shadow to photograph the filling of UDMH and then NTO from the side wall is shown in Figure 4 below.
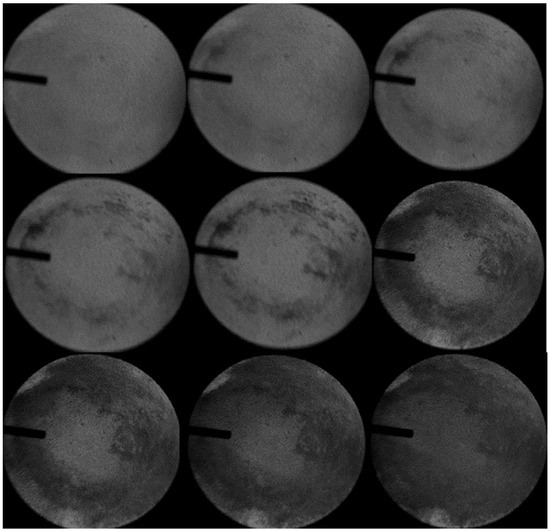
Figure 4.
Ripple-shadow NTO ignition experimental shadow measurements (Pure UDMH and NTO).
From Figure 5, it can be found that when the inner diameter of the inlet duct is increased from 3 mm to 6 mm, the ignition and stable flame propagation can be achieved by simultaneously charging UDMH and NTO into the combustion chamber. The gas volatilized by the two substances at room temperature can meet the experimental requirements, and the same results were obtained by repeating the experiment several times, which further confirmed that the experimental scheme is practical and feasible and can ensure the smooth operation of the experiment. G is the direction of gravity in the figure.
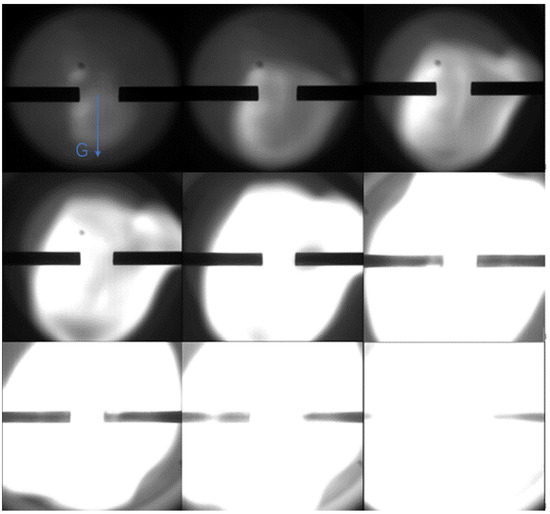
Figure 5.
Experimental results of UDMH/NTO hedge ignition under 6 mm inlet duct conditions (Pure UDMH and NTO).
This experiment includes the measurement of ignition delay time and the study of flame propagation characteristics. The ignition delay time measurement experiment uses a high-speed camera coupled with a grain shadow system to perform the measurement, and the high-speed camera of the coupled grain shadow can clearly capture the disturbance of the gas flow field in the combustion chamber, and the whole process of fuel and oxidizer entering the combustion chamber to meet until ignition can be clearly observed. It is defined as the ignition delay time of UDMH at room temperature. This process is defined as the ignition delay time of UDMH at room temperature. A clear picture of the gas flow field perturbation process captured by this experimental test setup is shown in Figure 6.
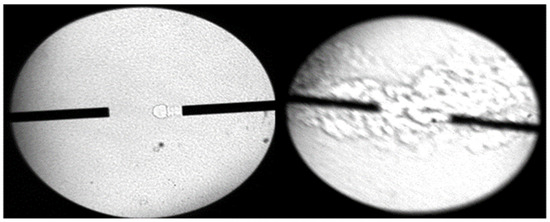
Figure 6.
Typical gas flow field disturbance process (Pure UDMH and NTO).
The flow field change after the gas comes out and the subsequent gas hedging process can be clearly seen in Figure 6. Based on this, it is feasible for this experiment to measure the ignition delay time by observing the flow field change during the process of gas coming out until the encounter by coupling a high-speed camera with ripple shadow. The following experiments were carried out on this experimental set-up:
The effect of impurities (H2O, FDH, DMA and mixtures of the three) on the ignition characteristics of UDMH/NTO was evaluated by using a high-speed camera to determine the ignition delay times of UDMH and its doping with different impurities when they met with NTO.
During the experiment, it was found that when the ignition of UDMH and NTO occurred, a large amount of H2O vapor was generated immediately to cover the optical window, which hindered the further recording of the combustion flow field by the high-speed camera. The ignition and combustion processes of UDMH and NTO were attempted to be filmed directly through an optical window with a high-speed camera in a dark environment. Although the moment of encounter between meta-hydrazine and tetroxide could not be captured, the ignition and subsequent flame propagation of the two could be clearly filmed. In this experiment, the ignition and combustion processes of UDMH and NTO were directly photographed using high-speed photography to study the flame propagation characteristics of the two species after ignition.
For hedge flame ignition delay time measurement experiments, the entire experimental system is first pumped to ultimate vacuum before the single experiment to ensure that the entire experiment is carried out under room temperature vacuum conditions. Afterwards, the filling of fuel and oxidizer tanks with gas at specified pressures and the ripple shadowing system was tuned to optimal conditions (Photron AX-200 high-speed camera with a maximum frame rate of 6400 Fps was selected for the experiments. 1000 Fps was selected for the ignition delay time measurements with a maximum resolution of 1024 × 1024). Subsequently, a synchronous trigger device was used to control the opening and closing of the battery valve for the ignition experiment, and the entire experiment was recorded by high-speed photography with coupled ripple shadows.
2.4. Experimental Procedure
The experiment was carried out under vacuum conditions at room temperature. The boiling point of UDMH is 61 °C and the saturated vapor pressure at room temperature is 16.4 KPa, and the gas volatilized at room temperature can meet the experimental requirements through experimental tests. NTO is a coexistence of NTO and nitrogen dioxide at room temperature, with a boiling point of 21 °C. Even at room temperature, a substantial volume of gas was generated, adequately meeting the experimental conditions.
Prior to initiating a single experiment, the entire experimental system was pumped to ultimate vacuum (below 1000 Pa), the solenoid valves on both sides of the combustion chamber were closed, and the fuel and oxidizer valves on both sides were opened to pressurize the fuel and oxidizer pre-storage tanks to the specified pressure. The ripple shadow and high-speed camera were adjusted to their appropriate positions, and the synchronization trigger was manually activated. UDMH and NTO were simultaneously introduced into the combustion chamber, where they met and reacted after a delay time before extinguishment. The high-speed camera recorded the entire experimental process. After each individual experiment, the exhaust system was opened to release residual waste gas from the combustion chamber into the fume hood. Nitrogen was used for washing more than three times to ensure the safety of the experiment and prevent any residue from previous reactions that could potentially impact the results of subsequent experiments.
After the preparation of the equipment and fuel, the experiment can be started. The specific experimental working conditions are shown in Table 2.

Table 2.
Experimental test conditions.
Based on the pre-designed experiments, ignition delay time measurements were carried out for UDMH with 1%, 5% and 10% H2O, 1%, 5% and 10% FDH, 1%, 5% and 10% DMA, and 3%, 15% and 30% of all three substances in a homogeneous mixture. The experimental procedure was always carried out ensuring that the fuel mixture pressure was at 9 KPa and NTO at 70 KPa. Under the same test conditions as in Table 1 above, a high-speed video camera was chosen to directly capture the ignition and flame propagation process. The frame rate was selected to be 4000 Fps, and the maximum resolution was 896 × 896. The synchronized triggering device was used to control the solenoid valves on the inlet pipeline of UDMH and NTO for the ignition experiments, and a high-speed video camera was used to directly film the whole ignition and combustion process, and the results of the experiments were analyzed in order to study the flame propagation characteristics.
2.5. Error Analysis
At least three experiments for the same working conditions, taking into account possible errors in a single experiment. During the pre-experiment, we made several single-point measurements of the ignition delay time for pure UDMH and nitrogen tetroxide hedge ignition, and the results obtained were all within 5% error. All three experimental results are considered normal when they deviate within 5% of each other. The average value of the three experiments was used as the ignition delay time of the fuel/oxidizer under this operating condition. Uncertainties in this process arise mainly from measurement errors and the non-ideal state of the gas.
3. Results and Discussion
3.1. Ignition Delay Time Measurement Results
Take the example of a hedge ignition experiment with pure UDMH and NTO, from which a time point is intercepted as time zero. It was observed that the two gases met at 266 ms and ignition occurred at 591 ms with a time interval of 325 ms. The time interval between the moment of encounter between UDMH and NTO and the onset of ignition is defined as the ignition delay time under these conditions. Based on this definition, the ignition delay time of UDMH and NTO under this condition is 325 ms. A typical experimental recording procedure is shown in Figure 7.
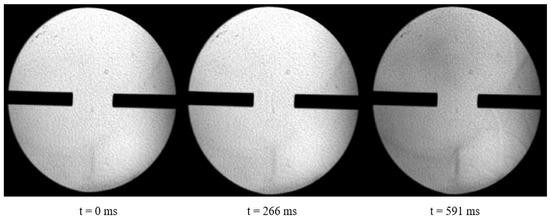
Figure 7.
Ripple-shadow record of the hedge ignition experiment with UDMH and NTO (Pure UDMH and NTO).
By the above experimental method, the pure UDMH and the experimental group adding different proportions of dopants were respectively hedged with NTO to measure the ignition delay time, and the experimental results were obtained as shown in Figure 8.
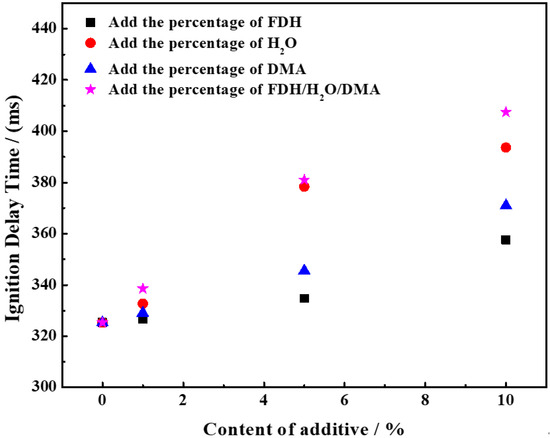
Figure 8.
Results of the effect of H2O, DMA and FDH content on the ignition delay time of UDMH/NTO.
From Figure 8, it can be found that the ignition delay time for the combustion of pure UDMH and NTO is the shortest, with an actual measured ignition delay time of 325.33 ms. The addition of H2O, FDH and DMA all increase the ignition delay time of UDMH to varying degrees. In particular, the addition of 1% H2O increased the ignition delay time of the system to 332.67 ms, an increase of 7.34 ms; the addition of 1% DMA increased the ignition delay time of the system by 3.67 ms and the ignition delay time of the system grew to 329 ms; the addition of 1% FDH increased the ignition delay time of the system slightly by 1.4 ms and the ignition delay time became 326.75 ms. It can be concluded that the ignition delay time for combustion with NTO increases significantly when the H2O content in UDMH reaches 1%, while the ignition delay time of UDMH remains almost unchanged when the content of FDH in UDMH increases to 1%, with the addition of 1% DMA having an in-between effect on the ignition delay time. The results indicate that the simultaneous addition of 1% H2O, 1% DMA, and 1% FDH to UDMH results in the longest ignition delay time compared to the cases where each impurity is added individually. The ignition delay time reaches 338.5 ms, showing an increase of 13.17 ms.
Elevating the FDH content in UDMH to 5% resulted in an increased ignition delay time of the mixture, reaching 334.75 ms. This effect closely paralleled the impact observed with the addition of 1% H2O. Notably, the ignition delay time experienced a mere 2.9% increase compared to that of pure UDMH/NTO. Conversely, the introduction of 5% H2O elevated the ignition delay time for UDMH to 378.33 ms, marking a substantial 16.3% increase relative to the pure UDMH/NTO ignition delay time. Meanwhile, the inclusion of 5% DMA led to a 6.2% rise in the ignition delay time compared to pure UDMH/NTO, reaching 345.5 ms. The longest ignition delay time observed was 381 ms when 5% H2O, 5% DMA, and 5% FDH were introduced simultaneously into the UDMH. While this duration closely approached the ignition delay time observed with 5% H2O alone, it surpassed the ignition delay time associated with the addition of 5% H2O.
The ignition delay time of the mixture increased by 32.33 ms, reflecting a rise of only 9.9% compared to pure UDMH when the FDH content reached 10%. Introducing 10% DMA resulted in a 45.67 ms increase in the ignition delay time, representing a 14.4% augmentation. With 10% H2O, the mixture exhibited an ignition delay time of 393.66 ms, marking a 68.33 ms increase, equivalent to a 21% rise compared to pure UDMH. Notably, the concurrent presence of 10% of each of the three substances led to an ignition delay time of 407.5 ms, signifying an 82.17 ms increase, and a 25.26% elevation compared to pure UDMH.
While all three substances influenced the ignition delay time of UDMH, H2O had the most pronounced effect, followed by DMA, with FDH exerting the least impact on the ignition delay time. The simultaneous presence of all three substances demonstrated a more significant effect on the ignition delay time of UDMH than the addition of any one substance alone.
The outcomes of this experiment underscore the pronounced influence of H2O on the ignition delay time of UDMH. Consequently, diligent monitoring of H2O content in UDMH is imperative during practical applications. Additionally, to meet usage requirements, the inclusion of a specific quantity of DMA (10%) demonstrates a noteworthy impact on the ignition delay of UDMH/NTO, warranting vigilant scrutiny of its content during practical utilization. In contrast, the presence of a certain amount of FDH (1%) exerts minimal influence on the ignition process of UDMH/NTO.
3.2. Measurement Results of Flame Propagation Characteristics
Based on the same experimental conditions as in Table 2 above, a study of flame propagation characteristics of hedge ignition with UDMH and NTO mixed with different proportions of impurities under different operating conditions was carried out. It has been found in the previous experiments that the ripple shadow could not continue to record the subsequent flame propagation process because the H2O produced during the combustion process covered the optical window. In order to study the flame propagation characteristics of UDMH under the conditions of doping with different impurities, the experiments were chosen to directly capture the ignition and flame propagation processes using a high-speed camera. Taking the ignition and combustion experiments of pure UDMH and NTO as an example, the whole process from the beginning of ignition to the complete extinction of the flame was clearly obtained by high-speed camera. The experimental results are shown in Figure 9.
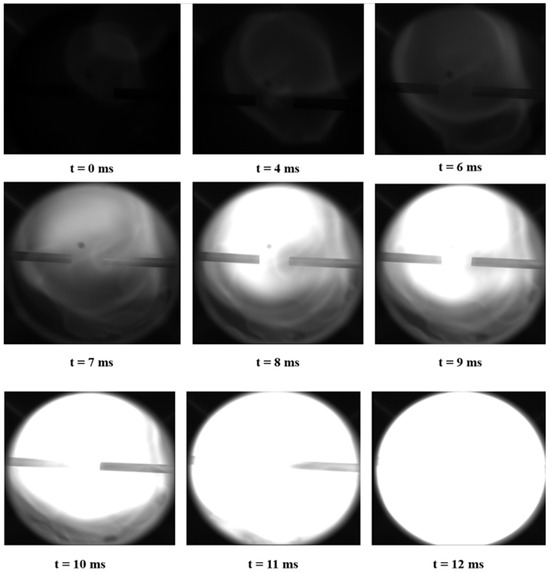
Figure 9.
Results of UDMH/NTO hedge ignition and combustion experiments (Pure UDMH and NTO).
Through the experimental results captured by the high-speed camera, the experimental data processing, selected just occurred weak ignition time point as the starting point of ignition occurrence, set as time zero. It was found that the initial combustion flame of UDMH at room temperature and the hedonic combustion of NTO was relatively weak. As the combustion progressed, a bright and blinding flame was formed inside the initial combustion flame, and the flame gradually expanded to the entire glass viewport of this experimental setup, and the flame was gradually extinguished after the fuel was consumed.
The flame propagation characteristics of this experiment under hedging conditions are different from the spherical outward propagation characteristics of the flame of a typical hydrocarbon fuel after a central ignition. The outward flame propagation process exhibits greater intricacy, and the derived flame propagation paths from the experiments manifest a higher degree of irregularity compared to those observed in other hydrocarbon fuels. The time between the formation of a weak flame in the first stage after the fuel and oxidizer meet and the bright flame formed in the second stage first reaches the edge of the optical window is defined as the shortest flame propagation time. The average flame propagation speed corresponding to this is defined as the maximum flame propagation speed.
From the figure, it can be observed that pure UDMH and NTO ignite in two stages, with the first stage first forming a weak flame of low intensity, and with the continuous entry of both, the reaction rate is further accelerated. The second stage of high-intensity bright flame first forms near the combustion chamber inlet duct (centre of the combustion chamber). The flame spreads outward continuously, completely covering the entire area of the optical viewport (110 mm) at 12 ms.
Compared to the flame propagation characteristics of pure UDMH with nitrogen oxide hedge combustion, the flame propagation characteristics of UDMH with 1% H2O are shown in Figure 10 below.
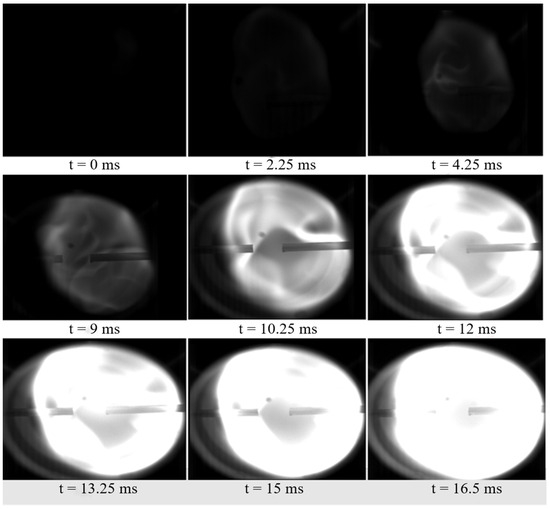
Figure 10.
Results of 1% H2O/99% UDMH/NTO hedge combustion experiments (1% H2O/99% UDMH and NTO).
The above figure shows the hedonic combustion of UDMH and NTO with 1% H2O, flame propagation is significantly hindered, and the involvement of H2O has a significant effect on the flame propagation of UDMH. Under the same experimental conditions with the addition of meta-hydrazone and DMA, the flame could still fill the entire experimental window. The experimental results of adding different proportions of H2O, DMA and FDH are shown in Figure 11.
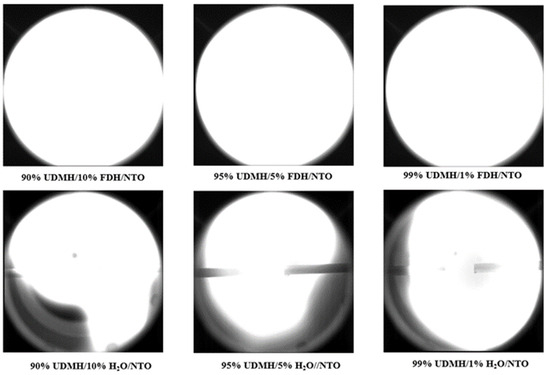
Figure 11.
Results of the effect of H2O and FDH content on the flame propagation characteristics of UDMH/NTO.
Figure 11 shows that when 1% of H2O is present in UDMH, its flame propagation after combustion with NTO is already hindered, and the addition of H2O severely hinders the flame propagation. Under the present experimental test conditions, when 1% of H2O was added to UDMH, the flame was unable to spread over the entire optical window range (110 mm), and as the amount of H2O contained increased, the farthest distance of flame propagation became shorter and shorter. Compared to H2O, the addition of FDH has little effect on the flame propagation of UDMH, and the flame can still fill the entire optical window after the mixture burns with the addition of 10% FDH.
Figure 12 shows that the addition of DMA has a smaller effect on flame propagation, and with 10% DMA the flame can still spread to fill the entire optical window. However, when all three substances are added to UDMH at the same proportion at the same time, the flame cannot spread to the whole range of the optical window, when the proportion is all 1%, and the farthest distance of flame propagation becomes shorter and shorter as the proportion of the three substances keeps increasing. The maximum flame propagation velocities for UDMH/NTO hedge ignition under different experimental conditions are shown in Figure 13.
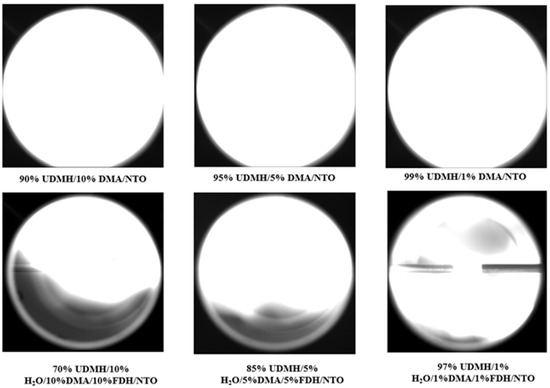
Figure 12.
Results of the effect of DMA content and the combined presence of DMA, H2O and FDH on the flame propagation characteristics of UDMH/NTO.
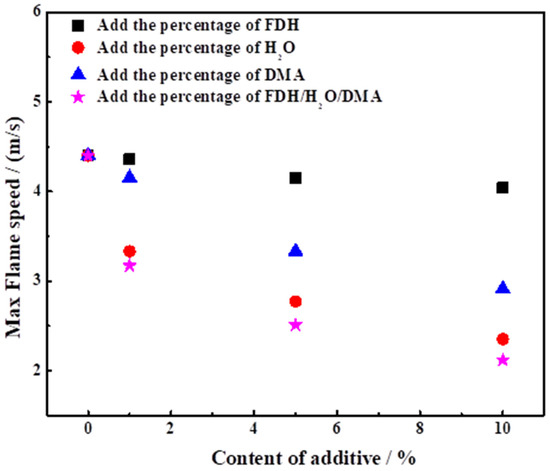
Figure 13.
Effect of H2O, DMA and FDH content on the maximum flame propagation velocity of UDMH/NTO.
It can be found that the maximum flame propagation velocity of pure UDMH is about 4.4 m/s, and when 1% H2O is added, its maximum flame propagation velocity decreases to 3.33 m/s. The addition of H2O obviously affects the maximum flame propagation velocity of UDMH. When 5% of H2O was added to UDMH, its flame propagation velocity was only 2.77 m/s. The addition of 10% H2O directly reduced the maximum flame propagation velocity of UDMH to 2.35 m/s. When H2O was added in the amount of 1%, 5% and 10%, the maximum flame propagation velocity of hedonic combustion of UDMH/NTO was reduced by 24.3%, 37.1% and 46.6%.
When 1% DMA was added, its maximum flame propagation speed decreased to 4.15 m/s, which was 5.7% lower compared to pure UDMH, and when 5% DMA was added, the flame propagation speed was 3.3 m/s, and when 10% DMA was added its flame propagation speed decreased to 2.91 m/s, a decrease of 33.86%. The addition of 10% DMA has an effect on the flame propagation of UDMH to a similar extent to that of 5% H2O.
From Figure 13 it can also be noticed that the addition of FDH did not significantly affect the maximum flame propagation velocity of UDMH/NTO. The maximum flame propagation velocity drops to 4.36 m/s when 1% FDH is included in UDMH, which is almost unchanged compared to pure UDMH after taking into account measurement errors. When 5% of FDH was added to UDMH, the maximum flame propagation velocity of the mixture decreased to 4.15 m/s, a decrease of 5.7%, while 10% of FDH only decreased the maximum flame propagation velocity of UDMH by 9.0%, and the maximum flame propagation velocity still reached 4.04 m/s. Thus, it can be seen that the addition of FDH had less effect on the maximum flame propagation velocity of UDMH/NTO after hedge ignition, The effect was relatively small.
When the three substances were added to UDMH in the same proportion, the flame propagation speed was 3.17 m/s at 1% of each substance, which was 27.95% lower than that of pure UDMH; the maximum flame propagation speed was 2.51 m/s for each of the three substances at 5%, a decrease of 42.95%, which was 42.95% lower; with 10% of each of the three substances, the maximum flame propagation speed drops to 2.12 m/s, a reduction of 51.2%. propagation velocity dropped to 2.12 m/s, a drop of 51.2%. In comparison with the previous experiments of adding one substance alone, it was found that the three substances added to UDMH in the same proportion at the same time had the greatest effect on the flame propagation velocity of UDMH/NTO compared to the addition of a single component to UDMH. Comparing the experimental results with the single component H2O, it is mainly H2O that plays a dominant role when the three substances are mixed, and its flame propagation speed is slightly lower than that of H2O alone.
The results of this experiment show that H2O has a great influence on the flame propagation characteristics of UDMH, followed by DMA. In practice, in order to meet the requirements of use, special attention should be paid to monitoring the content of H2O and DMA in UDMH; DMA (10%) has a greater influence on the flame propagation, whereas a certain amount of FDH (1%) will not have too much influence on the flame propagation of UDMH/NTO.
4. Conclusions
In this experiment, a set of experimental equipment which can be used to study the hedge ignition of self-igniting fuels was built by ourselves and its reliability was verified. On the basis of this experiment, under the determined conditions of oxidizing agent and fuel pressure, the effects of the participation of different proportions of impurities into the UDMH on the combustion characteristics of the UDMH were investigated by adding different proportions of water, DMA and FDH into the UDMH, and the following conclusions were drawn:
(1) The combustion process of UDMH can be divided into four stages, namely, the white smoke generation stage, the weak combustion stage, the intense combustion stage and the final extinguishing stage, but it was found that a large amount of white smoke would be generated only when the ratio of the two was not optimal in the actual experimental process, and a large number of comparative experiments were done in the present experiments, and when the UDMH was overdosed, a large amount of white smoke would be generated. When the amount of UDMH and NTO is in the right proportion, the white smoke generation in the combustion process is very rapid, and the ignition has already occurred when the process of white smoke generation is almost not captured.
(2) The degradation products of UDMH, such as H2O, DMA, and FDH, contribute to an increase in the ignition delay time of UDMH. Among these substances, H2O exerts the most substantial impact on the ignition delay time, followed by DMA, while FDH has a relatively minor effect. Nevertheless, when the FDH concentration reaches a specific level (10%), its influence on the ignition delay time becomes noticeably significant. Across various conditions designed in this experiment, the ignition delay times ranged from 325 milliseconds to 407 milliseconds.
(3) The three UDMH deterioration products, H2O, DMA and FDH, all inhibit the flame propagation rate of UDMH/NTO combustion to a certain extent, but in comparison, H2O has the greatest effect on the flame propagation rate of UDMH, followed by DMA, and FDH has a lesser effect on the flame propagation rate. In this experiment, the flame propagation speed was between 2.1–4.4 m/s.
It can be seen from this experiment that the impurity H2O should be avoided as much as possible during the actual use, and the content of DMA and FDH should be controlled, and the effect of reaching a certain amount of DMA (5%) and FDH (10%) should be taken into account. The tests carried out in this experiment provide a reference for the storage and use of UDMH/NTO binary propellants.
Author Contributions
Methodology, R.H., Y.L. and J.L.; Software, Y.F. and R.M.; Validation, R.H.; Investigation, X.W., J.Z. and J.W.; Data curation, X.W.; Writing—original draft, X.W.; Supervision, J.L.; Project administration, J.L.; Funding acquisition, J.L. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge the financial foundations from the National Natural Science Foundation of China (No.12172335), the Scientific Activities of Selected Returned Overseas Professionals in Shanxi Province (No.20230014).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhou, F.; Reng, X.H.; Qiang, H.F.; Zeng, Y.Z.; Fan, M.M. GO enhancement of Visible Light Response of g-C3N4 Aerogel and Photocatalytic Degradation of Undimethylhydrazine Wastewater. Mater. Eng. 2021, 49, 171–178. [Google Scholar]
- Hao, Z.W.; Jia, Y.; Yin, L.F.; Su, J.; Wang, H.Y. Study on Degradation of Undimethylhydrazine Wastewater by Electrocatalytic Oxidation. Appl. Chem. Ind. 2022, 51, 1261–1266. [Google Scholar]
- Huang, L.C.; Ye, J.F.; Wang, D.K.; Zheng, Y.Z. Experimental Study on Degradation of Undimethylhydrazine Wastewater by Low Temperature Plasma. Energet. Mater. 2022, 30, 1013–1021. [Google Scholar]
- Guo, Z.; Cheng, Y.X.; Zhang, Z.J. Ozone-micro Nano Bubble Advanced Oxidation Coupling Process for Hydrazine Wastewater Treatment. Ind. Water Treat. 2021, 41, 94–98. [Google Scholar]
- Luo, S.T.; Fu, Y.W.; Zhang, M.Y.; Liu, Y.F.; Wang, D.K.; Zhang, J.W.; Liu, D.X.; Rong, M.Z. Theoretical Study on the Degradation Pathways of Unsymmetrical Dimethylhydrazine by Aqueous. Plasma Chem. Plasma Process. 2023, 43, 81–97. [Google Scholar] [CrossRef]
- Hou, R.M.; Jia, Y.; Huang, Y.Z.; Shen, K.K.; Zhu, H.X. TiO2 reduced graphene oxide for the removal of gas-phase unsymmetrical dimethylhydrazine. New J. Chem. 2021, 45, 394–402. [Google Scholar]
- Koroleva, T.V.; Semenkov, I.N.; Lednev, S.A.; Soldatova, O.S. Unsymmetrical Dimethylhydrazine (UDMH) and Its Transformation Products in Soils: A Review of the Sources, Detection, Behavior, Toxicity, and Remediation of Polluted Territories. Eurasian Soil Sci. 2023, 56, 210–225. [Google Scholar] [CrossRef]
- Angaji, M.T.; Ghiaee, R. Decontamination of unsymmetrical dimethylhydrazine waste water by hydrodynamic cavitation-induced advanced Fenton process. Ultrason. Sonochem. 2015, 23, 257–265. [Google Scholar] [CrossRef]
- Yu, L.W. Study of eu-doped nano-zno Photocatalytic Degradation of Unsymmetrical Dimethyl-Hydrazine. 2009. Available online: https://webofscience.clarivate.cn/wos/alldb/full-record/PQDT:67185869 (accessed on 23 May 2023).
- Liang, M.L.; Li, W.J.; Qi, Q.; Zeng, P.C.; Zhou, Y.C.; Zheng, Y.P.; Wu, M.; Ni, H.M. Catalyst for the degradation of 1,1-dimethylhydrazine and its by-product N-nitrosodimethylamine in propellant wastewater. RSC Adv. 2016, 6, 5677–5687. [Google Scholar] [CrossRef]
- Zhan, X.; Cui, C.Y.; Zhou, X.D.; Xin, T.D.; Han, X.Y. Numerical Simulation of Reaction between Undimethylhydrazine and Nitrogen Dioxide Gas. Missiles Space Veh. 2019, 1, 59–63+79. [Google Scholar]
- Xu, Z.L.; Chen, F.; Huang, L.Z.; Guo, W.Z. Determination of Oxidation Products of Undimethylhydrazine in Storage by Gas Chromatography-Mass Spectrometry. J. Explos. Explos. 2018, 41, 523–530. [Google Scholar]
- Bhosale, V.K.; Karnik, S.; Kulkarni, P.S. Ignition Study of Amine Borane/Cyanoborane Based Green Hypergolic Fuels. Combust. Flame 2019, 210, 1–8. [Google Scholar] [CrossRef]
- He, B.; Nie, W.; Feng, S.; Su, L.; Zhuang, F. Effects of NTO Oxidizer Temperature and Pressure on Hypergolic Ignition Delay and Life Time of UDMH Organic Gel Droplet. Propellants Explos. Pyrotech. 2013, 38, 665–684. [Google Scholar] [CrossRef]
- Feng, S.; He, B.; He, H.; Su, L.; Hou, Z.; Nie, W.; Guo, X. Experimental Studies the Burning Process of Gelled Unsymmetrical Dimethylhydrazine Droplets under Oxidant Convective Conditions. Fuel 2013, 111, 367–373. [Google Scholar] [CrossRef]
- Salvador, C.A.V.; Costa, F.S. Vaporization Lengths of Hydrazine Fuels Burning with NTO. J. Propuls. Power 2006, 22, 1362–1372. [Google Scholar] [CrossRef]
- Swami, U.; Senapathi, K.; Srinivasulu, K.M.; Desingu, J.; Chowdhury, A. Ignition Delays of Mixtures of the Non-Hypergolic Energetic Ionic Liquid Hydroxyethylhydrazinium Nitrate Blended with Unsymmetrical Dimethylhydrazine. Propellants Explos. Pyrotech. 2019, 44, 1139–1146. [Google Scholar] [CrossRef]
- Wang, S.; Thynell, S. An Experimental Study on the Hypergolic Interaction between Monomethylhydrazine and Nitric Acid. Combust. Flame 2012, 159, 438–447. [Google Scholar] [CrossRef]
- Dennis, J.D.; Son, S.F.; Pourpoint, T.L. Critical Ignition Criteria for Monomethylhydrazine and Red Fuming Nitric Acid. J. Propuls. Power 2015, 31, 1184–1192. [Google Scholar] [CrossRef]
- Zhang, D.; He, C.; Zhang, P.; Tang, C. Mass Interminglement and Hypergolic Ignition of TMEDA and WFNA Droplets by Off-Center Collision. Combust. Flame 2018, 197, 276–289. [Google Scholar] [CrossRef]
- Liu, W.-G.; Wang, S.; Dasgupta, S.; Thynell, S.T.; Goddard, W.A.; Zybin, S.; Yetter, R.A. Experimental and Quantum Mechanics Investigations of Early Reactions of Monomethylhydrazine with Mixtures of NO2 and N2O4. Combust. Flame 2013, 160, 970–981. [Google Scholar] [CrossRef]
- Catoire, L.; Chaumeix, N.; Pichon, S.; Paillard, C. Visualizations of Gas-Phase NTO/MMH Reactivity. J. Propuls. Power 2006, 22, 120–126. [Google Scholar] [CrossRef]
- Shi, Y.H.; Hu, Y.P.; Li, M.H.; Guo, L.L. Numerical Simulation of Thermal Dynamic Characteristics of UDMH Pool Fire. Sci. Technol. Eng. 2023, 23, 8464–8471. [Google Scholar]
- Jiang, Y.D.; Xu, Q.Y.; Ma, Y.L.; Cheng, Y. Dynamic Response Study of Hydrazine-Based Monocomponent Rocket Engine. J. Aeronaut. 2023, 1–13. Available online: http://kns.cnki.net/kcms/detail/11.1929.v.20230921.1021.002.html (accessed on 12 November 2023).
- He, B.; Feng, S.J.; Nie, W.S. Numerical Simulation of Chemical Delay Time of Liquid Rocket Hypergolic Propellant. J. Syst. Simul. 2013, 25, 612–615+625. [Google Scholar]
- Huang, Z.Y.; Luo, F.; Shi, H.W.; Wang, X.J. Numerical Simulation of Diffusion of Unsymmetrical Dimeythylhrazine Under Storage Condition. Sci. Technol. Rev. 2011, 29, 67–70. [Google Scholar]
- Wang, F.S.; Yao, Z.P.; Liu, Y.; Zhang, Z.; Cai, K.; Wang, P.; Mao, X.F.; Yang, L. Numerical and Experimental Analysis of Pulse Mode Characteristics in a MMH/NTO Rocket Engine. Aerosp. Control Appl. 2021, 47, 56–62. [Google Scholar]
- Wu, R.; Nie, W.S.; Cai, H.H.; Qiao, Y.; Feng, W. Three-dimensional Simulation Study of UDMH/NTO Rocket Engine Plume Flow Field Characteristics. Missiles Space Veh. 2016, 5, 74–79. [Google Scholar]
- Feng, S.J.; He, B.; Nie, W.S. Progress in combustion characteristic research of liquid rocket gelled propellants. J. Rocket Propuls. 2009, 35, 1–7+13. [Google Scholar]
- Fu, Q.; Du, Z.; Lan, H.; Yu, S.; Yang, C. Preparation and properties research of UDMH/NTO gel propellant. J. Rocket Propuls. 2006, 32, 48–53. [Google Scholar]
- Han, W.; Du, Z.G.; Fu, Q.J.; Lan, H.P.; Yang, C.; Wu, J.; Yu, J. Study on the formulation of NTO gelled propellant. J. Rocket Propuls. 2008, 34, 54–58. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).