Abstract
The demand for biodiesel worldwide is skyrocketing as the need to replace fossil diesel with renewable energy sources becomes increasingly pressing. In this context, biocatalysis is emerging as an environmentally friendly and highly efficient alternative to chemical catalysis. When combined with the utilization of waste materials, it has the potential to make the process of biodiesel production sustainable. In the study, the potential of an extract rich in lipase produced by an Amazonian endophytic fungus as a biocatalyst in the transesterification of waste cooking oil for biodiesel production has been systematically investigated. The fungus Endomelanconiopsis endophytica exhibited an enzyme production of 11,262 U/mL after 120 h of cultivation. The lipolytic extract demonstrated its highest catalytic activity at 40 °C and a pH of 5.5. Using soybean oil and frying residue as raw materials, biodiesel was produced through biocatalytic transesterification, and yields of 91% and 89% (wt.), respectively, were achieved. By evaluating the process parameters, a maximum biodiesel yield of 90% was achieved using ethanol at a ratio of 3:1 ratio within 120 min. The experimental results demonstrate the feasibility and sustainability of applying a fungal enzymatic extract as a biocatalyst in the production of ethyl esters using waste cooking oil as a raw material.
1. Introduction
Fossil diesel is a non-renewable energy source that generates pollutants and is directly associated with global warming, climate change and even some incurable diseases [1]. Biodiesel, being a renewable fuel, has become an attractive alternative to replace diesel or be blended with it in order to mitigate pollution problems [2]. The advantages of biofuel over diesel include the fact that it is safe, non-toxic and biodegradable; contains no sulfur; and is a better lubricant. In addition, the global demand for biodiesel has been increasing considerably in recent years due to public policies that demand increased incorporation of biodiesel in diesel [3]. The global biodiesel market is estimated to be worth around US $54.8 billion in 2028, with a compound expected annual growth of around 5.8% between 2021 and 2028 [4].
In Brazil, soybean oil is the main raw material used to produce biodiesel and, as such, represents 70 to 80% of the total cost of the production of the biofuel [5]. In addition, soybean oil is a widely used product in the food industry, which fuels the debate regarding the need to prioritize its use for food rather than fuel [6,7]. One solution to decrease this cost and the impasse over the use of food oils is to replace the soybean oil used in fuel production with waste cooking oil [7,8,9,10,11].
Waste cooking oil is a valuable source of raw materials that have considerable potential for biodiesel production and stand out for their lower cost when compared to refined vegetable oils [12,13,14,15,16]. These waste oils constitute one of the main categories of organic waste generated in commercial establishments in the food sector and in households and are a widely available raw material. In addition, waste cooking oil represents an environmental threat, since it contributes to soil and water pollution and causes clogging in sewage systems [14,17].
By virtue of its remarkable energy density, waste cooking oil can play a key role in the production of biodiesel [7,18]. Therefore, the use of cooking oil as a raw material in the manufacture of biodiesel can be considered an environmentally responsible and sustainable approach to the management of such waste [7,19]. Additionally, approximately 20% of all biofuels used in Europe currently originate from waste cooking oil, the use of which has grown when compared to other biomass-based feedstocks [20]. This finding reinforces the need to explore and improve new processes for biodiesel production.
The main method for commercial biodiesel production is alkaline catalysis, which involves base-catalyzed transesterification (such as NaOH or KOH) to convert long-chain fatty acids to esters [17,21]. However, this process generates a highly alkaline effluent, requires significant water consumption in the purification steps and faces challenges in the recovery of glycerol [22]. In addition, when waste cooking oil is used as a raw material, preliminary treatment is required, which increases the costs of biodiesel production [16,17,20]. Therefore, alternatives are actively sought to optimize this process, such as the adoption of biocatalysis with the use of lipases [17,23,24].
Lipases are enzymes whose biological function is to catalyze the hydrolysis of fats and oils, releasing free fatty acids, diacylglycerols, monoacylglycerols and glycerol [25]. Nonetheless, depending on the reaction conditions, these enzymes can also act as catalysts for transesterification reactions (acidolysis, aminolysis and alcoholysis), esterification and interesterification [26]. These enzymes generated 1.94 billion US dollars in 2019, with an estimated market increase of 5.9% per year until 2026 [27]. Lipases demonstrate considerable levels of activity and stability in non-aqueous environments, which favors the catalysis of reactions such as esterification and transesterification [28].
Lipases of microbial origin have numerous industrial applications, such as in the pharmaceutical, food and chemical industries [29,30]. Some studies have shown that endophytic fungi are promising sources of these enzymes [31,32,33,34], and microorganisms associated with tropical hosts are still little explored for this application.
The transesterification process for conversion of the oily feedstock into long-chain fatty acid esters is a kinetically controlled reaction, and therefore, the biodiesel yield depends on the catalyst [35]. The performance of lipase, in turn, depends on several factors, including adsorption characteristics of the active center of the enzyme, specificity, and inhibitors of the reaction, among others [26]. As a result, the search for new sources of obtaining this enzyme becomes interesting [4].
The choice of the biocatalytic approach in this study aims to improve biodiesel production by using waste cooking oil as a raw material, making it more efficient and ecologically responsible. This is possible thanks to the use of an extract enriched with lipase obtained from an Amazonian endophytic fungus. This fungus is recognized as a promising source of enzymes of industrial interest and offers a valuable alternative to traditional sources of lipolytic enzymes. Specifically, this research uses the fungus Endomelaconiopsis endophytica as a catalyst in the production of biodiesel from waste cooking oil in order to evaluate its viability. This approach not only contributes to the sustainable and efficient production of biodiesel but also strengthens the industry by diversifying the sources of raw materials and offering an additional source of industrial enzymes.
2. Materials and Methods
The present study was conducted following the steps described in the flowchart shown in Figure 1. Initially, the enzyme extract was produced from the Amazonian endophytic fungus E. endophytica QAT_7AC in submerged culture. The lipase produced was characterized in terms of optimal pH and temperature ranges. Simultaneously, the waste cooking oil was collected, filtered and characterized in terms of density, acidity index, saponification index and peroxide index. Enzymatic transesterification of the waste cooking oil was performed in the presence of alcohol (ethanol or methanol) and lipase, resulting in biodiesel that was purified and then analyzed using GC-MS. The process was studied using comparative evaluations, and with the aid of an experimental design, the influence of alcohol type, reaction time and alcohol:oil ratio on biodiesel yield was evaluated.
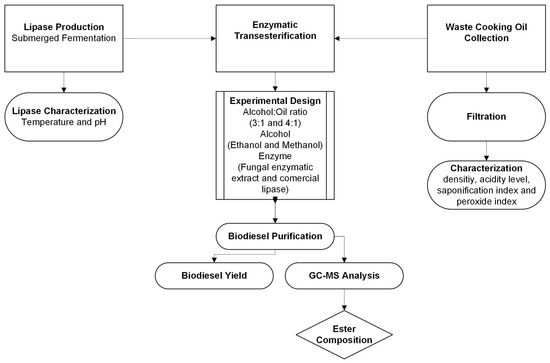
Figure 1.
Flowchart of the steps taken to obtain biodiesel via enzymatic transesterification.
2.1. Waste Cooking Oil and Soybean Oil
The waste cooking oil was provided by a restaurant located in the city of Manaus. The waste oil was collected in a sufficient quantity to perform all the experiments. After filtration, it was stored in bottles at room temperature. Unused soybean oil, used for comparison purposes, was purchased in a local supermarket.
2.2. Physicochemical Characterization of Waste Cooking Oil
Density was determined according to the AOCS [36], using a 5 mL pycnometer. For the determination of the acidity index, the methodology described by the Instituto Adolfo Lutz [37] was followed; 5 g of residue were homogenized in an ether:alcohol solution (2:1) v/v and titrated with 0.1 M of potassium hydroxide solution (previously standardized). The peroxide index was defined by the ability of the residue to oxidize potassium iodide, as described by the AOCS [38], as well as the saponification index, which was determined by the KOH mass required to neutralize the free fatty acids obtained from the hydrolysis of 1 g of the residue [39].
2.3. Microorganism
The endophytic fungus Endomelaconiopsis endophytica QAT_7AC, isolated from Aniba canelilla (Lauraceae), was used in the present study for lipase production. The fungus is part of the Central Microbiological Collection (CMC) of the Universidade do Estado do Amazonas (UEA) and was previously selected as a good producer of the enzyme [33]. Its reactivation was carried out in a potato dextrose agar (PDA) medium, in a BOD chamber (Tecnal, TE-39I, Piracicaba, Brazil) at 30 °C for 11 days.
2.4. Lipase Production
The fungus was cultured in Erlenmeyer flasks containing 100 mL of liquid medium composed of NH2NO3 (1.0 g/L), MgSO4·7H2O (0.6 g/L), KH2PO4 (1.0 g/L), peptone (20 g/L) and olive oil (1.0%), pH 6.0 [40]. The fungus E. endophytica was inoculated with three mycelial discs (5 mm in diameter) taken from the edge of the colony grown in PDA. The vials were incubated in a shaker (Tecnal, TE-4200, Piracicaba, Brazil) for 7 days at 28 °C under 160 rpm agitation. The experiments were conducted in triplicate. Every 24 h, 1 mL aliquots were taken and filtered for further measurement of enzymatic activity. From the determination of the enzymatic activity during the cultivation time, the time to obtain the enzyme was defined, which was later used in the production of the biodiesel.
2.5. Determination of Lipase Enzyme Activity
The lipolytic activity was quantified according to the methodology of Winkler and Stuckmann [41], whereby a p-nitrophenyl palmitate (pNPP) emulsion was prepared by adding (dropwise) 1 mL of solution A (30 mg of pNPP dissolved in 10 mL of isopropanol) in 9 mL of solution B (0.4 g of Triton X-100; 0.1 g of gum arabic and 90 mL of 50 mM tris HCl buffer, pH 7.0) under intense agitation. The emulsion obtained and the previously filtered enzyme extract samples were stabilized for 5 min at 37 °C. An aliquot of 0.2 mL of the supernatant was added to 1.8 mL of the substrate emulsion and the mixture was incubated for 15 min at 40 °C. The absorbance of the mixtures was measured in a spectrophotometer (Shimadzu, UV-1800, Kyoto, Japan) at 410 nm [42]. One unit (U) of enzyme activity was defined as the amount of enzyme required to release 1.0 µmol of p-nitrophenol per minute under assay conditions.
2.6. Characterization of Enzyme Extract
To determine the pH that best favors the highest enzymatic activity, the extract of the fungal enzyme was incubated in a 50 mM citrate buffer (pH 5.5 and 6.0) and sodium phosphate buffer (pH 7.0, 8.0 and 8.5). The determination of the optimal temperature for the lipolytic extract was carried out via its incubation at 35, 37, 40, 45 and 50 °C [33,43].
2.7. Determination of Protein Concentration
The dosage of proteins present in the enzyme extract was determined by using the Bradford method [44], in which 100 µL of enzyme extract was mixed with 1.0 mL of Bradford reagent, followed by the reading of the absorbance in a spectrophotometer at 595 nm. Bovine serine albumin (BSA) was used as the reference standard.
2.8. Enzymatic Transesterification—Biodiesel Production
Initially, enzymatic transesterification was performed with two different raw materials: unused soybean oil (used as a control for comparison purposes) and waste cooking oil, in order to verify the feasibility of using the waste oil via the biocatalytic route. The reaction time was 360 min and the ethanol:oil ratio was 3:1. The enzyme extract was added to the reaction medium at a concentration of 3% (m/v) [45,46]. The reactions were carried out in an adapted bench reactor, with constant stirring at 150 rpm and at a temperature of 40 °C, ideal conditions for the enzyme to act [47,48]. The adaptation of the reactor was carried out as follows: raw materials totaling 100 mL were added to 250 mL sealable borosilicate glass Erlenmeyer flasks. The flasks were placed in a shaker (Tecnal, TE-4200, Piracicaba, Brazil), with precise control of temperature and agitation. Figure 2 schematically illustrates the procedure used in the assay.
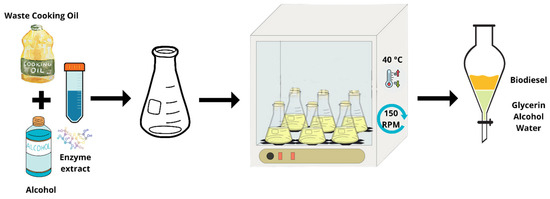
Figure 2.
Illustration of the procedure used in the enzymatic transesterification reaction to obtain biodiesel.
After this, the ethanol:oil ratios of 2:1 and 3:1 were evaluated, using the waste oil and the enzyme extract. The commercial enzyme Candida rugosa lipase (Sigma-Aldrich, L1754, St. Louis, MI, USA) was used for comparison with the fungal extract. The biocatalytic reaction of the waste oil was also evaluated from a 23 experimental design, in which the influence of the reaction time (120 and 360 min), the type of short-chain alcohol (methanol and ethanol) and the alcohol:oil ratio (3:1 and 4:1) were determined. The reaction time was chosen based on the reaction time used to produce biodiesel under alkaline or acid catalysis [49,50]. The alcohol:waste cooking oil ratio was set considering amounts of alcohol that would not denature the enzyme [48,51]. The tests were performed in triplicate.
2.9. Purification of the Biodiesel
At the end of the reaction, the mixture obtained was transferred to a separation funnel and allowed to stand for 24 h. After phase separation, the biodiesel was washed with 50 mL water [52] (approximately 1 mL of water to 2 mL of biodiesel) and then subjected to gas chromatography–mass spectrometry (GC–MS) analysis. The mass of biodiesel obtained after purification was used in the calculation of the yield.
2.10. Chromatographic Analysis
To confirm the production of biodiesel, the methodology described by Naser et al. [53] with adaptations was used. One mL of acetone was added to 100 µL of the sample obtained after the purification process. The sample was then analyzed on a chromatograph (Agilent Technologies, CG-7890B, Santa Clara, CA, USA) coupled to a mass spectrometer (Agilent Technologies, MS-5977a, Santa Clara, CA, USA). The column used was a Carboxen 1010 (30 m × 0.53 mm). The carrier gas was H2 with a flow of 1.2 mL/min. The initial temperature of the column was 100 °C, maintained for 1 min, with a heating rate of 5 °C/min up to 290 °C, maintained at this temperature for another 21 min. The temperatures of the injector and detector remained at 300 °C. The amount of sample injected was 3 µL. The resulting mass spectra were compared with those of the NIST Standard Reference Database 1A library. This comparison allowed the identification of the compounds present in the biodiesel samples.
2.11. Biodiesel Yield
The yield of biodiesel produced was calculated from the mass of biodiesel obtained after the transesterification reaction (mbiodisel), as a function of the mass of oil or waste oil (moil) used in the reaction (Equation (1)) [21,54,55].
2.12. Statistical Analysis
The results were expressed as mean and standard deviations and submitted to the analysis of normality and homogeneity of data, analysis of variance (ANOVA) and Student’s t and Tukey tests (p < 0.05). The data from the experimental design were analyzed with the aid of Statistica 10.0 software (p < 0.05).
3. Results and Discussion
3.1. Characterization of the Waste Oil
The results obtained for the characterization of the waste cooking oil, used as raw material for biodiesel production, are presented in Table 1, which also shows results found by other authors for the characterization of this type of oil.

Table 1.
Characterization of waste cooking oil used for biodiesel production.
High values are observed for the acidity and peroxide indices when compared to the specific values for refined oil, which is the main raw material used for biodiesel production in Brazil [61]. However, these values are in accordance with those obtained for waste cooking oil, as can be seen in Table 1. According to Castro et al. [56] and Plata et al. [62], the high values of the acidity index and peroxide index indicate the thermal and oxidative degradation of the oil, caused by the high temperature of the frying process in contact with atmospheric oxygen. The low saponification index value may be associated with the occurrence of hydrolysis during the frying process, releasing unsaponifiable impurities. It is also observed that the residue presented a density that is close to that of commercial soybean oil (0.9205 g/cm3) [63], which, according to Castro et al. [56], may be an indication that there are no significant amounts of water or impurities in the waste oil.
The parameters analyzed presented values similar to those of other studies [56,57,58,59,60,64,65], in which waste oil was used for biodiesel production. This indicates that the waste oil used in this study also presents adequate characteristics for the lipase-catalyzed transesterification reaction. However, it is important to note that, unlike other studies [56,59,66], the waste oil used in our research did not undergo any type of treatment; it was only filtered and used directly in the enzymatic transesterification reaction.
3.2. Enzyme Production
Lipase enzyme activity was evaluated daily during submersed culture of the endophytic fungus E. endophytica QAT_7AC (Figure 3). It can be observed that the highest lipase production, of 11,262 U/mL, occurred after 120 h of fungal culture. The enzymatic extract presented a protein concentration of 1.85 mg/mL, which means that the extract has a high specific enzymatic activity of 6087 U/mg.
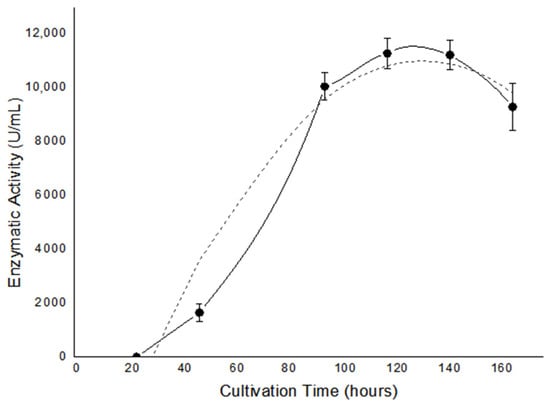
Figure 3.
Lipase production of the Amazonian fungus Endomelanconiopsis endophytica QAT_7AC in submerged culture (●) and polynomial curve (--).
From the adjustment of the experimental data, it was possible to draw a polynomial curve and, from this, generate an equation that could mathematically describe the enzymatic production of E. endophytica, which is presented in Equation (2) (R2 = 0.9538):
Y = enzyme activity (%)
X = growing time in hours
Abu et al. [67], as well as Dutta et al. [68] and Behera et al. [69], also used mathematical models to describe the production of microbial lipase. The equation derived from the polynomial curve provides a quantitative and modeled approach to understand the performance of enzyme production over time. This approach not only helps to identify the point of maximum enzymatic activity but also provides a tool for optimizing the time to obtain the enzyme. Using the equation, it is possible to anticipate and predict enzyme production over time [67,68,69].
The results of enzyme production can be considered promising. When compared to the result obtained by Carvalho Neto [70], who used the same fungal species, it is noted that E. endophytica isolated from A. canelilla presents greater enzymatic activity. In addition, when compared to other endophytic fungi [71,72] and even bacteria [35], E. endophytica QA7_AC demonstrates excellent potential as a source of lipase, with high extracellular enzyme production.
However, when evaluating the time taken to obtain the highest lipolytic activity, it is noted that QAT_7AC begins enzymatic production only after 24 h of culture, with a peak at 120 h. In the study by Sopalun et al. [71], the enzyme extract with the highest activity (82.22 U/mL) was obtained in 72 h using the endophytic fungus Colletotrichum gloeosporioides XmL-02. Oliveira, Silva and Hirata [72] obtained an enzyme extract rich in lipase (28 U/mL), which was produced using the fungus Preussia africana in only 48 h. Rocha et al. [73] obtained the highest lipolytic activity in 96 h using the endophytic fungi Stemphylium lycopersici (397 U/mL) and Sordaria sp. (286 U/mL). On the other hand, Sena et al. [74] obtained their extract with the highest lipase activity in 144 h using the endophytic fungus Aspergillus sp., which is closer in time taken to that obtained using E. endophytica QAT_7AC, although it presented lower enzyme activity.
Thus, in order to reduce the cultivation time, there is a need to optimize the production of the fungal enzyme. Factors such as culture temperature and pH of the medium are crucial for lipolytic production [74] and were previously evaluated in the study by Matias et al. [33]. However, other factors should be studied, such as agitation, components of the medium and inoculum concentration, in order to reduce the bioprocess time [33,75].
3.3. Characterization of the Enzyme Extract
The enzymatic extract obtained after 120 h of culture of E. endophytica QT_7AC was characterized regarding the temperature and pH ranges in which it presents the highest enzymatic activity. The results obtained are shown in Table 2.

Table 2.
Effect of temperature and pH on lipase activity of the enzyme extract produced by the fungus Endomelanconiopsis endophytica QAT_7AC.
The enzyme extract of E. endophytica QAT_7AC showed the maximum lipase activity when incubated at 40 °C. Although the enzyme extract showed high activity throughout the evaluated temperature range, there was a statistically significant difference (p < 0.05) between the analyzed temperatures. Therefore, the temperature of 40 °C was considered the optimal temperature for obtaining lipase using E. endophytica QAT_7AC. These results are in agreement with the study by Colla et al. [76], for which the temperature between 30 and 40 °C was reported as the optimal range for the activity of lipase produced by Aspergillus niger. As for the pH, a significant difference (p < 0.05) was also observed between the analyzed samples, and it was determined that pH 5.5 was the best among those evaluated for the performance of the lipase obtained using E. endophytica QAT_7AC.
The conditions for the lipase produced by E. endophytica QAT_7AC are optimal for the production of biodiesel using enzymatic transesterification, especially when the raw materials have a high acidity index, as in the case of waste cooking oil. The acidity value is a limiting factor for producing biodiesel using the transesterification method in the presence of homogeneous alkaline catalysts (NaOH, KOH), since acid values lower than 0.5 mg NaOH/g prevent the conversion process [77], and the use of lipase for these raw materials may be a promising alternative for the production of the biofuel [78,79].
3.4. Biodiesel Production
The formation of ethyl esters was observed in the samples from the biocatalytic reactions using soybean oil and waste cooking oil as raw materials. The linoleic acid ester (retention time = 19.920 min) was produced in greater quantity, followed by oleic acid ester (retention time = 23.512 min) (Figure 4). Esters of palmitic acid and stearic acid were identified in smaller proportions. The yields obtained were 92% and 89% for the enzymatic transesterification of soybean oil and waste cooking oil, respectively.
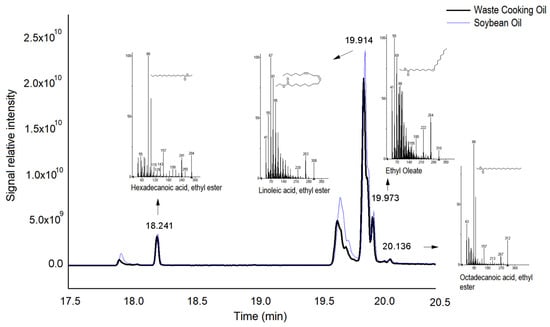
Figure 4.
Chromatogram of biodiesel sample produced by enzymatic transesterification of unused soybean oil and waste cooking oil. The reactions were performed for 360 min, with 3% (w/v) of the fungal enzyme extract, at 40 °C, with an ethanol:oil ratio of 3:1.
The biodiesel yield obtained from the waste cooking oil was comparable to that obtained with the unused soybean oil. This suggests that both the biocatalytic system (lipase from E. endophytica QAT_7AC) and the use of the waste cooking oil are viable for obtaining ethyl esters. This finding highlights not only the effectiveness of the process, but also the possibility of contributing to the reuse of resources that have no further use.
The biodiesel yields obtained using the enzymatic extract of the fungus E. endophytica QAT_7AC are comparable to those of other studies that used lipase-mediated biocatalytic transesterification reactions [80,81,82,83,84,85]. Table 3 shows the biodiesel yields obtained via the transesterification of different oily raw materials under different reaction conditions.

Table 3.
Biodiesel yields obtained by biocatalytic transesterification under different reaction conditions.
3.5. Biodiesel Production—Evaluation of the Parameters Involved in the Reaction
The biocatalytic reaction was evaluated for the ethanol:waste oil ratio, using the commercial lipase for comparison with the fungal biocatalytic system. The chromatograms of Figure 5 demonstrate the production of ethyl esters under all the reaction conditions evaluated. The fatty acid ethyl ester (FAEE) composition of the raw material and of the biodiesel samples produced by enzymatic transesterification using Candida rugosa lipase (CRL) and Endomelanconiopsis endophytica lipase (EEL), determined using GC-MS, can be found in Table S1.
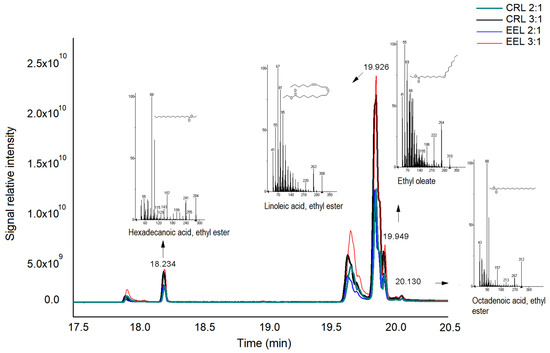
Figure 5.
Chromatogram of biodiesel samples obtained in the transesterification reactions of waste cooking oil catalyzed using commercial Candida rugosa lipase (CRL) and using the enzymatic extract produced using the fungus Endomelanconiopsis endophytica QAT_7AC (EEL), with ethanol:waste cooking oil ratios of 2:1 and 3:1. The reactions were carried out for 360 min, with 3% (w/v) of fungal enzyme extract, at 40 °C.
The biodiesel yields obtained in the transesterification reactions with 2:1 and 3:1 ratios of ethanol:waste cooking oil, using the enzymatic extract and the commercial enzyme, are shown in Figure 6. The enzymatic extract produced by the Amazonian endophytic fungus made it possible to obtain yields comparable to those obtained with the commercial lipase of Candida rugosa, an enzyme that underwent a purification process. It is also noted that the increase in the proportion of ethanol in relation to the residue promoted the increase in biodiesel yield. According to Cavalcante et al. [86], regarding the production of biodiesel, it is necessary to use between three and six times the amount of alcohol to that of soybean oil in order to guarantee the conversion to product in the transesterification reaction.
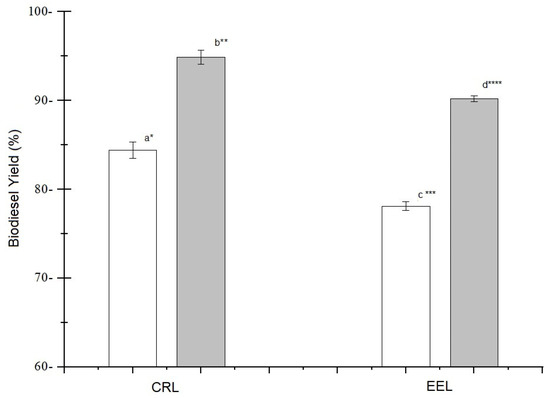
Figure 6.
Biodiesel yield obtained from the transesterification of the waste cooking oil catalyzed using the commercial enzyme (Candida rugosa lipase—CRL) and using the enzymatic extract produced by the endophytic fungus Endomelanconiopsis endophytica QAT_7AC (EEL), with an ethanol:waste cooking oil ratio of (□) 2:1 and ( ) 3:1. The reaction was carried out for 360 min at 40 °C. Experiments that do not share the same letter and symbol (*) are significantly different according to the Tukey test (p < 0.05).
) 3:1. The reaction was carried out for 360 min at 40 °C. Experiments that do not share the same letter and symbol (*) are significantly different according to the Tukey test (p < 0.05).
 ) 3:1. The reaction was carried out for 360 min at 40 °C. Experiments that do not share the same letter and symbol (*) are significantly different according to the Tukey test (p < 0.05).
) 3:1. The reaction was carried out for 360 min at 40 °C. Experiments that do not share the same letter and symbol (*) are significantly different according to the Tukey test (p < 0.05).
The biodiesel yields obtained in the present study are comparable to those of other studies that used lipase-mediated biocatalytic transesterification reactions. Table 4 shows the biodiesel yields obtained for the transesterification of the waste cooking oil under different reaction conditions.

Table 4.
Biodiesel yields obtained by biocatalytic transesterification of the waste cooking oil under different reaction conditions.
When compared with the results obtained by other authors, the yield values observed here are promising, since 90% was obtained using the fungal enzyme extract, a result which is similar to that achieved in studies that used purified enzymes for the biocatalytic reaction [54,82,88,89].
According to Geris et al. [92], for a stoichiometrically complete transesterification, a molar ratio of 3:1 alcohol:triacylglyceride is required. However, due to the reversible character of the reaction, the transesterifying agent (alcohol) is usually added in excess in these reactions, thus contributing to increasing the yield of the ester, as well as allowing its separation from the glycerol formed. However, excess alcohol can impair enzymatic activity, as it can destabilize the enzyme structure, compromising its three-dimensional conformation and, consequently, its catalytic function [92]. In addition, alcohol can act as a non-competitive inhibitor of the enzyme; in other words, it binds to sites other than the active site of the enzyme, interfering with its ability to bind to the substrate and carry out catalysis. These unwanted interactions between alcohol and the enzyme can result in a significant reduction of enzymatic activity [93,94]. Therefore, it is essential to avoid excess alcohol in the biocatalytic reaction in order to preserve the correct structure and functioning of the enzyme, thus ensuring efficiency in the transesterification process [95].
Considering the promising performance of the fungal biocatalyst system in the production of biodiesel from the transesterification of waste cooking oil, via an experimental design, we evaluated the influence of the parameters that affect the yield of the reaction: reaction time, alcohol type and alcohol:waste oil ratio. The results obtained in each assay are shown in Table 5.

Table 5.
Biodiesel yields obtained from the transesterification of the waste cooking oil catalyzed using the enzymatic extract produced by the endophytic fungus Endomelanconiopsis endophytica QAT_7AC under different reaction conditions.
The biodiesel yields varied between 81.43% and 89.53%, which confirms the satisfactory performance of the fungal biocatalytic system used in the transesterification reactions. When analyzing Table 5, it is observed that there is no significant difference between experiments 2, 6, 7 and 8 in terms of yield. Of these, only experiment 2 was performed with methanol. Methanol is widely employed as a solvent and reagent in the production of biodiesel using enzymatic transesterification. Its high reactivity allows the rapid conversion of triglycerides into methyl esters, resulting in an efficient and fast execution process [96,97]. In a study conducted by Muanruksa and Kaewkannetra [48], the authors obtained a yield of 91.3% of biodiesel using the ratio of 3:1 methanol:sludge palm oil at 40 °C in just 4 h of reaction using a commercial lipase.
The variables studied via the experimental design had a significant influence (p < 0.05) on biodiesel yield, with the reaction time being the independent factor with the greatest effect (Figure 7). In other words, the longer reaction times were favorable for obtaining higher biodiesel yields. These results corroborate the conclusions of the study conducted by Bessa et al. [98]. However, when using an ethanol ratio of 4:1, both in a reaction time of 120 and 360 min, equivalent yields were obtained. These results indicate, therefore, that the increase in reaction time is not necessary for the biocatalytic system employed.
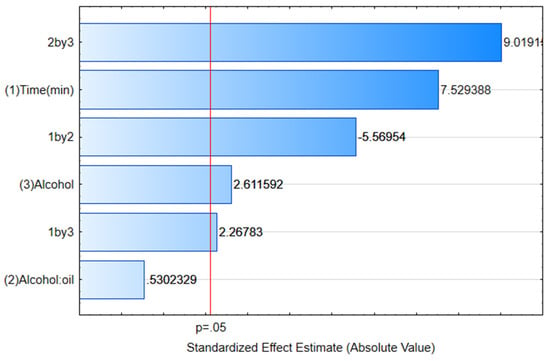
Figure 7.
Pareto plot for biodiesel yield obtained from transesterification of waste cooking oil catalyzed by the enzyme extract produced by the endophytic fungus Endomelanconiopsis endophytica QAT_7AC.
The reaction time is an important variable that should be evaluated in order to ensure the conversion of triglycerides into methyl or ethyl esters. An insufficient reaction time can lead to a low conversion of esters, while a prolonged reaction time can lead to the formation of unwanted byproducts, which can reduce the quality of the biodiesel produced [87]. Parandi et al. [54] used methanol in the production of biodiesel and evaluated the reaction time (between 12 and 36 h), with the highest yield occurring after 30 h (1800 min).
The type of alcohol also significantly (p < 0.05) influenced the biodiesel yield, and ethanol is preferable to methanol. The use of the ethyl route to replace the use of methanol in biodiesel production is important, because ethyl esters have several advantages over methyl esters, such as their higher cetane number, higher oxidative stability, lower iodine number and better lubrification properties; in addition, the ethyl route produces 100% renewable biodiesel and gives a better energy balance because it is a fuel synthesized from biomass fermentation, in addition to having the advantages of non-toxicity and biodegradability [99].
The proportion of alcohol, i.e., the amount of methanol or ethanol used in relation to the waste cooking oil, is also an important factor to be considered in the production of biodiesel. However, this factor, individually, did not present statistical significance (Figure 7). On the other hand, the interactions between the studied variables showed a significant effect, and their behavior can be visualized in the response surface graphs (Figure 8).
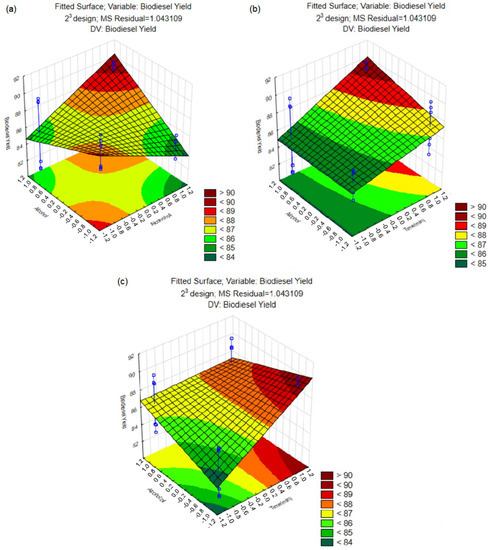
Figure 8.
Response surface graphs for biodiesel yield as a function of: (a) alcohol:waste oil ratio and alcohol type; (b) reaction time and alcohol type; and (c) reaction time and alcohol:waste oil ratio. Blue circles indicate the tested conditions.
The interaction between the alcohol:waste cooking oil ratio and the type of alcohol has the greatest influence on biodiesel yield (Figure 8a). The correct proportion of the alcohol to be used can ensure the high efficiency of the reaction and minimize the formation of unwanted by-products. However, it is important to evaluate the influence of the alcohol ratio under these different experimental conditions. For methanol, in higher proportions, it is possible to observe a decrease in biodiesel yield, a result that corroborates those obtained by Rosset et al. [51]. This behavior can be attributed to an inhibitory effect of methanol on lipase, accelerating the denaturation of the enzyme and, consequently, impairing the yield of the process. Ren, Li and Liu [93] demonstrated that the ethanol:oil ratio for biodiesel conversion using lipase as biocatalyser can be increased to 4:5.
In addition, when comparing the results obtained in this study with other methodologies that used cooking oil residue for biodiesel production, similar or higher yields are observed. For example, Corral-Bobadilha et al. [21] achieved a maximum yield of 92.7% using alkaline catalysis. Lin et al. [7] and Peng et al. [16] obtained maximum yields of 87.3% and 87.8%, respectively, when adopting CaO as a catalyst in a microwave heating system. Additionally, Banchapattanasakda et al. [100] achieved a maximum yield of 81.7% with direct pyrolysis and 83.5% when using activated carbon as a catalyst. These results reinforce the effectiveness of the biocatalysis methodology used in this study and demonstrate that it provides yields comparable to those obtained with classical methodologies.
From the statistical analysis, it was possible to observe that, in this study, the use of ethanol resulted in a significant increase in biodiesel yield (Figure 7). The multivariate function that describes the biodiesel yield for the transesterification of the waste oil with ethanol, considering the reaction time and the alcohol:waste oil ratio is presented in Equation (3) (R2 = 0.98167).
Y = 42.93 + 0.12x1 + 11.52x2 − 0.03x1x2
Y = biodiesel yield (%).
X1 = time in minutes (ranging from 120 to 360 min).
X2 = number of moles of alcohol in the alcohol:waste oil ratio (ranging between 3 and 4 moles).
Anwar et al. [101] conducted a study on the production of biodiesel from Carica papaya oil and investigated the relationship between the percentage of biodiesel in blends, the load and the engine speed. The authors developed a multivariate function that provided important answers in engine tests. Thus, the multivariate function developed in this study represents a useful tool to predict the performance of the transesterification process. By simultaneously considering the reaction time and the alcohol:waste oil ratio, the model allows us to understand the complex interactions between these variables and their impact on the final result. The reaction time is a critical factor that influences the formation of ethyl esters, while the ratio between alcohol and residual oil has a fundamental role in determining the amount of biodiesel produced and in the enzymatic performance. By synthesizing these elements into a conjoint function, researchers and engineers can more accurately predict the yield of the transesterification process under different experimental conditions.
The results obtained via the experimental design highlight the importance of this step in the evaluation of the efficiency of biodiesel production and in determining the optimal reaction conditions to ensure higher yields of the biofuel. In addition, the results indicate that the use of ethanol for biodiesel production is more appropriate, which is an advantage when considering the environmental benefits associated with the choice of ethanol, and it contributes to the reduction of the dependence on fossil fuels and the reduction of greenhouse gas emissions, thus strengthening the sustainability of the biodiesel production process. Therefore, the results show not only the efficiency of biodiesel production using the enzyme produced by the endophytic fungus but also the possibility of achieving a sustainable process.
4. Conclusions
The Amazonian endophytic fungus E. endophytica is a promising source of lipase, an enzyme of great interest for industrial applications. The lipolytic extract derived from this fungus was used as a biocatalyst, enabling the use of waste cooking oil as raw material in the production of biodiesel, with yields between 81.43 and 90.00%. The study also revealed the identification of important parameters to be considered in biodiesel production, such as reaction time and the alcohol:waste oil ratio. In addition, it was found that the use of ethanol presented advantages when compared to methanol, highlighting the potential of the investigated biocatalytic system for a sustainable production of biodiesel.
These findings underline the potential of the biocatalytic system studied for enabling sustainable biodiesel production, since the biocatalysis methodology used in this study demonstrated the achievement of yields comparable to those obtained with classical methodologies. However, it is critical to recognize that this process still faces challenges and limitations that require additional considerations. Some of these obstacles include the continuous need for process optimization, covering variables such as temperature, reactant concentration and reaction time, in order to achieve higher yields in shorter reaction times. In addition, variability in the composition and quality of the waste cooking oil can impact the efficiency of transesterification, thus requiring a detailed analysis on how this variability affects the process. Considerations related to the cost and availability of the lipolytic enzyme produced by the fungus E. endophytica should also be weighed, given that large-scale production may be costly and logistically challenging.
In summary, while the results of this study are promising and represent an innovative approach to sustainable biodiesel production, it is imperative to recognize that significant challenges and limitations persist. Continuous progress and dedicated research are crucial in order to overcome these barriers and maximize the benefits of this method. Thus, this work not only contributes to the advancement of the biodiesel industry by offering a more effective and ecologically conscious solution but also emphasizes the importance of continuing to investigate new approaches to the production of sustainable energy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/en16196937/s1, Table S1: Fatty acid ethyl ester (FAEE) composition of raw material and of biodiesel samples produced by enzymatic transesterification using Candida rugosa lipase (CRL) and Endomelanconiopsis endophytica lipase (EEL), determined using GC-MS.
Author Contributions
Conceptualization, P.M.A., J.G.C.R. and S.D.J.; investigation, J.G.C.R., F.V.C., C.C.d.S. and R.R.M.; methodology: J.G.C.R., P.M.A. and S.D.J.; formal analysis, P.M.A., N.T.M. and S.D.J.; data curation, J.G.C.R.; validation, J.G.C.R.; writing—original draft preparation, J.G.C.R.; writing—review and editing, P.M.A.; N.T.M.; project administration, P.M.A. and S.D.J.; resources: P.M.A. and S.D.J.; funding acquisition, P.M.A. and S.D.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação de Amparo à Pesquisa do Estado do Amazonas (FAPEAM) (grant number 01.02.016301.00568/2021-05 and 062.00165/2020), by POSGRAD/FAPEAM 2022 and by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (finance code 001 and grant number 88881.510151/2020-01—PDPG Amazônia Legal). The article processing charge was funded by Universidade do Estado do Amazonas (UEA).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors gratefully acknowledge Universidade do Estado do Amazonas—UEA, FAPEAM and CAPES for supporting this research.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Aransiola, E.F.; Ojumu, T.V.; Oyekola, O.O.; Madzimbamuto, T.F.; Ikhu-Omoregbe, D.I.O. A review of current technology for biodiesel production: State of the art. Biomass Bioenergy 2014, 61, 276–297. [Google Scholar] [CrossRef]
- Tiong, A.N.T.; Khan, Z.; Chin, Z.; Wahid, O.A.; Wachira, R.M.; Kung, S.M. Plant design of biodiesel production from waste cooking oil in Malaysia. Biofuels 2023, 14, 353–364. [Google Scholar] [CrossRef]
- Carpio, L.G.T. Transmission of variations in the biodiesel mandate for Brazilian biodiesel market. Biofuels 2023, 14, 373–386. [Google Scholar] [CrossRef]
- Grand View Research: Biodiesel Market Size, Share & Trends Analysis Report by Feedstock (Vegetable Oils, Animal Fats), By Application (Fuel, Power Generation), by Region (Europe, APAC), and Segment Forecasts, 2022–2030. Available online: https://www.grandviewresearch.com/industry-analysis/biodiesel-market (accessed on 4 May 2023).
- Naylor, R.L.; Higgins, M.M. The rise in global biodiesel production: Implications for food security. Glob. Food Secur. 2018, 16, 75–84. [Google Scholar] [CrossRef]
- Ávila, M.T.; Gazzoni, D.L. Biocombustível: Embrapa—Empresa Brasileira de Pesquisa Agropecuária: Soja. 8 December 2021. Available online: https://www.embrapa.br/agencia-de-informacao-tecnologica/cultivos/soja/pos-producao/agroenergia/biocombustiveis (accessed on 3 May 2022).
- Lin, Y.; Amesho, K.T.T.; Chen, C.; Cheng, P.; Chou, F. A cleaner process for green biodiesel synthesis from waste cooking oil using recycled waste oyster shells as a sustainable base heterogeneous catalyst under the microwave heating system. Sustain. Chem. Pharm. 2020, 17, 100310. [Google Scholar] [CrossRef]
- Sahar, S.S.; Iqbal, J.; Ullah, I.; Bhatti, H.N.; Nouren, S.; Rehman, H.; Nisar, J.; Iqbal, M. Biodiesel production from waste cooking oil: An efficient Technique to convert waste into biodiesel. Sustain. Cities Soc. 2018, 41, 220–226. [Google Scholar] [CrossRef]
- Tambor, J.H.M.; Cwejgorn, F.V.; Santos, A.G.; Lopes, G.C.; Lescano, V.P. Biodiesel production from the used kitchen oil: A sustainable alternative. Rev. Caleidosc. 2019, 11, 545–548. [Google Scholar]
- Sarno, B.; Iuliano, M. Biodiesel production from waste cooking oil. Green Process. Synth. 2019, 8, 828–836. [Google Scholar] [CrossRef]
- Thilakarathne, D.; Miyuranga, K.A.V.; Arachchige, U.S.P.R.; Weerasekara, N.A.; Jayasinghe, R.A. Production of biodiesel from Waste Cooking Oil Laboratory Scale: A Review. Int. J. Sci. Eng. Sci. 2021, 5, 28–34. [Google Scholar]
- Fonseca, J.M.; Taleken, J.G.; Almeida, V.C.; Silva, C. Biodiesel from waste frying oils: Methods of production and purification. Energy Convers. Manag. 2019, 184, 205–218. [Google Scholar] [CrossRef]
- Kasirajan, R. Biodiesel production by two step process from an energy source of Chrysophyllum albidum oil using homogeneous catalyst. S. Afr. J. Chem. Eng. 2021, 37, 161–166. [Google Scholar] [CrossRef]
- Chen, H.X.; Xia, W.; Wang, S. Biodiesel production from waste cooking oil using a waste diaper derived heterogeneous magnetic catalyst. Braz. J. Chem. Eng. 2022, 40, 511–520. [Google Scholar] [CrossRef]
- Khan, H.M.; Iqbal, T.; Ali, C.H.; Yasin, S.; Jamil, F. Waste quail beaks as renewable source for synthesizing novel catalysts for biodiesel production. Renew. Energy 2020, 154, 1035–1043. [Google Scholar] [CrossRef]
- Peng, Y.-P.; Amesho, K.T.T.; Chen, C.-E.; Jhang, S.-R.; Chou, F.-C.; Lin, Y.-C. Optimization of Biodiesel Production from Waste Cooking Oil Using Waste Eggshell as a Base Catalyst under a Microwave Heating System. Catalysts 2018, 8, 81. [Google Scholar] [CrossRef]
- Ferrusca, M.C.; Romero, r.; Martínez, S.L.; Remírez-Serrano, A.; Natividade, R. Biodiesel production from waste cooking oil: A pespective on catalytic processes. Processes 2023, 11, 1952. [Google Scholar] [CrossRef]
- Husain, I.A.; Alkhatib, M.F.; Jammi, M.S.; Mirghani, M.E.; Bin Zainudin, Z.; Hoda, A. Problems, control and treatment of fat, oil and grease (FOG): A review. J. Oleo Sci. 2014, 36, 747–752. [Google Scholar] [CrossRef]
- Gaur, A.; Mishra, S.; Chowdhury, S.; Baredar, P.; Verma, P. A review on factor affecting biodiesel production from waste cooking oil: An Indian perspective. Mater. Today Proc. 2021, 46, 5594–5600. [Google Scholar] [CrossRef]
- Al-Muhtaseb, A.H.; Osman, A.I.; Murphin Kumar, P.S.; Jamil, F.; Al-Haj, L.; Al Nabhani, A.; Kyaw, H.H.; Myint, M.T.Z.; Mehta, N.; Rooney, D.W. Circular economy approach of enhanced bifunctional catalytic system of CaO/CeO2 for biodiesel production from waste loquat seed oil with life cycle assessment study. Energy Convers. Manag. 2021, 236, 114040. [Google Scholar] [CrossRef]
- Corral-Bobadilla, M.; Lostado-Lorza, R.; Somovilla-Gómez, F.; Íñiguez-Macedo, S. Life cycle assessment multi-objective optimization for eco-efficient biodiesel production using waste cooking oil. J. Clean. Prod. 2022, 359, 132113. [Google Scholar] [CrossRef]
- Claeys, C. Used Cooking Oil (UCO) feedstock now accounts for one-fifth of all european biofuels. In Proceedings of the ACI Oleofuels Conference, Marseille, France, 18–19 May 2022. [Google Scholar]
- Mandari, V.; Devarai, S.K. Biodiesel production using homogeneous, heterogeneous, and enzyme catalysts via transesterification and esterification reactions: A critical review. Bioenergy Res. 2021, 15, 935–961. [Google Scholar] [CrossRef]
- Avhad, M.R.; Marchetti, J.M. Uses of Enzymes for Biodiesel Production. In Advanced Bioprocessing for Alternative Fuels, Biobased Chemicals, and Bioproducts; Woodhead Publishing: Sawston, UK, 2019; pp. 135–152. [Google Scholar] [CrossRef]
- Ramos, L.P.; Kothe, V.; Aparecida, M.; Muniz-Wypych, A.S.; Nakagaki, S.; Krieger, N.; Wypych, F.; Cordeira, C. Biodiesel: Raw materials, production technologies and fuel properties. Rev. Virtual Quím. 2017, 9, 317–369. [Google Scholar] [CrossRef]
- Norjannah, B.; Ong, H.C.; Masjuki, H.H.; Juan, J.C.; Chong, W.T. Enzymatic transesterification for biodiesel production: A comprehensive review. RSC Adv. 2016, 6, 60034–60055. [Google Scholar] [CrossRef]
- Oliveira, N.M.L.; Vieira-Almeida, E.C.; Silva, L.P.; Paula, C.B.C.; Bastos, A.C.M.; Santos, I.L.; Paula-Elias, F.C.; Almeida, A.F. Processos Químicos e Biotecnológicos—Lipases microbianas. In Bioprocessos e Aplicações Industriais, 1st ed.; Andrade, D.F., Souza, A.A., Andrade, D.E., Oliveira, E.J., Santos, F., Lopes, J.E.F., Neves, O.F., Lima, L.C., Ferreira Filho, N., Oliveira, V.A., Eds.; Poisson: Belo Horizonte, Brazil, 2020; Volume 5, pp. 2–45. [Google Scholar] [CrossRef]
- Marotti, B.S.; Cortez, D.V.; Gonçalves, D.B.; Castro, H.F. Screening of species from the genus Penicillium producing cell bound lipases to be applied in the vegetable oil hydrolysis. Quím. Nova 2017, 40, 427–430. [Google Scholar] [CrossRef]
- Chandra, P.; Enespa, S.R.; Arora, P.K. Microbial lipases and their industrial applications: A comprehensive review. Microb. Cell Fact. 2020, 9, 169. [Google Scholar] [CrossRef]
- Zambare, V.; Patankar, R.; Bhusare, B.; Christopher, L. Recents advances in feedstock and lipase research and development towards commecialization of enzymatic biodiesel. Processes 2021, 9, 1743. [Google Scholar] [CrossRef]
- Martinez-Klimova, E.; Rodríguez-Peña, K.; Sánchez, S. Endophytes as sources of antibiotics. Biochem. Pharmacol. 2017, 134, 1–17. [Google Scholar] [CrossRef]
- Matias, R.R.; Sepúlveda, A.M.G.; Batista, B.N.; Lucena, J.M.V.M.; Albuquerque, P.M. Degradation of Staphylococcus aureus biofilm using hydrolytic enzymes produced by amazonian endophytic fungi. Appl. Biochem. Biotechnol. 2021, 193, 2145–2161. [Google Scholar] [CrossRef] [PubMed]
- Matias, R.R.; Rodrigues, J.G.C.; Procópio, R.E.L.; Matte, C.R.; Duvoisin Junior, S.; Soares, R.M.D.; Albuquerque, P.M. Lipase production from Aniba canelilla endophytic fungi, characterization, and application of the enzymatic extract. Res. Soc. Dev. 2022, 11, e180111234326. [Google Scholar] [CrossRef]
- Batista, B.N.; Matias, R.R.; Oliveira, R.L.; Albuquerque, P.M. Hydrolytic enzyme production from açai palm (Euterpe precatoria) endophytic fungi and characterization of the amylolytic and cellulolytic extracts. World J. Microbiol. Biotechnol. 2022, 38, 30–45. [Google Scholar] [CrossRef]
- Rana, Q.U.A.; Irfan, M.; Ahmed, S.; Hasan, F.; Shah, A.A.; Khan, S.; Rehman, F.U.; Khan, H.; Ju, M.; Li, W.; et al. Bio-catalytic transesterification of mustard oil for biodiesel production. Biofuels 2019, 13, 69–76. [Google Scholar] [CrossRef]
- AOCS: American Oil Chemists’ Society. A.O.C.S. Official Method Cc 10a-25. In Official Methods and Recommended Practices of the American Oil Chemists’ Society, 4th ed.; AOCS: Champaign, IL, USA, 1990. [Google Scholar]
- Instituto Adolfo Lutz. Métodos Físico-Químicos para Análise de Alimentos; Instituto Adolfo Lutz: São Paulo, Brazil, 2008. [Google Scholar]
- AOCS: American Oil Chemists’ Society. AOCS Official Method Cd 8-53. In Official Methods and Recommended Practices of the American Oil Chemists’ Society, 4th ed.; AOCS: Champaign, IL, USA, 1990. [Google Scholar]
- AOCS: American Oil Chemists’ Society. A.O.C.S. Official Method Cd 3-25. In Official Methods and Recommended Practices of the American Oil Chemists’ Society, 4th ed.; AOCS: Champaign, IL, USA, 1990. [Google Scholar]
- Gurgel, R.S.; Rodrigues, J.G.C.; Matias, R.R.; Batista, B.N.; Oliveira, R.L.; Albuquerque, P.M. Biological activity and production of metabolites from Amazon endophytic fungi. Afr. J. Micrbiol. Res. 2020, 14, 85–93. [Google Scholar] [CrossRef]
- Winkler, U.K.; Stuckmann, M. Glycogen, hyaluronate and some other polysaccharides greatly enhance the formation of exolipase by Serratia marcescens. J. Bacteriol. 1979, 138, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Tombini, J. Selection of Lipolytic Microorganisms and Lipase Production from Soy Processing by Products. Master’s Thesis, Federal Technological University of Paraná, Pato Branco, Brazil, 29 May 2015. [Google Scholar]
- Dantas, A. Imobilização e Caracterização da Lipase Ns-40116 em Poliestireno. Master’s Thesis, Federal University of Santa Catarina, Florianópolis, Brazil, 24 February 2017. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248. [Google Scholar] [CrossRef]
- Cruz Junior, A. Imobilização de Lipase de Candida antarctica B em Quitosana para Obtenção de Biodiesel por Transesterificação do Óleo de Mamona. Master’s Thesis, Federal University of Santa Catarina, Florianópolis, Brazil, 18 July 2007. [Google Scholar]
- Marder, F.; Celin, M.M.; Mazuim, M.S.; Scneider, R.C.S.; Macganan, M.T.; Carbellini, V.A. Produção de biodiesel por biocatálise utilizando método alternativo de imobilização da lipase em hidrogel. Tecno-Lógica 2008, 12, 56–64. [Google Scholar]
- Parawira, W. Biotechnological production of biodiesel fuel using biocatalysed transesterification: A review. Crit. Rev. Biotechnol. 2009, 29, 82–93. [Google Scholar] [CrossRef]
- Muanruksa, P.; Kaewkannetra, P. Combination of fatty acids extraction and enzymatic esterification for biodiesel production using sludge palm oil as a low-cost substrate. Renew. Energy 2020, 146, 901–906. [Google Scholar] [CrossRef]
- Burmana, A.D.; Tambun, R.; Haarynato, B.; Alexander, V. Effect of reaction time on biodiesel production from palm fatty acid distillate by, using PTSA as a catalyst. IOP Conf. Ser. Mater. Sci. Eng. 2020, 1003, 12134. [Google Scholar] [CrossRef]
- Pedro, K.C.N.R.; Parreira, J.M.; Correia, I.N.; Henriques, C.A.; Langone, M.A.P. Enzymatic biodiesel synthesis from acid oil using a lipase mixture. Quim. Nova 2018, 41, 284–291. [Google Scholar] [CrossRef]
- Rosset, D.V.; Wancura, J.H.C.; Mazutti, M.A.; Jahn, S.L. Produção de biodiesel catalisada por lipases solúveis: Influência do excesso de metanol e da concentração de água na reação. In Proceedings of the Anais do XII Congresso Brasileiro de Engenharia Química, São Carlos, Brazil, 16–19 July 2017. [Google Scholar]
- Aquino, I.P. Evaluation of Biodiesel Corrosiveness by Gravimetric and Eletrochemical Techniques. Doctoral Thesis, University of Sao Paulo, São Paulo, Brazil, 20 March 2012. [Google Scholar]
- Naser, J.; Avbenake, O.P.; Dabai, F.N.; Jibril, B.Y. Regeneration os spent bleaching earth and conversion of recovered oil to biodiesel. Waste Manag. 2021, 126, 258–265. [Google Scholar] [CrossRef]
- Parandi, E.; Safaripour, M.; Abdellatif, M.H.; Saidi, M.; Bozorgian, A.; Nodeh, H.R.; Rezania, S. Biodiesel production from waste cooking oil using a novel biocatalyst of lipase enzyme immobilized magnetic nanocomposite. Fuel 2022, 313, 123057. [Google Scholar] [CrossRef]
- Jamil, N.; Zairi, M.N.M.; Nasim, N.A.M.; Pa’ee, F. Influences of Enviromental Conditions to Phytoconstituents in Clitoria ternatea (Butterfly Pea Flower)—A review. J. Sci.Technol. 2018, 10, 208–228. [Google Scholar] [CrossRef]
- Castro, S.V.F.; Silva, C.V.; Previdi, D.; Portela, F.M.; Gomes, M.F. Caracterização Estrututral e físico-química de biodiesel produzido a partir de óleo residual do reifeitório do IF Goiano—Campus Urutaí. Multi-Sci. J. 2018, 1, 47–53. [Google Scholar] [CrossRef]
- Aworanti, O.A.; Ajani, A.O.; Agarry, S.E. Process parameter estimation of biodiesel production from waste frying oil (vegetable and palm oil) using homogeneous catalyst. J. Food Process. Technol. 2019, 10, 811. [Google Scholar] [CrossRef]
- Al-Saadi, A.; Mathan, B.; He, Y. Biodiesel production via simultaneous transesterification and esterification reactions over SrO–ZnO/Al2O3 as a bifunctional catalyst using high acidic waste cooking oil. Chem. Eng. Res. Des. 2020, 162, 238–248. [Google Scholar] [CrossRef]
- Farooq, M.; Ramli, A.; Naeem, A. Biodiesel production from low FFA waste cooking oil using geterogeneous catalyst derived from chicken bones. Renew. Energy 2015, 76, 362–368. [Google Scholar] [CrossRef]
- Siqueira, A.F.; Vidigal, I.G.; Melo, M.P.; Giordani, D.S.; Batista, P.S.; Ferreira, A.L.G. assessing waste cooking oils for the production of quality biodiesel using na eletronic nose and a stochastic model. Energy Fuels 2019, 33, 3221–3226. [Google Scholar] [CrossRef]
- Agência Nacional de Vigilância Sanitária (ANVISA). Resolução RDC nº 270, de 22 de Setembro de 2005. 2005. Available online: http://www.anvisa.gov.br/e-legis/ (accessed on 12 December 2021).
- Plata, V.; Ferreira-Beltrán, D.; Gauthier-Maradei, P. Effect of cooking conditions on selected properties of biodiesel produced from Palm-Based waste cooking oil. Energies 2022, 15, 908. [Google Scholar] [CrossRef]
- Morais, V.S.; Castro, E.V.R.; Carneiro, M.T.W.D.; Brandão, G.P.; Fabri Junior, R.; Sena, D.R. ASTM color: A simple and fast method for determining quality of biodiesel produced from used cooking oils. Quim. Nova 2013, 36, 587–592. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.H.; Kim, D.S.; Yoo, H.Y.; Park, C.; Kim, S.W. Biodiesel production by lipases co-immobilized on the functionalized activated carbon. Bioresour. Technol. Rep. 2019, 7, 100248. [Google Scholar] [CrossRef]
- Binhayeedung, N.; Lomklao, S.; Prasertsan, P.; Sangkharak, K. Improvement of biodiesel production using waste cooking oil and applying single and mixed immobilized lipases on polyhydroxyalkanoate. Renew. Energy 2020, 162, 1819–1827. [Google Scholar] [CrossRef]
- Rovere, B.O.; Rodrigues, J.H.; Teleken, J.G. Reduction of the acidity index through neutralization and esterification for biodiesel production. Braz. J. Dev. 2020, 6, 24678–24686. [Google Scholar] [CrossRef]
- Abu, M.L.; Nooh, H.M.; Oslan, S.N.; Salleh, A.B. Optimization of physical conditions for the production of thermostable T1 lipase in Pichia guilliermondii strain SO using response surface methodology. BMC Biotechnol. 2017, 17, 78–87. [Google Scholar] [CrossRef]
- Dutta, B.; Shamekh, S.; Deska, J.; Bandopadhyay, R. Statistical optimization of media components for production of extracellular lipase from edible mushroom Cantharellus cibarius. Biol. Futur. 2022, 73, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Behera, A.R.; Veluppal, A.; Dutta, K. Optimization of physical parameters for enhanced production of lipase from Staphylococcus hominis using response surface methodology. Environ. Sci. Pollut. Res. 2019, 26, 34277–34284. [Google Scholar] [CrossRef]
- Carvalho Neto, F.G.M.R. Isolamento e Clonagem do Gene que Codifica a Lipase do Fungo Endomelanconiopsis Endophytica. Master’s Thesis, Amazonas State University, Manaus, Brazil, 25 October 2013. [Google Scholar]
- Sopalun, K.; Laosripaiboon, W.; Wachirachaikarn, A.; Iamtham, S. Biological potential and chemical composition of bioactive compounds from endophytic fungi associated with thai mangrove plants. S. Afr. J. Bot. 2021, 141, 66–76. [Google Scholar] [CrossRef]
- Oliveira, G.F.; Silva, M.R.L.; Hirata, D.B. Production of new lipase from Preussia africana and a partial characterization. Prep. Biochem. Biotechnol. 2021, 52, 942–949. [Google Scholar] [CrossRef]
- Rocha, K.S.C.; Queiroz, M.S.R.; Gomes, B.S.; Dallago, R.; Souza, R.O.M.A.; Guimarães, D.O.; Itabaiana, I., Jr.; Leal, I.C.R. Lipases of Endophytic Fungi Stemphylium lycopersici and Sordaria sp.: Application in the synthesis of solketal derived monoacylglycerols. Enzyme Microb. Technol. 2020, 142, 109664. [Google Scholar] [CrossRef]
- Sena, I.S.; Ferreira, A.M.; Marinho, V.H.; Holanda, F.H.; Borges, S.F.; Souza, A.A.; Koga, R.C.R.; Lima, A.L.; Florentino, A.C.; Ferreira, I.M. Euterpe oleracea Mart (Açaizeiro) from the Brazilian Amazon: A Novel Font of Fungi for Lipase Production. Microorganisms 2022, 10, 2394. [Google Scholar] [CrossRef] [PubMed]
- Szymczak, T.; Cybulska, J.; Podlesny, M.; Frac, M. Various Perspectives on Microbial Lipase Production Using Agri-Food Waste and Renewable Products. Agriculture 2021, 11, 540. [Google Scholar] [CrossRef]
- Colla, L.M.; Ficanha, A.M.M.; Rizzardi, J.; Bertolin, T.E.; Reinehr, C.O.; Costa, J.A.V. Production and Characterization of Lipases by Two New Isolates of Aspergillus through Solid-State and Submerged Fermentation. BioMed Res. Int. 2015, 2015, 725959. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, M.C.; Liao, P.H.; Lan, N.V.; Hou, S.S. Enhancement of biodiesel production from high-acid-value waste cooking oil via a microwave reactor using a homogeneous alkaline catalyst. Energies 2021, 14, 437. [Google Scholar] [CrossRef]
- Talukder, M.M.R.; Wu, J.C.; Fen, N.M.; Melissa, Y.L.S. Two-step lipase catalysis for production of biodiesel. Biochem. Eng. J. 2010, 49, 207–212. [Google Scholar] [CrossRef]
- Lampi, A.M.; Yang, Z.; Mustonen, O.; Piironen, V. Potential of faba bean lipase and lipoxygenase to promote formation of volatile lipid oxidation products in food models. Food Chem. 2020, 311, 125982. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.A. Estudo da Produção de Biodiesel Utilizando Etanol e Óleo de Soja ou De macaúba, Catalisada por Lipase de Mamona e de Thermomyces lanuginosus. Master’s Thesis, São Carlos Federal University, São Carlos, Brazil, 25 February 2015. [Google Scholar]
- Aguieiras, E.C.G.; Barros, D.S.N.; Sousa, H.; Fernandez-Lafuente, R.; Freire, D.M.G. Influence of the raw material on the final properties of biodiesel produced using lipase from Rhizomucor miehei grown on babassu cake as biocatalyst of esterification reactions. Renew. Energy 2017, 113, 112–118. [Google Scholar] [CrossRef]
- Aguieiras, E.C.G.; Oliveira, E.D.C.; Castro, A.M.; Langone, M.A.P.; Freire, D.M.G. Biodiesel production from Acrocomia aculeata acid oil by (enzyme/enzyme) hydroesterification process: Use of vegetable lipase and fermented solid as low-cost biocatalysts. Fuel 2014, 135, 1–7. [Google Scholar] [CrossRef]
- Li, W.; Chen, B.; Tan, T. Esterification synthesis of ethyl oleate in solvent-free system catalyzed by lipase mebrane from fermentation broth. Appl. Biochem. Biotechnol. 2011, 163, 102–111. [Google Scholar] [CrossRef]
- Raita, M.; Laothanachareon, T.; Champreda, V.; Laosiripojana, N. Biocatalytic esterification of palm oil fatty acids for biodiesel production using glycine-based cross-linked protein coated microcrystalline lipase. J. Mol. Catal. B Enzym. 2011, 73, 74–79. [Google Scholar] [CrossRef]
- Ávila Vázquez, V.; Aguilera Flores, M.M.; Hernández Casas, L.F.; Medellín Castillo, N.A.; Rocha Uribe, A.; Correa Aguado, H.C. Biodiesel production catalyzed by lipase extract powder of Leonotis nepetifolia (Christmas Candlestick) seed. Energies 2023, 16, 2848. [Google Scholar] [CrossRef]
- Cavalcanti, F.T.T.; Simao Neto, F.; Falcao, I.R.A.; Souza, J.E.S.; Moura Junior, L.S.; Sousa, P.S.; Rocha, T.G.; Sousa, I.G.; Gomes, P.H.L.; Souza, M.C.M.; et al. Opportunities for improving biodiesel production via lipase catalysis. Fuel 2020, 288, 119577. [Google Scholar] [CrossRef]
- Yan, J.; Yan, Y.; Liu, S.; Hu, L.J.; Wang, G. Praparation of cross-linked lipase-coated micro-crystals for biodiesel production from waste cooking oil. Bioresour. Technol. 2011, 102, 4755–4758. [Google Scholar] [CrossRef]
- Abdulla, R.; Derman, E.; Mathialagan, T.; Yaser, A.Z.; Samah, M.A.A.; Gansau, J.A.; Najmuddin, S.U.F.S. Biodiesel production from waste palm cooking oil using immobilized Candida rugosa lipase. Sustainability 2022, 14, 13632. [Google Scholar] [CrossRef]
- Taher, H.; Nashef, E.; Anvar, N.; Al-Zuhair, S. Enzymatic production of biodiesel from waste oil in ionic liquid medium. Biofuels 2017, 10, 463–472. [Google Scholar] [CrossRef]
- Gong, H.; Gao, L.; Nie, K.; Wang, M.; Tan, T. A new reactor for enzymatic synthesis of biodiesel from waste cooking oil: A static-mixed reactor pilot study. Renew. Energy 2020, 15, 270–277. [Google Scholar] [CrossRef]
- Geris, R.; Santos, N.A.C.; Amaral, B.A.; Maia, I.S.; Castro, V.D.; Carvalho, J.R.M. Biodiesel from soybean oil: Experimental procedure of transesterification for organic chemistry laboratories. Quím. Nova 2007, 30, 1369–1373. [Google Scholar] [CrossRef]
- Ren, H.; Li, Y.; Liu, D. Free lipase-catalyzed esterefication of oleic acid fatty acid ethyl ester preparation with response surface optimization. J. Am. Oil Chem. Soc. 2013, 90, 73–79. [Google Scholar] [CrossRef]
- José, C.; Bonetto, R.D.; Gambaro, L.A.; Torres, M.P.G.; Foresti, M.L.; Ferreira, M.J.; Birand, L.R. Investigation of the causes of deactivation degradation of the commercial biocatalyst Novozym 432 in ethanol and ethanol-aqueous media. J. Mol. Catal. B Enzymat. 2011, 71, 95–107. [Google Scholar] [CrossRef]
- Aguieiras, E.C.G.; Souza, S.L.; Langone, M.P. Study of immobilized lipase Lipozyme RM IM esterification reactions for biodiesel synthesis. Quím. Nova 2013, 36, 646–650. [Google Scholar] [CrossRef]
- Queiroz, D.S.; Parreira, J.M.; Bastos, C.M. Efeito do solvente na atividade enzimática de lipases comerciais imobilizadas. In Proceedings of the Anais do XX Congresso Brasileiro de Engenharia Química, Florianópolis, Brazil, 19–22 October 2014. [Google Scholar]
- Nielsen, M.; Brask, J.; Fjerbaek, L. Enzymatic biodiesel production: Technical and economical considerations. Eur. J. Lipid Sci. Technol. 2008, 100, 692–700. [Google Scholar] [CrossRef]
- Silva, A.; Resende, R.J.; Costa, T.C.; Sousa, B.V.O.; Santos, A.K.; Marotti, B.S.; Silva, S.L.; Cancelier, A.; Gonçalves, D.B. Análise do potencial biocatalítico de lipase de Candida rugosa imobilizada em diferentes suportes. Rev. Acta Ambient. Catarin. 2021, 18, 10–23. [Google Scholar] [CrossRef]
- Bessa, D.H.R.F.; Flumignan, D.L.; Souza, A.O.; Gonçalves, M.C.M.; Castro, C.F.S. Crude enzymatic broth from lipoliptic fungi for the production of metylic esters. Rev. Agronegócio Meio Ambiente 2022, 15, e9279. [Google Scholar] [CrossRef]
- Almeida, T.S.; Erazo, R.G.T.P.; Ramos, R.A.V.; Dias Filho, N.L. Transesterificação de óleo de soja e de pinhão-manso por metanólise e etanólise empregando diversos catalisadores. In Proceedings of the 6th Congresso da Rede Brasileira de Tecnologia de Biodiesel, Natal, Brazil, 22–25 November 2016. [Google Scholar]
- Banchapattanasakda, W.; Asavatesanupap, C.; Santikunaporn, M. Conversion of waste cooking oil into bio-fuel via pyrolysis using activated carbon as a catalyst. Molecules 2023, 28, 3590. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.; Rasul, M.G.; Ashwath, N. A Systematic multivariate analysis of Carica papaya biodiesel blends and their interactive effect on performance. Energies 2018, 11, 2931. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).