A Review of Pyrolysis Technologies and the Effect of Process Parameters on Biocarbon Properties
Abstract
:1. Introduction
2. Pyrolysis Methods and Technologies
2.1. Pyrolysis Stages and Biocarbon Formation
- Cellulose decomposes into pyrolysis oil, gaseous products, and biocarbon at 315–400 °C [19,41]. The decomposition products depend on the feedstock heating rate. At slow heating rates, the process favors biocarbon formation. Rapid volatilization occurs at high heating rates, leading to the formation of levoglucosan, which breaks down further into liquid and gas products [40].
2.2. Pyrolysis Methods
2.2.1. Slow Pyrolysis
2.2.2. Fast Pyrolysis
2.2.3. Flash Pyrolysis
2.2.4. Intermediate Pyrolysis
2.2.5. Segmented Heating
2.3. Pyrolysis Technologies
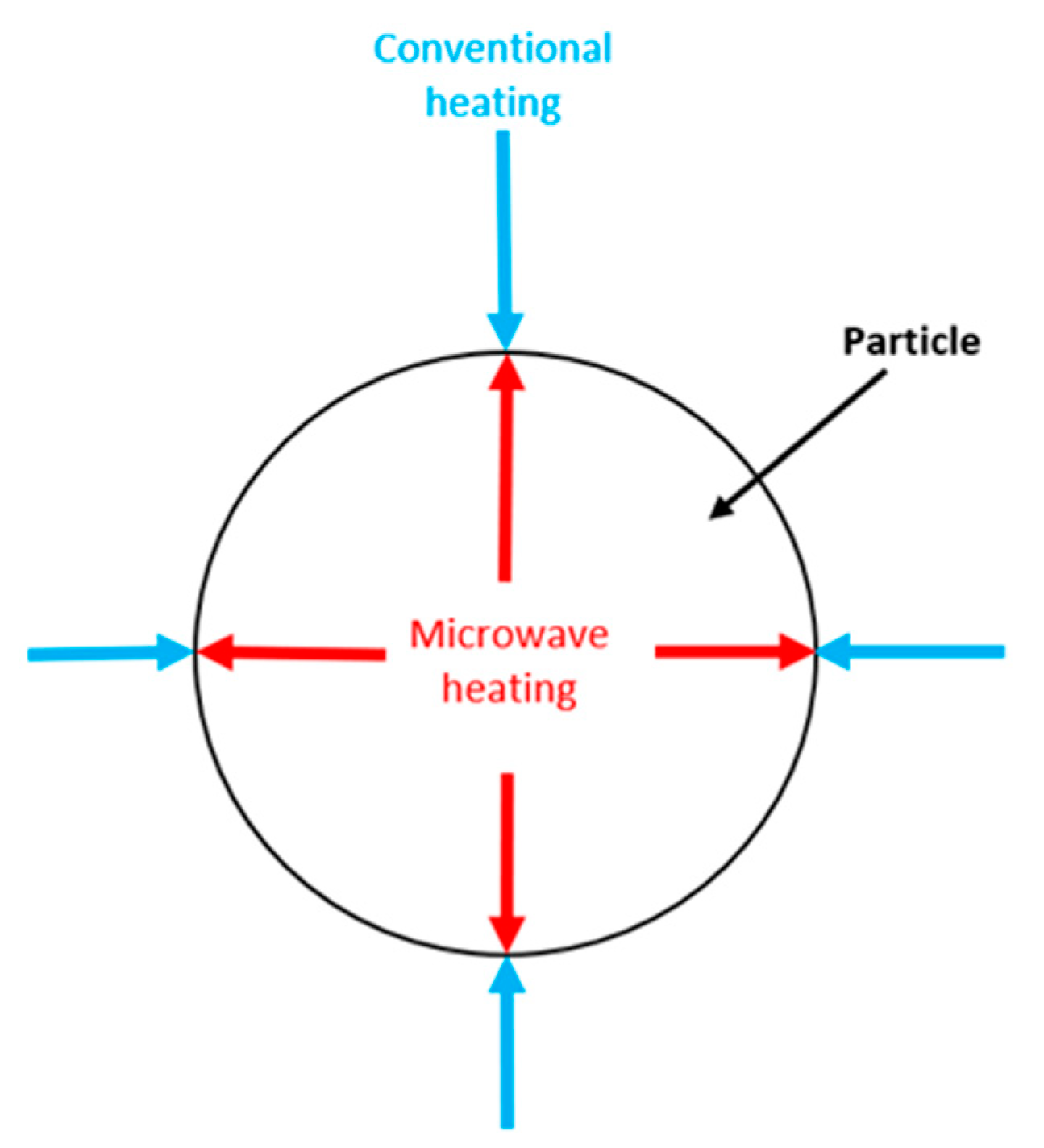
2.3.1. Microwave Pyrolysis
2.3.2. Solar Pyrolysis
2.3.3. Plasma Pyrolysis
2.3.4. Vacuum Pyrolysis
2.4. Reactor Types
2.4.1. Fluidized Bed Reactor
2.4.2. Ablative Plate Reactor
2.4.3. Auger Reactor
2.4.4. Rotating Cone Reactor
2.4.5. Cyclone/Vortex Reactor
3. The Effect of Reaction Conditions and Process Parameters
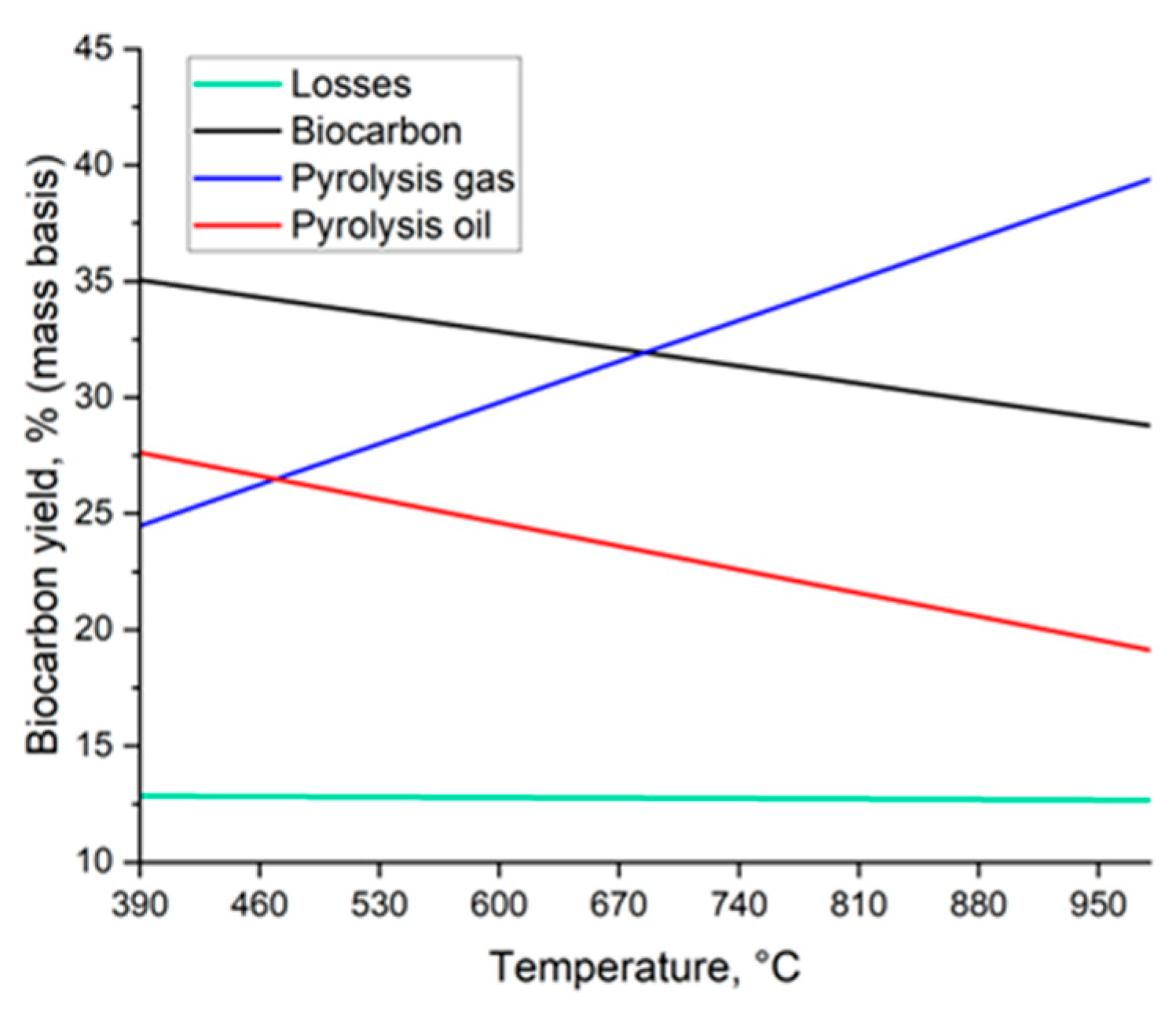
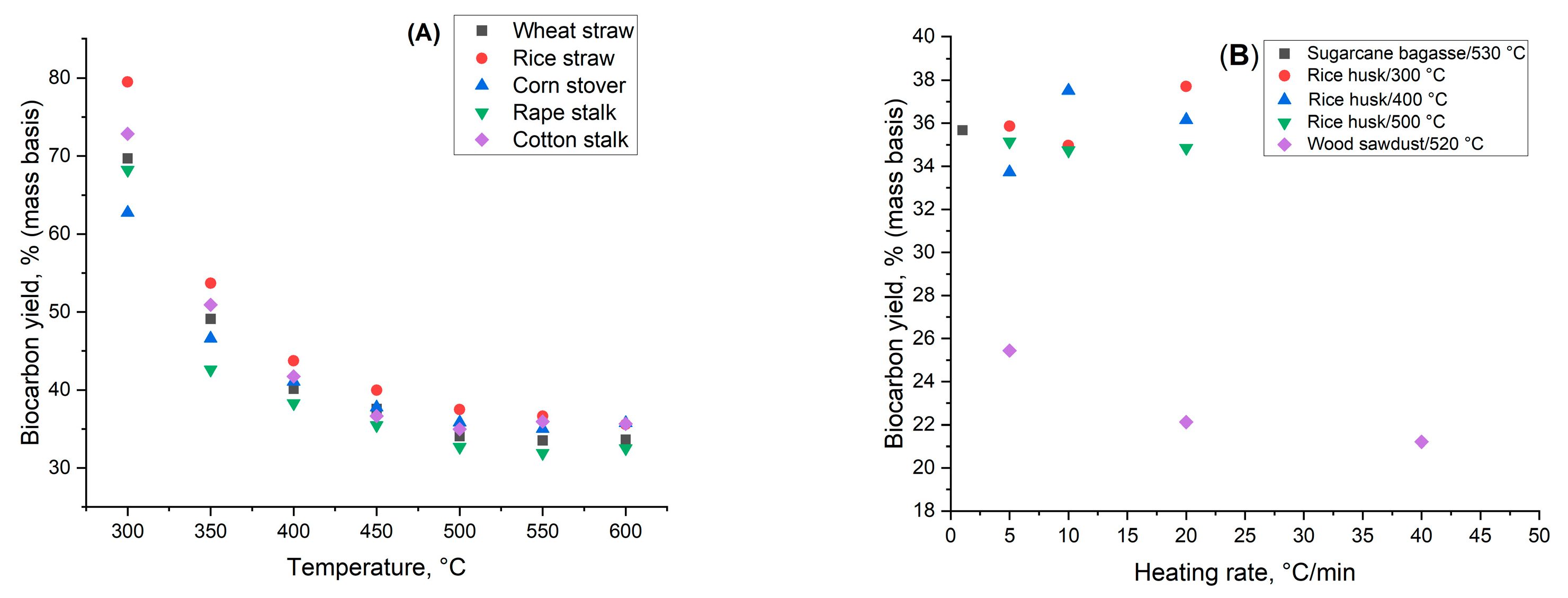
3.1. Effect of Final Temperature and Heating Rate
3.2. Vapor and Biomass Residence Time
3.3. Feedstock Particle Size
3.4. Reaction Atmosphere
3.5. Pressure
3.6. Catalyst
3.7. Binders
4. Discussion
- Slow heating rates, below 1 °C/min.
- Low pyrolysis temperatures, below 500 °C.
- Batch or auger reactors.
- Nitrogen as a purge gas.
- Use of small particle size, below 0.2 cm.
- Use of atmospheric pressure.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- IEA. Net Zero by 2050. Available online: https://www.iea.org/reports/net-zero-by-2050 (accessed on 1 June 2022).
- OECD. Climate Change: Meeting the Challenge to 2050. Available online: https://www.oecd.org/env/39762914.pdf (accessed on 23 June 2022).
- International Energy Agency.Global Energy Review: CO2 Emissions in 2021. Available online: https://www.iea.org/reports/global-energy-review-co2-emissions-in-2021-2 (accessed on 27 July 2022).
- Hoffman, C.; Van Hoey, M.; Zeumer, B. Decarbonization in Steel. Available online: https://www.mckinsey.com/industries/metals-and-mining/our-insights/decarbonization-challenge-for-steel (accessed on 22 June 2022).
- United Nations. Adoption of the Paris Agreement—Paris Agreement Text English. Available online: https://unfccc.int/sites/default/files/english_paris_agreement.pdf (accessed on 25 May 2023).
- Shukla, P.R.; Skea, J.; Slade, R.; Al Khourdajie, A.; Vyas, P.; Luz, S.; Fradera, R.; Belkacemi, M.; Hasija, A.; Malley, J.; et al. Climate Change 2022: Mitigation of Climate Change; Contribution of Working Group III to the Sixth ASSESSMENT report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2022; p. 10. ISBN 9781009157926. [Google Scholar]
- Pardo, N.; Moya, J.A. Prospective Scenarios on Energy Efficiency and CO2 Emissions in the European Iron & Steel Industry. Energy 2013, 54, 113–128. [Google Scholar] [CrossRef]
- Suopajärvi, H.; Umeki, K.; Mousa, E.; Hedayati, A.; Romar, H.; Kemppainen, A.; Wang, C.; Phounglamcheik, A.; Tuomikoski, S.; Norberg, N.; et al. Use of Biomass in Integrated Steelmaking—Status Quo, Future Needs and Comparison to Other Low-CO2 Steel Production Technologies. Appl. Energy 2018, 213, 384–407. [Google Scholar] [CrossRef]
- Mathieson, J.G.; Somerville, M.A.; Deev, A.; Jahanshahi, S. Utilization of Biomass as an Alternative Fuel in Ironmaking. In Iron Ore: Mineralogy, Processing and Environmental Sustainability; Lu, L., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 581–613. ISBN 9781782421597. [Google Scholar]
- Mousa, E.; Wang, C.; Riesbeck, J.; Larsson, M. Biomass Applications in Iron and Steel Industry: An Overview of Challenges and Opportunities. Renew. Sustain. Energy Rev. 2016, 65, 1247–1266. [Google Scholar] [CrossRef]
- Huang, H.; Reddy, N.G.; Huang, X.; Chen, P.; Wang, P.; Zhang, Y.; Huang, Y.; Lin, P.; Garg, A. Effects of Pyrolysis Temperature, Feedstock Type and Compaction on Water Retention of Biochar Amended Soil. Sci. Rep. 2021, 11, 7419. [Google Scholar] [CrossRef] [PubMed]
- Dhyani, V.; Bhaskar, T. A Comprehensive Review on the Pyrolysis of Lignocellulosic Biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Nanda, S.; Mohammad, J.; Reddy, S.N.; Kozinski, J.A.; Dalai, A.K. Pathways of Lignocellulosic Biomass Conversion to Renewable Fuels. Biomass. Convers. Biorefin. 2014, 5, 157–191. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of Wood/Biomass for Bio-Oil: A Critical Review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Wang, G.; Dai, Y.; Yang, H.; Xiong, Q.; Wang, K.; Zhou, J.; Li, Y.; Wang, S. A Review of Recent Advances in Biomass Pyrolysis. Energy Fuels 2020, 34, 15557–15578. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, P.; Liu, S.; Peng, P.; Min, M.; Cheng, Y.; Anderson, E.; Zhou, N.; Fan, L.; Liu, C.; et al. Effects of Feedstock Characteristics on Microwave-Assisted Pyrolysis—A Review. Bioresour. Technol. 2017, 230, 143–151. [Google Scholar] [CrossRef]
- Akhtar, A.; Krepl, V.; Ivanova, T. A Combined Overview of Combustion, Pyrolysis, and Gasification of Biomass. Energy Fuels 2018, 32, 7294–7318. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Choo, M.Y.; Oi, L.E.; Ling, T.C.; Ng, E.P.; Lee, H.V.; Juan, J.C. Conversion of Microalgae Biomass to Biofuels. In Microalgae Cultivation for Biofuels Production; Yousuf, A., Ed.; Elsevier: London, UK, 2019; pp. 149–161. ISBN 9780128175361. [Google Scholar]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. Torrefaction of Wood. Part 2. Analysis of Products. J. Anal. Appl. Pyrolysis 2006, 77, 35–40. [Google Scholar] [CrossRef]
- Piloni, R.V.; Brunetti, V.; Urcelay, R.C.; Daga, I.C.; Moyano, E.L. Chemical Properties of Biosilica and Bio-Oil Derived from Fast Pyrolysis of Melosira Varians. J. Anal. Appl. Pyrolysis 2017, 127, 402–410. [Google Scholar] [CrossRef]
- Li, Y.; Xing, B.; Ding, Y.; Han, X.; Wang, S. A Critical Review of the Production and Advanced Utilization of Biochar via Selective Pyrolysis of Lignocellulosic Biomass. Bioresour. Technol. 2020, 312, 123614. [Google Scholar] [CrossRef] [PubMed]
- Altamer, D.H.; Al-Irhayim, A.N.; Saeed, L.I. Bio-Based Liquids and Solids from Sustainable Feedstock: Production and Analysis. J. Anal. Appl. Pyrolysis 2021, 157, 105224. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a Sorbent for Contaminant Management in Soil and Water: A Review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Yaashikaa, P.R.; Kumar, P.S.; Varjani, S.; Saravanan, A. A Critical Review on the Biochar Production Techniques, Characterization, Stability and Applications for Circular Bioeconomy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar] [CrossRef]
- Wang, Y.; He, T.; Liu, K.; Wu, J.; Fang, Y. From Biomass to Advanced Bio-Fuel by Catalytic Pyrolysis/Hydro-Processing: Hydrodeoxygenation of Bio-Oil Derived from Biomass Catalytic Pyrolysis. Bioresour. Technol. 2012, 108, 280–284. [Google Scholar] [CrossRef]
- Carrier, M.; Hardie, A.G.; Uras, Ü.; Görgens, J.; Knoetze, J. Production of Char from Vacuum Pyrolysis of South-African Sugar Cane Bagasse and Its Characterization as Activated Carbon and Biochar. J. Anal. Appl. Pyrolysis 2012, 96, 24–32. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, Y.; Chen, P.; Liu, S.; Zhou, N.; Ding, K.; Fan, L.; Peng, P.; Min, M.; Cheng, Y.; et al. Gasification Technologies and Their Energy Potentials. In Sustainable Resource Recovery and Zero Waste Approaches; Elsevier: Amsterdam, The Netherlands, 2019; pp. 193–206. [Google Scholar] [CrossRef]
- Luque, R.; Men, J.A.; Arenillas, A.; Cot, J. Microwave-Assisted Pyrolysis of Biomass Feedstocks: The Way Forward? Energy Environ. Sci. 2012, 5, 5481–5488. [Google Scholar] [CrossRef]
- Babu, B.V. Biomass Pyrolysis: A State-of-the-Art Review. Biofuels Bioprod. Biorefin. 2008, 2, 393–414. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.; Karim, A.M.; Sun, J.; Wang, Y. Catalytic Fast Pyrolysis of Lignocellulosic Biomass. Chem. Soc. Rev. 2014, 43, 7594–7623. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Rasul, M.G.; Chowdhury, A.A.; Ashwath, N. Biofuels Production through Biomass Pyrolysis—A Technological Review. Energies 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Sobek, S.; Werle, S. Solar Pyrolysis of Waste Biomass: Part 1 Reactor Design. Renew. Energy 2019, 143, 1939–1948. [Google Scholar] [CrossRef]
- Yin, C. Microwave-Assisted Pyrolysis of Biomass for Liquid Biofuels Production. Bioresour. Technol. 2012, 120, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Lee, X.J.; Lee, L.Y.; Gan, S.; Thangalazhy-Gopakumar, S.; Ng, H.K. Biochar Potential Evaluation of Palm Oil Wastes through Slow Pyrolysis: Thermochemical Characterization and Pyrolytic Kinetic Studies. Bioresour. Technol. 2017, 236, 155–163. [Google Scholar] [CrossRef]
- Cárdenas- Aguiar, E.; Gascó, G.; Paz-Ferreiro, J.; Méndez, A. The Effect of Biochar and Compost from Urban Organic Waste on Plant Biomass and Properties of an Artificially Copper Polluted Soil. Int. Biodeterior. Biodegrad. 2017, 124, 223–232. [Google Scholar] [CrossRef]
- Demirbas, A. Effects of Temperature and Particle Size on Bio-Char Yield from Pyrolysis of Agricultural Residues. J. Anal. Appl. Pyrolysis 2004, 72, 243–248. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, S. The Structural and Thermal Characteristics of Wheat Straw Hemicellulose. J. Anal. Appl. Pyrolysis 2010, 88, 134–139. [Google Scholar] [CrossRef]
- Amalina, F.; Razak, A.S.A.; Krishnan, S.; Zularisam, A.W.; Nasrullah, M. A Comprehensive Assessment of the Method for Producing Biochar, Its Characterization, Stability, and Potential Applications in Regenerative Economic Sustainability—A Review. Clean. Mater. 2022, 3, 100045. [Google Scholar] [CrossRef]
- Xia, C.; Cai, L.; Zhang, H.; Zuo, L.; Shi, S.Q.; Shiung Lam, S. A Review on the Modeling and Validation of Biomass Pyrolysis with a Focus on Product Yield and Composition. Biofuel Res. J. 2021, 29, 1296–1315. [Google Scholar] [CrossRef]
- Brebu, M.; Vasile, C. Thermal Degradation of Lignin-A Review. Cellul. Chem. Technol. 2010, 44, 353–363. [Google Scholar]
- Nachenius, R.W.; Ronsse, F.; Venderbosch, R.H.; Prins, W. Biomass Pyrolysis. In Advances in Chemical Engineering; Marin, G.B., West, D.H., Li, J., Narasimhan, S., Eds.; Academic Press: Cambridge, MA, USA, 2013; Volume 42, pp. 75–139. [Google Scholar]
- Antal, M.J.; Grønli, M. The Art, Science, and Technology of Charcoal Production. Ind. Eng. Chem. Res. 2003, 42, 1619–1640. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of Process Parameters on Production of Biochar from Biomass Waste through Pyrolysis: A Review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Fahmy, T.Y.A.; Fahmy, Y.; Mobarak, F.; El-Sakhawy, M.; Abou-Zeid, R.E. Biomass Pyrolysis: Past, Present, and Future. Environ. Dev. Sustain. 2020, 22, 17–32. [Google Scholar] [CrossRef]
- Kazawadi, D.; Ntalikwa, J.; Kombe, G. A Review of Intermediate Pyrolysis as a Technology of Biomass Conversion for Coproduction of Biooil and Adsorption Biochar. J. Renew. Energy 2021, 143, 1939–1948. [Google Scholar] [CrossRef]
- Hornung, A. Intermediate Pyrolysis of Biomass. In Biomass Combustion Science, Technology and Engineering; Rosendahl, L., Ed.; Woodhead Publishing: Cambridge, UK, 2013; Volume 143, pp. 172–186. ISBN 9780857091314. [Google Scholar]
- Lam, K.-L.; Lee, C.-W.; Hui, C.-W. Multi-Stage Waste Tyre Pyrolysis: An Optimisation Approach. Chem. Eng. Trans. 2010, 21, 853. [Google Scholar] [CrossRef]
- Cheung, K.Y.; Lee, K.L.; Lam, K.L.; Chan, T.Y.; Lee, C.W.; Hui, C.W. Operation Strategy for Multi-Stage Pyrolysis. J. Anal. Appl. Pyrolysis 2011, 91, 165–182. [Google Scholar] [CrossRef]
- Oyedun, A.O.; Lam, K.-L.; Gebreegziabher, T.; Lee, H.K.M.; Hui, C.-W. Optimisation of Operating Parameters in Multi-Stage Pyrolysis. Chem. Eng. Trans. 2012, 29, 655–660. [Google Scholar] [CrossRef]
- Oyedun, A.O.; Lam, K.L.; Hui, C.W. Charcoal Production via Multistage Pyrolysis. Chin. J. Chem. Eng. 2012, 20, 455–460. [Google Scholar] [CrossRef]
- Cai, W.; Liu, Q.; Shen, D.; Wang, J. Py-GC/MS Analysis on Product Distribution of Two-Staged Biomass Pyrolysis. J. Anal. Appl. Pyrolysis 2019, 138, 62–69. [Google Scholar] [CrossRef]
- Babinszki, B.; Sebestyén, Z.; Jakab, E.; Kőhalmi, L.; Bozi, J.; Várhegyi, G.; Wang, L.; Skreiberg, Ø.; Czégény, Z. Effect of Slow Pyrolysis Conditions on Biocarbon Yield and Properties: Characterization of the Volatiles. Bioresour. Technol. 2021, 338, 125567. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Wang, Q.; Zhao, J.; Luo, K.H.; Li, H.; Chen, Y.; Lu, C. Combustion Pattern, Characteristics, and Kinetics of Biomass and Chars from Segmented Heating Carbonization. Asia-Pac. J. Chem. Eng. 2016, 11, 812–822. [Google Scholar] [CrossRef]
- Qi, J.; Zhao, J.; Xu, Y.; Wang, Y.; Han, K. Segmented Heating Carbonization of Biomass: Yields, Property and Estimation of Heating Value of Chars. Energy 2018, 144, 301–311. [Google Scholar] [CrossRef]
- Ethaib, S.; Omar, R.; Mazlina, S.; Kamal, M.; Radiah, D.; Biak, A.; Zubaidi, S.L. Microwave-Assisted Pyrolysis of Biomass Waste: A Mini Review. Processes 2020, 8, 1190. [Google Scholar] [CrossRef]
- Mushtaq, F.; Mat, R.; Ani, F.N. A Review on Microwave Assisted Pyrolysis of Coal and Biomass for Fuel Production. Renew. Sustain. Energy Rev. 2014, 39, 555–574. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, Y.; Liu, S.; Fan, L.; Zhou, N.; Peng, P.; Wang, Y.; Guo, F.; Min, M.; Cheng, Y.; et al. Fast Microwave-Assisted Pyrolysis of Wastes for Biofuels Production—A Review. Bioresour. Technol. 2020, 297, 122480. [Google Scholar] [CrossRef]
- Macquarrie, D.J.; Clark, J.H.; Fitzpatrick, E. The Microwave Pyrolysis of Biomass. Biofuels Bioprod. Biorefin. 2012, 6, 549–560. [Google Scholar] [CrossRef]
- Omar, R.; Mokhtar, N.M.; Ethaib, S. Effect of Microwave Absorbers on the Products of Microwave Pyrolysis of Oily Sludge. J. Eng. Sci. Technol. 2018, 13, 3313–3330. [Google Scholar]
- Yu, Y.; Yu, J.; Sun, B.; Yan, Z. Influence of Catalyst Types on the Microwave-Induced Pyrolysis of Sewage Sludge. J. Anal. Appl. Pyrolysis 2014, 106, 86–91. [Google Scholar] [CrossRef]
- Budarin, V.L.; Shuttleworth, P.S.; De Bruyn, M.; Farmer, T.J.; Gronnow, M.J.; Pfaltzgraff, L.; Macquarrie, D.J.; Clark, J.H. The Potential of Microwave Technology for the Recovery, Synthesis and Manufacturing of Chemicals from Bio-Wastes. Catal. Today 2015, 239, 80–89. [Google Scholar] [CrossRef]
- Motasemi, F.; Afzal, M.T. A Review on the Microwave-Assisted Pyrolysis Technique. Renew. Sustain. Energy Rev. 2013, 28, 317–330. [Google Scholar] [CrossRef]
- Klinger, J.L.; Westover, T.L.; Emerson, R.M.; Williams, C.L.; Hernandez, S.; Monson, G.D.; Ryan, J.C. Effect of Biomass Type, Heating Rate, and Sample Size on Microwave-Enhanced Fast Pyrolysis Product Yields and Qualities. Appl. Energy 2018, 228, 535–545. [Google Scholar] [CrossRef]
- Ingole, P.M.; Ranveer, A.C.; Deshmukh, S.M.; Deshmukh, S.K. Microwave Assisted Pyrolysis of Biomass: A Review. Int. J. Adv. Technol. Eng. Sci. 2016, 4, 78–84. [Google Scholar]
- Wu, C.; Budarin, V.L.; Gronnow, M.J.; De Bruyn, M.; Onwudili, J.A.; Clark, J.H.; Williams, P.T. Conventional and Microwave-Assisted Pyrolysis of Biomass under Different Heating Rates. J. Anal. Appl. Pyrolysis 2014, 107, 276–283. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, M.; Liu, H.; Zhao, C.; Ma, C.; Song, Z. Effect of Temperature and Additives on the Yields of Products and Microwave Pyrolysis Behaviors of Wheat Straw. J. Anal. Appl. Pyrolysis 2013, 100, 49–55. [Google Scholar] [CrossRef]
- Wallace, C.A.; Afzal, M.T.; Saha, G.C. Effect of Feedstock and Microwave Pyrolysis Temperature on Physio-Chemical and Nano-Scale Mechanical Properties of Biochar. Bioresour. Bioprocess. 2019, 6, 33. [Google Scholar] [CrossRef]
- Mašek, O.; Budarin, V.; Gronnow, M.; Crombie, K.; Brownsort, P.; Fitzpatrick, E.; Hurst, P. Microwave and Slow Pyrolysis Biochar—Comparison of Physical and Functional Properties. J. Anal. Appl. Pyrolysis 2013, 100, 41–48. [Google Scholar] [CrossRef]
- Shirvanimoghaddam, K.; Czech, Z.; Abdikheibari, S.; Brodie, G.; Ko, M.; Krzyszczak, A.; Al-Othman, A.; Naebe, M. Microwave Synthesis of Biochar for Environmental Applications. J. Anal. Appl. Pyrolysis 2022, 161, 105415. [Google Scholar] [CrossRef]
- Abas, F.Z.; Ani, F.N. Comparing Characteristics of Oil Palm Biochar Using Conventional and Microwave Heating. J. Teknol. 2014, 68, 33–37. [Google Scholar] [CrossRef]
- Mohd, N. Conventional and Microwave Pyrolysis of Empty Fruit Bunch and Rice Husk Pellets. Ph.D. Thesis, University of Sheffield, Sheffield, UK, 2017. [Google Scholar]
- Ge, S.; Foong, S.Y.; Ma, N.L.; Liew, R.K.; Wan Mahari, W.A.; Xia, C.; Yek, P.N.Y.; Peng, W.; Nam, W.L.; Lim, X.Y.; et al. Vacuum Pyrolysis Incorporating Microwave Heating and Base Mixture Modification: An Integrated Approach to Transform Biowaste into Eco-Friendly Bioenergy Products. Renew. Sustain. Energy Rev. 2020, 127, 109871. [Google Scholar] [CrossRef]
- Md Said, M.S.; Azni, A.A.; Wan Ab Karim Ghani, W.A.; Idris, A.; Ja’afar, M.F.Z.; Mohd Salleh, M.A. Production of Biochar from Microwave Pyrolysis of Empty Fruit Bunch in an Alumina Susceptor. Energy 2022, 240, 122710. [Google Scholar] [CrossRef]
- Zhang, L.; Tan, Y.; Cai, D.; Sun, J.; Zhang, Y.; Li, L.; Zhang, Q.; Zou, G.; Song, Z.; Bai, Y. Enhanced Pyrolysis of Woody Biomass under Interaction of Microwave and Needle-Shape Metal and Its Production Properties. Energy 2022, 249, 123667. [Google Scholar] [CrossRef]
- Morales, S.; Miranda, R.; Bustos, D.; Cazares, T.; Tran, H. Solar Biomass Pyrolysis for the Production of Bio-Fuels and Chemical Commodities. J. Anal. Appl. Pyrolysis 2014, 109, 65–78. [Google Scholar] [CrossRef]
- Bashir, M.; Yu, X.; Hassan, M.; Makkawi, Y. Modeling and Performance Analysis of Biomass Fast Pyrolysis in a Solar-Thermal Reactor. ACS Sustain. Chem. Eng. 2017, 5, 3795–3807. [Google Scholar] [CrossRef]
- Rahman, M.A.; Parvej, A.M.; Aziz, M.A. Concentrating Technologies with Reactor Integration and Effect of Process Variables on Solar Assisted Pyrolysis: A Critical Review. Therm. Sci. Eng. Prog. 2021, 25, 100957. [Google Scholar] [CrossRef]
- Li, R.; Zeng, K.; Soria, J.; Mazza, G.; Gauthier, D.; Rodriguez, R.; Flamant, G. Product Distribution from Solar Pyrolysis of Agricultural and Forestry Biomass Residues. Renew. Energy 2016, 89, 27–35. [Google Scholar] [CrossRef]
- Zeng, K.; Gauthier, D.; Soria, J.; Mazza, G.; Flamant, G. Solar Pyrolysis of Carbonaceous Feedstocks: A Review. Sol. Energy 2017, 156, 73–92. [Google Scholar] [CrossRef]
- Ayala-Cortés, A.; Lobato-Peralta, D.R.; Arreola-Ramos, C.E.; Martínez-Casillas, D.C.; Pacheco-Catalán, D.E.; Cuentas-Gallegos, A.K.; Arancibia-Bulnes, C.A.; Villafán-Vidales, H.I. Exploring the Influence of Solar Pyrolysis Operation Parameters on Characteristics of Carbon Materials. J. Anal. Appl. Pyrolysis 2019, 140, 290–298. [Google Scholar] [CrossRef]
- Giwa, A.; Yusuf, A.; Ajumobi, O.; Dzidzienyo, P. Pyrolysis of Date Palm Waste to Biochar Using Concentrated Solar Thermal Energy: Economic and Sustainability Implications. Waste Manag. 2019, 93, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, P.; Al-Ansari, T.; Mackey, H.R.; Sheeba Narayanan, K.; McKay, G. A Review on Prominent Animal and Municipal Wastes as Potential Feedstocks for Solar Pyrolysis for Biochar Production. Fuel 2022, 316, 123378. [Google Scholar] [CrossRef]
- Nzihou, A.; Flamant, G.; Stanmore, B. Synthetic Fuels from Biomass Using Concentrated Solar Energy—A Review. Energy 2012, 42, 121–131. [Google Scholar] [CrossRef]
- Chintala, V. Production, Upgradation and Utilization of Solar Assisted Pyrolysis Fuels from Biomass—A Technical Review. Renew. Sustain. Energy Rev. 2018, 90, 120–130. [Google Scholar] [CrossRef]
- Zeng, K.; Soria, J.; Gauthier, D.; Mazza, G.; Flamant, G. Modeling of Beech Wood Pellet Pyrolysis under Concentrated Solar Radiation. Renew. Energy 2016, 99, 721–729. [Google Scholar] [CrossRef]
- Weldekidan, H.; Strezov, V.; Town, G.; Kan, T. Production and Analysis of Fuels and Chemicals Obtained from Rice Husk Pyrolysis with Concentrated Solar Radiation. Fuel 2018, 233, 396–403. [Google Scholar] [CrossRef]
- Chen, D.; Cen, K.; Cao, X.; Zhang, J.; Chen, F.; Zhou, J. Upgrading of Bio-Oil via Solar Pyrolysis of the Biomass Pretreated with Aqueous Phase Bio-Oil Washing, Solar Drying, and Solar Torrefaction. Bioresour. Technol. 2020, 305, 123130. [Google Scholar] [CrossRef]
- Chen, D.; Cen, K.; Chen, F.; Zhang, Y. Solar Pyrolysis of Cotton Stalks: Combined Effects of Torrefaction Pretreatment and HZSM-5 Zeolite on the Bio-Fuels Upgradation. Energy Convers. Manag. 2022, 261, 115640. [Google Scholar] [CrossRef]
- Singh, Y.; Singla, A.; Singh, K.; Sharma, A. Production and Feasibility Characterization of Bio-Oil from Jojoba Seed-Based Biomass through Solar Thermal Energy Pyrolysis Process. Biomass Convers. Biorefin. 2022, 1, 1–13. [Google Scholar] [CrossRef]
- Ciuta, S.; Tsiamis, D.; Castaldi, M.J. Fundamentals of Gasification and Pyrolysis. In Gasification of Waste Materials: Technologies for Generating Energy, Gas, and Chemicals from Municipal Solid Waste, Biomass, Nonrecycled Plastics, Sludges, and Wet Solid Wastes; Ciuta, S., Tsiamis, D., Castaldi, M.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 13–36. ISBN 9780128127162. [Google Scholar]
- Hrabovsky, M. Thermal Plasma Gasification of Biomass. In Progress in Biomass and Bioenergy Production; Shaukat, S.S., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 39–62. ISBN 978-953-307-491-7. [Google Scholar]
- Shie, J.L.; Tsou, F.J.; Lin, K.L.; Chang, C.Y. Bioenergy and Products from Thermal Pyrolysis of Rice Straw Using Plasma Torch. Bioresour. Technol. 2010, 101, 761–768. [Google Scholar] [CrossRef]
- Cheng, Y.; Yan, B.H.; Cao, C.X.; Cheng, Y.; Jin, Y. Experimental Investigation on Coal Devolatilization at High Temperatures with Different Heating Rates. Fuel 2014, 117, 1215–1222. [Google Scholar] [CrossRef]
- Sturmn, G.S.J.; Muños, A.N.; Aravind, P.V.; Stefanidis, G.D. Microwave-Driven Plasma Gasification for Biomass Waste Treatment at Miniature Scale. IEEE Trans. Plasma Sci. 2016, 44, 670–678. [Google Scholar] [CrossRef]
- Tang, L.; Huang, H. Biomass Gasification Using Capacitively Coupled RF Plasma Technology. Fuel 2005, 84, 2055–2063. [Google Scholar] [CrossRef]
- Huang, H.; Tang, L. Treatment of Organic Waste Using Thermal Plasma Pyrolysis Technology. Energy Convers. Manag. 2007, 48, 1331–1337. [Google Scholar] [CrossRef]
- Soria-Verdugo, A. Pyrolysis of Sludge and Biomass Residues. In Wastewater Treatment Residues as Resources for Biorefinery Products and Biofuels; Olivares, J.A., Puyol, D., Melero, J.A., Dufour, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 155–181. ISBN 9780128162040. [Google Scholar]
- Liu, L.; Xiong, B.; Zhang, X.; Ye, L. Vacuum Pyrolysis of Ammonium Paratungstate: Study on Reaction Mechanism and Morphology Changes of Product. J. Anal. Appl. Pyrolysis 2021, 157, 105168. [Google Scholar] [CrossRef]
- Ruan, J.; Huang, J.; Qin, B.; Dong, L. Heat Transfer in Vacuum Pyrolysis of Decomposing Hazardous Plastic Wastes. ACS Sustain. Chem. Eng. 2018, 6, 5424–5430. [Google Scholar] [CrossRef]
- Carrier, M.; Hugo, T.; Gorgens, J.; Knoetze, H. Comparison of Slow and Vacuum Pyrolysis of Sugar Cane Bagasse. J. Anal. Appl. Pyrolysis 2011, 90, 18–26. [Google Scholar] [CrossRef]
- Dusso, D.; Téllez, J.F.; Fuertes, V.C.; De Paoli, J.M.; Moyano, E.L. Vacuum Pyrolysis of Chia Flour Residues: An Alternative Way to Obtain Omega-3/Omega-6 Fatty Acids and Calcium-Enriched Biochars. J. Anal. Appl. Pyrolysis 2022, 161, 105379. [Google Scholar] [CrossRef]
- Zaman, C.Z.; Pal, K.; Yehye, W.A.; Sagadevan, S.; Shah, S.T.; Adebisi, G.A.; Marliana, E.; Rafique, R.F.; Johan, R.B. Pyrolysis: A Sustainable Way to Generate Energy from Waste. In Pyrolysis; Samer, M., Ed.; IntechOpen: Rijeka, Croatia, 2017; pp. 3–36. ISBN 978-953-51-3312-4. [Google Scholar]
- Collard, F.X.; Carrier, M.; Görgens, J.F. Fractionation of Lignocellulosic Material With Pyrolysis Processing. In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Mussatto, S.I., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 81–101. ISBN 9780128025611. [Google Scholar]
- Yadav, K.; Jagadevan, S.; Yadav, K.; Jagadevan, S. Influence of Process Parameters on Synthesis of Biochar by Pyrolysis of Biomass: An Alternative Source of Energy. In Recent Advances in Pyrolysis; Hassan, A.-H.I., Ed.; IntechOpen: Rijeka, Croatia, 2019; ISBN 978-1-78984-064-3. [Google Scholar]
- Shah, A.T.; Attique, S.; Batool, M.; Godini, H.R.; Goerke, O. Role of Polyoxometalates in Converting Plastic Waste into Fuel Oil. In Advanced Technology for the Conversion of Waste into Fuels and Chemicals: Volume 2: Chemical Processes; Khan, A., Pizzi, A., Jawaid, M., Azum, N., Asiri, A., Isa, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 2, pp. 333–355. ISBN 9780323901505. [Google Scholar]
- Dhyani, V.; Bhaskar, T. Pyrolysis of Biomass. In Biomass, Biofuels, Biochemicals: Biofuels: Alternative Feedstocks and Conversion Processes for the Production of Liquid and Gaseous Biofuels; Pandey, A., Larroche, C., Dussap, C.-G., Gnansounou, E., Khanal, S.K., Ricke, S., Eds.; Elsevier: London, UK, 2019; pp. 217–244. ISBN 9780128168561. [Google Scholar]
- Rizzo, A.M.; Nistri, R.; Buffi, M.; Marsili Libelli, I.; Bettucci, L.; Prussi, M.; Chiaramonti, D. Effect of Feedstock Composition on Quality and Yield of Bio-Oil from the Pyrolysis of Three Microalgae Species from Open Pond and Closed Photobioreactor. In Proceedings of the 21st European Biomass Conference and Exhibitions, Copenghagen, Denmark, 3 June 2013; pp. 494–499. [Google Scholar]
- Wang, X.; Kersten, S.R.A.; Prins, W.; Van Swaaij, W.P.M. Biomass Pyrolysis in a Fluidized Bed Reactor. Part 2: Experimental Validation of Model Results. Ind. Eng. Chem. Res. 2005, 44, 8786–8795. [Google Scholar] [CrossRef]
- Bermudez, J.M.; Fidalgo, B. Production of Bio-Syngas and Bio-Hydrogen via Gasification. In Handbook of Biofuels Production: Processes and Technologies; Lugue, R., Lin, C.S.K., Wilson, K., Clark, J., Eds.; Woodhead Publishing: Duxford, UK, 2016; pp. 431–494. ISBN 9780081004562. [Google Scholar]
- Ram, M.; Mondal, M.K. Biomass Gasification: A Step toward Cleaner Fuel and Chemicals. In Biofuels and Bioenergy: Opportunities and Challenges; Gurunathan, B., Sahadevan, R., Zakaria, Z.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 253–276. ISBN 978-0-323-85269-2. [Google Scholar]
- Mallick, D.; Sharma, S.D.; Kushwaha, A.; Brahma, H.S.; Nath, R.; Bhowmik, R. Emerging Commercial Opportunities for Conversion of Waste to Energy: Aspect of Gasification Technology. In Waste-to-Energy Approaches Towards Zero Waste; Hussain, C.M., Singht, S., Goswami, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 105–127. ISBN 978-0-323-85387-3. [Google Scholar]
- Zhu, Y.; Frey, H.C. Integrated Gasification Combined Cycle (IGCC) Power Plant Design and Technology. In Advanced Power Plant Materials, Design and Technology; Roddy, D., Ed.; Woodhead Publishing: Cambridge, UK, 2010; pp. 54–88. ISBN 978-1-84569-515-6. [Google Scholar]
- Khuenkaeo, N.; Tippayawong, N. Bio-Oil Production from Ablative Pyrolysis of Corncob Pellets in a Rotating Blade Reactor. IOP Conf. Ser. Earth Environ. Sci. 2018, 159, 012037. [Google Scholar] [CrossRef]
- Mei Wu, L.; Hui Zhou, C.; Shen Tong, D.; Hua Yu, W. Catalytic Thermochemical Processes for Biomass Conversion to Biofuels and Chemicals. In Bioenergy Research: Advances and Applications; Gupta, V.K., Tuohy, M.G., Kubicek, C.P., Saddler, J., Xu, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 243–254. ISBN 9780444595614. [Google Scholar]
- Hu, X.; Gholizadeh, M. Biomass Pyrolysis: A Review of the Process Development and Challenges from Initial Researches up to the Commercialisation Stage. J. Energy Chem. 2019, 39, 109–143. [Google Scholar] [CrossRef]
- Brassard, P.; Godbout, S.; Raghavan, V.; Palacios, J.H.; Grenier, M.; Zegan, D. The Production of Engineered Biochars in a Vertical Auger Pyrolysis Reactor for Carbon Sequestration. Energies 2017, 10, 288. [Google Scholar] [CrossRef]
- Campuzano, F.; Brown, R.C.; Martínez, J.D. Auger Reactors for Pyrolysis of Biomass and Wastes. Renew. Sustain. Energy Rev. 2019, 102, 372–409. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of Fast Pyrolysis of Biomass and Product Upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Nunez Manzano, M.; Gonzalez Quiroga, A.; Perreault, P.; Madanikashani, S.; Vandewalle, L.A.; Marin, G.B.; Heynderickx, G.J.; Van Geem, K.M. Biomass Fast Pyrolysis in an Innovative Gas-Solid Vortex Reactor: Experimental Proof of Concept. J. Anal. Appl. Pyrolysis 2021, 156, 105165. [Google Scholar] [CrossRef]
- Nam, H.; Capareda, S.C.; Ashwath, N.; Kongkasawan, J. Experimental Investigation of Pyrolysis of Rice Straw Using Bench-Scale Auger, Batch and Fluidized Bed Reactors. Energy 2015, 93, 2384–2394. [Google Scholar] [CrossRef]
- Coates, R.L.; Coates, B.R.; Coates, J.L. Method and Apparatus for Fast Pyrolysis of Biomass in Rotary Kilns. U.S. Patent US20120063965A1, 7 April 2015. [Google Scholar]
- Williams, P.T. Pyrolysis of Waste Tyres: A Review. Waste Manag. 2013, 33, 1714–1728. [Google Scholar] [CrossRef]
- Mohabeer, C.; Guilhaume, N.; Laurenti, D.; Schuurman, Y. Microwave-Assisted Pyrolysis of Biomass with and without Use of Catalyst in a Fluidised Bed Reactor: A Review. Energy 2022, 15, 3258. [Google Scholar] [CrossRef]
- Tang, L.; Huang, H.; Hao, H.; Zhao, K. Development of Plasma Pyrolysis/Gasification Systems for Energy Efficient and Environmentally Sound Waste Disposal. J. Electrost. 2013, 71, 839–847. [Google Scholar] [CrossRef]
- Joardder, M.U.H.; Halder, P.K.; Rahim, A.; Paul, N. Solar Assisted Fast Pyrolysis: A Novel Approach of Renewable Energy Production. J. Eng. 2014, 2014, 252848. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.S. A Review on Operating Parameters for Optimum Liquid Oil Yield in Biomass Pyrolysis. Renew. Sustain. Energy Rev. 2012, 16, 5101–5109. [Google Scholar] [CrossRef]
- Angin, D. Effect of Pyrolysis Temperature and Heating Rate on Biochar Obtained from Pyrolysis of Safflower Seed Press Cake. Bioresour. Technol. 2013, 128, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Solar, J.; de Marco, I.; Caballero, B.M.; Lopez-Urionabarrenechea, A.; Rodriguez, N.; Agirre, I.; Adrados, A. Influence of Temperature and Residence Time in the Pyrolysis of Woody Biomass Waste in a Continuous Screw Reactor. Biomass Bioenergy 2016, 95, 416–423. [Google Scholar] [CrossRef]
- Al Arni, S. Comparison of Slow and Fast Pyrolysis for Converting Biomass into Fuel. Renew. Energy 2018, 124, 197–201. [Google Scholar] [CrossRef]
- Mlonka-Mędrala, A.; Evangelopoulos, P.; Sieradzka, M.; Zajemska, M.; Magdziarz, A. Pyrolysis of Agricultural Waste Biomass towards Production of Gas Fuel and High-Quality Char: Experimental and Numerical Investigations. Fuel 2021, 296, 120611. [Google Scholar] [CrossRef]
- Penzik, M.V.; Kozlov, A.N.; Zhang, S.; Badenko, V.V.; Sosnovsky, I.K.; Shamansky, V.A. A Segmental Analysis of Pyrolysis of Woody Biomass. Thermochim. Acta 2022, 711, 179209. [Google Scholar] [CrossRef]
- He, X.; Liu, Z.; Niu, W.; Yang, L.; Zhou, T.; Qin, D.; Niu, Z.; Yuan, Q. Effects of Pyrolysis Temperature on the Physicochemical Properties of Gas and Biochar Obtained from Pyrolysis of Crop Residues. Energy 2018, 143, 746–756. [Google Scholar] [CrossRef]
- Anand, A.; Gautam, S.; Ram, L.C. Feedstock and Pyrolysis Conditions Affect Suitability of Biochar for Various Sustainable Energy and Environmental Applications. J. Anal. Appl. Pyrolysis 2023, 170, 105881. [Google Scholar] [CrossRef]
- Vieira, F.R.; Romero Luna, C.M.; Arce, G.L.A.F.; Ávila, I. Optimization of Slow Pyrolysis Process Parameters Using a Fixed Bed Reactor for Biochar Yield from Rice Husk. Biomass Bioenergy 2020, 132, 105412. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic Biomass Pyrolysis: A Review of Product Properties and Effects of Pyrolysis Parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Principles and Practice of Biomass Fast Pyrolysis Processes for Liquids. J. Anal. Appl. Pyrolysis 1999, 51, 3–22. [Google Scholar] [CrossRef]
- Liu, R.; Liu, G.; Yousaf, B.; Abbas, Q. Operating Conditions-Induced Changes in Product Yield and Characteristics during Thermal-Conversion of Peanut Shell to Biochar in Relation to Economic Analysis. J. Clean. Prod. 2018, 193, 479–490. [Google Scholar] [CrossRef]
- Mlonka-Mędrala, A.; Magdziarz, A.; Dziok, T.; Sieradzka, M.; Nowak, W. Laboratory Studies on the Influence of Biomass Particle Size on Pyrolysis and Combustion Using TG GC/MS. Fuel 2019, 252, 635–645. [Google Scholar] [CrossRef]
- Kirubakaran, V.; Sivaramakrishnan, V.; Nalini, R.; Sekar, T.; Premalatha, M.; Subramanian, P. A Review on Gasification of Biomass. Renew. Sustain. Energy Rev. 2009, 13, 179–186. [Google Scholar] [CrossRef]
- Şensöz, S.; Angin, D.; Yorgun, S. Influence of Particle Size on the Pyrolysis of Rapeseed (Brassica Napus L.): Fuel Properties of Bio-Oil. Biomass Bioenergy 2000, 19, 271–279. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L.; Berrueco, C.; Fidalgo, B.; Paterson, N.; Millan, M. Influence of Temperature and Particle Size on Structural Characteristics of Chars from Beechwood Pyrolysis. J. Anal. Appl. Pyrolysis 2018, 130, 249–255. [Google Scholar] [CrossRef]
- Somerville, M.; Deev, A. The Effect of Heating Rate, Particle Size and Gas Flow on the Yield of Charcoal during the Pyrolysis of Radiata Pine Wood. Renew. Energy 2020, 151, 419–425. [Google Scholar] [CrossRef]
- Mellin, P.; Yu, X.; Yang, W.; Blasiak, W. Influence of Reaction Atmosphere (H2O, N2, H2, CO2, CO) on Fluidized-Bed Fast Pyrolysis of Biomass Using Detailed Tar Vapor Chemistry in Computational Fluid Dynamics. Ind. Eng. Chem. Res. 2015, 54, 8344–8355. [Google Scholar] [CrossRef]
- Minkova, V.; Razvigorova, M.; Bjornbom, E.; Zanzi, R.; Budinova, T.; Petrov, N. Effect of Water Vapour and Biomass Nature on the Yield and Quality of the Pyrolysis Products from Biomass. Fuel Process. Technol. 2001, 70, 53–61. [Google Scholar] [CrossRef]
- Bach, Q.V.; Trinh, T.N.; Tran, K.Q.; Thi, N.B.D. Pyrolysis Characteristics and Kinetics of Biomass Torrefied in Various Atmospheres. Energy Convers. Manag. 2017, 141, 72–78. [Google Scholar] [CrossRef]
- Özbay, N.; Uzun, B.B.; Varol, E.A.; Pütün, A.E. Comparative Analysis of Pyrolysis Oils and Its Subfractions under Different Atmospheric Conditions. Fuel Process. Technol. 2006, 87, 1013–1019. [Google Scholar] [CrossRef]
- Önal, E.P.; Uzun, B.B.; Pütün, A.E. Steam Pyrolysis of an Industrial Waste for Bio-Oil Production. Fuel Process. Technol. 2011, 92, 879–885. [Google Scholar] [CrossRef]
- Aladin, A.; Modding, B.; Syarif, T.; Dewi, F.C. Effect of Nitrogen Gas Flowing Continuously into the Pyrolysis Reactor for Simultaneous Production of Charcoal and Liquid Smoke. J. Phys. Conf. Ser. 2021, 1763, 012020. [Google Scholar] [CrossRef]
- Lee, J.; Yang, X.; Song, H.; Ok, Y.S.; Kwon, E.E. Effects of Carbon Dioxide on Pyrolysis of Peat. Energy 2017, 120, 929–936. [Google Scholar] [CrossRef]
- Manyà, J.J.; Azuara, M.; Manso, J.A. Biochar Production through Slow Pyrolysis of Different Biomass Materials: Seeking the Best Operating Conditions. Biomass Bioenergy 2018, 117, 115–123. [Google Scholar] [CrossRef]
- Pilon, G.; Lavoie, J.-M. Biomass Char Production at Low Severity Conditions under CO2 and N2 Environments. WIT Trans. Ecol. Environ. 2011, 143, 109–121. [Google Scholar] [CrossRef]
- Basile, L.; Tugnoli, A.; Stramigioli, C.; Cozzani, V. Influence of Pressure on the Heat of Biomass Pyrolysis. Fuel 2014, 137, 277–284. [Google Scholar] [CrossRef]
- Qin, L.; Wu, Y.; Hou, Z.; Jiang, E. Influence of Biomass Components, Temperature and Pressure on the Pyrolysis Behavior and Biochar Properties of Pine Nut Shells. Bioresour. Technol. 2020, 313, 123682. [Google Scholar] [CrossRef]
- Gouws, S.M.; Carrier, M.; Bunt, J.R.; Neomagus, H.W.J.P. Co-Pyrolysis of Torrefied Biomass and Coal: Effect of Pressure on Synergistic Reactions. J. Anal. Appl. Pyrolysis 2022, 161, 105363. [Google Scholar] [CrossRef]
- Ren, X.; Shanb Ghazani, M.; Zhu, H.; Ao, W.; Zhang, H.; Moreside, E.; Zhu, J.; Yang, P.; Zhong, N.; Bi, X. Challenges and Opportunities in Microwave-Assisted Catalytic Pyrolysis of Biomass: A Review. Appl. Energy 2022, 315, 118970. [Google Scholar] [CrossRef]
- Nishu; Liu, R.; Rahman, M.M.; Sarker, M.; Chai, M.; Li, C.; Cai, J. A Review on the Catalytic Pyrolysis of Biomass for the Bio-Oil Production with ZSM-5: Focus on Structure. Fuel Process. Technol. 2020, 199, 106301. [Google Scholar] [CrossRef]
- Wan, Y.; Chen, P.; Zhang, B.; Yang, C.; Liu, Y.; Lin, X.; Ruan, R. Microwave-Assisted Pyrolysis of Biomass: Catalysts to Improve Product Selectivity. J. Anal. Appl. Pyrolysis 2009, 86, 161–167. [Google Scholar] [CrossRef]
- Chen, W.; Fang, Y.; Li, K.; Chen, Z.; Xia, M.; Gong, M.; Chen, Y.; Yang, H.; Tu, X.; Chen, H. Bamboo Wastes Catalytic Pyrolysis with N-Doped Biochar Catalyst for Phenols Products. Appl. Energy 2020, 260, 114242. [Google Scholar] [CrossRef]
- Wang, Y.; Akbarzadeh, A.; Chong, L.; Du, J.; Tahir, N.; Awasthi, M.K. Catalytic Pyrolysis of Lignocellulosic Biomass for Bio-Oil Production: A Review. Chemosphere 2022, 297, 134181. [Google Scholar] [CrossRef] [PubMed]
- Aho, A.; Kumar, N.; Eränen, K.; Salmi, T.; Hupa, M.; Murzin, D.Y. Catalytic Pyrolysis of Biomass in a Fluidized Bed Reactor: Influence of the Acidity of h-Beta Zeolite. Process Saf. Environ. Prot. 2007, 85, 473–480. [Google Scholar] [CrossRef]
- Mohamed, B.A.; Ellis, N.; Kim, C.S.; Bi, X. Microwave-Assisted Catalytic Biomass Pyrolysis: Effects of Catalyst Mixtures. Appl. Catal. B 2019, 253, 226–234. [Google Scholar] [CrossRef]
- Chai, M.; Liu, R.; He, Y. Effects of SiO2/Al2O3 Ratio and Fe Loading Rate of Fe-Modified ZSM-5 on Selection of Aromatics and Kinetics of Corn Stalk Catalytic Pyrolysis. Fuel Process. Technol. 2020, 206, 106458. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, Y.; Xu, Y. Review of Briquette Binders and Briquetting Mechanism. Renew. Sustain. Energy Rev. 2018, 82, 477–487. [Google Scholar] [CrossRef]
- Kataki, R.; Kataki, M.D. Weeds as a Renewable Bioresource: Prospects for Bioconversion to Biofuels and Biomaterials through a Cascade of Approaches. In Biofuels and Bioenergy; Gurunathan, B., Sahadevan, R., Zakaria, Z.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 437–461. ISBN 978-0-323-85269-2. [Google Scholar]
- Ahn, B.J.; Chang, H.S.; Lee, S.M.; Choi, D.H.; Cho, S.T.; Han, G.S.; Yang, I. Effect of Binders on the Durability of Wood Pellets Fabricated from Larix kaemferi C. and Liriodendron tulipifera L. Sawdust. Renew. Energy 2014, 62, 18–23. [Google Scholar] [CrossRef]
- Hu, Q.; Shao, J.; Yang, H.; Yao, D.; Wang, X.; Chen, H. Effects of Binders on the Properties of Bio-Char Pellets. Appl. Energy 2015, 157, 508–516. [Google Scholar] [CrossRef]
- Lu, D.; Tabil, L.G.; Wang, D.; Wang, G.; Emami, S. Experimental Trials to Make Wheat Straw Pellets with Wood Residue and Binders. Biomass Bioenergy 2014, 69, 287–296. [Google Scholar] [CrossRef]
- Obi, O.F.; Pecenka, R.; Clifford, M.J. A Review of Biomass Briquette Binders and Quality Parameters. Energies 2022, 15, 2426. [Google Scholar] [CrossRef]
- Wang, T.; Tang, L.; Feng, X.; Xu, J.; Ding, L.; Chen, X. Influence of Organic Binders on the Pyrolysis Performance of Rice Straw Pellets. J. Anal. Appl. Pyrolysis 2022, 161, 105366. [Google Scholar] [CrossRef]
- Kumar, J.A.; Kumar, K.V.; Petchimuthu, M.; Iyahraja, S.; Kumar, D.V. Comparative Analysis of Briquettes Obtained from Biomass and Charcoal. Mater. Today Proc. 2021, 45, 857–861. [Google Scholar] [CrossRef]
- Ugwu, K.E.; Agbo, K.; Ugwu, K. Evaluation of Binders in the Production of Briquettes from Empty Fruit Bunches of Elais Guinensis. Int. J. Renew. Sustain. Energy 2013, 2, 176–179. [Google Scholar] [CrossRef]
- Riva, L.; Nielsen, H.K.; Skreiberg, Ø.; Wang, L.; Bartocci, P.; Barbanera, M.; Bidini, G.; Fantozzi, F. Analysis of Optimal Temperature, Pressure and Binder Quantity for the Production of Biocarbon Pellet to Be Used as a Substitute for Coke. Appl. Energy 2019, 256, 113933. [Google Scholar] [CrossRef]
- Riva, L.; Cardarelli, A.; Andersen, G.J.; Buø, T.V.; Barbanera, M.; Bartocci, P.; Fantozzi, F.; Nielsen, H.K. On the Self-Heating Behavior of Upgraded Biochar Pellets Blended with Pyrolysis Oil: Effects of Process Parameters. Fuel 2020, 278, 118395. [Google Scholar] [CrossRef]
- Kang, K.; Zhu, M.; Sun, G.; Qiu, L.; Guo, X.; Meda, V.; Sun, R. Codensification of Eucommia Ulmoides Oliver Stem with Pyrolysis Oil and Char for Solid Biofuel: An Optimization and Characterization Study. Appl. Energy 2018, 223, 347–357. [Google Scholar] [CrossRef]
- Sarker, T.R.; Azargohar, R.; Dalai, A.K.; Meda, V. Characteristics of Torrefied Fuel Pellets Obtained from Co-Pelletization of Agriculture Residues with Pyrolysis Oil. Biomass Bioenergy 2021, 150, 106139. [Google Scholar] [CrossRef]
- Wakchaure, G.C.; Mani, I. Effect of Binders and Pressures on Physical Quality of Some Biomass Briquettes. J. Agric. Eng. 2009, 46, 24–30. [Google Scholar]
- Monedero, E.; Portero, H.; Lapuerta, M. Pellet Blends of Poplar and Pine Sawdust: Effects of Material Composition, Additive, Moisture Content and Compression Die on Pellet Quality. Fuel Process. Technol. 2015, 132, 15–23. [Google Scholar] [CrossRef]
- Arous, S.; Koubaa, A.; Bouafif, H.; Bouslimi, B.; Braghiroli, F.L.; Bradai, C. Effect of Pyrolysis Temperature and Wood Species on the Properties of Biochar Pellets. Energies 2021, 14, 6529. [Google Scholar] [CrossRef]
- Wang, Z.; Zhai, Y.; Wang, T.; Wang, B.; Peng, C.; Li, C. Pelletizing of Hydrochar Biofuels with Organic Binders. Fuel 2020, 280, 118659. [Google Scholar] [CrossRef]
- Zhang, T.; Qiu, L.; Wang, Y.; Zhang, C.; Kang, K. Comparison of Bio-Oil and Waste Cooking Oil as Binders during the Codensification of Biomass: Analysis of the Pellet Quality. Bioenergy Res. 2019, 12, 558–569. [Google Scholar] [CrossRef]
- Wang, T.; Meng, D.; Zhu, J.; Chen, X. Effects of Pelletizing Conditions on the Structure of Rice Straw-Pellet Pyrolysis Char. Fuel 2020, 264, 116909. [Google Scholar] [CrossRef]
- Lohmeier, L.; Thaler, C.; Harris, C.; Wollenberg, R.; Schröder, H.-W.; Braeuer, A.S. Use of Bentonite and Organic Binders in the Briquetting of Particulate Residues from the Midrex Process for Improving the Thermal Stability and Reducibility of the Briquettes. Steel Res. Int. 2021, 19, 2100210. [Google Scholar] [CrossRef]
- Russell, S.H.; Turrion-Gomez, J.L.; Meredith, W.; Langston, P.; Snape, C.E. Increased Charcoal Yield and Production of Lighter Oils from the Slow Pyrolysis of Biomass. J. Anal. Appl. Pyrolysis 2017, 124, 536–541. [Google Scholar] [CrossRef]
- Adeleke, A.A.; Odusote, J.K.; Ikubanni, P.P.; Olabisi, A.S.; Nzerem, P. Briquetting of Subbituminous Coal and Torrefied Biomass Using Bentonite as Inorganic Binder. Sci. Rep. 2022, 12, 8716. [Google Scholar] [CrossRef]
- Dou, G.; Goldfarb, J.L. In Situ Upgrading of Pyrolysis Biofuels by Bentonite Clay with Simultaneous Production of Heterogeneous Adsorbents for Water Treatment. Fuel 2017, 195, 273–283. [Google Scholar] [CrossRef]
- Emerhi, E.A. Physical and Combustion Properties of Briquettes Produced from Sawdust of Three Hardwood Species and Different Organic Binders. Adv. Appl. Sci. Res. 2011, 2, 236–246. [Google Scholar]
- Hernando, H.; Ochoa-Hernández, C.; Shamzhy, M.; Moreno, I.; Fermoso, J.; Pizarro, P.; Coronado, J.M.; Čejka, J.; Serrano, D.P. The Crucial Role of Clay Binders in the Performance of ZSM-5 Based Materials for Biomass Catalytic Pyrolysis. Catal. Sci. Technol. 2019, 9, 789–802. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, L.; Surgot Meulien, E.; Bi, X.T.; Lim, J.C.; Chen, W.-H. Waste Plastics as an Effective Binder for Biochar Pelletization. Energy Fuels 2021, 35, 13840–13846. [Google Scholar] [CrossRef]
- Olugbade, T.; Ojo, O.; Mohammed, T. Influence of Binders on Combustion Properties of Biomass Briquettes: A Recent Review. Bioenergy Res. 2019, 12, 241–259. [Google Scholar] [CrossRef]
- Adeleke, A.A.; Odusote, J.K.; Ikubanni, P.P.; Orhadahwe, T.A.; Lasode, O.A.; Ammasi, A.; Kumar, K. Ash Analyses of Bio-Coal Briquettes Produced Using Blended Binder. Sci. Rep. 2021, 11, 547. [Google Scholar] [CrossRef]

| Slow Pyrolysis | Fast Pyrolysis | Flash Pyrolysis | Reference | |
|---|---|---|---|---|
| Temperature (°C) | 300–700 | 400–800 | 800–1000 | [14] |
| Heating rate (°C/s) | 0.1–1 | 10–200 | >1000 | [14,30,31] |
| Vapor residence time | 5–30 min 10–100 min | <5 s | <0.5 s | [14,30,31,32] |
| Particle size (mm) | 5–50 | <1 | <0.2 | [33] |
| Products (%) | [14] | |||
| Biocarbon | 35 | 20 | 12 | |
| Pyrolysis oil | 30 | 50 | 75 | |
| Pyrolysis gas | 35 | 30 | 13 |
| Pyrolysis Type | Advantages | Challenges |
|---|---|---|
| Slow pyrolysis | Leads to a high yield of biocarbon | Energy consumption when the temperature is greater than 600 °C |
| Easy to scale up | ||
| Diverse range of possible feedstock materials | ||
| Fast pyrolysis | Leads to a high yield of pyrolysis gas | |
| Easy to scale up | ||
| Diverse range of possible feedstock materials | ||
| Flash pyrolysis | Leads to high yield of pyrolysis oil | |
| Easy to scale up | ||
| Diverse range of possible feedstock materials |
| Pyrolysis Technology | Advantages | Challenges | Reference |
|---|---|---|---|
| Microwave pyrolysis | Lower energy consumption than traditionally used technologies | Exact temperature of the reactor is hard to determinate. Scaling problems from laboratory to industrial scale. | [30,66,67] |
| Flexible heating rates | |||
| Process can be optimized to produce biocarbon, pyrolysis gas, or pyrolysis oil | |||
| No need to dry the biomass before pyrolysis | |||
| Solar pyrolysis | Less environmental pollution compared to conventional pyrolysis | Uniform heat flux throughout the reactor is difficult to achieve. Heat losses on the reactor surface, especially when the wind is strong. Generated heat flux depends highly on the time of day and the season. | [77,78,79,80] |
| More flexible heating rates and pyrolysis temperatures than in conventional pyrolysis | |||
| Plasma pyrolysis | High heating rate | High energy consumption and because of this decreased process efficiency. | [93,99] |
| Short residence time | |||
| Because of short residence time, fewer side reactions occur | |||
| Vacuum pyrolysis | Short residence time | High maintenance and investment costs. | [101,103] |
| Reduced energy consumption compared to traditionally used pyrolysis technologies |
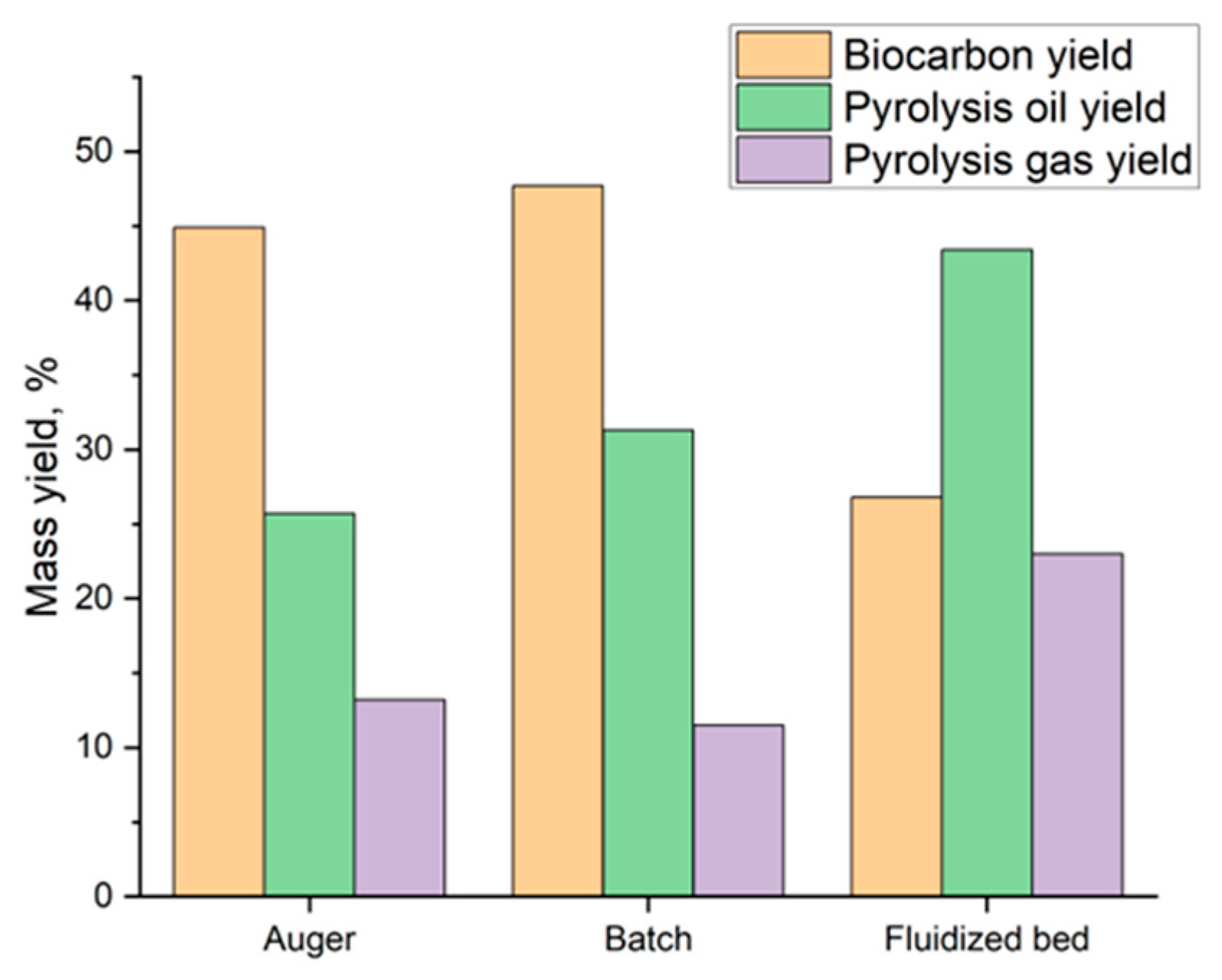
| Reactor Type | Pyrolysis Type | Feedstock Material | Biocarbon Yield (%) | Temperature (°C) | Reference |
|---|---|---|---|---|---|
| Batch | Fast | Rice straw | 47.7 | 500 | [122] |
| Auger | Slow | Rice straw | 44.9 | 500 | [122] |
| Rotating cone | Fast | Wood waste pellets | 29.1 | 450 | [123] |
| Fluidized bed | Fast | Rice straw | 26.8 | 500 | [122] |
| Ablative plate | Fast | Corncob | 24.5 | 500 | [115] |
| Cyclone/vortex | Fast | Pine | 13.9 | 500 | [121] |
| Organic Binders | Inorganic Binders | Composite Mixtures |
|---|---|---|
| Peach starch [171] Sodium carboxymethylcellulose [171] Cassava starch [172] Asphalt [173] Pyrolysis oil [174,175,176,177] Molasses [178] Distillers dry grain [178] Crude glycerol [169] Lignosulfonate [169,179] Lignin [168] Lignin powder [167] Pinecones [167] Pyrolytic Lignin [180] Protein, starch, lignin, and molasses [181] Waste cooking oil [182] Deionized water [183] | Bentonite [184,185,186,187] Ash [188] Limestone [165] Clay [165] Magnesium oxide [165] Calcium oxide [165] Calcium hydroxide [168] Sodium hydroxide [168] Attapulgite [189] Sodium silicate [165] Magnesium chloride [165] Waste Plastic [190] | Kaolin-bentonite-sodium humate [191] Corn straw-sodium hydroxide-MgCl/MgO [191] Coal tar pitch phenolic resins [191] Coal tar pitch and molasses [192] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pahnila, M.; Koskela, A.; Sulasalmi, P.; Fabritius, T. A Review of Pyrolysis Technologies and the Effect of Process Parameters on Biocarbon Properties. Energies 2023, 16, 6936. https://doi.org/10.3390/en16196936
Pahnila M, Koskela A, Sulasalmi P, Fabritius T. A Review of Pyrolysis Technologies and the Effect of Process Parameters on Biocarbon Properties. Energies. 2023; 16(19):6936. https://doi.org/10.3390/en16196936
Chicago/Turabian StylePahnila, Mika, Aki Koskela, Petri Sulasalmi, and Timo Fabritius. 2023. "A Review of Pyrolysis Technologies and the Effect of Process Parameters on Biocarbon Properties" Energies 16, no. 19: 6936. https://doi.org/10.3390/en16196936
APA StylePahnila, M., Koskela, A., Sulasalmi, P., & Fabritius, T. (2023). A Review of Pyrolysis Technologies and the Effect of Process Parameters on Biocarbon Properties. Energies, 16(19), 6936. https://doi.org/10.3390/en16196936






