Abstract
The main purpose of the article is to present a comprehensive and critical review of the challenges and risks associated with the use of green ammonia as an alternative fuel in land transport. The review is motivated by the clear trend toward phasing out fossil fuel vehicles and replacing them with emission-free alternatives. Topics covered include safety aspects such as safety of powering of vehicles, the production of green ammonia, the use of ammonia in the context of various fuel solutions (combustion engines and fuel cell engines), and the discussion of ammonia-powered vehicles in the context of air pollution. The paper offers new insights into identifying the challenges and obstacles that may arise in the case of the massive use of green ammonia as a fuel for land transport. In addition, the review presents the latest information on the technological readiness of the necessary infrastructure for the production, transport, storage, and utilization of green ammonia in internal combustion or electric engines.
1. Introduction
In recent years, the global trend to phase out fossil fuel vehicles can be seen in many countries and cities, as summarized in [1]. The most ambitious plans assume a ban on the registration of gas-powered cars by 2025 (Norway and South Korea), 2026 (Belgium), and 2027 (Austria). By September 2021, 28 countries had declared this action on the horizon of 2050 [2]. One of the most influential regulations was established in the European Union (EU). Road transport is reported to be responsible for 15% of EU CO emissions [3]; therefore, in June 2022, the EU Parliament agreed to a complete ban on the registration of vehicles with non-zero emissions starting 2035 and a reduction in new registrations by half by 2030 [3]. The aim of these regulations is to achieve the climate neutrality of road transport in terms of CO emissions by 2050 [4].
It should also be noted that similar regulations are being introduced in other parts of the world. China has announced that all new vehicles sold in China will have to run on alternative fuels by 2035 [5]. Half of them will have to be electric and half will have to fuel cells or plug-in hybrid. Moreover, California regulators passed legislation in 2022 banning the sale of new gas-powered cars by 2035 [6]. In addition, other international regulations, such as the GHG Strategy by the International Maritime Organization (IMO), which assumes a 40% reduction in average emissions by 2030 (compared to 2008), force the transition to zero- or low-emission fuels in maritime transport [7].
The withdrawal of gas-powered vehicles occurs in conjunction with the popularization and strong promotion of electromobility. This is well illustrated by the Chinese policy mentioned above that requires the registration of electric, fuel cell, and plug-in hybrid cars [5]. Another similar example is the Electric Vehicles Initiative (EVI) [8], which is an intergovernmental policy forum created in 2010 as part of the Clean Energy Ministerial (CEM) program. In 2017, EVI launched the EV30@30 campaign, which aims to achieve a 30% share of the electric vehicle market in total sales of all vehicles (except two-wheelers) by 2030. The campaign was endorsed by 11 countries and is supported by 29 companies and organizations [8].
In addition to the very strong trend towards electromobility, other alternative fuels are being considered and developed, such as hydrogen, ammonia [9], and biomethane [10]. Several other fuels are listed as helping reduce emissions on the road to zero-emission transport. These are liquefied petroleum gas (LPG), compressed natural gas (CNG), liquefied natural gas (LNG), and synthetic and paraffinic fuels (made from biomass or natural gas, as well as vegetable oils or animal fats) [9]. Some of these fuels are permanent gases (hydrogen, biomethane, and natural gas), which makes their storage challenging as it requires elevated pressure (in the case of hydrogen, 700 bar) and low temperature (approximately 110 K for natural gas and biomethane and 30 K for hydrogen). This will result in the high complexity of storage and fueling systems, which will be demanding in terms of safe exploitation. This can be a limiting factor in the massive application of these fuels.
This paper is focused on the application of ammonia, for which numerous aspects promote its application as fuel in onshore transportation and is summarized as follows.
- As a carbon-free emission fuel:
- –
- It can be used directly in the combustion process [11];
- –
- It is a igh-density hydrogen carrier (liquid ammonia contains 1.77 times as much as liquid hydrogen (by volume) [12], and hydrogen can be converted to ammonia [13])
- –
- As a non-fossil fuel, its production is not limited by natural deposits.
- As a good economic solution:
- –
- Availability: NH is one of the most produced and distributed chemicals in the world [14];
- –
- Technological feasibility: infrastructure already exists for the production, transport, and distribution [15];
- –
- Low cost of ammonia as fuel: it is comparable to the cost of diesel fuel on an energy basis [16].
- –
- Volume expander to current fuels: as a mixture, ammonia can be blended with, for example, gasoline [13];
- –
- Low storage costs: ammonia can be stored at room temperature (300 K) with relatively low pressure (more-less 10 bar) [16];
- –
- Cheap long-distance transportation: ammonia’s energy cost is far lower than for transporting electricity over long distances (>2000 km) [13];
However, at this point, it should be emphasized that the success of the green transformation depends not only on the setting of ambitious goals and regulations but also on the technological readiness of the solutions required for this. This factor concerns, in particular, the field of alternative fuels and supporting infrastructure (responsible for fuel production, storage, and logistics). This article aims to critically assess the state of technological readiness for the use of green ammonia as a zero-emission alternative fuel. The work presents a comprehensive review of the technological process chain schematically presented in Figure 1. It starts with green ammonia production technology and then the possibilities of its storage and logistics are analyzed, taking into account the accompanying processes such as regasification and charging. Subsequently, the possibility of using ammonia as a fuel in available solutions dedicated to road transport is discussed and analyzed.
Figure 1.
Illustration of the ammonia process chain.
The review also concerns the safety aspects of the discussed processes, as they are an integral element of each link in the above-mentioned process chain for green ammonia. In addition, the work highlights and identifies potential roadblocks and dangerous interference with other industries that use ammonia. Attention is also drawn to the most important challenges related to the use of green ammonia as an alternative emission-free fuel in road transport.
2. Current Status of Ammonia
Besides the important future role of ammonia in decarbonization, it is already the second most widely produced chemical with an annual production ranging from 150 [17] to 183 Mts [18] and a production capacity of 243 Mts [18]. Between 80% [17] and 85% [18] of ammonia is used in the fertilizer industry as a fixed nitrogen source, and it is estimated that from 50% [18] to 70% [17] of the global population is fed with the support of ammonia-based fertilizers. Other applications of ammonia are refrigeration [19] (ammonia is a common refrigerant in large industrial refrigeration systems) and feedstock for the production of explosives, textiles, and pharmaceuticals [20].
Although 90% is consumed on-site as feedstock, transport and storage infrastructure are also well developed [18]. Around 18–20 Mt of ammonia is transported by ship (with a fleet of 170 ammonia vessels, 40 in continuous operation), and 7–10 Mt by road, train, or via pipelines. The ammonia shipping infrastructure is also well developed with loading and unloading facilities. There are around 120 ammonia terminals around the world located in every industrialized region [18,21].
The above-mentioned review of the current status of ammonia shows that it is already a well-developed and high-maturity industry (in terms of production, storage, and transport), as ammonia has been playing a crucial industrial role for decades. However, the currently available infrastructure and production capacity can become far insufficient for the development of new applications of ammonia, especially those as demanding as road transport. It is estimated that due to the increasing interest in ammonia as a maritime fuel and in power generation, its demand may triple by 2050 compared to 2020 levels [17]. If the potential demand for onshore transportation is included this forecast may be exceeded. Moreover, increasing the application of ammonia can negatively interfere with the production of fertilizers and as a result with the prices and availability of food, which can have severe social and economic consequences.
3. Safety Aspects, Also Related to the Safe Powering of Vehicles
According to the Regulation of the European Parliament and of the Council No. 1272/2008 on the classification, labeling, and packaging of substances and mixtures, ammonia is registered in the EU list of existing commercial substances. Ammonia is registered under number 231-635-3 and is classified as a hazardous substance. This, in turn, imposes on all entities placing ammonia on the European market the obligation to prepare and make available a safety data sheet prepared in accordance with the Regulation of the EU Parliament and of the Council No. 1907/2006 on the registration, evaluation, authorization, and restriction of chemicals, commonly known as the REACH regulation. According to the sample ammonia safety data sheet [22], the following hazards have been identified:
- Physical threats
- Flammable gas;
- Gas under pressure, which may explode if heated.
- Health hazards
- Toxic if inhaled;
- Causes severe skin burns and eye damage;
- Corrosive to the respiratory tract.
- Environmental hazards
- Very toxic to aquatic life, with long-lasting effects.
3.1. Human Toxicity
Ammonia is a colorless, toxic gas with a strong pungent odor that can be felt at concentrations as low as 5–30 ppm. In contact with the skin, liquid ammonia can cause irritation and serious burns. At concentrations of 1700 ppm, health problems such as coughing and swelling of the laryngeal area can appear. Exposure to concentrations of 2500 to 4500 ppm for about 30 min can be fatal [23]. Recommendations from the US National Institute for Occupational Safety and Health (NIOSH) indicate that the concentration at which exposure longer than 15 min is potentially dangerous is 35 ppm, and the level immediately harmful to life or health is 300 ppm [24]. Because ammonia vapors dissolve well in water and also produce strong reflexes that immediately hold, effect of breathing ammonia vapors into the respiratory organs is usually limited to the upper respiratory tract. Very high concentrations of ammonia can enter the lower respiratory tract. The consequences can be very serious, such as lung damage, which is an indirect threat to life. In contact with the skin, ammonia vaporizes as a result of its very high affinity for water, and ammonia causes severe dehydration of tissues and, as a consequence of this reaction, ammonium hydroxide, which can then cause severe burns. Ammonia in liquid form also poses a serious risk of frostbite: at ambient pressure, ammonia’s saturation temperature is C. With the release of liquefied ammonia, because of the presence of water vapor in the air (e.g., in high humidity), vapors heavier than air are formed. These toxic fumes may spread at ground level or in poorly ventilated areas in low-lying areas, potentially exposing people to harmful (fatal) concentrations. Thus, if large amounts of ammonia are released, this may cause long-range ammonia clouds from the spill site. Table 1 summarizes the health effects for humans when exposed to ammonia depending on the concentration.

Table 1.
Effects of ammonia concentration in the air on human health [25].
3.2. Environmental Toxicity
Ammonia is a toxic substance that poses a threat to the natural environment. Its leakage may lead to contamination of the air, soil, and, in particular, the water environment. When analyzing the impact of ammonia on the environment, the most important factor is its reaction with water. Ammonia in contact with water, found in any environment, undergoes an equilibrium reaction in which a nontoxic ammonium ion and a hydroxide ion are formed [28]. The equilibrium depends on the reaction conditions, for example, the water’s temperature and pH. The reaction of dissolving ammonia in water is highly exothermic, generating 2000 kJ of heat per kilogram of ammonia dissolved in water. Dissolving 1 kg of ammonia releases enough energy to evaporate nearly 1.5 kg of water.
Ammonia in the air is a pollutant and a secondary precursor to particulate matter. Some ammonia forms ammonium, and some of it combines with other compounds in the atmosphere, such as nitric and sulfuric acids, to form ammonium salts, a form of fine particulate matter that is harmful to humans [29]. However, air polluted with ammonia is quickly cleaned by rainfall, which transfers pollutants to the soil or water reservoirs.
After an uncontrolled release of ammonia into the soil, some will evaporate immediately. Both the vapor and the part remaining in the soil will react with moisture from the air and the soil. The evaporated part of the ammonia, which forms ammonium, eventually returns to the soil in the rain. Ammonium binds rapidly to negatively charged soil organic matter and soil clays. Ammonium rarely accumulates in the soil as it is quickly taken up by plant roots, and the remainder is converted by bacteria to nitrates. Nitrates can also be absorbed by plant roots [30].
If ammonia is released into water, particularly small bodies of stagnant water, the dissolved ammonia poses a serious threat to the aquatic environment. As a result of exceeding the lethal concentration, aquatic organisms in the immediate vicinity of the spill are killed. Due to the exothermic reaction with water, ammonia will evaporate at a high rate and, entering the gas phase, will contaminate the air, significantly increasing the range of environmental impact. According to recommendations, the ammonia concentration should not exceed 3–6 mg/L for short-term exposure and 0.3–2 mg/L for long-term exposure [31]. It is also assumed that a concentration less than 0.02 mg/L is safe for fish reproduction [32].
The actual effects of ammonia leakage into the environment are difficult to predict due to several factors. First, the course of the water–ammonia reaction depends on the pH of the medium and its temperature. The higher the pH of the water and its temperature, the higher the concentration of ammonia in relation to the ammonium ion. In addition to the concentration of ammonia, its negative impact on living organisms depends on the species but mainly on the exposure time. There is no information in the literature on the lethal concentration of ammonia in water or air for humans, but there are a lot of data for farm animals and aquatic organisms. Toxicity to living organisms of chemical substances is defined using two indicators: lethal dose (LD) and lethal concentration (LC).
- LD50 is the amount of material administered at one time that causes the death of 50% (half) of a group of test animals. LD50 is one way to measure the short-term poisoning potential (acute toxicity) of a material.
- LC values usually refer to the concentration of a chemical in air, but in environmental tests, they can also mean the concentration of a chemical in water. According to the Organization for Economic Cooperation and Development (OECD) guidelines for the testing of chemicals, a traditional experiment involves groups of animals exposed to a concentration (or series of concentrations) for a specified period of time (usually 4 h). The animals are clinically observed for up to 14 days. The concentration of a chemical in the air that kills 50% of test animals during the observation period is the LC50 value. Other exposure times (as opposed to the traditional 4 h) may apply depending on specific regulations.
Table 2 shows the selected values of the LC and LD parameters available in the literature.

Table 2.
Values of LD and LC for selected animal species.
In Table 2, it can be seen that ammonia is particularly dangerous for the aquatic environment; the lethal concentration of LC ammonia for aquatic organisms is several orders of magnitude lower than for terrestrial animals.
Although ammonia can have a negative impact on the environment, the risk of using it as a fuel appears to be low. This is due to several factors. First, the large difference between the normal saturation temperature of ammonia and the ambient temperature causes a significant amount of ammonia to evaporate when spilled. Second, the ratio of the amount of ammonia used as fuel to the amount of surrounding water or soil is so small that even in the case of a complete fuel spill, the lethal concentration will only be exceeded in the immediate vicinity of the spill and should not exceed the limit values at a greater distance. The last argument is that ammonia, as a nitrogen carrier, is a nutrient, and over time, through natural processes called the nitrogen cycle, it will turn into nitrides, which are a fertilizer for plants [35].
3.3. Flammability
Due to the low flammability of ammonia compared to hydrocarbon fuels and other chemical substances, the hazards resulting from its flammability are definitely lower than, for example, from toxicity. In air, the limit of flammability of ammonia is 15% and the upper limit is 28%, and in oxygen, these values are 25% and 75%, respectively. The minimum ignition energy is 8 mJ, which is about 30 times lower than in the case of methane. The auto-ignition temperature is relatively high at 651 °C.
Due to the high heat of vaporization of 1371 kJ/kg ( for methane it is only 510 kJ/kg), liquid ammonia is not flammable. This is due to the fact that the heat obtained in the combustion process is usually not sufficient to evaporate enough liquid to sustain the combustion process. This situation changes significantly if the heat for evaporation is supplied from another source. For example, if a leak occurs, the large amounts of heat supplied from the ground or water will cause rapid evaporation to sustain the combustion process. The combustion of ammonia in air is problematic, and an additional source of ignition is usually required [36].
3.4. Explosiveness
Although ammonia is a slow-burning agent, under specific conditions, it can form an explosive mixture. The minimum ignition energy is 680 mJ (about 10,000 times more than hydrogen). An explosion can occur when a large amount of vapor is ignited in an enclosed or semi-enclosed space. Poorly ventilated rooms, in particular, are at risk of explosion.
3.5. Corrosivity
Ammonia, when mixed with a small amount of water, becomes highly corrosive to a range of materials, including zinc, copper and brass, but is not corrosive to iron or steel within ammonia storage temperatures. However, ammonia corrosion in special situations can occur even in materials resistant to the corrosive effects of ammonia, which is related to the mechanism of stress corrosion. This type of corrosion can occur when components are subjected to simultaneous stresses and a corrosive environment. Steel-made pressure tanks are particularly susceptible to this type of corrosion. Factors that have a direct impact on the occurrence of stress corrosion cracking of steel in an ammonia environment are
- The yield strength of the material;
- Residual stresses, e.g., after welding processes;
- Oxygen content;
- Water content
It is recommended that the components in contact with ammonia are made of steel with relatively low yield strength MPa. All elements in which residual stresses may occur, e.g., after welding, heat treatment, and plastic forming processes, are tempered to reduce stresses. Additionally, for safety reasons, air should be removed from the installation before starting, for example, by blowing with inert gas, and ammonia should be used in a hydrated form with a minimum water content of 0.2%.
3.6. Gas under Pressure
Ammonia is a refrigerant that can be stored in liquid and gaseous forms at ambient temperature under significant pressure. Pressure depends on temperature, and at 25 C, it is 10 bar. The storage of any medium under pressure is inherently associated with the risk of bursting the storage tank in the event of an uncontrolled increase in pressure caused by an increase in temperature. Therefore, the design pressure of liquid ammonia storage tanks always has values corresponding to the saturation pressure at much higher temperatures, e.g., 55 C, where the pressure is 23 bar. In particular, tanks are exposed to an uncontrolled increase in temperature during emergency situations such as fire, during which the external temperature of the tank can increase to several hundred C, which corresponds to a pressure increase of several hundred bars. In this case, the tank must be protected in a different way than a sufficiently high design pressure; for this purpose, safety valves are used. Each space, including the tank, in which liquid ammonia may be contained should be protected with a device that protects against excessive pressure increase.
3.7. Chilled Refrigerant
The second method for storing liquid ammonia is saturation storage at ambient pressure, for which the temperature of ammonia is −33 C. In addition to the risk of chemical burns described above, contact with the medium at this temperature is associated with a risk of severe frostbite. The same risk exists when in contact with pressurized ammonia; if for any reason the pressure drops, the liquid will cool down quickly. Therefore, any work on installations with saturated liquid ammonia should be carried out with special precautions and with the use of personal protective equipment, i.e., gloves for contact with cold agents, protective glasses, long-sleeved clothing, and trousers.
3.8. Summary
The presented physicochemical properties of ammonia and the associated hazards mean that its use as a fuel should be carried out while maintaining the appropriate safety measures. Unfortunately, in the case of land applications, there are currently no relevant regulations in this area.
It can be expected that the use of ammonia as an energy carrier for vehicles and land-based equipment will require legislation in the near future that will include
- Design rules;
- Specifications of the valve in relation to the required safety level;
- Specifications of the valve in relation to the performance of the installation;
- Requirements for all installation components to have certificates confirming compliance with the relevant regulations;
- Installation and maintenance requirements;
- Supervision requirements; and
- Requirements for periodic inspection.
The maritime transport segment is the only legally regulated transport sector with respect to the use of ammonia as fuel [37,38]. This is due to the response of the maritime industry to the GHG reduction target defined by the IMO [39]. In the Emission Control Areas (ECAs) introduced by the IMO, the emissions of SOx and NOx are severely limited. Ammonia, apart from, e.g., LNG, is one of the few fuels that can meet these criteria.
4. Production of Green Ammonia
As mentioned above, ammonia is one of the most produced chemicals, and therefore, the technologies for its production are mature and well developed in terms of production capacity. However, most ammonia is produced from natural gas (via steam methane reforming and Haber–Bosch process) and in the current production pathway cannot be considered green [40]. Therefore, the main challenge concerning the production of green ammonia is not related to production technologies but to the availability of green substrates for its production: green hydrogen and green nitrogen.
Figure 2 shows the map of ammonia production pathways. One can distinguish a path based on conventional sources, i.e., coal or biomass gasification and steam methane reforming of natural gas. This path is not green as it leads to additional greenhouse gas emissions. The second path is based on the use of electricity from emission-free sources or the use of biogas for reforming. It should be noted that both in the first and in the second case, the key element remains the Haber–Bosch process, which is the high-temperature and high-pressure synthesis of N and H. This process can be considered as green as long as the components of synthesis are achieved in a zero-emission manner. In practice, it requires the application of renewable energy sources such as wind, solar, hydro, and nuclear combined with hydrogen from electrolysis and nitrogen from the air separation unit [40] or biogas (as a one-to-one replacement for natural gas) in the steam methane reforming process [41,42].
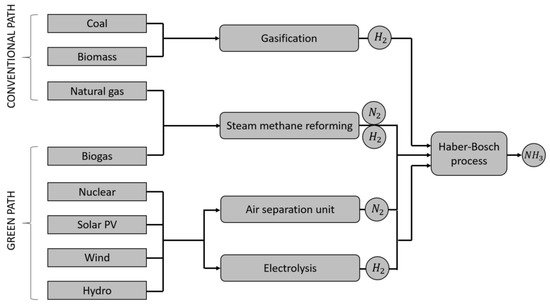
Figure 2.
Possible ammonia production pathways. The conventional path, resulting in additional net greenhouse gas emissions, and the emission-free green path.
Examples of green ammonia plants include Norway’s Rjukan from 1927 to 1988 and Glomfjord from 1949 to 1993, which used hydropower. The process was not competitive with ammonia produced from natural gas and therefore did not proceed. Additionally, in Pilbara in Australia, plans have been proposed to build electrolyzers, powered by solar energy, to produce a small fraction of the large plant’s capacity of 840,000 tons per year. The mix of plants based on fossil and renewable electricity has been called hybrid green ammonia [43].
Numerous researchers have analyzed the techno-economic assessment of the viability of green ammonia production plants, which is summarized in Table 3. Several renewable production pathways have been analyzed, including onshore and offshore wind [44,45,46,47,48,49], solar [47,48,49,50], and hydro [49,51]. The application of biogas is also suggested by [41,42], but is not yet supported by techno-economic analysis.
The reported production cost of ammonia ranges from 430 to 1248 EUR/t of NH. It is expected that it can be improved to 282 EUR/t of NH (from the initial 430 EUR/t of NH) [47] and to 410 EUR/t of NH (from the initial 630 EUR/t of NH) [50]. The authors [46] reported a breakdown of CAPEX of the 300 t/day green ammonia plant, where the electrolyzer corresponds to 65% of the total CAPEX. The report [40] compared energy cost efficiency and CAPEX for various feedstocks for ammonia production: natural gas, coal, and wind. Green ammonia produced from renewable sources is approximately 5 times more expensive in terms of energy cost and 3–4 times more expensive in terms of CAPEX than ammonia from natural gas. Green ammonia was also shown to cost approximately 400–850 USD/t of NH (current ammonia pricing varies from 200–700 USD/t of NH), which is similar to costs reported by IEA [52]: 450–700 USD/t of NH and IRENA [53]: 650–850 USD/t of NH.

Table 3.
Summary of studies focused on the techno-economic assessment of the viability of green ammonia production plants.
Table 3.
Summary of studies focused on the techno-economic assessment of the viability of green ammonia production plants.
| Reference [44] | Production Path Wind | LCOA 455–637 EUR | Remarks |
|---|---|---|---|
| [45] | wind +/ solar | 842 and 759 EUR | for off-grid and on-grid solutions analyzed for multiple locations |
| [46] | offshore wind | 1114 EUR | |
| [51] | hydro | 1248 EUR | |
| [47] | wind, solar | 430 EUR | 534 locations in 70 countries studied future improvement to 282 EUR possible island operation |
| [48] | solar, wind | 664 EUR | |
| [49] | solar, wind, hydro | 431–528 EUR | |
| [50] | solar | 653 EUR | future price 410 EUR |
In the report [40], a very important issue is highlighted on the scale of green ammonia production. It was prepared with reference to the marine sector. It indicates that in order to produce 650 million tons of ammonia (the demand of the marine sector), 6.5 PWh of renewable electricity produced from solar, wind, and hydro is required, which corresponds to the current electricity generation in China. CAPEX of the electricity generation infrastructure is estimated at 3.2 trillion USD, with an additional 1.3 trillion USD for ammonia plants. According to [54], the fuel consumption of road transport is approximately two times greater than for the marine sector, which corresponds to investments in renewable energy and ammonia plant infrastructure at the level of 10 trillion USD (in comparison, the US budget for 2022 was1.6 trillion USD [55]). Very high investment costs can be a roadblock for the application of ammonia as a fuel on a large scale.
Another important aspect is interference with food production, as it is also dependent on ammonia. As mentioned above, 80% [17] to 85% [18] ammonia is utilized in the fertilizer industry as a fixed nitrogen source, and it is estimated that 50% [18] to 70% [17] of the global population is fed with the support of ammonia-based fertilizers. This can lead to a shortage of ammonia for food production, an increase in food prices, or local food shortages. A similar scenario has already taken place in the past, when a large proportion of agricultural producers switched to the production of biofuels, which caused very large price increases and consequently famine in the poorest countries [56,57].
The facts presented above form a very strong argument against any other large-scale application of ammonia, including its application as a fuel. However, the food market will suffer from green transformation anyway because current emissive ammonia sources will have to be replaced by green ones, which, as shown above, require significant capital investments and will result in higher ammonia production costs.
Summary
The most significant advantage of ammonia is that it is a mature chemical in terms of production. However, currently produced ammonia cannot be considered green. There are several green ammonia production pathways based on a variety of renewable sources, including biogas, wind, solar, hydro, and nuclear. The available techno-economic analyses show that the prices of green ammonia will be at least double those of conventional ammonia. Moreover, it is indicated that the application of green ammonia in transport will force significant development of renewable sources and ammonia plants, which require capital investments on the order of tens of trillions of USD. Last, expanding the application of ammonia to new areas can be very influential for other ammonia-based sectors, especially the agriculture and food sector. This is, in the authors’ opinion, the greatest threat related to the application of green ammonia as a fuel in onshore transportation. This interference is actually challenging and dangerous, even in the case of replacing conventional natural-gas-based ammonia with green ammonia, regardless of the application.
5. Storage (Refrigeration and Pressure) and Regasification of Ammonia
The technology for the storage of ammonia and transport infrastructure for ammonia are at a high level of maturity due to the large-scale use of this substance in the production of inorganic fertilizers. Ammonia has an established infrastructure for trade and transportation around the world, with a network of ports equipped to handle large quantities of ammonia [58]. This existing infrastructure provides a solid foundation for the rapid adoption of ammonia as a viable energy carrier. A vital system of ports, pipelines, and storage facilities is in place to facilitate the trade of ammonia. The United States has more than 10,000 ammonia storage sites, which are linked through a pipeline network spanning more than 3000 km and connecting the Gulf of Mexico to the Midwest [59]. The Tolyatti–Odessa pipeline, which spans an impressive 2471 km, is currently the longest ammonia pipeline in the world and runs from Russia to Ukraine [59]; however, the transfer was stopped in February 2022 due to the military conflict between the two countries.
The storage of ammonia is carried out under increased pressure or reduced temperature, as described in Section 3. The substance is typically stored in the form of a liquid [60]. Figure 3 shows the phase equilibrium diagram of ammonia. The triple point [61] of this substance occurs for an absolute pressure of 6.060 kPa and a temperature of 195.4 K. The normal boiling point of ammonia (saturation temperature at 1 bar) is about −33.4 C. The critical parameters of ammonia are 113.57 bar and 132.41 C, respectively [61]. This means that ammonia can be stored as a liquid under subcritical conditions at saturation. It is possible to periodically store ammonia as a subcooled liquid, resulting, for example, from the mode of the current operation, but conditions in the tank will tend toward the saturated state. The storage of liquid ammonia at a reduced temperature is advantageous because of the significant change in the density of the liquid depending on the saturation temperature. For a temperature of −33 C, the density is 682 kg/m, while at a saturation temperature of 53 C, the density is 555 kg/m. To store liquefied ammonia under atmospheric pressure, it is necessary to cool the medium to saturation temperature (point 1 bar, −33 C) (Figure 4). The capacity of an atmospheric tank can reach 50,000 t NH [60]. Atmospheric storage characterizes steel consumption at a rate 4–10 times lower than other types of tanks [62]. However, the use of atmospheric storage requires the continuous withdrawal of ammonia vapors as a result of heat input caused by low saturation temperature. These tanks are used mainly in industry. Both atmospheric and semi-refrigerated tanks require the application of a refrigeration compressor [62], which is used to recompress the boil-off as well as to cool the incoming ammonia [60].
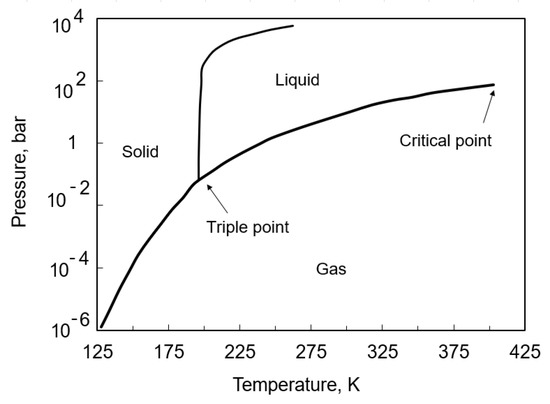
Figure 3.
Phase diagram of ammonia [63].
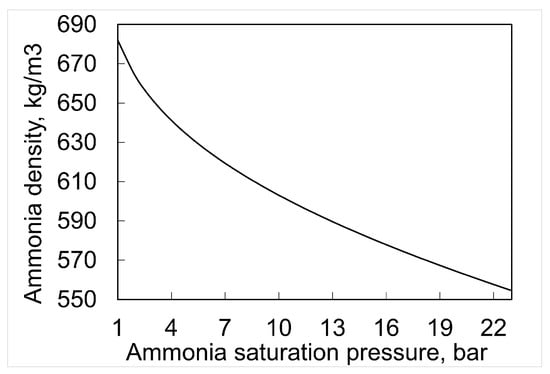
Figure 4.
Density of ammonia as a function of saturation temperature and pressure [64].
The absorption process can also be applied to the accumulation of ammonia. This method may be advantageous for safety reasons, since ammonia desorption occurs under heating or pressure drop. Metal halides are proposed for the absorption material [65,66,67,68,69]. This kind of ammonia storage is found in TRL 3–4 [69].
In onshore transport, pressurized storage conditions are the most common [60]. Gaseous ammonia is compressed to a saturation pressure corresponding to the ambient temperature. This allows the ammonia vapor to condense under high pressure in the heat exchanger and deliver the liquid to the tank. This method is also applied for other fuels such as CNG or compressed hydrogen (H compressed gas) (Figure 5); however, the pressure required for ammonia is much lower.
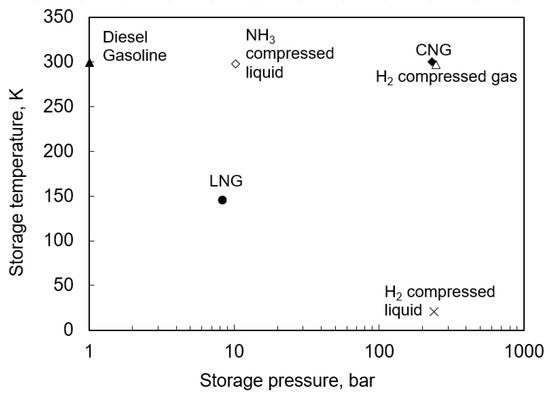
Figure 5.
Storage parameters of different possible substances to apply as a fuel in onshore transport [70,71,72,73].
Ammonia has a density and normal boiling point comparable to that of LPG. As a result, some process solutions, such as storage tanks or fueling systems, can be simply adapted to ammonia but with additional safety systems included. Furthermore, as a result of the corrosive properties of ammonia, appropriate materials should also be considered in their production. An example of such a storage system was proposed in [74] and illustrates an example of an ammonia tank system, which can be installed in onshore transport such as a car.
Preventing ammonia leakage requires a combination of predictive modeling, advanced sensing technologies, and efficient detection methods. A hydraulic model was developed to predict ammonia outflow from damaged pipelines [75]. In addition to predictive modeling, the development of advanced sensing technologies can also play a crucial role in preventing ammonia leakage. In [76], the development of a reentrant thorny ZnO/graphene hybrid nanowall structure that can detect NH gas while repelling liquid contamination was presented. This sensor exhibits excellent sensitivity, selectivity, repeatability, and stability, even after bacterial contamination, making it a promising tool for monitoring ammonia levels in various environments.
Ammonia needs to be converted from a liquid to a gas for use as a fuel. This is typically performed using a heated vaporizer with a pressure regulator [12,14,77] or high-pressure common rail injection [78]. Spark-ignition engines are well suited for use with ammonia due to their high compression ratios. Adding a small amount of H or using a catalyst can speed up the combustion process, but modifications are needed to prevent backfiring [71].
Summary
Ammonia storage tanks (refrigerated, semi-refrigerated, and non-refrigerated) are in TRL 9 [62], which means that this technology is well developed. However, these solutions mainly concern the refrigeration and fertilizer industries. To use ammonia for energy production in onshore transport, it is necessary to adapt and modernize the existing infrastructure. As a result of the similarity of the thermodynamic parameters of ammonia and LPG, it is possible to use LPG facilities for ammonia storage. However, they should be equipped with additional safety systems.
6. Ammonia Fueling Solutions
The use of ammonia as a fuel source has been known for years. During World War II, ammonia was used as a fuel in vehicles when diesel fuel became limited due to the ongoing war. In 1942, Belgium’s transport department was informed that diesel fuel would not be available, which led to a shortage of fuel for buses. A common solution at the time was to mix coal gas with ammonia because coal gas acted as a combustion promoter due to ammonia’s difficulty burning. However, these buses were decommissioned as soon as fossil fuels became available again [79].
The idea of using ammonia as fuel also appeared in the US military, which realized that 65% of the total tonnage required for combat and transportation operations during the war consisted of fuels and lubricants. As a result, they researched alternative fuels and proposed using mobile nuclear reactors to locally produce ammonia for their vehicles. This would reduce their transportation costs and increase their independence from third parties. Although these mobile reactors were supposed to be used in the 1980s, they were never realized [79].
Today, the main concerns are more focused on carbon-free emission fuels, and for that reason, ammonia is again becoming an interesting area for development [79]. Ammonia is widely recognized as the most viable option to reduce GHG emissions in large-scale internal combustion engines and power generation, which is listed in the related literature [80]. This is because the low energy density of current battery technologies makes electric propulsion impractical in these sectors [14].
As an emission-free fuel, ammonia has a lower volumetric and mass density than most commonly used hydrocarbons in the fuel industry (Figure 6). However, the difference is within one order of magnitude. Its gravimetric energy density is also lower than that of hydrogen, but it has lower storage and safety costs [14]. These characteristics increase its competitiveness against hydrocarbons and H. Ammonia is the primary nitrogen fuel currently being studied in automotive powertrains [79]. Most research focuses on ammonia alone or dual fuel solutions for both types of engines: spark ignition engines (SI) and compression ignition engines (CI) (see Figure 7). Because ammonia is also considered a good source of hydrogen carriers, it was also researched for use in fuel cells (ammonia fuel cells and hydrazine fuel cells). In the following section, the focus is on NH-fueled combustion engines and their optimizations.
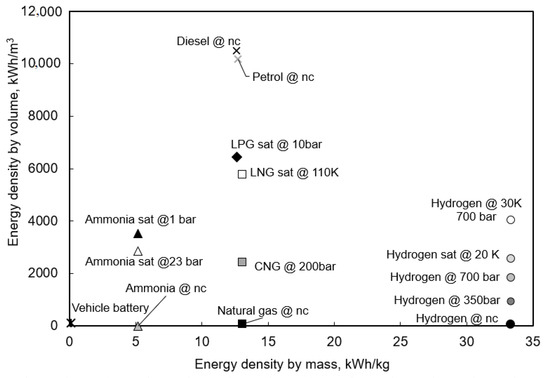
Figure 6.
Comparison of ammonia energy densities to other fuels [64].
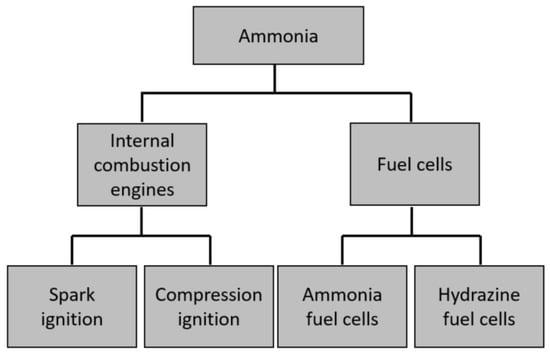
Figure 7.
Different technologies paths to be used for vehicular propulsion using nitrogen-based fuels.
6.1. Hydrogen-Based Fuels in Transport Industry
Currently, alternative fuels such as ammonia, methanol, and hydrogen face marginal use in the transportation industry. In onshore transportation, the use of ammonia is completely unheard of, and the most popular solution is hydrogen, which is mainly used in fuel cell systems. Currently, hydrogen-powered cars or trains with internal combustion engines are not available on the market. However, such attempts have been made in the past, but none of the solutions have taken off on a large scale, and they are not available on the market. Fuel cells are more popular in car transportation. PEMFC has already been applied, and it is the only fuel cell technology used on an industrial scale in that sector. However, in 2019, only three public cars powered by hydrogen-fed PEMFC were available on the market: (i) Hyundai ix35 FCEV (100 kW), (ii) Toyota Mirai (113 kW), and (iii) Honda Clarity (100 kW) [81].
Buses have slightly higher propulsion power requirements than cars. In this sector, the power demand is up to 300 kW, and the use of hydrogen and fuel cells is more common there than in public cars. The reason is more support programs, for example, JIVE I, JIVE II [82], that affect the popularization of hydrogen-powered urban transportation. This stimulates the industry to introduce new solutions. The last onroad area is heavy-duty transport. The power demand in that sector varies from 30 kW to 500 kW. This large power range is related to the fact that some of the supporting systems can be powered by a separate energy source, which makes it possible to reduce CO without making global technological changes in the truck. In 2023, in connection with the H2Haul EU co-financed project, 16 new heavy-duty hydrogen PEMFC trucks manufactured by IVECO and VDL should appear on EU roads [83].
When discussing onshore transportation, the railway sector should not be omitted. Non-electrified sections of railway lines are a significant problem across Europe. In 2013, a map of nine main EU rail transport lines was presented, almost 50% of which are used by trains powered by diesel engines [84]. The power of diesel engines used in locomotives ranges from 1to 3 MW [85,86]. Reducing fossil fuel consumption requires technological changes in this part of the transportation sector as well. The capacity needed to power locomotives is up to several times higher than that required for road transport. In 2018, the first German hydrogen-powered passenger train entered service. The total power rating of the two fuel cell installations is more than 600 kW. The solution is based on compressed hydrogen, and, as shown above, it has a lower volumetric power density than ammonia.
The power requirements for land transportation are in the range of 30–500 kW for road solutions and up to several MW for rail solutions (Figure 8). Currently, this market is occupied by internal combustion engines fueled by fossil fuels, but the latest trend (in addition to unannounced battery solutions) points to the slow deployment of hydrogen-powered PEM fuel cell systems. However, as outlined in earlier chapters, ammonia does not appear to be a worse fuel than hydrogen in terms of power density. On the other hand, the use of ammonia faces technological problems from the perspective of implementation, whether in fuel cells or in internal combustion engines. The following subsections discuss the potential advantages and disadvantages of the selected ammonia-fueled energy conversion technologies.
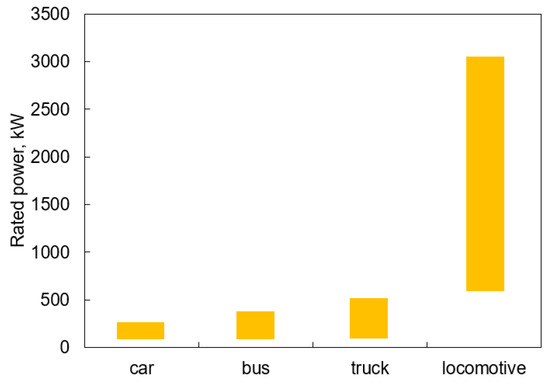
Figure 8.
Power demand for selected types of ground transportation from the perspective of fuel cell deployment based on [81,82,83,84,85,86].
6.2. Internal Combustion Engines (ICE)—Issues Related with Ammonia as Fuel Source
The use of ammonia as a fuel in combustion systems faces several practical challenges. These challenges arise due to NH’s poor ignition quality, high autoignition temperature, and low flame speed. NH has a Research Octane Number (RON) greater than 130, which is considered poor and a high autoignition temperature of 924 K. Its flame speed is also significantly lower than that of conventional fuels and alcohols, about one order of magnitude, and three times lower than that of H. Furthermore, NH has narrow flammability limits in air and a very high Minimum Ignition Energy (MIE) of 680 mJ compared to other fuels (Table 4). Furthermore, NH has a high latent heat of vaporization of 1370 kJ/kg, which is much higher than that of ethanol, LH, and gasoline. This high latent heat of vaporization can lead to a drastic decrease in combustion temperature when NH is injected in engines, resulting in incomplete combustion and loss of engine efficiency [14].

Table 4.
Combustion properties of ammonia and other fuels [14].
Under normal conditions, the combustion of ammonia typically leads to the formation of higher levels of nitrogen oxides compared to other fuels. These nitrogen oxides consist mainly of nitric oxide (NO) and nitrogen dioxide (NO). These pollutants are both chemically and radioactively active, contributing to the greenhouse gas effect and playing a significant role in the formation of acid rain [87]. The issue of NO emissions is described in detail in the next section.
To achieve stable combustion in the chamber, it is necessary that ammonia be present in vapor form. This requires the design and implementation of onboard systems capable of vaporizing and cracking ammonia to increase the flame speed and combustion ratio [88]. Such systems may include various components, such as vaporizers, catalysts, and reactors, that work together to ensure efficient and effective combustion of ammonia in the desired application. As described in Section 3, ammonia can also react with materials and lead to corrosion. The proper design and operation of these systems are critical to ensuring safe and reliable performance [13].
6.3. Spark Ignition Engines
Internal combustion engines (ICEs) can be divided into spark ignition engines (SIs) and compression ignition engines (CI). Both engine types were tested with ammonia as a fuel. One of the first research papers dedicated to SI was Cornelius et al. [89], who examined the combustion of neat gaseous NH in a SI engine. Liquid ammonia was vaporized and then injected into the intake manifold to mix with the intake air. The start of ignition (SOI) of the NH engine had to be advanced by a crank angle (CA) of approximately 100° before the top dead center (bTDC) to achieve stable engine operation. However, even with advanced SOI, the indicated thermal efficiency (ITE) of the ammonia engine was approximately 12% lower than that of neat gasoline engines running at 2400 RPM. Furthermore, the NH-fueled engine was only capable of operating up to 2400 RPM due to the low reactivity of NH.
These findings suggest that further research and development are necessary to improve the performance and efficiency of NH-fueled engines, which are listed and described in Table 5.

Table 5.
Spark Ignition engines optimization summary [11].
Recent research has demonstrated that a mixture of ammonia and hydrogen can power an SI engine. In this mixture, ammonia serves as fuel, while hydrogen acts as a combustion promoter generated by an on-board catalytic reactor [93]. The heat produced by the engine exhaust gases maintains the hydrogen at a working temperature, preventing the cracking of ammonia. This process is significantly more beneficial than pure ammonia injection, but it leads to higher combustion temperatures and correspondingly higher NO emissions [13].
6.4. Compression Ignition Engines
The CI engine is a type of piston machine that is commonly used for power generation, and it has a higher compression ratio (CR) (14–25) and thermal efficiency (eta: 45–55%) than SI engines (CR: 8–12, : 28–42%). The annual installed capacity for CI engines is approximately ten times higher than that of SI engines. Unlike the SI engine, combustion in the CI engine occurs in four distinct stages: ignition delay, premixed burning, mixing-controlled combustion, and afterburning. The presence of NH influences the combustion process at each stage and the subsequent reaction [11].
In recent years, many developments have been implemented in CI engines and new solutions have been designed for better engine performance; a summarized list of different solutions and their corresponding advantages and disadvantages is presented in Table 6.

Table 6.
Compression Ignition Engine optimization summary [11].
Using a retarded fuel injection timing for aTDC is not an effective way to improve NH combustion in CI engines. While this method can significantly reduce NO emissions, it also causes a significant increase in unburned NH. Aqueous ammonia is a better option to improve engine performance and emissions. However, it is important to note that the use of aqueous ammonia is likely to increase engine noise because of the higher heat release in the premixing burning stage. When NH is blended with fossil diesel, it often leads to an improved engine heat release rate (HRR) but higher NO emissions. In general, combustion and emissions of NH can be improved by optimizing the mass flow and timing for the pilot and main injection, resulting in a simultaneous reduction in N-based emissions and an increase in engine HRR [11].
6.5. Summary
To aid the decarbonization of the energy sector and encourage the widespread use of hydrogen, the use of ammonia directly as fuel, partially decomposed or mixed with other fuels, can have numerous advantages. Ammonia is an attractive, sustainable fuel and energy carrier due to its high volumetric energy density (in comparison to hydrogen) and well-established production and transportation infrastructure. However, the use of ammonia in combustion presents several significant challenges, such as low flame speeds, narrow flammability limits, and a tendency to form NO.
There are no mass-produced vehicles that use ammonia as a fuel source today, but there are already some working solutions using this carbon-free fuel. However, the concern of MAN, which is also the main manufacturer of trucks in Europe, works on improving combustion engines to burn ammonia [110]. The research and development of the technology are being performed with a view of implementation in the maritime industry, but it involves internal combustion engines, which raises the potential for implementation in other transportation sectors as well.
There are some working automobile solutions with ammonia as a fuel source. At a news conference held on 27 April 2015 at Pinehurst Farm in Blairstown, Iowa, the first tractor operating on renewable fuels was unveiled. This tractor has the ability to run on hydrogen gas or ammonia, which is mixed with a small amount of hydrogen [111].
Another ammonia-powered car was the Marangoni GT 86-R Eco Explorer presented at the Geneva Motor Show in 2013. The Eco Explorer was a modification that enhances the appearance and performance of the Toyota sports coupe while also bolstering its eco-friendly features. Marangoni achieved this by installing an LPG tank below the boot, allowing GT 86 to utilize compressed ammonia as fuel, resulting in a range of 111 miles without generating any CO as ammonia does not contain carbon. Additionally, the vehicle can be switched back to gasoline power for an exhilarating driving experience [112].
Looking at TRL makes it evident that, during WW2, ammonia-powered buses were on the TRL = 9, as proven in operation in the environment. Still, it was only temporary until the return to fossil fuels was more efficient and less hazardous. Today, this technology has been demonstrated in single prototypes and can be considered at TRL = 7.
6.6. Fuel Cells—Current State of Development
During the past two decades, the popularity of fuel cells in the industry has been steadily increasing [113]. The most popular types of fuel cells are proton exchange membranes, alkaline, phosphoric acid, direct methanol, molten carbonite, and solid oxide, which ae abbreviated as PEMFC, AFC, PAFC, DMFC, MCFC, and SOFC, respectively. Each of the technologies is characterized by a different purpose and method of operation, and their strengths and weaknesses are widely described in the literature [114].
In 2021, the total capacity of fuel cell systems was estimated to be 2330.4 MW [115]. As shown in a report from 2019 [113], a number of large-scale (>200 kW) stationary fuel cell units has grown continuously. Between 2007 and 2017, a 10-fold increase (from around 80 MW to almost 800 MW) in installed capacity was recorded. During this period, an increase in power was observed in stationary installations, mainly for MCFC, PAFC, and SOFC [113]. The highest increase was recorded for SOFC. Fuel cell technologies are the most popular in the USA and Korea [113]. Only in 2019 were two stationary SOFC system projects with power 80 and 100 MW assigned in Korea [116]. However, when fuel cells are discussed, one cannot forget the huge market share occupied by PEMFCs. This technology is used more often in smaller units, portable applications, or in transportation [114,117]. In 2022, according to Polaris Market Research, the global fuel cell vehicle market was estimated at 1.51 billion USD and was occupied mainly by PEMFCs. It has been predicted to increase to 62.88 billion USD by 2032 [118]. The possible use of fuel cells is being considered in almost all modes of transportation, considering trains, ships, and even airplanes [21,114,117,119]. The use of fuel cells is in line with the growing popularity of using hydrogen and other alternative fuels such as ammonia.
6.7. Selected Methods of Supplying Fuel Cells with Ammonia
As mentioned above, the energy source in NH is hydrogen. During combustion, it is accompanied by high temperatures that allow ammonia to decompose and oxidize. Because of the mentioned problems related to poor ignition quality, high auto-ignition temperature, and low flame speed, fuel cells are an interesting technology to consider for converting ammonia to electricity since they do not involve typical combustion but electrochemical reactions. In the case of fuel cells, NH must be decomposed into molecular hydrogen, which is then involved in electrochemical processes. The decomposition of ammonia (cracking), depending on the catalyst, occurs at temperatures ranging from 350 C to 800 C [120]. For this reason, unlike SOFCs, low-temperature fuel cells (e.g., PEMFCs, AFCs) require an external reactor to decompose ammonia to (H and N) and a cooler to deliver them to cells at operating temperature [121].
The use of NH in the selected fuel cell technology requires various auxiliary components in the fuel processing system. Figure 9 shows possible configurations of selected ammonia-fueled fuel cells. Ammonia decomposition can be carried out in a reactor as an endothermic cracking process or as an autothermal process with partial hydrogen combustion. In the second case, nitrogen and sulfur oxides or CO can be supplied with the air to the reactor. If the gases are not purified, they can poison the fuel cells. Furthermore, in the autothermic decomposition process, that is, partial combustion, there is a chance of the appearance of nitrogen oxides, which can adversely affect the operation of PEM-type cells [122]. CO can be dangerous for AFC, and sulfur can poison SOFC [123]. For this reason, additional filters are required in installations.
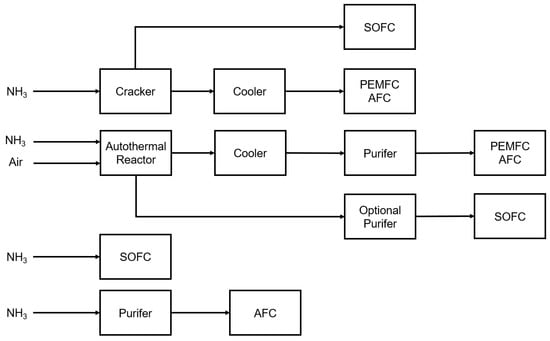
Figure 9.
Suggested possible configurations of selected ammonia-fed fuel cells based on collected information on limitations in selected technologies [120,121,122,123,124,125,126,127,128,129].
The main advantage of SOFC cells is that the high nickel content of the anode substrates means they do not require a cracker [124,125,126,127,128]. Likewise with the AFC, however, if the performance of SOFCs fueled by ammonia is very similar to that of a hydrogen-fueled one, then the AFC performance is rather low [129]. On the other hand, ammonia poisons PEM cells [130]. On this basis, it can be concluded that in order to use NH in AFC or PEMFC systems, additional components are required to enable proper fuel processing. In addition, the cells themselves do not require technological changes, which allows us to conclude that the PEMFC technology implemented already in transportation is ready for the use of NH, while the entire system needs to be redesigned, and this can block ammonia implementations in these solutions. On the other hand, taking into account the number of additional elements which must be used in such a system, as well as the NH cracking and operating temperatures of the fuel cells, it seems optimal to use SOFC technology powered indirectly or directly by ammonia. According to the IEA, ammonia-fed SOFC electric vehicles are at a 4–5 TRL level [131], but no TRL information was found for ammonia-fed PEMFC or AFC systems.
6.8. Ammonia-Fed Solid Oxide Fuel Cells
Currently, there are few companies on the market that produce SOFCs or energy systems based on them. Most of the proposed SOFCs are on a small scale of 1–10 kW, that is, in a range that does not meet the current requirements in the transport sector. Based on the sources found, only five companies’ SOFC systems offer solutions below and above 100 kW [132,133,134,135]. A market review paper appeared in the NAUTILIUS project, which collected data on the power density of fuel cell stacks, as well as systems offered on the market. A comparison of SOFC and PEM technologies and a comparison with a typical 500 kW diesel engine is shown in Table 7. The summary notes that PEMFC and SOFC systems are competitive with diesel engines in terms of power density. It is worth noting, however, that the NAUTILUS comparison encountered limited data on the gravimetric energy density of both PEMFC and SOFC fuel cell stacks. This can be seen, among other things, by comparing the energy density of PEMFC systems versus stacks. The maximum energy density of PEMFC stacks from sources found by NAUTILUS is lower than the maximum energy density of the systems. This is related to the limited information provided by manufacturers. However, the comparison shows what power densities can be expected from fuel cell systems, which are likely to become more common on the market. The maximum achieved electrical efficiencies of both cell-based systems exceed 50% for SOFC stacks reaching up to 65%. However, the performance of hydrogen-powered stacks is compared with that of an example 500kW diesel engine. Due to the absence of fuel cell systems as well as ammonia-fueled engines on the market, this comparison only indicates that cell technology has a high potential to replace combustion technologies [115].
Referring to previously gathered information about the construction of NH-powered and fuel-cell-based systems, installing additional equipment including an NH reactor will lower their power density. Therefore, due to the fact that a standard method of ammonia decomposition occurs in the presence of a nickel catalyst at >550 C, SOFC technology is more convenient for discussion than PEMFC [136].

Table 7.
Comparison of SOFC and PEMFC technologies fed by hydrogen with an example 500 kW Diesel engine [115,137].
Table 7.
Comparison of SOFC and PEMFC technologies fed by hydrogen with an example 500 kW Diesel engine [115,137].
| Technology | Volumetric Power Density, kW/m | Gravimetric Power Density, kW/t | Efficiency, % |
|---|---|---|---|
| SOFC stack | 200–400 | <100 | - |
| SOFC system | 2–100 | <70 | 35–65 |
| PEMFC stack | 50–200 | 100–600 | - |
| PEMFC system | 4–500 | 10–500 | 40–60 |
| Diesel engine (500 kW) | 235 | 330 | - |
Studies of ammonia-fed SOFC stacks are constantly appearing in the literature. In 2015, Cinti and Desideri [138] presented an experimental analysis with a 0-dimensional model (including heat exchangers, an SOFC stack, and an afterburner) to determine the efficiency of a system based on an SOFC cell stack fueled by urea, which decomposes into ammonia, among other things, during the decomposition process. The total efficiency of the modeled system, depending on the fuel utilization factor, reached 65% at 800 °C. Okanishi et al. [139] conducted a comprehensive study on a cell stack (NiO-YSZ anode materials, ZrO-based electrolyte) at 770 C. In the study, the analysis was carried out for three methods of fuel processing—direct stack supply (DIC), ammonia cracker, and auto-thermal cracker, in which ammonia is decomposed to hydrogen in the presence of trace oxygen, which leads to the partial conversion of hydrogen to steam, allowing it to maintain constant temperature in the reactor. Due to the steam supply to the stack, the performance with the autothermal cracker was lowest, especially for low currents. However, at high loads, they showed that power densities were comparable to the case with an internal cracker. The stack showed the highest performance for hydrogen. In a 1000 h test, the operation of the ammonia-fueled stack was stable and did not show more degradation over time than hydrogen [139]. The researchers then conducted analyses on a 30-cell 1 kW class stack; both tests did not show negative effects of ammonia on the cells, and the researchers did not observe NH emissions [125]. In 2018, results were published [140] on a short stack of SOLIDpower S.p.A. in cells with a Ni-YSZ/YSZ/GDC/LSCF-GDC configuration. The stack was fed with ammonia distributed in a cracker to N and H. Experiments were conducted at 700 C, 750 C, and 800 C. A power density of 375 mW/cm was achieved, and the energy conversion efficiency of the cell based on the numerical model was 67%. One of the most recent studies published by Quach et al. [141] was also performed on a mixture of N- and H-simulated ammonia. The experiments were performed on a 2.5 kW class stack. This shows interest in the use of ammonia in SOFC stacks. This coincides with an increase in the number of cells used to build the stacks under study. This indicates an ever-increasing interest in the subject and carries great potential for technology development in the coming years. Table 8 collects the data of tests performed on ammonia. It can be seen that the electrical efficiency of the NH-fueled stacks matches the electrical efficiency of the H2-fueled SOFC systems compiled in Table 7. It is assumed that the efficiency of the systems is lower, as power is required for auxiliary equipment. However, this does not change the fact that the efficiency of ammonia-fueled SOFC stacks can exceed 60% efficiency.

Table 8.
Studies on ammonia-fueled stacks.
6.9. Summary
As shown, the transition from hydrogen to NH for fuel cell systems may not be straightforward, as it involves problems such as the requirement of additional equipment to reduce the energy density of the system, fuel cell poisoning, and temperature incompatibility. SOFCs are the best candidate for using NH as fuel. The IEA states that the ammonia-fed SOFC fuel cells that power electric vehicles are at a readiness level of 4–5 [131], and there is no information on TRLs for other types of fuel cells. However, the use of SOFC stacks in short-distance transportation appears to be impossible with the current development of this technology. SOFC stacks work mainly in stationary solutions, where frequent load shifting or frequent blanking is not required. Therefore, this technology has much more potential for implementation in the far sea or in rail freight transportation. Currently, it seems that the use of ammonia in land transport using fuel cells brings great challenges, especially from the perspective of powering road transport, while not bringing many benefits.
7. Ammonia Fuel in the Context of Clean Air Policies
For many of the reasons described above (see Section 1), ammonia is being researched and developed as a carbon-emission-free fuel. In addition to removing CO emissions from transport, reducing emissions of air pollutants is an equally great challenge, as they are one of the greatest threats to humanity today [145]. Exposure to air pollution of particulate matter up to 2.5 m in diameter (PM2.5), nitrogen dioxide (NO), and ozone (O) is associated with a reduced life expectancy due to the increased risk of heart disease, stroke, and chronic obstructive pulmonary disease. There is also an apparent link between exposure to air pollution and diabetes and Alzheimer’s disease [146]. According to the latest recommendations of the World Health Organization (WHO) air pollution recommendations, in 2019, 97% of the urban population in Europe was exposed to PM2.5, 94% to NO, and 99% to O [147].
People who live near highways with high traffic experience increased health risks from traffic pollution [148,149]. According to calculations based on 2014 data [150], the transportation sector accounted for 8.6% of global PM2.5 emissions, contributing to 330,000 premature deaths and 891 billion USD in economic losses annually [151]. Despite the absence of tailpipe emissions of PM from electric or fuel cell cars, it is still emitted primarily through resuspension, brake wear, and friction with the road surface [152]. Studies show that the non-exhaust emission of PM in electric cars is higher than the total dust emissions from gasoline or diesel cars with EURO 6 standards. Considering the different types of roads, it can be higher by 7–12%, with reference to internal combustion engines [153]. It is related to the increased weight of electric cars due to the application of batteries. Given that cars using ammonia will also have fuel cells installed along with the battery, the weight of the car may be similar to or even greater than that of an EV. This may contribute to even greater non-exhaust emissions. However, due to the inability to compare the weight of similar vehicles, the lack of knowledge of the technology used in the braking system and other variables, estimating emissions is affected by a high level of uncertainty. It is worth remembering, however, that emissions from pre-EURO 5 passenger cars have significantly higher particulate matter emissions into the air than those with fuel cells, as a result of fuel combustion, mainly from diesel engines [154].
In Europe, the transportation sector accounts for 48% of total nitrogen oxide emissions, and in the US, the rate is 41% [155]. It is notable that diesel vehicles are responsible for most of these concentrations due to their relatively higher share of NO emissions compared to gasoline-powered engines, especially in cars with emission standards lower than EURO 5 [156]. NO is a pollutant of particular importance because it acts as a precursor to the formation of O and secondary PM2.5 particles. Studies indicate that vehicles that use ammonia as fuel in the catalytic combustion process emit nitrogen oxides [157]. NO concentrations in untreated diesel exhaust are typically between 50 and 1000 ppm (particles per million). In vehicles using ammonia combustion, NO emissions range from 0 to 6 ppm, depending on the combustion temperature and catalyst used [158]. With the technology of using ammonia as a fuel for fuel cells, there are no emissions of nitrogen oxides [143]. This type of technology would be more beneficial in terms of reducing NO in the atmosphere.
Although ammonia is being explored for use as a low-carbon fuel for transportation, it is important to remember that atmospheric ammonia emissions are considered a political and scientific problem because they pose health and environmental risks. NH is extremely volatile and highly toxic to humans, as mentioned in Section 3. Agriculture is the largest source of global NH emissions, accounting for 81%. Ammonia emissions pose a significant threat to urban air quality due to their important role in secondary particle formation [159]. Chemical reactions in the atmosphere involving ammonia contribute significantly to the formation of PM2.5 [160]. NH is responsible for half of the PM2.5 pollution in Europe and 30% in the US [161], and ammonia-derived aerosols account for a large share of total PM [162]. Given these toxic effects of ammonia, NH emissions from the road transport sector are significant. The new emission standards being introduced for vehicles also have a direct impact on reducing ammonia pollution [163]. NH emission rates for gasoline engines range from 300 to 470 ppm, for diesel vehicles from 340 to 500 ppm, and for vehicles powered by other fuels from 290 to 600 ppm. In the case of using a dual-fuel compression ignition engine with ammonia and diesel, the concentration of unburned ammonia in wet exhaust ranged from 7 ppm for the diesel-only case to 14,800 ppm for the maximum contribution of ammonia energy (84.2% of the energy input) [164]. Using solid oxide fuel cells, ammonia emissions have been observed in the range of 40–250 ppm [143]. It is important to note that SOFC-based systems typically use an additional post-reaction gas afterburner, such as a catalytic burner, where the ammonia can further decompose. In addition, most of the studies mentioned in the previous section using ammonia as fuel also presented a solution with an additional ammonia reactor upstream of the fuel cell stack. Such solutions mean that there should not be any NH emissions in the target applications.
Summary
The use of ammonia in combination with another fuel (gasoline or diesel) does not solve the problem of total reduction in nitrogen oxides, and in some cases, these are higher for combustion cars meeting EURO 6 emission standards. If ammonia fuel cells are applied, NO emissions do not occur, which is beneficial from the point of view of improving air quality. Cars powered by fuel cells can contribute to the same or even higher PM emissions compared to combustion cars with EURO 5 standard and above, as mentioned before. This is caused by the greater non-exhausted emission due to the significantly higher weight of the cars powered by fuel cells. In addition, the amount of PM emissions resulting from the combustion of ammonia in conjunction with other fuels has not yet been estimated. Due to the strong toxicity of ammonia indicated above, a more detailed analysis of NH air emissions from cars using ammonia as fuel is needed. There is still great uncertainty in assessing the impact of these types of vehicles on air pollution. Some indirect emissions, for example, the health effects of ammonia fumes escaping during vehicle refueling, are, to the best of our knowledge, unknown.
8. Summary: Indication of the Strengths and Weaknesses of Green Ammonia as a Fuel and Further Research and Optimization Directions Required
The article presents an analysis of the challenges associated with the use of ammonia as a fuel. Despite the demonstration of a large number of challenges, it should be noted that all of them are already technically mastered in other industrial sectors. These challenges are no greater than those associated with other types of fuels commonly used today, but they are simply different and require a different approach. The main barrier blocking the widespread use of ammonia as a fuel is not the risks associated with it but the lack of appropriate legislation regulating such a process. Therefore, the immediate step should be to develop such regulations; unfortunately, the maritime sector remains the only sanctioned one in this respect so far, and there is no information about ongoing work in this direction in other transport sectors.
In terms of ammonia production, it has to be pointed out that current ammonia technologies are completely mature, but in the current pathway, they cannot be considered green. Therefore, the challenge is mainly related to providing renewable energy for the production of green ammonia. The CAPEX of this conversion is reported to be very high, which can be considered a potential roadblock. Furthermore, based on the available techno-economic analyses of green ammonia plants, it can be expected that the price of green ammonia will exceed the current ammonia prices by 100–200%. This will definitely limit the application of ammonia as a fuel in road transport. Despite the significant challenges summarized above, the greatest threat, in the authors’ opinion, remains possible interference with other ammonia-dependent markets, especially the agriculture and food sector, which have a very severe impact on food prices and availability. To proceed safely with the development of new ammonia-demanding sectors, the protection of ammonia supplies can be required for such strategic and high-priority sectors as agriculture and food production.
Ammonia storage tanks indicate an advanced maturity of the technology. However, these solutions are primarily tailored for the refrigeration and fertilizer industries. For the use of ammonia in onshore transport energy production, existing infrastructure needs adaptation and modernization. Given the similarity of the thermodynamic parameters between ammonia and LPG, LPG facilities could potentially be repurposed for ammonia storage, provided they are equipped with additional safety systems. This adaptation is crucial for the safe and efficient use of ammonia in the energy sector. Despite the high TRL of current ammonia storage solutions, their conversion to new energy applications will require further innovation and modification.
Ammonia as a fuel source has already been implemented on a mass scale during WW2, and nowadays, there are single-working prototypes with better engine efficiency. Most of those implemented automobiles were based on dual fuel solutions due to poor ammonia combustion properties that lead to low engine stability. Due to this fact, vehicles with combustion engines working only on ammonia do not seem to also be introduced for mass transportation. The dual-fuel operation brings with it an increase in system complexity; however, it is not exclusionary, as best exemplified by cars running on gasoline and LPG. Although in these solutions the engine usually operates interchangeably in single-fuel modes, it has the equipment to process both fuels. However, the downside to this solution can be drawn from the fact that it will not result in an elimination of CO emissions but only in their reduction, even contributing to an increase in NO emissions. Therefore, the use of ammonia has benefits but does not appear to be a game changer.
Fuel cells are increasingly resonating in the energy market. However, they are currently adapted to operate with hydrogen and possibly methane. As the review showed, design changes are required to implement ammonia in fuel cell systems. The expansion of current systems used in the on-road industry seems irrational. The first reason is that the market is mainly taken over by PEM cells, which are not temperature-compatible with the ammonia cracking process required to be carried out before the fuel is delivered to the cell stacks. The second is the required expansion of the system by several components, which ultimately reduces the gravimetric and volumetric energy density, acting negatively on the potential size of the cars, as well as the economics of their production. The difference in the operating temperature of cells and the cracking of ammonia can be eliminated using SOFC cells. However, the operating characteristics of SOFC-based systems appear to be unsuitable for on-road applications. The long system start-up time and the limited ability to operate in the dynamic change mode make SOFC a candidate for long-distance transportation applications, mainly marine and possibly rail, rather than on-road solutions. Compiling information on the powering of internal combustion engines and fuel cells, it seems that the use of ammonia in land transportation, especially road transportation, brings far more challenges than gain from the transformation of the fuel sector. However, it should be noted that if the changes aim to reduce the amount of unhealthy substances released into the atmosphere a few centimeters above the ground, it is understandable to push for new, safer solutions.
This paper also discusses the use of ammonia as a potential carbon-free fuel in the transportation sector, with a focus on its impact on air pollution. Hazardous pollutants such as PM2.5 and NO are considered, which are associated with many health problems. The transportation sector is identified as a significant contributor to these pollutants. Ammonia as a fuel significantly reduces CO emissions and could be a step towards carbon-neutral transportation. Vehicles using ammonia fuel in catalytic combustion processes produce very low NO emissions compared to diesel and gasoline vehicles, which can also help reduce ozone and secondary PM2.5 particles. The use of SOFC technology eliminates NO emissions, providing another potential advantage of ammonia over conventional fuels. However, co-firing with other fuels does not solve the problem of reducing NO concentrations, reducing the effectiveness of ammonia as a low-emission fuel. Moreover, fuel cell vehicles may emit more particulate matter than vehicles with internal combustion engines with EURO 5 and older due to higher non-exhaust emissions. In addition, due to the high toxicity of ammonia, their increased use in transportation could pose a significant risk to public health and the environment. More research is needed to quantify and reduce non-exhaust emissions from heavy electric or fuel cell vehicles, including those powered by ammonia. Further studies are required to assess the impacts of NH emissions from vehicles that use ammonia as fuel due to its high toxicity. While SOFC technology is promising, more work is needed to optimize its performance and reduce the potential for PM emissions. Since co-firing does not adequately reduce NO emissions, other methods of combining ammonia with conventional fuels need to be investigated. This could also include exploring advanced after-treatment technologies to reduce NO emissions from co-fired engines.
The prospects of using green ammonia as a fuel are not obvious. Although ammonia has already been used to power internal combustion engines for many decades, its properties are incomparably less favorable than those of crude oil, gasoline, methane, or LPG, in terms of both possible performance and safety. This requires the installation of additional systems and more expensive materials, which may affect the price, lifetime, and comfort of use. An interesting alternative seems to be the use of ammonia to power fuel cells, but further research is required here. However, the most important issue may be the negative impact of the massive use of ammonia as a fuel on food prices, as ammonia is essential for food production.
Author Contributions
Conceptualization, M.C., M.L., K.M., Z.M., D.P., P.P., Z.R., M.S., M.L., K.M., Z.M., D.P., P.P., Z.R. and M.S.; writing—original draft preparation, M.L., K.M., Z.M., D.P., P.P., Z.R. and M.S.; writing—review and editing, M.L., K.M., Z.M., D.P., P.P., Z.R. and M.S.; visualization, M.L., K.M. and Z.R.; supervision, Z.R. and Z.M. All authors have read and agreed to the published version of the manuscript.
Funding
The work has been partly funded by National Fund for Environmental Protection and Water Management in Warsaw, Poland. The work has been supported by statutory funds.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
From the Polish Ministry of Science and Higher Education.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AFC | Alkaline Fuel Cell |
| AIT | Autoignition |
| CAPEX | Capital Expenditures |
| CEM | Clean Energy Ministerial |
| CI | Compression Ignition |
| CNG | Compressed Natural Gas |
| CR | Compression Ratio |
| DMFC | Direct Methanol Fuel Cell |
| EU | European Union |
| EVI | Electric Vehicles Initiative |
| FC | Fuel cell |
| GHG | Green House Gases |
| HRR | Heat Release Rate |
| ICE | Internal Combustion Engine |
| IEA | International Energy Agency |
| IMO | International Marine Organization |
| IRENA | International Renewable Energy Agency |
| LC | Lethal Concentration |
| LCOA | Levelized Cost of Ammonia |
| LD | Lethal Dose |
| LNG | Liquefied Natural Gas |
| LPG | Liquid Petroleum Gas |
| MCFC | Molten Carbonite Fuel Cell |
| MIE | Minimum Ignition Energy |
| NIOSH | National Institute for Occupational Safety and Health |
| OECD | Organization for Economic Cooperation and Development |
| PAFC | Phosphoric Acid Fuel Cell |
| PEMFC | Proton Exchange Membrane Fuel Cell |
| PM | Particulate Matter |
| RON | research Octane Number |
| SI | Spark Ignition |
| SOFC | Solid Oxide Fuel Cell |
| TRL | Technology Readiness Level |
| US | United States |
References
- Phase-Out of Fossil Fuel Vehicles. 2023. Available online: https://en.wikipedia.org/wiki/Phase-out_of_fossil_fuel_vehicles (accessed on 10 May 2023).
- More Than 25 Countries & US States Are Planning Gas Car Bans. 2021. Available online: https://insideevs.com/news/534890/countries-states-gas-car-bans/ (accessed on 11 May 2023).
- CO2 Emissions from Cars: Facts and Figures (Infographics). 2019. Available online: https://www.europarl.europa.eu/news/en/headlines/society/20190313STO31218/co2-emissions-from-cars-facts-and-figures-infographics (accessed on 11 May 2023).
- Unijny Zakaz Sprzedaży Nowych Samochodów Spalinowych od 2035 r.—co to Oznacza? 2022. Available online: https://www.europarl.europa.eu/news/pl/headlines/economy/20221019STO44572/unijny-zakaz-sprzedazy-nowych-samochodow-spalinowych-od-2035-r-co-to-oznacza (accessed on 11 May 2023).
- China Joins List of Nations Banning the Sale of Old-Style Fossil-Fuelled Vehicles. 2020. Available online: https://europeansting.com/2020/11/17/china-joins-list-of-nations-banning-the-sale-of-old-style-fossil-fuelled-vehicles/ (accessed on 11 May 2023).
- Powell, A. California Dreaming? Nope. 2022. Available online: https://environment.harvard.edu/news/california-dreaming-nope (accessed on 11 May 2023).
- DNV. Alternative Fuels for Containerships; Technical Report 4; DNV GL: Bærum, Norway, 2021. [Google Scholar]
- IEA. Global EV Outlook 2020: Technology Report; IEA: Paris, France, 2020; pp. 39–85. [Google Scholar]
- How to Increase the Use of Alternative Fuels for Cars. 2022. Available online: https://www.europarl.europa.eu/news/en/headlines/economy/20221013STO43019/how-to-increase-the-use-of-alternative-fuels-for-cars (accessed on 11 May 2023).
- European Biogas Association. The Potential of Biomethane for a Faster Decarbonisation of Transport Should Not Go to Waste Photo Credits: Deva Darshan; Technical Report; European Biogas Association: Brussels, Belgium, 2020. [Google Scholar]
- Chiong, M.C.; Chong, C.T.; Ng, J.H.; Mashruk, S.; Chong, W.W.F.; Samiran, N.A.; Mong, G.R.; Valera-Medina, A. Advancements of combustion technologies in the ammonia-fuelled engines. Energy Convers. Manag. 2021, 244, 114460. [Google Scholar] [CrossRef]
- Frigo, S.; Gentili, R. Analysis of the behaviour of a 4-stroke Si engine fuelled with ammonia and hydrogen. Int. J. Hydrogen Energy 2013, 38, 1607–1615. [Google Scholar] [CrossRef]
- Chehade, G.; Dincer, I. Progress in green ammonia production as potential carbon-free fuel. Fuel 2021, 299, 120845. [Google Scholar] [CrossRef]
- Tornatore, C.; Marchitto, L.; Sabia, P.; De Joannon, M. Ammonia as Green Fuel in Internal Combustion Engines: State-of-the-Art and Future Perspectives. Front. Mech. Eng. 2022, 8, 944201. [Google Scholar] [CrossRef]
- Elbaz, A.M.; Wang, S.; Guiberti, T.F.; Roberts, W.L. Review on the recent advances on ammonia combustion from the fundamentals to the applications. Fuel Commun. 2022, 10, 100053. [Google Scholar] [CrossRef]
- Ryu, K.; Zacharakis-Jutz, G.E.; Kong, S.C. Performance enhancement of ammonia-fueled engine by using dissociation catalyst for hydrogen generation. Int. J. Hydrogen Energy 2014, 39, 2390–2398. [Google Scholar] [CrossRef]
- Yüzbaşıoğlu, A.E.; Avşar, C.; Gezerman, A.O. The current situation in the use of ammonia as a sustainable energy source and its industrial potential. Curr. Res. Green Sustain. Chem. 2022, 5, 100307. [Google Scholar] [CrossRef]
- IRENA; AEA. Innovation Outlook: Renewable Ammonia; Technical Report; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2022. [Google Scholar]
- Pearson, A. Refrigeration with ammonia. Int. J. Refrig. 2008, 31, 545–551. [Google Scholar] [CrossRef]
- Khudhur, D.A.; Tuan Abdullah, T.A.; Norazahar, N. A Review of Safety Issues and Risk Assessment of Industrial Ammonia Refrigeration System. Acs Chem. Health Saf. 2022, 29, 394–404. [Google Scholar] [CrossRef]
- Machaj, K.; Kupecki, J.; Malecha, Z.; Morawski, A.; Skrzypkiewicz, M.; Stanclik, M.; Chorowski, M. Ammonia as a potential marine fuel: A review. Energy Strategy Rev. 2022, 44, 100926. [Google Scholar] [CrossRef]
- Linde. Ammonia Datasheet; Technical Report; Linge AG: Dublin, Ireland, 2023. [Google Scholar]
- Birken, G.A.; Fabri, P.J.; Carey, L.C. Acute ammonia intoxication complicating multiple trauma. J. Trauma 1981, 21, 820–822. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Occupational Safety and Health. NIOSH recommended standards for occupational exposure to ammonia and benzene. Int. J. Occup. Health Saf. 1974, 43, 32–33. [Google Scholar]
- The Fertilizer Institute. Health Effects of Ammonia; Technical Report; The Fertilizer Institute: Arlington, VA, USA, 2020. [Google Scholar]
- EC-European Commission. Commission Regulation (EU) No 582/2011 of 25 May 2011 Implementing and Amending Regulation (EC) No 595/2009 of the European Parliament and of the Council with Respect to Emissions from Heavy Duty Vehicles (Euro VI) and Amending Annexes I and III to Directive 2007/46/EC of the European Parliament and of the Council; Technical Report; EC-European Commission: Brussels, Belgium, 2011. [Google Scholar]
- EC-European Commission. Commission Regulation (EU) No 64/2012 of 23 January 2012 Amending Regulation (EU) No 582/2011 Implementing and Amending Regulation (EC) No 595/2009 of the European Parliament and of the Council with Respect to Emissions from Heavy Duty Vehicles (Euro VI) Text with EEA Relevance; Technical Report; EC-European Commission: Brussels, Belgium, 2012. [Google Scholar]
- Ziegler, B.; Trepp, C. Equation of state for ammonia-water mixtures. Int. J. Refrig. 1984, 7, 101–106. [Google Scholar] [CrossRef]
- Patterson, P.; Adrizal. Management strategies to reduce air emissions: Emphasis—Dust and ammonia. J. Appl. Poult. Res. 2005, 14, 638–650. [Google Scholar] [CrossRef]
- Wang, X.B.; Xiang, Y.; Li, X.Y. Environmental risk for application of ammonia-soda white mud in soils in China. J. Integr. Agric. 2020, 19, 601–611. [Google Scholar] [CrossRef]
- United States Environmetnal Protection Agency. 1999 Update Of Ambient Water Quality Criteria For Ammonia. EPA-822-R-99-014; Technical Report; USEPA: Washington, DC, USA, 1999.
- Wicks, B.; Joensen, R.; Tang, Q.; Randall, D. Swimming and ammonia toxicity in salmonids: The effect of sub lethal ammonia exposure on the swimming performance of coho salmon and the acute toxicity of ammonia in swimming and resting rainbow trout. Aquat. Toxicol. 2002, 59, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Tepper, J.S.; Weiss, B.; Wood, R.W. Alterations in behavior produced by inhaled ozone or ammonia. Fundam. Appl. Toxicol. 1985, 5, 1110–1118. [Google Scholar] [CrossRef]
- Johnson, I.; Sorokin, N.; Atkinson, C.; Rule, K.; Hope, S.J. Proposed EQS for Water Framework Directive Annex VIII Substances: Ammonia (un-Ionised); Technical Report; Environment Agency: Bristol, UK, 2007.
- Levit, S.M. Review of the Literature on the Effects of Ammonia on Fish; Technical Report; Center for Science in Public Participation: Bozeman, MT, USA, 2010. [Google Scholar]
- Kobayashi, H.; Hayakawa, A.; Somarathne, K.; Okafor, E. Science and technology of ammonia combustion. Proc. Combust. Inst. 2019, 37, 109–133. [Google Scholar] [CrossRef]
- American Bureau of Shipping, Guide for Ammonia Fueled Vessels September 2021; Technical Report; American Bureau of Shipping: Houston, TX, USA, 2021.
- DNV. RU-SHIP Part 6 Additional Class Notations Chapter 2 Propulsion, Power Generation and Auxiliary Systems Section 14 Gas Fuelled Ship Installations—Gas Fuelled Ammonia; Technical Report; DNV: Bærum, Norway, 2021. [Google Scholar]
- International Maritime Organization. IMO Strategy on Reduction of GHG Emissions from Ships. Available online: https://unfccc.int/sites/default/files/resource/250_IMO%20submission_Talanoa%20Dialogue_April%202018.pdf (accessed on 10 May 2023).
- Group Technology & Research, D&G. Ammonia as a Marine Fuel; Technical Report; DNV-GL: Bærum, Norway, 2020. [Google Scholar]
- Rogala, Z.; Stanclik, M.; Łuszkiewicz, D.; Malecha, Z. Perspectives for the Use of Biogas and Biomethane in the Context of the Green Energy Transformation on the Example of an EU Country. Energies 2023, 16, 1911. [Google Scholar] [CrossRef]
- Bertilsson, O.B.; Kirchmann, H. Sustainable N fertilizer production based on a loop: Straw—Biogas—’Haber-Bosch’ process. Agric. Syst. 2021, 190, 103100. [Google Scholar] [CrossRef]
- Laval, A.; Hafnia, H.T.; Vestas, S.G. Ammonfuel—An Industrial View of Ammonia as a Marine Fuel. Hafnia 2020, 7, 32–59. [Google Scholar]
- Ofori-Bah, C.O.; Amanor-Boadu, V. Directing the wind: Techno-economic feasibility of green ammonia for farmers and community economic viability. Front. Environ. Sci. 2023, 10, 1070212. [Google Scholar] [CrossRef]
- Campion, N.; Nami, H.; Swisher, P.R.; Vang Hendriksen, P.; Münster, M. Techno-economic assessment of green ammonia production with different wind and solar potentials. Renew. Sustain. Energy Rev. 2023, 173, 113057. [Google Scholar] [CrossRef]
- Morgan, E.R. Techno-Economic Feasibility Study of Ammonia Plants Powered by Offshore Wind. Ph.D. Thesis, University of Massachusetts Amherst, Amherst, MA, USA, 2013. [Google Scholar]
- Nayak-Luke, R.M.; Bañares-Alcántara, R. Techno-economic viability of islanded green ammonia as a carbon-free energy vector and as a substitute for conventional production. Energy Environ. Sci. 2020, 13, 2957–2966. [Google Scholar] [CrossRef]
- Nayak-Luke, R.; Bañares-Alcántara, R.; Wilkinson, I. “Green” Ammonia: Impact of Renewable Energy Intermittency on Plant Sizing and Levelized Cost of Ammonia. Ind. Eng. Chem. Res. 2018, 57, 14607–14616. [Google Scholar] [CrossRef]
- Ikäheimo, J.; Kiviluoma, J.; Weiss, R.; Holttinen, H. Power-to-ammonia in future North European 100 % renewable power and heat system. Int. J. Hydrogen Energy 2018, 43, 17295–17308. [Google Scholar] [CrossRef]
- Osman, O.; Sgouridis, S.; Sleptchenko, A. Scaling the production of renewable ammonia: A techno-economic optimization applied in regions with high insolation. J. Clean. Prod. 2020, 271, 121627. [Google Scholar] [CrossRef]
- Sousa, J.; Waiblinger, W.; Friedrich, K.A. Techno-economic Study of an Electrolysis-Based Green Ammonia Production Plant. Ind. Eng. Chem. Res. 2022, 61, 14515–14530. [Google Scholar] [CrossRef]
- IEA. The Future of Hydrogen; Technical Report June; IEA: Paris, France, 2019. [Google Scholar] [CrossRef]
- IRENA. Renewable Power Generations Costs; IRENA: Masdar City, United Arab Emirates, 2018; p. 160. [Google Scholar]
- U.S. Energy Information Administration. Transportation sector energy consumption. In International Energy Outlook 2016; U.S. Energy Information Administration: Washington, DC, USA, 2016; Volume 2016, pp. 127–137. [Google Scholar]
- Office of Management and Bugdet. Budget of the U.S. Government Budget of the U.S. Government; Technical Report; Office of Management and Bugdet: Washington, DC, USA, 2022.
- Ivanic, M.; Martin, W.; Zaman, H. Estimating the Short-Run Poverty Impacts of the 2010–2011 Surge in Food Prices. World Dev. 2012, 40, 2302–2317. [Google Scholar] [CrossRef]
- Lomborg, B. False Alarm: How Climate Change Panic Costs Us Trillions, Hurts the Poor, and Fails to Fix the Planet; Basic Books: Hachette, UK, 2020. [Google Scholar]
- Patonia, A.; Poudineh, R. Ammonia as a Storage Solution for Future Decarbonized Energy Systems; Technical Report; OIES Paper: Oxford, UK, 2020. [Google Scholar]
- IEA. Ammonia Technology Roadmap: Towards More Sustainable Nitrogen Fertilizer Production; International Energy Agency: Paris, France, 2021; p. 163. [Google Scholar]
- Appl, M. Ammonia; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006. [Google Scholar] [CrossRef]
- Engineering Toolbox: Fuels—Higher and Lower Calorific Values. Available online: https://www.engineeringtoolbox.com/fuels-higher-calorific-values-d_169.html (accessed on 15 May 2023).
- Rouwenhorst, K.H.; der Ham, A.G.V.; Mul, G.; Kersten, S.R. Islanded ammonia power systems: Technology review & conceptual process design. Renew. Sustain. Energy Rev. 2019, 114, 109339. [Google Scholar] [CrossRef]
- Richter, T.M.; Niewa, R. Chemistry of ammonothermal synthesis. Inorganics 2014, 2, 29–78. [Google Scholar] [CrossRef]
- Lemmon, E.W.; Bell, I.H.; Huber, M.L.; McLinden, M.O. NIST Standard Reference Database 23: Reference Fluid Thermodynamic and Transport Properties-REFPROP, Version 10.0; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2018. [CrossRef]
- Christensen, C.H.; Johannessen, T.; Sørensen, R.Z.; Nørskov, J.K. Towards an ammonia-mediated hydrogen economy? Catal. Today 2006, 111, 140–144. [Google Scholar] [CrossRef]
- Sørensen, R.Z.; Hummelshøj, J.S.; Klerke, A.; Reves, J.B.; Vegge, T.; Nørskov, J.K.; Christensen, C.H. Indirect, reversible high-density hydrogen storage in compact metal ammine salts. J. Am. Chem. Soc. 2008, 130, 8660–8668. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.B.; Lysgaard, S.; Quaade, U.J.; Vegge, T. Designing mixed metal halide ammines for ammonia storage using density functional theory and genetic algorithms. Phys. Chem. Chem. Phys. 2014, 16, 19732–19740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Miyaoka, H.; Miyaoka, H.; Ichikawa, T.; Kojima, Y. Review on Ammonia Absorption Materials: Metal Hydrides, Halides, and Borohydrides. Acs Appl. Energy Mater. 2018, 1, 232–242. [Google Scholar] [CrossRef]
- Malmali, M.; Le, G.; Hendrickson, J.; Prince, J.; McCormick, A.V.; Cussler, E.L. Better Absorbents for Ammonia Separation. ACS Sustain. Chem. Eng. 2018, 6, 6536–6546. [Google Scholar] [CrossRef]
- Langmi, H.W.; Engelbrecht, N.; Modisha, P.M.; Bessarabov, D. Hydrogen storage. In Electrochemical Power Sources: Fundamentals, Systems, and Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 455–486. [Google Scholar] [CrossRef]
- Dimitriou, P.; Javaid, R. A review of ammonia as a compression ignition engine fuel. Int. J. Hydrogen Energy 2020, 45, 7098–7118. [Google Scholar] [CrossRef]
- Barelli, L.; Bidini, G.; Perla, M.; Pilo, F.; Trombetti, L. Boil-off gas emission from the fuel tank of a LNG powered truck. Fuel 2022, 325, 124954. [Google Scholar] [CrossRef]
- Farzaneh-Gord, M.; Saadat-Targhi, M.; Khadem, J. Selecting optimal volume ratio of reservoir tanks in CNG refueling station with multi-line storage system. Int. J. Hydrogen Energy 2016, 41, 23109–23119. [Google Scholar] [CrossRef]
- Duijm, N.; Markert, F.; Paulsen, J.L. Safety Assessment of Ammonia as a Transport Fuel; Technical Report; Forskningscenter Risoe: Roskilde, Denmark, 2005. [Google Scholar]
- Amelina, L.; Biliaiev, M.; Berlov, O.; Verhun, O.; Rusakova, T. Modeling of Environmental Pollution by Ammonia Emission from a Damaged Pipeline. Sci. Transp. Prog. 2021, 1, 5–14. [Google Scholar] [CrossRef]
- Hang, T.; Wu, J.; Xiao, S.; Li, B.; Li, H.; Yang, C.; Hu, N.; Xu, Y.; Zhang, Y.; Xie, X. Anti-biofouling NH3 gas sensor based on reentrant thorny ZnO/graphene hybrid nanowalls. Microsystems Nanoeng. 2020, 6, 41. [Google Scholar] [CrossRef]
- Ezzat, M.F.; Dincer, I. Development and assessment of a new hybrid vehicle with ammonia and hydrogen. Appl. Energy 2018, 219, 226–239. [Google Scholar] [CrossRef]
- Willmann, M.; Berger, I.; Barrow, E. Woodward L’Orange’s New Injector Generation—An Ideal Platform for the Combustion of E-Fuels in Large Engines; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Brohi, E.A. Ammonia as Fuel for Internal Combustion Engines? An Evaluation of the Feasibility of Using Nitrogen-Based Fuels in ICE; Chalmers University of Technology: Gothenburg, Sweden, 2014. [Google Scholar]
- Kurien, C.; Mittal, M. Review on the production and utilization of green ammonia as an alternate fuel in dual-fuel compression ignition engines. Energy Convers. Manag. 2022, 251, 114990. [Google Scholar] [CrossRef]
- Pielecha, I. Modeling of Fuel Cells Characteristics in Relation to Real Driving Conditions of FCHEV Vehicles. Energies 2022, 15, 6753. [Google Scholar] [CrossRef]
- JIVE 2 under way to accelerate commercial start of fuel cell buses. Fuel Cells Bull. 2018, 2018, 2. [CrossRef]
- Available online: https://cordis.europa.eu/project/id/826236 (accessed on 13 March 2023).
- Auditors, E.C. Rail Freight Transport in the EU: Still Not on the Right Track; Special Report No 08; Publications Office: Luxembourg, 2016. [Google Scholar]
- Ibrahim Ozigis, I.; Oche, J.; Lawal, N. Locomotive Engines and the Future of Railway Automotive Power in Africa: A Review. Niger. J. Technol. 2021, 40, 660–673. [Google Scholar] [CrossRef]
- García-Garre, A.; Gabaldón, A. Analysis, Evaluation and Simulation of Railway Diesel-Electric and Hybrid Units as Distributed Energy Resources. Appl. Sci. 2019, 9, 3605. [Google Scholar] [CrossRef]
- Grennfelt, P.; Engleryd, A.; Forsius, M.; Hov, Ø.; Rodhe, H.; Cowling, E. Acid rain and air pollution: 50 years of progress in environmental science and policy. Ambio 2020, 49, 849–864. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Morris, S.; Runyon, J.; Pugh, D.G.; Marsh, R.; Beasley, P.; Hughes, T. Ammonia, Methane and Hydrogen for Gas Turbines. Energy Procedia 2015, 75, 118–123. [Google Scholar] [CrossRef]
- Cornelius, W.; Huellmantel, L.W.; Mitchell, H.R. Ammonia as an Engine Fuel. In Proceedings of the 1965 International Automotive Engineering Congress and Exposition; SAE International: Warrendale, PA, USA, 1965. [Google Scholar] [CrossRef]
- Lhuillier, C.; Brequigny, P.; Rousselle, C.; Contino, F. Performance and Emissions of an Ammonia-Fueled SI Engine with Hydrogen Enrichment. In Proceedings of the 14th International Conference on Engines & Vehicles; SAE International: Warrendale, PA, USA, 2019. [Google Scholar] [CrossRef]
- Grannell, S.; Assanis, D.; Bohac, S.; Gillespie, D. The Operating Features of a Stoichiometric, Ammonia and Gasoline Dual Fueled Spark Ignition Engine. In ASME International Mechanical Engineering Congress and Exposition; ASME: New York, NY, USA, 2006. [Google Scholar] [CrossRef]
- Haputhanthri, S.; Maxwell, T.; Fleming, J.; Austin, C. Ammonia Gasoline-Ethanol/Methanol Tertiary Fuel Blends as an Alternate Automotive Fuel. In ASME International Mechanical Engineering Congress and Exposition, Proceedings (IMECE); American Society of Mechanical Engineers: New York, NY, USA, 2014; Volume 6. [Google Scholar] [CrossRef]
- Comotti, M.; Frigo, S. Hydrogen generation system for ammonia–hydrogen fuelled internal combustion engines. Int. J. Hydrogen Energy 2015, 40, 10673–10686. [Google Scholar] [CrossRef]
- Frigo, S.; Gentili, R.; Doveri, N. Ammonia Plus Hydrogen as Fuel in a S.I. Engine: Experimental Results. SAE Tech. Pap. 2012, 4. [Google Scholar] [CrossRef]
- Ryu, K.; Zacharakis-Jutz, G.E.; Kong, S.C. Effects of gaseous ammonia direct injection on performance characteristics of a spark-ignition engine. Appl. Energy 2014, 116, 206–215. [Google Scholar] [CrossRef]
- Niki, Y.; Nitta, Y.; Sekiguchi, H.; Hirata, K. Diesel Fuel Multiple Injection Effects on Emission Characteristics of Diesel Engine Mixed Ammonia Gas Into Intake Air. J. Eng. Gas Turbines Power 2019, 141, 061020. [Google Scholar] [CrossRef]
- Tay, K.L.; Yang, W.; Li, J.; Zhou, D.; Yu, W.; Zhao, F.; Chou, S.K.; Mohan, B. Numerical investigation on the combustion and emissions of a kerosene-diesel fueled compression ignition engine assisted by ammonia fumigation. Appl. Energy 2017, 204, 1476–1488. [Google Scholar] [CrossRef]
- Lamas Galdo, M.I.; Castro-Santos, L.; Rodriguez Vidal, C.G. Numerical Analysis of NOx Reduction Using Ammonia Injection and Comparison with Water Injection. J. Mar. Sci. Eng. 2020, 8, 109. [Google Scholar] [CrossRef]
- Lamas, M.; Rodriguez, C. Numerical model to analyze Nox reduction by ammonia injection in diesel-hydrogen engines. Int. J. Hydrogen Energy 2017, 42, 26132–26141. [Google Scholar] [CrossRef]
- Pyrc, M.; Gruca, M.; Jamrozik, A.; Tutak, W.; Juknelevičius, R. An experimental investigation of the performance, emission and combustion stability of compression ignition engine powered by diesel and ammonia solution (NH4OH). Int. J. Engine Res. 2021, 22, 2639–2653. [Google Scholar] [CrossRef]
- Schönborn, A. Aqueous solution of ammonia as marine fuel. Proc. Inst. Mech. Eng. J. Eng. Marit. Environ. 2021, 235, 142–151. [Google Scholar] [CrossRef]
- Şahin, Z.; Akcanca, İ.Z.; Durgun, O. Experimental investigation of the effects of ammonia solution (NH3OH) on engine performance and exhaust emissions of a small diesel engine. Fuel 2018, 214, 330–341. [Google Scholar] [CrossRef]
- Sivasubramanian, R.; Sajin, J.; Omanakuttan Pillai, G. Effect of ammonia to reduce emission from biodiesel fuelled diesel engine. Int. J. Ambient. Energy 2022, 43, 661–665. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lin, H.A. Engine performance and emission characteristics of a three-phase emulsion of biodiesel produced by peroxidation. Fuel Process. Technol. 2007, 88, 35–41. [Google Scholar] [CrossRef]
- Zacharakis-Jutz, G.; Kong, S.C.; Ryu, K.H. Effects of Fuel Compositions on Diesel Engine Performance Using Ammonia-DME Mixtures. In Proceedings of the SAE 2013 World Congress & Exhibition; SAE International: Warrendale, PA, USA, 2013. [Google Scholar] [CrossRef]
- Gross, C.W.; Kong, S.C. Performance characteristics of a compression-ignition engine using direct-injection ammonia–DME mixtures. Fuel 2013, 103, 1069–1079. [Google Scholar] [CrossRef]
- Wang, W.; Herreros, J.M.; Tsolakis, A.; York, A.P. Ammonia as hydrogen carrier for transportation; investigation of the ammonia exhaust gas fuel reforming. Int. J. Hydrogen Energy 2013, 38, 9907–9917. [Google Scholar] [CrossRef]
- Gill, S.; Chatha, G.; Tsolakis, A.; Golunski, S.; York, A. Assessing the effects of partially decarbonising a diesel engine by co-fuelling with dissociated ammonia. Int. J. Hydrogen Energy 2012, 37, 6074–6083. [Google Scholar] [CrossRef]
- Pochet, M.; Jeanmart, H.; Contino, F. A 22:1 Compression Ratio Ammonia-Hydrogen HCCI Engine: Combustion, Load, and Emission Performances. Front. Mech. Eng. 2020, 6, 43. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, D.; Yang, W.; Qian, Y.; Mao, Y.; Lu, X. Investigation on the potential of using carbon-free ammonia in large two-stroke marine engines by dual-fuel combustion strategy. Energy 2023, 263, 125748. [Google Scholar] [CrossRef]
- World’s First Tractor Powered by Renewable Fuels with Zero Carbon Emissions Debuts on IOWA Farm. Available online: https://solarhydrogensystem.com/2016/01/06/worlds-first-tractor-powered-by-renewable-fuels-with-zero-carbon-emissions-debuts-on-iowa-farm/ (accessed on 13 April 2023).
- Toyota GT86-R Marangoni Eco Explorer: Sport and Technology in the New 2013 Show Car. Available online: https://marangonipress.com/2013/02/28/toyota-gt86-r-marangoni-eco-explorer-sport-and-technology-in-the-new-2013-show-car-fitted-with-marangoni-m-power-evored-tyres/?cookie-state-change=1670785787773 (accessed on 28 February 2023).
- Commission, E.; Centre, J.R.; Ortiz Cebolla, R.; Davies, J.; Weidner, E. Global Deployment of Large Capacity Stationary Fuel Cells: Drivers of, and Barriers to, Stationary Fuel Cell Deployment; Publications Office: Luxembourg, 2019. [Google Scholar] [CrossRef]
- Fan, L.; Tu, Z.; Chan, S.H. Recent development of hydrogen and fuel cell technologies: A review. Energy Rep. 2021, 7, 8421–8446. [Google Scholar] [CrossRef]
- The Comparison of Different Technologies for On-Board Power Supply of Cruise Ships; Nautilius Technical Report; Fuel Cells and Hydrogen Observatory: Brussels, Belgium, 2020; Available online: https://www.fchobservatory.eu/sites/default/files/reports/Chapter_1_Technology_and_Market_070920.pdf (accessed on 15 May 2023).
- Ministry of Ecnomic Affairs and Climate Policy. Hydrogen Economy Development in Korea; Ministry of Economy and Affairs and Climate Policy: Seoul, Republic of Korea, 2020.
- E4tech. Fuel Cell Industry Review 2019; E4tech: London, UK, 2019. [Google Scholar]
- Hydrogen Fuel Cell Vehicle Market. Available online: https://www.polarismarketresearch.com/industry-analysis/hydrogen-fuel-cell-vehicle-market (accessed on 13 February 2023).
- Hoelzen, J.; Silberhorn, D.; Zill, T.; Bensmann, B.; Hanke-Rauschenbach, R. Hydrogen-powered aviation and its reliance on green hydrogen infrastructure—Review and research gaps. Int. J. Hydrogen Energy 2022, 47, 3108–3130. [Google Scholar] [CrossRef]
- Lucentini, I.; Garcia, X.; Vendrell, X.; Llorca, J. Review of the Decomposition of Ammonia to Generate Hydrogen. Ind. Eng. Chem. Res. 2021, 60, 18560–18611. [Google Scholar] [CrossRef]
- Mekhilef, S.; Saidur, R.; Safari, A. Comparative study of different fuel cell technologies. Renew. Sustain. Energy Rev. 2012, 16, 981–989. [Google Scholar] [CrossRef]
- Shabani, B.; Hafttananian, M.; Khamani, S.; Ramiar, A.; Ranjbar, A. Poisoning of proton exchange membrane fuel cells by contaminants and impurities: Review of mechanisms, effects, and mitigation strategies. J. Power Sources 2019, 427, 21–48. [Google Scholar] [CrossRef]
- Zarabi Golkhatmi, S.; Asghar, M.I.; Lund, P.D. A review on solid oxide fuel cell durability: Latest progress, mechanisms, and study tools. Renew. Sustain. Energy Rev. 2022, 161, 112339. [Google Scholar] [CrossRef]
- Hagen, A.; Langnickel, H.; Sun, X. Operation of solid oxide fuel cells with alternative hydrogen carriers. Int. J. Hydrogen Energy 2019, 44, 18382–18392. [Google Scholar] [CrossRef]
- Kishimoto, M.; Muroyama, H.; Suzuki, S.; Saito, M.; Koide, T.; Takahashi, Y.; Horiuchi, T.; Yamasaki, H.; Matsumoto, S.; Kubo, H.; et al. Development of 1 kW-class Ammonia-fueled Solid Oxide Fuel Cell Stack. Fuel Cells 2020, 20, 80–88. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Y.; Zhang, H.; Yang, J.; Wang, J.; Guan, W.; Chen, J.; Chi, B.; Jia, L.; Muroyama, H.; et al. Efficient and durable ammonia power generation by symmetric flat-tube solid oxide fuel cells. Appl. Energy 2020, 270, 115185. [Google Scholar] [CrossRef]
- Fuerte, A.; Valenzuela, R.; Escudero, M.; Daza, L. Ammonia as efficient fuel for SOFC. J. Power Sources 2009, 192, 170–174. [Google Scholar] [CrossRef]
- Hung, Y.; Shy, S. A pressurized ammonia-fed planar anode-supported solid oxide fuel cell at 1–5 atm and 750–850 °C and its loaded short stability test. Int. J. Hydrogen Energy 2020, 45, 27597–27610. [Google Scholar] [CrossRef]
- Lan, R.; Tao, S. Ammonia as a Suitable Fuel for Fuel Cells. Front. Energy Res. 2014, 2, 35. [Google Scholar] [CrossRef]
- Zhao, J.F.; Liang, Q.C.; Liang, Y.F. Simulation and Study of PEMFC System Directly Fueled by Ammonia Decomposition Gas. Front. Energy Res. 2022, 10, 819939. [Google Scholar] [CrossRef]
- IEA. ETP Clean Energy Technology Guide. Available online: https://www.iea.org/data-and-statistics/data-tools/etp-clean-energy-technology-guide?search=ammonia (accessed on 13 February 2023).
- Mukerjee, S.; Leah, R.; Selby, M.; Stevenson, G.; Brandon, N.P. Chapter 9—Life and Reliability of Solid Oxide Fuel Cell-Based Products: A Review. In Solid Oxide Fuel Cell Lifetime and Reliability; Brandon, N.P., Ruiz-Trejo, E., Boldrin, P., Eds.; Academic Press: New York, NY, USA, 2017; pp. 173–191. [Google Scholar] [CrossRef]
- Ghezel-Ayagh, H.; Borglum, B.P. Review of Progress in Solid Oxide Fuel Cells at FuelCell Energy. ECS Trans. 2017, 78, 77. [Google Scholar] [CrossRef]
- Bloom completes first international project, for SoftBank in Japan. Fuel Cells Bull. 2013, 2013, 6. [CrossRef]
- Corigliano, O.; Pagnotta, L.; Fragiacomo, P. On the Technology of Solid Oxide Fuel Cell (SOFC) Energy Systems for Stationary Power Generation: A Review. Sustainability 2022, 14, 15276. [Google Scholar] [CrossRef]
- Kishimoto, M.; Furukawa, N.; Kume, T.; Iwai, H.; Yoshida, H. Formulation of ammonia decomposition rate in Ni-YSZ anode of solid oxide fuel cells. Int. J. Hydrogen Energy 2017, 42, 2370–2380. [Google Scholar] [CrossRef]
- Engines, M. On-Road Diesel Engines, Axles and Transfer Cases for Trucks. Available online: https://pdf.directindustry.com/pdf/man-engines-division-man-truck-bus/brochure-truck/81737-302605.html (accessed on 13 February 2023).
- Cinti, G.; Desideri, U. SOFC fuelled with reformed urea. Appl. Energy 2015, 154, 242–253. [Google Scholar] [CrossRef]
- Okanishi, T.; Okura, K.; Srifa, A.; Muroyama, H.; Matsui, T.; Kishimoto, M.; Saito, M.; Iwai, H.; Yoshida, H.; Saito, M.; et al. Comparative Study of Ammonia-fueled Solid Oxide Fuel Cell Systems. Fuel Cells 2017, 17, 383–390. [Google Scholar] [CrossRef]
- Tan, W.C.; Iwai, H.; Kishimoto, M.; Brus, G.; Szmyd, J.S.; Yoshida, H. Numerical analysis on effect of aspect ratio of planar solid oxide fuel cell fueled with decomposed ammonia. J. Power Sources 2018, 384, 367–378. [Google Scholar] [CrossRef]
- Quach, T.Q.; Giap, V.T.; Keun Lee, D.; Pineda Israel, T.; Young Ahn, K. High-efficiency ammonia-fed solid oxide fuel cell systems for distributed power generation. Appl. Energy 2022, 324, 119718. [Google Scholar] [CrossRef]
- Cinti, G.; Discepoli, G.; Sisani, E.; Desideri, U. SOFC operating with ammonia: Stack test and system analysis. Int. J. Hydrogen Energy 2016, 41, 13583–13590. [Google Scholar] [CrossRef]
- Barelli, L.; Bidini, G.; Cinti, G. Operation of a Solid Oxide Fuel Cell Based Power System with Ammonia as a Fuel: Experimental Test and System Design. Energies 2020, 13, 6173. [Google Scholar] [CrossRef]
- Stoeckl, B.; Preininger, M.; Subotić, V.; Megel, S.; Folgner, C.; Hochenauer, C. Towards a wastewater energy recovery system: The utilization of humidified ammonia by a solid oxide fuel cell stack. J. Power Sources 2020, 450, 227608. [Google Scholar] [CrossRef]
- WHO Air Pollution. 2019. Available online: https://www.who.int/health-topics/air-pollution (accessed on 7 March 2023).
- Mulholland, E.; Miller, J.; Bernard, Y.; Lee, K.; Rodríguez, F. The role of NOx emission reductions in Euro 7/VII vehicle emission standards to reduce adverse health impacts in the EU27 through 2050. Transp. Eng. 2022, 9, 100133. [Google Scholar] [CrossRef]
- European Environment Agency. Report No. 15/2021: Air Quality in Europe—2021 Report; Technical Report; EEA: Copenhagen, Denmark, 2021. [Google Scholar]
- Calderón-Garcidueñas, L.; Villarreal-Ríos, R. Living close to heavy traffic roads, air pollution, and dementia. Lancet 2017, 389, 675–677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Batterman, S. Air pollution and health risks due to vehicle traffic. Sci. Total Environ. 2013, 450, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Weagle, C.L.; Snider, G.; Li, C.; Donkelaar, A.V.; Philip, S.; Bissonnette, P.; Burke, J.; Jackson, J.; Latimer, R.; Stone, E.; et al. Global Sources of Fine Particulate Matter: Interpretation of PM2.5 Chemical Composition Observed by SPARTAN using a Global Chemical Transport Model. Environ. Sci. Technol. 2018, 52, 11670–11681. [Google Scholar] [CrossRef] [PubMed]
- Anenberg, S.C.; Miller, J.; Henze, D.K.; Minjares, R.; Achakulwisut, P. The global burden of transportation tailpipe emissions on air pollution-related mortality in 2010 and 2015. Environ. Res. Lett. 2019, 14, 094012. [Google Scholar] [CrossRef]
- Timmers, V.R.; Achten, P.A. Non-exhaust PM emissions from electric vehicles. Atmos. Environ. 2016, 134, 10–17. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Gao, J.; Li, Y.; Dave, K.; Chen, J.; Federici, M.; Perricone, G. Comparative analysis of non-exhaust airborne particles from electric and internal combustion engine vehicles. J. Hazard. Mater. 2021, 420, 126626. [Google Scholar] [CrossRef]
- Beddows, D.C.; Harrison, R.M. PM10 and PM2.5 emission factors for non-exhaust particles from road vehicles: Dependence upon vehicle mass and implications for battery electric vehicles. Atmos. Environ. 2021, 244, 117886. [Google Scholar] [CrossRef]
- Shaw, S.; Heyst, B.V. Nitrogen Oxide (NOx) emissions as an indicator for sustainability. Environ. Sustain. Indic. 2022, 15, 100188. [Google Scholar] [CrossRef]
- Lozhkina, O.V.; Lozhkin, V.N. Estimation of nitrogen oxides emissions from petrol and diesel passenger cars by means of on-board monitoring: Effect of vehicle speed, vehicle technology, engine type on emission rates. Transp. Res. Transp. Environ. 2016, 47, 251–264. [Google Scholar] [CrossRef]
- Hinokuma, S.; Kiritoshi, S.; Kawabata, Y.; Araki, K.; Matsuki, S.; Sato, T.; Machida, M. Catalytic ammonia combustion properties and operando characterization of copper oxides supported on aluminum silicates and silicon oxides. J. Catal. 2018, 361, 267–277. [Google Scholar] [CrossRef]
- Weissenberger, T.; Zapf, R.; Pennemann, H.; Kolb, G. Effect of the Active Metal on the NOx Formation during Catalytic Combustion of Ammonia SOFC Off-Gas. Catalysts 2022, 12, 1186. [Google Scholar] [CrossRef]
- Farren, N.J.; Davison, J.; Rose, R.A.; Wagner, R.L.; Carslaw, D.C. Characterisation of ammonia emissions from gasoline and gasoline hybrid passenger cars. Atmos. Environ. 2021, 11, 100117. [Google Scholar] [CrossRef]
- Giannakis, E.; Kushta, J.; Bruggeman, A.; Lelieveld, J. Costs and benefits of agricultural ammonia emission abatement options for compliance with European air quality regulations. Environ. Sci. Eur. 2019, 31, 93. [Google Scholar] [CrossRef]
- Wyer, K.E.; Kelleghan, D.B.; Blanes-Vidal, V.; Schauberger, G.; Curran, T.P. Ammonia emissions from agriculture and their contribution to fine particulate matter: A review of implications for human health. J. Environ. Manag. 2022, 323, 116285. [Google Scholar] [CrossRef]
- Werner, M.; Kryza, M.; Dore, A.J. Differences in the Spatial Distribution and Chemical Composition of PM10 Between the UK and Poland. Environ. Model. Assess. 2014, 19, 179–192. [Google Scholar] [CrossRef]
- Zhang, Q.; Wei, N.; Zou, C.; Mao, H. Evaluating the ammonia emission from in-use vehicles using on-road remote sensing test. Environ. Pollut. 2021, 271, 116384. [Google Scholar] [CrossRef] [PubMed]
- Nadimi, E.; Przybyła, G.; Lewandowski, M.T.; Adamczyk, W. Effects of ammonia on combustion, emissions, and performance of the ammonia/diesel dual-fuel compression ignition engine. J. Energy Inst. 2023, 107, 101158. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).