Abstract
In solid propellants, the combustion of aluminum particles often occurs in a hydrocarbon combustion atmosphere. In order to study the combustion energy release process of aluminum particles during propellant combustion, we carried out a study of the combustion behavior of aluminum particles in the combustion atmosphere of hydrocarbon fuels and conducted experiments using a plane flame burner to observe the combustion process of aluminum particles in a methane plane flame combustion atmosphere. High-speed microscopy revealed a new special combustion phenomenon: ejection combustion with the release of internal components from a point on the particle at high speed, in addition to the already observed particle microexplosions. Both phenomena show faster-than-normal combustion with short combustion energy release times. The experiments also showed that the combustion behavior of aluminum particles changes with the combustion environment. As the ambient effective oxidizer mole fraction increases from 13% to 29%, the basic combustion behavior of aluminum particles changes from vapor evaporation combustion to multiphase surface combustion. In addition, the percentage of aluminum particles burned by ejection increases from 18.2% to 49.2%, which becomes the dominant mechanism in the special combustion phenomenon of aluminum particles. This paper argues that the multiphase surface combustion provides higher heating rates due to the heat production collected on the particles and the diffusion combustion in the air around the aluminum particles, compared with the evaporation combustion. Therefore, the rate of temperature rise within the particle is affected by the ambient oxidant concentration, leading to a transformation from microexplosion to ejection combustion. The effect of the temperature of the combustion environment on this phenomenon has also been investigated through experiments conducted under different conditions.
1. Introduction
Metal powder fuels have excellent mass and volume calorific values of combustion, and therefore are widely used in solid rocket motors [1] and powder-fueled ramjets [2]. In many applications, the combustion of aluminum particles occurs in hydrocarbon fuel combustion atmosphere. The combustion mechanism of aluminum particles is different from gas combustion [3] and liquid combustion [4]. Compared to gas fuels, which are easily mixed with oxygen, the combustion reaction of aluminum particles can only occur near the surface of the particles, resulting in a huge difference in the combustion rate between the two. Compared to liquid fuels, which burn as small droplets, the combustion process is very different due to the presence of an oxide film on the surface of aluminum particles that blocks the passage of reactants. The combustion characteristics of aluminum particles are not only very different from those of common gas and liquid fuels, but the combustion process is also characterized by agglomeration, which is unique to the combustion of metal particles [5], leading to inadequate combustion and increased two-phase flow losses [6], resulting in a large gap between the actual energy performance and the theoretical performance. In order to solve this problem, a large group of researchers and scholars have conducted studies on the ignition combustion characteristics of aluminum particles [7,8]. One of the microexplosion phenomena that occurs during the combustion of metal particles can improve the combustion efficiency and reduce the two-phase flow loss [9], which reduces the energy loss to a certain extent. Therefore, a comprehensive understanding of the combustion behavior of aluminum particles in a hydrocarbon combustion atmosphere is very important for understanding the combustion behavior of aluminum particles in a real combustion environment.
With regard to the combustion phenomena of metal particles in a hydrocarbon fuel combustion environment, the main focus is on the conventional gas-phase/diffusion combustion behavior of the particles, as well as the special combustion phenomenon of microexplosions, which has now been widely observed.
Various studies have investigated the combustion behavior of aluminum particles in different atmospheres. For example, M. Soo et al. [10,11,12] found that the combustion behavior of aluminum particles differs in different atmospheres. They observed a gas diffusion flame around the aluminum particles and high concentrations of aluminum vapor in the space around the particles when burning in air. However, in a methane/air flame, the concentration of aluminum vapor around the particles was low and no elevated diffusion microflame was formed, proving that the combustion process is kinetically controlled.
Y. Feng et al. investigated the combustion of some metallic fuels in a propellant combustion atmosphere and conducted experiments on the combustion of aluminum single particles [13,14] and aluminum–magnesium alloy single particles in a methane flame [15], and found that the combustion time of micron-sized aluminum particles was greatly reduced with the increase of the effective oxidation concentration in the combustion environment, and the ignition delay time of the particles was significantly reduced with the increase of the combustion environment temperature, but the effect of temperature increase on the combustion time of the particles is limited.
Huan Liua et al. [16] found that the agglomerate size showed an increase followed by a decrease with increasing particle size of the initial metallic aluminum powder. A. Braconniera [17] conducted a combustion study of aluminum particles under different oxidizing mixtures and found that, with the increase of oxidizing agent in the environment, aluminum oxide on the droplet surface could form and accumulate rapidly, which eventually led to a shorter duration of symmetric combustion. Hongqi Nie et al. [18] found that aluminum–magnesium alloys have a shorter ignition delay time and higher combustion temperature than pure metallic aluminum particles. Jiarui Zhang et al. [19] experimentally studied turbulent jet flames of aluminum particles using high-speed photography and Mie scattering techniques to obtain turbulent jet flame structures and found that increasing the oxidation capacity of the environment could significantly increase the flame temperature and reduce the height of lift and visible length of the jet flame.
As for the combustion model of aluminum particles, Xiangrui Zou developed a predictive model for the ignition of nano- to micron-sized aluminum metal particles [20,21]. This model is based on the coupling of heat transfer and aluminum metal oxidation, which can obtain more accurate ignition parameters of aluminum metal particles in a high-temperature environment, and the ignition of micron aluminum metal particles is affected by gas flow rate, ambient radiation and oxygen concentration. Jinyun Wang [22] established a combustion model for prolate and oblate spheroidal aluminum particles to study the effect of nonspherical particles on the combustion behavior, and the results of numerical simulation are consistent with the experimental results. Paul E. DesJardin et al. [23] developed a model for the ignition combustion of aluminum particles in an air atmosphere, which models the ignition process and combustion process of aluminum particles separately and considers the transition from kinetically controlled surface reaction combustion in the ignition phase to diffusion-controlled evaporation combustion in the combustion phase as the combustion process proceeds. Ephraim B. Washburn [24] added the transition mechanism from diffusion control to chemical reaction kinetic control to the model and explored the effects of environmental parameters such as gas pressure, components, and ambient temperature on the combustion behavior of aluminum particles, and found that the combustion mechanism of aluminum particles changed to surface reaction kinetic-controlled combustion when the diameter of aluminum particles was less than 10 μm.
Microexplosion is a special type of combustion behavior that can occur during the combustion of liquid droplets or metal particles. Microexplosions have been extensively studied for multicomponent liquid hydrocarbon fuels [25,26,27], and it has been shown to reduce fuel consumption, improve air–fuel mixing and reduce emissions. However, similar work has not been properly investigated for metal powder fuels. Microexplosion of metal particles would shorten the combustion time, prevent the reduction of aluminum powder agglomeration, and allow adequate combustion while obtaining smaller-sized combustion products and reducing two-phase flow losses.
For microexplosion of particles, M.A. Rubio [28], who studied the laser ignition combustion performance of polymer-coated aluminum particles, found that for Al/PTFE particles, the laser fluence required for microexplosion to occur under laser heating decreased as the mass fraction of PTFE contained increased. Under the same heating conditions, Al/PTFE particles produced smaller combustion product fragments than Al/LDPE particles.
B.C. Terry [9] proposed a kinetic mechanism for the combustion of mixed/alloy metal droplets. For metal alloy fuels with different component volatilities, such as aluminum–-lithium alloys, a diffusion layer begins to form near the droplet surface as the more volatile component begins to burn/evaporate. As the diffusion layer forms and the internal temperature of the molten droplet continues to rise, the temperature of the aggregated droplet at the diffusion layer interface will eventually reach the superheat limit and nucleation and vaporization will begin within the particle, resulting in dispersive boiling or crushing microexplosions.
E.R. Wainwright [29] used X-ray phase-contrast imaging to study bubble nucleation and growth within particles during combustion of Al/Zr particles in solid propellants, characterizing heterogeneous nucleation, slow bubble growth and coalescence, and rapid bubble growth leading to microexplosions, and found that microexplosions of Al/Zr molten droplets generated from the propellant surface lead to much higher combustion rates and can contribute to a reduction in two-phase flow losses and an increase in combustion efficiency.
When Huang [30] et al. studied laser-induced ignition combustion of Al/Mg alloy powders, they found that the combustion process involved decomposition of the intermetallic compound phase, overflow of the metal boiling gas phase inside the particle, and multiple microexplosion processes of the same particle with microexplosion time intervals of about 300–600 μs.
H. Belal [31] found that the microexplosion tendency of Al/Mg alloy particles added to the propellant decreases at higher pressures due to reduced bubble growth, and monitored the microexplosion phenomenon during combustion by burning individual alloy particles in an oxidizing atmosphere, and found that Al/Mg alloy particles had superior performance in terms of combustion rate and product size compared to pure aluminum particles in the same environment.
In response to the various combustion mechanisms and special phenomena observed in aluminum particles during combustion, this paper investigates the combustion mechanism of aluminum particles in methane flames. Through experiments, a new special combustion behavior, ejection combustion, was observed in addition to the previously observed microexplosion combustion phenomenon. Unlike microexplosion combustion, ejection combustion involves the ejection and burning of liquid metal in a specific direction without complete rupture of the oxide layer or crushing disintegration of the particles. This paper examines both the microexplosion behavior of monolithic aluminum particles and the factors that influence ejection combustion behavior.
2. Material and Methods
The aluminum particles were obtained from Shanghai Shuitian Materials Technology Co., Ltd. (Shanghai, China), with a nominal particle size of 50 μm, and the particle size d50 obtained by laser particle size analysis was 38.639 μm, as shown in Figure 1.
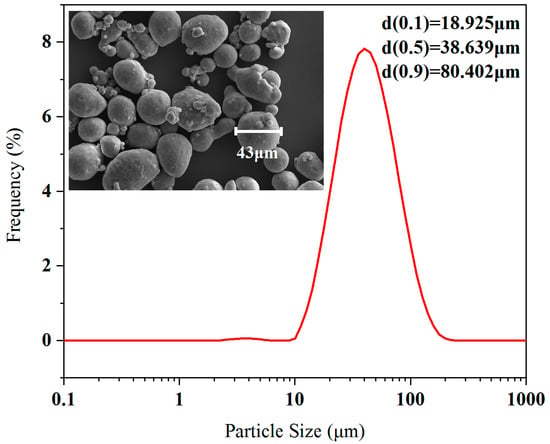
Figure 1.
Particle size distribution and SEM results.
The methane cone flame is generated as the combustion atmosphere for aluminum particle using the Central South University flat-flame combustion system, and the oxidation concentration and temperature of the aluminum particle combustion environment are controlled by controlling the flow rate of the methane air oxygen component supplied to the flat-flame furnace.
During the experiments, a high-speed camera (Photron FASTCAM MINI UX50, Produced by Photron Corporation, Japan and provided by CSU, Changsha, China) equipped with a Nikon AF-S 50mm f/1.8G fixed focal length lens was used to capture images of the burning aluminum particles in the combustion environment and to monitor the morphological changes of the particles during the combustion process. The camera was set to record in color mode at a resolution of 1280*488 with a frame rate of 5000 fps.
Aluminum powders are placed in a fluidization chamber and blown into the combustion environment using nitrogen fluidization with a controlled nitrogen flow rate of 10 L/min.
In this study, assuming adequate combustion of premixed fuel gas, the definition of effective oxidizer mole fraction, Xeff, proposed by M.W. Beckstead [32] was used to describe the ambient oxidant concentration for combustion, and CEA was used to calculate the adiabatic flame temperature of premixed fuel gas at atmospheric pressure as the reference temperature T. Two sets of tests were designed and conducted. The first set of tests T1–T4 controls the ambient effective oxidizer mole fraction Xeff at 21% and changing the reference temperature from 1600 K to 2200 K. The second set of tests O1–O5 was designed to control the reference temperature at 2000 K and changing the ambient effective oxidizer mole fraction from 13% to 29%. Both sets of tests are shown in Table 1. The T3 and O3 operating conditions were the same in both sets of tests and were not repeated, and a total of 8 operating conditions were tested.
The gas supply parameters used in this study were as follows:

Table 1.
Experimental conditions.
Table 1.
Experimental conditions.
| Case | Methane (L/min) | Oxygen (L/min) | Air (L/min) | Carrier Gas (L/min) | Effective Oxidizer Mole Fraction of Post-Combustion Gas Xeff | Reference Temperature T (K) |
|---|---|---|---|---|---|---|
| T1 | 2.30 | 9.94 | 28.76 | 10 | 21% | 1600 K |
| T2 | 2.74 | 10.20 | 28.06 | 10 | 21% | 1800 K |
| T3 | 3.21 | 10.47 | 27.32 | 10 | 21% | 2000 K |
| T4 | 3.75 | 10.79 | 26.46 | 10 | 21% | 2200 K |
| O1 | 3.18 | 7.17 | 30.65 | 10 | 13% | 2000 K |
| O2 | 3.19 | 8.82 | 28.99 | 10 | 17% | 2000 K |
| O3 | 3.21 | 10.47 | 27.32 | 10 | 21% | 2000 K |
| O4 | 3.22 | 12.12 | 25.66 | 10 | 25% | 2000 K |
| O5 | 3.23 | 13.76 | 24.01 | 10 | 29% | 2000 K |
3. Results and Discussion
3.1. Gas-Phase Combustion Flame and Combustion Behavior Transition of Aluminum Particles
The combustion of aluminum particles has been experimentally observed with the following basic combustion behavior.
The combustion test images of aluminum particles at 13%, 17%, and 21% of the effective oxidizer mole fraction and combusting ambient temperature at 2000 K were analyzed and compared in turn, as shown in Figure 2, and it was found that there was a gas-phase flame around the aluminum particles when they were burned in the methane combustion atmosphere. Since the brightness of the gas-phase flame around the particles in the original image is weak, the brightness and contrast of the original particle combustion image were increased by using the same parameters to increase the brightness and contrast of the original particle combustion image, so that the particle combustion spot and the gas-phase flame can be more visually observed. In the enhanced image, the particle burn spot itself appears white, while the color of the gas0phase flame is red. It can be seen in the figure that, as the oxygen concentration of the burning environment of the particle increases, its gas-phase flame gradually shrinks. When the oxygen concentration increases to 21% and 25%, the gas-phase flame disappears and is replaced by the particle’s own luminescence, that phenomenon is consistent with the absence of gas-phase microdiffusion flames in aluminum-methane-air flames observed previously [10].
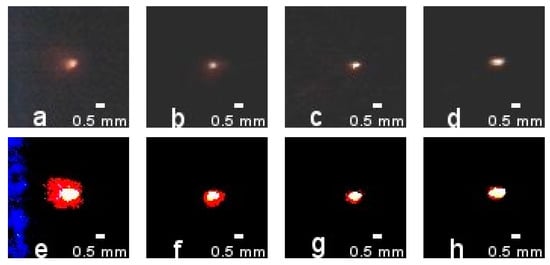
Figure 2.
Burning particles and their gas-phase flames under different Xeff at 2000 K: (a–d): particles combustion at 13%, 17%, 21%, and 25% of Xeff; (e–h): particle combustion after image enhancement.
The reduction and even disappearance of the gas-phase flame of aluminum particles burning in a methane flame is shown in Figure 3. This is because, on the one hand, the evaporation rate of aluminum at a certain temperature is not affected by the oxygen concentration and is relatively fixed, and on the other hand, as the effective oxidizer mole fraction of the environment increases, the oxidation capacity of the atmosphere around the aluminum particles and the diffusion capacity of oxygen increase. When the oxidation concentration increases, the aluminum–oxygen reaction surface is pushed to the side near the aluminum particles, so that the gas-phase flame area is reduced. When the reaction surface is pushed to the surface of the aluminum particle, the reaction mechanism changes from evaporation of aluminum vapor from the particle to diffusion of oxygen through the oxide film on the surface of the aluminum particle into the particle to react with its aluminum, i.e., from vapor combustion to multiphase surface combustion.
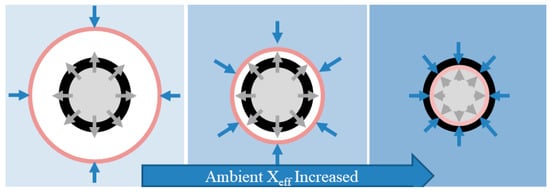
Figure 3.
The transformation process of combustion process when Xeff increased. The black layer, pink layer, blue arrow, and gray arrow indicate the surface oxide film, the Al-O reaction surface, the oxidant diffusion, and the Al (g) diffusion, respectively.
3.2. Special Combustion Phenomenon of Aluminum Particle
The use of a larger fluidized gas flow rate can prevent the complete and continuous single-particle combustion process of aluminum particles, which helps to investigate the special combustion mechanism of aluminum particles. The combustion phenomena observed in the experiments are basically special combustion phenomena, and it is rarely observed that the aluminum particles undergo a complete and continuous single-particle combustion process. This is because the experiments were designed taking into account that the conventional gas-phase combustion flame of aluminum particles affects and blocks the special combustion signal, and the particles cannot maintain the conventional combustion in a high fluidized gas flow environment, and because the special combustion is fast and short, and the fluidized gas flow rate has little effect, so the special combustion phenomenon was separately screened by using a high flow rate fluidized gas method and used to conduct the study. The experimental results also show that most of the captured combustion phenomena are special combustion phenomena, indicating the effectiveness of the high flow rate fluidized gas method.
Two special combustion phenomena are distinguished and defined: shattering microexplosion and ejection, and gas-phase combustion of particles and surface reaction combustion are called basic combustion phenomena. In this paper, the special combustion phenomena captured by high-speed photography are categorized according to the following bases: the combustion behavior that satisfies particle breakage and releases internal molten aluminum in a non-single direction is called shattering microexplosion; the combustion behavior that releases several smaller droplets in one direction is called dispersion boiling; the combustion behavior that generates an ejection of aluminum particles with a eject velocity much higher than the original particle motion velocity is called ejection.
3.3. Microexplosion Phenomenon of Aluminum Particles
The process of microexplosion is as follows, which is mainly characterized by the rupture of the entire aluminum particle and the release of the internal liquid-phase molten aluminum component.
Figure 4 and Figure 5. are the complete microexplosion combustion records of two cases of aluminum particles. The microexplosion combustion process is illustrated in Figure 6. Aluminum particles glow, while its surrounding gas-phase flame glows; then the spot area increased significantly, the central brightness increases, and the release of internal components in all directions, smoke-like, is thus called microexplosion.

Figure 4.
Continuous images of the microexplosion process of aluminum particles with a particle size of 50 μm at Xeff of 13%, 2000 K, Δt = 200 μs.

Figure 5.
Continuous images of the microexplosion process of aluminum particles with a particle size of 50 μm at Xeff of 17%, 2000 K, Δt = 200 μs.
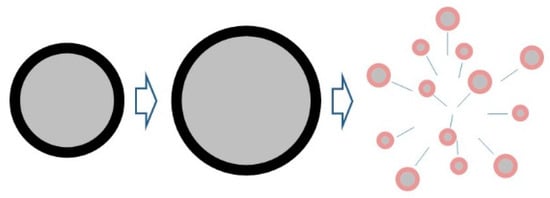
Figure 6.
Diagram of the particle microexplosion process. The black layer indicates the surface oxide film, the gray layer indicates the internal aluminum, and the red layer indicates the oxidation reaction surface.
The microexplosion mechanism of monolithic metal particles is as follows: metal particles with a surface oxide film in a high-temperature oxidation environment, due to their own heat and their own oxidation heat generation and heating, internal aluminum melting and even evaporation, volume increase; surface oxide film for the melting point boiling point higher metal oxide phase, at the temperature is still a solid phase and relatively rigid, difficult to deform, will prevent the internal components of the volume increase, while the volume of internal components by Tension is generated in the oxide film by the internal component volume stress. When the surface oxide film can withstand the stress, continues to increase, so the tension on the oxide film to reach the limit of the oxide film can withstand, the oxide film broken, the internal aluminum components to the direction of the spray, thus microexplosion occurs.
3.4. Ejection Combustion Phenomenon of Aluminum Particles
The process of spraying is as follows, the main feature of which is that the aluminum particles break from a point on the surface and eject the internal molten aluminum composition in a bundle.
Figure 7 and Figure 8 are the complete ejection combustion records of two cases of aluminum particles. The ejection combustion process is illustrated in Figure 9: the aluminum particle glows, then ejects the hot material inside at high speed in a particular direction, then the ejected material slows down.
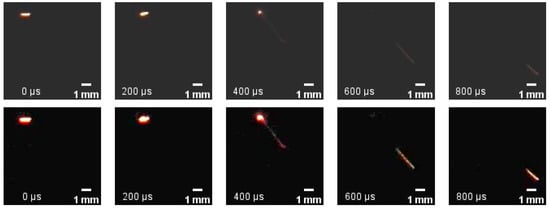
Figure 7.
Ejection combustion process at Xeff of 17% and 2000 K with a shot interval of Δt = 200 μs. Downstream is image after enhancement of upstream image.

Figure 8.
Ejection combustion process at Xeff of 21% and 2200 K with a shot interval of Δt = 200 μs. Downstream is image after enhancement of upstream image.
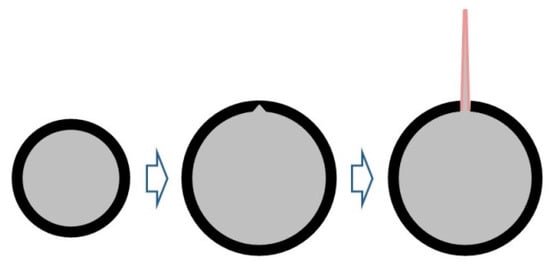
Figure 9.
Diagram of the particle ejection process. The black layer indicates the surface oxide film, the gray layer indicates the internal aluminum, and the red layer indicates the oxidation reaction surface.
The process of ejection combustion of aluminum particles at 2200 K in the diagram is as follows: the aluminum particle first heats up and glows, performing the basic combustion behavior, and then when the temperature reaches the ejection critical temperature for that particle, the internally molten aluminum is ejected from a point in the burning aluminum particle. After ejection, the ejected portion decelerates and burns rapidly, and the original aluminum particle disappears from view. The ejection mechanism of the monolithic metal particles is similar to that of a microexplosion, except that as the stress on the oxide film continues to increase due to expansion of the internal constituents, the internal aluminum constituents are ejected from the fracture, and combustion occurs due to localized fracture of the oxide film at a defect in the aluminum oxide organization on the film or at a reduced thickness due to stress concentration.
The phenomenon photographed here is an ejection of tissue within the particle, rather than a mere particle motion resulting in a trailing shadow, since the ejection beam speed was calibrated to reach an average of 20 m/s and a maximum of over 40 m/s, while the average motion speed of aluminum particles in the current environment is only 1–2 m/s. The speed of the ejection beam is an order of magnitude greater than the original speed of particle motion, which rules out the possibility that the ejection beam image is simply a trailing shadow of the particle’s own motion. The possibility of a trailing shadow of the particles themselves can be ruled out. The velocity of the ejected beam is suddenly accelerated from a low velocity to a high velocity, and then decelerated back to a low velocity due to the sudden acceleration caused by the ejection of the internal components of the particles, after which the velocity of the ejected material is much greater than the velocity of the air stream and is subject to the drag of the air stream in the opposite direction to that of the motion, resulting in a deceleration to the pre-ejection velocity level.
3.5. Factors Influencing the Unique Combustion Phenomenon of Aluminum Particle
The root cause of particle microexplosion and ejection of two special combustion phenomena with the same particle heating, but there are differences in the phenomenon. In order to investigate the causes, the following study was conducted.
Statistics in a period of time (1000 frame, 200 ms) appeared in the high-speed camera field of view of all special combustion phenomena, according to certain rules to distinguish their combustion phenomena and categorize the count, in order to study the dominant mechanism of aluminum particles in different environments when burning.
When the oxidation concentration of the combustion environment is 13%, the presence of gas-phase flame on the particle surface is seen when the sprayed aluminum particles are burned before spraying. This indicates that, at low oxidation concentrations, the aluminum particles are ejection-burned according to the heat-evaporation combustion-breakout ejection process. The intensity of the gas-phase flame decreases when the oxidation concentration of the combustion environment increases to 17%. When the oxidation concentration increases to 21%, the gas-phase flame disappears. The phenomenon that the gas-phase flame becomes weaker and finally disappears as the effective oxidizer mole fraction of the combustion environment increases indicates that the basic combustion mechanism of aluminum particles changes from vaporization combustion to diffusion combustion as the oxidation concentration increases.
Observing and counting the combustion phenomenon of aluminum particles in each working condition, it was found that, with different combustion environments, the proportion of aluminum particles combusted according to the microexplosion combustion; dispersive boiling combustion and ejection combustion mode has changed.
First, the temperature of the combustion environment was set at 2000 K. The effect of the effective oxidizer mole fraction of the combustion environment on the composition of the peculiar phenomenon of aluminum particle combustion was studied. With the change of effective oxidizer mole fraction, the change of percentage of special combustion of aluminum particles is shown in Figure 10.
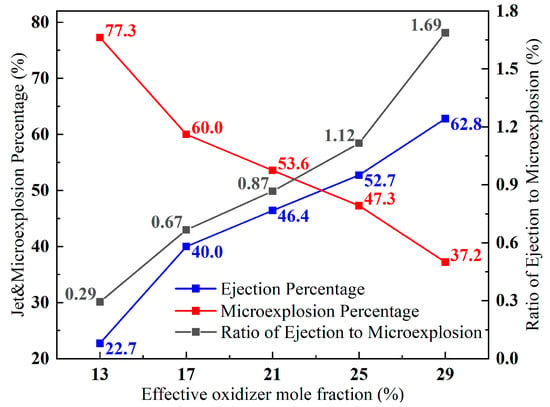
Figure 10.
Relationship between the effective oxidizer mole fraction and the percentage of two combustion phenomena.
At the effective oxidizer mole fraction of 13%, according to the microexplosion mechanism for the combustion of aluminum particles accounting for 77.3% of all special combustion of aluminum particles, while the ejection and the phenomenon accounted for 22.7% of the total, the ratio of the number of the two is 0.29, indicating that the microexplosion is the main special combustion phenomenon under the oxidation concentration. With the increase of oxidation concentration of the combustion environment, in all aluminum particles of special combustion phenomena, the proportion of microexplosion decreased, and the proportion of ejections increased. Oxidation concentration increases to 25%, microexplosions accounted for 47.3%, while the proportion of ejections accounted for 52.7%, more than microexplosions, so the ejections become the main special combustion phenomenon under high oxidation concentration. When the effective oxidizer mole fraction of the environment reaches 29%, the ejection phenomenon accounts for 62.8%, and the ratio of the number of ejections to microexplosions reaches 1.69.
Considering the phenomenon that the combustion mechanism of aluminum particles changes from evaporation combustion to multiphase surface reaction combustion when the ambient oxidation concentration increases, and the phenomenon that the particles change from microexplosion combustion to ejection combustion, changes that occur with the increase of the ambient oxidation concentration, there should be a correlation between them.
The basic reason for the occurrence of the special combustion phenomenon of aluminum particles is the heating of the particles. After the aluminum particles enter the combustion environment, the special combustion of aluminum particles occurs mainly according to the development of the process of heated heating–basic combustion–special combustion–end of combustion cooling. In the process of heated heating, the source of heat required for heating comes mainly from convection and radiation heat exchange in the background of the ambient temperature higher than the temperature of the particles, followed by the heat generated by the slow oxidation of the particles themselves; in the process of basic combustion, the particles undergo intense combustion and glow when the temperature of the particles themselves is higher than the ambient temperature, the heat required for heating comes from the difference between the heat released by oxidation and the heat lost by the action of the environment. At this stage, evaporation combustion and surface reaction combustion result in the difference in particle heating rate. In evaporative combustion, aluminum vapor leaves the particle and enters the environment, where the oxidation reaction occurs. When the reaction occurs in the environment, only part of the heat released is absorbed by the particles, causing the particles to heat up, while when the particles are subjected to surface reaction combustion, the reaction occurs on the surface of the particles, and the proportion of the heat obtained for the particles to the exothermic heat of the reaction is greater than the proportion of the heat obtained by the particles to the exothermic heat of the reaction when the aluminum vapor leaves the particles and reacts in the environment. Therefore, the heating rate of the particles by the surface reaction combustion process is faster compared to the combustion of the particles by the evaporation combustion process. Thus, compared with the particles burned by the evaporation combustion process, the particles burned by the surface reaction combustion process, the heating rate of the particles is faster.
While the aluminum particles for evaporation combustion, because the rate of evaporation combustion is subject to the evaporation rate, and the evaporation rate is only affected by the particle temperature and the particle itself size composition and other factors, so the temperature is a certain case, the rate of evaporation combustion of aluminum particles is constant. At this time, with the effective oxidizer mole fraction of the environment in which the particles are located, the reaction surface of aluminum vapor and the oxidant in the environment moves to the side of the particles, and the closer the reaction surface is to the particles, the higher the proportion of reaction heat release absorbed by the particles, the faster the heating rate. Therefore, when the particles are burned by evaporation, the heating rate of the particles increases with the increase of the ambient oxidant concentration.
When the aluminum particles are burned by surface reaction, the reaction rate is controlled by the reaction kinetics, and increasing the effective oxidizer mole fraction will directly increase the reaction rate, resulting in the increase of the particle heating rate.
In summary, as the ambient oxidation concentration increases, the combustion process of the particles themselves exists from evaporation combustion—evaporation combustion, but the reaction surface moves to the side of the particles—the reaction surface moves to the surface of the particles, the reaction mechanism changes to multiphase surface reaction combustion–surface reaction combustion process, and the particle heating rate. In turn, the rate of particle heating increases.
Particles of the special combustion phenomenon occur, because the thermal stress during the heating process exceeds the degree of tolerance of the oxide film on the surface of aluminum particles, so as the oxidation concentration increases, the special combustion phenomenon from the ejection to microexplosion shifts, which is due to the different heating rates of particles. The occurrence of ejections on the temperature sensitivity compared to the occurrence of microexplosion on the temperature sensitivity is higher; with the increase in oxidation concentration, the particle heating rate also increased, resulting in the particle special combustion phenomenon from microexplosion to ejections.
In order to test this conjecture, a series of experiments was designed to keep the effective oxidizer mole fraction of the environment constant, only changing the temperature of the combustion environment. Since the particle heating rate increases with increasing ambient temperature, and no other effects are introduced when only the ambient temperature is increased, experiments T1–T4 were conducted to verify the effect of the heating rate on the specific combustion phenomenon of aluminum particles. When the ambient temperature increases, the particle combustion occurs when the ejection phenomenon increases; it can be verified that the particle temperature rise rate leads to the particle special combustion mechanism reason for the microexplosion to ejection transformation.
With the change in ambient temperature, the change in percentage of special combustion of aluminum particles is shown in Figure 11.
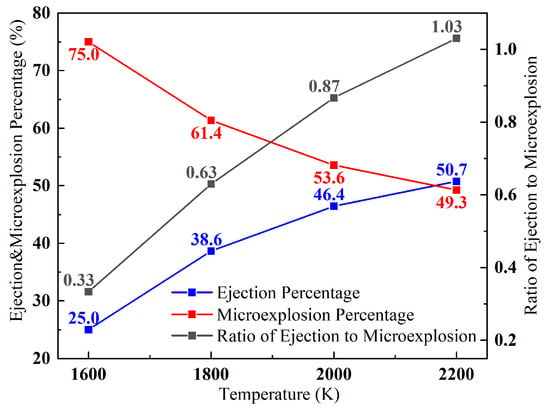
Figure 11.
Relationship between the ambient temperature and the percentage of two combustion phenomena.
At an ambient temperature of 1600 K, microexplosion aluminum particles accounted for 75% of all special combustion aluminum particles for the current combustion temperature of the dominant combustion phenomenon with the ambient temperature increases, the proportion of ejections increases, and the proportion of microexplosion decreases. When the ambient temperature reaches 2200 K, the proportion of ejection phenomenon reaches 50.7%, and the ratio of the number of ejections to microexplosion reaches 1.03. The change in the pattern of ejections and microexplosion as the ambient temperature increases is the same as in the conjecture, which confirms that the particle heating rate leads to a shift from microexplosion to ejections.
In addition, comparing the special combustion ejection/microexplosion ratios at equivalent oxidation concentrations from 13% to 17% and ambient temperatures from 1600 K to 2200 K shows a high correlation between the two (Figure 12). Statistical analysis of the Pearson’s correlation coefficient between the two factors Pearson’s R reached 0.99582 with a p-value of 0.00418, indicating an extremely strong linear correlation between the two. Thus, for the ejection/microexplosion ratio indicator, increasing the temperature alone and increasing the ambient oxidant concentration alone have very similar effects in this range, with oxidant concentrations of 13% to 25% for each 4% increase in the ratio of the number of particle ejection/microexplosion combustion phenomena having essentially the same effect as the temperature from 1600 K to 2200 K for each 200 K increase, indicating that, in this interval, the temperature factor and the effect of the oxidation concentration factor on the ratio of the special combustion phenomena of the two particles are in accordance with a certain equivalence relationship, said equivalence relationship being T = 5000 Xeff + 950.
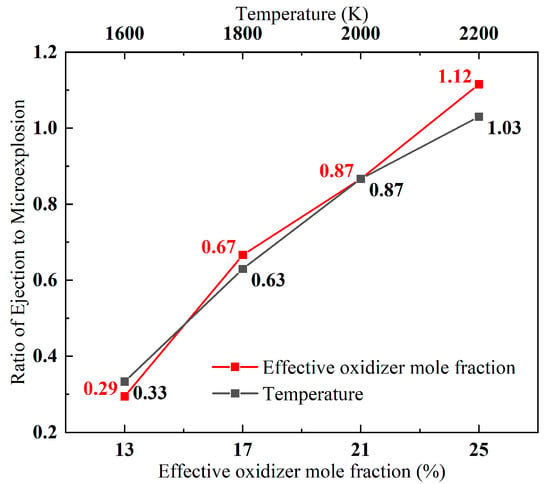
Figure 12.
Effect of oxidation concentration and temperature on combustion phenomena.
4. Conclusions
In this study, we focused on the combustion behavior of aluminum particles under methane–air flame and observed the normal combustion phenomenon of single-particle gas-phase combustion, as well as the special combustion phenomenon of microexplosion and ejection by high-speed photography. The effects of combustion ambient temperature and effective oxidant molar concentration on the combustion behavior of aluminum particles were analyzed and obtained.
It was found that the gas-phase flame of aluminum particle combustion decreases as the oxidation concentration of the combustion environment increases, and the gas-phase flame disappears when the oxidation concentration increases to 21% at 2000 K. On the other hand, as the oxidation concentration of the combustion environment increases, the proportion of ejections in the special combustion phenomenon of particles decreases and the proportion of microexplosion increases. The heating rate of aluminum particles increases with the oxidation concentration of the environment, and the increase in the heating rate leads to the occurrence of particle ejections, which are more sensitive to the heating rate, in preference to microexplosion. Experiments with a single temperature variable have verified that changes in particle heating rate lead to changes in particle-specific combustion phenomena, and that temperature and equivalent oxygen concentration have a similar effect on the change in ejection/microexplosion ratio, and that the equivalent relationship between these two factors is T = 5000Xeff + 950.
This research helps to better utilize the microexplosion and ejection behavior of aluminum particles, improve the combustion efficiency of aluminum particles, and take advantage of the energy of aluminum particles, thus improving the thrust and specific impulse performance of the engine. Due to the limitation of experimental conditions, this study only investigated the effect of combustion environment parameters on the macroscopic ratio of the two phenomena for the injection of aluminum particles and the micro-explosion phenomenon, while the combustion energy release process of aluminum particles under the two mechanisms was not studied deeply enough. Future research should start from the visualization and quantification of the special combustion process of aluminum particles.
Author Contributions
Conceptualization, S.X.; data curation, K.M.; formal analysis, S.X.; investigation, Y.L.; methodology, S.X.; project administration, H.L.; resources, W.X.; software, W.X.; supervision, H.L.; validation, K.M.; visualization, Y.L.; writing—original draft, S.X.; writing—review and editing, S.X. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Defense Science and Technology Key Laboratory Fund (Fund No. 6142603032103).
Data Availability Statement
Calculation data are available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yuan, J.; Liu, J.; Zhou, Y.; Wang, J.; Xv, T. Aluminum agglomeration of AP/HTPB composite propellant. Acta Astronaut. 2019, 156, 14–22. [Google Scholar] [CrossRef]
- Smirnova, M. Mars transportation vehicle concept. Acta Astronaut. 2014, 103, 250–256. [Google Scholar] [CrossRef]
- Yan, Z.; Brendan, H.; Shubham, S.; Valeriy, S.; Vincent, M. Decarbonized combustion performance of a radiant mesh burner operating on pipeline natural gas mixed with hydrogen. Int. J. Hydrog. Energy 2022, 47, 18551–18565. [Google Scholar]
- Safiullah; Samir, C.R.; Keiya, N.; Vincent, M.; Yoichi, O. Effects of full transient Injection Rate and Initial Spray Trajectory Angle profiles on the CFD simulation of evaporating diesel sprays- comparison between singlehole and multi hole injectors. Energy 2023, 263, 125796. [Google Scholar] [CrossRef]
- Chu, Q.; Shi, B.; Liao, L.; Zou, X.; Luo, K.H.; Wang, N. Reaction Mechanism of the Aluminum Nanoparticle: Physicochemical Reaction and Heat/Mass Transfer. J. Phys. Chem. C 2020, 124, 3886–3894. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Yang, Z.-L.; Wang, M.-J. Investigation of nozzle two-phase flow characteristics for nanometer aluminum powder combustion in a metal fuel motor. Powder Technol. 2018, 339, 446–458. [Google Scholar] [CrossRef]
- Jin, B.-N.; Wang, Z.-X.; Xu, G.; Ao, W.; Liu, P.J. Three-dimensional spatial distributions of agglomerated particles on and near the burning surface of aluminized solid propellant using morphological digital in-line holography. Aerosp. Sci. Technol. 2020, 106, 106066. [Google Scholar] [CrossRef]
- Li, L.-B.; Chen, X.; Zhou, C.-S.; Zhu, M.; Musa, O. Experimental and numerical investigations of the effect of pressure and oxygen concentration on combustion characteristics of Al/Mg fuel-rich propellants. Appl. Therm. Eng. 2020, 167, 114695. [Google Scholar] [CrossRef]
- Terry, B.C.; Gunduz, I.E.; Pfeil, M.A.; Sippel, T.R.; Son, S.F. A mechanism for shattering micro explosions and dispersive boiling phenomena in aluminum–lithium alloy based solid propellant. Proc. Combust. Inst. 2016, 36, 2309–2316. [Google Scholar] [CrossRef]
- Soo, M.; Julien, P.; Goroshin, S.; Bergthorson, J.M.; Frost, D.L. Stabilized flames in hybrid aluminum–methane–air mixtures. Proc. Combust. Inst. 2013, 34, 2213–2220. [Google Scholar] [CrossRef]
- Julien, P.; Soo, M.; Goroshin, S.; Frost, D.L.; Bergthorson, J.M.; Glumac, N.; Zhang, F. Combustion of aluminum suspensions in hydrocarbon flame products. J. Propuls. Power 2013, 30, 1047–1054. [Google Scholar] [CrossRef]
- Soo, M.; Goroshin, S.; Glumac, N.; Kumashiro, K.; Vickery, J.; Frost, D.L.; Bergth-Orson, J.M. Emission and laser absorption spectroscopy of flat flames in aluminum suspensions. Combust. Flame 2017, 180, 230–238. [Google Scholar] [CrossRef]
- Feng, Y.; Xia, Z.; Huang, L.; Ma, L. Ignition and combustion of a single aluminum particle in hot gas flow. Combust. Flame 2018, 196, 35–44. [Google Scholar] [CrossRef]
- Feng, Y.; Xia, Z.; Huang, L.; Ma, L. Effect of ambient temperature on the ignition and combustion process of single aluminium particles. Energy 2018, 162, 618–629. [Google Scholar] [CrossRef]
- Feng, Y.; Ma, L.; Xia, Z.; Huang, L. Ignition and combustion characteristics of single gas-atomized Al-Mg alloy particles in oxidizing gas flow. Energy 2020, 196, 117036. [Google Scholar] [CrossRef]
- Liu, H.; Ao, W.; Hu, Q.; Liu, P.; Hu, S.; Liu, L.; Wang, Y. Effect of RDX content on the agglomeration, combustion and condensed combustion products of an aluminized HTPB propellant. Acta Astronaut. 2020, 170, 198–205. [Google Scholar] [CrossRef]
- Braconnier, A.; Chauveau, C.; Halter, F.; Gallier, S. Experimental investigation of the aluminum combustion in different O2 oxidizing mixtures: Effect of the diluent gases. Exp. Therm. Fluid Sci. 2020, 117, 110110. [Google Scholar] [CrossRef]
- Nie, H.; Pisharath, S.; Hng, H.H. Combustion of fluoropolymer coated Al and Al–Mg alloy powders. Combust. Flame 2020, 220, 394–406. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, Z.; Ma, L.; Huang, L.; Feng, Y.; Yang, D. Experimental study on aluminum particles combustion in a turbulent jet. Energy 2021, 214, 118889. [Google Scholar] [CrossRef]
- Zou, X.; Wang, N.; Liao, L.; Chu, Q.; Shi, B. Prediction of nano/micro aluminum particles ignition in oxygen atmosphere. Fuel 2020, 266, 116952. [Google Scholar] [CrossRef]
- Zou, X.; Wang, N.; Wang, J.; Feng, Y.; Shi, B. A numerical investigation on heterogeneous combustion of aluminum nanoparticle clouds. Aerosp. Sci. Technol. 2021, 112, 106604. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Z.; Wang, M. Effect of non-spherical particles on burning behavior during aluminum combustion. Particuology 2020, 51, 173–183. [Google Scholar] [CrossRef]
- Bojko, B.T.; DesJardin, P.E.; Washburn, E.B. On modeling the diffusion to kinetically controlled burning limits of micron-sized aluminum particles. Combust. Flame 2014, 161, 3211–3221. [Google Scholar] [CrossRef]
- Washburn, E.; Gross, M.; Smith, S.; Balachandar, S. Fundamental Simulation of Aluminum Droplet Combustion. In Proceedings of the 46th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit 2010, Nashville, TN, USA, 25–28 July 2010; AIAA: Reston, VA, USA, 2010; p. 6677. [Google Scholar]
- Antonov, D.V.; Fedorenko, R.M.; Strizhak, P.A. Micro-Explosion Phenomenon: Conditions and Benefits. Energies 2022, 15, 7670. [Google Scholar] [CrossRef]
- Won, J.; Baek, S.W.; Kim, H.; Lee, H. The Viscosity and Combustion Characteristics of Single-Droplet Water-Diesel Emulsion. Energies 2019, 12, 1963. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, H.; He, Y.; Zhu, Y.; Wang, Z. Evaporation, Autoignition and Micro-Explosion Characteristics of RP-3 Kerosene Droplets under Sub-Atmospheric Pressure and Elevated Temperature. Energies 2022, 15, 7172. [Google Scholar] [CrossRef]
- Rubio, M.A.; Gunduz, I.E.; Groven, L.J.; Sippel, T.R.; Han, C.W.; Unocic, R.R.; Ortalan, V.; Son, S.F. Microexplosions and ignition dynamics in engineered aluminum/polymer fuel particles. Combust. Flame 2017, 176, 162–171. [Google Scholar] [CrossRef]
- Wainwright, E.R.; Lakshman, S.V.; Leong, A.F.T. Viewing internal bubbling and microexplosions in combusting metal particles via x-ray phase contrast imaging. Combust. Flame 2019, 199, 194–203. [Google Scholar] [CrossRef]
- Huang, X.; Yang, Y.; Hou, F.; Li, S.; Qin, Z.; Zhao, F. Laser-Induced Ignition and Combustion of Al Mg Alloy Powder Prepared by Melt Atomization. Propellants Explos. Pyrotech. 2020, 45, 1645–1653. [Google Scholar] [CrossRef]
- Belal, H.; Han, C.W.; Gunduz, I.E.; Ortalan, V.; Son, S.F. Ignition and combustion behavior of mechanically activated Al-Mg particles in composite solid propellants. Combust Flame 2018, 194, 410–418. [Google Scholar] [CrossRef]
- Beckstead, M.W. Correlating aluminum burning times. Combust. Explos. Shock Waves 2005, 41, 533–546. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).