Abstract
The physical and mechanical properties of rocks can be reduced significantly by an acidic environment, resulting in engineering weaknesses, such as building foundation instability, landslides, etc. In order to investigate the mechanical properties of rocks after hydrochemical erosion, a chemical damage constitutive model was established and used to analyze chemical damage variables and energy transformation. It is assumed that the strength of the rock elements obeyed Weibull distribution, considering the nonuniformity of rock. The chemical damage variable was proposed according to the load-bearing volume changes in the rock under water–rock chemical interactions. The chemical damage constitutive model was derived from coupling the mechanical damage under the external load and the chemical damage under hydrochemical erosion. In order to verify the accuracy of the model, semi-immersion experiments and uniaxial compression experiments of black sandy dolomite were carried out with different iron ion concentrations. Compared with the experimental data, the chemical damage constitutive model proposed could predict the stress–strain relationship reasonably well after water–rock interaction. The effects of water–rock interaction on the rock were a decrease in peak stress and an increase in peak strain. The peak strain increased by 4.96–29.58%, and the deterioration rate of peak strength was 0.19–4.18%. The energy transformation of the deterioration process was analyzed, and the results showed that the decrease in releasable elastic energy, Ue, is converted into dissipated energy, Ud, after hydrochemical erosion.
1. Introduction
Water–rock interaction, also known as hydrochemical corrosion, refers to a variety of physical and chemical interactions between the aqueous ions and the rocks [1,2]. The effect of water–rock interactions on the rocks is realized by changing the composition of the chemical elements and influencing their fine microstructure [3,4]. Under the effect of dissolved oxygen and water, the sulfide minerals and organic matters produce acidic water and form an acidic-water environment through the water–rock chemical interactions. [5,6] The acidic water environment causes the rapid weathering of the rock-forming minerals and the instability of the material structures, causing changes in volume, cementation force, and the internal stress of the rocks [7,8]. The rebalancing of the resultant force and the internal stress changes the internal structure and mechanical properties of the rocks [9,10,11]. Rock corrosion in an acidic environment accelerates the water–rock chemical reaction in the rock pores, forming the dominant weathering surfaces and the high energy zones of the fracture or joints in the pores, such as the mineral cleavage surfaces, fractured surfaces, and mechanical damage zones. Under the chemical interaction between water and rock, the structure and composition of the black rocks change and the mechanical properties deteriorate, leading to a series of geological disasters, such as rock avalanches, landslides, etc. [12,13,14].
The constitutive models of rock are the basis for the structure design, and the establishment of constitutive models relays the macro- and micromechanics, such as stress-strain relations, energy transformation, etc. [15,16,17]. Based on the maximum loss principle, Vasarhelyi and Davarpanah established an elastic-plastic damage model, achieving a strong coupling between plasticity and damage [18]. Some scholars have revealed the mechanism of rock damage and failure processes via a continuum and fracture mechanics [19,20]. Rocks contain a large number of randomly distributed defects. These defects complicate their mechanical properties and constitutive relations. Thus, some constitutive models are mainly derived through microcracks or crack propagation under the load effect [21]. Meanwhile, the long-term effects of water–rock interaction will eventually lead to chemical weathering and the physical and mechanical performance degradation of the rocks [22,23]. Compression tests were conducted to evaluate the relationship between water content and the strength of different stones, and the results showed that the uniaxial compressive strength of sedimentary rock decreased obviously with water content [24,25,26]. In previous studies, there have been a lot of developments in rock damage constitutive models [27,28,29,30]. However, the study of the chemical constitutive models of black dolomite is lacking and needs further exploration.
According to the laws of thermodynamics, the deformation and failure process of rock under external loads is essentially an energy absorption process, which transforms energy from the outside to the inside. Chen et al. explained the rock deformation and failure process and the energy conversion characteristics (from the perspective of nonequilibrium thermodynamics), and they divided the energy absorbed and transformed from the outside into the dissipated energy and the released elastic energy [31,32]. The dissipated energy was defined as a unidirectional irreversible process used to produce the plastic deformation of a rock mass and new internal damage, which was the main cause of rock strength loss [33]. At peak strength, the energy input into the rock exceeds the limit of the maximum bearing capacity of the elastic energy, causing sudden failure and energy release. Elastic energy release is also the main cause of rock failure [34]. The water–rock interaction has a great influence on rock energy, but the mechanism needs more discussion.
In order to better analyze the mechanism of water–rock interactions and predict mechanical rock behavior, a chemical damage model was proposed for black rocks. The constitutive model of rock under uniaxial compression was established, and chemical damage was considered via a chemical damage variable. The uniaxial compression tests were carried out on the rock samples under the water–rock interaction to verify the accuracy of the model. The energy transformation after water–rock interaction was analyzed to explain the chemical damage mechanism.
2. Chemical Damage Model Establishment
2.1. Damage Constitutive Model
The rock damage constitutive model was established by damage theory. The relationship between the stress–strain curves and the damage variables is often established from the random distribution of rock microelement strength to obtain the Lemaitre-J strain equivalent hypothesis of the damage constitutive model [35]. According to this hypothesis, the strain with the damage is equivalent to that without damage under effective stress. The strain produced by the isotropic and elastic materials under the effective stress is
where E and E′ are the Young’s moduli for the undamaged and damaged rocks, respectively, and σ and σ′ are the stress and the effective stress, and ε is the strain.
The material deforms under the external load until its failure. The crack porosity changes cannot be described by the internal porosity of crack interactions and mutual influence using geometry due to no single controlling factor. Therefore, the numerous scattered regions of the microcracks are regarded as nonhomogeneous fields. An irreversible field variable is defined to describe the damage state of the entire uniform field. This field variable is defined as the damage variable D to characterize the deterioration degree [36]. The relationship between the elastic moduli of the material before and after damage is proposed below.
where E and E′ are the Young’s moduli for the undamaged and damaged rocks. According to Equations (1) and (2), the damage constitutive model under uniaxial compression can be obtained as
The rock damage variable is the degree of material damage, and it is related to the defects in each primitive body, which directly affects its strength. The material inside the mesoscopic structure has obvious heterogeneity, and it contains a variety of defects. The distribution of defects and damage are randomly distributed in the rock and make a significant difference, and thus the mechanical property of the rock is a random variable. The rock can be divided into some microelements containing several defects. The relationship between the statistical distribution density of the microelements and the damage variables is assumed as:
where, P() is a damage measure function of a microelement under loading. In this paper, it is assumed that the microelement strength in the loading process follows the Weibull distribution, and its probability density function is proposed:
where is the average elemental maximum deformation and ξ is the Weibull distribution shape coefficient. Considering Equations (3) and (4), the following relation can be obtained:
By integrating Equation (6), the damage variable D can be obtained as follows:
From Equation (3), the damage constitutive model under uniaxial compression can be expressed as:
2.2. Chemical Damage Constitutive Model
According to the equivalent strain principle, the strain under the hydrochemical reaction is equivalent to that of the undamaged material.
where E and E″ are the Young’s moduli for the undamaged and damaged rocks under oxidative acid corrosion, respectively, σ and σ″ are the effective stress of the undamaged and damaged materials under corrosion, respectively, and ε is the strain of the rocks. Similar to Equation (2), the damage constitutive model of the rocks under uniaxial compression can be deduced as follows:
where Dn is the damage variable of the rock under an external load after oxidation and acid etching. Compared with the uncorroded rocks, the mechanical properties of the rocks under the water–rock interaction are degraded. In this case, the damage caused by the external load can be regarded as the total damage of the rock caused by the coupling of the chemical damage and the load damage [37]. Therefore, a chemical damage variable, Dc, should be introduced.
The chemical mineral sensitivity of rock is consumed under the water–rock interaction, and the cementing force between the components weakens, causing the rock mechanical performance degradation. The detailed chemical reaction process will be discussed in Section 4.2. Therefore, the effects of the water–rock interaction on the rock mainly manifest as the chemical dissolution of the rock minerals and cement. On a macrolevel, that is reflected in the loss of rock mass. According to the relationship between the change in the effective bearing volume and the mass, the chemical damage variable, Dc, is expressed as [38]:
where V and ΔV are the initial effective bearing volume of the rocks and the change in the effective bearing volume, respectively, m0 is the mass of the rock material, and m is the mass of the rock material after water–rock interaction.
Based on the generalized equivalent strain principle of rocks, the material begins to damage and expand under the external load [39]. Its damage state changes as the interaction progresses. The strain of the first damage state applied to the material in the second damage state is equivalent to that of the second damage state applied to the material in the first damage state. The damage states in the hydrochemical interaction and the natural state are defined as the first and the second damage states, respectively. The following results can be obtained:
where σ′ and σ″ are the effective stress under the natural damage state and the oxidized acid corrosion damage state, respectively, E′ and E″ are the elastic moduli of the natural damage state and the oxidized acid corrosion damage state, and Dc is the chemical damage variables of the rocks subjected to oxidative acid etching. From Equations (2), (10), and (12), the following can be obtained:
According to Equations (6), (7), and (11), the following can be obtained:
At the initial stage of the uniaxial compression, the internal microcracks are pressed and closed under the external load when the stress–strain curve is in the nonlinear change stage. In the case of no chemical corrosion, the stress–strain relationship of the rock at this stage can be taken as [40]
where σA and εA are the maximum stress and strain in the initial nonlinear change stage.
In the nonlinear change stage, the energy stored in the rock is less than its storage capacity limit, and the internal damage caused by the external load in the rock can be ignored. Only the damage caused by the chemical corrosion exists in this stage. Therefore, the strain subjected to chemical corrosion in the nonlinear change stage should be
Furthermore, the stress–strain relationship of the chemically eroded rock at this stage is modified as follows:
According to Equations (16) and (19), the chemical damage constitutive model of rock is as follows:
and in the above formula:
where σA and εA are the maximum stress and strain in the initial nonlinear change stage, respectively, εP is the peak strain of the sample, and ξ is the shape parameter of the distribution function, defined as the uniformity coefficient of the material medium.
3. Validation of the Model
3.1. Validation Preparation
The outcrop of black rock of the Cambrian in Chongqing, China, was analyzed as the research object, for which the main lithology is black sandy dolomite. It contains a certain amount of pyrite, which is easily oxidized and intensifies the water–rock interaction [41]. In a natural environment, the black sandy dolomite interacts with water easily to produce acid water, dissolving the rock and releasing an amount of Fe3+ into the pore [42]. As one of the main controlling factors affecting the water–rock interaction, Fe3+ plays an important role in the oxidation reaction. It can induce a series of complex physical and chemical reactions within the rock, leading to microscopic changes in the rock mass. The cementation characteristics and mechanical properties of the rock skeleton change under water–rock interaction. This causes a deterioration in the macro mechanical properties [43]. In order to explain the rock chemical degradation mechanism and verify the proposed constitutive model, the semi-immersion tests were carried out under different concentrations of Fe3+ in an acidic medium.
The rock blocks with a relatively consistent lithology were selected to reduce the sample differences. The composition analysis was performed on three black dolomite samples: CK-01, CK-02, and CK-03. The X-ray diffraction (XRD) results are shown in Figure 1, and the mineral compositions are demonstrated in Table 1. The black sandy dolomite contained 31.70–36.80% of quartz, dolomite (46.60–52.40%), a small amount of pyrite (3.60–5.30%), plagioclase layers (1.60–2.90%), a mixed-layer of illite/smectite (4.50–6.40%), and trace amounts of illite (1.90–3.60%), kaolinite (0.3–0.46%) and chlorite (0.36–0.59%).
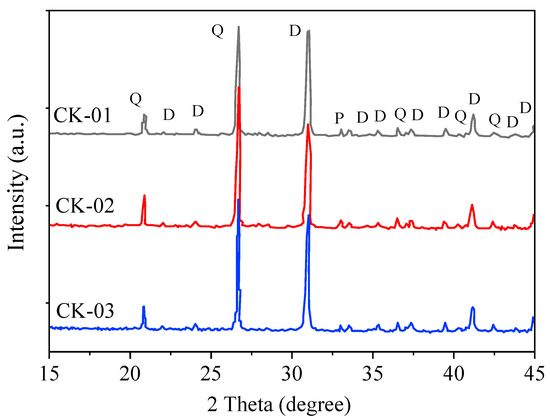
Figure 1.
X-ray diffraction results (Q: quartz, D: dolomite, and P: pyrite).

Table 1.
Physical and chemical properties of the samples.
From Table 2, the main chemical compositions measured by X-ray fluorescence (XRF) were SiO2 (32.00–36.37%), CaO (16.45–18.55%), and MgO (11.55–12.95%). The Al2O3, SO3, and Fe2O3 contents were 2.17–3.30%, 2.25–2.8%, and 1.32–1.61%, respectively, indicating that the rock contained many sulfates. The other chemical components were relatively low. The loss of ignition value of the sample was large, ranging from 25.64–28.98%, showing that there were many inorganic carbonates in the rocks.

Table 2.
Chemical composition of the samples.
These samples were made into φ 50 mm × 100 mm standard cylindrical shapes. The concentration of the iron ions was analyzed as the main controlling factor affecting the water–rock interaction. These samples were soaked in different solutions of various concentrations of Fe2(SO4)3 after drying and vacuuming. The containers were acid-resistant plastic boxes. The solutions with Fe3+ concentrations of 1.0, 1.5, 2.0, and 2.5 g/L were selected for the immersion tests.
The samples were kept semi-immersed for 41 days until the water–rock interaction was complete. Then, these samples were subjected to the uniaxial compression tests by the rock uniaxial testing machine (YZW-Y). The loading rate was 0.5 MPa/s. After the samples were completely destroyed, the test was stopped, and the stress–strain mechanical parameters of each sample were obtained.
3.2. Validation Results
The uniaxial compression test results after water–rock interaction with different Fe3+ concentrations are shown in Figure 2. The calculation results can be obtained from the parameters in Table 3. The established chemical damage constitutive model could reflect the stress–strain relationship of the rock after water–rock interaction. The goodness of fit (R2) of the four samples was 0.915, 0.775, 0.815, and 0.686, respectively. The most theoretical curves in the prepeak zone showed good agreement with the experimental results. In the postpeak stage, some theoretical curves are in agreement with the measured data when the deterioration was large.
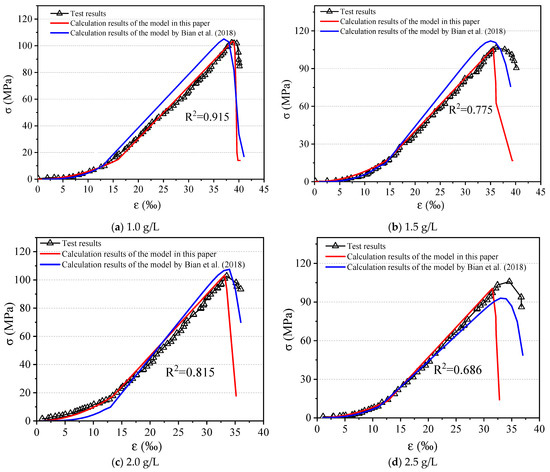
Figure 2.
Comparison of the experimental and theoretical results with different Fe3+ concentrations.

Table 3.
Model parameters.
The model of Bian et al. (2018) was used for the comparison [44]. According to the results, the model established in this paper had a better performance in the nonlinear deformation stage and the linear deformation stage. It is more consistent with the actual elastic moduli of the rock after water–rock interaction. The trends of the postpeak curves of the Bian et al. model is more reasonable in describing the strain-softening phenomenon. In practical applications, the nonlinear and elastic stages of rocks are mainly taken into account. The rocks are usually considered to have been damaged after the peak stress has been exceeded, and thus the residual strength is not considered in the design.
The theoretical value of the model is very close to the actual elastic modulus of the sample. The theoretical curve established by using the change in rock quality before and after water–rock interaction can match the experimental curve of each sample. This indicated that the change in the stored elastic energy, which is characterized by a change in mass, was the main reason for the change in the mechanical properties. The corrosion effect of the water–rock interaction on the rocks was mainly the consumption of diagenetic minerals and the dissolution of the cement. Macroscopically, the corrosion effect was expressed as an increase in the pores and a decrease in rock quality. The internal pores decreased the effective bearing volume, and the bond between the rock components weakened, which changed the mechanical properties of the rocks [45].
The results in Figure 2 showed that the R2 of the model proposed in this paper was only 0.686 when the concentration was 2.5 g/L. This low R2 was mainly caused by the small peak strain in the calculation. The reasons for the low fit may come from various factors, such as unreasonable model assumptions, inaccurate parameter selection, and experimental data errors. When the iron ion concentration was 2.5 g/L, the value of m/m0 was only 0.93, indicating that the chemical damage at this stage was small and mainly occurred on the sample surface. At this time, the mass loss of the specimen was not proportional to the effective bearing volume, and thus it did not satisfy the assumption and caused the error. However, the error in the calculated peak strain was only 11%, which was still acceptable in practice.
The aqueous chemical solution had a strong softening effect, generating strain softening in the samples. The softening effect of the chemical solution increased the peak strain of the samples. Moreover, the elastic modulus could reflect the softening effect of the chemical solution on the samples, as the curve slopes changed obviously in the prepeak stage. As shown in Figure 3, for the Fe3+ concentrations of 1.0, 1.5, 2.0, and 2.5 g/L, the peak strength of the samples decreased by 4.18%, 0.19%, 2.93%, and 0.64% when compared with the nonsoaked sample, respectively. Meanwhile, their peak strain increased by 29.58%, 14.11%, 12.42%, and 4.96%, respectively. This showed that the corrosion effect of different concentrations of the soaking solution on the samples was different. Iron ions formed a film on the sample surfaces to inhibit the sample, which inhibited the reaction. The corrosion effect and deterioration degree decreased with concentration [46,47].
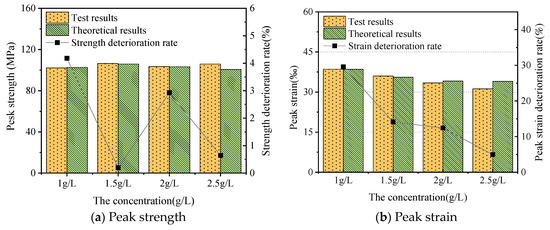
Figure 3.
Mechanical parameters and deterioration of the samples.
Before the rock enters the yield stage, the main reason for the decrease in the mechanical properties is the development of secondary pore microcracks in the rock caused by water–rock chemical interaction. This leads to the dissolution of the diagenetic minerals and cement in the rock, weakening the connection between the rock components [48,49]. At the peak point, the main composition, and properties of the rock itself, did not change after water–rock interaction.
4. Mechanism Analysis
4.1. Effect of Solution on the Mechanical Properties of Rock
Under the action of O2 and H2O in the surrounding environment, pyrite is oxidized to release an amount of H+. This provides a large number of hydrogen ions for the subsequent water–rock chemical reactions of other minerals [50]. The overall reaction formula is
Dolomite is consumed, which manifests as an increase in the fractures in the rock mass and a loss of quality. In the process of soaking, the generated gypsum and epsomite are dissolved in water. The main reaction formula is shown as follows:
The effect of feldspar minerals in chemical corrosion generates kaolinite and other products, leading to a change in the rock skeleton structure and the degradation of rock’s mechanical properties. The chemical reaction formulas of feldspar minerals in black sandy dolostone under water–rock interactions are shown as follows:
The clay minerals generally fill the pores in the rock, acting as cementation, and are consumed after chemical corrosion; K+, Mg2+, Al3+, and other metal ions are released through the chemical reaction and will continue to interact with the mineral composition of the rock, accelerating the water–rock chemical interaction. Some of the relevant equations are
Other than the H+ produced from the pyrite oxidation within the black dolostone, iron ions play an important role in the water–rock interactions as well. The effect of its property transformation on the mechanical properties of a rock mass should also be considered. In the process of water–rock interactions, pyrite interacts with oxygen and water, releasing ferrous ions. Then, trivalent iron participates in the oxidation of pyrite to produce ferrous ions and sulfur elements, after which the chemical reaction begins to cycle and accelerate the oxidation of pyrite. At the same time, ferric iron generates iron hydroxide during the hydrolysis reaction in water, and iron hydroxide will be oxidized to brown iron oxide precipitates.
4.2. Energy Conversion of Rock under Compression
In the process of the rock failures in the uniaxial compression tests, the work done to the rock by the outside includes the releasable elastic strain energy and the dissipated energy: U = Ue + Ud, where Ue is the releasable elastic strain energy and Ud is the dissipated energy. The dissipative energy of the rock represents the internal damage and plastic deformation of the element body, and its value is equal to the area formed by the unloading elastic modulus and the stress–strain curve [51,52]. The sample was only subjected to axial stress σ1 in order to do work, so the releasable elastic strain energy in the principal stress space is:
where (i = 1, 2, 3) is the elastic strain in three principal stress directions. In the same way, the work done by the principal stress in the direction of the principal stress is
According to the uniaxial compression test results of each sample and the analysis of Equations (30)–(32), the stress–strain curve data of the sample under different conditions were processed to calculate the strain energy, as shown in Table 4.

Table 4.
Each characteristic point corresponds to the strain energy.
The relationship between each energy index and the concentration when the sample reaches the peak value is shown in Figure 4.
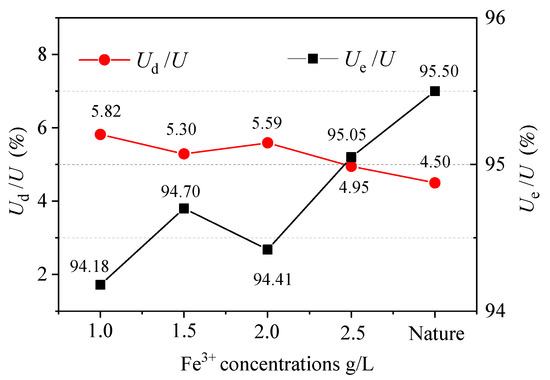
Figure 4.
Relationship between sample energies and concentrations.
After chemical etching with the solution concentration, the peak of the strain energy value was less, suggesting that the soaking process by dissolution increased the internal damage of the sample. With the corrosion effect, the internal damage became more severe and more likely to cause new damage. At the same time, the internal part of the rock minerals affected by the chemical changes reduced the brittleness of the samples [53,54,55]. From Figure 4 and Table 4, the concentration decreased the total energy, U, and the value of Ud/U. The energy storage form of the sample did not fundamentally change after corrosion by the aqueous chemical solution. The absorbed energy was mainly stored as the releasable elastic strain energy, Ue, in the sample. The solution concentration had a significant influence on the strain energy of the samples. The transformation rate of Ud/U was faster than that of Ue/U. This transformation trend was gradually strengthened with the load.
Figure 5 shows the energy changes of the natural samples and the severe water–rock interactions (1 g/L). The stress–strain curves in Figure 2 can be divided into four stages considering the energy transformation: the nonlinear-change stage, the linear-elastic stage, the yield and failure stage, and the energy trend and the causes of each stage are discussed, respectively.
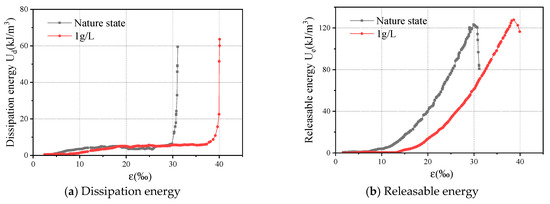
Figure 5.
The changes in the energy curves.
Nonlinear change stage: due to the defects in the sample in the natural state, at the initial stage of the test, the microcracks in the sample would close under the action of the external force, and some energy would escape. The curves were concave (upward), and the dissipated energy, Ud, increased with axial strain. At the same time, the sample began to accumulate energy, and its releasable strain energy, Ue, increased with the increase in axial strain. From Figure 5, the increase rate of dissipated energy, Ud, is smaller than the change rate of the releasable strain energy, Ue, in the initial stage of the nonlinear change in strain energy. This indicates that the sample is mainly accumulating releasable elastic strain energy, Ue, at this time. The dissipated energy mainly came from the damage of internal microstructure. After the sample was corroded by the oxidized acid, the accumulated energy decreased. The growth rate of the released elastic energy, Ue, decreased with solution concertation.
Linear elastic change stage: the accumulated strain energy inside the sample increased with axial strain, while the energy escaping only increased slightly. Meanwhile, most of the energy was stored inside the rock as elastic strain energy, Ue, and the curves changed approximately linearly. The increased dissipated energy, Ud, was speculated to be caused by the compaction of the remaining microcracks in the sample under the external forces. The deterioration of the mechanical properties was caused by oxidative acid corrosion, the generation of new cracks, and the nonmovement of the closed pores.
Yield stage: the sample was about to reach the limit of its stored energy. The released elastic strain energy, Ue, and dissipated energy, Ud, increased with axial strain. The growth rate of the strain energy started to slow down, while it increased rapidly later. Under the external force, more cracks were generated inside the sample, and they continued to develop until they formed through macrocracks and produced plastic deformation. The energy escaping reflected the strength of the mechanical properties of the sample itself. The growth rate of the dissipated energy, Ud, and the value of the dissipated energy, Ud, decreased at the final peak.
Failure stage: after reaching this stage, the accumulated energy of the sample reached its self-storage limit. Although the external force continued to work on the sample, the dissipated energy, Ud, of the sample increased rapidly at this stage. The growth rate of Ue saw negative growth. On the macrolevel, the bearing capacity of the sample was gradually lost, and crack propagation and coalescence occurred, causing the sudden brittle failure.
5. Conclusions
In this paper, a chemical damage model for rocks was proposed and verified by the semi-immersion tests and uniaxial compression tests. The degradation law of the mechanical properties of black sandy dolomite under aqueous chemical action was studied in combination with the energy mechanism. The main conclusions are as follows:
- (1)
- Based on the chemical damage mechanism of the water–rock interaction on rocks, a uniaxial compression chemical damage model for rocks was established. When considering the heterogeneity of the rock itself, the chemical damage relationship of rocks was established based on the change in mass of the samples before and after water immersion by using damage theory, the strain equivalence principle, and the generalized strain equivalence principle;
- (2)
- The theoretical curves were compared with the uniaxial compression test results after the deterioration of the water–rock interaction of each sample. The test curves were in general agreement with the theoretical curves, which verified that the proposed model could reflect the stress–strain relationship after water–rock interaction;
- (3)
- The dissipative energy, Ud, of the natural black sandy dolomite samples was slightly different from that of the chemically corroded sample. In the late yield stage and after the peak, the sharp growth point in the dissipative energy, Ud, of the chemically corroded sample was relatively lower. This indicated that the brittleness of the sample under a chemical solution decreased, and the elastoplasticity increased. The released elastic strain energy saw little change at its peak. The water–rock interaction was mainly dominated by the dissolution effect, and the composition and properties of the rocks did not change greatly. The Ue/U value of the sample decreased, while the Ud/U value increased with solution concertation.
Author Contributions
Conceptualization, methodology, formal analysis, software, writing—original draft preparation, A.T., Q.X.; validation, X.L. (Xinyu Luo), X.L. (Xin Liao), Q.T.; writing—review and editing, Q.T., X.L. (Xin Liao). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (52078317), Natural Science Foundation of Jiangsu Province for Excellent Young Scholars (BK20211597), project from the Bureau of Housing and Urban-Rural Development of Suzhou (2021-25; 2021ZD02; 2021ZD30), Bureau of Geology and Mineral Exploration of Jiangsu (2021KY06), China Tiesiju Civil Engineering Group (2021-19), CCCC First Highway Engineering Group Company Limited (KJYF-2021-B-19) and CCCC Tunnel Engineering Company Limited (8gs-2021-04).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pasek, M. An Increase in Phosphorus Availability from Redox-Induced Changes by Water–Rock Interactions. First Billion Years Habitability 2019, 2134, 1027. [Google Scholar]
- Antunes, M.; Teixeira, R.; Albuquerque, T.; Valente, T.; Carvalho, P.; Santos, A. Water–Rock Interaction and Potential Contamination Risk in a U-Enriched Area. Geosciences 2021, 11, 217. [Google Scholar] [CrossRef]
- Sisodiya, M.; Singh, S.; Thomas, D.; Zhang, Y. Effect of Water–Rock Interaction on the Axial Capacity of Drilled Caissons Socketed in Claystone Bedrock. J. Geotech. Geoenviron. Eng. 2021, 10, 147. [Google Scholar] [CrossRef]
- Guo, H.; Gao, Z.; Xiu, W. Research status and trend of coupling between nitrogen cycle and arsenic migration and transformation in groundwater systems. Hyd. Eng. Geol. 2022, 49, 153–163. [Google Scholar]
- Yin, Q.; Wu, J.; Jiang, Z.; Zhu, C.; Su, H.; Jing, H.; Gu, X. Investigating the effect of water quenching cycles on mechanical behaviors for granites after conventional triaxial compression. Geomech. Geophys. Geo-Energy Geo-Resour. 2022, 8, 77. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, C.; He, M.; Wang, X. Macro-meso dynamic fracture behaviors of Xinjiang marble exposed to freeze thaw and frequent impact disturbance loads: A lab-scale testing. Geomech. Geophys. Geo-Energy Geo-Resour. 2022, 8, 154. [Google Scholar] [CrossRef]
- Chen, G.; Chang, X.; Guo, X.; Pang, Y.; Zhang, P. Geochemical characteristics and organic matter enrichment mechanism of Permian black mudstone in the South Yellow Sea Basin, China. J. Pet. Sci. Eng. 2022, 208, 109248. [Google Scholar] [CrossRef]
- Tang, Q.; Tang, X.W.; Li, Z.Z.; Wang, Y.; Hu, M.M.; Zhang, X.J.; Chen, Y.M. Zn(II) Removal with Activated Firmiana Simplex Leaf: Kinetics and Equilibrium Studies. J. Environ. 2012, 138, 190–199. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, L.; Sun, X. Experimental study of the influence of water and temperature on the mechanical behavior of mudstone and sandstone. Bull. Eng. Geol. Environ. 2017, 76, 645–660. [Google Scholar] [CrossRef]
- Zhang, L.; Mao, X.; Li, M.; Liu, R. Experimental study of thermal expansion characteristics of coaly mudstone at high temperatures. Geotech. Geol. Eng. 2018, 36, 521–529. [Google Scholar] [CrossRef]
- Owusu, E.B.; Gebretsadik, H.T.; Sum, C.W.; Padmanabhan, E. Organic geochemical analyses of the Belata black shale, Peninsular Malaysia; implications on their shale gas potential. J. Nat. Gas Sci. Eng. 2019, 69, 102945. [Google Scholar] [CrossRef]
- Editorial Department of China Journal of Highway and Transport. Review on China’s Subgrade Engineering Research. China J. Highw. Transp. 2021, 34, 1–49. [Google Scholar]
- Xu, H.; Ren, X.; Chen, J. Centrifuge model tests of geogrid-reinforced slope supporting a high embankment. Geosynth. Int. 2019, 26, 629–640. [Google Scholar] [CrossRef]
- Liao, X.; Zhang, W.; Chen, J.; Wang, Q.; Wu, X.; Ling, S.; Guo, D. Deterioration and Oxidation Characteristics of Black Shale under Immersion and Its Impact on the Strength of Concrete. Materials 2020, 13, 2515. [Google Scholar] [CrossRef]
- Jeong, U.; Yoon, W.S.; Choi, J.W.; Kim, J.H. Influence of weathering depth and fracture intensity to cut slope movement. Geosci. J. 2005, 9, 47–52. [Google Scholar] [CrossRef]
- Liao, X.; Wu, X.; Zhu, B.; Feng, J. Study on the characteristics of black strata geochemical weathering and its disaster-causing mechanism. Disa. Adv. 2012, 5, 1558–1562. [Google Scholar]
- Tang, Q.; Wang, H.; Chen, H.; Tang, X. A characterization study of hydraulic conductivity of compacted clay and fine sand treated with landfill leachate and nutrient solution. Electron. J. Geotech. Eng. 2015, 20, 1–14. [Google Scholar]
- Nazarenko, L.; Stolarski, H.; Altenbach, H. A statistical interphase damage model of random particulate composites. Int. J. Plast. 2019, 116, 118–142. [Google Scholar] [CrossRef]
- Tang, S.; Li, J.; Ding, S.; Zhang, L. The influence of water-stress loading sequences on the creep behavior of granite. Bull. Eng. Geol. Environ. 2022, 81, 482. [Google Scholar] [CrossRef]
- Antony, S.J.; Olugbenga, A.; Ozerkan, N.G. Sensing, measuring and modelling the mechanical properties of sandstone. Rock Mech. Rock Eng. 2018, 51, 451–464. [Google Scholar] [CrossRef]
- Vasarhelyi, B.; Davarpanah, S.M. Influence of Water Content on the Mechanical Parameters of the Intact Rock and Rock Mass. Period. Polytech. 2018, 62, 1060–1066. [Google Scholar] [CrossRef]
- Moses, C.O.; Nordstrom, D.K.; Herman, J.S.; Mills, A.L. Aqueous pyrite oxidation by dissolved oxygen and by ferric iron. Geochim. Cosmochim. Acta 1987, 51, 1561–1571. [Google Scholar] [CrossRef]
- Hung, A.; Muscat, J.; Yarovsky, I.; Russo, S.P. Density-functional theory studies of pyrite FeS2 (111) and (210) surfaces. Surf. Sci. 2002, 520, 111–119. [Google Scholar] [CrossRef]
- Meng, M.; Ge, H.; Shen, Y.; Wang, L. Influence of rock fabric on salt ion diffusion behavior in upper cretaceous lacustrine shale from Songliao Basin. J. Pet. Sci. Eng. 2022, 208, 109355. [Google Scholar] [CrossRef]
- Lemaitre, J. How to use damage mechanics. Nucl. Eng. Des. 1984, 80, 233–245. [Google Scholar] [CrossRef]
- Malyarenko, A.; Ostoja-Starzewski, M. Towards stochastic continuum damage mechanics. Int. J. Solids Struct. 2020, 184, 202–210. [Google Scholar] [CrossRef]
- Tang, Q.; Chu, J.; Wang, Y.; Zhou, T.; Liu, Y. Characteristics and factors influencing Pb(II) desorption from a Chinese clay by citric acid. Sep. Sci. Technol. 2016, 51, 2734–2743. [Google Scholar] [CrossRef]
- Li, X.; Qu, D.; Luo, Y.; Ma, R.; Xu, K.; Wang, G. Damage evolution model of sandstone under coupled chemical solution and freeze-thaw process. Cold Reg. Sci. Technol. 2019, 162, 88–95. [Google Scholar] [CrossRef]
- Khan, D.; Singh, S.; Needleman, A. Finite deformation analysis of crack tip fields in plastically compressible hardening–softening–hardening solids. Acta Mech. Sin. 2017, 33, 148–158. [Google Scholar] [CrossRef]
- Tutluoğlu, L.; İbrahim, F.; Karpuz, C. Relationship between pre-failure and post-failure mechanical properties of rock material of different origin. Rock Mech. 2015, 48, 121–141. [Google Scholar] [CrossRef]
- Wen, Y.; Xin, C.; Zhang, X. The Stability Analysis of Tunnel Lining Structure with Seismic Excitation Based on the Energy Evaluation Principle. Shock. Vib. 2021, 9995682. [Google Scholar] [CrossRef]
- Chen, J.; Dai, Y. Expressions of stored and dissipated energy densities. Optik 2020, 207, 163493. [Google Scholar] [CrossRef]
- Shiozawa, D.; Inagawa, T.; Washio, T. Accuracy improvement in dissipated energy measurement by using phase information. Meas. Sci. Technol. 2017, 28, 044004. [Google Scholar] [CrossRef]
- Souissi, S.; Hamdi, E.; Sellami, H. Microstructure effect on hard rock damage and fracture during indentation process. Geotech. Geol. Eng. 2015, 33, 1539–1550. [Google Scholar] [CrossRef]
- Gu, F.; Presti, D.; Heitzman, M.; Powell, B.; Allison, V. Feasibility of using more polishable aggregates in dense-graded asphalt surface mixture: Case study of dolomite. Constr. Build. Mater. 2022, 342, 127915. [Google Scholar] [CrossRef]
- Dhar, S.; Dixit, P.M.; Sethuraman, R. A continuum damage mechanics model for ductile fracture. Int. J. Press. Vessel. Pip. 2000, 77, 335–344. [Google Scholar] [CrossRef]
- Ma, J.; Du, W.; Gao, W.; Wriggers, P.; Xue, X. Multiscale finite element analysis of uncertain-but-bounded heterogeneous materials at finite deformation. Finite Elem. Anal. Des. 2018, 149, 15–31. [Google Scholar] [CrossRef]
- Atkinson, B.K. Subcritical crack propagation in rocks: Theory, experimental results and applications. J. Struct. Geol. 1982, 4, 41–56. [Google Scholar] [CrossRef]
- Leclerc, J.; Nguyen, V.D.; Pardoen, T.; Noels, L. A micromechanics-based non-local damage to crack transition framework for porous elastoplastic solids. Int. J. Plast. 2020, 127, 102631. [Google Scholar] [CrossRef]
- Higuchi, K.; Chigira, M.; Lee, D.H. Pore-water chemistry and its influence on rock mechanical properties and hydrogeophysical processes in a mudstone slope in the southwestern Taiwan badlands. Catena 2020, 190, 104533. [Google Scholar] [CrossRef]
- Kang, B.; Jian, L.; Wei, Z.; Zheng, X.; Ni, S.; Liu, Z. Mechanical behavior and damage constitutive model of rock subjected to water-weakening effect and uniaxial loading. Rock Mech. Rock Eng. 2019, 52, 97–106. [Google Scholar]
- Deng, J.; Gu, D. On a statistical damage constitutive model for rock materials. Comput. Geosci. 2011, 37, 122–128. [Google Scholar] [CrossRef]
- Tu, Z.; Wan, J.; Guo, C.; Fan, T.; Zhang, G.; Lu, J.R. Electrochemical oxidation of pyrite in pH 2 electrolyte. Electrochim. Acta. 2017, 239, 25–35. [Google Scholar] [CrossRef]
- Lin, Y.; Zhou, K.; Gao, F.; Li, J. Damage evolution behavior and constitutive model of sandstone subjected to chemical corrosion. Bull. Eng. Geol. Environ. 2019, 78, 5991–6002. [Google Scholar] [CrossRef]
- Tang, Q.; Tang, X.W.; Li, Z.Z.; Chen, Y.M.; Kou, N.Y.; Sun, Z.F. Adsorption and desorption behaviour of Pb(II) on a natural kaolin: Equilibrium, kinetic and thermodynamic studies. J. Chem. Technol. Biotechnol. 2009, 84, 1371–1380. [Google Scholar] [CrossRef]
- Tang, Q.; Tian, A.; Ling, C.; Huang, Y.; Gu, F. Physical and mechanical properties of recycled aggregates modified by microbially induced calcium carbonate precipitation. J. Clean. Prod. 2022, 135409. [Google Scholar] [CrossRef]
- Gu, F.; Sahin, H.; Luo, X.; Luo, R.; Lytton, R. Estimation of resilient modulus of unbound aggregates using performance-related base course properties. J. Mater. Civ. Eng. 2015, 27, 04014188. [Google Scholar] [CrossRef]
- Tang, Q.; Zhou, Y.; Gao, Y.; Fan, G. Use of cementchelated solidified (MSWI) fly ash for pavement material: Mechanical and environmental evaluations. Can. Geotech. J. 2017, 54, 1553–1566. [Google Scholar] [CrossRef]
- Vasyukova, E.V.; Oliva, P.; Viers, J.; Martin, F.; Dupré, B.; Pokrovsky, O.S. Chemical weathering of mafic rocks in boreal subarctic environment (northwest Russia) under influence of glacial moraine deposits. Chem. Geol. 2019, 509, 115–133. [Google Scholar] [CrossRef]
- Udagedara, D.T.; Oguchi, C.T.; Gunatilake, J.K. Evaluation of geomechanical and geochemical properties in weathered metamorphic rocks in tropical environment: A case study from Samanalawewa hydropower project, Sri Lanka. Geosci. J. 2017, 21, 441–452. [Google Scholar] [CrossRef]
- Sergeeva, N.M.; Korsakov, V.G. Self-organization of precipitates in oxidative-hydrolytic precipitation of iron ions. Russ. J. Appl. Chem. 2000, 73, 941–946. [Google Scholar]
- Ling, S.; Wu, X.; Zhao, S.; Liao, X. Evolution of porosity and clay mineralogy associated with chemical weathering of black shale: A case study of Lower Cambrian black shale in Chongqing, China. J. Geochem. Explor. 2018, 188, 326–339. [Google Scholar] [CrossRef]
- Marques, E.A.G.; Barroso, E.V.; Menezes, A.P.; Vargas, E.D. Weathering zones on metamorphic rocks from Rio de Janeiro—Physical, mineralogical and geomechanical characterization. Eng. Geol. 2010, 111, 1–18. [Google Scholar] [CrossRef]
- Munoz, H.; Taheri, A.; Chanda, E.K. Rock drilling performance evaluation by an energy dissipation based rock brittleness index. Rock Mech. Rock Eng. 2016, 49, 3343–3355. [Google Scholar] [CrossRef]
- Babaie, H.A.; Davarpanah, A. Semantic modeling of plastic deformation of polycrystalline rock. Comput. Geosci. 2018, 111, 213–222. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).