Abstract
A method has been developed for producing mixed actinide oxides suitable for fabricating mixed nitride uranium plutonium fuel for fast neutron reactors. The method is based on the use of microwave radiation for the direct denitration of actinide nitrate solutions. The possibility of producing uranium, plutonium, and neptunium-mixed oxides was shown. A pilot installation for preparing actinide oxides by microwave denitration was designed and tested. Mixed oxides of uranium and cerium (for plutonium imitation) were successfully used to synthesize uranium cerium nitrides and produce fuel pellets. Compared with the precipitation (ammonia) method of producing mixed oxides, microwave denitration reduces the generation of secondary liquid radioactive waste by more than six times.
1. Introduction
To develop industrial technologies to close the nuclear fuel cycle in the Russian Federation, the PRORYV project began constructing the BREST-OD-300 fast neutron reactor at the Siberian Chemical Combine (Seversk, Russia). The fabrication/re-fabrication module of mixed nitride uranium–plutonium (MNUP) fuel is already constructed, and the construction of the spent nuclear fuel (SNF) reprocessing module is planned [1]. A combined (pyro + hydro) technology (the PH process), as well as its hydrometallurgical variant, is being developed to reprocess this type of SNF. To produce MNUP fuel powders, carbothermic synthesis is used with mixed U–Pu–Np oxides as starting materials.
The most popular methods for preparing actinide oxides were reviewed, and several experiments were performed. There are many methods for preparing uranium and plutonium oxides, and all of them have different stages of implementation, from conceptual design to commercial use. These methods can be classified as reagent-based methods with precipitation and thermal methods. Reagent oxide preparation methods involve the following stages: (1) precipitation of the insoluble compounds, (2) their filtration, (3) washing of the precipitate, (4) drying and calcination (usually in the presence of a reductant), and (5) control of the mother solution. Studies have described the use of ammonia [2,3], oxalic acid [4,5], hydrogen peroxide [6], hydroxylamine [7], and hydrazine [8] for precipitation. Among the mentioned precipitators, only hydroxylamine and hydrazine are able to reduce actinides to hydrated actinide dioxides. All the other methods require a stage of calcination at 700–1000 °C in the presence of a reductant, usually hydrogen.
Thermal methods are based on the ability of actinide nitrates to decompose into actinide oxides when heated. There is a way to directly prepare actinide dioxides by thermal methods. The main thermal denitration methods are spray drying in a reduction medium [9], microwave denitration of solutions or solid nitrates [10,11,12], and gas-flame denitration in a hydrogen media [13,14].
During the preliminary studies, the following variants were tested: gas-flame denitration, co-precipitation of actinides as oxalates, reduction to dioxide in alkaline media by hydrazine, and denitration by microwave radiation. For further research, a method of microwave denitration under the action of ultrahigh frequency radiation was chosen. Via this method, solid solutions of mixed oxides can be obtained in one stage and in one apparatus from the actinide nitrate solution, without the formation of mother liquors. In our earlier research [15,16,17], we used microwave radiation to directly produce uranium dioxide powder or a mixture of actinide dioxides of ceramic quality from actinide nitrate solutions with preliminary decomposition and removal of nitrate ions.
Microwave radiation has long been used to produce metal oxides for various purposes, e.g., to produce uranium and thorium oxides [18,19]. Several variants of both methods and devices have been proposed to carry out the denitration process directly from solutions and from the solid salt of uranyl nitrate hexahydrate [20,21,22,23,24]. All stages of uranyl hexahydrate decomposition under microwave radiation are described in detail in [25,26]. Chemical, thermogravimetric, and X-ray analyses show that the decomposition proceeds in two stages and that the precursor of the formation of UO3 is uranyl nitrate hydroxyl. During microwave denitration of uranium nitrate solutions, the solution is evaporated to form UO2(NO3)2·6H2O, further heating of which leads to its dehydration, with the formation of uranium trioxide in accordance with Reaction (1). At the same time, UO3 formed during the decomposition of uranyl nitrate in a reducing atmosphere is partially reduced to U3O8 according to Reaction (2), which, unlike UO3, absorbs microwave radiation energy, which makes it possible to heat UO2(NO3)2·6H2O to a temperature of at least 600 °C, at which its decomposition proceeds according to Reaction (3). The U3O8 formed under the action of hydrogen in a mixture with argon with further microwave exposure quantitatively transforms into UO2 according to Reaction (4).
UO2(NO3)2·6H2O → UO3 + NO2 + NO + O2 + 6H2O
3UO3 → U3O8 + 1/2O2
3UO2(NO3)2·6H2O → U3O8 + 6NO2 + 18H2O + 2O2
U3O8 + 2H2 → 3UO2 + 2H2O
The thermochemical denitration of U and Ce (as Pu or Am simulant) nitric acid solutions was investigated [27]. Experiments were carried out on a microwave heating apparatus with 800 W power and 2.45 GHz frequency; the installation is described in [17]. Nitric acid solutions (0.5 mol/L) containing 400.0 g/L of U and up to 44.4 g/L of Ce were used. Uranium and cerium mixture solutions were denitrated in two stages. In the first stage, the solution was evaporated for 35–40 min and metal nitrates were decomposed. The experiment was carried out in the following order. (1) A quartz flask containing salts in a nitric acid solution was purged with a reducing gas mixture (RGM) of argon with 5% hydrogen for 5 min at room temperature. (2) Without stopping the RGM feed, microwave heating was switched on at 800 W. (3) The microwave unit was switched off when the release of water vapor and nitrogen oxides was completed. The powders formed in the first stage contained from 13 to 33% U(IV). Before starting the second stage of the process, the powders were stirred, the system was blown cold with the RGM, and then microwave heating at a radiation power of 180–800 W was switched on, without terminating the RGM feeding. Quantitative (at least 99%) recovery of U was achieved at this stage. Thus, uranium recovery by denitration of the initial 250 mL solutions was achieved at 800 W power in 3 h, at 450 W in 4.5 h, and at 180 W in 6 h. The obtained powders contained 60–70 wt% of particles larger than 400 µm, which is higher than the required particle size in fuel pellet production. After additional milling of coarse particles on a 400 µm cell size vibrating screen, powders consisting of aggregates of particles no larger than 400 µm were obtained and the fraction of particles smaller than 25 µm did not exceed 1 wt%. XRD data show that the obtained powders are solid solutions of uranium dioxide with cerium (U,Ce)O2 (see Supplementary Materials Figure S1). A diffractogram of the powder showed characteristic peaks of UO2 (uraninite), and the U3O8 impurity content did not exceed 1 wt%, which meets the requirements for powders for fuel production. The bulk density of the powders with shrinkage was 2.3–2.5 g/cm3. With a decrease in the microwave radiation power, the specific surface area of the powders obtained with different heating powers increased significantly: from ~ 0.3 m2/g at 800 W up to 1.0 m2/g at 450 W and up to 2.2–2.5 m2/g at 180 W. An increase in the specific surface area of the powders with a decrease in the microwave radiation power relates to a decrease in the average size of crystalline particles. SEM data confirmed the presence of a significant amount of large crystalline aggregates compacted due to intergrown crystals of about 1 µm size in the powders produced at 800 W power (see Supplementary Materials Figure S2). At the same time, powder particles obtained by 180 W power consisted of crystals no more than about 0.2 μm in size. Crystal size enlargement was related to the increase in the temperature, i.e., the temperature to which the powders were heated, with an increase in microwave radiation power.
This paper presents the results of developing a method for producing mixed uranium and plutonium oxide powders, as well as testing pilot-scale equipment, by microwave denitration.
2. Experimental Section
2.1. Production of U and Pu Oxides in a Laboratory Setup
In the studies to produce U and Pu oxides in a boxed laboratory setup using nitric acid solutions (1 mol/L) of actinides, the following equipment and methods of analysis were used. An apparatus was housed in a glove box. The magnetron and the resonator chamber were located under the bottom of the glove box. The rotary bolt holder unit, the gas inlet and outlet unit, the pyrometer, and the video camera, as well as bodies of loading and unloading of material, were located on the lid of the unit. Figure 1 shows the scheme of the unit.
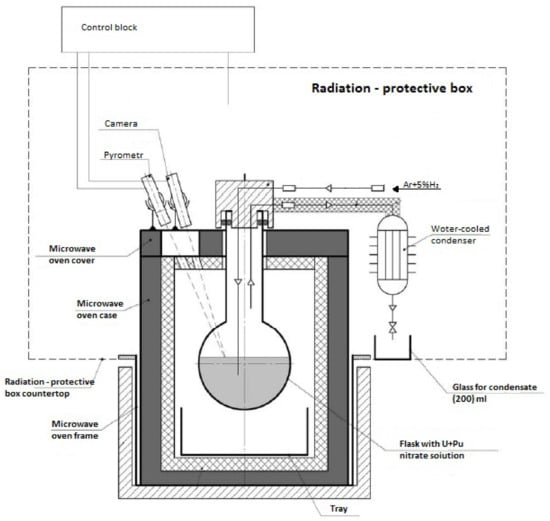
Figure 1.
Scheme of the laboratory microwave denitration unit in the glove box.
To monitor the process, the reaction zone was illuminated by an external light source and a video camera was placed in that area. A pyrometer was used to measure the temperature in the reaction zone. The temperature measurement zone was located at the bottom of the flask. The unit was equipped with a mechanical stirrer that worked during all stages of the process. The unit could produce actinide oxide samples of a total metal mass up to 250 g. To test the denitration process in a laboratory setup, two solution compositions were used with a plutonium content of 20 and 30% with respect to the sum of U and Pu. To obtain the required mass of oxide during denitration, the solution was poured into the flask several times.
Phase composition analysis was carried out on the URS-2,0 X-ray apparatus (copper radiation with a nickel filter). The phase composition was identified with the JCPDS database and the RENTGEN complex.
The resulting oxides were dissolved in a non-oxidizing medium (hydrochloric acid) to chemically analyze uranium oxides. Next, the optical spectrum of the resulting solution was recorded, and the concentrations of U(VI) and U(IV), respectively, were determined from the characteristic peaks at 414 and 670 nm. The plutonium content was analyzed by the α-radiometric method.
2.2. Mixed U and Ce Oxides Produced in a Microwave Denitration Pilot Unit
To study the scaling effect, a mock-up of the microwave denitration pilot unit was created. The mock-up included a resonator chamber with a crucible, a feed solution dispensing vessel, a bubbler for primary gas cleaning and vapor condensation, a coarse filter, a microwave radiation generator (connected to the resonator by three waveguides), and an argon–hydrogen mixture heating system. The rated power of the unit was 6 kW. Smooth power adjustment was provided by six magnetrons, controlled by a specifically created program. Figure 2 shows the layout.

Figure 2.
General view of the installation: (a) 1, the resonator chamber with the crucible; 2, the bubbler; 3, the dispensing vessel; 4, the waveguide) and (b) the magnetron control box.
Research was carried out using 7.5 L of uranyl nitrate and Ce (as Pu simulant) mixture solutions (4 kg for metals). The share of cerium in the total (U + Ce) was 12%. An aluminum oxide crucible was placed in the lower part of the resonator chamber. The upper part accommodated an electro-mechanical stirrer for not only mixing the solution, but also grinding the powder in the second stage of denitration.
The design of the resonator chamber is described in more detail in our patent [28]. To visually observe the processes taking place in the resonator chamber, a video camera was mounted on top of it. To ensure radiation safety, a vacuum of 10–20 kPa was created inside the resonator chamber. The powder formed was manually unloaded from the crucible after completion of each experiment.
The obtained powders and convertible solutions were analyzed using chemical and physical analysis methods: the ferro-phosphate-vanadate method was used to determine the total uranium content in solid samples and in solutions, GOST 25279-93 helped determine the bulk density (before and after shrinkage), the diffractometer “DRON-3” was used to determine the phase composition, ICP-AES Shimadzu “ICPE-9000” spectrometrically identified the cerium content, and the titrimetric method identified the nitric acid content in the presence of a complexing agent.
3. Results and Discussion
3.1. Results of Experiments in a Boxed Setup
Experiments were carried out in two stages similar to the method described in our paper [27]. The difference was that, in the first stage, the U–Pu nitrate melt was first obtained by removing water and acid by microwave heating. The microwave generator power was then varied between 200 and 400 °C in the reaction zone. At this stage, the quartz flask was purged with argon to create the reaction volume. The final product of this stage is a “denitrated speck” of uranium trioxide and hydrated plutonium oxides.
Waste steam and gas stream entered a water-cooled condenser to condense water and partially capture nitrogen oxides. The condensate was collected in a receiver tank.
In the second stage, the resulting speck was crushed with the mechanical stirrer inside the flask and microwave power was increased to 2 kW. An argon–hydrogen mixture was fed into the reaction zone. In the course of treatment, the temperature was increased to 700–800 °C (in some cases up to 1000 °C). After cooling, the obtained dioxide powder was manually unloaded from the quartz flask, crushed, and transferred for fractional and chemical composition analyses. The appearance of the powders obtained in both stages is shown in Figure 3.

Figure 3.
Appearance of the uranium powders obtained in the laboratory microwave denitration unit during the denitration of the uranyl nitrate solution: (a) after uranium trioxide sintering and (b) the final stage.
The influence of the processing time, the microwave radiation power, and the rate of feeding RGM into the reaction volume on the phase composition of the obtained oxides was investigated. Since calcination of Pu nitrate produces only Pu dioxide, the bulk of the experiments were carried out with only uranyl nitrate, achieving uranyl dioxide of uniform composition.
All experiments displayed the “wall effect”, i.e., the formation of the U oxide undercooled mass, mainly α-UO3, because the α-UO3 that formed on the walls had not been stirred and heated to the temperature at which reduction to UO2 occurs. After determining the above-mentioned conditions that maximize the yield of UO2, mixed oxides of U and Pu were obtained. The initial solution mimicked the mixed re-extract in the SNF reprocessing technology [1]. As a result, two batches of powder containing 38–43% UO2, 31–36% U3O8, and 20–30% PuO2 were obtained. The incomplete reduction of uranium to dioxide probably occurred due to the large mass of the oxide and the thickness of the layer. Perhaps, the reducing gaseous medium did not reach the lower layers of the oxide; as a result, complex oxides of the gross composition (U0.75Pu0.25)O2.4 and (U0.7Pu0.3)O2.2 were obtained. Thus, it can be assumed that the mixing of the solid phase during denitration is a necessary condition for obtaining a complete reduction and a uniform composition of the oxide powder.
Another important result for the technology was obtained in the laboratory plant: the residual actinide concentration in the condensate is less than 10 mg/L. This value is many times less than what we obtained in the joint oxalate deposition of uranium and thorium (simulant plutonium). In the best experiments, the uranium content in the mother lode was 5–6 g/L and the thorium content up to 0.25 mg/L.
3.2. Experimental Results in the Microwave Denitration Pilot Unit
On testing the unit on a solution of uranyl nitrate, it was found that evaporation and denitration proceeded according to the same regularities as in the laboratory tests and the box unit cases. However, it was not possible to complete the process with uranium dioxide formation. In most cases, the final product was uranium trioxide, with a small fraction of U3O8. To increase the temperature of the calcined powder, it was proposed to add extra microwave-absorbing material to the crucible material. Therefore, a new crucible was made to which up to 20% silicon carbide was added. Silicon carbide itself was not in contact with uranyl nitrate solution. Therefore, the powder temperature could be raised during exposure to microwave radiation to 600–650 °C. In this calcination regime, the final form was U3O8. Traces (less than 5%) of uranium dioxide were found in some samples, as recorded by IR spectrometry.
In all experiments, the solution volume and the initial metal concentration were the same, i.e., 4 L and 450 g/L of metal, respectively. During all stages of the process, the crucible temperature was recorded. Figure 4 displays the temperature dependence plots. As shown in Figure 4a, evaporation, removal of crystallization water, and denitration both for the individual solution of uranyl nitrate and for solutions of uranyl nitrate containing 10 to 30% of Ce nitrate heat up the product in the crucible homogeneously, indicating the absence of phase transitions. The heating rate depends only on the applied microwave power and the heat loss. During oxide production, the maximum achieved temperature was observed to be directly dependent on the cerium content. This dependence is shown in Figure 4b. This is probably due to different thermal conductivity values of U and Ce oxides.
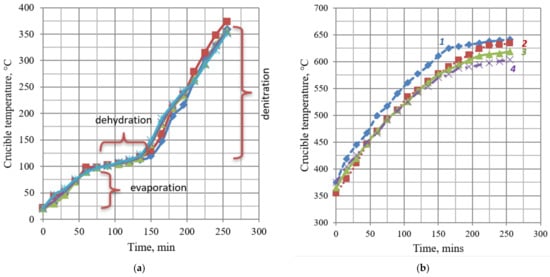
Figure 4.
Time-varying crucible temperature graph during evaporation and denitration of cerium and uranyl nitrates by microwave heating (a) and a plot of crucible temperature versus time during microwave heating of uranyl and cerium nitrate denitration products in an argon–hydrogen medium (b): 1, crucible temperature with products of uranyl nitrate denitration; 2, temperature in the presence of 10% cerium; 3, temperature in the presence of 20% cerium; and 4, temperature in the presence of 30% cerium.
As a result of all research on the mock-up, a regime of producing mixed UO3 and U3O8 with CeO2 was developed. It was not possible to produce UO2 in one stage, even in the reducing gas atmosphere (5% H2–Ar). To demonstrate the possibility of using the products of microwave denitration to fabricate refabricated nuclear fuel, the obtained oxide mixture was reduced in a hydrogen medium in a separate unit. Pellets of nitride fuel were produced from the obtained dioxide powder. In terms of physical and chemical parameters (density, homogeneity, chemical composition), the fuel pellets fully met the requirements for nitride fuel.
In a pilot plant, it was determined that during evaporation, 0.66 L of condensate per 1 L of stock solution is produced. In the powder stage, a further 0.056 L per 1 L of condensate is produced. Chemical analysis of the condensate showed that its uranium content changes from 9 to 70 mg/L and its cerium content from 7 to 8.3 mg/L. Thus, losses of these elements with condensate are from 2 × 10−3 to 1.6 × 10−2% wt. for uranium and (3.7–4.4) × 10−3% wt. for cerium. Compared with the precipitation (ammonia) method of mixed oxide production, the use of microwave denitration reduces the generation of secondary liquid waste by more than six times. This calculation is based on the condition that the ammonia precipitation is fed to a solution with a metal concentration not exceeding 100 g/L. After precipitation by the introduction of gaseous ammonia, the resulting sludge is filtered and washed with a diluted solution of ammonium hydroxide. In this process, liquid waste is also obtained. In general, the specific volume of liquid waste is up to 12 mL per 1 g of deposited metal, compared to 2.22 mL per 1 g of deposited metal in microwave denitration.
The microwave denitration products of actinide solutions have been demonstrated to be suitable for fabricating nitride fuel pellets.
4. Conclusions
A method of direct thermal denitration in the field of microwave radiation was developed that makes it possible to obtain mixed actinide oxides suitable for producing mixed nitride uranium–plutonium fuel. A pilot mock-up for producing mixed uranium–plutonium–neptunium oxides was developed. The developed unit is suitable in principle for producing mixed uranium–plutonium–neptunium and americium oxides. Industrial implementation of the developed method will significantly reduce the amount of secondary radioactive waste.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/en15186618/s1: Figure S1: Diffractogram of (U, Ce)O2 powder obtained by microwave denitration of 10% wt. Ce solution (*-Uraninite, UO2, #65-0285 in PDF-2), Figure S2: SEM images of the (U,Ce)O2 powders obtained by microwave radiation with power of 800 W (a) and 180 W (b).
Author Contributions
All the authors (K.D., Y.K., S.V., B.M., M.D., O.U., Y.M., A.S. and P.S.) have accepted responsibility for the entire content of this submitted manuscript and approved its submission. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out within the framework of the PRORYV project with the financial support of the State Atomic Energy Corporation of the Russian Federation (State Corporation “Rosatom”). The work of the Vernadsky Institute of Geochemistry and Analytical Chemistry of RAS was financed under contract no. 26/7133-D.
Conflicts of Interest
The authors declare no conflict of interest regarding this article.
References
- Shadrin, A.Y.; Dvoeglazov, K.N.; Maslennikov, A.G.; Kashcheev, V.A.; Tret’yakova, S.G.; Shmidt, O.V.; Vidanov, V.L.; Ustinov, O.A.; Volk, V.I. PH process as a technology for reprocessing mixed uranium–plutonium fuel from BREST-OD-300 reactor. Radiochemistry 2016, 58, 271–279. [Google Scholar] [CrossRef]
- Andryshin, V.G.; Borisov, L.M.; Zakharkin, B.S.; Makarov, V.M.; Morkovnikov, V.E.; Nikiforov, A.S.; Raginsky, L.S.; Revyakin, V.V.; Khaustov, O.V.; Yaroshinsky, V.M.; et al. Development of production processes for mixed (U-Pu) oxides suitable for fast and thermal reactor fuel. At. Energy 1992, 72, 440–443. [Google Scholar]
- Fillipov, E.A.; Karelin, A.I.; Lobas, O.P.; Papkov, A.S.; Zhiganov, A.N.; Shamin, V.I. Sostoyanie i tendentsiya razvitiya fiziko-khimicheskikh metodov polucheniya mikrosfericheskikh chastits yadernogo topliva. Radiochemistry 1984, 26, 225–239. (In Russian) [Google Scholar]
- Grandjean, A.; Beres, A.; Maillard, C.; Rousselle, J. Procede de Coprecipitation D’actinides a des Etats D’oxydation Distincts et Procede de Preparation de Composes Mixtes d’ Actinides. Patent FR2870841 (A1), 28 May 2004. [Google Scholar]
- Amokrane, A. Oxalate precipitation in emulsion: From the drop scale to the industrial process. Procedia Chem. 2012, 7, 456–459. [Google Scholar] [CrossRef][Green Version]
- Ramazanov, L.M.; Suslov, Y.P.; Antakov, G.M.; Bobyilev, A.I. Razrabotka peroksidnoy tekhnologii konversii oruzheynogo plutoniya v smeshannye uran-plutonievye oksidy, prigonye dlya izgotovleniya yadernogo topliva. In Proceedings of the 4th Russian Conference on Radiochemistry—‘Radiochemistry-2003’, Ozersk, Russia, 20–25 October 2003; pp. 139–140. (In Russian). [Google Scholar]
- Ilyin, E.G.; Beykharov, A.G.; Kulyako, Y.M.; Trofimov, T.I.; Samsonov, M.D.; Myasoedov, B.F. A new procedure for preparing mixed uranium-plutonium dioxide. Radiochemistry 2010, 52(4), 350–353. [Google Scholar] [CrossRef]
- Myasoedov, B.F.; Kulyako, Y.M.; Trofimov, T.I.; Perevalov, S.A.; Vinokurov, S.E.; Samsonov, M.D.; Fedoseev, A.M.; Bessonov, A.A.; Shadrin, A.Y. Preparation of Np, Pu, and U dioxides in nitric acid solutions in the presence of hydrazine hydrate. Radiochemistry 2013, 55, 574–580. [Google Scholar] [CrossRef]
- Dicter, V.; Horst, W.; Elmar, G. Process for the Preparation of Nuclear Fuel Mixed Oxides from a Nitrate Solution. DE Patent DE3805063 (A1), 18 February 1988. [Google Scholar]
- Oshima, H.; Tomura, T.; Koizumi, M. Outline of the conversion facility of Pu-U nitrate solution to the mixed oxide powder using a microwave heating method. Trans. Am. Nucl. Soc. 1982, 40, 48–50. [Google Scholar]
- Kulyako, Y.M.; Trofimov, T.I.; Perevalov, S.A.; Malikov, D.A.; Vinokurov, S.E.; Samsonov, M.D.; Myasoedov, B.F.; Travnikov, S.S.; Dvoeglazov, K.N.; Shadrin, A.Y. Preparation of uranium oxides by reductive denitration of uranyl nitrate under microwave heating. Radiochemistry 2015, 57, 251–254. [Google Scholar] [CrossRef]
- Weimin, B.; Xuejun, W.; Xuquan, M.; Miaoyi, S.; Zhicheng, Z.; Tian, B.Z. The research of technology and equipment for a microwave denitration process of the uranyl nitrate solution. In Proceedings of the Third International Conference on Nuclear Fuel Reprocessing and Waste Management, RECOD’91(II), Sendai, Japan, 14 April 1991; pp. 1075–1080. [Google Scholar]
- Pchelin, I.V. Sintez modifitsirovannykh poroshkov dioksida urana keramicheskogo sorta metodom gazoplamennoy konversii uranylnitrata. Graduate work, Mendeleev University of Chemical Technology of Russia, Moscow, Russia, 2011. (In Russian). [Google Scholar]
- Missorin, D.S.; Kamordin, S.I.; Chizhevskaya, S.V. Sintez modifitsirovannykh poroshkov dioksida urana keramicheskogo sorta metodom gazoplamennoy konversii. Uspekhi v khimii I khimicheskoy tekhnologii 2009, 13, 39–42. (In Russian) [Google Scholar]
- Shadrin, A.; Mochalov, Y.; Dvoeglazov, K.; Kashceev, V. Reprocessing of fast reactors mixed U-Pu used nuclear fuel: Studies and industrial test. In Proceedings of the International Conference on Fast Reactors and Related Fuel Cycles, Yekaterinburg, Russia, 26–29 June 2017; p. 68. [Google Scholar]
- Kulyako, Y.M.; Trofimov, T.I.; Pilyushenko, K.S.; Malikov, D.A.; Perevalov, S.A.; Vinokurov, S.E.; Savel’ev, B.V.; Myasoedov, B.F. Preparation of powdered uranium oxides by denitration of nitric acid uranium solutions using UHF radiation. Radiochemistry 2017, 61, 1–4. [Google Scholar] [CrossRef]
- Kulyako, Y.M.; Trofimov, T.I.; Pilyushenko, K.S.; Vinokurov, S.E.; Myasoedov, B.F. Production of Mixed Powders of Actinide Dioxides by Thermal Reductive Denitration Using Microwave Heating. Phys. At. Nucl. 2020, 83, 1396–1399. [Google Scholar] [CrossRef]
- Vanetsev, A.S.; Tretyakov, Y.D. Microwave synthesis of individual and multicomponent oxides. Adv. Chem. 2007, 76, 435–453. [Google Scholar] [CrossRef]
- Chandramouli, V.; Anthonysamy, S.; Vasudeva Rao, P.R.; Divakar, R.; Sundararaman, D. Microwave synthesis of solid solutions of urania and thoria—A comparative study. J. Nucl. Mater. 1998, 254, 55–64. [Google Scholar] [CrossRef]
- Ohtsuka, K.; Ohuchi, J.; Takahashi, Y. Method for Producing Oxide Powder. US Patent US4364859, 14 July 1980. [Google Scholar]
- Simonovich, M. Method and Apparatus for Reducing Uranium Trioxide. US Patent US200100140305A1, 16 August 2001. [Google Scholar]
- Yoshiharu, T. Continuous Denitration Apparatus. US Patent US5589140, 31 December 1996. [Google Scholar]
- Kulyako, Y.M.; Vinokurov, S.E.; Trofimov, T.I.; Perevalov, S.A.; Samsonov, M.D.; Myasoedov, B.F.; Malikov, D.A.; Travnikov, S.S.; Zevakin, E.A.; Shadrin, A.Y.; et al. Method of Producing Uranium Oxide. RU Patent RU2603359, 27 November 2016. [Google Scholar]
- Koizumi, M.; Ohtsuka, K.; Isagawa, H.; Akiyama, H.; Todokoro, A. Development of a process for the co-conversion of Pu-U nitrate mixed solutions to mixed-oxide powder using a microwave heating method. Nucl. Technol. 1983, 61, 55–70. [Google Scholar] [CrossRef]
- Kulyukhin, S.A.; Kamenskaya, A.N.; Lavrikov, V.A. The mechanism of decomposition of UO2(NO3)2·6H2O under the action of microwave radiation. Part 1. Radiochemistry 2009, 51, 228–233. [Google Scholar] [CrossRef]
- Kulyukhin, S.A.; Kamenskaya, A.N.; Romer, I.A. The mechanism of decomposition of UO2(NO3)2·6H2O under the action of microwave radiation. Part 2. Radiochemistry 2009, 51, 441–418. [Google Scholar] [CrossRef]
- Kulyako, Y.M.; Vinokurov, S.E.; Trofimov, T.I.; Pilyushenko, K.S.; Malikov, D.A.; Perevalov, S.A.; Savel’ev, B.V.; Dvoeglazov, K.N.; Shadrin, A.Y.; Myasoedov, B.F. Preparation of solid solutions of uranium and cerium oxides from their nitric acid solutions using microwave radiation. Radiochemistry 2019, 61, 661–664. [Google Scholar] [CrossRef]
- Krylosov, S.M.; Korotkov, V.S.; Shadrin, A.Y.; Dvoeglazov, K.N. Batch-Type Microwave Material Treatment Device. RU Patent RU2693820, 24 December 2018. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).