Application of Moving Bed Biofilm Reactor and Fixed Bed Hybrid Biological Reactor for Oilfield Produced Water Treatment: Influence of Total Dissolved Solids Concentration
Abstract
1. Introduction
2. Materials and Methods
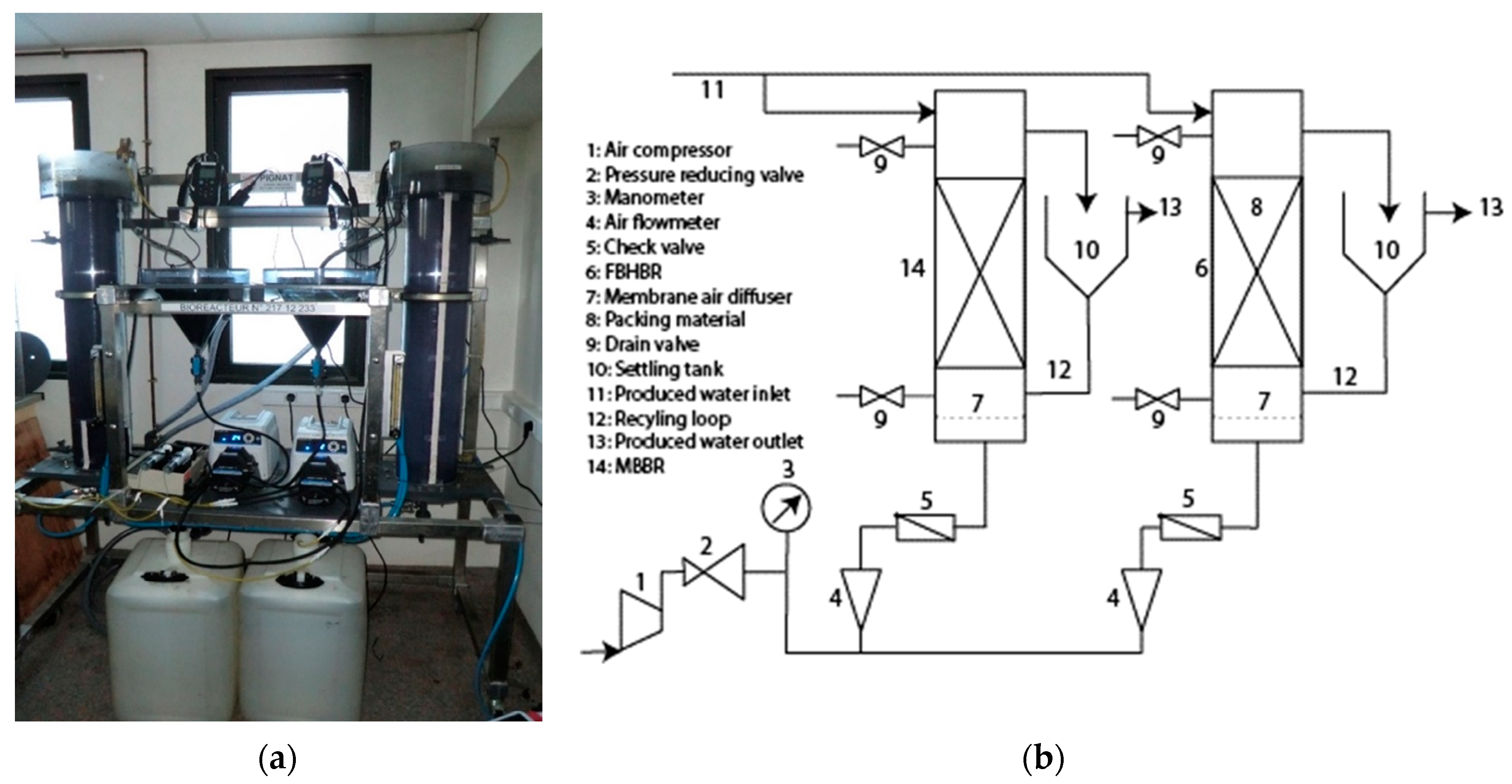
2.1. Experimental Setup
2.2. Microbial Inoculum
2.3. Composition of the Synthetic Produced Water
2.4. Experimental Schedule
2.5. Analytical Methods
2.5.1. Solids Concentration Measurements
2.5.2. Physical-Chemical Parameters
2.5.3. Evaluation of Water Ecotoxicity
2.5.4. Characterization of the Microbial Population
3. Results and Discussion
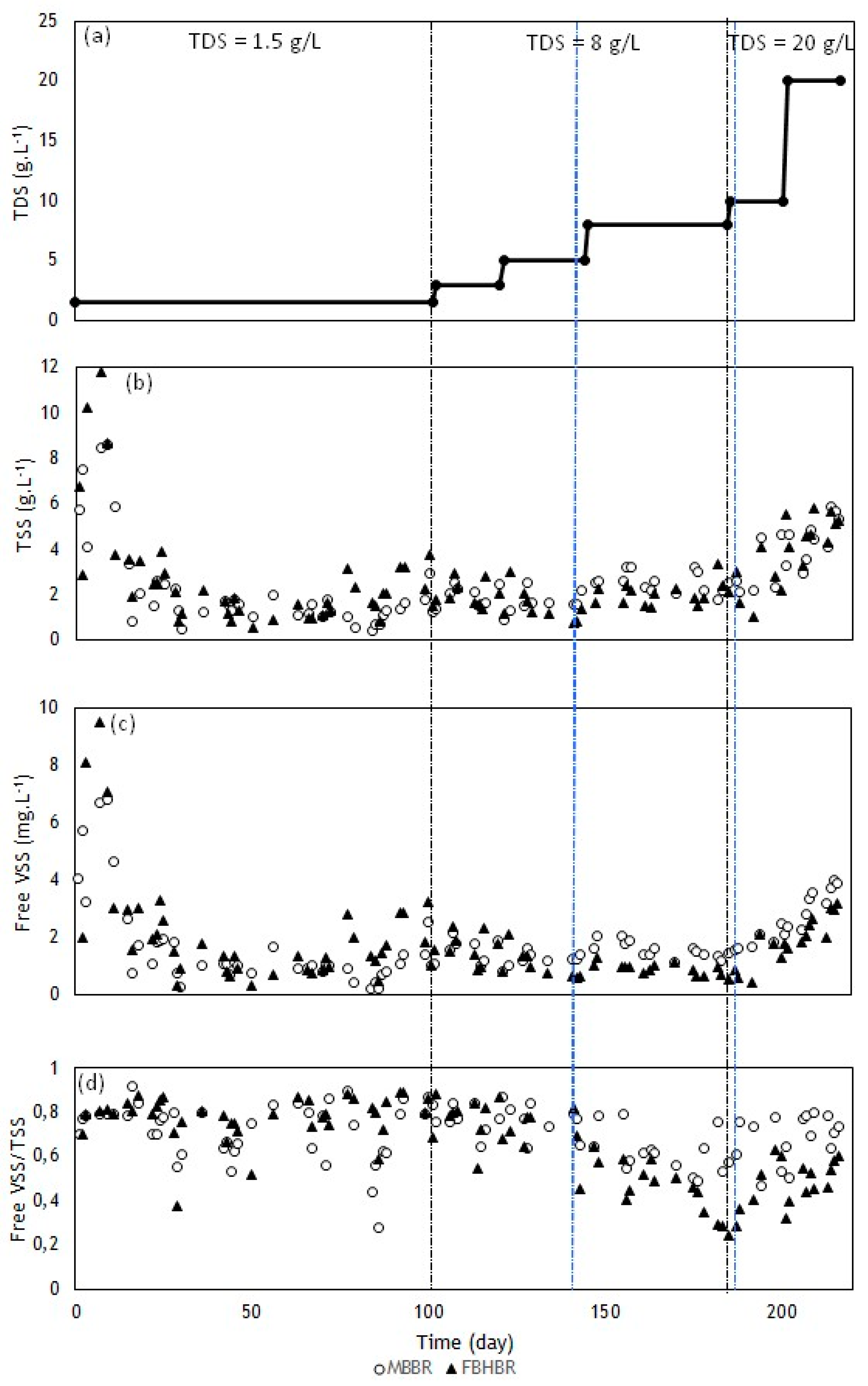
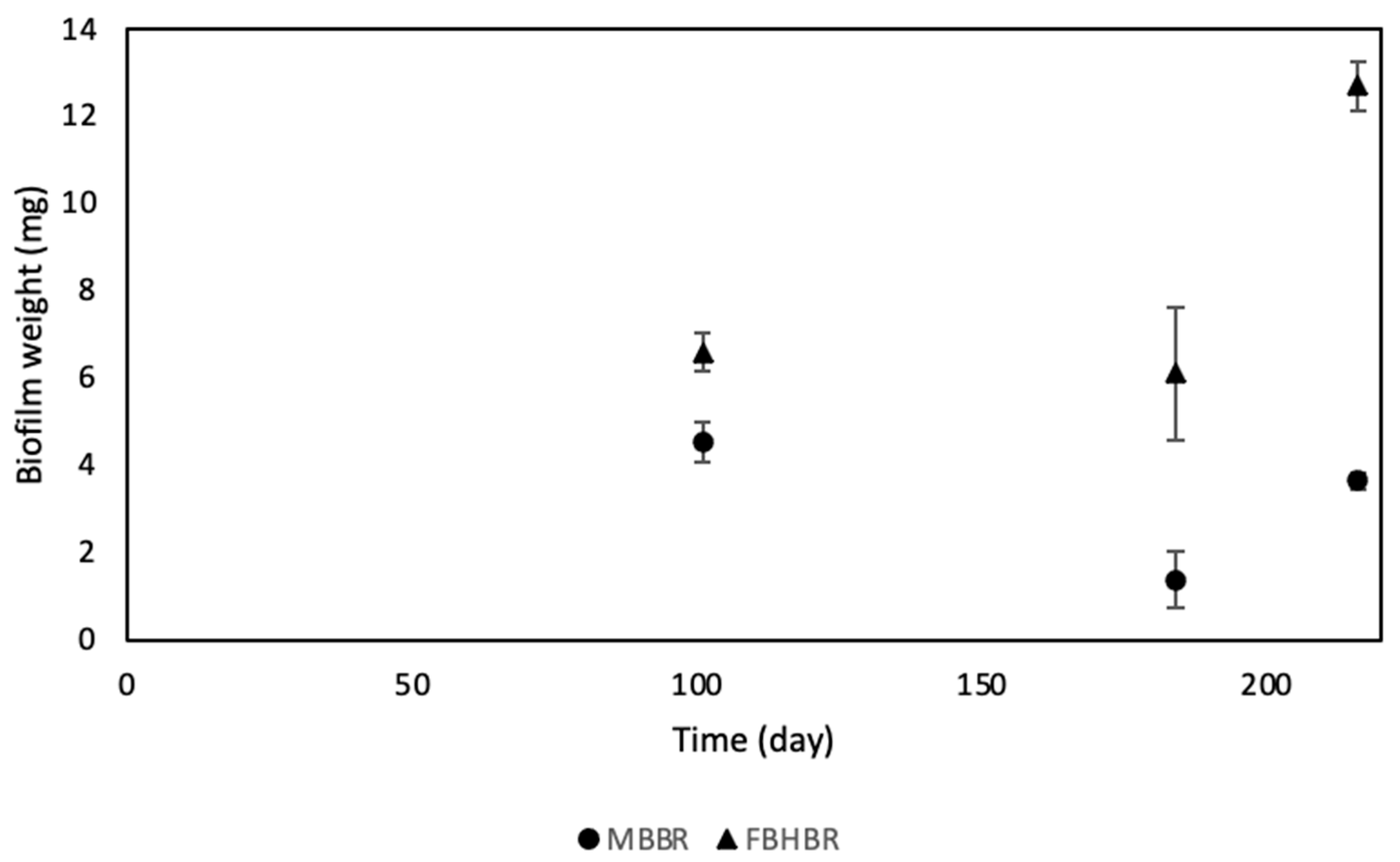
3.1. Evolution of Solids Concentrations
3.2. Removal of Pollutants
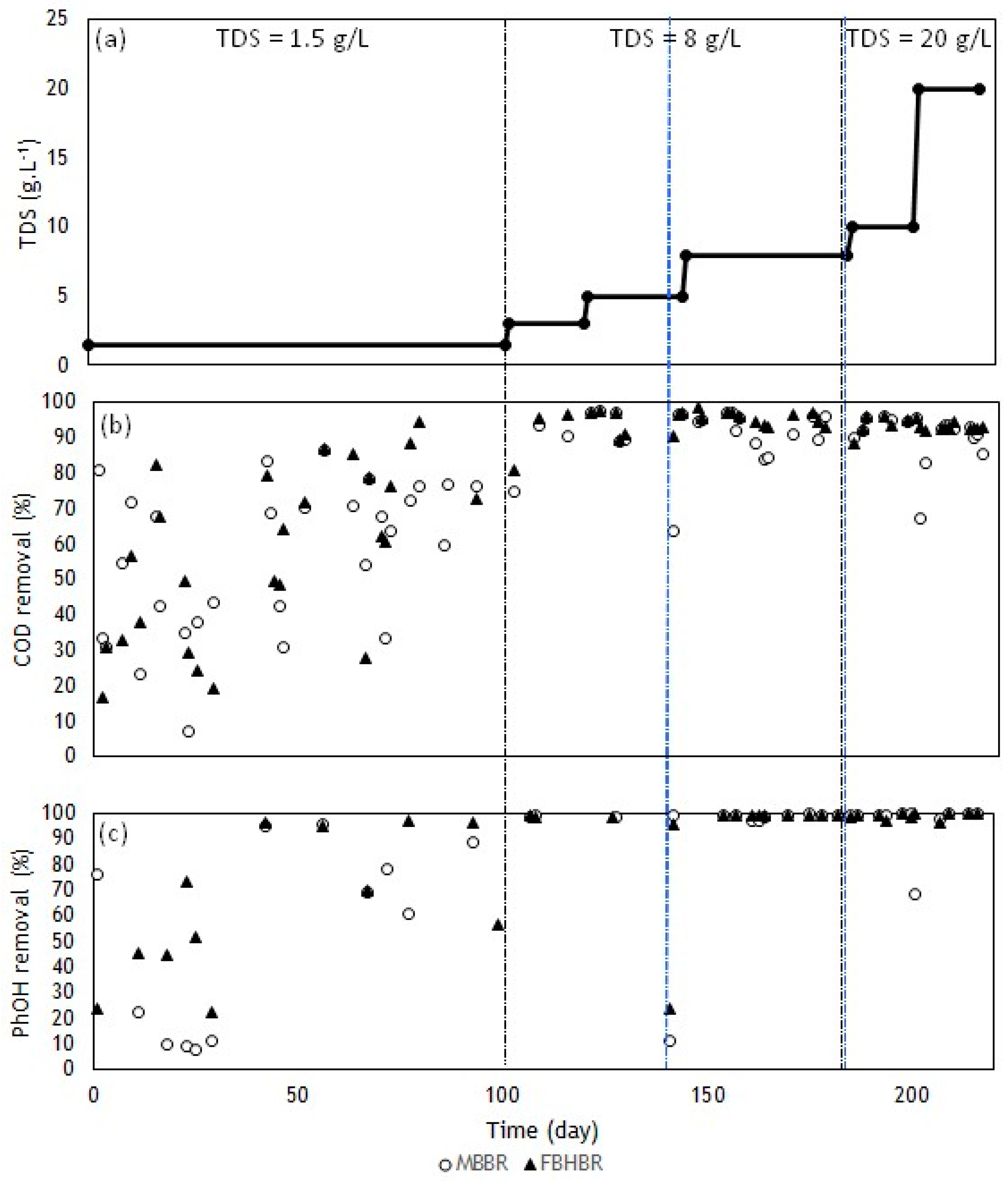
3.2.1. Chemical Oxygen Demand Removal
3.2.2. Pollutant Removal Performance
3.3. Ecotoxicity Assessments
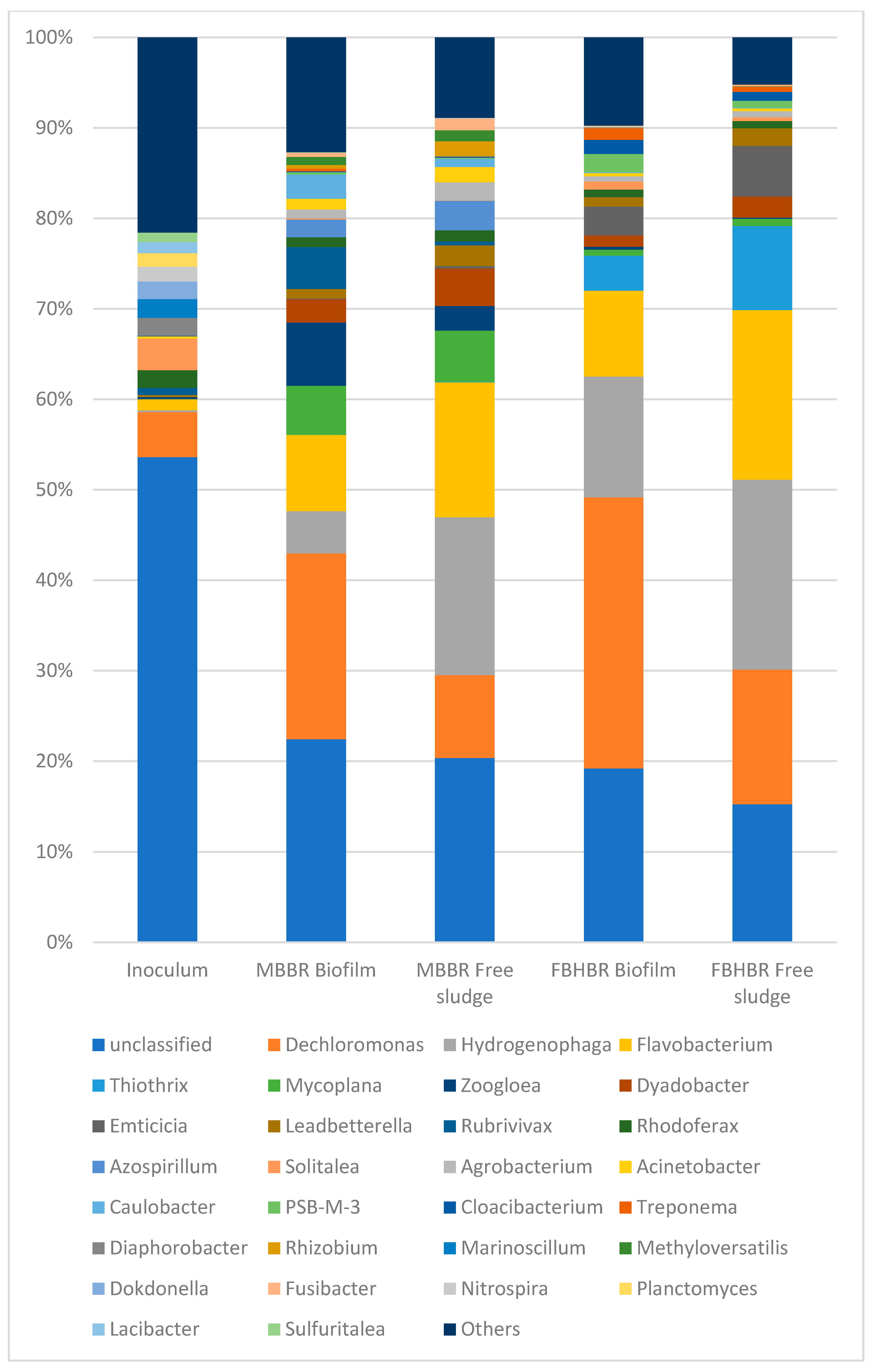
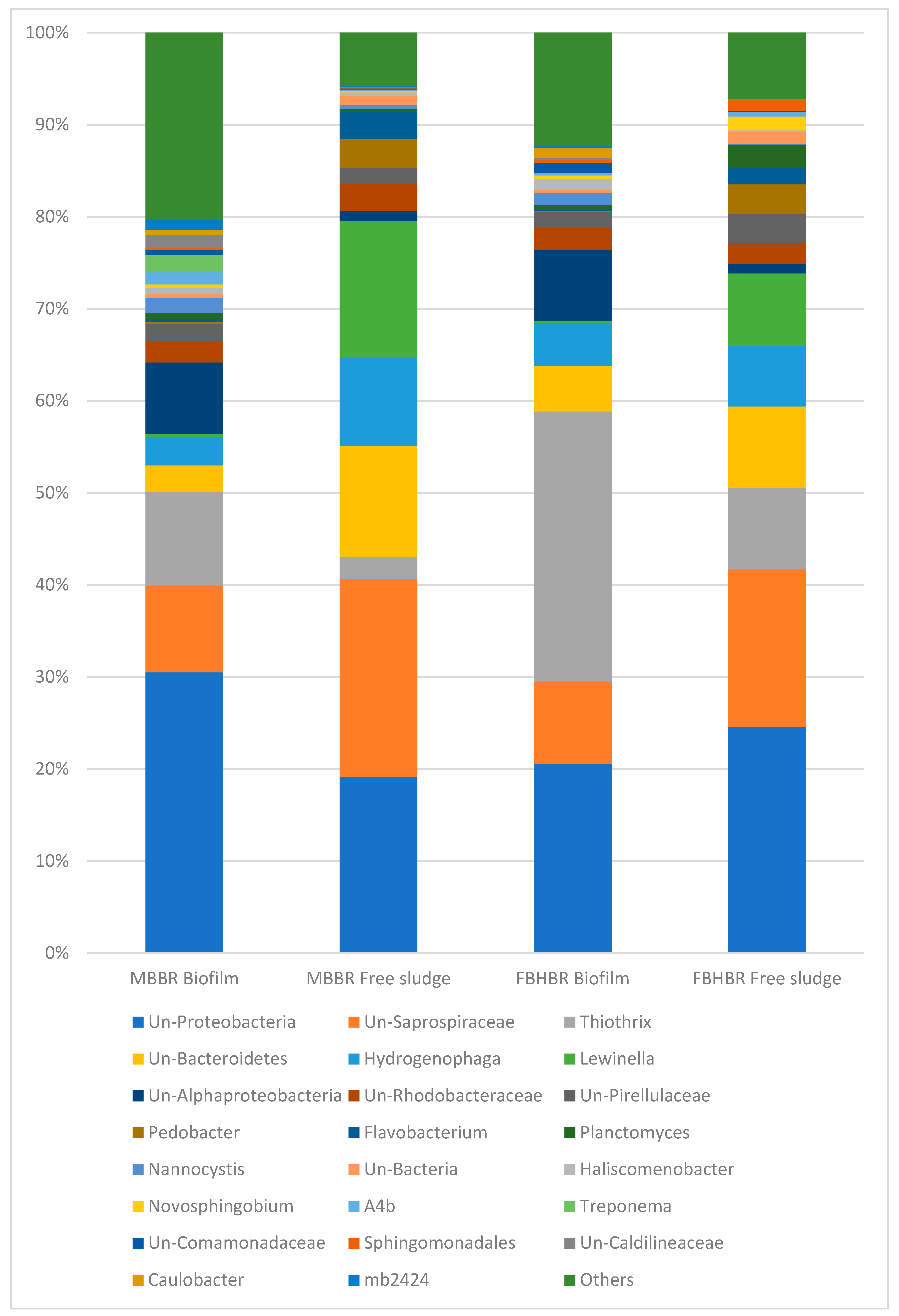
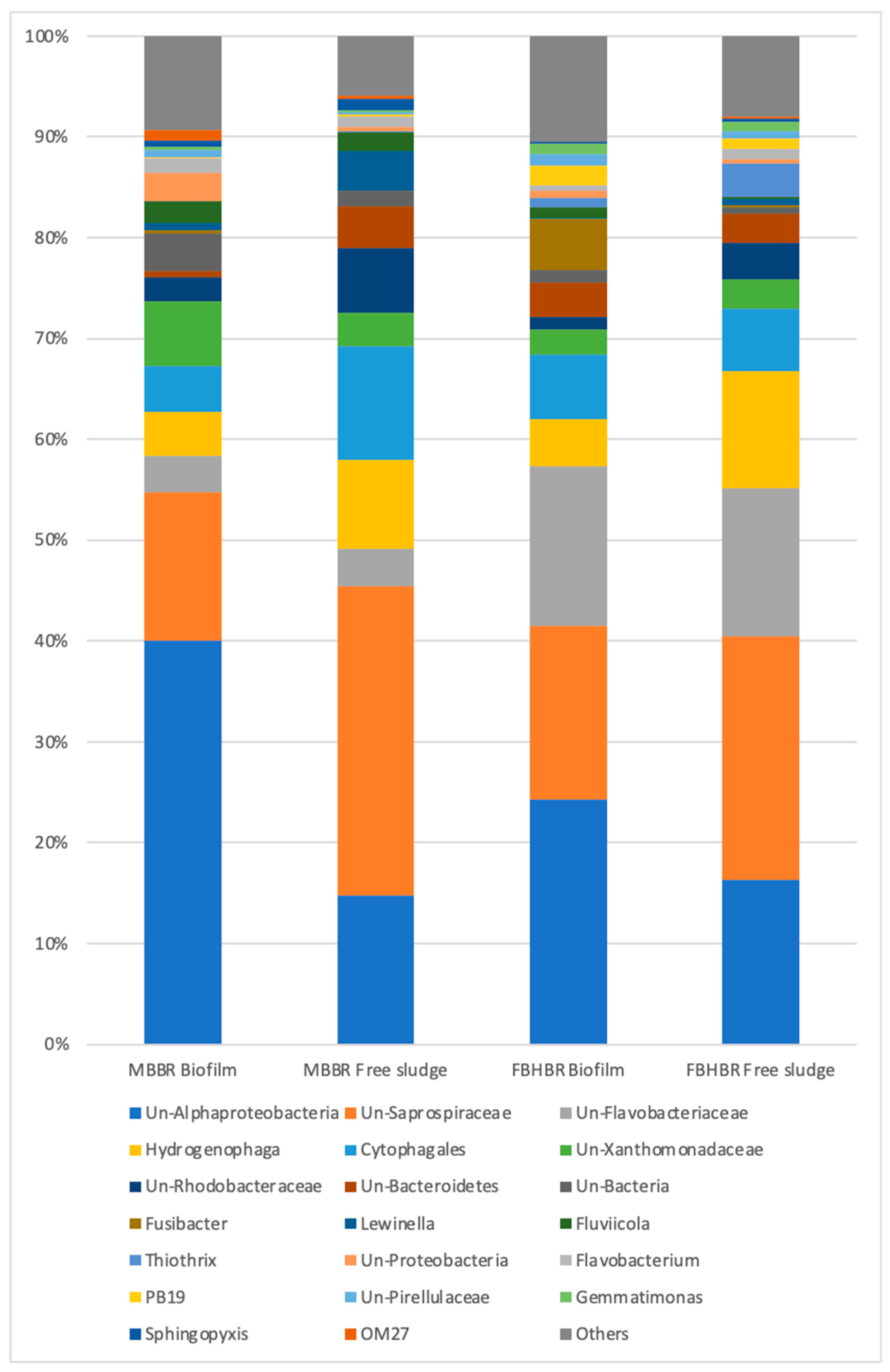
3.4. Assessment of Bacterial Populations
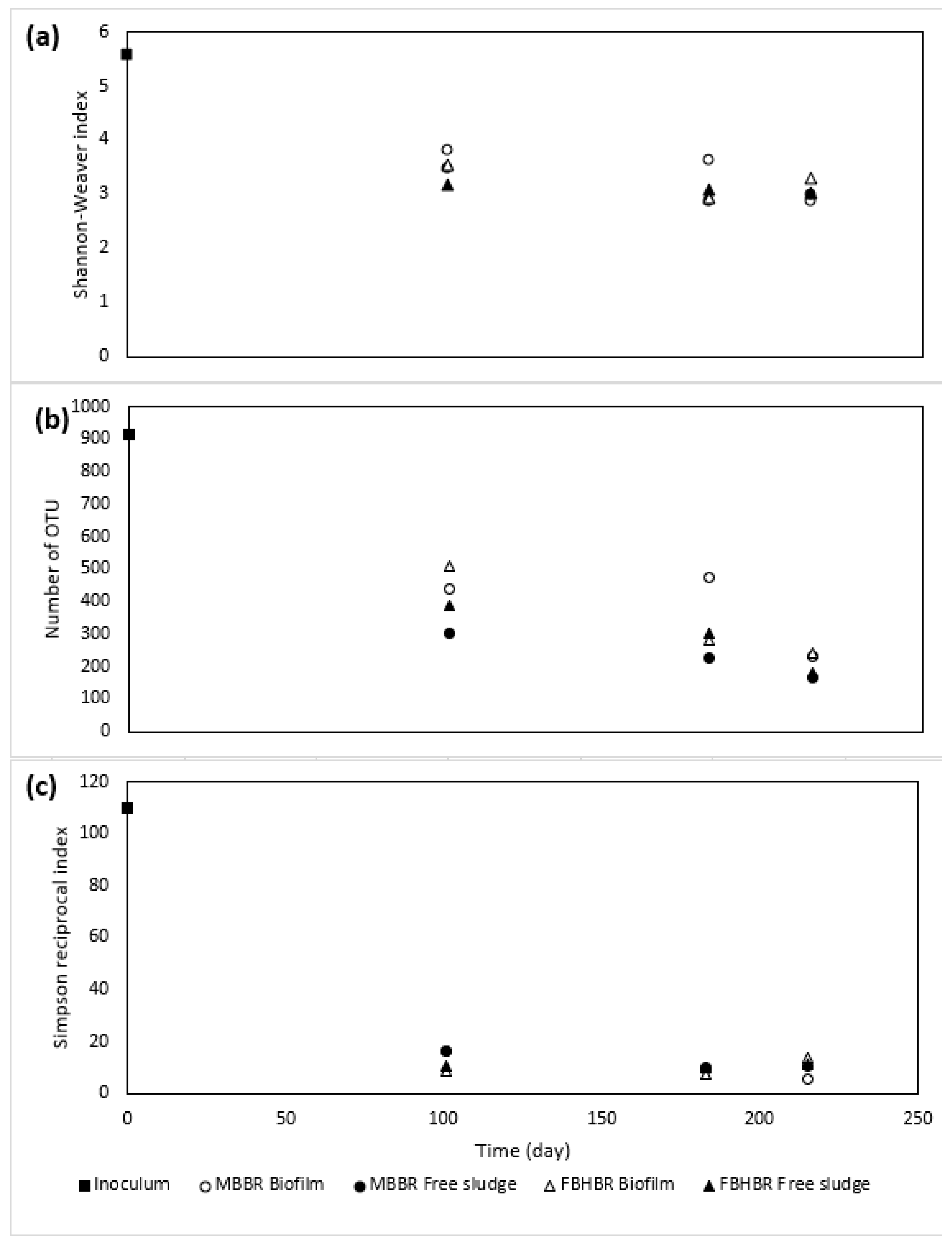
3.4.1. Evolution of Bacterial Diversity
3.4.2. Bacterial Population at TDS = 1.5 g·L−1
3.4.3. Bacterial Population at TDS = 8 g·L−1
3.4.4. Bacterial Population at TDS = 20 g·L−1
4. Conclusions
- Both bioreactors exhibited an increase in TSS concentrations, once the acclimation phase was over, with the increase in TDS concentrations. At the same time, the free VSS/TSS ratio tended to decrease in both bioreactors, which suggests an accumulation of inorganic solids in the free suspended sludge.
- Both bioreactors were proved to be efficient to remove the COD from the influent as well as VOCs and PAHs. An absence of toxicity was noticed in the outlet water performing tests with different microorganisms.
- A decrease in the bacterial diversity indices was observed with respect to the inoculum, leading to the predominance of a lower number of bacterial species. Despite a large part of unclassified gena, some gena, such as Lewinella sp. seem to indicate a logical shift of the bacterial community from freshwater bacteria towards saline bacteria.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aroswoshola, L. Global Water Intelligence Produced Water Market: Opportunities in the Oil, Shale and Gas Sectors in North America; Media Analytics: Oxford, UK, 2011; ISBN 978-1-907467-14-1. [Google Scholar]
- Dores, R.; Hussain, A.; Katebah, M.; Adham, S.S. Using advanced water treatment technologies to treat produced water from the petroleum industry. In Proceedings of the SPE International Health, Safety & Environment Conference, Abu Dhabi, UAE, 2–4 April 2012. SPE-157108-MS. [Google Scholar]
- Veil, J.A.; Puder, M.G.; Elcock, D.; Redweik, R.J., Jr. A White Paper Describing Produced Water from Production of Crude Oil, Natural Gas, and Coal Bed Methane; US Department of Energy: Argonne, IL, USA, 2004.
- Al-Ghouti, M.A.; Al-Kaabi, M.A.; Ashfaq, M.Y.; Da’na, D.A. Produced water characteristics, treatment and reuse: A Review. J. Water Process Eng. 2019, 28, 222–239. [Google Scholar] [CrossRef]
- Alley, B.; Beebe, A.; Rodgers, J.; Castle, J.W. Chemical and physical characterization of produced waters from conventional and unconventional fossil fuel resources. Chemosphere 2011, 85, 74–82. [Google Scholar] [CrossRef]
- Arthur, J.D.; Dillon, L.W.; Drazan, D.J. Management of Produced Water from Oil and Gas Wells. Working Document of the NPC North American Resource Development Study United States; 2011; pp. 1–32. [Google Scholar]
- Munirasu, S.; Haija, M.A.; Banat, F. Use of membrane technology for oil field and refinery produced water treatment—A review. Process Saf. Environ. Prot. 2016, 100, 183–202. [Google Scholar] [CrossRef]
- Liang, Y.; Ning, Y.; Liao, L.; Yuan, B. Special focus on produced water in oil and gas fields. In Formation Damage During Improved Oil Recovery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 515–586. ISBN 978-0-12-813782-6. [Google Scholar]
- Clark, C.E.; Veil, J.A. Produced Water Volumes and Management Practices in the United States; ANL/EVS/R-09-1; Argonne National Lab. (ANL): Argonne, IL, USA, 2009; p. 1007397.
- OSPAR Commission OSPAR Recommendation 2012/5 for a Risk Based Approach to the Management of Roduced Water Discharges from Offshore Installations. Available online: https://www.ospar.org/convention/agreements (accessed on 29 May 2018).
- Zheng, J.; Chen, B.; Thanyamanta, W.; Hawboldt, K.; Zhang, B.; Liu, B. Offshore produced water management: A review of current practice and challenges in Harsh/Arctic environments. Mar. Pollut. Bull. 2016, 104, 7–19. [Google Scholar] [CrossRef]
- OSPAR Commission Establishment of a List of Predicted No Effect Concentrations (PNECs) for Naturally Occuring Substances in Produced Water (OSPAR Agreement 2014/5). Available online: https://www.ospar.org/convention/agreements (accessed on 29 May 2018).
- Jiménez, S.; Micó, M.M.; Arnaldos, M.; Medina, F.; Contreras, S. State of the art of produced water treatment. Chemosphere 2018, 192, 186–208. [Google Scholar] [CrossRef]
- Innocenzi, V.; Zueva, S.B.; Vegliò, F.; De Michelis, I. Pilot-scale experiences with aerobic treatment and chemical processes of industrial wastewaters from electronics and semiconductor industry. Energies 2021, 14, 5340. [Google Scholar] [CrossRef]
- Morais, B.P.; Martins, V.; Martins, G.; Castro, A.R.; Alves, M.M.; Pereira, M.A.; Cavaleiro, A.J. Hydrocarbon toxicity towards hydrogenotrophic methanogens in oily waste streams. Energies 2021, 14, 4830. [Google Scholar] [CrossRef]
- Lusinier, N.; Seyssiecq, I.; Sambusiti, C.; Jacob, M.; Lesage, N.; Roche, N. Biological treatments of oilfield produced water: A comprehensive review. SPE J. 2019, 24, 2135–2147. [Google Scholar] [CrossRef]
- Lusinier, N.; Seyssiecq, I.; Sambusiti, C.; Jacob, M.; Lesage, N.; Roche, N. A comparative study of conventional activated sludge and fixed bed hybrid biological reactor for oilfield produced water treatment: Influence of hydraulic retention time. Chem. Eng. J. 2020, 420, 127611. [Google Scholar] [CrossRef]
- Sharghi, E.A.; Bonakdarpour, B.; Pakzadeh, M. Treatment of hypersaline produced water employing a moderately halophilic bacterial consortium in a membrane bioreactor: Effect of salt concentration on organic removal performance, mixed liquor characteristics and membrane fouling. Bioresour. Technol. 2014, 164, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Sharghi, E.A.; Bonakdarpour, B. the study of organic removal efficiency and halophilic bacterial mixed liquor characteristics in a membrane bioreactor treating hypersaline produced water at varying organic loading rates. Bioresour. Technol. 2013, 149, 486–495. [Google Scholar] [CrossRef]
- Fakhru’l-Razi, A.; Pendashteh, A.; Abidin, Z.Z.; Abdullah, L.C.; Biak, D.R.A.; Madaeni, S.S. Application of membrane-coupled sequencing batch reactor for oilfield produced water recycle and beneficial re-use. Bioresour. Technol. 2010, 101, 6942–6949. [Google Scholar] [CrossRef]
- Pendashteh, A.R.; Abdullah, L.C.; Fakhru’l-Razi, A.; Madaeni, S.S.; Zainal Abidin, Z.; Awang Biak, D.R. Evaluation of membrane bioreactor for hypersaline oily wastewater treatment. Process Saf. Environ. Prot. 2012, 90, 45–55. [Google Scholar] [CrossRef]
- Tong, K.; Zhang, Y.; Liu, G.; Ye, Z.; Chu, P.K. Treatment of heavy oil wastewater by a conventional activated sludge process coupled with an immobilized biological filter. Int. Biodeterior. Biodegrad. 2013, 84, 65–71. [Google Scholar] [CrossRef]
- Di Bella, G.; Di Prima, N.; Di Trapani, D.; Freni, G.; Giustra, M.G.; Torregrossa, M.; Viviani, G. Performance of membrane bioreactor (MBR) systems for the treatment of shipboard slops: Assessment of hydrocarbon biodegradation and biomass activity under salinity variation. J. Hazard. Mater. 2015, 300, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Sambusiti, C.; Saadouni, M.; Gauchou, V.; Segues, B.; Ange Leca, M.; Baldoni-Andrey, P.; Jacob, M. Influence of HRT reduction on pilot scale flat sheet submerged membrane bioreactor (SMBR) Performances for oil&gas wastewater treatment. J. Membr. Sci. 2020, 594, 117459. [Google Scholar] [CrossRef]
- Tellez, G.T.; Nirmalakhandan, N.; Gardea-Torresdey, J.L. Performance evaluation of an activated sludge system for removing petroleum hydrocarbons from oilfield produced water. Adv. Environ. Res. 2002, 6, 455–470. [Google Scholar] [CrossRef]
- Baldoni-Andrey, P.; Pedenaud, P.; Dehaene, P.-L.; Segues, B. Impact of high salinity of produced water on the technical feasibility of biotreatment for E & P on shore applications. In Proceedings of the SPE International Health, Safety & Environment Conference, Abu Dhabi, UAE, 2–4 April 2006. SPE-98751-MS. [Google Scholar]
- Woolard, C.R.; Irvine, R.L. Treatment of hypersaline wastewater in the sequencing batch reactor. Water Res. 1995, 29, 1159–1168. [Google Scholar] [CrossRef]
- Woolard, C.R.; Irvine, R.L. Biological treatment of hypersaline wastewater by a biofilm of halophilic bacteria. Water Environ. Res. 1994, 66, 230–235. [Google Scholar] [CrossRef]
- Sun, C.; Leiknes, T.; Weitzenböck, J.; Thorstensen, B. Salinity effect on a biofilm-mbr process for shipboard wastewater treatment. Sep. Purif. Technol. 2010, 72, 380–387. [Google Scholar] [CrossRef]
- Lefebvre, O.; Moletta, R. Treatment of organic pollution in industrial saline wastewater: A literature review. Water Res. 2006, 40, 3671–3682. [Google Scholar] [CrossRef]
- Grandclément, C.; Seyssiecq, I.; Piram, A.; Wong-Wah-Chung, P.; Vanot, G.; Tiliacos, N.; Roche, N.; Doumenq, P. From the conventional biological wastewater treatment to hybrid processes, the evaluation of organic micropollutant removal: A review. Water Res. 2017, 111, 297–317. [Google Scholar] [CrossRef] [PubMed]
- Tseng, L.Y.; Gonsior, M.; Schmitt-Kopplin, P.; Cooper, W.J.; Pitt, P.; Rosso, D. Molecular characteristics and differences of effluent organic matter from parallel activated sludge and integrated fixed-film activated sludge (IFAS) processes. Environ. Sci. Technol. 2013, 130827102639005. [Google Scholar] [CrossRef]
- Ødegaard, H.; Rusten, B.; Westrum, T. A New Moving Bed Biofilm Reactor—Applications and Results. Water Sci. Technol. 1994, 29, 157–165. [Google Scholar] [CrossRef]
- Cohen, Y. Biofiltration—The treatment of fluids by microorganisms immobilized into the filter bedding material: A review. Bioresour. Technol. 2001, 77, 257–274. [Google Scholar] [CrossRef]
- Machat, H.; Boudokhane, C.; Roche, N.; Dhaouadi, H. Effects of C/N ratio and DO concentration on carbon and nitrogen removals in a hybrid biological reactor. Biochem. Eng. J. 2019, 151, 107313. [Google Scholar] [CrossRef]
- Ruys, V.S.; Zerari, K.; Seyssiecq, I.; Roche, N. Study of carbonaceous and nitrogenous pollutant removal efficiencies in a hybrid membrane bioreactor. J. Chem. 2017, 2017, 4080847. [Google Scholar] [CrossRef]
- Zerari, K.; Seyssieq, I.; Akretche, D.-E.; Roche, N. Enhancement of oxygen mass transfer coefficients in a hybrid membrane bioreactor: Enhancement of oxygen mass transfer coefficients in a HMBR. J. Chem. Technol. Biotechnol. 2013, 88, 1007–1013. [Google Scholar] [CrossRef]
- Dong, Z.; Lu, M.; Huang, W.; Xu, X. Treatment of oilfield wastewater in moving bed biofilm reactors using a novel suspended ceramic biocarrier. J. Hazard. Mater. 2011, 196, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Hasanzadeh, R.; Abbasi Souraki, B.; Pendashteh, A.; Khayati, G.; Ahmadun, F.-R. Application of isolated halophilic microorganisms suspended and immobilized on walnut shell as biocarrier for treatment of oilfield produced water. J. Hazard. Mater. 2020, 400, 123197. [Google Scholar] [CrossRef]
- Federation, W.E.; American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 21st ed.; centennial ed; Eaton, A.D., American Public Health Association, American Water Works Association, Water Pollution Control Federation, Eds.; American Public Health Association: Washington, DC, USA, 2005; ISBN 978-0-87553-047-5. [Google Scholar]
- Abtahi, S.M.; Petermann, M.; Juppeau Flambard, A.; Beaufort, S.; Terrisse, F.; Trotouin, T.; Joannis Cassan, C.; Albasi, C. Micropollutants removal in tertiary moving bed biofilm reactors (MBBRs): Contribution of the biofilm and suspended biomass. Sci. Total Environ. 2018, 643, 1464–1480. [Google Scholar] [CrossRef] [PubMed]
- AFNOR NF ISO 11423-1 (T90-155) of 1997-09-01. Water Quality—Determination of Benzene and Some Derivatives—Part 1: Head-Space Gas Chromatographic Method; ISO: Geneva, Switzerland, 1997. [Google Scholar]
- AFNOR DIN ISO 28540 of 2014-05-01. Water Quality—Determination of 16 Polycyclic Aromatic Hydrocarbons (PAH) in Water—Method Using Gas Chromatography with Mass Spectrometric Detection (GC-MS) (ISO 28540:2011); ISO: Geneva, Switzerland, 2014. [Google Scholar]
- AFNOR NF EN ISO 11348-3 (T90-320-3) of 2009-02-01. Water Quality—Determination of the Inhibitory Effect of Water Samples on the Light Emission of Vibrio Fischeri (Luminescent Bacteria Test)—Part 3: Method Using Freeze-Dried Bacteria; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- AFNOR NF EN ISO 8692 (T90-304) of 2012-05-01. Water Quality—Fresh Water Algal Growth Inhibition Test with Unicellular Green Algae; ISO: Geneva, Switzerland, 2012. [Google Scholar]
- AFNOR NF EN ISO 6341 (T90-301) of 2012-12-01. Water Quality—Determination of the Inhibition of the Mobility of Daphnia Magna Straus (Cladocera, Crustacea)—Acute Toxicity Test; ISO: Geneva, Switzerland, 2012. [Google Scholar]
- AFNOR NF ISO 20666 (T90-334) of 2009-01-01. Water Quality—Determination of the Chronic Toxicity to Brachionus Calyciflorus in 48 h; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- AFNOR NF EN ISO 10253 of 2016-12-01. Water Quality—Marine Algal Growth Inhibition Test with Skeletonema sp. and Phaeodactylum Tricornutum; ISO: Geneva, Switzerland, 2016. [Google Scholar]
- AFNOR FD ISO 14669 of 2003-08-01. Water Quality—Determination of Acute Lethal Toxicity to Marine Copepods (Copepoda, Crustacea); ISO: Geneva, Switzerland, 2003. [Google Scholar]
- Ludzack, F.J.; Noran, D.K. Tolerance of high salinities by conventional wastewater treatment processes. J. Water Pollut. Control Fed. 1965, 37, 1404–1416. [Google Scholar] [PubMed]
- Fatone, F.; Di Fabio, S.; Bolzonella, D.; Cecchi, F. Fate of aromatic hydrocarbons in italian municipal wastewater systems: An overview of wastewater treatment using conventional activated-sludge processes (CASP) and membrane bioreactors (MBRs). Water Res. 2011, 45, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; O’Connor, S.M.; Chan, E.; Coates, J.D. Anaerobic degradation of benzene, toluene, ethylbenzene, and xylene compounds by dechloromonas strain RCB. Appl. Environ. Microbiol. 2005, 71, 8649–8655. [Google Scholar] [CrossRef]
- Lewin, R.A. New herpetosiphon species (flexibacterales). Can. J. Microbiol. 1970, 16, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Sly, L.I.; Taghavit, M.; Fegan, M. Phylogenetic heterogeneity within the genus herpetosiphon: Transfer of the marine species herpetosiphon cohaerens, herpetosiphon nigricans and herpetosiphon persicus to the genus lewinella gen. Nov. in the flexibacter-bacteroides-cytophaga phylum. Int. J. Syst. Bacteriol. 1998, 48, 731–737. [Google Scholar] [CrossRef][Green Version]
- Koshlaf, E.; Shahsavari, E.; Haleyur, N.; Mark Osborn, A.; Ball, A.S. Effect of biostimulation on the distribution and composition of the microbial community of a polycyclic aromatic hydrocarbon-contaminated landfill soil during bioremediation. Geoderma 2019, 338, 216–225. [Google Scholar] [CrossRef]
- Sun, F.; Hu, J.; Zhou, Y.; Mei, R.; Wang, C.; He, Y.; Wu, W. High efficient alternating anaerobic/aerobic process for polyester resin wastewater treatment: Performance and microbial community structure. Biochem. Eng. J. 2018, 138, 121–130. [Google Scholar] [CrossRef]

| Compound | Concentration (mg·L−1) |
|---|---|
| COD (adjusted with ethanol, sodium acetate, urea and peptone) | 1600 |
| TOC | 379 |
| TN (from NH4Cl, urea and peptone) | 20.6 |
| TP (from KH2PO4) | 4 |
| Phenol | 12 |
| Toluene | 8 |
| o-Xylene | 1 |
| m-Xylene | 3 |
| Naphthalene | 0.2 |
| Phenanthrene | 0.05 |
| Benzo[a]pyrene | 0.0002 |
| Phase | I | II | III |
|---|---|---|---|
| Duration (days) | 1–120 | 121–180 | 181–216 |
| HRT (h) | 12 | 12 | 12 |
| OLR (kgCOD·m−3·d−1) | 3.2 | 3.2 | 3.2 |
| Salinity (g·L−1) | 1.5 | 8 | 20 |
| Microorganism | Tested Water | ||
|---|---|---|---|
| Inlet PW | Outlet MBBR | Outlet FBHBR | |
| Daphnia magna | 1.6 | NT | NT |
| Brachionus calyciflorus (rotifer) | 22.7 | >90 | >90 |
| Pseudokirchneriella subcapitata (freshwater algae) | 35 | >90 | >90 |
| Vibrio fischeri (microtox test) | 29.9 | NT | NT |
| Microorganism | Tested Water | ||
|---|---|---|---|
| Inlet PW | Outlet MBBR | Outlet FBHBR | |
| Vibrio fischeri (microtox test) | 48.5 | NT | NT |
| Phaeodactylum tricornutum (marine algae) | 29.7 | 38.4 | 60.6 |
| Artemia salina (crustaceans) | 50.7 | NT | NT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lusinier, N.; Seyssiecq, I.; Sambusiti, C.; Jacob, M.; Lesage, N.; Roche, N. Application of Moving Bed Biofilm Reactor and Fixed Bed Hybrid Biological Reactor for Oilfield Produced Water Treatment: Influence of Total Dissolved Solids Concentration. Energies 2021, 14, 7297. https://doi.org/10.3390/en14217297
Lusinier N, Seyssiecq I, Sambusiti C, Jacob M, Lesage N, Roche N. Application of Moving Bed Biofilm Reactor and Fixed Bed Hybrid Biological Reactor for Oilfield Produced Water Treatment: Influence of Total Dissolved Solids Concentration. Energies. 2021; 14(21):7297. https://doi.org/10.3390/en14217297
Chicago/Turabian StyleLusinier, Nicolas, Isabelle Seyssiecq, Cecilia Sambusiti, Matthieu Jacob, Nicolas Lesage, and Nicolas Roche. 2021. "Application of Moving Bed Biofilm Reactor and Fixed Bed Hybrid Biological Reactor for Oilfield Produced Water Treatment: Influence of Total Dissolved Solids Concentration" Energies 14, no. 21: 7297. https://doi.org/10.3390/en14217297
APA StyleLusinier, N., Seyssiecq, I., Sambusiti, C., Jacob, M., Lesage, N., & Roche, N. (2021). Application of Moving Bed Biofilm Reactor and Fixed Bed Hybrid Biological Reactor for Oilfield Produced Water Treatment: Influence of Total Dissolved Solids Concentration. Energies, 14(21), 7297. https://doi.org/10.3390/en14217297







