Abstract
Biolubricants refer to eco-friendly, biodegradable, and non-toxic lubricants. Their applications are still limited compared to mineral oils; however, their sustainable credentials are making them increasingly attractive. Vegetable oils are frequently used for this purpose. However, vegetable oils have issues of low lipid productivity, dependence on climatic conditions, and need for agricultural land. Microbial oils represent a more sustainable alternative. To ensure their widespread applicability, the suitability of microbial oils from a physicochemical point of view needs to be determined first. In this study, oils obtained from various oleagenic microbes—such as microalgae, thraustochytrids, and yeasts—were characterized in terms of their fatty acid profile, viscosity, friction coefficient, wear, and thermal stability. Oleaginous microalgal strains (Auxenochlorella protothecoides and Chlorella sorokiniana), thraustochytrids strains (Aurantiochytrium limacinum SR21 and Aurantiochytrium sp. T66), and yeast strains (Rhodosporidium toruloides and Cryptococcus curvatus) synthesized 64.5%, 35.15%, 47.89%, 47.93%, 56.42%, and 52.66% of lipid content, respectively. Oils from oleaginous microalgae (A. protothecoides and C. sorokiniana) and yeasts (R. toruloides and C. curvatus) possess excellent physicochemical and tribological qualities due to high amount of monounsaturated fatty acids (oleic acid C18:1 content, 56.38%, 58.82%, 46.67%, 38.81%) than those from oleaginous thraustochytrids (A. limacinum SR21 and Aurantiochytrium sp. T66; 0.96%, 0.08%, respectively) supporting their use as renewable and biodegradable alternatives to traditional mineral oil-based lubricants. Oil obtained from microalgae showed a lower friction coefficient than oils obtained from yeasts and thraustochytrids.
1. Introduction
Along with industrialization and automation, a growing global population is contributing to a rise in energy usage. The heavy dependence on fossil fuels over the past century has led to a steady decline in non-renewable fuel stocks, whose depletion is estimated for the medium term. As a result of this situation, researchers are looking for renewable sources to replace conventional fossil fuels and chemicals [1]. Among various renewable alternatives, biomass is the only one that can replace petroleum both as an energy source and for the synthesis of organic products [2].
A lubricant is added to reduce friction, minimize wear, transfer heat, remove contaminants, and generally improve processes across two contacting surfaces. The worldwide demand for lubricants amounted to 39 million tons in 2017, of which more than 60% was used as automotive lubricants [3]. Around 95% of the global supply of lubricants is petroleum-based. Mineral oils are composed of diverse combination of paraffins, olefins, naphthenes, and aromatic hydrocarbons of 20 to 50 carbon atoms. They are non-biodegradable, non-renewable, highly toxic or carcinogenic to humans, as well as a source of greenhouse gas emissions and water pollution [4]. Given the increasing demand for vehicles and mechanical industrialization, it is projected that the consumption of lubricants will be increased by 2% annually [5]. To comply with new environmental standards, more biodegradable and less toxic lubricants are being developed. Biolubricants—i.e., lubricants derived from bio-based sources—have emerged as prospective replacements for traditional mineral oils [6]. This is encouraged by the rising costs of oil supplies, legislative restrictions to the use of petroleum-based products, and environmental protection concerns [7]. Bio-based lubricants can be categorized into natural and synthetic oils based on their chemical composition. The former are usually of plant or animal origin; whereas the latter rely on further modification—such as ester synthesis—of natural oils [8]. The starting materials for oil extraction and distillation have included plants and animal sources [9]. While vegetable oils are more readily accessible, and display better biodegradability than mineral oils [5], the most economical feedstock for renewable energy generation has now become microbial oil, known also as single-cell oil [10]. In spite of their similarity to vegetable oils, microbial oils offer better lipid productivity (in g/L/day) and the ability of microorganisms to grow in a controlled environment, thus reducing dependence on climatic fluctuations [11]. Oleaginous microorganisms comprise microalgae, bacteria, fungi, and yeasts; they are defined by the ability to accumulate high lipids amounts in their cellular compartment (>20%) [12], which can be more than 60% of their cell dry biomass [13]. The majority of lipids synthesized by oleaginous bacteria have an unbranched chain of 4 to 28 carbon atoms, and can include saturated or unsaturated fatty acids, which in turn can be mono- or polyunsaturated depending on the number of double bonds [14]. Lipids from microalgal or yeast strains have been already studied as a possible source of biodiesel; whereas thraustochytrids are being considered for commercial-scale docosahexaenoic acid (C22:6) production. Some oleaginous microorganisms are not suitable for renewable energy (biofuel) uses due to a high proportion of polyunsaturated fatty acids (PUFAs) [15], whose multiple double bonds are prone to auto-oxidation and foul odor [16]. Hence, to ensure the applicability of microbial oils, it is first necessary to determine the suitability of their fatty acid profiles and physicochemical properties.
In this study, we assessed the fatty acid composition, as well as the physicochemical and tribological properties, such as friction coefficient (FC), viscosity, wear volume, and thermal stability of oils obtained from a diverse group of oleaginous microorganisms, including microalgae, thraustochytrids, and yeasts.
2. Materials and Methods
2.1. Microbial Strains and Cultivation Conditions
2.1.1. Microalgae Cultivation
Two strains of microalgae namely Auxenochlorella protothecoides (SAG 211-13) and Chlorella sorokiniana (SAG 211-8k) were acquired from SAG (Sammlung von Algenkulturen der Universität Göttingen, Göttingen, Germany) located at Göttingen University, Germany. These strains were selected based on their heterotrophic cultivation characteristics that give high biomass and lipid productivity than those obtained with obligatory autotrophic microalgae. Initially, these strains were cultivated with agar dishes prepared with Bold’s basal medium (BBM), glucose as carbon source and yeast extract as nitrogen source and kept at 16 °C for their long survival according to our previous manuscript with A. protothecoides [16]. All chemicals for the cultivation of microalgae including BBM were bought from Sigma Aldrich (St. Louis, MO, USA).
Cultivations were carried out according to our previous manuscript with some modifications [16]. To obtain the maximum lipid production from these microalgal strain, the medium was prepared with high C/N ratio, where the BBM was mixed with glucose (20 g/L) and yeast extract that gives a C/N ratio of 60. All cultivation experiments were carried out in Erlenmeyer flasks (5000 mL) with 1 L working solution and kept at room temperature at 180 rpm in an incubator under dark conditions for 5 days. Sampling for residual carbon in medium, biomass and lipid accumulation was performed daily at 24 h of intervals by taking out 5 mL of cultures from flasks.
2.1.2. Thraustochytrids Cultivation
Two thraustochytrids strains such as Aurantiochytrium sp. T66 (ATCC PRA-276) and Aurantiochytrium limacinum SR21 were ordered from ATCC, Manassas, VA, USA and were maintained on medium containing yeast extract, peptone, and glucose in artificial seawater as described previously [17]. Here, it was kept on ATCC 790 By+ culture medium which contains yeast extract (1 g), peptone (1 g), and glucose (5 g) in 1 L of artificial seawater (ASW). The latter consisted of NaCl (18 g/L), MgSO4·7H2O (2.44 g/L), KCl (0.6 g/L), NaNO3 (1.0 g/L), CaCl2·2H2O (0.3 g/L), KH2PO4 (1.0 g/L), Tris buffer (1.0 g/L), NH4Cl (0.027 g/L), vitamin B12 (15 × 10−8 g/L), chelated iron solution (3 mL) containing Na2EDTA·2H2O (26 µM), 0.1M HCl (45 mM), FeCl3·6H2O (3 µM), and metal solution (10 mL) containing Na2EDTA·2H2O (2.7 mM), H3BO3 (18.4 mM), FeCl3·6H2O (0.18 mM), MnSO4·H2O (0.97 mM), ZnSO4·7H2O (0.07 mM), and CoCl2·6H2O (0.02 mM). The cultures were revived twice for cultivation experiments and seed cultures were prepared with the abovementioned medium in 250-mL Erlenmeyer flasks, which were then incubated at 25 °C and 180 rpm for 24 h. Batch cultivations were performed with 60 g/L and 40 g/L of glucose for A. limacinum SR21 and Aurantiochytrium sp. T66, respectively, whereas C/N ratio was maintained at 10 with the yeast extract and mixed in ASW. The flasks were incubated at 25 °C at 180 rpm in an incubator shaker under dark conditions.
2.1.3. Yeast Cultivation
Two oleaginous yeasts Rhodosporidium toruloides NCYC 1576 and Cryptococcus curvatus DSM-101032 were obtained from the NCYC; Norwich, UK and the Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany), respectively. Initially, both yeasts were grown on YPD agar plates (glucose, 20 g/L; peptone, 20 g/L; yeast extract, 10 g/L) according to protocol mentioned in our previous studies for yeast cultivation [18,19]. Inoculum was prepared in YPD broth and flasks were incubated at 28 °C for 48 h. Batch cultivations of C. curvatus were performed in 5-L Erlenmeyer flasks with 1000 mL working volume consisted of glucose (40 g/L), yeast extract (1 g/L), while the C/N 40 was maintained with (NH4)2SO4. Batch cultivations of R. toruloides were carried out in 5-L Erlenmeyer flasks with 1000 mL working volume consisted of glucose (40 g/L), yeast nitrogen base without ammonium sulphate and amino acids (0.76 g/L), yeast extract (0.1 g/L), and C/N 400 was adjusted with (NH4)2SO4. The flasks were inoculated with 2% seed culture and placed in a rotary incubator at 28 °C until the stationary phase was achieved.
2.2. Monitoring of Lipid Accumulation in Cultures
Lipid accumulation during the cultivation of oleaginous microorganisms was monitored by fluorescence microscopy. Sampling was carried out before stationary phase by mixing 1 mL of growing culture with 2 μL of BODIPY493/503 stock solution at 0.1 mg/mL in DMSO. The samples were mixed thoroughly and incubated in the dark for 5 min. Images were taken using a fluorescence microscope (EVOS-FL; Thermo Fisher Scientific, Waltham, MA, USA).
2.3. Lipid Extraction from Biomass
Once fermentation experiments were completed, the slurry was centrifuged and the biomass from each sample was heated at 45 ± 2 °C until the constant weight was achieved. The obtained biomass was pulverized into paste by using solution of chloroform: methanol (2:1, v/v). The mixture was kept at 25 °C at 100 rpm. The slurry was transferred to a conical glass tube followed by centrifugation at 8000 rpm for 10 min at 25 °C. The lower solvent layer containing lipids was transferred to a pre-weighed glass tube and dried under vacuum. Once dry, the tube was weighed, and the lipid concentration (g/L) was evaluated. Following formula was considered to calculate the lipid content (%, w/w)
Lipid content (%) = (Total Lipid concentration (g/L)/(Cell dry weight (g/L)
2.4. Determination of Fatty Acid Profiles by Gas Chromatography-Mass Spectrometry (GC-MS) and Thin-Layer Chromatography (TLC)
The lipids extracted from mentioned microbes were analyzed for their contents such as TAG, MAG, DAG, PL, and SL by thin-layer chromatography (TLC). The lipids were mixed with n-hexane and spotted on the ready to use chromatographic plate (silica gel 60) and run with n-hexane: diethyl ether: acetic acid (85:15:1; v/v/v). After finishing this step, the plates were dried by hot air and dipped for few seconds in the staining solution containing 0.63 g MnCl2·4H2O, 60 mL water, 60 mL methanol, and 4 mL concentrated H2SO4). The plate was kept in an oven at 125 ± 5 °C for five minutes for the development of bands. The presence of triacylglycerol (TAG) was assessed using triolein as control.
The lipids were also analyzed for its fatty acids profile by GC-MS. Initially, lipid was subjected to transesterification by using acid catalyst as mentioned in the Laboratory Analytical Procedure (LAP) by NREL, USA [20]. Briefly, 100 mg of lipids were mixed with 2 mL of chloroform: methanol (2:1, v/v) in Ace pressure tube, (bushing type, Front seal, capacity 120 mL; Sigma-Aldrich) and further mixed with methanolic HCl. The Ace tubes were kept in a water bath at 85 ± 2 °C for 60 min and shaken every 10 min interval for proper mixing. After finishing the reaction, the tubes were cooled at room temperature and n-hexane (3 mL) was added in it followed by centrifugation for phase separation as described previously [10]. The fatty acid methyl esters (FAMEs) containing upper layer of n-hexane was transferred in GC glass vails for the analysis. GC-MS analysis was performed with Clarus 690 MS coupled to Clarus SQ8 series GC (PerkinElmer, Waltham, MA, USA) using capillary column (Elite-FFAP Capillary Column—30 m × 0.25 mm I.D. × 0.25 µm, N9316352).
2.5. Infrared Spectroscopy (IR) Analysis of Microbial Oils
A Vertex 80v FT-IR spectrometer (Bruker, Billerica, MA, USA) with a deuterated lanthanum-α-alanine-doped triglycine sulfate detector and a platinum-attenuated total reflection accessory containing a diamond crystal was used to capture infrared spectra. Samples were scanned as single beam background by combined 128 scans with an optical resolution of 4 cm−1. The resultant interferograms were then Fourier-transformed using the Mertz phase correction mode, a Blackman-Harris three-term apodization function, and a zero-filling factor of 2. The double-side forward-backward acquisition technique was used to acquire all spectra in vacuum.
2.6. Rheological Properties of Microbial Oils
Using a CVO 100 rheometer (Bohlin Instruments, Cirencester, UK), the average viscosity of lipids extracted from oleaginous microorganisms was measured at 25 °C with shear rates ranging from 1/s to 100/s. A cone-on-plate geometry was utilized, with the cone having a 1° cone angle and a 20 mm diameter, and the lower plate having a 60 mm diameter.
2.7. Determination of Lubrication Properties
The tribological characteristics of extracted lipids from oleaginous microorganisms were estimated with protocol mentioned in ASTM D 6425 using an Optimol SRV-III oscillating friction and wear tester (Optimol Instruments Prüftechnik GmbH, München, Germany). A steel ball (52100 bearing steel, diameter 10 mm, surface roughness (Ra) 20 nm; SKF, Göteborg, Sweden) was moved under continuous rotation condition against a static steel disc (100CR6 ESU toughened steel, diameter 24 7.9 mm, Ra 120 nm; Optimol Instruments Prüftechnik GmbH). Equipments were wiped with acetone before the experiments, and microbial lipid samples (0.5 mL) was evenly applied to the steel disc using a glass rod. The experiments were carried out at 25 °C with a load of 77 N and 150 N (maximum Hertzian pressures of 2.0 GPa and 2.5 GPa), a 1 mm amplitude, and a 50-Hz sliding frequency. The temperature (±1 °C) was regulated by a cooling/heating mechanism under the steel disk, which also functioned as a sample container. A data acquisition device connected to the SRV-III tester was used to automatically capture FC curves. After the evaluations, an optical profiling device (Zygo 7300; Zygo Corporation, Middlefield, CT, USA) was used to measure the ball wear diameter and wear volume of the bottom disc. To reduce experimental error, three replications of each experiment were performed where comparison was made up with PEG 200.
2.8. Thermogravimetric Analysis (TGA) of Microbial Oils
The thermal stability of microbial oils was assessed using a Thermogravimetric analyzer (TGA 8000, PerkinElmer, Waltham, MA, USA). The tests were carried out with 10-mg samples, supplied at 40 mL/min in N2 flow and a temperature ramp of 10 °C/min (from 35 °C to 600 °C). The samples were then maintained at 600 °C for 20 min while 10 mL/min of oxygen was introduced during 5–19 min to complete combustion of the residue.
2.9. Statistical Analysis
All experiments were carried out in triplicates to calculate the average value and errors bars expressing the ± standard deviation. Fatty acid profiles of microbial oils are presented as average value of three individual experiments.
3. Results and Discussion
3.1. Biomass and Lipid Production by Oleaginous Microalgae, Yeasts, and Thraustochytrids
Biomass and lipid production differed among the oleaginous microorganisms tested. The results of cell dry weight (CDW) g/L, total lipids (g/L) and lipid content (%, w/w) of all oleaginous microorganisms used in this study is compiled in Figure 1A, where the data for A. protothecoides, S. limacinum, Aurantiochytrium sp. T66, and C. curvatus are reproduced from our previous studies [16,17,19,21]. As it was mentioned in our previous manuscript, A. protothecoides synthesized maximum CDW of 8.40 g/L and 5.43 g/L lipids which corresponded to 64.52% of lipids content when cultivation was performed with 20 g/L glucose and C/N 60 [16]. In this study, the maximum CDW and lipid concentration obtained in microalgal strain C. sorokiniana were 7.23 g/L and 2.54 g/L, respectivly that corresponded to 35.13% w/w lipid content when cultivated on 20 g/L glucose and C/N 60 (Figure 1A). With thraustochytrids, a maximum CDW of 26.87 g/L and lipid yield of 12.87 g/L were reported when S. limacinum was cultivated on 60 g/L glucose at C/N 10 [17]. The other strain, Aurantiochytrium sp. T66, synthesized maximum CDW and total lipids of 10.39 g/L and 4.98 g/L, respectively—which corresponded to 47.93% lipid content after being grown on 30 g/L glucose at C/N 10 [21]. The yeasts R. toruloides and C. curvatus accumulated 12.21 g/L and 9.93 g/L of CDW, and 6.89 g/L and 5.23 g/L total lipids, respectively [22].
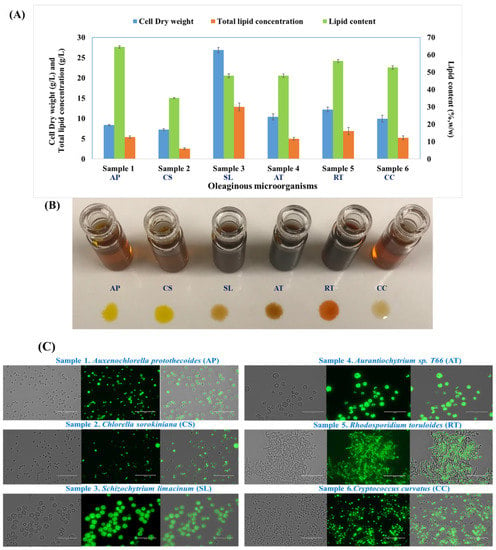
Figure 1.
(A) Biomass and lipid accumulation from oleaginous microalgae A. protothecoides (AP) and C. sorokiniana (CS); thraustochytrids S. limacinum (SL) and Aurantiochytrium sp. T66 (AT); and yeasts R. toruloides (RT) and C. curvatus (CC). (B) Lipids were extracted from the corresponding microorganisms mentioned in panel A. (C) Fluorescence micrographs showing the morphology and lipid accumulation in oleaginous microorganisms.
The lipids were extracted by a chloroform: methanol solution, which also caused the extraction of some pigments from the cells, as shown in Figure 1B. Pigment removal was not attempted owing to the complexity and additional cost of the procedure.
Based on the above observations, biomass and lipid accumulation appeared to be species-specific. The initial stages toward producing microbial lipids are the identification of suitable candidates and the optimization of their growth and lipid accumulation medium. These microorganisms have the capacity to synthesize unique classes of fatty acids depending on culture conditions, such as temperature, cultivation mode (autotrophic, mixotrophic, or heterotrophic), type of carbon source, and its concentration and ratio with other nutrient sources (C/N and C/P) and pH; as well as the microbial strain itself [14]. The productivity of lipids from oleaginous microalga C. protothecoides was observed to be 4 times higher when cultivated in heterotrophic conditions than those reported under autotrophic conditions [23,24]. Specific microalgal lipids are suitable for target industrial applications [25,26].
Morphological analysis and lipid accumulation in oleaginous microorganisms was performed by fluorescence microscopy (Figure 1C). All selected oleaginous microorganisms showed a single-cell structure and accumulated lipid droplets inside the cellular compartment.
3.2. Fatty Acid Profile of Oleaginous Microorganisms
Oleaginous microorganisms usually synthesize lipids in the form of lipid droplets containing TAGs, diacylglycerols, monoacylglycerols, and sterols.
The fatty acid profile of the A. protothecoides revealed presence of myristic acid (C14:0; 1.60%), palmitic acid (C16:0; 18.51%), palmitoleic acid (C16:1; 0.62%), margaric acid (C17:0; 0.42%), stearic acid (C18:0; 4.93%), oleic acid (C18:1; 56.38%), linoleic acid (C18:2; 14.15%), γ-Linolenic acid (C18:3; 2.57%), and arachidic acid (C20:0; 0.82%)—which was similar to our previous work [16] (Figure 2). The fatty acid profile was similar in C. sorokiniana, which contained C14:0 (1.45%), C16:0 (17.97%), C16:1 (0.60%), C17:0 (0.39%), C18:0 (4.09%), C18:1 (58.82%), C18:2 (13.77%), C18:3 (2.42%), and C20:0 (0.48%).
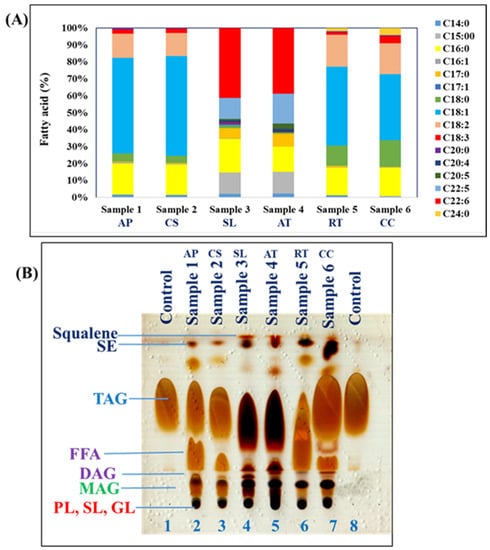
Figure 2.
(A) Lipid profile of oils extracted from various oleaginous microorganisms such as A. protothecoides (AP) and C. sorokiniana (CS); thraustochytrids S. limacinum (SL) and Aurantiochytrium sp. T66 (AT); and yeasts R. toruloides (RT) and C. curvatus (CC). (B). Estimation of total lipids by TLC. Lane 1, TAG standard; lanes 2 and 3, microalgal lipids; lanes 4 and 5, thraustochytrid lipids; lanes 6 and 7, yeast lipids; lane 8, TAG standard.
The fatty acid profile of thraustochytrids, presented in Figure 2A, was similar to previously published profiles of these microorganisms [27]. The important fatty acids were pentadecanoic acid, margaric acid, myristic acid, palmitic acid, stearic acid, oleic acid, linoleic acid, γ-linolenic acid, and docosahexaenoic acid and their content changed with the strain. S. limacinum usually produced C14:0 (2.07%), C15:0 (12.59%), C16:0 (19.89%), C16:1 (0.19%), C17:0 (6.08%), C18:0 (1.24%), C18:1 (0.96%), C18:3 (0.60%), C20:0 (0.61%), arachidonic acid (C20:4; 1.27%), eicosapentaenoic acid (C20:5; 0.84%), docosapentaenoic acid (C22:5; 12.41%), and docosahexaenoic acid C22:6 (41.26%). Aurantiochytrium sp. T66 showed a similar fatty acid profile: C14:0 (2.25%), C15:0 (12.95%), C16:0 (14.80%), C16:1 (0.19%), C17:0 (6.08%), C18:0 (0.82%), C18:1 (0.08%), C18:3 (0.08%), C20:0 (0.00%), C20:4 (1.87%), C20:5 (3.12%), C22:5 (17.66%), and C22:6 (38.81%).
It has been reported that oleaginous yeasts synthesized C16, C18, C18:1, C18:2, and C18:3 after cultivated in medium having glucose as carbon source [12]. Here, the oleaginous yeast R. toruloides produced C14:0 (1.23%), C15:0 (0.15%), C16:0 (16.19%), C16:1 (0.32%), C17:0 (0.71%), C18:0 (11.95%), C18:1 (46.67%), C18:2 (18.75%), C18:3 (1.51%), C20:0 (0.50%), and C24:0 (2.02%); whereas C. curvatus generated C14:0 (0.48%), C15:0 (0.48%), C16:0 (16.57%), C16:1 (0.24%), C17:0 (0.33%), C18:0 (15.74%), C18:1 (38.81%), C18:2 (18.30%), C18:3 (4.29%), C20:0 (0.55%), and tetracosanoic acid (C24:0; 4.21%) (Figure 2B).
The composition of lipids extracted from oleaginous microorganisms cultivated on glucose was analyzed by TLC. As can be seen from the chromatogram presented in Figure 2B, all oleaginous microorganisms synthesized high amounts of TAGs. Additionally, A. protothecoides (lane 2) and R. toruloides (lane 6) showed an elevated amount of free fatty acids, whereas C. sorokiniana and C. curvatus contained abundant TAG but very low free fatty acid levels.
3.3. IR
Spectroscopy is a rapid, non-destructive, and non-invasive tool for investigating biomolecules, such as proteins and lipids. Overall, all oleaginous microorganisms displayed similar vibration modes (Figure 3). Methylene asymmetric and symmetric stretches belonging to the long alkyl chains of fatty acids were assigned to the strong and wide vibrational peaks at 2923.89 cm−1 and 2854.46 cm−1. The presence of an ester group in all oils was indicated by C=O stretches at 1743 cm−1, while that of free fatty acids was indicated by a C=O stretch in a low-intensity band at 1706.89 cm−1. The presence of unsaturated fatty acids was suggested by =C-H and -C=C- stretches, as well as =C-H bending at 3006 cm−1, 1662 cm−1, and 930 cm−1, respectively. The results displayed by IR spectroscopy for microbial lipids have strong correlation with the results obtained by GC-MS for saturated, unsaturated, and polyunsaturated fatty acids from microalgae, yeast, and thraustochytrid samples.
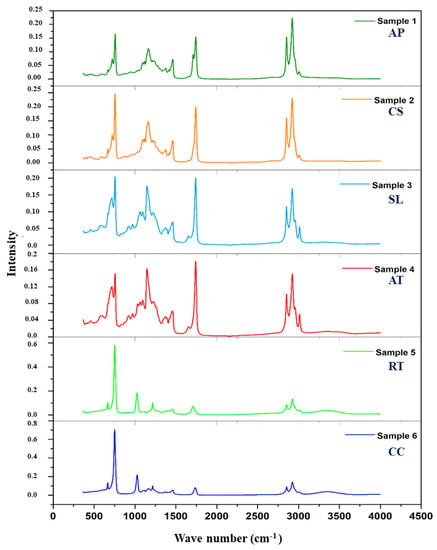
Figure 3.
IR spectra of microbial oils such as A. protothecoides (AP) and C. sorokiniana (CS); thraustochytrids S. limacinum (SL) and Aurantiochytrium sp. T66 (AT); and yeasts R. toruloides (RT) and C. curvatus (CC).
3.4. Tribological Studies of Microbial Lipids
3.4.1. Friction Coefficient
The fundamental function of lubricating oils is to form and maintain a stable lubricating layer at the metal-to-metal contact area. Microbial oils have great lubricant properties because of their ester functional group [28,29]. Figure 4 shows the FC variation over time and under two different pressures (2.0 GPa and 2.5 GPa). For all microbial oils, there was a substantial reduction in FC at the start of each trial, following which the FC attained a steady-state condition. Oil obtained from microalgae showed a lower FC than oils obtained from yeasts and thraustochytrids. This could be explained by the fact that microalgal oil contains more unsaturated fatty acids and fewer saturated fatty acids per total lipids (see Figure 2). Because organic acids—e.g., oleic acid—are often used as friction modifiers to get lower friction through the polishing effect and a better adsorption behavior than the base oils. At 2 GPa, the FC of microbial oils from A. protothecoides (AP), C. sorokiniana (CS); thraustochytrids S. limacinum (SL), Aurantiochytrium sp. T66 (AT) and yeasts R. toruloides (RT), and C. curvatus (CC) has lower than that of the PEG 200. At 2.5 GPa, the FC of oil obtained from thraustochytrids displayed a similar value as the control, which can be explained by an elevated amount of saturated and polyunsaturated fatty acids per total lipids. TAGs are polar ester molecules found mainly in vegetable and microbial oils. A proportionally longer fatty acid chain in TAGs improves the strength of the lubricating layer and minimizes friction between contacting surfaces [30]. At the same time, the presence of PUFAs in oils imparts poor oxidative stability, caused by the interaction between oxygen and the double bonds following exposure to air. The degree of unsaturation, as well as position have been shown to significantly affect the rate of auto-oxidation. Here, oils from C. sorokiniana and R. toruloides were more effective at reducing friction from sliding contact surfaces compared to oils from other samples (Figure 4), which can be ascribed to the presence of free fatty acids. A similar trend was reported for Millettia pinnata oil, which is also rich in free fatty acids, compared to other vegetable oils [31,32]. It has been mentioned that COOH groups present in fatty acids can be adsorbed on metallic surfaces while its phosphate group containing negative charged polar head faced outward to surface [30,33].
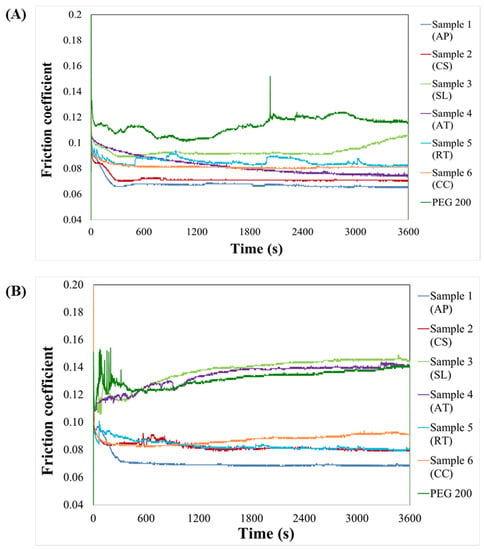
Figure 4.
Friction coefficient of different microbial oils (A. protothecoides (AP), C. sorokiniana (CS); thraustochytrids S. limacinum (SL), Aurantiochytrium sp. T66 (AT) and yeasts R. toruloides (RT) and C. curvatus (CC) and PEG 200 as control, determined under load of (A) 2.0 GPa or (B) 2.5 GPa.
3.4.2. Viscosity
Viscosity must be taken into consideration when conventional petroleum-based lubricants are replaced with bio-based alternatives. The viscosity of most biolubricants is high, and this is influenced by the length of the carboxylic acid chain, or the alcohol hydrocarbon chain in ester biolubricants. The higher viscosity of biolubricants can be seen as an advantage. Because in many cases for mineral oil-based lubricants, it is necessary to add thickeners to increase the viscosity of the lubricant. Interactions occurring via hydrogen bonds present in fatty acids can modify the viscosity index that is defined by fluid characteristics for viscosity changes relative to temperature changes [34,35]. Overall, an increased viscosity value suggests that lubricating oils have some contamination or have undergone oxidation, whereas a low value points to reduced thinning properties of the oil [36]. The viscosity of all microbial lipids reduced consistently with increasing shear rate in the current research (Figure 5), indicating a characteristic ‘shear-thinning’ phenomenon. In tribology, shear thinning is known as the non-Newtonian behavior of fluids where viscosity of fluids reduces after shear strain. It is also referred to as pseudoplastic behavior [37]. To increase the viscosity index of any lubricant for crankcase engine, some viscosity modifier additives are required that can experience shear thinning at the high shear rates found in lubricated contacts, and consequently decrease the viscosity which resulted in thinner lubricant coatings and lower hydrodynamic friction than would be expected if shear thinning did not occur [38,39].
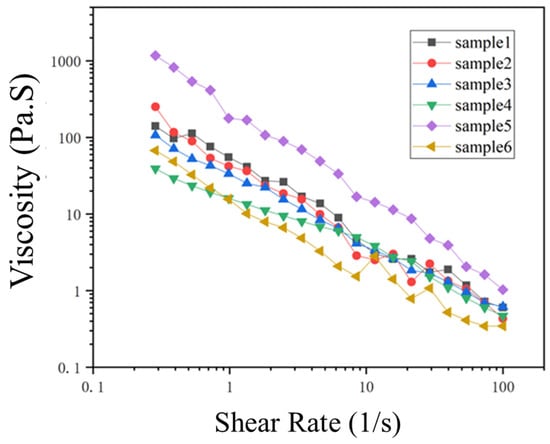
Figure 5.
Rheological properties of microbial oils A. protothecoides (AP) and C. sorokiniana (CS); thraustochytrids S. limacinum (SL) and Aurantiochytrium sp. T66 (AT); and yeasts R. toruloides (RT) and C. curvatus (CC) obtained in this study. Log-log plot of viscosity as a function of shear rate is a figure. Schemes follow the same formatting.
3.4.3. TGA of Microbial Oils
Thermal stability is an important feature when the oil is used as a lubricant at very high temperature [40,41]. Here, TGA and DTG were performed to estimate the thermal stability of microbial oils (Figure 6). The decomposition was started at a temperature of 200–250 °C for the microalgal oil from A. protothecoides and yeast R. toruloides, at 300–330 °C for oil from S. limacinum and Aurantiochytrium sp. T66, and finally at 350–380 °C for oil from the microalga C. sorokiniana and the yeast C. curvatus. The last two microbial oils had a greater breakdown temperature than the others. The greater thermal stability of these two oils is due to the presence of more unsaturated fatty acids in the form of TAG (Figure 2B). In contrast, oils from A. protothecoides and R. toruloides have a high proportion of free fatty acids, which lowers their thermal stability. A previous study reported that vegetable oils (Millettia pinnata, coconut oil, rice bran oil) presented better thermal stability related to mineral oil-based lubricants (SAE40). The starting decomposition temperature determines the thermal stability of any lubricant, which depends mostly on chemical structure and free fatty acid content [42], as well as length of the fatty acid chain, branching, and degree of unsaturation [43]. The presence of oleic acid improves thermal stability, as suggested by the higher thermal stability of M. pinnata oil compared to coconut and rice bran oils [44]. It can also be seen from the DTG results in Figure 6B that there is a main peak of CC, CS, and AP at 420–430 °C, while the peaks of AP and RT with faster decomposition rate appear at about 250 °C. With the increase of carbon atom in fatty acid, its decomposition temperature will also increase [45]. As compared with other four samples, the S. limacinum and Aurantiochytrium sp. T66 have two relatively higher decomposition temperatures (380–390 and 450–480 °C). This is due to the total amount of substances above C20 being more than 50%, which leads to the higher decomposition temperature.
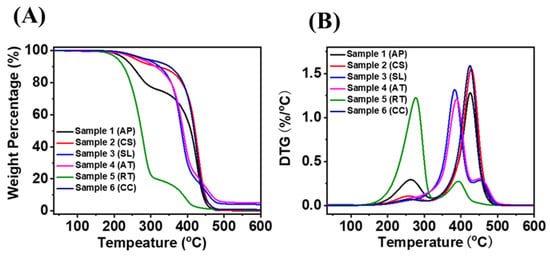
Figure 6.
TGA (A) and DTG (B) results of microbial oils A. protothecoides (AP) and C. sorokiniana (CS); thraustochytrids S. limacinum (SL) and Aurantiochytrium sp. T66 (AT); and yeasts R. toruloides (RT) and C. curvatus (CC) under air atmosphere.
3.4.4. Wear Characteristic
There are many types of wear—such as corrosive, abrasive, adhesive, and fatigue wear—generated on metal contact surfaces in a mechanical device. Moreover, they increase consecutively due to peroxide formation at higher temperatures. The decreased thickness of the lubricant layer makes the surfaces nearer, which will be responsible for greater wear. Three-dimensional surface profiles of the wear tracks on a metal disc and metal ball surface after friction tests with different types of microbial oils and PEG 200 as control are presented in Figure 7. Two different conditions of disc wear volume are presented for each microbial oil. Morphological analysis of the surface indicated wear triggered by the reciprocating balls shifting in a gliding direction. The wear track left on the disc after using PEG 200 was markedly deeper than the tracks left when using microbial oils, which highlights the superior anti-wear properties of these bio-based oil lubricants. Some micro cracks and wear debris were visible on the surface lubricated with oils obtained from S. limacinum, Aurantiochytrium sp. T66, and R. toruloides at 2 GPa (Figure 7A). At 2.5 GPa, wear debris were visible on the surface lubricated with all oils; however, they were more intense and deeper in the case of R. toruloides and C. curvatus (Figure 7A,B), although still less visible than in the control. The reason for microbial oils causing less wear due to having more molecular groups than a standard lubricating oil such as PEG 200 might be linked to these lubricated surfaces experiencing less oxidative or chemical wear [46].
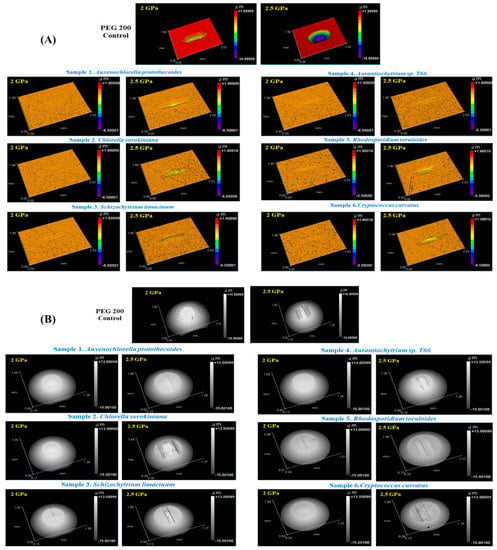
Figure 7.
Three-dimensional surface images showing (A) disc wear and (B) ball wear of lipid samples and PEG 200 as controls at loads of 2.0 GPa or 2.5 GPa.
Figure 8 depicts the variance in disc and ball wear diameter (mm) for different microbial oils. Oil from the thraustochytrid S. limacinum exhibited a higher ball wear diameter (0.511 mm at 2 GPa) than any of the other microbial oils and displayed a similar value as the PEG 200 control. Microalgal oils presented a smaller ball wear diameter (0.427 mm), indicating maximum capability to protect metal against other metal contacting surfaces. Increasing the length of fatty acids in lubricating oil enhances the absorbed film thickness as well as protective area of metal contact surfaces [33]. Oxidation occurs as a result of chemical interaction between fatty acids and peroxides, which might reduce lubricity [47]. However, this phenomenon can be mitigated by using anti-wear additives, such as tyrosine derivatives, molybdate ester with zinc dialkyldithiophosphate, tricresyl phosphate, and zinc dithiophosphate [48,49,50].
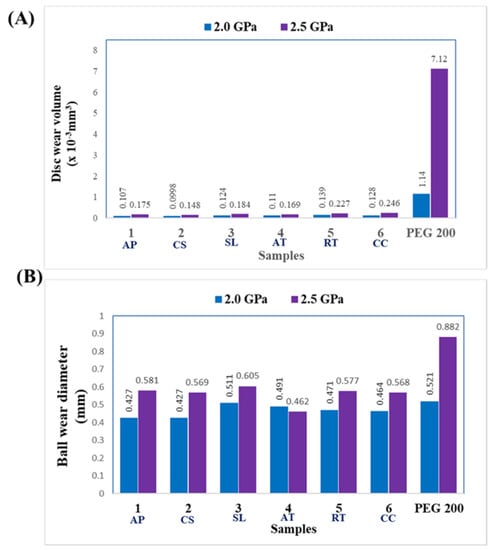
Figure 8.
Performance indicators showing (A) disc wear volume and (B) ball wear diameter while using PEG 200 or microbial fatty acids as lubricants at loads of 2.0 GPa and 2.5 GPa, over a test duration of 1 h, and at room temperature.
4. Conclusions
Several vegetable oils—such as soyabean, palm, and rapeseed—are now being studied for their tribological usages; however, these feedstocks as biolubricants are unsustainable in long term while being direct competition with food sources. The present assessment of fatty acid profiles and tribological characteristics of oleaginous microorganisms reveals the promising anti-wear and friction-reducing properties, as well as thermal stability of oleaginous microalgae and yeast oils compared to oils obtained from thraustochytrids. A lower degree of friction and surface protection against wear are key advantages of microalgal and yeast oils, which can be attributed to an optimal combination of saturated and unsaturated fatty acids. In particular, comparison with a traditional mineral-based lubricant such as PEG 200 revealed the much lower friction coefficient, and wear of metallic surfaces of microalgal and yeast oils. Fatty acids present in the feedstock affect its tribological properties mostly—e.g., presence of double bonds in fatty acid chain makes it highly susceptible for autooxidation after being contact with air. Moreover, some limitation of using microbial oils as biolubricants—such their oxidative and hydrolytic stability—can be further improved by chemical modifications inactive sites present in the oil such as acyl group and double bonds or by blending with some oils having different fatty acid profiles.
Author Contributions
Conceptualization, A.P., U.R., P.C. and L.M. (Leonidas Matsakas); Methodology, A.P. and L.M. (Liwen Mu); Validation, A.P., L.M. (Leonidas Matsakas) and L.M. (Liwen Mu); Investigation, A.P. and L.M. (Liwen Mu); Data curation, A.P., L.M. (Liwen Mu) and Y.S.; Writing—original draft preparation, A.P.; Writing—review and editing, L.M. (Liwen Mu), Y.S., U.R., P.C. and L.M. (Leonidas Matsakas). All authors have read and agreed to the published version of the manuscript.
Funding
The authors want to thank the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (Formas, no. 2019-01162), and the Swedish Research Council (no. 2019-04941) for supporting this work.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank Kentaro Umeki from the division of Energy Science, Department of Engineering Sciences and Mathematics and Manish Kumar Shimpi from the division of Chemical Engineering, Department of Civil, Environmental and Natural Resources Engineering, Luleå University of Technology, Sweden for providing the TGA analyzer and FTIR instruments used in this study, respectively.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ASTM | American Society for Testing and Materials |
| BBM | Bold’s basal medium |
| CDW | Cell dry weight |
| ATCC | American Type Culture Collection |
| C/N | Carbon/nitrogen |
| DAG | Diacylglycerols |
| DHA | Docosahexaenoic acid |
| FAMEs | Fatty acid methyl esters |
| FC | Friction coefficient |
| FFA | Free fatty acids |
| GC-MS | Gas chromatography-mass spectrometry |
| h | Hour(s) |
| LD | Lipid droplets |
| MAG | Monoacylglycerols |
| MUFAs | Mono-unsaturated fatty acids |
| NCYC | National collection of yeast cultures |
| NREL | National Renewable Energy Laboratory |
| OD | Optical density |
| PEG | Polyethylene glycol |
| PL | Phospholipids |
| PUFAs | Poly-unsaturated fatty acids |
| SFAs | Saturated fatty acids |
| SL | Sphingolipids |
| TAGs | Triacylglycerols |
| TLC | Thin layer chromatography |
| YPD | Yeast extract peptone dextrose |
References
- Sheldon, R.A. The Road to Biorenewables: Carbohydrates to Commodity Chemicals. ACS Sustain. Chem. Eng. 2018, 6, 4464–4480. [Google Scholar] [CrossRef] [Green Version]
- Sheldon, R.A. Metrics of Green Chemistry and Sustainability: Past, Present, and Future. ACS Sustain. Chem. Eng. 2018, 6, 32–48. [Google Scholar] [CrossRef] [Green Version]
- Hamdan, S.H.; Chong, W.W.F.; Ng, J.H.; Chong, C.T.; Zhang, H. Nano-tribological characterisation of palm oil-based trimethylolpropane ester for application as boundary lubricant. Tribol. Int. 2018, 127, 1–9. [Google Scholar] [CrossRef]
- Schneider, M.P. Plant-oil-based lubricants and hydraulic fluids. J. Sci. Food Agric. 2006, 86, 1769–1780. [Google Scholar] [CrossRef]
- Singh, Y.; Farooq, A.; Raza, A.; Mahmood, M.A.; Jain, S. Sustainability of a non-edible vegetable oil based bio-lubricant for automotive applications: A review. Process Saf. Environ. Prot. 2017, 111, 701–713. [Google Scholar] [CrossRef]
- Shah, R.; Woydt, M.; Zhang, S. The Economic and Environmental Significance of Sustainable Lubricants. Lubricants 2021, 9, 21. [Google Scholar] [CrossRef]
- Nagendramma, P.; Kaul, S. Development of ecofriendly/biodegradable lubricants: An overview. Renew. Sustain. Energy Rev. 2012, 16, 764–774. [Google Scholar] [CrossRef]
- Cecilia, J.A.; Plata, D.B.; Saboya, R.M.A.; de Luna, F.M.T.; Cavalcante, C.L.; Rodríguez-Castellón, E. An overview of the biolubricant production process: Challenges and future perspectives. Processes 2020, 8, 257. [Google Scholar] [CrossRef] [Green Version]
- Lovell, M.; Higgs, C.F.; Deshmukh, P.; Mobley, A. Increasing formability in sheet metal stamping operations using environmentally friendly lubricants. J. Mater. Process. Technol. 2006, 177, 87–90. [Google Scholar] [CrossRef]
- Patel, A.; Rova, U.; Christakopoulos, P.; Matsakas, L. Assessment of Fatty Acids Profile and Omega-3 Polyunsaturated Fatty Acid Production by the Oleaginous Marine Thraustochytrid Aurantiochytrium sp. T66 Cultivated on Volatile Fatty Acids. Biomolecules 2020, 10, 694. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Lipids of oleaginous yeasts. Part II: Technology and potential applications. Eur. J. Lipid Sci. Technol. 2011, 113, 1052–1073. [Google Scholar] [CrossRef]
- Patel, A.; Arora, N.; Sartaj, K.; Pruthi, V.; Pruthi, P.A. Sustainable biodiesel production from oleaginous yeasts utilizing hydrolysates of various non-edible lignocellulosic biomasses. Renew. Sustain. Energy Rev. 2016, 62, 836–855. [Google Scholar] [CrossRef]
- Patel, A.; Pruthi, V.; Pruthi, P.A. Innovative screening approach for the identification of triacylglycerol accumulating oleaginous strains. Renew. Energy 2019, 135, 936–944. [Google Scholar] [CrossRef]
- Ratledge, C. Fatty acid biosynthesis in microorganisms being used for Single Cell Oil production. Biochimie 2004, 86, 807–815. [Google Scholar] [CrossRef]
- Patel, A.; Matsakas, L.; Hrůzová, K.; Rova, U.; Christakopoulos, P. Biosynthesis of Nutraceutical Fatty Acids by the Oleaginous Marine Microalgae Phaeodactylum tricornutum Utilizing Hydrolysates from Organosolv-Pretreated Birch and Spruce Biomass. Mar. Drugs 2019, 17, 119. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.; Matsakas, L.; Rova, U.; Christakopoulos, P. Heterotrophic cultivation of Auxenochlorella protothecoides using forest biomass as a feedstock for sustainable biodiesel production. Biotechnol. Biofuels 2018, 11, 169. [Google Scholar] [CrossRef]
- Patel, A.; Liefeldt, S.; Rova, U.; Christakopoulos, P.; Matsakas, L. Co-production of DHA and squalene by thraustochytrid from forest biomass. Sci. Rep. 2020, 10, 1992. [Google Scholar] [CrossRef]
- Patel, A.; Pruthi, V.; Singh, R.P.; Pruthi, P.A. Synergistic effect of fermentable and non-fermentable carbon sources enhances TAG accumulation in oleaginous yeast Rhodosporidium kratochvilovae HIMPA1. Bioresour. Technol. 2015, 188, 136–144. [Google Scholar] [CrossRef]
- Patel, A.; Mikes, F.; Bühler, S.; Matsakas, L. Valorization of brewers’ spent grain for the production of lipids by oleaginous yeast. Molecules 2018, 23, 3052. [Google Scholar] [CrossRef] [Green Version]
- Van Wychen, S.; Ramirez, K.; Laurens, L.M. Determination of Total Lipids as Fatty Acid Methyl Esters (FAME) by In Situ Transesterification (NREL/TP-5100-60958); Laboratory Analytical Procedure (LAP), National Renewable Energy Laboratory: Golden, CO, USA, 2013; Volume 303.
- Patel, A.; Rova, U.; Christakopoulos, P.; Matsakas, L. Simultaneous production of DHA and squalene from Aurantiochytrium sp. grown on forest biomass hydrolysates. Biotechnol. Biofuels 2019, 12, 255. [Google Scholar] [CrossRef]
- Patel, A.; Matsakas, L. A comparative study on de novo and ex novo lipid fermentation by oleaginous yeast using glucose and sonicated waste cooking oil. Ultrason. Sonochem. 2019, 52, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Wu, Q. High yield bio-oil production from fast pyrolysis by metabolic controlling of Chlorella protothecoides. J. Biotechnol. 2004, 110, 85–93. [Google Scholar] [CrossRef]
- Xu, H.; Miao, X.; Wu, Q. High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J. Biotechnol. 2006, 126, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.A.K.; Sahoo, P.K.P.K.; Singhal, S.; Patel, A. Impact of various media and organic carbon sources on biofuel production potential from Chlorella spp. 3 Biotech 2016, 6, 116. [Google Scholar] [CrossRef] [Green Version]
- Jin, M.; Slininger, P.J.; Dien, B.S.; Waghmode, S.; Moser, B.R.; Orjuela, A.; Sousa, L.D.C.; Balan, V. Microbial lipid-based lignocellulosic biorefinery: Feasibility and challenges. Trends Biotechnol. 2015, 33, 43–54. [Google Scholar] [CrossRef]
- Barclay, W.R.; Meager, K.M.; Abril, J.R. Heterotrophic production of long chain omega-3 fatty acids utilizing algae and algae-like microorganisms. J. Appl. Phycol. 1994, 6, 123–129. [Google Scholar] [CrossRef]
- Erhan, S.Z.; Sharma, B.K.; Liu, Z.; Adhvaryu, A. Lubricant base stock potential of chemically modified vegetable oils. J. Agric. Food Chem. 2008, 56, 8919–8925. [Google Scholar] [CrossRef] [PubMed]
- Mosarof, M.H.; Kalam, M.A.; Masjuki, H.H.; Alabdulkarem, A.; Ashraful, A.M.; Arslan, A.; Rashedul, H.K.; Monirul, I.M. Optimization of performance, emission, friction and wear characteristics of palm and Calophyllum inophyllum biodiesel blends. Energy Convers. Manag. 2016, 118, 119–134. [Google Scholar] [CrossRef]
- Jayadas, N.H.; Nair, K.P. Coconut oil as base oil for industrial lubricants-evaluation and modification of thermal, oxidative and low temperature properties. Tribol. Int. 2006, 39, 873–878. [Google Scholar] [CrossRef]
- Cermak, S.C.; Biresaw, G.; Isbell, T.A.; Evangelista, R.L.; Vaughn, S.F.; Murray, R. New crop oils-Properties as potential lubricants. Ind. Crops Prod. 2013, 44, 232–239. [Google Scholar] [CrossRef]
- Habibullah, M.; Masjuki, H.H.; Kalam, M.A.; Zulkifli, N.W.M.; Masum, B.M.; Arslan, A.; Gulzar, M. Friction and wear characteristics of Calophyllum inophyllum biodiesel. Ind. Crops Prod. 2015, 76, 188–197. [Google Scholar] [CrossRef]
- Fox, N.J.; Tyrer, B.; Stachowiak, G.W. Boundary lubrication performance of free fatty acids in sunflower oil. Tribol. Lett. 2004, 16, 275–281. [Google Scholar] [CrossRef]
- Saboya, R.M.A.; Cecilia, J.A.; García-Sancho, C.; Sales, A.V.; de Luna, F.M.T.; Rodríguez-Castellón, E.; Cavalcante, C.L. Assessment of commercial resins in the biolubricants production from free fatty acids of castor oil. Catal. Today 2017, 279, 274–285. [Google Scholar] [CrossRef]
- Saboya, R.M.A.; Cecilia, J.A.; García-Sancho, C.; Luna, F.M.T.; Rodríguez-Castellón, E.; Cavalcante, C.L. WO3-based catalysts supported on porous clay heterostructures (PCH) with Si-Zr pillars for synthetic esters production. Appl. Clay Sci. 2016, 124–125, 69–78. [Google Scholar] [CrossRef]
- Kalam, M.A.; Masjuki, H.H.; Shahabuddin, M.; Mofijur, M. Tribological characteristics of amine phosphate and octylated/butylated diphenylamine additives infused bio-lubricant. Energy Educ. Sci. Technol. Part A Energy Sci. Res. 2012, 30, 123–136. [Google Scholar]
- Slatter, T.; Lewis, P.R. Thesis Investigation into Tribological Performance of Vegetable Oils as Biolubricants at Severe Contact Conditions. Ph.D. Theis, University of Sheffield, Sheffield, UK, 2017. [Google Scholar]
- Marx, N.; Fernández, L.; Barceló, F.; Spikes, H. Shear Thinning and Hydrodynamic Friction of Viscosity Modifier-Containing Oils. Part I: Shear Thinning Behaviour. Tribol. Lett. 2018, 66, 92. [Google Scholar] [CrossRef] [Green Version]
- Ashrafi, N. Effects of temperature on rheology of olive oils. Appl. Rheol. 2012, 22, 342031–342037. [Google Scholar] [CrossRef]
- Garcia, C.C.; Franco, P.I.B.M.; Zuppa, T.O.; Filho, N.R.A.; Leles, M.I.G. Thermal stability studies of some cerrado plant oils. J. Therm. Anal. Calorim. 2007, 87, 645–648. [Google Scholar] [CrossRef]
- Santos, J.C.O.; Lima, L.N.; Santos, I.M.G.; Souza, A.G. Thermal, spectroscopic and rheological study of mineral base lubricating oils. J. Therm. Anal. Calorim. 2007, 87, 639–643. [Google Scholar] [CrossRef]
- Dasari, S.R.; Goud, V.V. Effect of pre-treatment on solvents extraction and physico-chemical properties of castor seed oil. J. Renew. Sustain. Energy 2014, 6, 063108. [Google Scholar] [CrossRef]
- Garcia-Perez, M.; Adams, T.T.; Goodrum, J.W.; Geller, D.; Das, K.C. Production and fuel properties of pine chip bio-oil/biodiesel blends. Energy Fuels 2007, 21, 2363–2372. [Google Scholar] [CrossRef]
- Mosarof, M.H.; Kalam, M.A.; Masjuki, H.H.; Arslan, A.; Monirul, I.M.; Ruhul, A.M.; Shahir, S.A.; Khuong, L.S. Analysis of thermal stability and lubrication characteristics of: Millettia pinnata oil. RSC Adv. 2016, 6, 81414–81425. [Google Scholar] [CrossRef]
- Luo, S.; Feng, J.; Ng, K.M. Effect of fatty acid on the formation of ITO nanocrystals via one-pot pyrolysis reaction. CrystEngComm 2015, 17, 1168–1172. [Google Scholar] [CrossRef]
- Shi, Y.; Larsson, R. Non-corrosive and Biomaterials Protic Ionic Liquids with High Lubricating Performance. Tribol. Lett. 2016, 63, 1. [Google Scholar] [CrossRef]
- Wu, Y.; Li, W.; Zhang, M.; Wang, X. Oxidative degradation of synthetic ester and its influence on tribological behavior. Tribol. Int. 2013, 64, 16–23. [Google Scholar] [CrossRef]
- Jayadas, N.H.; Nair, K.P. Study of the anti-wear properties of coconut oil using quantum chemical calculations and tribological tests. J. Tribol. 2006, 128, 654–659. [Google Scholar] [CrossRef]
- Hu, J.Q.; Wei, X.Y.; Dai, G.L.; Fei, Y.W.; Xie, F.; Zong, Z.M. Tribological behaviors and mechanism of sulfur- and phosphorus-free organic molybdate ester with zinc dialkyldithiophosphate. Tribol. Int. 2008, 41, 549–555. [Google Scholar] [CrossRef]
- Zeng, X.; Wu, H.; Yi, H.; Ren, T. Tribological behavior of three novel triazine derivatives as additives in rapeseed oil. Wear 2007, 262, 718–726. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).