Abstract
Nowadays, the rapidly growing population, climate change, and environment pollution put heavy pressure on fresh water resources. The atmosphere is the immense worldwide and available water source. The Adsorptive Water Harvesting from the Atmosphere (AWHA) method is considered a promising alternative to desalination technologies for remote arid regions. The development of novel adsorbents with advanced water-adsorption properties is a prerequisite for practical realization of this method. Metal–organic frameworks (MOFs) are a novel class of porous crystalline solids that bring a great potential for AWHA due to their extremely high specific surface area, porosity, and tailored adsorption properties. This work addresses MIL-160 as a water adsorbent for AWHA. The water-adsorption equilibrium of MIL-160 was studied by volumetric method, the isosteric heat of adsorption was calculated, and finally, the potential of MIL-160 for AWHA was evaluated for climatic conditions of the deserts of Saudi Arabia, Mongolia, the Sahara, Atacama, and Mojave as reference arid regions. MIL-160 was shown to ensure a maximum specific water productivity of 0.31–0.33 gH2O/gads per cycle. High fractions of water extracted (0.90–0.98) and collected (0.48–0.97) could be achieved at a regeneration temperature of 80 °C with natural cooling of the condenser by ambient air. The specific energy consumption for water production varied from 3.5 to 6.8 kJ/g, which is acceptable if solar heat is used to drive the desorption. The AWHA method employing MIL-160 is a promising way to achieve a fresh water supply in remote arid areas.
1. Introduction
Climate change leading to the desertification of a vast area, environmental pollution, and a rapidly growing world population make the fresh water supply one of the major issues of our time [1]. Although the worldwide resources of fresh water are quite abundant (1·105 km3), most parts of them are in the form of hard-to-reach glaciers and deep underground water. The rest (rivers, lakes, and shallow underground water) is distributed very unevenly, which puts enormous pressure on water resources in arid regions. Nowadays, 1.5 billion people are facing portable water scarcity, and by 2025 this number is expected to grow to 3 billion. The most water-scarce regions include North Africa, the Near and Middle East, Northern China, India, Eastern Australia, Mexico, northeastern Brazil, and the west coast of South America [2,3].
At the same time, the total amount of moisture in the Earth’s atmosphere—about 13,000 km3—significantly exceeds the world’s requirements [4,5]. Due to its worldwide availability, interest in atmospheric water harvesting has been observed since ancient times. Artificial springs and ponds were described in [6] that accumulated moisture from the air during dewfall. Currently, water harvesting from the air is considered a promising method for water supply in arid and inland regions [7]. There are two basic types of atmospheric water extraction: passive, which does not require additional energy input (rainwater, fog, and dew collection), and active, which can operate only with an external input of electric or thermal energy. The active method can be based on: (i) the air cooling below its dew point to condense water [8], and (ii) the sorption of moisture by solid [9,10] or liquid desiccants [11,12].
The Adsorptive Water Harvesting from the Atmosphere (AWHA) method is founded on the absolute humidity in arid regions, which is comparatively stable during the day. The air temperature and, consequently, the relative humidity opposite vary greatly. [13]. The AWHA process involves two main stages: (i) the adsorption of moisture on an adsorbent at night when relative humidity is high, and (ii) the desorption of water from the adsorbent during the day driven by an external heat source (e.g., solar heat) with subsequent vapor condensation on a cold surface [14]. The main performance indexes of the AWHA are the specific water productivity (SP) per cycle, or the ratio of <the mass of water collected>/<the adsorbent mass>, and the specific energy consumption (SEC), equal to <the energy input>/<the mass of water collected>. Considering a quite low moisture content of 4–15 g per 1 m3 of air in arid regions [15,16,17,18], hundreds of cubic meters of air have to be passed through the adsorbent to extract several liters of water. This emphasizes the primary importance of the efficiencies of water extraction during adsorption and collection during the desorption/condensation stages. These efficiencies can be expressed as fractions: δex = <amount of water adsorbed>/<amount of vapor passed through the adsorber> of water extraction, and δcol = <amount of water collected>/<amount of water desorbed> of water collection [13].
The performance of AWHA is closely related to the used adsorbent, or, to be more precise, to the harmonization of its properties with the climatic conditions of the region where the process is implemented. A thermodynamic analysis showed that to enhance the performance of AWHA, the adsorbent should have adsorption centers with different affinities for water [13]. Adsorption sites with a strong affinity for water vapor effectively adsorb moisture at low relative pressure during the adsorption stage, and ensure high efficiency of water extraction. Adsorption sites with weak affinity easily release vapor during desorption at high relative pressure, and promote high water collection efficiency. The affinity should be distributed over a wide range, corresponding to the climate conditions of the region where the AWHA is realized. Such an adsorbent exhibits a divariant adsorption equilibrium, with uptake rising between relative humidity (RH) values, corresponding to the adsorption and desorption stages.
A number of adsorbents have been proposed for AWHA, including common porous solids like silica gel [19,20], crystalline alumino-phosphate (zeotype) AQSOA Z01 [21], composite sorbents involving hygroscopic salts embedded inside solid matrix [10,14,22,23]. A low water-adsorption capacity of silica gels and a high regeneration temperature of conventional zeolites have restricted the practical realization of AWEA. Several advanced zeotypes possess S-shaped adsorption isotherms, and can be regenerated at a low temperature. However, an analysis showed that their adsorption properties did not match the climatic conditions of the arid regions very well. Their drawbacks were a quite low amount of water exchanged under conditions of the AWHA cycle in arid regions (below 0.23 g/g, see Section 3.3.1) and expansive synthesis [24]. The “salt/matrix” composites possess a large adsorption capacity and variable adsorption properties. However, their use in open AWHA systems may be obstructed by the potential leakage of the salt solution, which is formed during the moisture adsorption, from the pore space [25]. This could lead to the adsorbent degradation and the adsorber corrosion.
Metal–organic frameworks (MOFs) are a novel family of crystalline compounds that consist of metal ions or clusters and bridging polydentate organic linkers. MOFs are characterized by one-, two-, or three-dimensional frameworks with a large fraction of voids [26,27,28]. Due to their extraordinarily surface area and pore volume, and the presence of both hydrophilic and hydrophobic parts, MOFs possess unique adsorption properties such as a large water-adsorption capacity and a great diversity of water-adsorption isotherms. [29,30]. Furthermore, the possibility to control MOFs’ adsorption properties by modifying both organic linkers and inorganic clusters makes it possible to tailor the MOFs with required sorption properties and hydrolytic stability [31,32], which inspired high hopes for enhancement of AWHA performance [33].
Seo et al. [34] first proposed mesoporous MIL-100 and MIL-101 as desiccants for AWHA. Since then, the study of AWHA employing MOFs has been attracting steadily increasing interest [4,13,35,36]. For a humid climate at the relative humidity of ambient air RH > 40–50%, MIL-100(Cr) and MIL-101(Cr) are promising adsorbents that display a high working capacity of up to 1.2 mL/g [37]. A Co2Cl2BTDD designed for AWHA was able to deliver 0.82 mL/g of water under moderately humid conditions at RH = 30% [38]. The performance of MOFs as adsorbents for arid climates is essentially lower. Kim et al. [39] showed that at the ambient RH = 20%, potentially ~0.2 mL/g per cycle can be harvested with MOF-801 by utilizing solar thermal energy to drive desorption. A microporous MOF-303 has recently been developed and tested at AWHA [40], and the water harvester operating for several cycles per day generated 0.7 and 1.3 L/kg per day at 10 and 32% relative humidity, respectively. MIL-160(Al) was suggested as a promising adsorbent for AWHA regions with arid climates [13]. Chang et al. [41] evaluated the potential of application of MIL-160(Al) as a material for water harvesting in a bench-scale fixed-bed unit, and demonstrated its capable of maximum water productivity of 305 L/(day·ton) at regeneration and condensation temperatures of 80 °C and 10 °C, respectively.
This research focuses on the study of MIL-160 as an adsorbent for the AWHA process and evaluation of its potential under climatic conditions of extremely arid regions (the Mojave and Sahara Deserts, Chile, Algeria, the central part of Saudi Arabia, and Mongolia). The performance characteristics of AWHA employing MIL-160 were estimated in terms of the specific consumption of thermal energy for the water production, the specific mass of the water harvested, and the fractions of water extracted from the air and water collected. These fractions are important performance indexes, as they determine the specific electric energy consumption for air blowing through the fixed-bed adsorber, which can be significant due to a small moisture content in the atmosphere in arid regions.
The structure of MIL-160 is formed by inorganic AlO6 octahedra, which form cis- corner-sharing chains linked via carboxylate groups of the ligand (2,5-furandicarboxylic acid) (Figure 1) [42]. It possesses both strong and weak adsorption sites, which allows AWHS to promote high efficiencies of water extraction and collection.
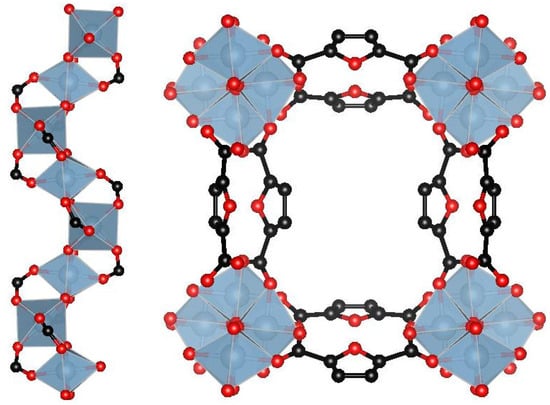
Figure 1.
Scheme of the MIL-160 structure: helical inorganic chains formed by AlO6 octahedra (left); channels in framework constructed from inorganic chains linked via carboxylate groups of the 2,5-furandicarboxylic acid (right). AlO6 octahedra (light gray), atoms C (black), O (red).
2. Materials and Methods
2.1. Sample Synthesis
MIL-160 was synthesized by a hydrothermal method according to a slightly modified procedure described in [42]. 2,5-furandircaboxilic acid (4.680 g, 30 mmol, >99%), AlCl3·6H2O (7.240 g, 30 mmol), and NaOH (1.212 g, 30 mmol) were dissolved in distilled H2O (60 mL) with stirring using a magnetic stirrer (~500 rpm) for 15 min. The obtained mixture of reagents was placed in a Teflon container inside a 100 mL cylindrical stainless-steel autoclave and heated at temperature T = 120 °C for 24 h in a convection oven. After cooling down to the ambient temperature, the mixture was separated from the solution with a small-pore paper filter using a water-jet pump and washed with hot water to remove the unreacted organic ligand. Finally, the obtained white solid precipitate was activated at 150 °C for 15 h under continuous evacuation.
2.2. Characterization
The structure of the obtained MIL-160 sample was confirmed by PXRD analysis using a Bruker D8 ADVANCE diffractometer with an XRK-900reaction chamber. The measurements were carried out using CuKα-radiation (λ = 1.54 Å) in scanning mode (2θ = 5–60°), with a step width of 2θ = 0.02° and an accumulation time of 10 s at each point.
IR-spectra were recorded on an Agilent Cary 660 FT-IR spectrometer using an attenuated total reflection (ATR) attachment, the 025-2018 MIRacle ZnSe Perf Crystal Plate, at 25 °C in the 500–4000 cm−1 range.
The BET surface area, the micropore volume, and total pore volume of the synthesized MIL-160 were determined by low-temperature nitrogen adsorption at 77 K using a Quantachrome Nova 1200e gas sorption analyzer. The samples were degassed in a vacuum at 150 °C for 3 h before measurement of N2 sorption isotherms. The specific surface area was calculated by the BET analysis of the adsorption branch of the isotherm in a relative pressure range of 0.01–0.025. The total pore volume was obtained from the amount of N2 adsorbed at a relative pressure close to unit P/P0 = 0.99. The micropore volume was calculated using the statistical thickness analysis of the isotherm adsorption branch and de Boer’s t-method. The pore-size distribution was calculated using the Dubinin–Astakhov method.
2.3. Water-Adsorption Equilibrium
The water-adsorption isotherms of MIL-160 were measured by a volumetric method at T = 50, 35, and 20 °C and in the water-vapor pressure range P = 0.8–40.0 mbar. Before the measurements, a preliminary degassing was carried out at 80 °C for 3 h under continuous evacuation (the residual pressure was 0.1 mbar) for drying the MIL-160 sample. The dry sample weight m0 was 0.50216 ± 0.00002 g. Afterward, the sample was cooled down to a fixed temperature, which was controlled by a thermostat connected to the adsorber with an accuracy of ±0.1 K. The temperature was measured with a K-type thermocouple placed at the bottom of the adsorber. Then, the measuring cell with the adsorbent was connected to the buffering vessel filled with water vapor, which resulted in a jump in water-vapor pressure over the sample. The vapor pressure was measured using an MKS Baratron® Type 626a pressure sensor with an accuracy of ± 0.01 mbar. The vapor-pressure jump initiated the adsorption, which led to a gradual decrease in the pressure. The sample was maintained at a fixed temperature for 1–4 h to reach equilibrium. The amount of water vapor sorbed (Δw (g/g)) was calculated from the temporal pressure dependence P(t) using the ideal gas equation:
where MH2O—the water molar mass, V—the system volume, —the universal gas constant, and T—the steam temperature. Subsequently, based on the data obtained, a set of water-sorption isotherms w(P) of the MIL-160 sample was calculated.
3. Results and Discussions
3.1. Structure Characterization of as-Prepared MIL-160
The texture characteristics of MOF-801 determined from the N2 sorption isotherm at 77 K (Figure 2a) belonged to the I-type according to the IUPAC classification, and the hysteresis loop was absent, which is typical for microporous solids. The synthesized MIL-160 possessed the specific surface area SBET = 830 m2/g and the total pore volume Vp = 0.40 cm3/g, which agreed with the literature data [42]. The micropore volume Vμ coincided with the Vp. Hence, the synthesized MIL-160 was microporous without meso- or macropores. The pore-size distribution obtained by the Dubinin–Astakhov method (Figure 2b) demonstrated a narrow peak in the micropore-size region. As shown in the curve obtained, the average pore diameter is 0.42 nm, which is consistent with the data presented in [42].
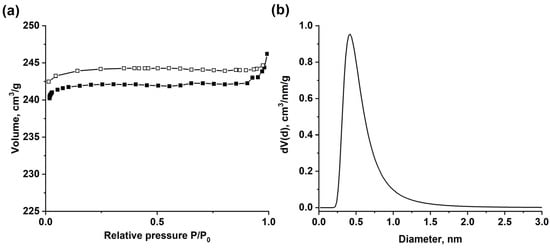
Figure 2.
N2 adsorption (solid symbols) and desorption (open symbols) isotherms (a) and pore-size distribution using DA model (b) of MIL-160.
The XRD pattern (Figure 3a) of the synthesized sample was consistent with that reported in the literature [42,43]. The FT-IR spectrum of the prepared MIL-160 sample (Figure 3b) agreed well with the literature data [41,43,44,45]. The characteristic bands with high intensity were attributed to asymmetric (1649 cm−1) and symmetric stretching (1405 cm−1) vibrations of carboxylate groups, along with the C=C bonds’ stretching vibrations of the furan ring at 1583 cm−1 [41,44,45]. The strong band at 630 cm−1 corresponded to the vibrations of the Al–O bond in the MIL-160 structure [41]. The peaks in the range of 1000–1300 cm−1 could be attributed to the asymmetric and symmetric stretching vibrations of the C–O–C in the furan rings [41]. A band at 782 cm−1 ascribed to the out-of-plane deformation vibrations of C–H bonds in the furan ring also was observed [44]. The broad band around 3500 cm−1 was assigned to the vibrations of the water molecules adsorbed from the ambient air, while the weak peak around 3200 cm−1 represented the vibration of bridging hydroxyl groups μ-OH in the metal–oxide clusters AlO6 [41,44]. It is worth noting that the lack of a band at 1700 cm−1 indicated the absence of residual free 2,5-furandicarboxylic acid in the pore-synthesized MIL-160 [44,45]. Thus, the characterization of the synthesized MIL-160 by XRD, nitrogen adsorption, and FTIR analysis verified its genuine structure and high purity.
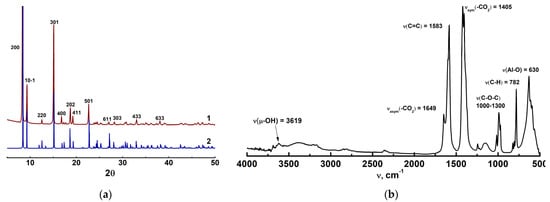
Figure 3.
XRD patterns (a) of the synthesized MIL-160 (2) and the curve calculated from CIF-file (1); FT-IR spectra (b) of MIL-160.
3.2. Water-Vapor Adsorption on MIL-160
The isotherms of water adsorption on the MIL-160 were S-shaped curves (Figure 4) showing a small water uptake (0.02 gH2O/gads) at low pressures, followed by a steep increase in uptake up to 0.25–0.30 gH2O/gads in a narrow pressure range, which depended on the temperature. Upon further increase in vapor pressure, the uptake gradually rose to 0.34 gH2O/gads, which was somewhat smaller than the specific volume of micropores Vμ = 0.40 cm3/g. This indicated that a fraction of the pore space in the vicinity of hydrophobic sites (organic linker) remained unoccupied by water molecules.
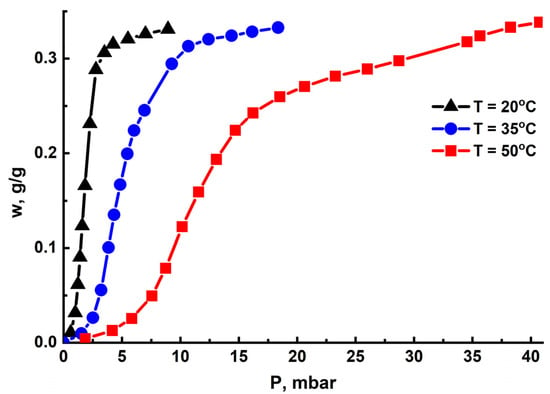
Figure 4.
Isotherms of water adsorption on the MIL-160 at T = 50 °C (red), 35 °C (blue), and 20 °C (black).
Based on the obtained data on water adsorption equilibrium on MIL-160, isosteres of water adsorption were plotted in ln(P) − 1/T coordinates (Figure 5a). The isosteric heat ΔHads of the water adsorption was calculated according to the Clausius–Clapeyron equation:
lnP = Qa∂c/(RT) + Const
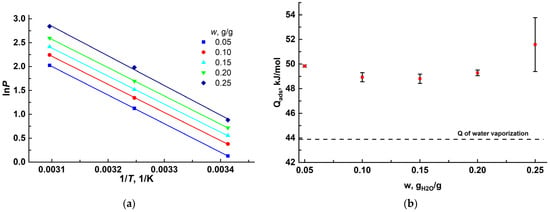
Figure 5.
Water adsorption isosteres (a) and isosteric enthalpy ΔHads (b) for the MIL-160.
The adsorption heat Qa∂c varied in the range of 49 to 52 ± 1 kJ/mol at w = 0.05–0.25 g/g (Figure 5b). The obtained values of adsorption heat somewhat exceeded the latent condensation heat of water ΔL = 43.9 kJ mol−1 at 303 K, which pointed to a moderately strong interaction of water molecules with the MIL-160′s surface.
Experimentally measured water adsorption isobars on the MIL-160 presented as a function of the Polanyi adsorption potential ΔF = −RTln[P/P0(T)], where P is the partial vapor pressure, P0 is the saturation vapor pressure at temperature T, merged into one characteristic curve w(ΔF) (Figure 6). This meant that the water adsorption equilibrium of the MIL-160 obeyed the Polanyi principle of temperature invariance [46]. The characteristic curve w(ΔF) was approximated by an empiric equation:
with the fitting parameters A = −1.321·10−3, B = 1.451·10−2, C = 0.321, k1 = −10.00, k2 = −1.470, x01 = 6.875, and x02 = 6.268.
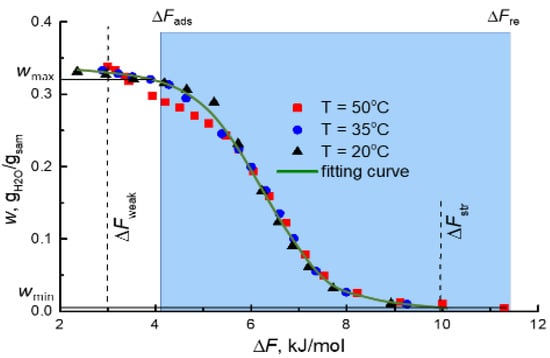
Figure 6.
Characteristic curve of water adsorption on the MIL-160 (symbols) and the fitting curve (solid line). Dashed lines indicate the values of adsorption potential, corresponding to the weakest (ΔFweak) and strongest (ΔFstr) adsorption sites. The blue area shows the ΔF range for the boundary conditions of the AWHA cycle in Riyadh-Old during the dry season (July).
3.3. Performance of AWHA Employing MIL-160
3.3.1. Specific Water Productivity
The characteristic curve w(ΔF) was used to evaluate the performance of AWHA employing MIL-160 for climatic conditions of several arid regions located on different continents (Figure 7). The Mojave Desert in North America; the Atacama in Chile, South America; Tamanrasset in Algeria; the Sahara Desert; Riyadh-Old in the central part of Saudi Arabia; and Noyon in Mongolia were selected as reference regions, characterized by their extremely arid climates. The climatic data (the average temperatures Tn and Td, and the average relative humidity RHn and RHd during night and day, respectively) were collected from the Meteonorm Global Climate Database. They were used to calculate the values of adsorption potential ΔFads and ΔFre (Table 1), corresponding to the boundary conditions of adsorption and desorption stages, respectively:
where Tre is the regeneration temperature.
ΔFad = −RTn[lnPam/P0(Tn)]
ΔFre = −RTre [lnPam/P0(Tre)],
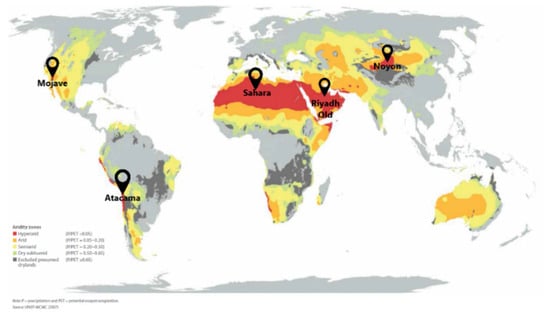
Figure 7.
Selected reference regions with extremely arid climates [47].

Table 1.
Climatic data for the selected regions.
The characteristic curve of water adsorption on the MIL-160 demonstrated that the strongest adsorption sites adsorbed water vapor at ∆Fstr ≈ 9.0–10.0 kJ/mol (Figure 6), which was lower than ∆Fre = 11.3–17.4 kJ/mol at Tre = 80 °C in all the reference regions (Table 1). Thus, the retained water could be desorbed completely at a quite low temperature of 80 °C, allowing usage of a simple solar collector for the adsorbent regeneration.
The maximum specific water productivity SPmax per cycle can be achieved in the AWHA process if water retained in the adsorbent during the adsorption stage is completely removed and collected during the desorption/condensation stage. The productivity SPmax was calculated from the characteristic curve (Figure 6) as the uptake variation Δw = wmax − wmin = w(ΔFad) − w(ΔFre). In all the selected regions, MIL-160 exchanged Δw = 0.31–0.33 gH2O/gads at Tre = 80 °C, which surpassed respective values for various adsorbents, both traditional and novel (Figure 8).
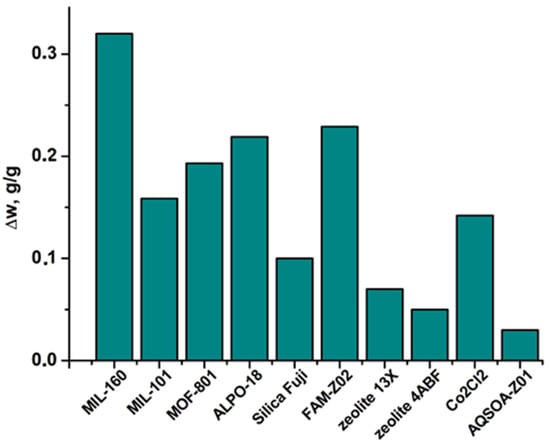
Figure 8.
Amount of water exchanged under climatic conditions of Riyadh-Old during the dry season for various adsorbents.
3.3.2. The Fractions of Water Extraction and Collection
Along with the specific water productivity, the fractions δex of water extracted from the air during adsorption and δcol of water collected during condensation are also the crucial performance indexes of AWHA. They determine the volume of air to be passed through the adsorber, for harvesting a unit mass of water, and consequently, the energy demand of the system for the air blowing.
The fractions of water extracted δex and collected δcol in a simple fixed-bed flowing adsorber (Figure 9a) with MIL-160 as the adsorbent can be evaluated as [13]:
where Pam—the partial pressure of water vapor in the ambient air; Pout.ad—the water vapor pressure in the outlet air during adsorption stage; Pout.re—the water vapor pressure in the outlet air during regeneration stage; and P0(Td)—the pressure of saturated water vapor at a day temperature of ambient air Td.
δex = (Pam − Pout.ad)/Pam = 1 − Pout.ad/Pam
δcol = [Pout.re − P0(Td)]/Pout.re = 1 − P0(Td)/Pout.re
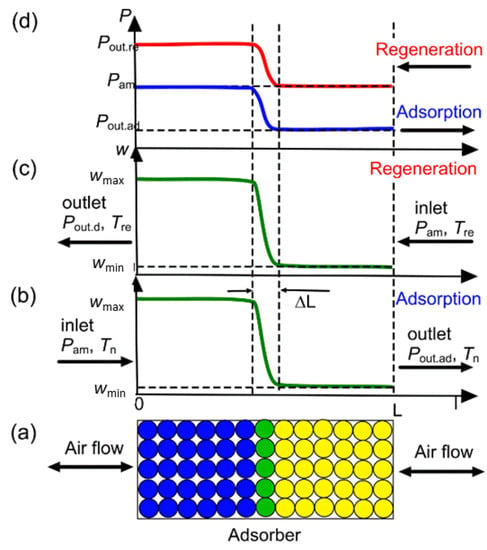
Figure 9.
Scheme of a fixed-bed flowing adsorber (a) and distribution of water uptake (b,c) and vapor partial pressure (d) along the adsorber.
Here, we assumed that the adsorption front thickness ΔL in a fixed-bed adsorber was negligible in comparison with the adsorber length L >> ΔL (Figure 9a–c). In this case, the dynamic adsorption capacity wd of the adsorbent equaled the equilibrium capacity w(P,T). Until the concentration front was inside the bed, the outlet vapor pressure Pout.ad during adsorption equaled the equilibrium pressure over the dry adsorbent (Figure 9d). The strongest adsorption centers of the MIL-160 adsorbed water vapor at ΔFstr ≈ 10.0 kJ/mol (Figure 6). Consequently, the outlet vapor pressure Pout.ad during the adsorption stage could be calculated as:
Pout.ad = P0(Tn) exp(−ΔFstr/RTn)
The δex values evaluated according to Equations (6) and (8) varied from 0.90 for Riyadh-Old to 0.96 for the Atacama regions during the dry season (Figure 10). During the humid season, δex was even higher due to the increase in RH of the ambient air (Figure 10), and reached 0.96–0.98 for the selected regions. Consequently, the affinity of the strongest adsorption sites MIL-160 to water vapor provided effective extraction of water vapor from the ambient air. That promoted a reduced air volume blown through the adsorber to extract a unit volume of water, and consequently, lowering energy consumption for the air purging.
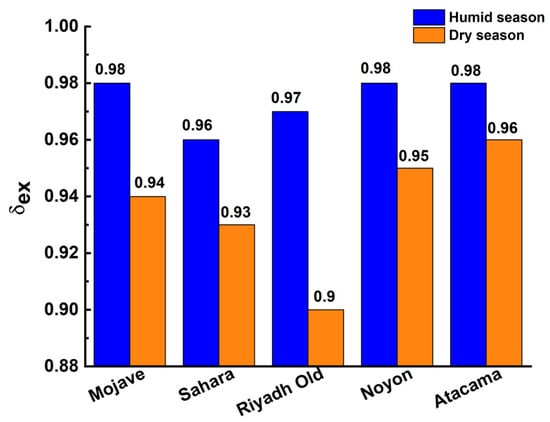
Figure 10.
Water-extraction fraction δex for the AWHA system based on MIL-160.
During the regeneration stage, the outlet air was in equilibrium with the adsorbent, saturated with water up to wmax right after the adsorption stage (Figure 9c,d). The weakest adsorption sites adsorbed water vapor at ΔFweak ≈ 3.0 kJ/mol (Figure 6). Consequently, at ΔF > ΔFweak, these sites released adsorbed water, and the outlet vapor pressure Pout.re during the desorption stage could be calculated as:
Pout.re = P0(Tre)exp(−ΔFweak/RTre)
It should be noted that for the dry season, the ΔFad > ΔFweak for all regions except Atacama (Table 1). This meant that the weakest adsorption sites remained unsaturated with water during the adsorption stage, and maximum uptake in the cycle equaled w = w(ΔFad). Accordingly, for the dry season, the pressure Pout.re was calculated using the following expression:
Pout.re = P0(Tre)exp(−ΔFad/RTre)
Then, the fraction δcol was evaluated as a function of temperature Tre using Equation (7). If the condenser was cooled by the ambient air at Td, δcol values for the selected regions varied in the range 0.48–0.95 and 0.88–0.97 during the dry (July) and humid (January) seasons, respectively, at Tre = 80 °C (Figure 11). The increase in the regeneration temperature enhanced the water collection fraction: at Tre = 100 °C, the collection fraction rose to 0.77–0.97 and 0.95–0.98 for the dry and humid seasons, respectively. On the contrary, lowering the regeneration temperature to 70 °C resulted in a dramatic reduction of this fraction, particularly for the dry season, down to 0.15–0.9. This showed the necessity of an external heat source for the water desorption, which could be solar or waste heat.
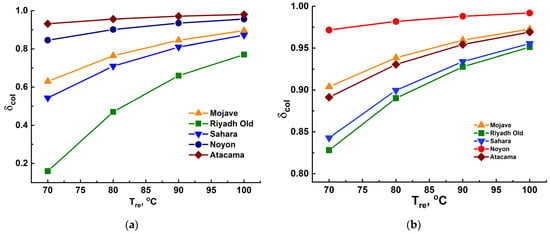
Figure 11.
Effect of the regeneration temperature on the water-collection fraction δcol of the AWHA system based on MIL-160 during the dry (a) and humid (b) seasons, with the condenser cooled by the ambient air.
Another efficient method of increasing the fraction δcol is a decrease in the condensation temperature Tcon. A tank with a heat exchanger located underground or a condenser connected to a heat pump or adsorptive chiller can be used as a condenser [48,49] instead of that cooled by the ambient air. Figure 12 shows that reducing Tcon from Td = 36.1 °C to 10 °C allowed the growth of δcol from 0.48–0.77 to 0.90–0.95 at Tre = 80–100 °C under the climatic conditions of Riyadh-Old during the dry season.
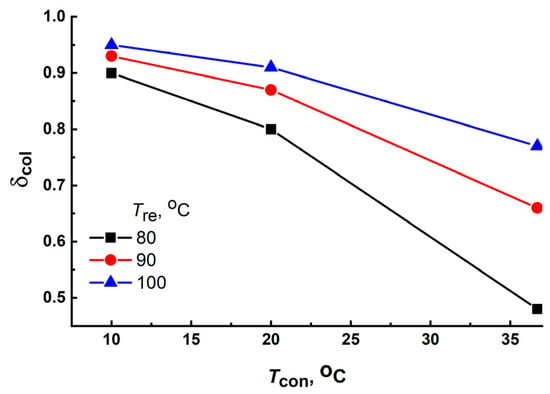
Figure 12.
Effect of the condensation temperature on the water-collection fraction δcol of the AWHA system based on MIL-160 during the dry season (July) for the Riyadh-Old region.
Thus, the calculated δex and δcol values showed a high performance of the AWHA system employing MIL-160 as an adsorbent. This MOF possessed both strong and weak adsorption sites, exchanged up to 0.33 gH2O/gads under climatic conditions of the selected arid regions, and provided high fractions δex = 0.90–0.98 and δcol = 0.48–0.97 at Tre = 80–100 °C.
3.3.3. The Specific Energy Consumption
An important performance index of the AWHA system is the specific consumption of thermal energy SEC for the water production, which can be calculated as:
where Cpad = 1.63 J/(g·K) is the specific heat of MIL-160 at 80 °C [50]), CpH2O = 4.19 J/(g·K) is the specific heat of water at 80 °C, and Qads = 2.84 kJ/g is the average isosteric heat of adsorption in the range w = 0.05–0.30 gH2O/gads.
SEC = Qsp.re/(Δw·δcol) = [Δw·Qads + (Cpad + wads·CpH2O)·(Tre − Tad)]/(Δw·δcol),
For the selected regions during the dry season, the SEC ranged from 3.7 to 6.8 kJ/gH2O at a regeneration temperature of 80 °C (Figure 13). The SEC was affected by the water-collection fraction δcol (Equation (11)). Accordingly, during the humid season (January), SEC decreased to 3.5–3.8 kJ/gH2O due to a higher water-collection fraction δcol. The decrease in condensation temperature was another way to reduce SEC. Thus, when the condenser was cooled to 10 °C, SEC for the Riyadh-Old region was reduced to 3.6 kJ/gH2O as compared to 6.8 kJ/gH2O for the condenser cooled by the ambient air at temperature Td (Table 2), owing to an appropriate increase in the water-collection fraction δcol.
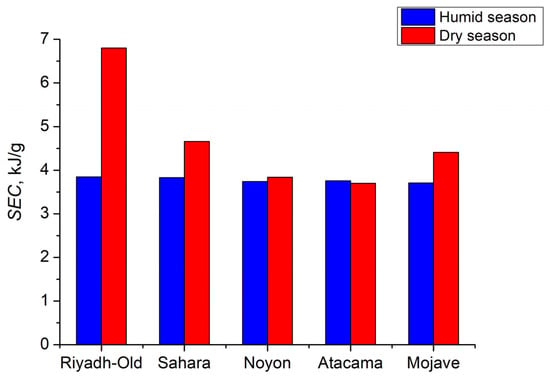
Figure 13.
SEC for water production in the AWHA process employing MIL-160 as an adsorbent at Tre = 80 °C and Tcon = Td.

Table 2.
SEC for water production in the AWHA process employing MIL-160 in the Riyadh-Old region at Tre = 80 °C (dry season).
These SEC values were higher than the heat of water vaporization (2.26 kJ/gH2O at 100 °C). Therefore, the energy consumption for AWEA was higher than the energy consumption for conventional water desalination. However, taking into account that in arid regions, solar heat is usually available in abundance and can be used for adsorbent regeneration, the obtained SEC-values for water production were quite acceptable. Accordingly, the AWEA method employing MIL-160 as an adsorbent is promising for arid regions remote from other sources of water.
4. Conclusions
In this paper, the water-vapor adsorption on MIL-160 was studied, and the assessment of the feasibility of MIL-160 for the AWHA process in arid climatic regions was carried out. The water-vapor adsorption isotherms were S-shaped curves with a maximum uptake of 0.34 g/g. The isosteric heat of water adsorption equaled 49–52 ± 1 kJ/mol at a water-uptake range of 0.05 to 0.25 g/g. Under conditions of several arid regions, namely Riyadh-Old (Saudi Arabia), the Sahara Desert, the Mojave Desert, Atacama (Chile), and Noyon (Mongolia), a high maximum specific water productivity of 0.31–0.33 gH2O/gads per cycle could be achieved with MIL-160. Employing MIL-160 allows a fraction of water extracted δex = 0.90–0.98 during the adsorption stage. The fraction of water collected varied in the range of 0.48–0.76 and 0.85–0.97 during the dry and humid seasons, respectively, at Tre = 80 °C with natural cooling of the condenser by ambient air. Further increase in the fraction of water collected could be achieved by lowering the condensation temperature to 10 °C and increasing the regeneration temperature to 100 °C. The specific energy consumption for water production varied from 3.5 to 6.8 kJ/g. This is quite acceptable if solar heat, available in abundance in arid regions, can be used to drive the desorption. The AWHA method employing MIL-160 is a promising way to achieve a fresh water supply in arid areas remote from coast-line.
Author Contributions
Conceptualization, L.G. and Y.A.; methodology, L.G. and Y.A.; formal analysis, M.S.; investigation, I.K. and M.S.; resources, L.G; data curation, L.G.; writing—original draft preparation, I.K. and M.S.; writing—review and editing, L.G. and Y.A.; visualization, I.K. and M.S.; funding acquisition, L.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Foundation for Basic Research (grant no. 18-29-04033) and by the Ministry of Science and Higher Education of the Russian Federation within the governmental order for Boreskov Institute of Catalysis (project AAAA-A21-121011390006-0).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank T.A. Krieger for XRD analysis of the synthesized MIL-160.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhuang, S.; Qi, H.; Wang, X.; Li, X.; Liu, K.; Liu, J.; Zhang, H. Advances in Solar-Driven Hygroscopic Water Harvesting. Glob. Chall. 2021, 5, 2000085. [Google Scholar] [CrossRef]
- Arnell, N.W. Climate change and global water resources: SRES emissions and socio-economic scenarios. Glob. Environ. Chang. 2004, 14, 31–52. [Google Scholar] [CrossRef]
- Vörösmarty, C.J.; Green, P.; Salisbury, J.; Lammers, R.B. Global water resources: Vulnerability from climate change and population growth. Science 2000, 289, 284–288. [Google Scholar] [CrossRef]
- Kalmutzki, M.J.; Diercks, C.S.; Yaghi, O.M. Metal–Organic Frameworks for Water Harvesting from Air. Adv. Mater. 2018, 30, 1–26. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Vandecasteele, C. Distillation vs. membrane filtration: Overview of process evolutions in seawater desalination. Desalination 2002, 143, 207–218. [Google Scholar] [CrossRef]
- Beysens, D.; Milimouk, I. The case for alternative fresh water sources. Sécheresse 2000, 11, 11–16. [Google Scholar]
- Tu, Y.D.; Wang, R.Z.; Zhang, Y.N.; Wang, J.Y. Progress and expectation of atmospheric water harvesting. Joule 2018, 2, 1452–1475. [Google Scholar] [CrossRef]
- Al-Farayedhi, A.A.; Ibrahim, N.I.; Gandhidasan, P. Condensate as a water source from vapor compression systems in hot and humid regions. Desalination 2014, 349, 60–67. [Google Scholar] [CrossRef]
- Alayli, Y.; Hadji, N.E.; Leblond, J. A new process for extraction of water from air. Desalination 1987, 67, 227–229. [Google Scholar] [CrossRef]
- Gordeeva, L.G.; Tokarev, M.M.; Parmon, B.N.; Aristov, Y.I. Selective water sorbents for multiple applications: 6. Fresh water production from the atmosphere. React. Kinet. Catal. Lett. 1998, 65, 153–160. [Google Scholar] [CrossRef]
- Talaat, M.A.; Awad, M.M.; Zeidan, E.B.; Hamed, A.M. Solar-powered portable apparatus for extracting water from air using desiccant solution. Renew. Energy 2018, 119, 662–674. [Google Scholar] [CrossRef]
- Hamed, A.M. Absorption-regeneration cycle for production of water from air-theoretical approach. Renew. Energy 2000, 19, 625–635. [Google Scholar] [CrossRef]
- Gordeeva, L.G.; Solovieva, M.V.; Aristov, Y.I. Potable water extraction from the atmosphere: Potential of MOFs. Renew. Energy 2020, 148, 72–80. [Google Scholar] [CrossRef]
- Aristov, Y.I.; Tokarev, M.M.; Gordeeva, L.G.; Snytnikov, V.N.; Parmon, V.N. New composite sorbents for solar-driven technology of fresh water production from the atmosphere. Sol. Energy 1999, 66, 165–168. [Google Scholar] [CrossRef]
- Arakawa, H. Climates of Northern and Eastern Asia. World Surv. Climatol. 1969, 8, 1–248. [Google Scholar]
- Takahashi, K.; Arakawa, H. Climates of Southern and Western Asia. World Surv. Climatol. 1981, 9, 1–333. [Google Scholar]
- Griffiths, J.F. Climates of Africa. World Surv. Climatol. 1972, 10, 1–604. [Google Scholar]
- Gentilli, J. Climates of Australia and New Zealand. World Surv. Climatol. 1971, 9, 1–405. [Google Scholar]
- Groth, W.; Hussmann, P. Process and system for recovering water from the atmosphere. U.S. Patent 4 146 372, 27 March 1979. [Google Scholar]
- Bennett, C.E. Heat energized vapor adsorbent pump. U.S. Patent 4,377,398, 22 March 1983. [Google Scholar]
- LaPotin, A.; Zhong, Y.; Zhang, L.; Zhao, L.; Leroy, A.; Kim, H.; Rao, S.R.; Wang, E.N. Dual-Stage Atmospheric Water Harvesting Device for Scalable Solar-Driven Water Production. Joule 2021, 5, 166–182. [Google Scholar] [CrossRef]
- Kumar, M.; Yadav, A. Experimental investigation of solar powered water production from atmospheric air by using composite desiccant material “CaCl2/saw wood”. Desalination 2015, 367, 216–222. [Google Scholar] [CrossRef]
- Wang, J.Y.; Liu, J.Y.; Wang, R.Z.; Wang, L.W. Experimental investigation on two solar-driven sorption based devices to extract fresh water from atmosphere. Appl. Therm. Eng. 2017, 127, 1608–1616. [Google Scholar] [CrossRef]
- Shimooka, S.; Oshima, K.; Hidaka, H.; Takewaki, T.; Kakkiuchi, H.; Kodama, A.; Kubota, M.; Matsuda, H. The evaluation of direct cooling and heating desiccant devices coated with FAM. J. Chem. Eng. Jpn. 2007, 40, 1330–1334. [Google Scholar] [CrossRef]
- Gordeeva, L.G.; Aristov, Y.I. Composites “salt inside porous matrix” for adsorption heat transformation: A current state of the art and new trends. Int. J. Low-Carbon Technol. 2012, 7, 288–302. [Google Scholar] [CrossRef]
- Férey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef]
- Horike, S.; Shimomura, S.; Kitagawa, S. Soft porous crystals. Nat. Chem. 2009, 1, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M.; O’Keeffe, M.; Ockwing, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef]
- Canivet, J.; Fateeva, A.; Guo, Y.; Coasne, B.; Farrusseng, D. Water adsorption in MOFs: Fundamentals and applications. Chem. Soc. Rev. 2014, 43, 5594–5617. [Google Scholar] [CrossRef]
- De Lange, M.F.; Verouden, K.J.F.M.; Vlugt, T.J.H.; Gascon, J.; Kapteijn, F. Adsorption driven heat pumps—The potential of Metal-Organic Frameworks. Langmuir 2015, 31, 12783–12796. [Google Scholar] [CrossRef]
- Song, D.; Bae, J.; Ji, H.; Kim, M.-B.; Bae, Y.-S.; Park, K.S.; Moon, D.; Jeong, N.C. Coordinative Reduction of Metal Nodes Enhances the Hydrolytic Stability of a Paddlewheel Metal−Organic Framework. J. Am. Chem. Soc. 2019, 141, 7853–7864. [Google Scholar] [CrossRef]
- Bae, J.; Lee, C.Y.; Jeong, N.C. Weak Coordination Bond of Chloromethane: A Unique Way to Activate Metal Node Within an Unstable Metal–Organic Framework DUT-34. Korean Chem. Soc. 2021, 42, 658–666. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.-K.; Yoon, J.W.; Lee, J.S.; Hwang, Y.K.; Jun, C.-H.; Chang, J.-S.; Wuttkee, S.; Bazin, P.; Vimont, M.; Daturi, M.; et al. Energy-efficient dehumidification over hierarchically porous metal-organic frameworks as advanced water adsorbents. Adv. Mater. 2012, 24, 806–810. [Google Scholar] [CrossRef] [PubMed]
- LaPotin, A.; Kim, H.; Rao, S.R.; Wang, E.N. Adsorption-based atmospheric water harveisting: Impact of material and component properties on system-level performance. Acc. Chem. Res. 2019, 52, 1588–1597. [Google Scholar] [CrossRef]
- Trapani, F.; Polyzoidis, A.; Loebbecke, S.; Piscopo, C.G. On the general water harvesting capability of metal-organic frameworks under well-defined climatic conditions. Microporous Mesoporous Mater. 2016, 230, 20–24. [Google Scholar] [CrossRef]
- Kim, S.-I.; Yoon, T.-U.; Kim, M.-B.; Lee, S.-J.; Hwang, Y.K.; Chang, J.-S.; Kim, H.-J.; Lee, H.-N.; Lee, U.-H.; Bae, Y.-S. Metal–organic frameworks with high working capacities and cyclic hydrothermal stabilities for fresh water production. Chem. Eng. J. 2016, 286, 467–475. [Google Scholar] [CrossRef]
- Rieth, A.J.; Yang, S.; Wang, E.N.; Dincǎ, M. Record Atmospheric Fresh Water Capture and Heat Transfer with a Material Operating at the Water Uptake Reversibility Limit. ACS Cent. Sci. 2017, 3, 668–672. [Google Scholar] [CrossRef]
- Kim, H.; Yang, S.; Rao, S.R.; Narayanan, S.; Kapustin, E.A.; Furukawa, H.; Umans, A.S.; Yaghi, O.M.; Wang, E.N. Water harvesting from air with metal-organic frameworks powered by natural sunlight. Science 2017, 356, 430–434. [Google Scholar] [CrossRef]
- Hanikel, N.; Prevot, M.S.; Fathieh, F.; Kapustin, E.A.; Lyu, H.; Wang, H.; Diercks, N.J.; Glover, T.G.; Yaghi, O.M. Rapid cycling and exceptional yield in a metal-organic framework water harvester. ACS Cent. Sci. 2019, 5, 1699–1706. [Google Scholar] [CrossRef]
- Silva, M.P.; Ribeiro, A.M.; Silva, C.G.; Nogueira, I.B.R.; Cho, K.-H.; Lee, U.-H.; Faria, J.L.; Loureiro, J.L.; Chang, J.-S.; Rodrigues, A.E.; et al. MIL-160(Al) MOF’s potential in adsorptive water harvesting. Adsorption 2021, 27, 213–226. [Google Scholar] [CrossRef]
- Cadiau, A.; Lee, J.S.; Borges, D.D.; Fabry, P.; Devic, T.; Wharmby, M.T.; Martineau, C.; Foucher, D.; Taulelle, F.; Jun, C.-H.; et al. Design of Hydrophilic Metal-Organic Framework Water Adsorbents for Heat Reallocation. Adv. Mater. 2015, 27, 4775–4780. [Google Scholar] [CrossRef]
- Permyakova, A.; Skrylnyk, O.; Courbon, E.; Affram, M.; Wang, S.; Lee, U.-H.; Valekar, A.H.; Nouar, F.; Mouchaham, G.; Devic, T.; et al. Synthesis Optimization, Shaping, and Heat Reallocation Evaluation of the Hydrophilic Metal–Organic Framework MIL-160(Al). ChemSusChem 2017, 10, 1419–1426. [Google Scholar] [CrossRef]
- Wahiduzzaman, M.; Lenzen, D.; Maurin, G.; Stock, N.; Wharmby, M.T. Rietveld refinement of MIL-160 and its structural flexibility upon H2O and N2 adsorption. Eur. J. Inorg. Chem. 2018, 32, 3626–3632. [Google Scholar] [CrossRef]
- Liu, Q.; Chapman, J.; Huang, A.; Williams, K.C.; Wagner, A.; Garapati, N.; Sierros, K.A.; Dinu, C.Z. User-Tailored Metal–Organic Frameworks as Supports for Carbonic Anhydrase. ACS Appl. Mater. Interfaces 2018, 10, 41326–41337. [Google Scholar] [CrossRef]
- Polanyi, M. Theories of the adsorption of gas. Trans. Faraday Soc. 1932, 28, 316–333. [Google Scholar] [CrossRef]
- Food and Agriculture Organisation of the United Nations. Available online: http://www.fao.org/fileadmin/user_upload/newsroom/docs/full-map.png (accessed on 2 April 2021).
- Bar, E. Extraction of water from air—An alternative solution for water supply. Desalination 2004, 165, 335. [Google Scholar] [CrossRef]
- Habeebullah, B.A. Potential use of evaporator coils for water extraction in hot and humid areas. Desalination 2009, 237, 330–345. [Google Scholar] [CrossRef]
- Cui, S.; Marandi, A.; Lebourleux, G.; Thimon, M.; Bourdon, M.; Chen, C.; Severino, M.I.; Steggles, V.; Nouar, F.; Serre, C. Heat properties of a hydrophilic carboxylate-based MOF for water adsorption applications. Appl. Therm. Eng. 2019, 161, 114135. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).