Effect of Cell Size on the Performance and Temperature Distribution of Molten Carbonate Fuel Cells
Abstract
1. Introduction
2. Simulation Model
2.1. Reaction Models
2.2. Flow Types
2.3. Simulation Model
2.4. Simulation Conditions
3. Results and Discussions
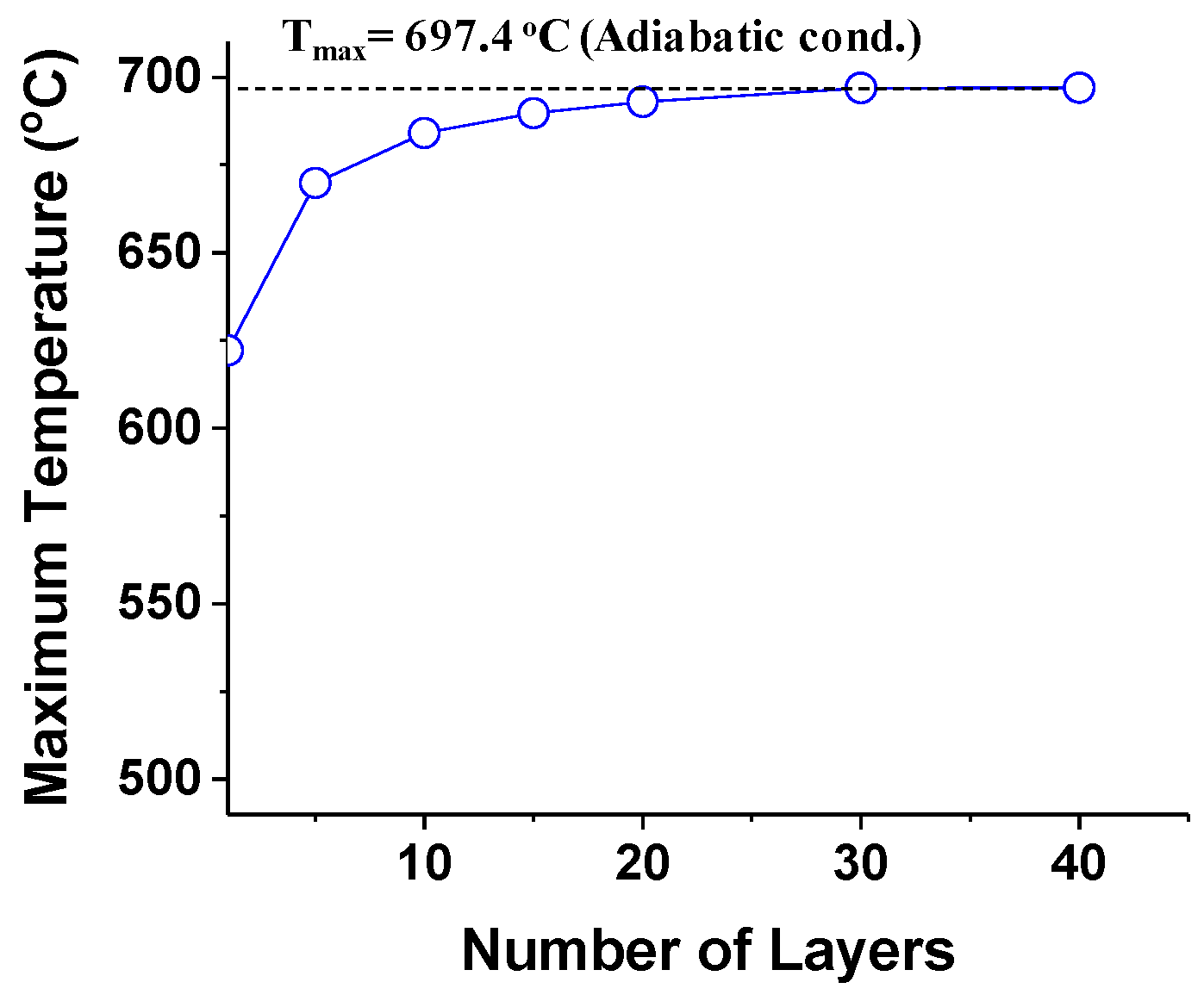
3.1. Stacking Effects
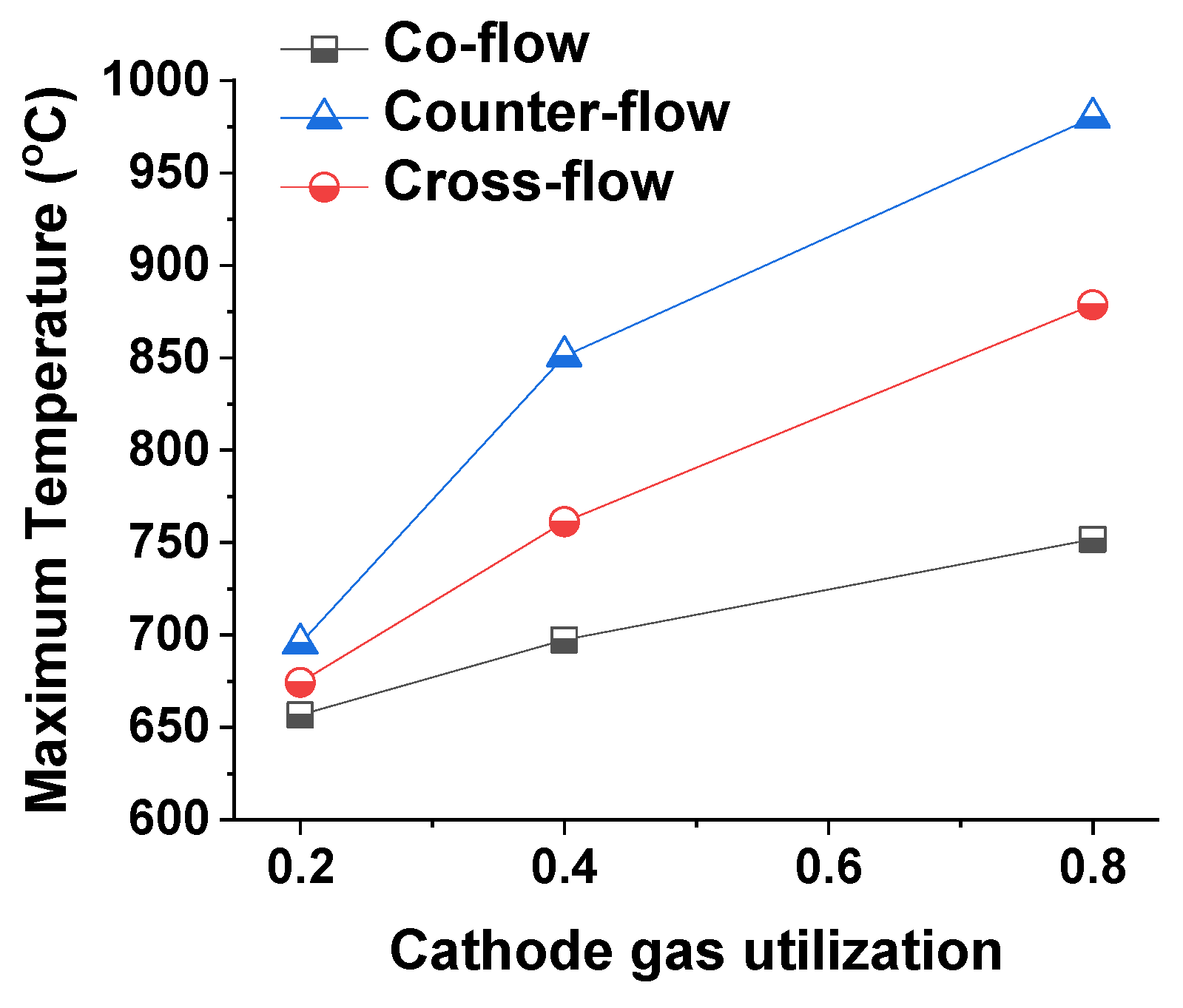
3.2. Effects of Cell Size with Respect to the Flow Types
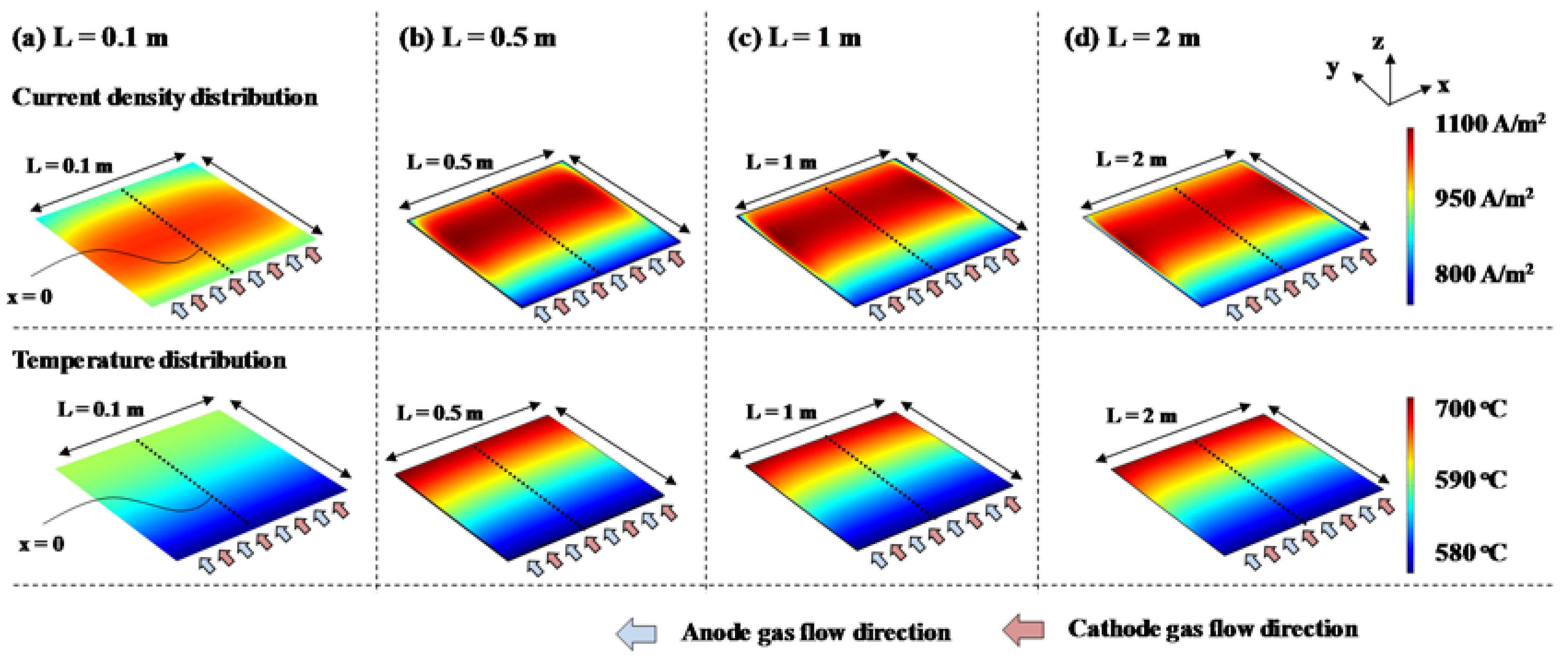
3.2.1. Co-Flow Cell
3.2.2. Counter-Flow Cell
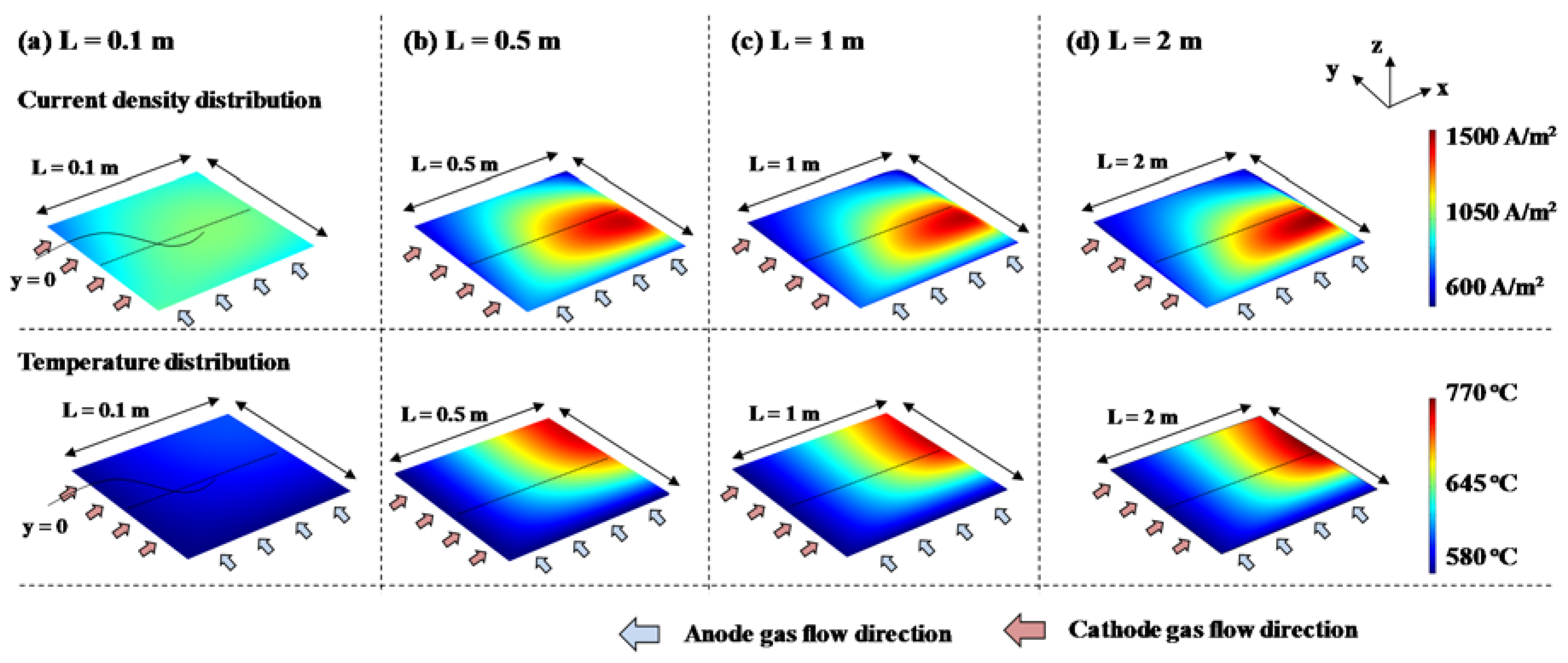
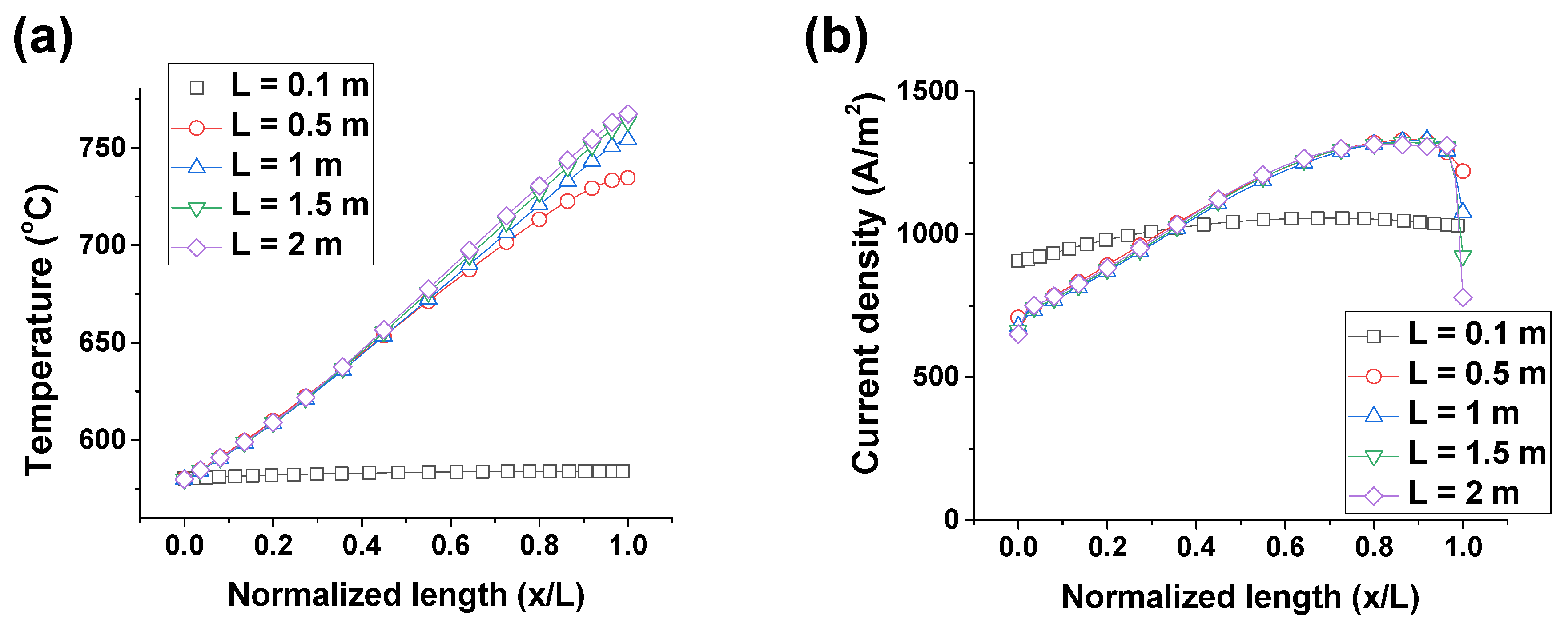
3.2.3. Cross-Flow Cell
3.3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| E | Nernst voltage (V) |
| ΔG | Related to the free energy change under standard state |
| F | Faraday’s constant (96485 As mol−1) |
| R | Universal gas constant (8.314 J mol−1 K−1) |
| P | Pressure (Pa) |
| V | Cell voltage (V) |
| Rtot | Total cell resistance (Ωm2) |
| T | Temperature (K) |
| i | Current density (Am−2) |
| K | Equilibrium constant of the WGS reaction |
| k | Conductivity (Wm−1K−1) |
| h | Heat transfer coefficient (Wm−2K−1) |
| q | Heat generation due to the chemical reaction (Wm−2) |
References
- Dicks, A.L. Molten carbonate fuel cells. Curr. Opin. Solid State Mater. Sci. 2004, 8, 379–383. [Google Scholar] [CrossRef]
- Yuh, C.; Colpetzer, J.; Dickson, K.; Farooque, M.; Xu, G. Carbonate fuel cell materials. J. Mater. Eng. Perform. 2006, 15, 457–462. [Google Scholar] [CrossRef]
- Morita, H.; Komoda, M.; Mugikura, Y.; Izaki, Y.; Watanabe, T.; Masuda, Y.; Matsuyama, T. Performance analysis of molten carbonate fuel cell using a Li/Na electrolyte. J. Power Sources 2002, 112, 509–518. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, M.C. Comparison of thermal performances of external and internal reforming molten carbonate fuel cells using numerical analyses. Int. J. Hydrog. Energy 2017, 42, 3510–3520. [Google Scholar] [CrossRef]
- Yoshiba, F.; Ono, N.; Izaki, Y.; Watanabe, T.; Abe, T. Numerical analyses of the internal conditions of a molten carbonate fuel cell stack: Comparison of stack performances for various gas flow types. J. Power Sources 1998, 71, 328–336. [Google Scholar] [CrossRef]
- Koh, J.-H.; Kang, B.S.; Lim, H.C. Effect of various stack parameters on temperature rise in molten carbonate fuel cell stack operation. J. Power Sources 2000, 91, 161–171. [Google Scholar] [CrossRef]
- Kim, H.; Bae, J.; Choi, D. An analysis for a molten carbonate fuel cell of complex geometry using three-dimensional transport equations with electrochemical reactions. Int. J. Hydrog. Energy 2013, 38, 4782–4791. [Google Scholar] [CrossRef]
- Kim, Y.J.; Chang, I.G.; Lee, T.W.; Chung, M.K. Effects of relative gas flow direction in the anode and cathode on the performance characteristics of a Molten Carbonate Fuel Cell. Fuel 2010, 89, 1019–1028. [Google Scholar] [CrossRef]
- Lee, C.-W.; Lee, M.; Lee, M.-J.; Chang, S.-C.; Yoon, S.-P.; Ham, H.C.; Han, J. Effect of the flow directions on a 100 cm2 MCFC single cell with internal flow channels. Int. J. Hydrog. Energy 2016, 41, 18747–18760. [Google Scholar] [CrossRef]
- Wolf, T.L.; Wilemski, G. Molten carbonate fuel cell performance model. J. Electrochem. Soc. 1983, 130, 48–55. [Google Scholar] [CrossRef]
- Yuh, C.; Selman, J. The Polarization of Molten Carbonate Fuel Cell Electrodes I. Analysis of Steady-State Polarization Data. J. Electrochem. Soc. 1991, 138, 3642–3648. [Google Scholar] [CrossRef]
- Park, J.M.; Kim, D.Y.; Baek, J.D.; Yoon, Y.-J.; Su, P.-C.; Lee, S.H. Numerical Study on Electrochemical Performance of Low-Temperature Micro-Solid Oxide Fuel Cells with Submicron Platinum Electrodes. Energies 2018, 11, 1204. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.M.; Srinivasan, S.; Chamberlin, C.E. Modeling of proton exchange membrane fuel cell performance with an empirical equation. J. Electrochem. Soc. 1995, 142, 2670–2674. [Google Scholar] [CrossRef]
- Liu, S.-F.; Chu, H.-S.; Yuan, P. Effect of inlet flow maldistribution on the thermal and electrical performance of a molten carbonate fuel cell unit. J. Power Sources 2006, 161, 1030–1040. [Google Scholar] [CrossRef]
- Kim, M.-H.; Park, H.-K.; Chung, G.-Y.; Lim, H.-C.; Nam, S.-W.; Lim, T.-H.; Hong, S.-A. Effects of water-gas shift reaction on simulated performance of a molten carbonate fuel cell. J. Power Sources 2002, 103, 245–252. [Google Scholar] [CrossRef]
- Hirata, H.; Nakagaki, T.; Hori, M. Effect of gas channel height on gas flow and gas diffusion in a molten carbonate fuel cell stack. J. Power Sources 1999, 83, 41–49. [Google Scholar] [CrossRef]
- Koh, J.-H.; Seo, H.-K.; Lee, C.G.; Yoo, Y.-S.; Lim, H.C. Pressure and flow distribution in internal gas manifolds of a fuel-cell stack. J. Power Sources 2003, 115, 54–65. [Google Scholar] [CrossRef]
- Kim, D.-W.; Kim, H.-Y.; Choi, J.-H.; Lee, C.-W. Study on the Effects of the Flow Characteristics and Size on the Peformance of Molten Carbonate Fuel Cells Using CFD. Trans. Korean Hydrog. New Energy Soc. 2019, 30, 147–154. [Google Scholar]
- Yang, F.; Zhu, X.-J.; Cao, G.-Y. Nonlinear fuzzy modeling of a MCFC stack by an identification method. J. Power Sources 2007, 166, 354–361. [Google Scholar] [CrossRef]
- Yoo, M.-J.; Kim, D.-P.; Chung, G.-Y.; Lim, H.-C. Studies on the Numerical Modeling of the Butterfly-type Unit Molten Carbonate Fuel Cell. J. Fuel Cell Sci. Technol. 2006, 3, 327–332. [Google Scholar] [CrossRef]
- Lee, C.W.; Yu, J.H.; Kim, H.W.; Ryu, B.H. Effect of the Size of Molten Carbonate Fuel Cells on the Temperature Distribution. Key Eng. Mater. 2018, 773, 118–122. [Google Scholar] [CrossRef]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.-H.; Lee, C.-W. Effect of Cell Size on the Performance and Temperature Distribution of Molten Carbonate Fuel Cells. Energies 2020, 13, 1361. https://doi.org/10.3390/en13061361
Yu J-H, Lee C-W. Effect of Cell Size on the Performance and Temperature Distribution of Molten Carbonate Fuel Cells. Energies. 2020; 13(6):1361. https://doi.org/10.3390/en13061361
Chicago/Turabian StyleYu, Jae-Hyeong, and Chang-Whan Lee. 2020. "Effect of Cell Size on the Performance and Temperature Distribution of Molten Carbonate Fuel Cells" Energies 13, no. 6: 1361. https://doi.org/10.3390/en13061361
APA StyleYu, J.-H., & Lee, C.-W. (2020). Effect of Cell Size on the Performance and Temperature Distribution of Molten Carbonate Fuel Cells. Energies, 13(6), 1361. https://doi.org/10.3390/en13061361




