Abstract
In view of the characteristics of the high content of SO42−, Fe2+ and Mn2+ in acid mine drainage (AMD) and low pH value, based on adsorption and biological methods, coal gangue was combined with sulfate-reducing bacteria (SRB). On this basis, four dynamic columns, including Column 1 (SRB combined with spontaneous combustion gangue from the Gaode coal mine), Column 2 (SRB combined with spontaneous combustion gangue from Haizhou), Column 3 (SRB combined with gangue from Haizhou), and Column 4 (SRB combined with gangue from Shanxi), were constructed. The efficacy of four columns was compared by the inflow of AMD with different pollution load. Results showed that the repair effect of four columns was: Column 3 > Column 2 > Column 1 > Column 4. In the second stage of the experiment, the repair effect of Column 3 was the best. The average effluent pH value and oxidation reduction potential (ORP) value were 9.09 and –262.83 mV, the highest removal percentages of chemical oxygen demand (COD) and SO42− were 84.41% and 72.73%, and the average removal percentages of Fe2+, Mn2+ were 98.70% and 79.97%, respectively. At the end of the experiment, when deionized water was injected, the fixed effect of AMD in the four columns was stable and no secondary release appeared.
1. Introduction
Coal mining destroys underground aquifers and produce a series of solid, liquid and gas pollutants such as coal gangue, acid mine drainage (AMD) and greenhouse gases [1]. AMD has low pH value, a high concentration of heavy metal ions and sulfate, and its pollution to the mine environment is one of the common problems in the world [2,3,4]. AMD is mainly formed by a series of biochemical and physicochemical reactions of metal sulfide in the mining area under the synergistic action of microorganisms, air and water, with low pH value and high content of Fe2+, Fe3+, Al3+, Mn2+, and sulfate [5,6]. AMD pollution has seriously damaged water resources in mining areas. Therefore, a large number of scholars have studied the pollution problem of AMD, and proposed methods of adsorption, neutralization, ion exchange, biological, coagulation and precipitation [7,8]. Among them, the adsorption method is to adsorb part of substances in AMD on the surface of porous solids. The adsorption material has good adsorption effect on metal cations, but is not ideal on sulfate. Therefore, the treatment of mining wastewater containing a large amount of sulfate is faced with severe challenges [9]. Sulfate-reducing bacteria (SRB) in microbial methods can use organic matter or H2 as an electron donor and reduce sulfate to sulfide under anaerobic conditions [10]. SRB is a kind of prokaryotic microorganism living in an anaerobic environment, which can reduce SO42− by using organics such as lactic acid [11]. First, in an anaerobic environment, lactic acid is degraded to pyruvate by lactate dehydrogenase in SRB, electrons and H+ [11,12,13]. Second, pyruvate is fermented in different ways, passing electrons and protons through the electron transport chain step by step to form acetate [11,12,14,15]. Third, C3 cytochrome transports the electrons produced by the oxidation of formic acid and H2 in the previous stage to reduce SO42− [11,12,16]. Finally, SO42− is activated to form 5ʹ-phosphosulfate (APS), which is reduced to sulfide (Equations (1)–(3)) [11,17,18].
AMP4− + SO42− + H+ → APS2− + HP2O73−
APS2− + 2e2− + H+ → HSO3− + AMP2−, E0’ = −60 mV
HSO3− + 6e2− + 6H+ → HS− + H2O, E0’ = −116 mV
However, microorganisms are susceptible to environmental pH and heavy metal ion concentration. Coal gangue is a kind of black gray rock with lower carbon content and harder than coal in the process of coal formation, which is produced in the process of tunneling, mining and coal washing [19]. Coal gangue production accounts for about 10%–15% of raw coal production, but the utilization rate of coal gangue is low and needs to be further improved [20,21]. It is found that coal gangue modified by physical or chemical methods can improve its adsorption and can be used for the pre-treatment of industrial wastewater [22]. Ruifang Qiu [23] modified the microstructure of coal gangue by a new calcination method, making coal gangue a low-cost adsorbent that can remove Mn2+ from industrial wastewater. Preliminary test results show that the spontaneous combustion coal gangue of Gaode coal mine in Fuxin has a certain adsorption effect on Fe and Mn in AMD, and the removal effect is as follows: SRB combined with spontaneous combustion coal gangue > NaOH modified spontaneous combustion coal gangue > unmodified spontaneous combustion coal gangue [24]. SRB combined with coal gangue from Gaode coal mine can remove Fe, Mn and SO42− in AMD effectively, but the repairing effect of SRB combined with different kinds of coal gangues on AMD is not clear. In addition, the ability of SRB with different kinds of coal gangues to resist AMD pollution load change is not clear.
Therefore, in view of the problem of AMD pollution, based on the mineral material adsorption method and microbial remediation technology, different types of coal gangue were combined with SRB to repair AMD. By changing the pH value, metal ion concentration and nutrient content of AMD, the repairing effect of SRB with different kinds of coal gangue on AMD and the ability of anti-pollution load change were studied. The mechanism of SRB combined with coal gangue to remove AMD pollutants was further revealed by low-temperature nitrogen adsorption, a scanning electron microscope equipped with an energy-dispersive X-ray spectroscopy probe (SEM/EDS), X-ray fluorescence (XRF), Fourier transform infrared spectroscopy (FTIR) and an X-ray diffractometer (XRD). In this study, a kind of SRB synergistic coal gangue material with high treatment efficiency, convenient, cheap materials and strong impact resistance will be obtained, which will provide a scientific foundation for its field application in mining areas.
2. Materials and Methods
2.1. Experimental Materials
Coal Gangue 1 is a spontaneous combustion coal gangue from the Gaode open-pit mine, Fuxin City, Liaoning Province; Coal Gangue 2 is a spontaneous combustion coal gangue from Haizhou open-pit mine, Fuxin City; Coal Gangue 3 is an original coal gangue from Haizhou open-pit mine; Coal Gangue 4 is an original coal gangue from a coal mine in Jincheng City, Shanxi Province. Four kinds of coal gangues were broken separately, sieved to a particle size of 0.42–6.70 mm, washed three times with distilled water, and dried at 80 °C. After drying, the four kinds of coal gangue were broken and screened to 0.074 mm in diameter respectively. The coal gangue samples with a diameter of 0.074 mm were placed in the sample storehouse of a XRF-1800 (Shimadzu, Kyoto, Japan). The samples were analysed under the conditions of X-ray tube, 4 kW thin window, Rh target and ultra-high speed scanning at 300 °/min. The chemical composition of four kinds of coal gangues are shown in Table 1.

Table 1.
Chemical composition of Coal Gangue 1–4 (%).
SRB: in order to improve the repair effect of SRB-combined coal gangue, wet mud at the foot of gangue piles of Haizhou open-pit mine in Fuxin City was taken as seed mud. According to the enrichment and culture method of SRB, the seed mud was inoculated into the sterilized Starkey medium for anaerobic culture [25]. When H2S and black precipitate appear, SRB is cultured [26]. Then the SRB with strong activity was enriched. Among them, the main components of the Starkey medium are: 0.5 g Na2SO4, 1.0 g NH4Cl, 0.5 g K2HPO4, 0.1 g CaCl2·H2O, 2.0 g MgSO4·7H2O, 1.2 g (NH4)2Fe(SO4)2·6H2O, 0.1 g ascorbic acid, 4.0 mL sodium lactate solution, 1.0 g yeast extract, 1 L distilled water, pH = 7.0, sterilized at 121 °C for 30 min [25].
AMD: according to the measured AMD water quality index of a coal mine in Hulunbuir City, Inner Mongolia Autonomous Region, and combined with the Fe2+, Mn2+, SO42− concentration and pH value of AMD simulated by Guo et al. [24]. In this experiment, AMD with different ion concentrations was simulated in the laboratory to study the treatment effect of SRB combined with coal gangue on AMD with different pollution concentration. Water quality indexes of simulated AMD are shown in Table 2.

Table 2.
Water quality index of dynamic experimental influent.
2.2. Experimental Method and Apparatus
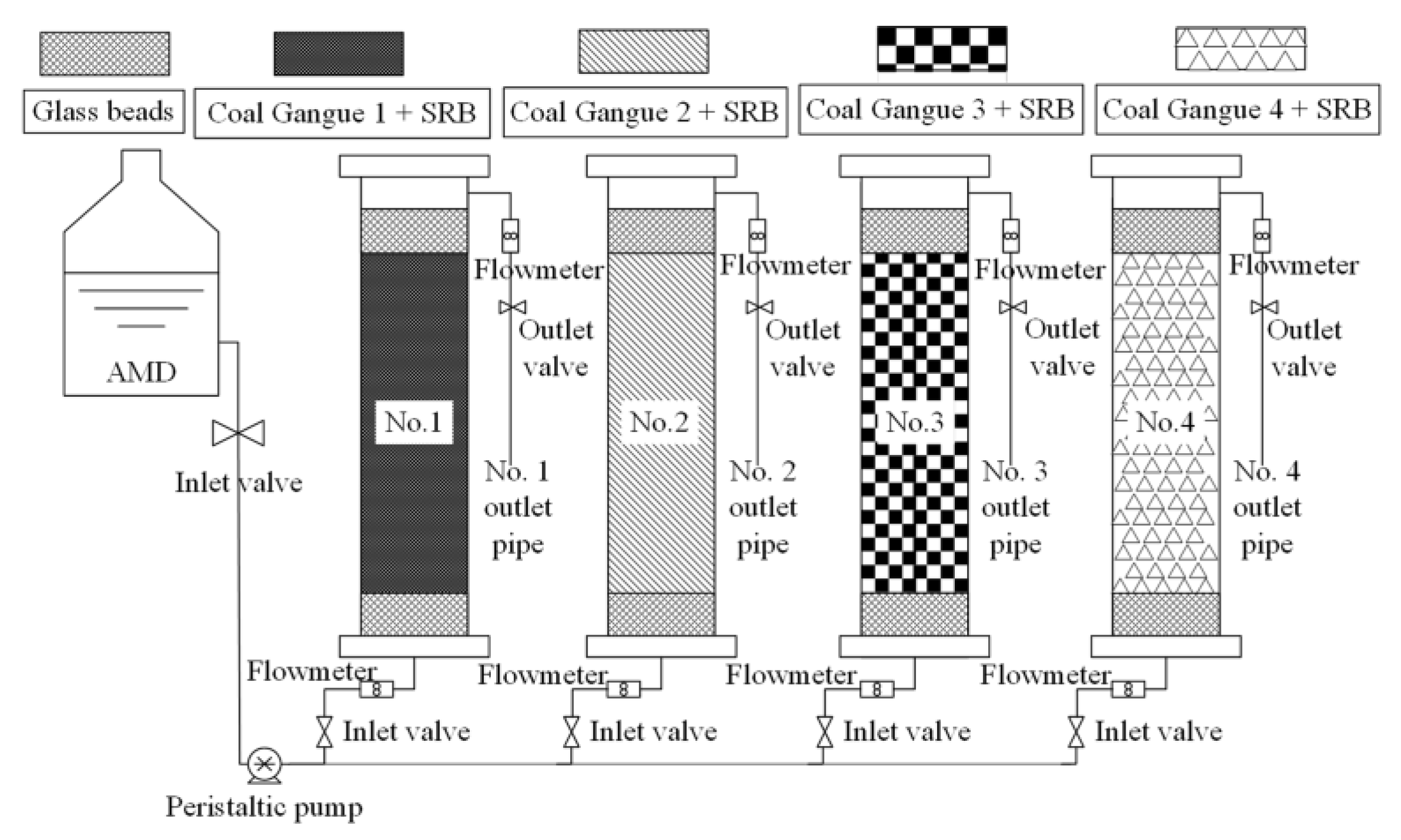
A dynamic leaching experiment of four kinds of coal gangues: Four plexiglass tubes were used as leaching experiment device, with an inner diameter of 56 mm and a height of 500 mm. 40 mm of glass beads, 320 mm of Coal Gangue 1 and 40 mm of glass beads were filled from top to bottom in Leaching Column 1; 40 mm of glass beads, 320 mm of Coal Gangue 2 and 40 mm of glass beads were filled from top to bottom in Leaching Column 2; 40 mm of glass beads, 320 mm of Coal Gangue 3 and 40 mm of glass beads were filled from top to bottom in Leaching Column 3; and 40 mm of glass beads, 320 mm of Coal Gangue 4 and 40 mm of glass beads were filled from top to bottom in Leaching Column 4. Glass beads are used to fix coal gangue. At the beginning of the experiment, four leaching columns were washed with deionized water from top to bottom, and the influent was set as 12 mL/h. The leachate of four leaching columns were collected once a day, and the pH value, ORP value and COD, SO42−, Fe2+ and Mn2+concentrations were measured. When the test indexes of leachate from four leaching columns tended to be stable, the experiment was finished.
The dynamic experimental method of SRB was combined with four kinds of coal gangues to repair AMD: four plexiglass tubes were used as the dynamic reaction column, with an inner diameter of 56 mm and a height of 500 mm; 40 mm of glass beads and 320 mm of Coal Gangue 1 were filled from bottom to top in Column 1; 40 mm of glass beads and 320 mm of Coal Gangue 2 were filled from bottom to top in Column 2; 40 mm of glass beads and 320 mm of Coal Gangue 3 were filled from bottom to top in Column 3; and 40 mm of glass beads and 320 mm of Coal Gangue 4 were filled from bottom to top in Column 4. Then, 200 mL SRB bacteria solution and 150 mL Starkey medium were added to the coal gangue layers of the four dynamic reaction columns, respectively. Finally, the upper of the four dynamic reaction columns were filled with a height of 40 mm of glass beads as the protective layer. The four dynamic reaction columns were placed in a 35 °C biochemical incubator for a week to realize SRB implantation growth and form SRB combined coal gangue materials. According to Table 2, the simulated AMD was injected into the four columns from the lower end of the column at the rate of 12 mL/h, and out from the upper end to ensure an anaerobic environment in the column. The test device is shown in Figure 1. The water samples of inlet water and outlet water of the four dynamic reaction columns were collected at 8:00 every morning, and the pH value, ORP value, SO42− concentration, COD concentration, Fe2+ concentration and Mn2+ concentration were measured. The removal percentages of SO42−, COD, Fe2+ and Mn2+ were calculated and analyzed. When the removal effect tended to be stable, AMD with higher concentration was injected in the next stage to study the efficacy of four dynamic columns to repair AMD and their ability of anti-pollution load change. At the end of the experiment, deionized water was injected into four dynamic columns to analyze its fixation effect on AMD and whether there was secondary release of pollutants.
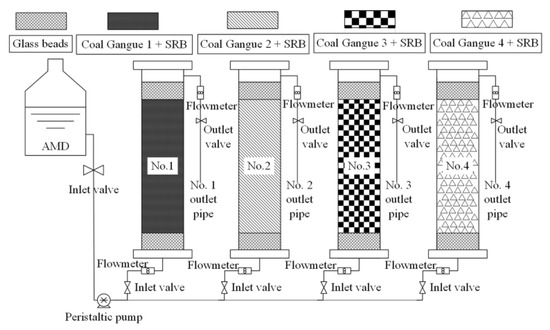
Figure 1.
System diagram of testing device.
Removal percentage = ((C0 − Ct)/C0) × 100%, where, C0 and Ct are the ions concentration before and after the treatment respectively (mg/L).
2.3. Detection and Analysis Methods
The water sample to be tested was left to stand for a while, and its supernatant was filtered with a 0.22 μm filter membrane. The pH value, ORP value, SO42− concentration, COD concentration, Fe2+ concentration and Mn2+ concentration of filtrate were measured according to the Water and wastewater monitoring and analysis method [27]. The pH value was determined by the glass electrode method (GB 6920-86); ORP value was measured by the potential method (SL 94-1994); SO42− was measured by barium chromate spectrophotometry (HJ/T 342-2007); COD was measured by the potassium dichromate method (HJ/T 399-2007); Fe2+ was measured by phenanthroline spectrophotometry (HJ/T 345-2007); Mn2+ was measured by potassium periodate spectrophotometry (GB 11906-89) [27]. A low-temperature nitrogen adsorption experiment was conducted by the ASAP 2020 automatic specific surface and porosity analyzer. The chemical substances of coal gangue were analyzed using XRF-1800. The morphology of the sample was observed by ZEISS Sigma 500 scanning electron microscope, and the chemical substances on the surface of the sample were measured by EDS. Stage analysis was carried out by Bruker D8 Advance X-ray Diffraction with a scan step of 5°–90°. The infrared spectrum test was conducted with Nicolet iS50 FTIR, and the test range was 4000–350 cm−1.
3. Results and Discussion
3.1. Dynamic Leaching Experiment Results of Four Kinds of Coal Gangues
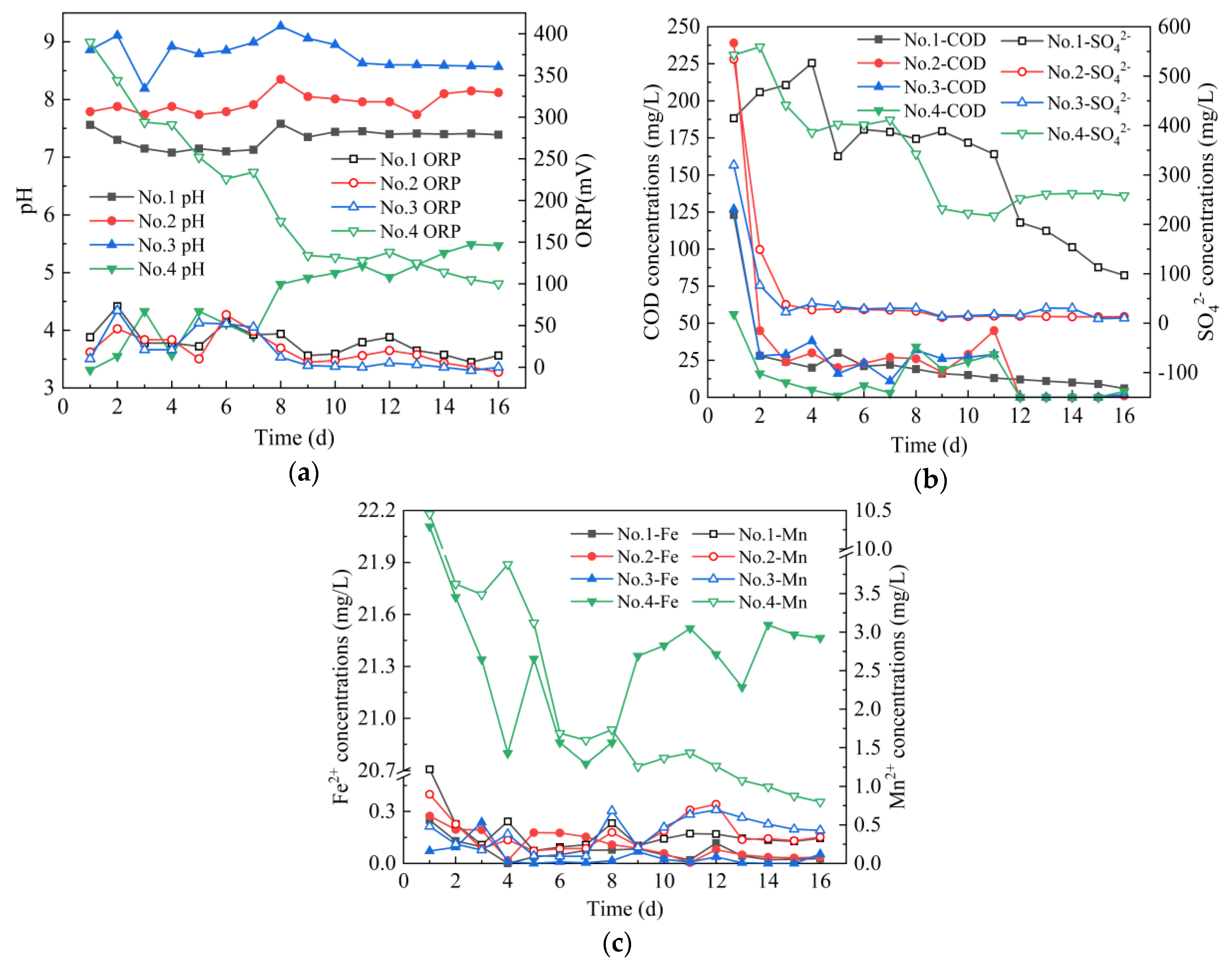
From Figure 2, on 1–16 days, the average pH values of the effluent of four leaching columns were 7.33, 7.95, 8.79 and 4.58, and the average ORP values were 30 mV, 21 mV, 18 mV and 199 mV, respectively. The effluent pH of Coal Gangue 2 was lower than that of Coal Gangue 3, which is consistent with the research results of Wang Xingming [28]. The effluent COD concentrations of four leaching columns decreased from 123, 239, 127 and 56 mg/L to 6, 1, 2 and 4 mg/L, and the average concentrations of released COD were 24, 33, 24 and 13 mg/L, respectively. The SO42− concentrations of the effluent of four leaching columns decreased from 527, 534, 320, and 543 mg/L to 97, 13, 10 and 258 mg/L, and the average concentrations of released SO42− were 327, 60, 5 and 341 mg/L, respectively. The amount of SO42− released by four leaching columns were different. The main reason for this difference was that the sulfur content in four kinds of coal gangues varied greatly. According to Table 1, the sulfur content in four kinds of coal gangues was Coal Gangue 4 > Coal Gangue 1 > Coal Gangue 3 > Coal Gangue 2. In addition, Coal Gangue 2 released more sulfate than Coal Gangue 3, which is consistent with the research results of Ran Zhou [29]. The average concentrations of Fe2+ released by four leaching columns were 0.07, 0.11, 0.04 and 21.32 mg/L, respectively. The average concentrations of Mn2+ released by four leaching columns were 0.39, 0.39, 0.39 and 2.42 mg/L respectively. Among them, the Mn2+ concentration of Coal Gangue 4 decreased from 10.45 mg/L to 2.42 mg/L.
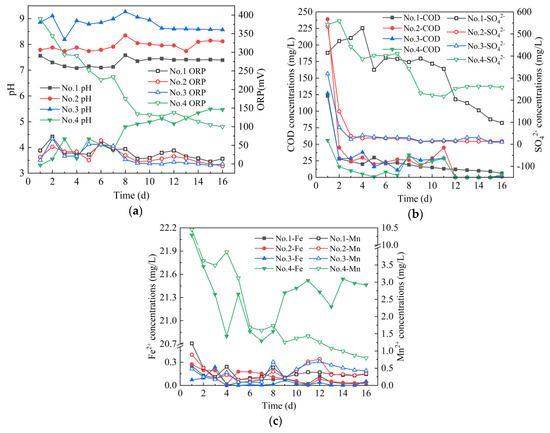
Figure 2.
Changes of dynamic leaching indexes of Coal Gangue 1–4. (a) pH and oxidation reduction potential (ORP) values; (b) chemical oxygen demand (COD) and SO42− concentrations; (c) Fe2+ and Mn2+ concentrations.
3.2. Dynamic Experimental Results of Sulfate-Reducing Bacteria (SRB) Combined with Four Coal Gangues to Repair Acid Mine Drainage (AMD)
3.2.1. Changes in pH and Oxidation Reduction Potential (ORP) Values
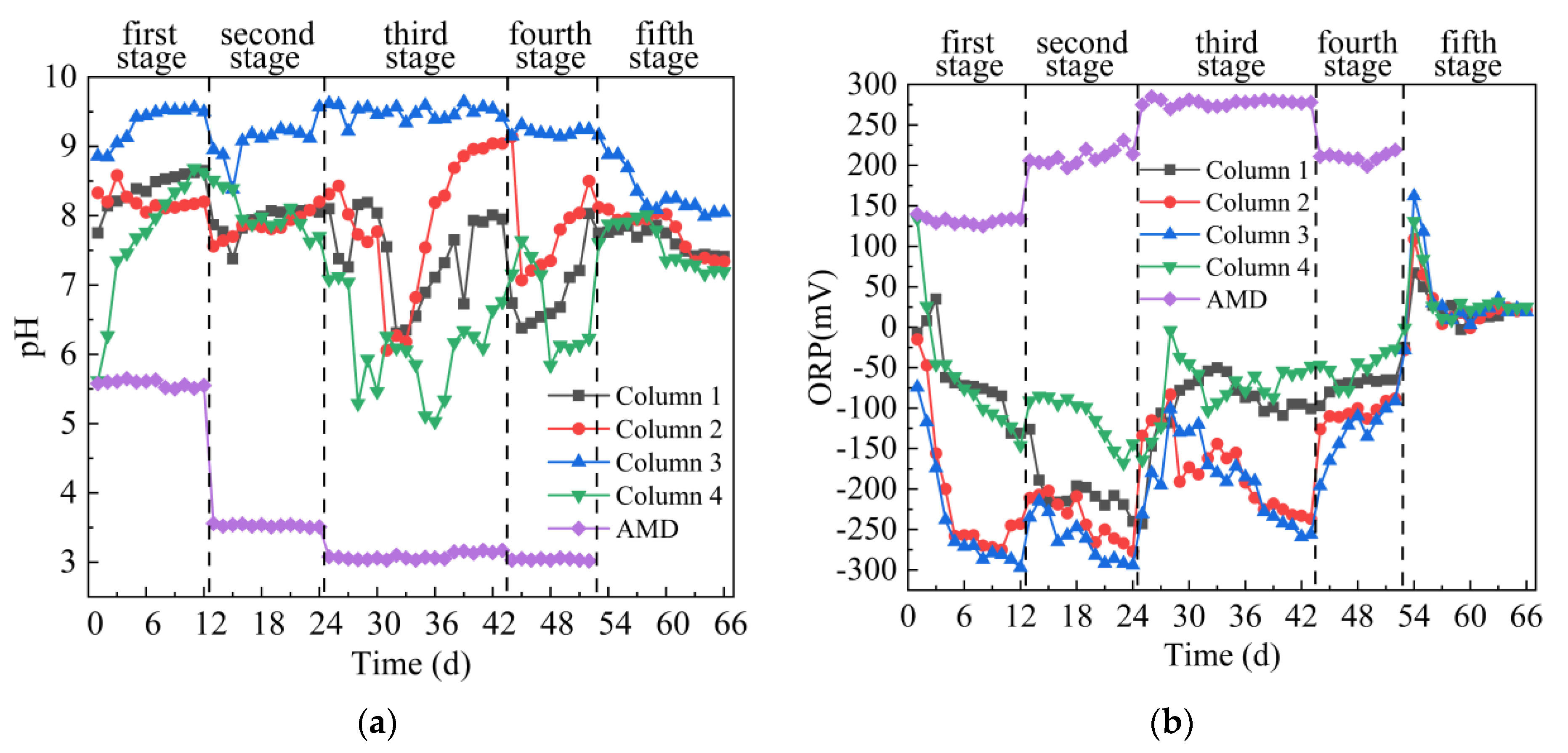
From Figure 3a,b, the average pH of the effluents of 4 columns were improved significantly, while the average ORP value decreased rapidly in the first stage. The average pH value of the effluent from the 4 columns were 8.38, 8.21, 9.32 and 7.70, and the average ORP value were −61.92 mV, −207.92 mV, −236.67 mV and −61.83 mV respectively. The changes in the effluent pH and ORP values of the 4 columns indicated that SRB-combined gangue could promote the growth of SRB and increase the pH of AMD. Previous studies have shown that the activity of anaerobic bacteria such as SRB can reduce the ORP value, and the sulfate metabolism process of SRB is accompanied by an increase in pH [30,31]. The difference of effluent pH and ORP values of the 4 columns were related to the four kinds of coal gangues. The leaching solution of Coal Gangue 1–3 was neutral or weak alkaline, and the leaching solution of Coal Gangue 4 was acidic. Neutral pH can promote SRB growth [32], accelerate the metabolic activity of SRB, increase pH and reduce ORP. In the second stage, the average pH values of the effluent from the 4 columns were 7.93, 7.86, 9.09 and 8.02, and the average ORP value were −204.33 mV, −236.92 mV, −262.83 mV and −112.83 mV, respectively. The highest pH values were 8.09, 8.2, 9.57 and 8.51, respectively. The lowest ORP values were −240 mV, −277 mV, −294 mV and –168 mV, respectively. Generally speaking, SRB organisms dominate when ORP < −100 mV and 5 < pH < 9 in the microbial growth environment [33]. Therefore, the pH value of the effluent from the 4 columns fluctuated steadily, and the ORP value decreased slightly. In the third stage, the average pH value of the effluent from the 4 columns were 7.44, 7.94, 9.49 and 6.10, and the average ORP value were −96.89 mV, −178.58 mV, −191.47 mV and −75.79 mV, respectively. The effluent pH of Column 1, 2 and 4 decreased first and then increased, while ORP increased first and then slowly decreased, indicating that a high concentration of metal ions and low pH in AMD inhibited the growth of SRB. With the extension of time, SRB gradually adapted to the environment with high metal ion concentration and low pH value, and could use its metabolism to improve pH value and precipitate metal ions, so that pH value increased and ORP decreased. The effluent pH of Column 3 fluctuated steadily in the trend, mainly because Coal Gangue 3 released more alkaline substances, which could neutralize the acidity of AMD and reduce the toxic effect of AMD on SRB. In the fourth stage, the average pH value of the effluent from the 4 columns were 6.86, 7.82, 9.20 and 6.64, and the average ORP value were −71.89 mV, −105.33 mV, −130.78 mV and −49.67 mV, respectively. The effluent pH of Column 1, 2 and 4 decreased first and then increased, and the ORP value showed an overall upward trend. It indicates that under the conditions of high acidity and high metal ions content, reducing the amount of carbon source would seriously affect the growth of SRB, and even lead to the death of SRB. Especially the average ORP values of Column 1 and 4 effluent were higher than −100 mV. Chang [34] reported that when ORP < −100 mV, SRB can grow normally, but when ORP value continues to increase, its reduction activity will decrease. In the fifth stage, deionized water was introduced instead of AMD. The effluent pH of the 4 columns decreased first and then stabilized, and the ORP value increased first and then decreased and finally stabilized. The average pH value of the effluent from the 4 columns were 7.64, 7.78, 8.36 and 7.57, and the average ORP value were 20.43 mV, 23.79 mV, 34.07 mV and 33.50 mV, respectively. In general, the effects of pH increase and ORP decrease of effluent of the 4 columns were as follows: Column 3 > Column 2 > Column 1 > Column 4. With the inflow of AMD, the average ORP values of Column 2 and 3 in the four stages were all < −100 mV.
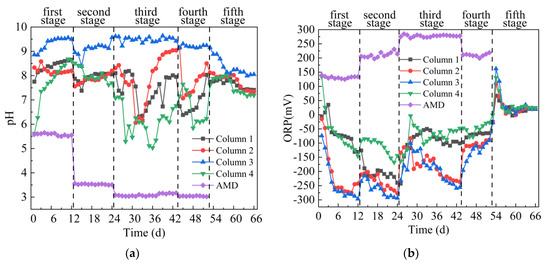
Figure 3.
Changes of pH and ORP values of sulfate-reducing bacteria (SRB) combined with Coal Gangue 1–4 to repair acid mine drainage (AMD). (a) pH value; (b) ORP value.
3.2.2. Analysis of Chemical Oxygen Demand (COD) Removal Effect
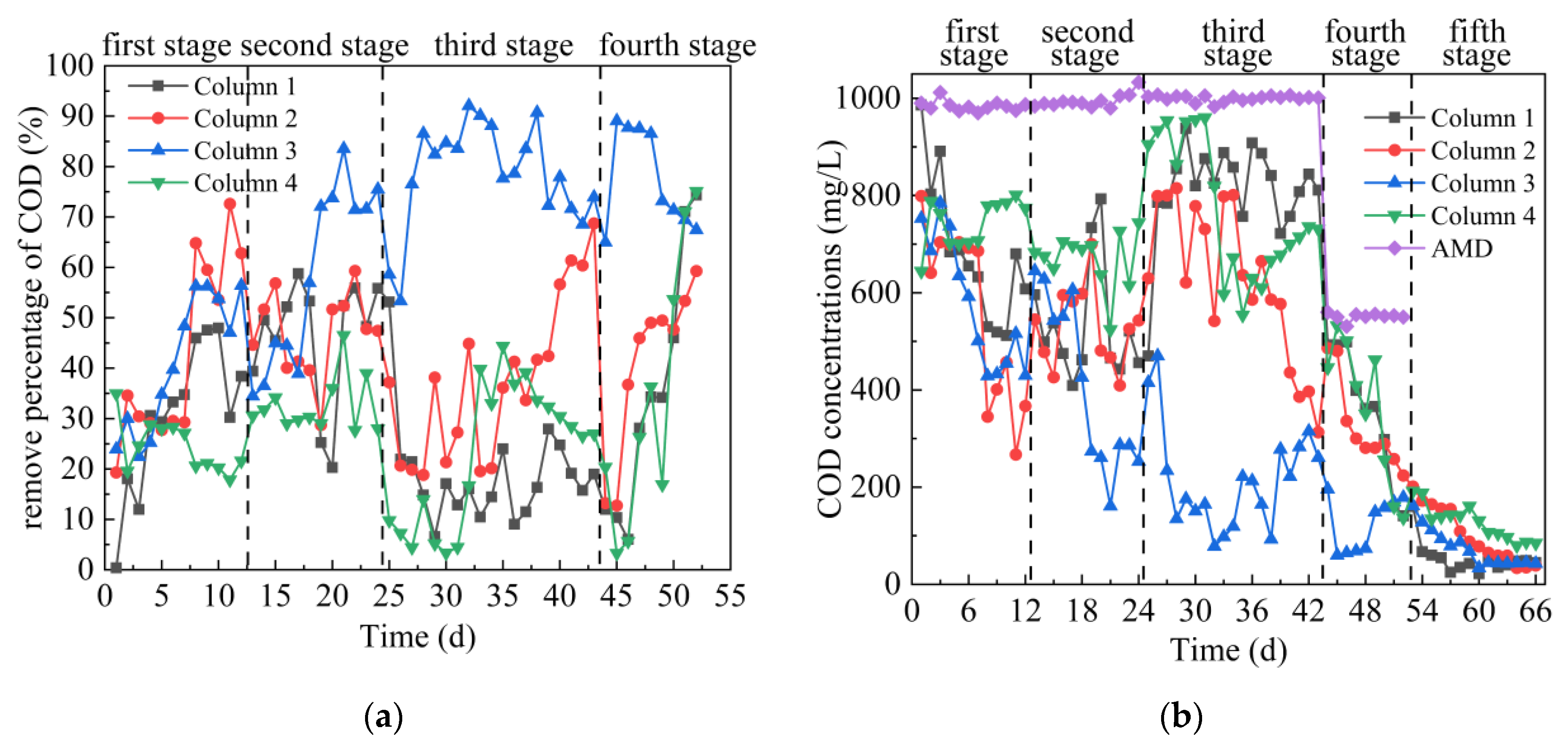
As can be seen from Figure 4a,b, in the first stage, the average COD removal percentage of the effluent from the 4 columns were 31%, 43%, 41% and 24%, respectively. The removal of COD from the 4 columns mainly relied on SRB growth and utilization. The process by which SRB converts S2− to SO42− in an anaerobic environment requires COD consumption [35]. In addition, the surface of gangue has pores, which can remove COD in AMD. In the second stage, the average COD removal percentages of effluent of Columns 1–4 were 46%, 47%, 59% and 33% respectively. The highest removal percentages of COD in effluent of Columns 1–4 were 60.41%, 60.41%, 84.41% and 49.27%, respectively. At this stage, the COD concentration only increased slightly, indicating that the increased concentrations of Fe2+, Mn2+ and SO42− in this stage had little influence on the removal effect of COD in the dynamic columns. The results were consistent with the change of pH and ORP values. Coal gangue could adsorb COD [36], ensuring the removal of COD by the dynamic column. It was reported that the reduction of 1 g sulfate by SRB consumes at least 0.67 g COD [37]. At this stage, the dynamic column can remove COD stably, indicating that SRB can be metabolized normally. In the third stage, the average COD removal percentages of the effluent of Columns 1–4 were 19%, 37%, 78% and 23%, respectively. The removal effect of Column 3 on COD was significantly higher than that of the other three columns, indicating that the SRB metabolic activity in the Column 3 was stronger. According to the changes of pH and ORP, it is indicated that SRB in Column 3 dominates at this time and can effectively convert SO42− and consume COD [33,35]. The percentages of COD removal of Column 1, 2 and 4 fluctuated greatly, mainly because the increased concentrations of Fe2+, Mn2+ and SO42− in this stage had an impact on the growth of SRB. The ability of SRB to metabolize COD decreased, and the adsorption of COD by coal gangue tended to be saturated. At the same time, sulfur compounds were accumulated in SRB metabolism in the early stage, and the removal efficiency of COD would drop sharply when the dissolved sulfur compounds were of high concentration [38]. In the fourth stage, the initial COD concentration in AMD was reduced, leading to a small increase in the removal of COD by some dynamic columns. Among them, the average COD removal percentages in the effluent of Columns 1–4 were 35%, 41%, 77% and 34%, respectively. When the deionized water was injected in the fifth stage, when the COD concentration released from the effluent of Columns 1–4 reached a stable level, COD concentration of Columns 1–3 was maintained at around 45 mg/L, and COD concentration of Column 4 maintained around 91 mg/L. Overall comparison of Columns 1–4 on COD removal effect was as follows: Column 3 > Column 2 > Column 1 ≥ Column 4.
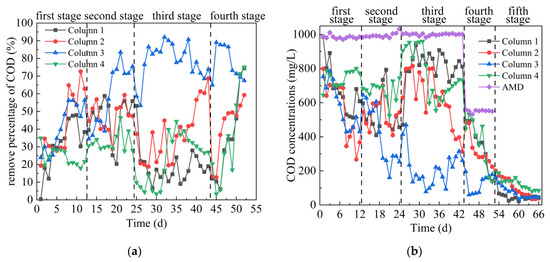
Figure 4.
Changes of COD removal effect of SRB combined with Coal Gangue 1–4 to repair AMD. (a) COD removal percentage; (b) COD concentration.
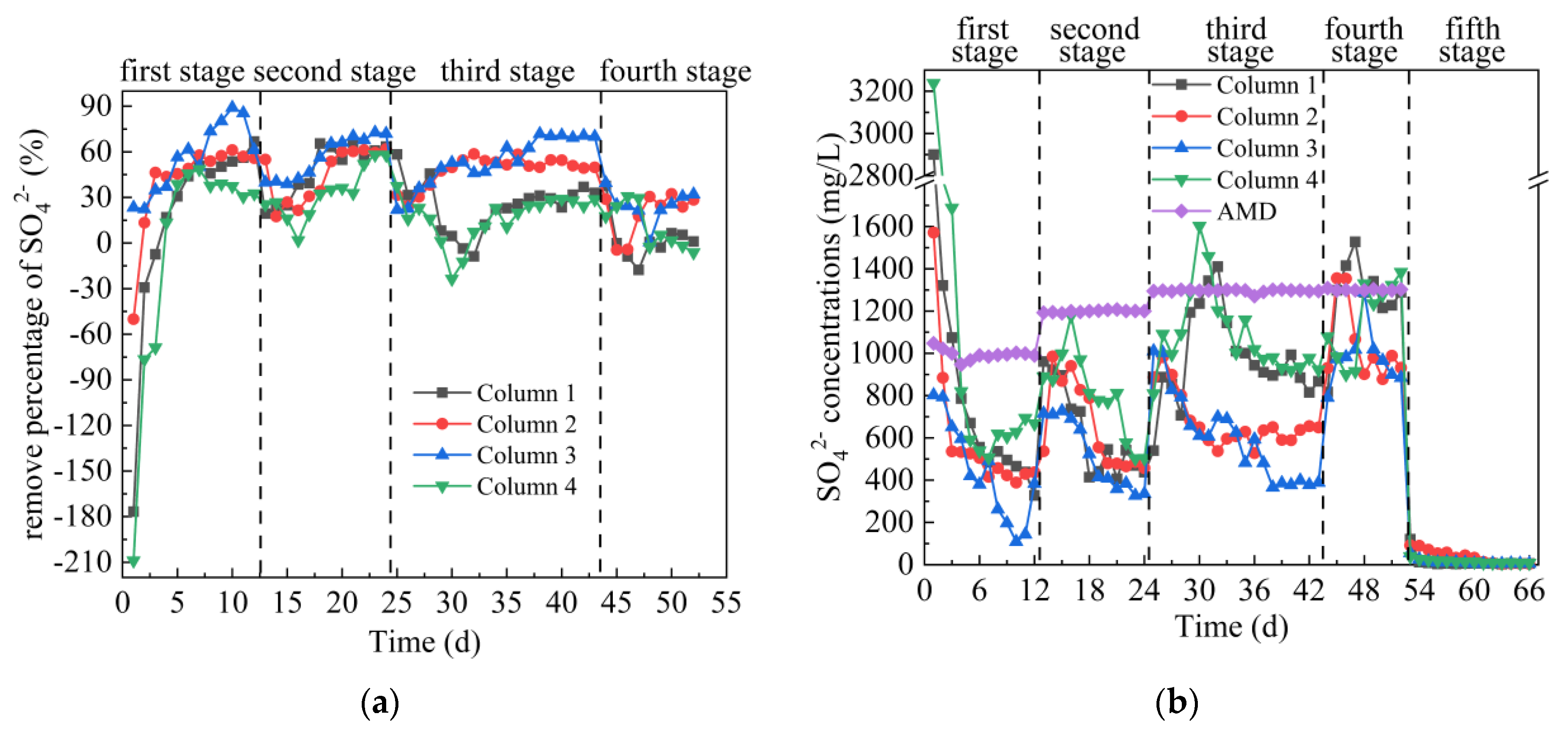
3.2.3. Analysis of SO42− Removal Effect
From Figure 5a,b, on the first day, the SO42− removal percentages of the effluent of Columns 1–4 were −177%, −50%, 23%, −209%, respectively. Among them, Column 1, Column 2 and Column 4 have a negative removal percentage of SO42−, while Column 3 is positive. According to Figure 2, the SO42− concentrations in the leachate of Coal Gangues 1–4 on the first day were 415 mg/L, 534 mg/L, 320 mg/L and 543 mg/L, respectively. In the early stage of the experiment, coal gangue released a large amount of SO42−, resulting in a higher concentration of SO42− in the effluent of Columns 1–4, even higher than that in AMD. Over time, SRB in Columns 1–4 gradually adapted to the wastewater environment, and SO42− in the system was reduced to S2− by self-metabolism. In the first stage, the average SO42− removal percentage of the effluent from Columns 1–4 were 16.82%, 40.95%, 56.32% and −2.55%, respectively. In the first stage of the experiment, the removal effect of SO42− by Columns 2 and 3 was significantly better than that by Columns 1 and 4, indicating that the SRB at the initial stage of the reaction was more suitable for the alkaline environment provided by coal gangue of Fuxin Haizhou open-pit mine (Coal Gangue 2 and Coal Gangue 3). In the second stage, the average SO42− removal percentage of the effluent from Columns 1–4 were 47.84%, 45.26%, 56.60% and 32.79%, respectively, and the corresponding residual SO42− removal concentration were 625 mg/L, 656 mg/L, 520 mg/L and 806 mg/L, respectively. The highest removal percentages of SO42− from Columns 1–4 were 66.32%, 61.90%, 72.73% and 58.11%, respectively. The SO42− concentration in the effluent of Columns 1–4 all showed a small increase first and then a decrease. It shows that the sudden increase of metal ion concentration in AMD had a slight effect on the AMD repairing effect of SRB combined with coal gangue. In the third stage, the average SO42− removal percentages of the effluent from Columns 1–4 were 24.65%, 48.03%, 53.80% and 16.69%, respectively. At this stage, the removal effect of Column 1, 2 and 4 on SO42− were lower than that in the second stage, because the H2S, low pH value and high concentration of metal ions accumulated in the early stage in the dynamic column affected the further metabolism of SRB. It was reported that when H2S concentration is too high, with the increase of H2S concentration, not only are the kinetic parameters of sulfate reduction inhibited proportionally, but also the biological diversity in the reactor is greatly reduced [39,40]. The effect of Column 3 on SO42− removal was better than the other three columns and higher than that of the second stage. The main reason was that Coal Gangue 3 released more alkaline substances, which promoted the transformation of H2S to S2− and accelerated the formation of FeS and MnS precipitation between S2− and Fe and Mn ions. In the fourth stage, the average SO42− removal percentages of the effluent of Columns 1–4 were 2.42%, 19.80%, 24.60% and 10.93%, respectively. The removal effect of Columns 1–4 on SO42− was significantly reduced, mainly because the continuous high concentration of metal interfered with the biological removal of sulfate by SRB [41]. It was reported that the reduction of 1 g sulfate by SRB consumes at least 0.67 g COD [37,42]. At this stage, the content of COD was reduced, leading to the reduction of carbon source and limiting the reduction of SO42− by SRB metabolism. In addition, the toxicity of sulfide to SRB is one of the reasons why SRB cannot effectively reduce SO42− [43,44]. In the fifth stage, deionized water was introduced instead of AMD. The release of SO42− tended to be stable, Columns 1–3 were stable around 5 mg/L, and Column 4 was stable around 8.6 mg/L. A comparison of the SO42− concentration (97 mg/L, 13 mg/L, 10 mg/L, 258 mg/L) released from Coal Gangue 1–4 at the end of the dynamic leaching experiment in Figure 2 showed that there was no secondary SO42− release after SRB combined with coal gangue to repair AMD. The overall comparison of Columns 1–4 on SO42− removal effect was as follows: Column 3 > Column 2 > Column 1 > Column 4.
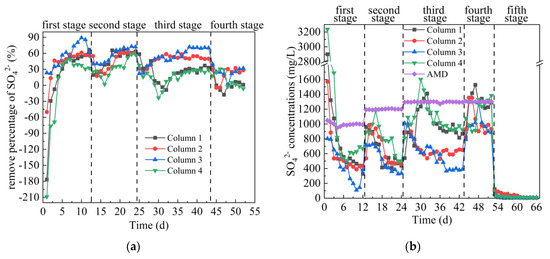
Figure 5.
Changes of SO42− removal effect of SRB combined with Coal Gangue 1–4 to repair AMD. (a) SO42− removal percentage; (b) SO42− concentration.
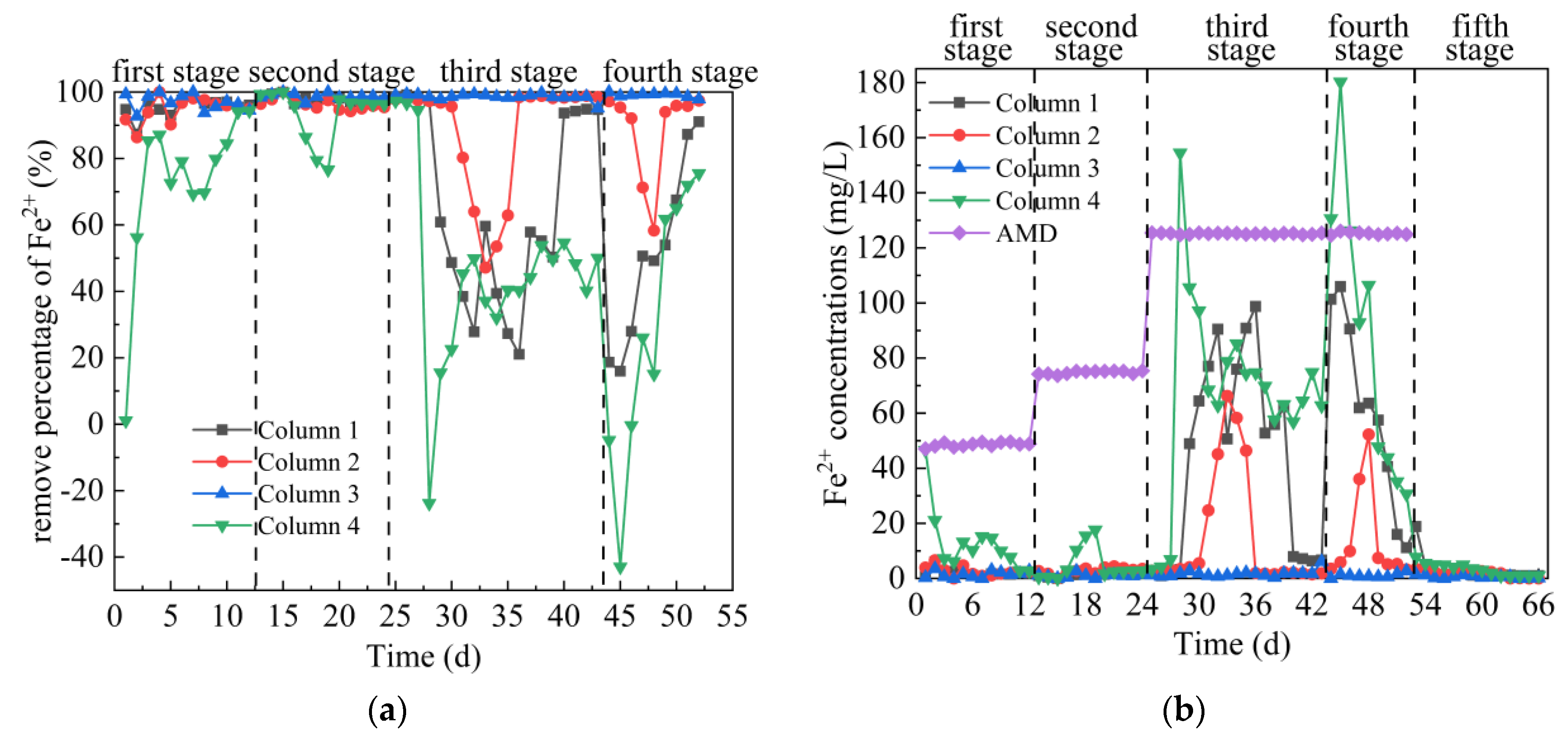
3.2.4. Analysis of Fe2+ Removal Effect
From Figure 6a,b, in the first stage, the average Fe2+ removal percentages of the effluent of Columns 1–4 were 95.45%, 94.77%, 96.98% and 72.76%, respectively. On days 1–6, the metabolic activity of SRB in Columns 1–4 was weak, and the removal of Fe2+ in AMD mainly relies on coal gangue to release alkaline precipitation, surface pore adsorption and ion exchange reaction [45]. On days 7–12, Fe removal in Columns 1–4 mainly relied on coal gangue to release alkalinity to form precipitation and SRB metabolic activity to form sulfide precipitation. The removal effect of Column 4 on Fe2+ was significantly lower than that of the other three columns, mainly because Coal Gangue 4 released more Fe. In addition, the initial water pH of Column 4 was acidic, which inhibited the growth of SRB and the formation of metal hydroxide. In the second stage, the average Fe2+ removal percentages of the effluent of Columns 1–4 were 97.72%, 96.33%, 98.70% and 93.39%, respectively, and the corresponding residual Fe2+ removal concentration were 1.71, 2.75, 0.98 and 4.96 mg/L, respectively. The initial pH = 3.5 of AMD had little effect on the removal of Fe in Columns 1–4, and the maximum removal percentage could reach 100%, indicating that the coal gangue could release alkalinity, pore adsorption and ion exchange at this time. Comparing Figure 5a,b and Figure 6a,b, it can be seen that the dynamic columns had different effects on the removal of Fe2+ and SO42− at this stage. It was reported that the presence of metals interferes with the biological removal of sulfates by SRB, but the resulting sulfide concentration is sufficient to remove more than 90% of metals from the environment [41]. Zhuxiang Liu [37] showed that under the conditions of [pH]0 = 6.0, [SO42−]0 = 2000 mg/L, hydraulic retention time = 15 h, liquid upflow velocity = 4.0 m/h, the removal efficiency of SRB on Fe was 95.2%–100%, and these high level removal effects could be attributed to the low solubility of FeS. Compared with the above results, SRB combined with coal gangue can repair AMD with higher acidity while ensuring high Fe removal percentage. In the third stage, the average Fe2+ removal percentage of the effluent of Columns 1–4 were 66.17%, 88.36%, 98.63% and 46.77%, respectively. Among them, the Fe removal percentage of Column 3 was stable at between 95.13% to 99.72%, indicating that both coal gangue and SRB could adapt to AMD with pH = 3 and a high concentration of metal ions at this stage. At the beginning of this stage, the Fe removal percentage of the other three columns was decreasing. This trend was consistent with the trend of pH, ORP and SO42− removal percentage, indicating that high concentration of metal ions and acidic pH inhibited SRB. At the later stage of this stage, basicity, adsorption and ion exchange generated by coal gangue reduced the inhibition of SRB by AMD, and the removal percentage of Fe increased first and then was stable. In the fourth stage, the average Fe2+ removal percentages of the effluent of Columns 1–4 were 51.36%, 88.59%, 99.14% and 29.67%, respectively. The removal efficiency of Fe2+ in Column 1, 2 and 4 decreased significantly first and then increased, indicating that under low pH and high metal concentration, reducing COD concentration had a greater impact on SRB. However, Fe removal percentage was maintained stable in Column 3, mainly because the relatively high alkalinity released by Coal Gangue 3 (average pH = 8.79), metal ions were removed in the form of hydroxide, and the acidity of AMD was neutralized and the toxic effect on SRB was reduced. In the fifth stage, when the release of Fe2+ tended to be stable, Columns 1 and 4 were stable around 1 mg/L, Column 2 was stable around 0.03 mg/L, and Column 3 was stable around 0.3 mg/L. The effluent Fe2+ concentration of Column 4 was significantly lower than that released by the dynamic leaching equilibrium of Coal Gangue 4 (21.32 mg/L), indicating that a small amount of SRB still existed in the device at the end of the test, ensuring the fixed effect of Fe in the system. The overall comparison of Columns 1–4 on Fe2+ removal effect was as follows: Column 3 > Column 2 > Column 1 > Column 4. The removal percentage of Fe2+ by Column 3 was between 92.72% and 100%, which was not affected by low pH value, high concentration of metal ions and low COD concentration in AMD influent of the first 4 stages.
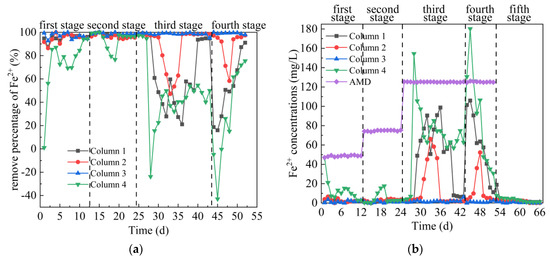
Figure 6.
Changes of Fe2+ removal effect of SRB combined with Coal Gangue 1–4 to repair AMD. (a) Fe2+ removal percentage; (b) Fe2+ concentration.
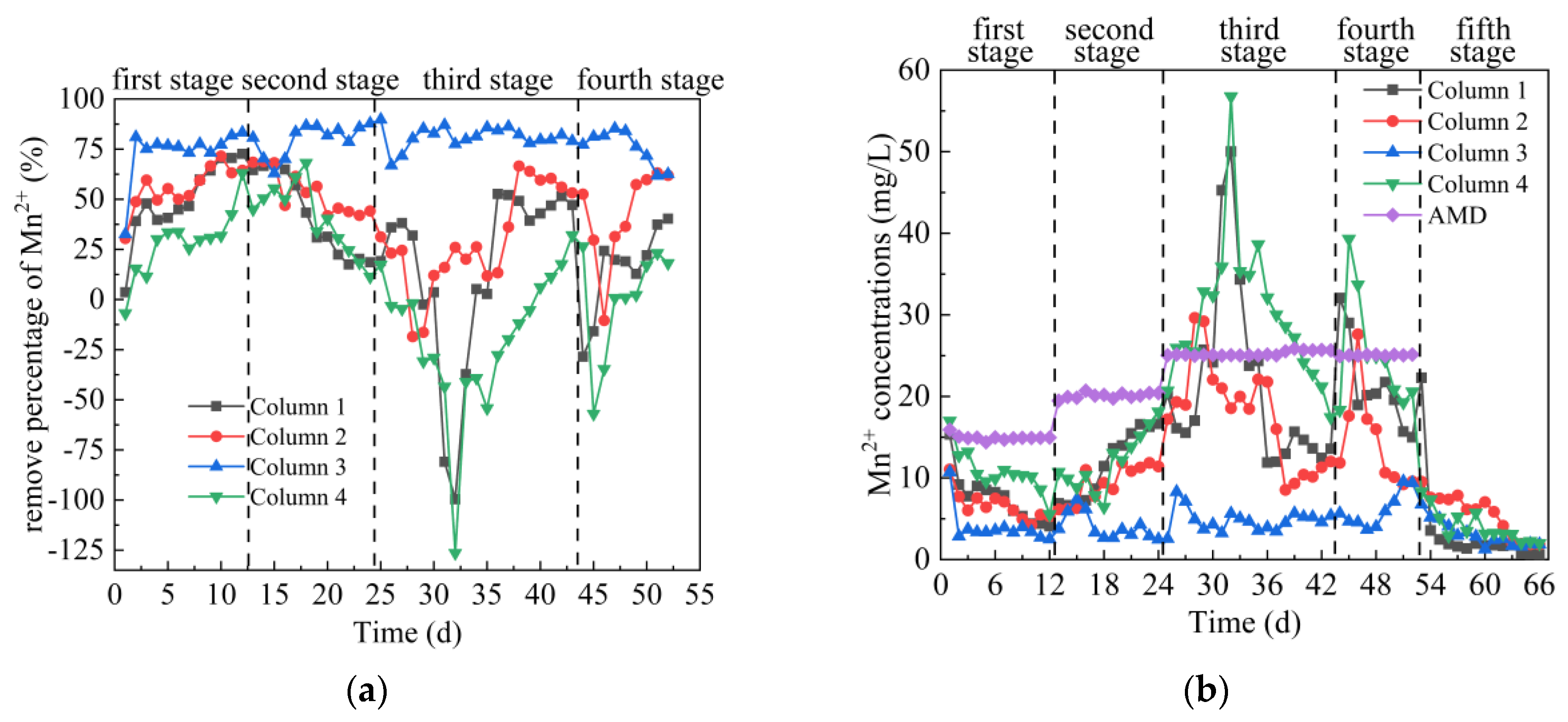
3.2.5. Analysis of Mn2+ Removal Effect
From Figure 7a,b, in the first stage, the Mn2+ concentration in the effluent of Columns 1–4 first decreased and then tended to be stable. The average percentages of Mn2+ removal were 50.04%, 55.90%, 73.81% and 28.36%, respectively. The main removal of Mn is that SRB reduces SO42− to S2−, and S2− reacts with Mn2+ to form MnS precipitation [46]. At the same time, SRB adhered to the surface of coal gangue, formed a biofilm, and secreted a large amount of extracellular polymeric substances (EPS). The hydroxyl and carboxyl groups contained in the EPS on the surface of SRB combined with the metals in AMD to improve the repair effect [47,48,49]. From the removal percentage, the removal effect of Mn2+ by Columns 1–4 was lower than that of Fe2+. Some studies have shown that there is exchange adsorption between metal sulfides, and metal sulfides with larger solubility can form metal sulfides with smaller solubility product [50]. Because Ksp(MnS) > Ksp(FeS) [51], it was not easy for Mn2+ to form stable and insoluble sulfide in the presence of Fe2+. In the second stage, the average Mn2+ removal percentages of the effluent of Columns 1–4 were 42.07%, 53.41%, 79.97% and 40.74%, respectively, and the corresponding residual Mn2+ removal concentration were 11.66, 9.39, 4.02 and 11.92 mg/L, respectively. The highest removal percentages of Mn2+ of Columns 1–4 were 67.57%, 68.79%, 87.86% and 68.10%, respectively. On 18–24 days, the removal of Mn2+ by Column 1, 2 and 4 decreased in varying degrees, indicating that the adsorption-desorption of Mn2+ occurred after the coal gangue reached saturation [52]. In addition, the decrease of removal percentage may be due to the reduction of adsorption sites of effective substances, which prevents H2S from forming HS− [46]. This caused S2−competition that was not conducive to Mn2+. However, the removal of Mn2+ by Column 3 shows a trend of first increasing and then stabilizing, indicating that Coal Gangue 3 had a stronger removal capacity of Mn2+ and was more suitable to repair AMD pollution with SRB. In the third stage, the average Mn2+ removal percentage of the effluent of Columns 1–4 were 15.69%, 29.75%, 81.03% and −18.67%, respectively. The removal of Mn from Columns 1–4 decreased first and then increased, which was consistent with the trend of pH value and the removal percentage of SO42− and Fe2+. This indicated that high concentration of metal ions, acidic pH and H2S were toxic to SRB when the initial AMD inflow conditions changed at this stage, while coal gangue could reduce the toxicity of AMD to SRB. In the fourth stage, the average Mn2+ removal percentages of the effluent of Columns 1–4 were 14.58%, 42.40%, 75.76% and −0.33%, respectively. In the fifth stage, when the release of Fe2+ tended to be stable, Columns 1–4 were stable around 1.09, 2.36, 1.94 and 2.52 mg/L. Overall comparison of Columns 1–4 on Mn2+ removal effect was as follows: Column 3 > Column 2 > Column 1 > Column 4.
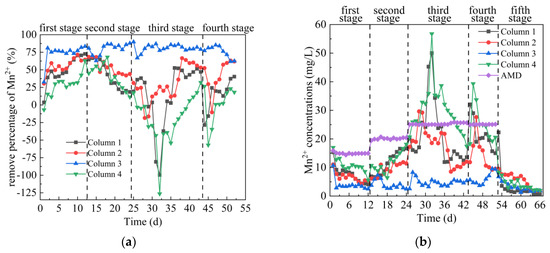
Figure 7.
Changes of Mn2+ removal effect of SRB combined with Coal Gangue 1–4 to repair AMD. (a) Mn2+ removal percentage; (b) Mn2+ concentration.
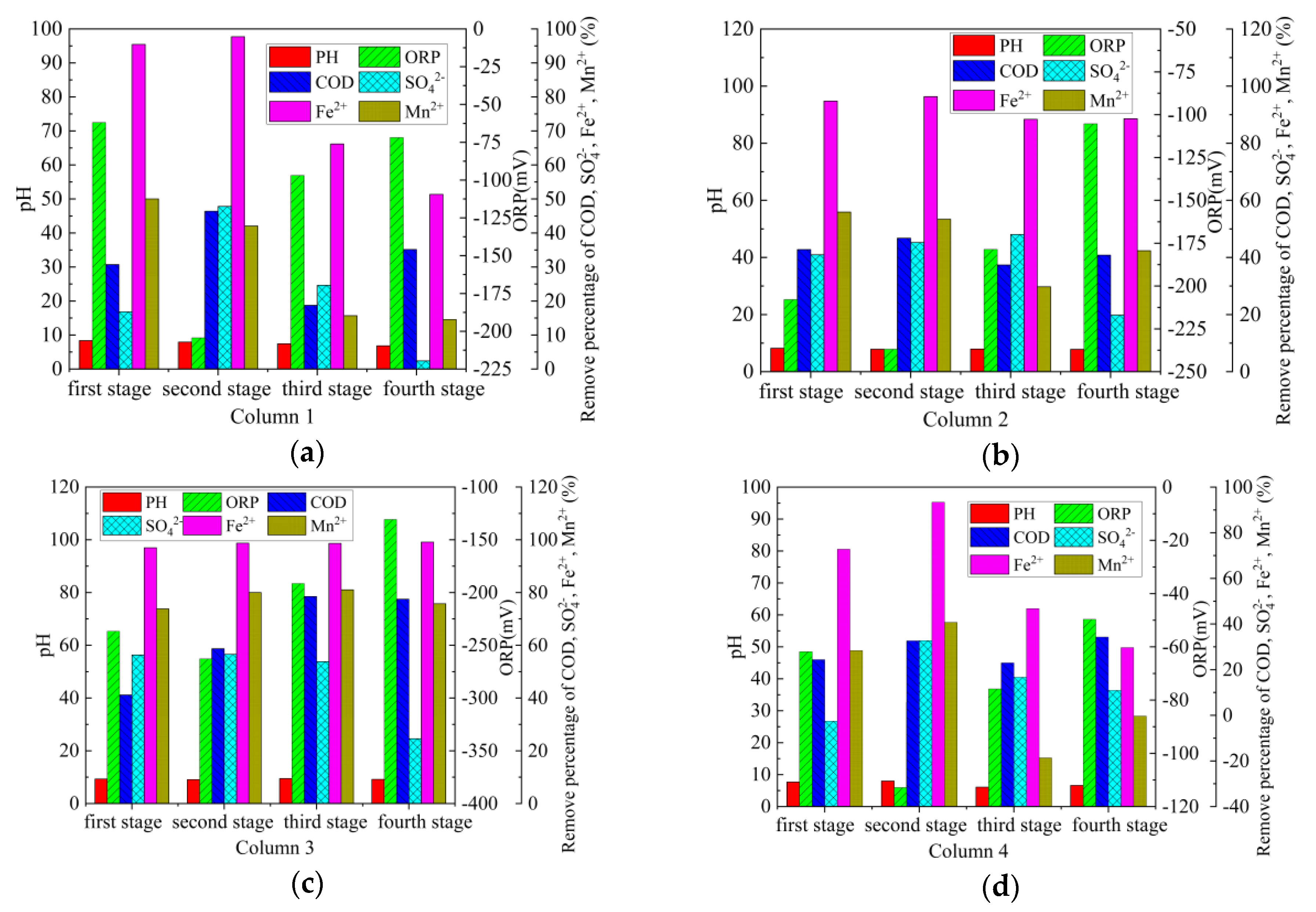
3.2.6. Repair Effect of Columns 1–4 on AMD with Different Pollution Load
The average repair effect of Columns 1–4 on AMD (first stage–fourth stage) with different pollution load is shown in Figure 8. From Figure 8a, the treatment effects of Column 1 on the AMD in the first and second stages are significantly better than those of the third and fourth stages. From Figure 8d, Column 4 and Column 1 have similar treatment effects. The main reasons for this phenomenon are as follows: on the one hand, the treatment effect of coal gangue on AMD gradually tends to saturation [52], leading to the decline of the repair effect. Meanwhile, SRB can be metabolized normally in AMD with low pollution load (stage 1 and stage 2), but AMD with high pollution load (stage 3 and stage 4) can inhibit the activity of SRB [41]. In addition, the accumulation of sulfide can poison SRB [38], which reduces the ability of SRB combined with coal gangue to repair AMD. From Figure 8b,c, the treatment effect of Column 2 and Column 3 on the AMD with different pollution load is relatively stable, and the treatment effect of Column 3 is better than that of Column 2. After the treatment of AMD with different pollution load by Column 3, the average pH value of the effluent was 9.09–9.49, the average ORP value was −131 mV–−263 mV, the average COD removal percentage was 41.19%–78.48%, the average SO42− removal percentage was 24.60%–56.60%, the average Fe2+ removal percentage was 96.98%–99.14%, the average Mn2+ removal percentage was 73.81%–81.03%, and the maximum sulfate reduction rate was 0.89 g/(L·d). Among them, the removal effect of COD is consistent with other research results. Zhang [53] takes sodium lactate as the carbon source and use SRB immobilized particles to repair AMD, the removal percentage of COD is about 36%–86%. Sahinkaya used a sulfate-reduction fluidized bed reactor to treat AMD, with a COD removal percentage of 75% [54]. The maximum sulfate reduction rate was different from the results in some literature. According to Zhang [53], the maximum sulfate reduction rate for treating AMD with SRB immobilized particles is 2.67 g/(L·d). Sahinkaya [55] showed that the maximum sulfate reduction rate of sulfate is approximately 3 g/(L·d) in an anaerobic baffle reactor. In order to promote the combination of SRB and coal gangue, the wet mud at the foot of gangue piles was used as seed mud to enrich SRB. However, SRB enriched in the soil of the mining area has not been purified and domesticated, and the reduction rate of sulfate is relatively low. According to Hwang [2], the indigenous SRB enriched in the soil samples of the mining area has the potential to remove heavy metals and sulfate from AMD, but the removal efficiency is lower than Desulfovibrio desulfuricans. Compared with the above studies [53,55], coal gangue, as a large amount of solid waste accumulated in mining areas, is used to treat AMD with SRB, which is easy to operate and obtain materials for, and has strong acid neutralization ability and high metal ions removal efficiency. In the experimental results, the removal percentage of Mn was significantly lower than that of Fe. Similar phenomena have been observed in other studies. Bai [56] showed that when AMD is treated by SRB and iron-reducing bacteria, the removal percentage of Mn is lower than that of other metals, and the removal percentage is only 20%–57%. Zhang [53] showed that it is difficult to remove Mn from AMD with SRB immobilized particles, and the removal percentage is 42%–99%.
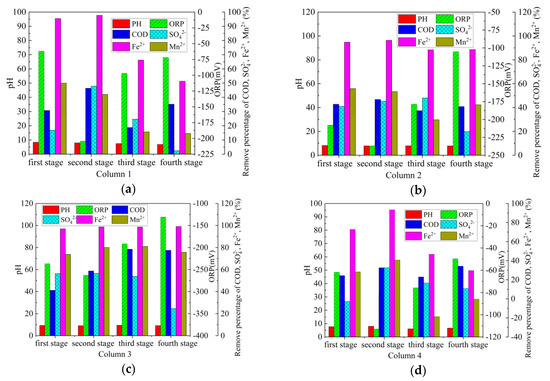
Figure 8.
Repair effect of Columns 1–4 on AMD with different pollution load. (a) Column 1; (b) Column 2; (c) Column 3; (d) Column 4.
4. Instrumental Analysis and Characterization
4.1. Low-Temperature Nitrogen Adsorption Analysis
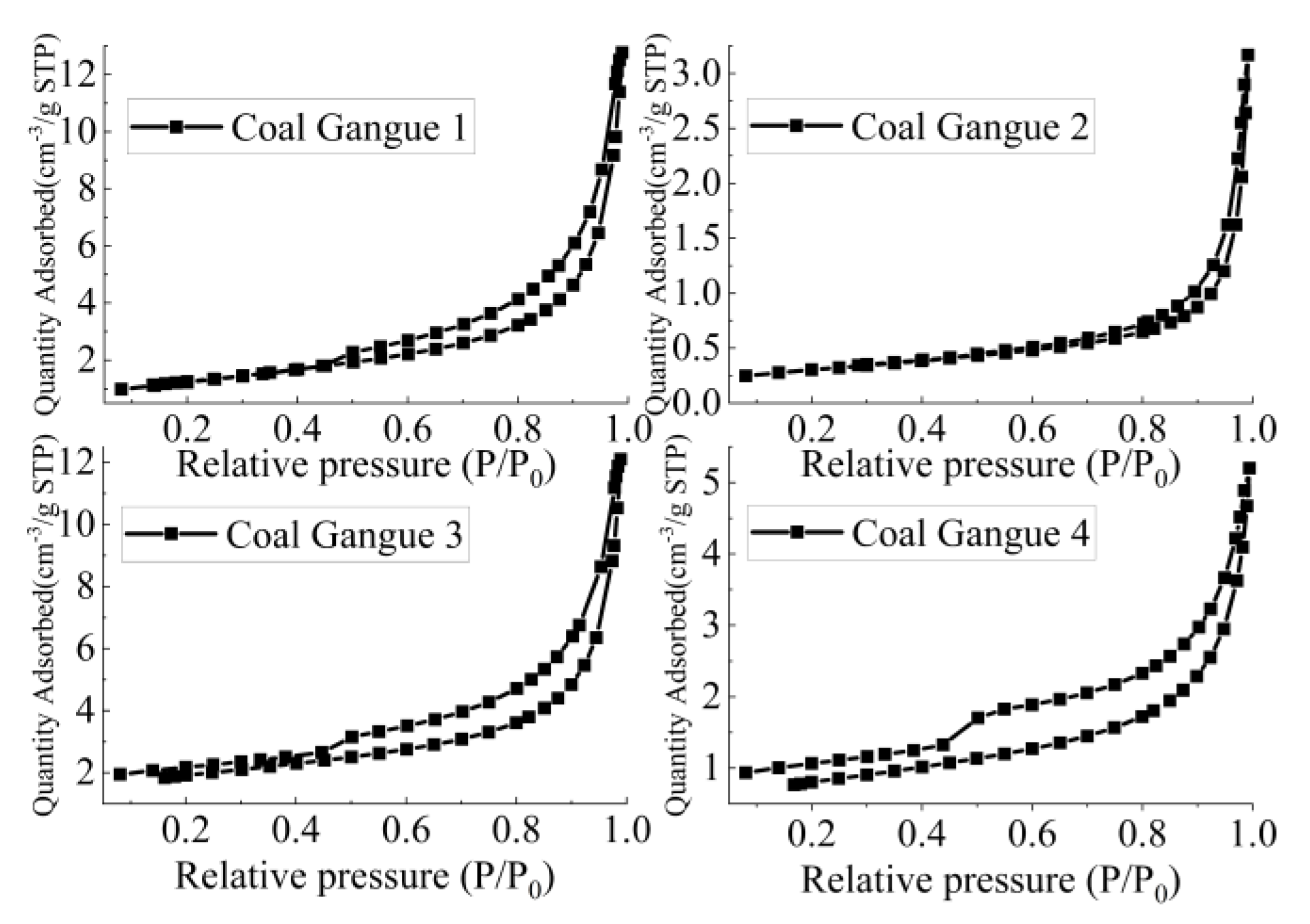
According to Figure 9 and Table 3, the specific surface area of Coal Gangue 1–4 was 3 > 1 > 4 > 2, and pore volume was 1 > 3 > 4 > 2. The specific surface areas of Coal Gangue 1–4 were 4.5486 m2/g, 1.0063 m2/g, 6.1727 m2/g and 2.7403 m2/g, respectively. Coal Gangue 3 had larger specific surface area and pore volume, larger contact area with AMD, and could adsorb and exchange more ions. Therefore, SRB combined with Coal Gangue 3 was the best way to repair AMD.
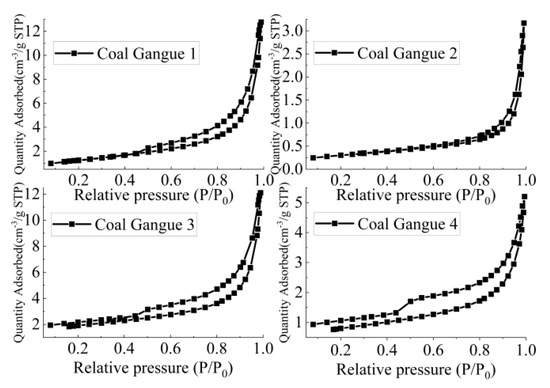
Figure 9.
Nitrogen adsorption desorption isotherm of Coal Gangue 1–4.

Table 3.
Comparison table of specific surface area and pore volume of Coal Gangue 1–4.
4.2. Scanning Electron Microscopy/Energy-Dispersive X-ray Spectroscopy (SEM/EDS) Analysis
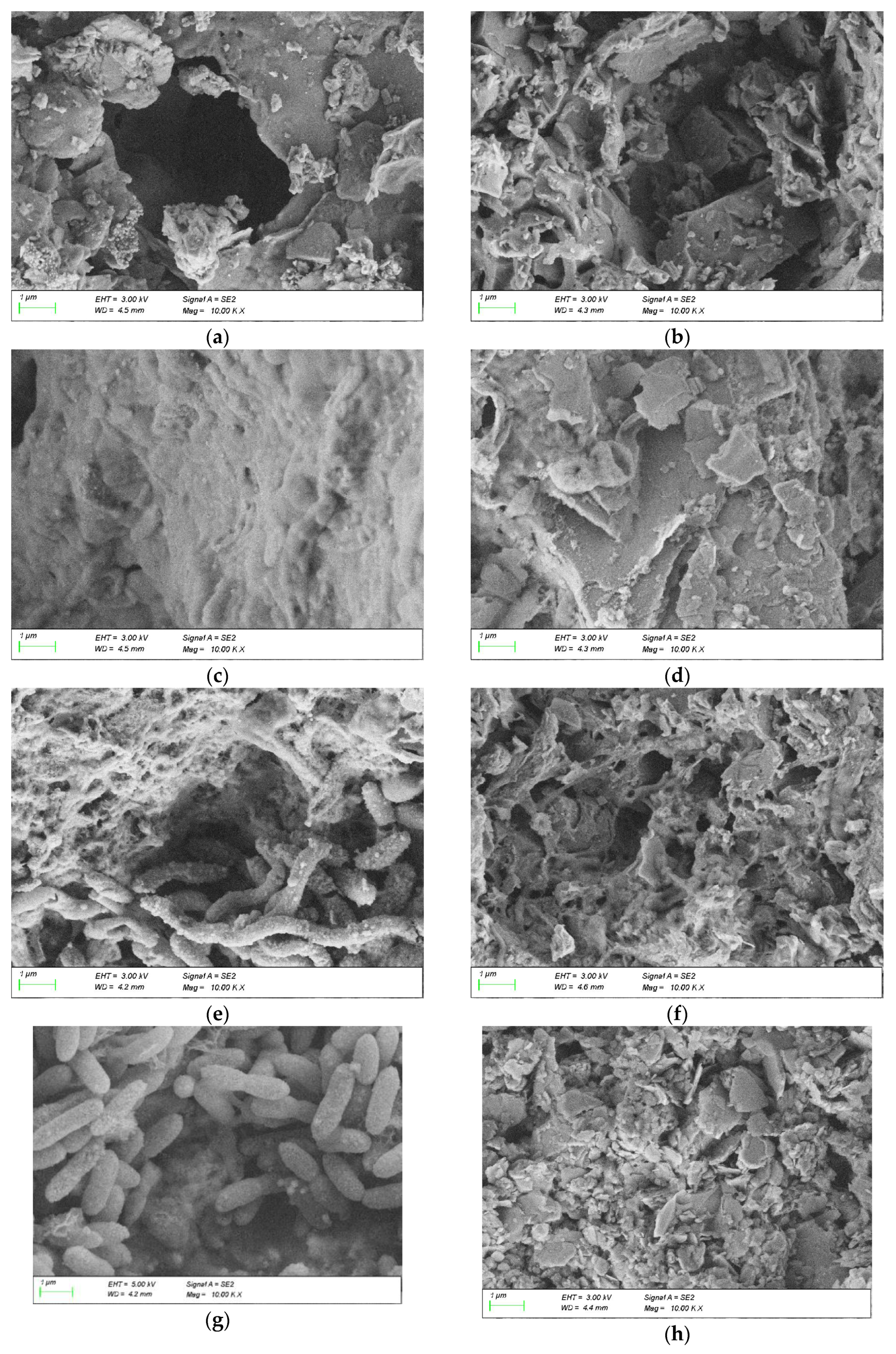
The SEM/EDS diagrams of Coal Gangue 1–4 before and after AMD treatment are shown in Figure 10. Compared with the SEM diagram of the original coal gangue, after AMD treatment, the surface of Coal Gangue 1 became rougher, many small black particles appeared on the brick-red surface, and the microstructure was rod-like structure similar to SRB bacteria. The surface of Coal Gangue 2 became irregular and rough, and there were many small pores. The particles attached to the surface of Coal Gangue 3 increased and presented a rod-shaped structure similar to SRB bacteria. The surface of Coal Gangue 4 became rougher, and a large number of small solid particles appeared. After AMD treatment, the surface of Coal Gangue 1-4 became relatively rough. This showed that SRB adhered to the surface of coal gangue and reacted with minerals on the surface, accelerated the dissolution of substances in coal gangue, changed the surface structure and produced more voids. The SEM image showed that the surface of Coal Gangue 3 was smooth before the experiment. Combined with the low temperature nitrogen adsorption experiment, this showed that there are many internal voids in Gangue 3. Therefore, the processing effect on AMD was better.
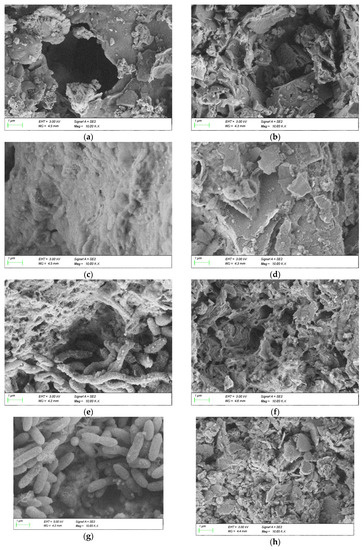
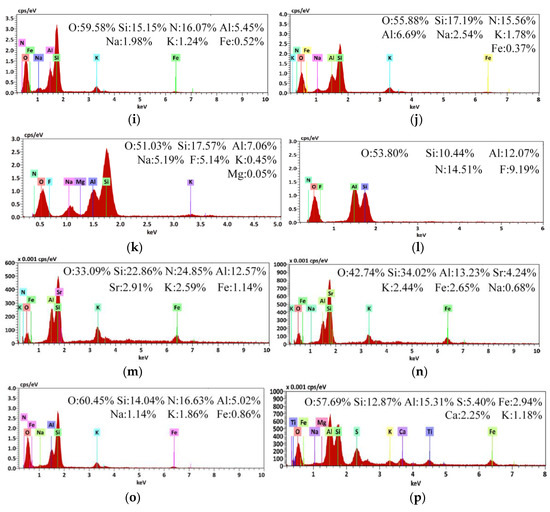
Figure 10.
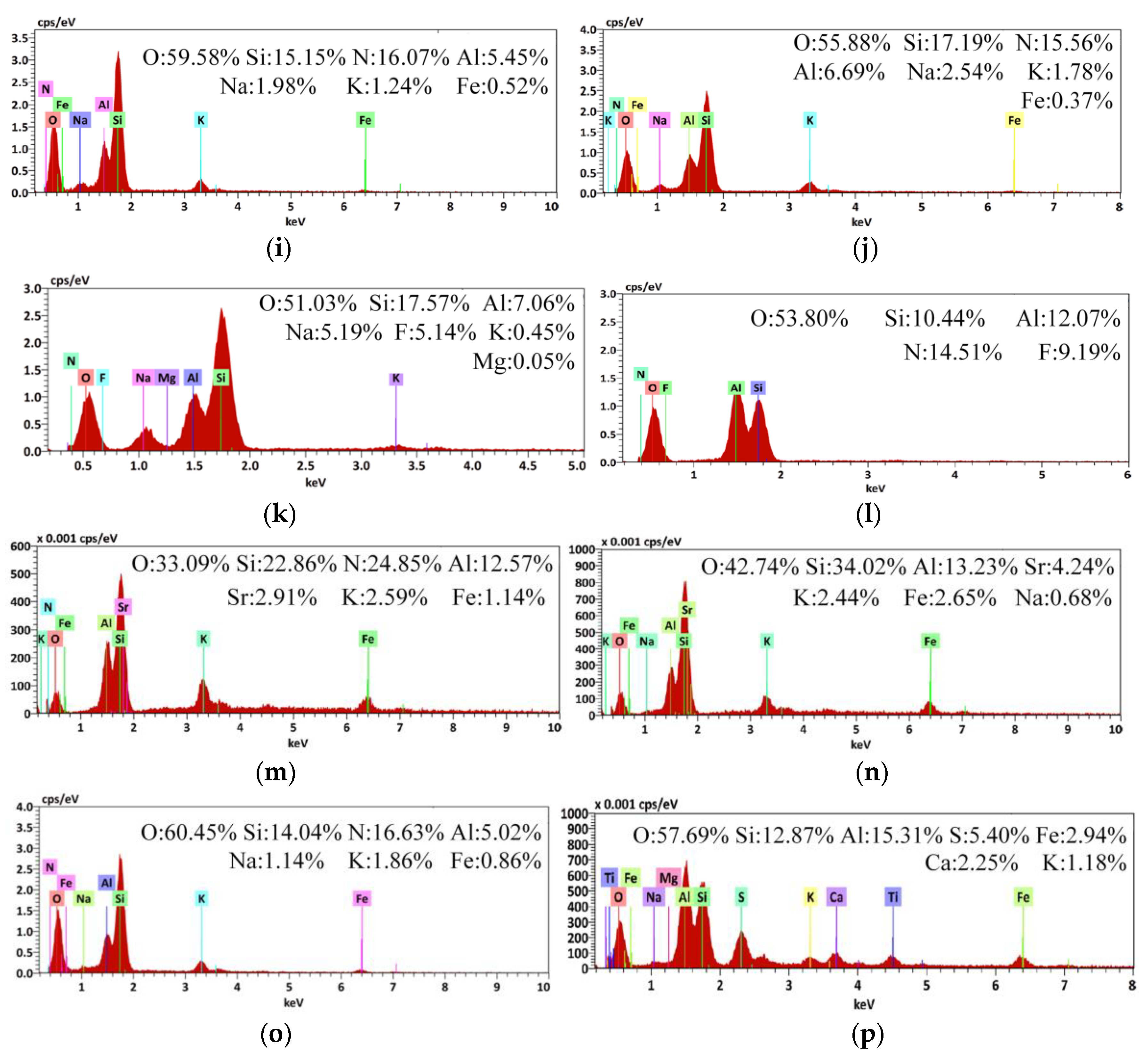
Scanning electron microscopy/energy-dispersive X-ray spectroscopy (SEM/EDS) diagram of Coal Gangue 1–4 before and after AMD repaired by dynamic experiment. (a) SEM diagram of Coal Gangue 1; (b) SEM diagram of Coal Gangue 2; (c) SEM diagram of Coal Gangue 3; (d) SEM diagram of Coal Gangue 4; (e) SEM diagram of Coal Gangue 1 after AMD repaired by dynamic experiment; (f) SEM diagram of Coal Gangue 2 after AMD repaired by dynamic experiment; (g) SEM diagram of Coal Gangue 3 after AMD repaired by dynamic experiment; (h) SEM diagram of Coal Gangue 4 after AMD repaired by dynamic experiment; (i) EDS diagram of Coal Gangue 1; (j) EDS diagram of Coal Gangue 2; (k) EDS diagram of Coal Gangue 3; (l) EDS diagram of Coal Gangue 4; (m) EDS diagram of Coal Gangue 1 after AMD repaired by dynamic experiment; (n) EDS diagram of Coal Gangue 2 after AMD repaired by dynamic experiment; (o) EDS diagram of Coal Gangue 3 after AMD repaired by dynamic experiment; (p) EDS diagram of Coal Gangue 4 after AMD repaired by dynamic experiment.
The EDS diagram showed that Fe content on the surface of Coal Gangue 1–4 increased after repairing AMD. Coal Gangue 1–4 increased from 0.52%, 0.37%, undetected and undetected, to 1.14%, 2.65%, 0.86% and 2.94%, respectively. After the treatment of AMD, a large number of small solid particles appeared on the surface of Coal Gangue 1–4, and the content of Fe on the surface increased significantly, which showed that SRB adsorbed on the surface of coal gangue. SRB transformed SO42− into S2−, and S2− forms metal sulfide solid particles with Fe2+, Mn2+ in wastewater. In addition, the alkaline released from coal gangue could precipitate metal ions in AMD, forming solid precipitation of metal hydroxides, which significantly increased the content of Fe element on the surface of coal gangue.
4.3. X-ray Fluorescence (XRF) Analysis
After the treatment of AMD by SRB combined with Coal Gangue 1–4, the main chemical components of coal gangue are shown in Table 4. By comparing Table 1 and Table 4, the main chemical composition changes of Coal Gangue 1–4 before and after repairing AMD are as follows. Among them, the content of Fe, Mn and S of Coal Gangue 1 in Column 1 decreased. According to the dynamic leaching experiment results of Coal Gangue 1, it can be seen that Fe, Mn, S and other elements in the coal gangue were released into the solution in the form of ions during the experiment, resulting in a decrease of Fe, Mn and S content in Coal Gangue 1 in XRF test results. The Mn content of Coal Gangue 2 in Column 2 decreased while the content of Fe and S increased. According to the dynamic leaching experiment and SEM/EDS test results of Coal Gangue 2, Fe, Mn, S and other elements in the coal gangue were released into the solution, which reduced the content of Fe, Mn and S. However, the SRB attached to the surface of Coal Gangue 2 reduced SO42− to S2−, which combined with Fe2+ to form FeS precipitation attached to the surface of Coal Gangue 2, leading to the increase of Fe and S content in Coal Gangue 2 in XRF test results. The Fe content of Coal Gangue 3 in Column 3 decreased while the content of Mn and S increased. According to the dynamic leaching experiment of Coal Gangue 3, Fe, Mn and S in the coal gangue were released into the solution, which reduced the content of Fe, Mn and S in Coal Gangue 3. According to the SEM/EDS test results, when SRB is combined with coal gangue to repair AMD, S2− generated by SRB metabolism and Fe2+, Mn2+ in AMD form precipitation and adhere to the surface of the coal gangue, increasing the content of Fe, Mn and S on the surface of Coal Gangue 3. Column 3 has a good removal effect on Fe2+ and SO42−, indicating that a large number of FeS particles were formed on the surface of Coal Gangue 3, and a large number of FeS particles gradually converged to form large particles, which were separated from the surface of the coal gangue and precipitated into solution, resulting in a decrease in Fe content of Coal Gangue 3 in XRF test results. The increase of Fe, Mn and S content of Coal Gangue 4 in Column 4 is due to the fact that when SRB is combined with coal gangue to repair AMD, S2− generated by SRB metabolism and Fe2+, Mn2+ in AMD form precipitation and adhere to the surface of the coal gangue. It was reported that there is a lot of carbonate cement in the void of coal gangue, and calcium carbonate and other dissolved materials show weak alkalinity [28]. Therefore, after the treatment of AMD by SRB combined with coal gangue, Ca content in Coal Gangue 1–4 decreased, indicating that calcium carbonate in gangue dissolved and participated in the repairing of AMD.

Table 4.
Main chemical constituents of Coal Gangue 1–4 after AMD repair by dynamic experiment (%).
4.4. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
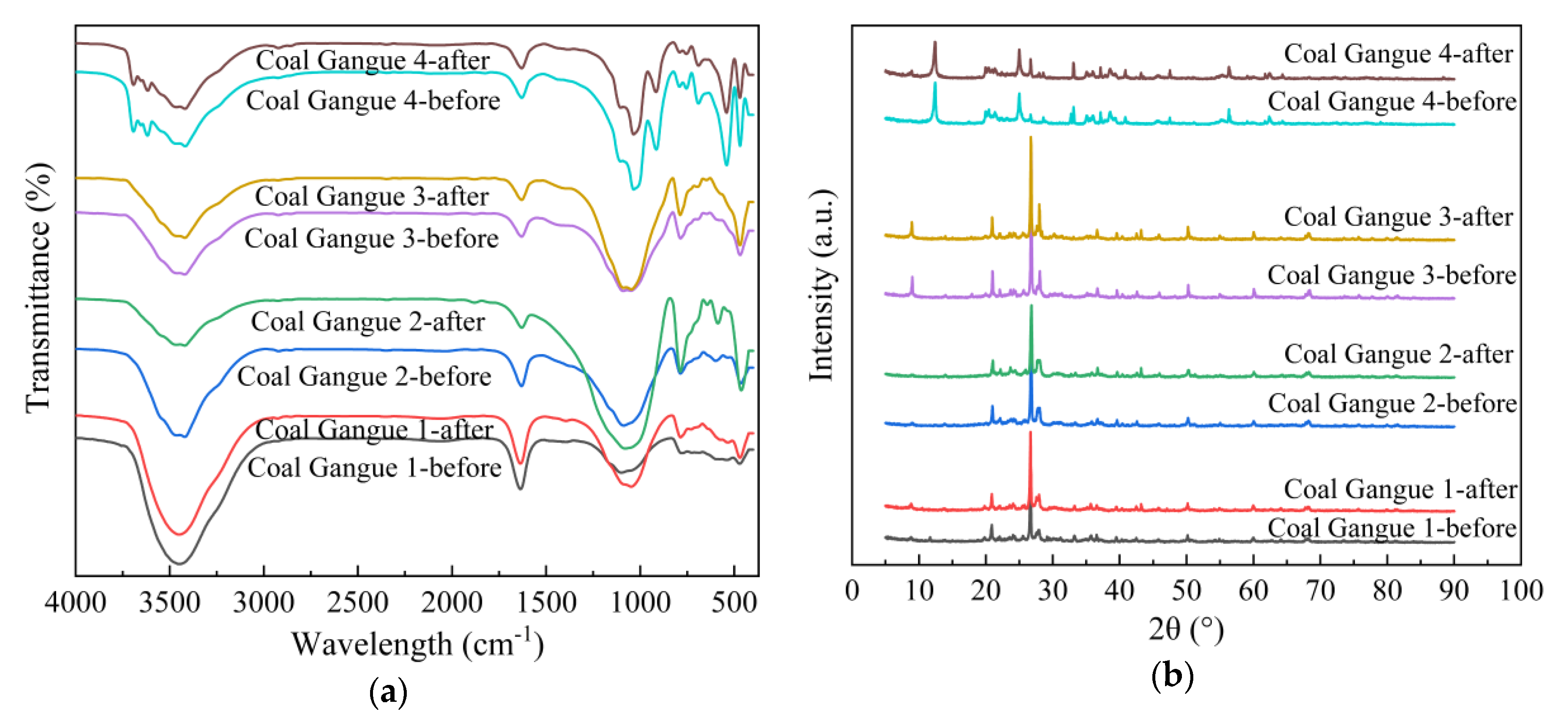
According to Figure 11a, FTIR peaks of Coal Gangue 1–4 were basically the same before and after AMD treatment, indicating that the same coal gangue had the same functional group. The absorption peaks at 1034, 1010, 799 and 466 cm−1 indicated the possible presence of quartz, and 3698, 3650, 1097, 1034, 1010, 799, 687, 533 and 466 cm−1 indicated the possible presence of kaolinite [23]. The absorption peaks at 2920, 2860 and 750–700 cm−1 indicated the presence of organic substances in coal gangue [57]. Si-O-Si tensile vibration peak at 1034 cm−1, 1000 cm−1 and Si-O-Si bending vibration peak at 798, 753, 471 cm−1 were characteristic absorption peaks of illite [58]. The characteristic peak at 432 cm−1 was pyrite, while the peak at 1438 cm−1 belonged to the CO32- stretching vibration peak of carbonate [59].
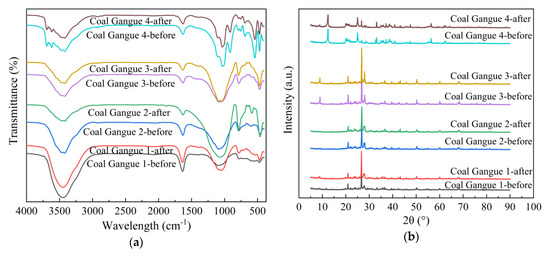
Figure 11.
Fourier transform infrared spectroscopy (FTIR) and X-ray diffraction (XRD) images of Coal Gangue 1–4 before and after AMD repaired by dynamic experiment. (a) FTIR image; (b) XRD image.
4.5. X-ray Diffraction (XRD) Analysis
From Figure 11b, there are differences in the diffraction peaks of Coal Gangue 1–4, indicating that the mineral components contained in the four types of gangue were different. Coal Gangue 1 mainly contained quartz, feldspar, zeolite, kaolin, pyrite, hematite, illite, calcite and other minerals. Coal Gangue 2 mainly contained quartz, feldspar, zeolite, illite, pyrite, calcite and other minerals. Coal Gangue 3 mainly contained quartz, feldspar, zeolite, chlorite, calcite, illite and other minerals. Coal Gangue 4 mainly contained kaolin, quartz, chlorite, sodium feldspar, illite, pyrite, hematite and other minerals. After the dynamic test of SRB combined with coal gangue treating AMD, diffraction peaks of hematite, calcite and kaolinite of Coal Gangue 1 decreased. Diffraction peaks of feldspar, calcite and kaolinite of Coal Gangue 2 decreased. The diffraction peaks of zeolite, calcite and chlorite in Coal Gangue 3 decreased, and the peak shape and peak intensity of feldspar diffraction peak changed. The diffraction peaks of pyrite and chlorite of Coal Gangue 4 decreased. The study of Peng [57] found that the diffraction peaks of calcite, pyrite and kaolinite in coal gangue decreased significantly under acidic conditions. Zeolite is a kind of aluminosilicate mineral of alkali metal or alkaline earth metal. The diffraction peaks of zeolite and calcite in Coal Gangue 3 became weaker, which indicated that zeolite and calcite took part in the reaction and released alkalinity when AMD was repaired. After treating AMD, diffraction peaks of MnS and FeS appeared in Coal Gangue 1–4. The FeS diffraction peaks at 2θ = 43.2° were all enhanced, among which Coal Gangue 3 was the most significant. It was reported that FeS and Fe3S4 are the main iron sulfide stages formed in SRB cultures [60]. The XRD patterns of Coal Gangue 1–4 before and after AMD treatment, indicated that some minerals participated in the ion exchange reaction, resulting in reduced crystallinity of minerals. In addition, the dissolution of alkaline minerals from coal gangue in the AMD environment increased the pH value and promoted the growth of SRB. In particular, Coal Gangue 3 had stronger promotion effect on SRB, with higher MnS and FeS diffraction peaks. The results were consistent with those of the water quality detection index, SEM/EDS, XRF and other analysis results.
5. Conclusions
- (1)
- After 16 days of leaching of Coal Gangue 1–4 under the condition of dynamic leaching capacity of 12 mL/h, the pH value, ORP value, SO42−, COD, Fe2+ and Mn2+ concentration of the leaching solution all tended to be stable. The average values of each index were as follows, Coal Gangue 4 > Coal Gangue 1 > Coal Gangue 2 > Coal Gangue 3. Among them, the average values of each index of Coal Gangue 4 leaching solution were 4.58, 199 mV, 13 mg/L, 341 mg/L, 21.32 mg/L and 2.42 mg/L, respectively.
- (2)
- The repair effect of SRB combined with Coal Gangue 1–4 on AMD was as follows: Column 3 > Column 2 > Column 1 > Column 4. In the second stage of influent, the removal efficiency of AMD by Column 1-4 was better and relatively stable. At this time, the average pH value and ORP value of the effluent of Column 3 were 9.09 and −262.83 mV, the highest removal percentage of COD and SO42− were 84.41% and 72.73%, respectively, the average removal percentage of Fe2+, Mn2+ were 98.70% and 79.97%, respectively. In the first four stages of influent, with the decrease of pH value, the increase of metal ion concentration and the decrease of nutrients in AMD, the growth activity of SRB was gradually inhibited. The alkalinity released by Coal Gangue 3 could effectively reduce the toxicity of AMD to SRB, which could remove AMD efficiently and steadily by SRB combined with Coal Gangue 3.
- (3)
- After introducing of the deionized water in the fifth stage, the indexes of the effluent of Column 1–4 tended to be stable, the pH was weak alkaline, and the average ORP value was stable at 20–34 mV. This system had a good fixation effect on SO42−, Fe2+ and Mn2+ ions in mine acid wastewater, and no secondary release of pollutants appeared.
- (4)
- Low temperature nitrogen adsorption, SEM/EDS, XRF, FTIR and XRD analysis showed that Coal Gangue 3 had the largest specific surface area and could adsorb and exchange more metal ions than the other three kinds of gangue. Coal Gangue 3 contained more alkaline minerals such as zeolite and calcite, which could not only precipitate metal ions in AMD, but also reduce the toxicity of AMD to SRB and ensure the normal growth of SRB. The SRB was attached to the surface of the coal gangue, and the SO42− in the AMD was converted into S2− by dissimilation. The S2− formed metal sulfide solid particle with the metal ions in the wastewater and adhered to the surface of the coal gangue. As a result, SRB combined with Coal Gangue 3 repaired AMD with the best result, and had the strongest anti-pollution load capacity.
Author Contributions
Y.D., J.D., Z.Y., Y.Z., X.W., X.G., Z.L. and G.J. are the main authors of this work. Conceptualization, Y.D. and J.D.; methodology, Y.D.; formal analysis, Y.D.; investigation Z.Y.; writing—original draft preparation, Y.D., J.D., Z.Y., Y.Z., X.W., X.G., Z.L. and G.J.; writing—review and editing, Y.D., J.D., Z.Y., Y.Z., X.W., X.G., Z.L. and G.J.; project administration, J.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (41672247, 41102157, 51304114), Liaoning Province’s “Program for Promoting Liaoning Talents”(XLYC1807159).
Acknowledgments
The author(s) would like to thank all editors and anonymous reviewers for their comments and suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fan, C.; Li, S.; Elsworth, D.; Han, J.; Yang, Z. Experimental investigation on dynamic strength and energy dissipation characteristics of gas outburst–prone coal. Energy Sci. Eng. 2019. [Google Scholar] [CrossRef]
- Hwang, S.K.; Jho, E.H. Heavy metal and sulfate removal from sulfate-rich synthetic mine drainages using sulfate reducing bacteria. Sci. Total Environ. 2018, 635, 1308–1316. [Google Scholar] [CrossRef]
- Giachini, A.J.; Sulzbach, T.S.; Pinto, A.L.; Armas, R.D.; Cortez, D.H.; Silva, E.P.; Buzanello, E.B.; Soares, A.G.; Soares, C.; Rossi, M.J. Microbially-enriched poultry litter-derived biochar for the treatment of acid mine drainage. Arch. Microbiol. 2018, 200, 1227–1237. [Google Scholar] [CrossRef]
- Masindi, V.; Chatzisymeon, E.; Kortidis, I.; Foteinis, S. Assessing the sustainability of acid mine drainage (AMD) treatment in South Africa. Sci. Total Environ. 2018, 635, 793–802. [Google Scholar] [CrossRef]
- Masindi, V.; Akinwekomi, V.; Maree, J.P.; Muedi, K.L. Comparison of mine water neutralisation efficiencies of different alkaline generating agents. J. Environ. Chem. Eng. 2017, 5, 3903–3913. [Google Scholar] [CrossRef]
- Crafton, E.; Pritchard, C.; Guo, L.; Senko, J.M.; Cutright, T.J. Dynamics of Mn removal in an acid mine drainage treatment system over 13 years after installation. Environ. Earth Sci. 2019, 78, 10. [Google Scholar] [CrossRef]
- Qureshi, A.; Jia, Y.; Maurice, C.; Ohlander, B. Potential of fly ash for neutralisation of acid mine drainage. Environ. Sci. Pollut. Res. Int. 2016, 23, 17083–17094. [Google Scholar] [CrossRef] [PubMed]
- Nordstrom, D.K.; Blowes, D.W.; Ptacek, C.J. Hydrogeochemistry and microbiology of mine drainage: An update. Appl. Geochem. 2015, 57, 3–16. [Google Scholar] [CrossRef]
- Wang, B.; Wu, D.; Ekama, G.A.; Tsui, T.H.; Jiang, F.; Chen, G.H. Characterization of a new continuous gas-mixing sulfidogenic anaerobic bioreactor: Hydrodynamics and sludge granulation. Water Res. 2018, 135, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Niu, Q.; Li, L.; Hu, Y.; Mribet, C.; Hojo, T.; Li, Y.Y. A gradual change between methanogenesis and sulfidogenesis during a long-term UASB treatment of sulfate-rich chemical wastewater. Sci. Total Environ. 2018, 636, 168–176. [Google Scholar] [CrossRef]
- Li, X.; Lan, S.; Zhu, Z.; Zhang, C.; Zeng, G.; Liu, Y.; Cao, W.; Song, B.; Yang, H.; Wang, S.; et al. The bioenergetics mechanisms and applications of sulfate-reducing bacteria in remediation of pollutants in drainage: A review. Ecotoxicol. Environ. Saf. 2018, 158, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Heidelberg, J.F.; Seshadri, R.; Haveman, S.A.; Hemme, C.L.; Paulsen, I.T.; Kolonay, J.F.; Eisen, J.A.; Ward, N.; Methe, B.; Brinkac, L.M. The genome sequence of the anaerobic, sulfate-reducing bacterium Desulfovibrio vulgaris Hildenborough. Nat. Biotechnol. 2004, 22, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Odom, J.M.; Peck, H.D. Hydrogen cycling as a general mechanism for energy coupling in the sulfate-reducing bacteria, Desulfovibrio sp. FEMS Microbiol. Lett. 1981, 12, 47–50. [Google Scholar] [CrossRef]
- Pieulle, L.; Magro, V.; Hatchikian, E.C. Isolation and analysis of the gene encoding the pyruvate-ferredoxin oxidoreductase of Desulfovibrio africanus, production of the recombinant enzyme in Escherichia coli, and effect of carboxy-terminal deletions on its stability. J. Bacteriol. 1997, 179, 5684–5692. [Google Scholar] [CrossRef]
- Voordouw, G. Carbon monoxide cycling by Desulfovibrio vulgaris Hildenborough. J. Bacteriol. 2002, 184, 5903–5911. [Google Scholar] [CrossRef]
- Aubert, C.; Brugna, M.; Dolla, A.; Bruschi, M.; Giudiciorticoni, M.T. A sequential electron transfer from hydrogenases to cytochromes in sulfate-reducing bacteria. Biochim. Biophys. Acta 2000, 1476, 85–92. [Google Scholar] [CrossRef]
- Keller, K.L.; Wall, J.D. Genetics and molecular biology of the electron flow for sulfate respiration in Desulfovibrio. Front. Microbiol. 2011, 2, 135. [Google Scholar] [CrossRef]
- Broco, M.; Rousset, M.; Oliveira, S.; Rodrigues-Pousada, C. Deletion of flavoredoxin gene in Desulfovibrio gigas reveals its participation in thiosulfate reduction. FEBS Lett. 2005, 579, 4803–4807. [Google Scholar] [CrossRef]
- Qian, T.; Li, J. Synthesis of Na-A zeolite from coal gangue with the in-situ crystallization technique. Adv. Powder Technol. 2015, 26, 98–104. [Google Scholar] [CrossRef]
- Wu, H.; Wen, Q.; Hu, L.; Gong, M.; Tang, Z. Feasibility study on the application of coal gangue as landfill liner material. Waste Manag. 2017, 63, 161–171. [Google Scholar] [CrossRef]
- Liu, X.; Yang, X.; Du, H.; Wu, Y.; Zhang, X.; Zhang, J. Preparation and characterization of a porous silicate material using a CO2-storage material for CO2 adsorption. Powder Technol. 2018, 333, 138–152. [Google Scholar] [CrossRef]
- Jabłońska, B.; Kityk, A.V.; Busch, M.; Huber, P. The structural and surface properties of natural and modified coal gangue. J. Environ. Manag. 2017, 190, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Qiu, R.; Cheng, F. Modification of waste coal gangue and its application in the removal of Mn2+ from aqueous solution. Water Sci. Technol. 2016, 74, 524–534. [Google Scholar] [CrossRef]
- Guo, X.; Yanrong, D.; Junzhen, D.; Ying, L.; Yang, D. Experimental treatment of acid mine drainage by the synergistic reaction with SRB and spontaneous combustion coal gangue. Non-Met. Min. 2016, 39, 28–31. [Google Scholar]
- Dong, Y.; Di, J.; Wang, X.; Xue, L.; Yang, Z.; Guo, X.; Li, M. Dynamic experimental study on treatment of acid mine drainage by bacteria supported in natural minerals. Energies 2020, 13, 439. [Google Scholar] [CrossRef]
- Dong, Y.; Di, J.; Wang, M.; Ren, Y. Experimental study on the treatment of acid mine drainage by modified corncob fixed SRB sludge particles. RSC Adv. 2019, 9, 19016–19030. [Google Scholar] [CrossRef]
- The State Environmental Protection Administration. Water and Wastewater Monitoring and Analysis Method, 4th ed.; China Environmental Science Press: Beijing, China, 2002.
- Wang, X.; Wang, Y.; Chu, Z.; Lu, X.; Chen, G.; Zha, F.; Cui, H.; Cheng, Y.; Zhang, R. Effects of coal gangue addition on the chemical fraction and bioavailability of heavy metals (Zn, Pb, Cd, Cr and Cu) in copper mine tailings. J. China Coal Soc. 2017, 42, 2688–2697. [Google Scholar]
- Ran, Z.; Liu, W.; Pan, Y.; Liu, W.; Gao, Z.; Zhao, Y. Influence of temperature on dynamic leaching characteristics of coal gangue. J. China Coal Soc. 2019, 44, 1239–1246. [Google Scholar]
- Sun, H.; Sheng, S.; Yang, Z.; Liu, H.; Jiao, X.; Wan, A. Analysis of Black-odor Factors of Lake Sediment and Elimination Strategies of Key Factor. Environ. Sci. Surv. 2019, 38, 12–20. [Google Scholar]
- Hao, T.; Xiang, P.; Mackey, H.R.; Chi, K.; Lu, H.; Chui, H.; van Loosdrecht, M.C.M.; Chen, G. A review of biological sulfate conversions in wastewater treatment. Water Res. 2014, 65, 1–21. [Google Scholar] [CrossRef]
- Hwang, T.; Neculita, C.M.; Han, J.I. Biosulfides precipitation in weathered tailings amended with food waste-based compost and zeolite. J. Environ. Qual. 2012, 41, 1857–1864. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, R.D.; Ropelewski, L.; Acheson, M. Sewage as a mixed organic substrate for desulfurization bacteria. Proc. Water Environ. Fed. 2012, 2012, 265–274. [Google Scholar] [CrossRef]
- Chang, Y.; Chang, Y.; Hung, C.; Lee, J.; Liao, H.; Chou, H. Microbial community analysis of anaerobic bio-corrosion in different ORP profiles. Int. Biodeterior. Biodegrad. 2014, 95, 93–101. [Google Scholar] [CrossRef]
- Wan, H.; Su, S.; Zhu, J.; Wan, X.; Ge, C. Study on the factors affecting growth of the sulphate-reducing bacteria and its biological desulfurization capability. J. Chem. Eng. Chin. Univ. 2004, 18, 218–223. [Google Scholar]
- Wang, G.; Zhang, R.; Fu, J.; Lu, Y.; Su, Y. Research on the constructed wetland modified substrates for the advanced treatment of sewage. Ind. Water Treat. 2016, 36, 73–78. [Google Scholar]
- Liu, Z.; Li, L.; Li, Z.; Tian, X. Removal of sulfate and heavy metals by sulfate reducing bacteria in an expanded granular sludge bed reactor. Environ. Technol. 2017, 39, 1814–1822. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, T.; Erdirencelebi, D.; Berktay, A. Effect of COD/SO42- ratio on anaerobic treatment of landfill leachate during the start-up period. Environ. Technol. 2012, 33, 313–320. [Google Scholar] [CrossRef]
- Bijmans, M.F.; Dopson, M.; Peeters, T.W.; Lens, P.N.; Buisman, C.J. Sulfate reduction at pH 5 in a high-rate membrane bioreactor: Reactor performance and microbial community analyses. J. Microbiol. Biotechnol. 2009, 19, 698–708. [Google Scholar] [CrossRef]
- Kushkevych, I.; Dordevic, D.; Vitezova, M. Toxicity of hydrogen sulfide toward sulfate-reducing bacteria Desulfovibrio piger Vib-7. Arch. Microbiol. 2019, 201, 389–397. [Google Scholar] [CrossRef]
- Barbosa, L.P.; Costa, P.F.; Bertolino, S.M.; Silva, J.C.C.; Guerra-Sá, R.; Leão, V.A.; Teixeira, M.C. Nickel, manganese and copper removal by a mixed consortium of sulfate reducing bacteria at a high COD/sulfate ratio. World J. Microbiol. Biotechnol. 2014, 30, 2171–2180. [Google Scholar] [CrossRef]
- Vossoughi, M.; Shakeri, M.; Alemzadeh, I. Performance of anaerobic baffled reactor treating synthetic wastewater influenced by decreasing COD/SO4 ratios. Chem. Eng. Process. Process Intensif. 2003, 42, 811–816. [Google Scholar] [CrossRef]
- Sabumon, P.C. Development of enhanced sulphidogenesis process for the treatment of wastewater having low COD/SO42- ratio. J. Hazard. Mater. 2008, 159, 616–625. [Google Scholar] [CrossRef]
- Lu, X.; Zhen, G.; Ni, J.; Hojo, T.; Kubota, K.; Li, Y.Y. Effect of influent COD/SO42- ratios on biodegradation behaviors of starch wastewater in an upflow anaerobic sludge blanket (UASB) reactor. Bioresour. Technol. 2016, 214, 175–183. [Google Scholar] [CrossRef]
- Li, D.; Chen, H. Study development of wastewater adsorbent prepared by coal gangue. J. Luoyang Inst. Sci. Technol. (Nat. Sci. Ed.) 2012, 22, 6–9. [Google Scholar]
- Miao, Z.; He, H.; Tan, T.; Zhang, T.; Tang, J.; Yang, Y.; Shi, K.; Tang, J. Biotreatment of Mn2+ and Pb2+ with Sulfate-Reducing Bacterium Desulfuromonas alkenivorans S-7. J. Environ. Eng. 2018, 144, 1–7. [Google Scholar] [CrossRef]
- Yue, Z.; Li, Q.; Li, C.; Chen, T.; Wang, J. Component analysis and heavy metal adsorption ability of extracellular polymeric substances (EPS) from sulfate reducing bacteria. Bioresour. Technol. 2015, 194, 399–402. [Google Scholar] [CrossRef]
- Comte, S.; Guibaud, G.; Baudu, M. Biosorption properties of extracellular polymeric substances (EPS) towards Cd, Cu and Pb for different pH values. J. Hazard. Mater. 2008, 151, 185–193. [Google Scholar] [CrossRef]
- Li, W.W.; Yu, H.Q. Insight into the roles of microbial extracellular polymer substances in metal biosorption. Bioresour. Technol. 2014, 160, 15–23. [Google Scholar] [CrossRef]
- Wang, L.; Chen, T.; Chang, D.; Liu, H.; Chen, D. Cd2+ adsorption from aqueous solutions by desulfurization products of manganese nodule leached residue. Acta Petrol. Mineral. 2011, 30, 1001–1006. [Google Scholar]
- Jia, J.; Lan, B.; Xie, X.; Wu, D.; Tang, Y.; Wang, J. Relationship between solubility of sulfides and solution pH value. J. Jilin Univ. (Earth Sci. Ed.) 2001, 31, 241–246. [Google Scholar]
- Guo, X.; Fan, G.; Di, J.; Dong, Y. Research on treatment of acid mine drainage containing Fe2+ and Mn2+ by modified spontaneous combustion coal gangue. Non-Met. Min. 2015, 38, 67–70. [Google Scholar]
- Zhang, M.; Wang, H. Preparation of immobilized sulfate reducing bacteria (SRB) granules for effective bioremediation of acid mine drainage and bacterial community analysis. Min. Eng. 2016, 92, 63–71. [Google Scholar] [CrossRef]
- Sahinkaya, E.; Gunes, F.M.; Ucar, D.; Kaksonen, A.H. Sulfidogenic fluidized bed treatment of real acid mine drainage water. Bioresour. Technol. 2011, 102, 683–689. [Google Scholar] [CrossRef]
- Sahinkaya, E.; Yucesoy, Z. Biotreatment of acidic zinc- and copper-containing wastewater using ethanol-fed sulfidogenic anaerobic baffled reactor. Bioprocess Biosyst. Eng. 2010, 33, 989–997. [Google Scholar] [CrossRef]
- Bai, H.; Kang, Y.; Quan, H.; Han, Y.; Sun, J.; Feng, Y. Treatment of acid mine drainage by sulfate reducing bacteria with iron in bench scale runs. Bioresour. Technol. 2013, 128, 818–822. [Google Scholar] [CrossRef]
- Peng, B.; Li, X.; Zhao, W.; Yang, L. Study on the release characteristics of chlorine in coal gangue under leaching conditions of different pH values. Fuel 2018, 217, 427–433. [Google Scholar] [CrossRef]
- Orrego-Ruiz, J.A.; Cabanzo, R.; Mejía-Ospino, E. Study of Colombian coals using photoacoustic Fourier transform infrared spectroscopy. Int. J. Coal Geol. 2011, 85, 307–310. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, G.; Yan, Z.; Fang, T.; Wang, R. Transformation behavior of mineral composition and trace elements during coal gangue combustion. Fuel 2012, 97, 644–650. [Google Scholar] [CrossRef]
- Gramp, J.P.; Bigham, J.M.; Sasaki, K.; Tuovinen, O.H. Formation of Ni- and Zn-sulfides in cultures of sulfate-reducing bacteria. Geomicrobiol. J. 2007, 24, 609–614. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).