PtPd Hybrid Composite Catalysts as Cathodes for Proton Exchange Membrane Fuel Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of PtPd Nanoparticles (NPs) Supported on Carbonaceous Nanomaterials
2.2. Physical Mixing of Carbonaceous Supports for the Synthesis of PtPd/(rGO:MWCNT) Electrocatalysts
2.3. Physical Mixing of Electrocatalysts to Obtain (PtPd/rGO) + (PtPd/MWCNT)
2.4. Physicochemical and Electrochemical Characterization
2.5. PEMFC Performance Test
3. Results and Discussion
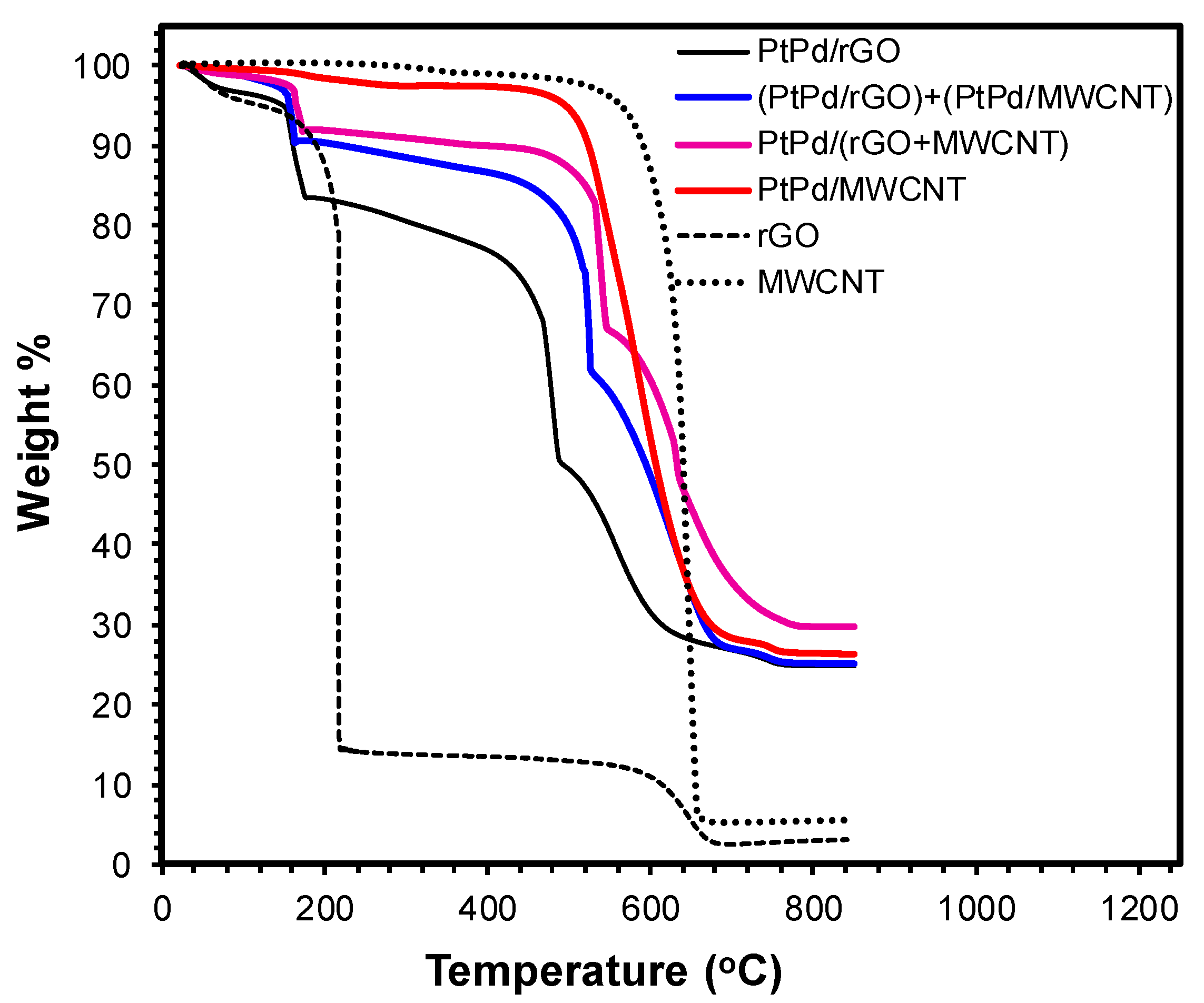
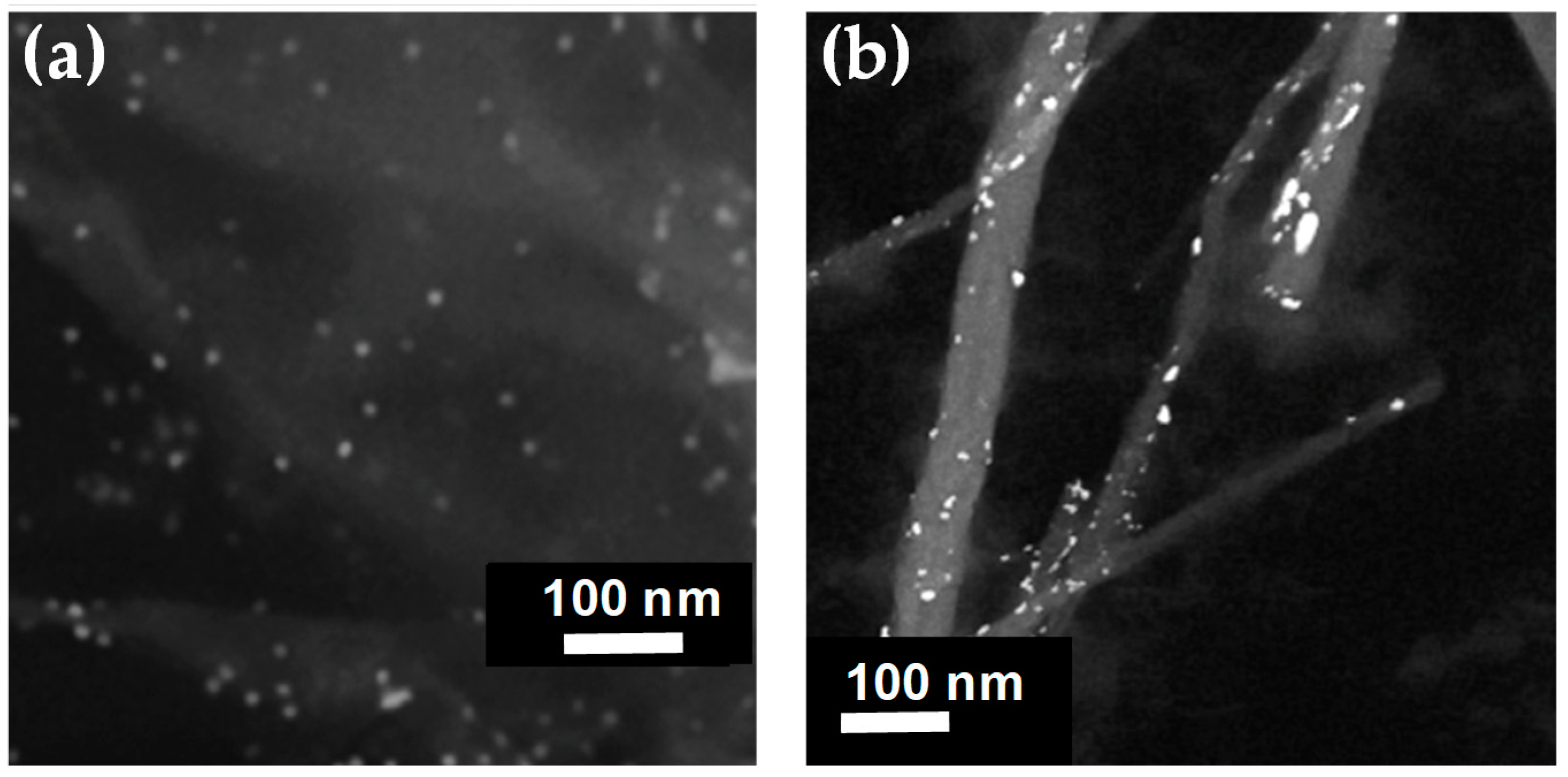
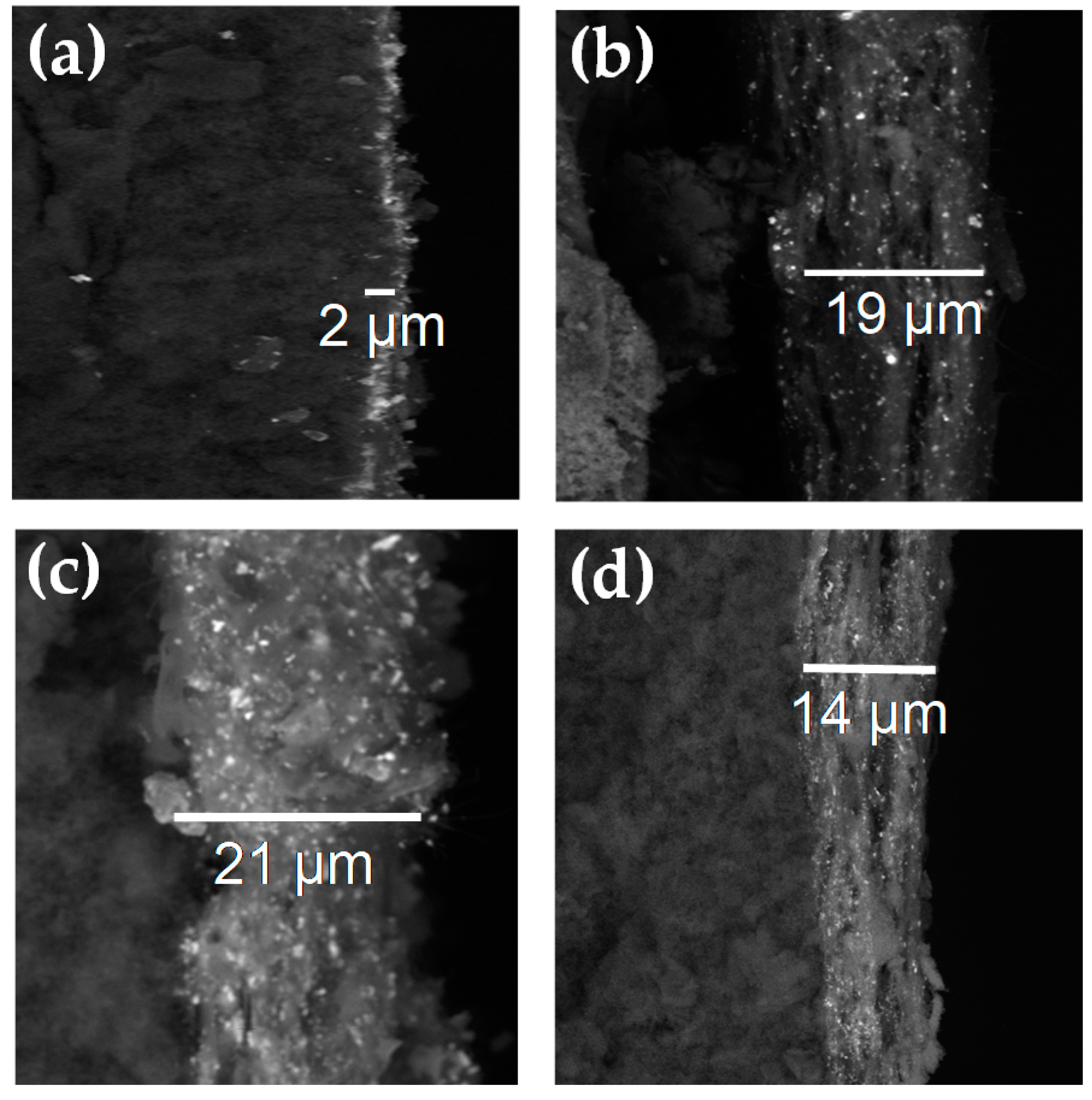
3.1. Physicochemical Characterization
3.2. Electrochemical Characterization
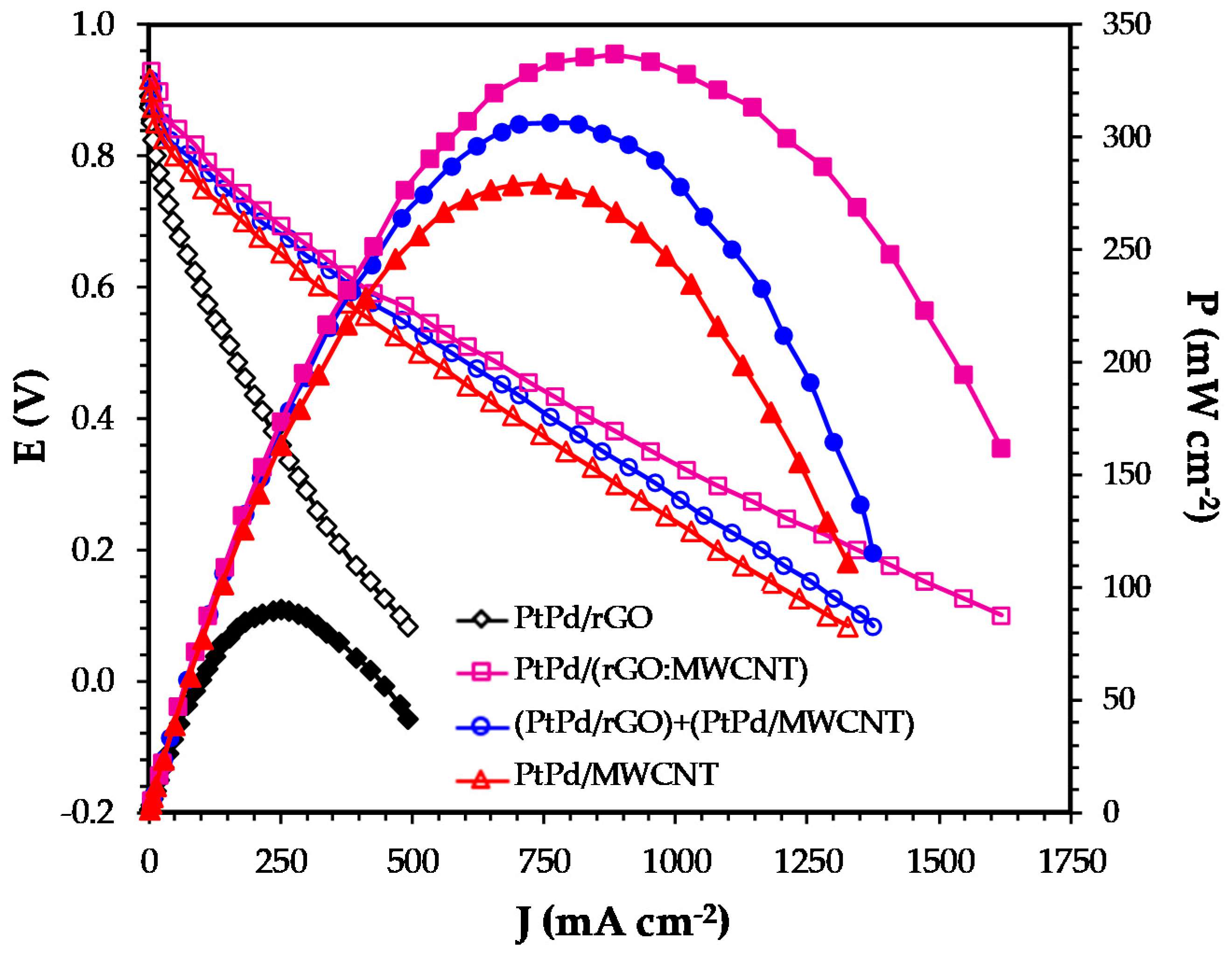
3.3. PEMFC Test
3.4. Accelerated Durability Tests (ADT)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zach, F.; Kretschmer, F.; Stoeglehner, G. Integrating energy demand and local renewable energy sources in smart urban development zones: New options for climate-friendly resilient urban planning. Energies 2019, 12, 3672. [Google Scholar] [CrossRef]
- Armeanu, D.Ş.; Gherghina, Ş.C.; Pasmangiu, G. Exploring the causal nexus between energy consumption environmental pollution and economic growth: Empirical evidence from central and eastern Europe. Energies 2019, 12, 3704. [Google Scholar] [CrossRef]
- Chub, A.; Vinnikov, D.; Stepenko, S.; Liivik, E.; Blaabjerg, F. Photovoltaic energy yield improvement in two-stage solar microinverters. Energies 2019, 12, 3774. [Google Scholar] [CrossRef]
- Jiménez-Buendía, F.; Villena-Ruiz, R.; Honrubia-Escribano, A.; Molina-García, Á.; Gómez-Lázaro, E. Submission of a WECC DFIG wind turbine model to Spanish operation procedure 12.3. Energies 2019, 12, 3749. [Google Scholar] [CrossRef]
- Jidin, R.; Othman, A.B. A computed river flow-based turbine controller on a programmable logic controller for run-off river hydroelectric systems. Energies 2017, 10, 1717. [Google Scholar] [CrossRef]
- Cadelano, G.; Cicolin, F.; Emmi, G.; Mezzasalma, G.; Poletto, D.; Galgaro, A.; Bernardi, A. Improving the energy efficiency, limiting costs and reducing CO2 Emissions of a museum using geothermal energy and energy management policies. Energies 2019, 12, 3192. [Google Scholar] [CrossRef]
- Yahhoub, M.; Jamalabadi, A.; Ghasemi, M.; Alamian, R.; Afshari, E.; Wongwises, S.; Rashidi, M.M.; Shadloo, M.S. A 3D simulation of single-channel high-temperature polymer exchange membrane fuel cell performances. Appl. Sci. 2019, 9, 3633. [Google Scholar] [CrossRef]
- Rivera-Lugo, Y.Y.; Salazar-Gastélum, M.I.; López-Rosas, D.M.; Reynoso-Soto, E.A.; Pérez-Sicairos, S.; Velraj, S.; Flores-Hernández, J.R. Effect of template, reaction time and platinum concentration in the synthesis of PtCu/CNT catalyst for PEMFC applications. Energy 2018, 148, 561–570. [Google Scholar] [CrossRef]
- Norskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Beltrán-Gastélum, M.; Salazar-Gastélum, M.I.; Flores-Hernández, J.R.; Botte, G.G.; Pérez-Sicairos, S.; Romero-Castañon, T.; Reynoso-Soto, E.A.; Félix-Navarro, R.M. Pt-Au nanoparticles on graphene for oxygen reduction reaction: Stability and performance on proton exchange membrane fuel cell. Energy 2019, 181, 1225–1234. [Google Scholar] [CrossRef]
- Todoroki, N.; Watanabe, H.; Kondo, T.; Kaneko, S.; Wadayama, T. Highly enhanced oxygen reduction reaction activity and electrochemical stability of Pt/Ir(111) bimetallic surfaces. Electrochim. Acta 2016, 222, 1616–1621. [Google Scholar] [CrossRef]
- Jackson, A.; Strickler, A.; Higgins, D.; Jaramillo, T.F. Engineering Ru@Pt core-shell catalysts for enhanced electrochemical oxygen reduction mass activity and stability. Nanomaterials 2018, 8, 38. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, T.; Guo, W. Surfactant-free synthesis of reduced graphene oxide supported well-defined polyhedral Pd-Pt nanocrystals for oxygen reduction reaction. Catalysts 2019, 9, 756. [Google Scholar] [CrossRef]
- Cai, Z.-X.; Liu, C.-C.; Wu, G.-H.; Chen, X.-M.; Chen, X. Green synthesis of Pt-on-Pd bimetallic nanodendrites on graphene via in situ reduction, and their enhanced electrocatalytic activity for methanol oxidation. Electrochim. Acta 2014, 127, 377–383. [Google Scholar] [CrossRef]
- Mirzaei, F.; Parnian, M.J.; Rowshanzamir, S. Durability investigation and performance study of hydrothermal synthesized platinum-multi walled carbon nanotube nanocomposite catalyst for proton exchange membrane fuel cell. Energy 2017, 138, 696–705. [Google Scholar] [CrossRef]
- Matsuda, N.; Nakashima, T.; Kato, T.; Shiroishi, H. Synthesis of multiwall carbon nanotube-supported platinum catalysts by solution plasma processing for oxygen reduction in polymer electrolyte fuel cells. Electrochim. Acta 2014, 146, 73–78. [Google Scholar] [CrossRef]
- Fortunato, G.; de Lima, F.; Maia, G. Oxygen-reduction reaction strongly electrocatalyzed by Pt electrodeposited onto graphene or graphene nanoribbons. J. Power Sources 2016, 302, 247–258. [Google Scholar] [CrossRef]
- Pothaya, S.; Regalbuto, J.R.; Monnier, J.R.; Punyawudho, K. Preparation of Pt/graphene catalysts for polymer electrolyte membran fuel cells by strong electrostatic adsorption technique. Int. J. Hydrogen Energy 2019, 44, 26361–26372. [Google Scholar] [CrossRef]
- Calderón, J.C.; Ndzuzo, L.; Bladergroen, B.J.; Pasupathi, S. Catalytic activity of carbon supported-Pt-Pd nanoparticles toward the oxygen reduction reaction. Mater. Today Proc. 2018, 5, 10551–10560. [Google Scholar] [CrossRef]
- Fu, K.; Wang, Y.; Mao, L.; Yang, X.; Jin, J.; Yang, S.; Li, G. Facile morphology controllable synthesis of PtPd nanorods on graphene-multiwalled carbon nanotube hybrid support as efficient electrocatalysts for oxygen reduction reaction. Mater. Res. Bull. 2018, 108, 187–194. [Google Scholar] [CrossRef]
- Yang, H.N.; Lee, D.C.; Park, K.W.; Kim, W.J. Platinum-boron doped graphene intercalated by carbon black for cathode catalyst in proton exchange membrane fuel cell. Energy 2015, 89, 500–510. [Google Scholar] [CrossRef]
- Sanli, L.I.; Yarar, B.; Bayram, V.; Gürsel, S.A. Electrosprayed catalyst layers based on Graphene-carbon black hybrids for the next-generation fuel cell electrodes. J. Mater. Sci. 2017, 52, 2091–2102. [Google Scholar] [CrossRef]
- Yang, H.N.; Ko, Y.D.; Kim, W.J. 3-D structured Pt/rGO-polyethyleneimine-functionalized MWCNT for proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2018, 43, 4439–4447. [Google Scholar] [CrossRef]
- Reyes-Cruzaley, A.P.; Félix-Navarro, R.M.; Trujillo-Navarrete, B.; Silva-Carrillo, C.; Zapata-Fernández, J.R.; Romo-Herrera, J.M.; Contreras, O.E.; Reynoso-Soto, E.A. Synthesis of novel Pd NP-PTH-CNTs hybrid materials as catalyst for H2O2 generation. Electrochim. Acta 2019, 296, 575–581. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Ratso, S.; Kruusenberg, I.; Joost, U.; Saar, R.; Tammeveski, K. Enhanced oxygen reduction reaction activity of nitrogen-doped graphene/multi-walled carbon nanotube catalysts in alkaline media. Int. J. Hydrogen Energy 2016, 41, 22510–22519. [Google Scholar] [CrossRef]
- Zhang, J.; Song, X.; Li, P.; Wu, Z.; Wu, Y.; Wang, S.; Liu, X. Sulfur, nitrogen co-doped nanocomposite of graphene and carbon nanotube as an efficient bifunctional electrocatalyst for oxygen reduction and evolution reactions. J. Taiwan Inst. Chem. Eng. 2018, 93, 336–341. [Google Scholar] [CrossRef]
- Beltrán-Gastélum, M.; Salazar-Gastélum, M.I.; Félix-Navarro, R.M.; Pérez-Sicairos, S.; Reynoso-Soto, E.A.; Lin, S.W.; Flores-Hernández, J.R.; Romero-Castañon, T.; Albarrán-Sánchez, I.L.; Paraguay-Delgado, F. Evaluation of PtAu/MWCNT (multiwalled carbon nanotubes) electrocatalyst performance as cathode of a proton exchange membrane fuel cell. Energy 2016, 109, 446–455. [Google Scholar] [CrossRef]
- Jeong, H.K.; Lee, Y.P.; Lahaye, R.J.W.E.; Park, M.H.; An, K.H.; Kim, I.J.; Yang, C.W.; Park, C.Y.; Ruoff, R.S.; Lee, Y.H. Evidence of nearly flat layers and AB stacking order of graphite oxides. J. Am. Chem. Soc. 2008, 130, 1362–1366. [Google Scholar] [CrossRef]
- Jeong, H.-K.; Lee, Y.P.; Jin, M.H.; Kim, E.S.; Bae, J.J.; Lee, Y.H. Thermal stability of graphite oxide. Chem. Phys. Lett. 2009, 470, 255–258. [Google Scholar] [CrossRef]
- Andersen, S.M.; Borghei, M.; Lund, P.; Elina, Y.-R.; Pasanen, A.; Kauppinen, E.; Ruiz, V.; Kauranen, P.; Skou, E.M. Durability of carbon nanofiber (CNF) & carbon nanotube (CNT) as catalyst support for Proton Exchange Membrane Fuel Cells. Solid State Ion. 2013, 231, 94–101. [Google Scholar] [CrossRef]
- Wepasnick, K.A.; Smith, B.A.; Schrote, K.E.; Wilson, H.K.; Diegelmann, S.R.; Fairbrother, D.H. Surface and structural characterization of multi-walled carbon nanotubes following different oxidative treatments. Carbon 2011, 49, 24–36. [Google Scholar] [CrossRef]
- Katsounaros, I.; Schneider, W.B.; Meier, J.C.; Benedikt, U.; Biedermann, P.U.; Auer, A.A.; Mayrhofer, K.J.J. Hydrogen peroxide electrochemistry on platinum: Towards understanding the oxygen reduction reaction mechanism. Phys. Chem. Chem. Phys. 2012, 14, 7384–7391. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Tang, J.; Ma, J.; Zhang, H.; Shinya, N.; Qin, L.-C. Graphene and carbon nanotube composite electrodes for supercapacitors with ultra-high energy density. Phys. Chem. Chem. Phys. 2011, 13, 17615–17624. [Google Scholar] [CrossRef]
- Yan, J.; Wei, T.; Shao, B.; Ma, F.; Fan, Z.; Zhang, M.; Zheng, C.; Shang, Y.; Qian, W.; Wei, F. Electrochemical properties of graphene nanosheet /carbon black composites as electrodes for supercapacitors. Carbon 2010, 48, 1731–1737. [Google Scholar] [CrossRef]
- Hyuck, C.; Wook, M.; Chang, H.; Hoon, J.; Ihl, S. Applied catalysis B : Environmental nitrogen-doped graphene/carbon nanotube self-assembly for efficient oxygen reduction reaction in acid media. Appl. Catal. B Environ. 2014, 144, 760–766. [Google Scholar] [CrossRef]
- Shaijumon, M.M.; Ramaprabhu, S. Platinum/multiwalled carbon nanotubes-platinum/carbon composites as electrocatalysts for oxygen reduction reaction in proton exchange membrane fuel cell. Appl. Phys. Lett. 2006, 88, 253105. [Google Scholar] [CrossRef]
- Matsumoto, T.; Komatsu, T.; Arai, K.; Yamazaki, T.; Kijima, M.; Shimizu, H.; Takasawa, Y.; Nakamura, J. Reduction of Pt usage in fuel cell electrocatalysts with carbon nanotube electrodes. Chem. Commun. 2004, 840–841. [Google Scholar] [CrossRef]
- Li, C.; Li, Z.; Zhu, H.; Wang, K.; Wei, J.; Li, X.; Su, P.; Zhang, H.; Wu, D. Graphene Nano-“patches” on a carbon nanotube network for highly transparent/conductive thin film applications. J. Phys. Chem. C 2010, 114, 14008–14012. [Google Scholar] [CrossRef]
- Tung, V.C.; Chen, L.-M.; Allen, M.J.; Wassei, J.; Nelson, K.; Kaner, R.B.; Yang, Y. Low-temperature solution processing of graphene-carbon nanotube hybrid materials for high-performance transparent conductors. Nano Lett. 2009, 9, 1945–1955. [Google Scholar] [CrossRef]
- King, P.J.; Khan, U.; Lotya, M.; De, S.; Coleman, J.N. Improvement of transparent conducting nanotube films by addition of small quantities of graphene. ACS Nano 2010, 4, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- Shim, W.; Kwon, Y.; Jeon, S.-Y.; Yu, W.-R. Optimally conductive networks in randomly dispersed CNT: Graphene hybrids. Sci. Rep. 2015, 5, 16568–16578. [Google Scholar] [CrossRef] [PubMed]
- Imran, J.R.; Arockiados, T.; Rajalakshmi, N.; Ramaprabhu, S. Nanostructured Pt disperse don graphene-multiwalled carbon nanotube hybrid nanomaterials as electrocatalyst for PEMFC. J. Electrochem. Soc. 2010, 157, B874–B879. [Google Scholar] [CrossRef]
- Du, H.Y.; Wang, C.H.; Hsu, H.C.; Chang, S.T.; Huang, H.C.; Chen, L.C.; Chen, K.H. Graphene nanosheet-CNT hybrid nanostructure electrode for a proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2012, 37, 18989–18995. [Google Scholar] [CrossRef]
- Xing, W.; Yin, G.; Zhang, J. Rotating Electrode Methods and Oxygen Reduction Electrocatalyst; Elsevier: Amsterdam, The Netherlands, 2014; Chapter 3; p. 109. [Google Scholar]
- Abedini, A.; Dabir, B.; Kalnasi, M. Experimental verification for simulation study of Pt/CNT nanostructured cathode catalyst layer for PEM fuel cells. Int. J. Hydrogen Energy 2012, 37, 8439–8450. [Google Scholar] [CrossRef]
- Yusof, M.S.M.; Jalil, A.A.; Ahmad, A.; Triwahyono, S.; Othman, M.H.D.; Abdullah, T.A.T.; Firmansyah, M.L.; Setiabudi, H.D.; Johari, A.; Nabgan, W. Effect of Pt-Pd/C coupled catalyst loading and polybenzimidazole ionomer binder on oxygen reduction reaction in high temperature PEMFC. Int. J. Hydrogen Energy 2019, 44, 20760–20769. [Google Scholar] [CrossRef]
- Ficicilar, B.; Bayrakceken, A.; Eroglu, I. Effect of Pd loading in Pd-Pt bimetallic catalysts doped into hollow core mesoporous shell carbon on performance of proton exchange membrane fuel cells. J. Power Sources 2009, 193, 17–23. [Google Scholar] [CrossRef]
- Jung, D.H.; Bae, S.J.; Kim, S.J.; Nahm, K.S.; Kim, P. Effect of the Pt precursor on the morphology and catalytic performance of Pt-impregnated on Pd/C for the oxygen reduction reaction in polymer electrolyte fuel cells. Int. J. Hydrogen Energy 2011, 36, 9115–9122. [Google Scholar] [CrossRef]
- Cho, Y.-H.; Choi, B.; Cho, Y.-H.; Park, H.-S.; Sung, Y.-E. Pd-based PdPt(19:1)/C electrocatalyst as an electrode in PEM fuel cell. Electrochem. Commun. 2007, 9, 378–381. [Google Scholar] [CrossRef]
- Hussain, S.; Erikson, H.; Kongi, N.; Tarre, A.; Ritslaid, P.; Rähn, M.; Matisen, L.; Merisalu, M.; Sammelselg, V.; Tammeveski, K. Pt nanoparticles sputter-deposited on TiO2/MWCNT composites prepared by atomic layer deposition: Improved electrocatalytic activity towards the oxygen reduction reaction and durability in acid media. Int. J. Hydrogen Energy 2018, 43, 4967–4977. [Google Scholar] [CrossRef]
- Steiger, B.; Anson, F.C. Examination of Cobalt “picket fence” prphyrin and its complex with 1-methylimidazole as catalysts for the electroreduction of dioxygen. Inorg. Chem. 2000, 39, 4579–4585. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, Y.; Chen, W. PtPd porous nanorods with enhanced electrocatalytic activity and durability for oxygen reduction reaction. Nano Energy 2013, 2, 836–844. [Google Scholar] [CrossRef]



| Reference | Current Density at 0.6 V (mA/cm2) | Material | Metal Loading (wt.%) | % Pt | % Pd | Pt Loading in MEA (mg/cm2) | Operating Temperature (°C) |
|---|---|---|---|---|---|---|---|
| This work | 351 | PtPd/(rGO:MWCNT) | 23.38 | 7.18 | 16.2 | 0.5 | 60 |
| [47] | 236 | PtPd/C | 31.47 | 15.92 | 15.55 | 0.1 | 170 |
| [48] | 265 | PtPd/HCMS | 20 | 15 | 5 | 0.4 | 70 |
| [49] | 424.4 | PtPd/C | 23.8 | 3.02 | 20.78 | 0.3 | 65 |
| [50] | 150 | PtPd/C | 60 | 3 | 57 | 0.2 | 70 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera-Lugo, Y.Y.; Pérez-Muñoz, K.I.; Trujillo-Navarrete, B.; Silva-Carrillo, C.; Reynoso-Soto, E.A.; Calva Yañez, J.C.; Lin, S.W.; Flores-Hernández, J.R.; Félix-Navarro, R.M. PtPd Hybrid Composite Catalysts as Cathodes for Proton Exchange Membrane Fuel Cells. Energies 2020, 13, 316. https://doi.org/10.3390/en13020316
Rivera-Lugo YY, Pérez-Muñoz KI, Trujillo-Navarrete B, Silva-Carrillo C, Reynoso-Soto EA, Calva Yañez JC, Lin SW, Flores-Hernández JR, Félix-Navarro RM. PtPd Hybrid Composite Catalysts as Cathodes for Proton Exchange Membrane Fuel Cells. Energies. 2020; 13(2):316. https://doi.org/10.3390/en13020316
Chicago/Turabian StyleRivera-Lugo, Yazmín Yorely, Kevin Isaac Pérez-Muñoz, Balter Trujillo-Navarrete, Carolina Silva-Carrillo, Edgar Alonso Reynoso-Soto, Julio Cesar Calva Yañez, Shui Wai Lin, José Roberto Flores-Hernández, and Rosa María Félix-Navarro. 2020. "PtPd Hybrid Composite Catalysts as Cathodes for Proton Exchange Membrane Fuel Cells" Energies 13, no. 2: 316. https://doi.org/10.3390/en13020316
APA StyleRivera-Lugo, Y. Y., Pérez-Muñoz, K. I., Trujillo-Navarrete, B., Silva-Carrillo, C., Reynoso-Soto, E. A., Calva Yañez, J. C., Lin, S. W., Flores-Hernández, J. R., & Félix-Navarro, R. M. (2020). PtPd Hybrid Composite Catalysts as Cathodes for Proton Exchange Membrane Fuel Cells. Energies, 13(2), 316. https://doi.org/10.3390/en13020316






