Abstract
Hydrogen is regarded as a promising energy carrier to substitute fossil fuels. However, storing hydrogen with high density remains a challenge. NaBH4 is a potential hydrogen storage material due to its high gravimetric hydrogen density (10.8 mass%), but the hydrogen kinetic and thermodynamic properties of NaBH4 are poor against the application needs. Nanosizing is an effective strategy to improve the hydrogen properties of NaBH4. In this context, we report on the direct synthesis of NaBH4 nanoparticles (~6–260 nm) from the NaOCH3 precursor. The hydrogen desorption properties of such nanoparticles are reported as well as experimental conditions that lead to the synthesis of (Na2B12H12) free NaBH4 nanoparticles.
1. Introduction
Sodium borohydride (NaBH4) has attracted significant interest as a potential hydrogen storage material, owing to its high hydrogen gravimetric capacity (10.8 mass%) [1,2]. However, the practical application of NaBH4 is difficult to achieve, because of its stability both in terms of hydrogen kinetics and thermodynamics. Typically, the dehydrogenation temperature of NaBH4 is above 500 °C and the kinetics of both the hydrogen release and uptake are too slow for practical use. Moreover, the inherent phase segregation of NaBH4 and partial evaporation of Na upon hydrogen release lead to poor hydrogen reversibility [3].
In order to enhance the hydrogen storage properties of NaBH4, the approach of nanosizing, i.e., reduction of particle size below 100 nm, has proven to lead to some improvements in the thermodynamics and kinetics. This is believed to result from several factors including an increase in the specific surface area and shorter hydrogen diffusion paths [4,5]. The typical method to downsize borohydrides is through their nanoconfinement in porous scaffolds [6,7], but the effective storage capacity achievable by such an approach remains limited, owing to the difficulty of filling the nanopores and the “dead volume and mass” of the host scaffold [4,8]. An alternative method is through the synthesis of isolated nanoparticles and their stabilization as core-shell nanostructures [3,9]. However, to date, this approach is significantly limited by the lack of synthetic methods for readily making NaBH4 nanoparticles of controlled properties.
Thus far, the synthesis methods for preparing isolated nanosized NaBH4 particles are rather limited [3,5]. A common method to synthesize nanoparticles is by growth in solution, but for NaBH4, this is not without challenges. NaBH4 is a strong reducing agent, and thus, many of the common reagents used for the synthesis and stabilization of conventional nanoparticles are out of scope. In addition, the solvents that can be used to synthesize NaBH4 nanoparticles are rather limited owing to the lack of solubility and/or reactivity of NaBH4 [2,3]. Oftentimes, solubilizing solvents form adduct compounds with NaBH4, and this brings additional complexity in terms of synthesizing solvent free nanoparticles [10]. Via a wet chemistry approach, Christian et al. reported that NaBH4 nanoparticles could be precipitated in solution with a size ranging from 2 to 60 nm via anti-precipitation with tetra-n-butylammonium bromide (TBAB) as a surfactant [1,5]. Solvent evaporation has also been reported as an effective method for the synthesis of borohydride nanoparticles. For example, Lai et al. have synthesized LiBH4 and NaBH4 nanoparticles with Ti doped by solvent evaporation [11] and we have also successfully synthesized LiBH4 nanoparticles of controllable sizes with various surfactants [5]. However, this approach leaves excessive amounts of surfactant around the nanoparticles. This significantly limits the effective hydrogen storage capacity and contaminates the hydrogen release with fragments of decomposing surfactants at elevated temperatures [5]. Removing such surfactants could be an option, but these are needed to minimize the surface energy of nanoparticles during their nucleation and growth.
A major drawback of current synthetic approaches is that they all rely on a source of NaBH4 as the starting material to lead to the formation of nanoparticles, and this is an expensive approach given the current cost of NaBH4 (95 USD$ kg−1) [2]. In this respect, the development of direct approaches for making NaBH4 nanoparticles from common precursors is more desirable. However, this is not without difficulties. As summarized in Table S1, the known synthetic methods of NaBH4 have drawbacks, such as: a) low yields, b) high temperatures and/or hydrogen pressures required, and c) low purity. Furthermore, direct production of nanoparticles from these synthetic methods is not evident, and to date, only Lai et al. reported on a thermal decomposition of sodium trimethyl borate (NaB(OCH3)4) to make NaBH4 nanoparticles. In this case, the approach first involves the formation of NaB(OCH3)4 through the reaction of NaH with trimethyl borate (B(OCH3)3) via reaction (1). This is then followed by the thermal decomposition of NaB(OCH3)4 in tetrahydrofuran to precipitate NaBH4 (reaction (2)) [3]:
NaH + B(OCH3)3 → NaBH(OCH3)3
However, the method was found to be difficult to control, the yield of NaBH4 was low, and the by-products of the reaction was difficult to remove [9].
In this work, we report on the development of two routes for making isolated NaBH4 nanoparticles through the reaction of NaOCH3 with B2H6 gas according to reaction (3):
3NaOCH3 + 2B2H6 → 3NaBH4 + B(OCH3)3
Considering that B(OCH3)3 has a low boiling point (68 °C) [12,13] and it is soluble in diethyl ether, in which NaBH4 is not soluble [14,15,16], B(OCH3)3 as a by-product can be removed by heating up or by diethyl ether washing. Thus, we investigated both routes for making NaBH4 nanoparticles, and in first instance, reaction (3) was carried out at 100 °C to facilitate the evaporation of B(OCH3)3, while B2H6 was reacted with NaBH4. To facilitate the dispersion of the reaction by-product, the feasibility of reaction (3) in solvents was also investigated. The morphology, purity and hydrogen properties of the NaBH4 nanoparticles obtained through these routes are reported. In particular, the main hydrogen release temperature of the NaBH4 nanoparticles synthesized by both methods has been reduced by more than 100 °C in comparison to commercial NaBH4.
2. Materials and Methods
Except the solid-gas synthesis of NaBH4 nanoparticles, all the other experiments were performed under an inert atmosphere in an argon filled LC-Technology glove box (O2 and H2O < 1 ppm).
2.1. Materials
NaOCH3 (95%), NaBH4 (98%), ZnCl2 (≥98%), tetra-n-butylammonium bromide (TBAB, >98%), methanol (anhydrous, 99.8%), and octadecylamine (ODA, ≥99%) were purchased from Sigma-Aldrich and hexane (99%, HPLC grade) was purchased from Honeywell. NaOCH3 was purified by solubilization in a large amount of anhydrous methanol and recrystallization of the centrifuged solution was done by evaporating the methanol. ZnCl2 was further dried at 120 °C overnight on a Schlenk line under vacuum (0.01 MPa). Hexane was dried with activated molecular sieve (4 Å) prior to use. The other chemicals were used as received.
2.2. Synthesis of the Diborane (B2H6) Precursor
NaZn2(BH4)5 as the precursor of B2H6 was synthesized by ball milling NaBH4 and ZnCl2 (2:1 molar ratio) in a stainless-steel vial (25 mL) containing a single stainless-steel ball (15 mm diameter). A Retsch MM301 mill operated at a frequency of 20Hz was used for this. The milling process was done twice for 10 min.
2.3. Synthesis of the NaBH4 Nanoparticles via Solid-Gas Reaction
The reaction was undertaken in an in-house built Sievert apparatus with a customized sample holder which had two compartments separated by a stainless-steel mesh (20 μm porosity). 100 mg of purified NaOCH3 was placed on the top of the mesh and variable amounts (0.2, 0.3, 0.4, 1.0 g) of NaZn2(BH4)5 were placed at the bottom of the sample holder. The reaction was carried out at 100 °C overnight. The materials synthesized by this solid-gas reaction (~72 mg) are denoted NaBH4-SGX, where X is the molar ratio of NaOCH3 and the B2H6 generated, X = 1.2, 1.8, 2.4, 5.9.
2.4. Synthesis of the NaBH4 Nanoparticles via Suspension in Hexane and Reaction with B2H6
NaOCH3 nanoparticles were first synthesized by a solvent evaporation method. To this aim, 0.2 M of purified NaOCH3 and 0.01 M TBAB acting as surfactant were dissolved in 10 mL methanol at room temperature. The solution was then placed in a Biotage V-10 evaporator and the methanol was evaporated at a controlled rate to trigger the nucleation and growth of NaOCH3 nanoparticles. The evaporation was carried out at 50 °C, at a centrifugal speed of 3000 rpm. The resulting NaOCH3 powered was then further dried overnight on a Schlenck line at 40 °C and under a vacuum level of 0.01 MPa to remove any methanol residue.
70 mg of as-synthesized NaOCH3 nanoparticles were then suspended in 10 mL hexane with 0.01 M octadecylamine acting as surfactant in a round flask. Variable amount of NaZn2(BH4)5 (0.5, 1, and 2 g) were placed in a separate round flask on a hot plate at 100 °C to generate B2H6. It is important to mention that the amount of B2H6 is much larger than that used in the solid-gas reaction. This is to compensate for the weak airtightness of the rubber seal on the glassware. Both round-bottomed flasks were sealed by a rubber septum and connected by a cannula to allow for the B2H6 generated from the decomposition of NaZn2(BH4)5 to react with the NaOCH3 nanoparticles in suspension. After reaction overnight, the resulting material was separated from the hexane by centrifugation, washed three times with diethyl ether to remove the by-product B(OCH3)3, and dried on a Schlenck line at room temperature under vacuum at 0.01 MPa. The materials synthesized for this suspension gas reaction (~42 mg) are denoted NaBH4-SUGX, where X is the molar ratio of NaOCH3 and the B2H6 generated, X = 4.2, 8.5, 16.9.
2.5. Characterization
The morphologies of the materials were determined by Transmission Electron Microscopy (TEM) on a Philips CM200 operated at 200 kV. The materials were dispersed in cyclohexane followed by ultrasonication and then dropped onto a carbon coated copper grid. The copper grid was then transferred to the TEM in a quick manner as to minimize air exposure. The mean particle size and distribution were collected from a minimum of 300 particles from a range of TEM images.
Crystalline phases were determined by X-Ray Diffraction (XRD) on a Philips X’pert Multipurpose XRD system operated at 40 mA and 45 kV with a monochromated Cu Kα radiation (λ = 1.541 Å) over a 2θ range from 10 to 70°. The materials were protected against oxidation from air by a Kapton foil.
Fourier Transform Infrared Spectroscopy (FTIR) was conducted on a Bruker Vertex 70 V. The materials were mixed with KBr in the glove box and loaded in an air-tight chamber fitted on a Harrick-Scientific Praying Mantis Diffuse Reflectance Infrared Fourier Transform Spectroscopy (DRIFT) accessory. The spectra were collected at room temperature from 600 to 3400 cm−1 over 124 scans with a resolution of 1 cm−1.
The hydrogen desorption properties of the materials were determined by Mass Spectrometry (MS) with an Omnistar instrument from Pfeiffer. The thermal stability of the surfactants was investigated by simultaneous Thermogravimetric Analysis/Differential Scanning Calorimetry (TGA/DSC) with a TGA/DSC1 from Mettler Toledo coupled to the MS. The characterizations were conducted under an argon flow at 20 mL min−1 from 20 to 700 °C with a heating rate of 10 °C min−1 and alumina crucibles (70 mg) were used.
The carbon content of the synthesized materials (NaBH4-SG1.8 and NaBH4-SUG8.5) that may result from remaining traces of NaOCH3 or B(OCH3)3 was determined with a total carbon analyzer (Multi N/C2100, Analytic Jena, Germany). The materials were dissolved in 10 mL of Milli-Q water and the resulting solution was filtered before total carbon analysis. Based on this analysis, both NaBH4-SG1.8 and NaBH4-SUG8.5 (which correspond to the optimum reaction conditions) were found to contain ~2.0 % of carbon per weight of material.
3. Results and Discussion
3.1. NaBH4 Nanoparticles Synthesized via Solid-Gas Interaction
3.1.1. Morphology of the As-Synthesized Materials
The morphology of the as-synthesized material was characterized by TEM (Figure 1). By exposing the NaOCH3 particles (Figure S1) to increasing amounts of B2H6, (from molar ratio 1.8 to 5.9), the morphology of the material synthesized evolved from large undefined particles of ~262 nm to spherical like particles of 6 nm. This reduction in size is probably due to a fragmentation of the NaOCH3 particles with B2H6 (reaction 3), and the subsequent release of gaseous B(OCH3)3 at 100 °C. This phenomenon has also been found to occur in reactions involving some gas release [17,18].
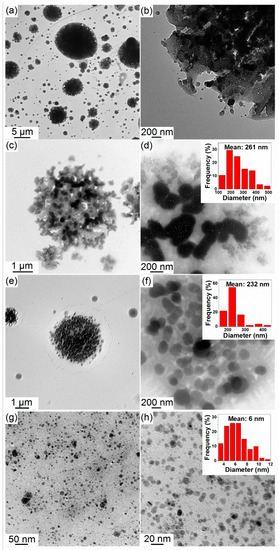
Figure 1.
Transmission Electron Microscopy (TEM) images of (a,b) NaBH4-SG1.2; (c,d) NaBH4-SG1.8; (e,f) NaBH4-SG2.4; and (g,h) NaBH4-SG5.9. The inset diagrams are the corresponding mean particle sizes and size distribution.
3.1.2. Composition of the As-Synthesized Materials
XRD and FTIR characterizations were performed to confirm the compositions of the as-synthesized material. From XRD analysis (Figure 2 and Figure S2), it is apparent that when the molar ratio of NaOCH3 and B2H6 is above 1.8, all the NaOCH3 is reacted because only diffraction peaks related to thermodynamically stable phase of NaBH4 were observed. FTIR analysis further confirmed this trend (Figure 3 and Figure S3). As the molar ratio evolved from 1.2 to 1.8, the vibrational peaks corresponding to NaOCH3 (Figure S3) disappeared, and this is in agreement with the XRD results (Figure 2). The peak at ~43 ° is assigned to the stainless steel sample holder.
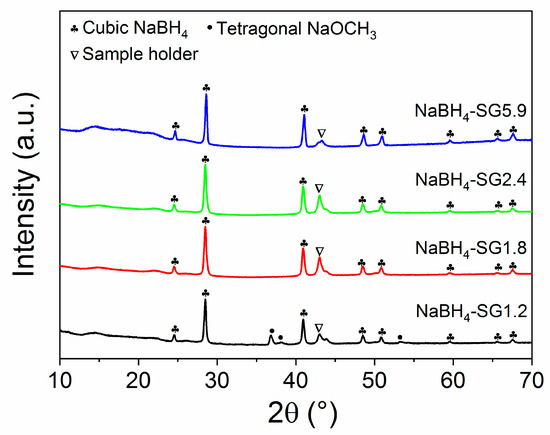
Figure 2.
XRD patterns the as-synthesized NaBH4 via solid-gas interaction.
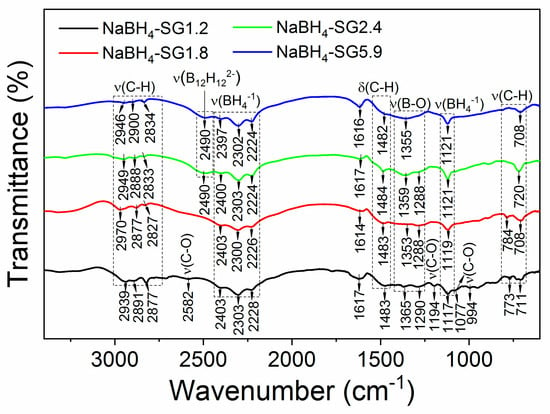
Figure 3.
FTIR spectra the as-synthesized NaBH4 via solid-gas interaction.
A typical FTIR spectrum of NaBH4 would display vibrational peaks associated with the BH4− group in the region of 2410–2200 cm−1 corresponding to the stretching mode of BH4−, whereas the bending modes of BH4− are to be found around 1120 cm−1 [19,20].
For NaBH4-SG1.2, the additional peaks observed at 2582, 1194, 1077, and 994 cm−1 were assigned to the C-O stretching modes associated with NaOCH3 [21,22,23,24,25,26,27,28,29]. Such vibrations were not observed at higher molar ratio of NaOCH3 and B2H6 and this confirmed that above a ratio of 1.2, NaOCH3 was fully reacted. However, when the molar ratio reached 2.4 and 5.9, a new vibration appeared at 2490 cm−1 (Figure 3). This vibration was assigned to the stretching mode of B12H122− [11,30]. Accordingly, traces of Na2B12H12 have been generated via the reaction of the newly formed NaBH4 with the excess of B2H6. Thus far, there have been no reports regarding the direct formation of Na2B12H12 via the reaction between NaBH4 and B2H6. Only the formation of NaB3H8 has been reported to occur according to (4) [31,32]:
NaBH4 + B2H6 → NaB3H8 + H2
Chen et al. reported that NaB3H8 can decompose to NaBH4, Na2B12H12, Na2B10H10 at 150 °C [33]. Given that our synthesis was performed at 100 °C, one possibility is that the Na2B12H12 phase observed by FTIR is the result of the decomposition of NaB3H8.
Finally, the broad peaks between 1420–1260 cm−1 that were observed in all the spectra of the as-synthesized NaBH4 nanoparticles were assigned to the stretching modes of B-O bond [26]. These vibrations are most likely the result of remaining traces of B(OCH3)3 in the as-synthesized NaBH4 nanoparticles. This was also confirmed by the presence of C-H stretching modes from B(OCH3)3 in all the synthesized materials (Figure 3).
3.2. NaBH4 Nanoparticles Synthesized from NaOCH3 Nanoparticles Reacted in Suspension
Although NaBH4 nanoparticles have been successfully obtained by the solid-gas method, the approach did not lead to nanoparticles of well-defined morphology. In order to better control the particle size and synthesize isolated NaBH4 nanoparticles, we investigated the possibility to first synthesize NaOCH3 nanoparticles by solvent evaporation method, and then suspended these NaOCH3 nanoparticles in hexane with octadecylamine as a stabilizer to retain the morphology during reaction with B2H6. After reaction at room temperature, the as-synthesized NaBH4 nanoparticles were collected by centrifugation and washed by diethyl ether to remove the solubilized B(OCH3)3 residue [14,15,16]. Figure 4a,b shows the typical TEM images of the as-synthesized NaOCH3 nanoparticles obtained by solvent evaporation method, and thus, such an approach was found to be effective in leading to nanoparticles of NaOCH3 with a mean particle size of 16 nm.
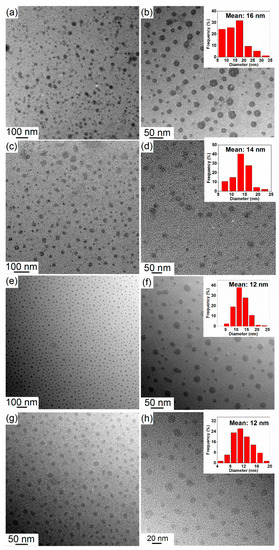
Figure 4.
TEM image of the as-synthesized (a,b) NaOCH3 nanoparticles; (c,d) NaBH4-SUG4.2; (e,f) NaBH4-SUG8.5; and (g,h) NaBH4-SUG16.9. The inset diagrams are the corresponding mean particle sizes and size distribution.
3.2.1. Morphology of the As-Synthesized NaBH4
After the reaction of the suspended NaOCH3 nanoparticles with B2H6, analysis by TEM of the resulting material revealed polydispersed particles with a mean size of 14 nm (NaBH4-SUG4.2, Figure 4c,d) and 12 nm (NaBH4-SUG8.5, Figure 4e,f, and NaBH4-SUG16.9, Figure 4g,h). This is slightly smaller than the particle size of NaOCH3 (Figure 4a,b), and this reduction in particle size could be the result of elemental loss in the form of B(OCH3)3 that is released during the synthesis (reaction 3).
3.2.2. Composition of the As-Synthesized Materials
Figure 5 shows the XRD patterns of the as-synthesized NaBH4 obtained from the suspension-gas reaction. Different to the cubic phase of NaBH4 synthesized by solid-gas reaction, the as-synthesized NaBH4 exhibited peaks of both tetragonal and cubic NaBH4. The peaks of NaOCH3 at ~11.5°, 30.7°, and 31.9° in NaBH4-SUG4.2 disappeared when the molar ratios of NaOCH3 and B2H6 were 8.5 and 16.9. This indicated that all the NaOCH3 was fully reacted with B2H6 to produce NaBH4.
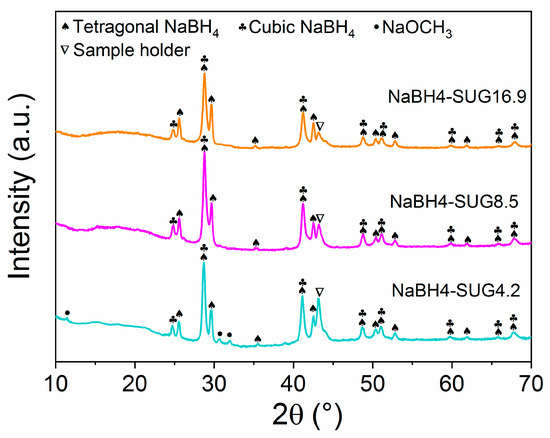
Figure 5.
XRD patterns of the as-synthesised NaBH4 via suspension-gas interaction.
The FTIR spectra of the NaBH4 synthesized by suspension-gas reaction also showed the typical stretching modes and bending modes of the BH4− groups in the range of 2400–2220 cm−1 and at ~1120 cm−1, respectively (Figure 6). Additional vibrational peaks assigned to C-H were observed between 2970–2680 cm−1 (stretching modes), ~1470 cm−1 (bending modes) and 780–710 cm−1 (stretching modes) [22].
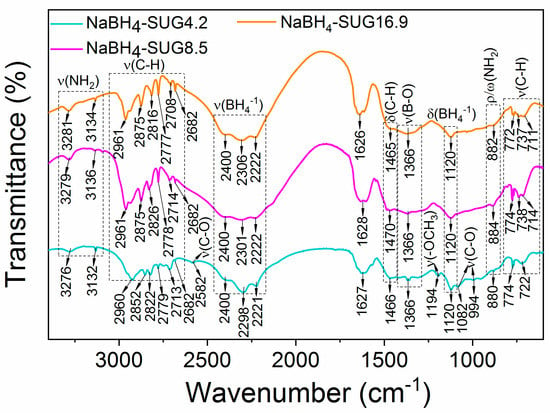
Figure 6.
FTIR spectra of the as-synthesized NaBH4 via suspension-gas interaction.
Compared with the FTIR spectrum of pristine octadecylamine (Figure S4), the stretching modes of the primary NH2 shifted from 3331, 3254 cm−1 to ~3280, 3135 cm−1 upon stabilization of the NaBH4 nanoparticles. In addition, the multiple peaks of NH2 rocking and wagging modes at 940–750 cm−1 in pristine octadecylamine [34,35,36] appeared as a weak peak at ~880 cm−1. This indicates that the interaction of NaBH4 and octadecylamine was through its head group (NH2).
FTIR analysis also proved the presence of the unreacted NaOCH3 in NaBH4-SUG4.2. For example, the peaks at around 2582, 1194, 1082, and 994 cm−1 in NaBH4-SUG4.2 were assigned to the stretching modes of C-O in NaOCH3 (Figure 6) [21,22,23,24,29]. These peaks were not observed in NaBH4-SUG8.5 and NaBH4-SUG16.9 by FTIR, and this proves that at NaOCH3 and B2H6 molar ratios higher than 8.5, NaOCH3 was fully reacted. However, a significant difference of the suspension-gas reaction as compared to the solid-gas reaction carried at 100 °C is the absence of any vibrations related to the formation NaB12H12 in NaBH4-SUG16.9, despite the excess of B2H6. This indicates that NaB12H12 cannot be formed at room temperature. This may be due to the high energy required for its formation, e.g., ΔfH0 = −1086.196 kJ mol−1 for its cubic phase, ΔfH0 = −1086.381 kJ mol−1 for its monoclinic phase [37].
3.3. Hydrogen Desorption Properties
The hydrogen desorption properties of the as-synthesized NaBH4 were characterized by TGA/DSC and MS. Figure 7 and Figure 8 show the TGA, DSC, and MS of NaBH4-SG1.8 and NaBH4-SUG8.5, which correspond to the optimum NaOCH3 and B2H6 molar ratios for both reaction paths according to the XRD and FTIR analyses.
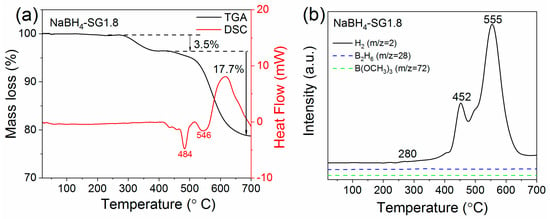
Figure 7.
(a) TGA/DSC under Ar of NaBH4-SG1.8; (b) H2 desorption from NaBH4-SG1.8 recorded by mass spectrometry and selected mass corresponding to the release of B2H6 and B(OCH3)3.
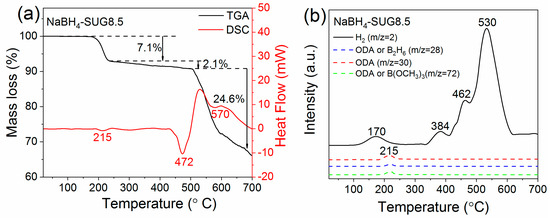
Figure 8.
(a) TGA/DSC under Ar of NaBH4-SUG8.5; (b) H2 desorption from NaBH4-SUG8.5 recorded by mass spectrometry and selected mass corresponding to the release of B2H6, B(OCH3)3, and octadecylamine.
The TGA analysis (Figure 7a) of NaBH4-SG1.8 first showed a mass loss of 3.5% between 280–440 °C. This is correlated to hydrogen released as observed by simultaneous MS analysis (Figure 7b). Within this temperature range, no obvious peak was observed by DSC. There is also no obvious evidence of other gases released from NaBH4-SG1.8.
For NaBH4-SUG8.5, the mass loss of 7.1 % from 175–240 °C resulted from the decomposition of octadecylamine (Figure 8 and Figure S5) [5,38,39], as confirmed by the endothermic peaks observed at 215 °C (Figure 8a) and octadecylamine fragments observed by MS (Figure 8b). It should be noted that in this case, the mass loss observed by TGA is much larger than the carbon amount determined by the total carbon analysis, because octadecylamine was removed by filtration before the carbon analysis [40]. At low temperatures, i.e., 170 °C, the small peak of hydrogen release observed by MS was assigned to the recombination of the protic hydrogen (NHδ+) in the octadecylamine with the hydridic hydrogen (BHδ-) from NaBH4. At higher temperatures, i.e., between 240 and 505 °C, the additional mass loss of 2.1% was assigned to hydrogen release as indicated by MS (Figure 8b).
The endothermic peaks observed for NaBH4-SG1.8 (Figure 7a) at 484 °C and NaBH4-SUG8.5 at 472 °C (Figure 8a) were assigned to the melting of the as-synthesized NaBH4 nanoparticles. Compare to the melting point of commercial NaBH4 (506 °C, Figure S6a), this indicates that the reduction in particle size influences the melting behavior of NaBH4 particles, and this result is in agreement with previous observations made for almost all free nanoparticles [41,42,43]. Furthermore, from the TGA/DSC/MS analysis, it can be observed that the temperatures at which the main hydrogen release start have been significantly reduced to 452 °C for NaBH4-SG1.8 and 384 °C for NaBH4-SUG8.5 in comparison to commercial NaBH4 at 586 °C (Figure S6). This is believed to be attributed to the reduction in particle size of the NaBH4 nanoparticles synthesized.
At temperatures >500 °C, the large mass losses of 17.7% for NaBH4-SG1.8 (Figure 7a) and 24.6% for NaBH4-SUG8.5 (Figure 8a) are believed to be the result of additional hydrogen release in conjunction with Na evaporation owing to the low melting point of Na (97.5 °C) and a high vapor pressure of 10−3 MPa at 500 °C [44,45].
4. Conclusions
We have synthesized NaBH4 nanoparticles with different sizes ranging from 6 to 260 nm through the direct reaction of NaOCH3 with B2H6. The particle size decreased as the amount of B2H6 increased during the solid-gas reaction. However, the formation of Na2B12H12 was found to occur when NaOCH3 was exposed to excessive amounts of B2H6 during the reaction at 100 °C. In contrast, reacting NaOCH3 nanoparticles in suspension with B2H6 at room temperature did not lead to the formation of Na2B12H12, and the purity of the resulting NaBH4 was ~98.0%. The start of hydrogen release from our NaBH4 nanoparticles is much lower than that of commercial NaBH4 (by at least 100 °C) and this was attributed to the nanosize nature of the materials synthesized. These results not only demonstrate the possibility to synthesize NaBH4 nanoparticle via a direct synthesis route with monodispersed particles, in particular through the reaction of NaOCH3 suspended in solution, but also the possibility to reduce the temperature at which hydrogen is released. Additional modification through a core-shell approach, for example, to confine NaBH4 and its elements upon hydrogen release, could lead to better hydrogen properties in particular in terms of hydrogen reversibility by avoiding elemental segregation. This will be at the core of future work.
Supplementary Materials
The following are available online at https://www.mdpi.com/1996-1073/12/23/4428/s1.
Author Contributions
All Authors contributed equally. Conceptualization, T.W. and K.-F.A.-Z.; Methodology, T.W. and K.-F.A.-Z.; Validation, T.W.; Formal Analysis, T.W.; Investigation, T.W. and K.-F.A.-Z.; Resources, K.-F.A.-Z.; Data Curation, T.W.; Writing-Original Draft Preparation, T.W.; Writing-Review & Editing, K.-F.A.-Z.; Supervision, K.-F.A.-Z.; Project Administration, K.-F.A.-Z.; Funding Acquisition, K.-F.A.-Z.
Funding
This research was funded by UNSW Internal Research Grant program and Office of Naval Research (Award No: ONRG - NICOP - N62909-16-1-2155).
Acknowledgments
We appreciate the use of instruments in the Mark Wainwright Analytical Centre at UNSW. The China Scholarship Council is gratefully acknowledged for the Scholarship granted to Ting Wang.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Christian, M.L.; Aguey-Zinsou, K.-F. Core–Shell strategy leading to high reversible hydrogen storage capacity for NaBH4. ACS Nano 2012, 6, 7739–7751. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Sun, Y.; Wang, T.; Modi, P.; Cazorla, C.; Demirci, U.B.; Ares Fernandez, J.R.; Leardini, F.; Aguey-Zinsou, K.F. How to design hydrogen storage materials? Fundamentals, synthesis, and storage tanks. Adv. Sust. Sys. 2019, 3, 1900043. [Google Scholar] [CrossRef]
- Lai, Q.; Wang, T.; Sun, Y.; Aguey-Zinsou, K.F. Rational design of nanosized light elements for hydrogen storage: Classes, synthesis, characterization, and properties. Adv. Mater. Technol. 2018, 3, 1700298. [Google Scholar] [CrossRef]
- De Jongh, P.E.; Adelhelm, P. Nanosizing and nanoconfinement: New strategies towards meeting hydrogen storage goals. ChemSusChem 2010, 3, 1332–1348. [Google Scholar] [CrossRef]
- Wang, T.; Aguey-Zinsou, K.F. Controlling the growth of LiBH4 nanoparticles for hydrogen storage. Energy Technol. 2019, 7, 1801159. [Google Scholar] [CrossRef]
- Lai, Q.; Yang, Y.; Aguey-Zinsou, K.-F. Nanoconfinement of borohydrides in hollow carbon spheres: Melt infiltration versus solvent impregnation for enhanced hydrogen storage. Int. J. Hydrogen Energy 2019, 44, 23225–23238. [Google Scholar] [CrossRef]
- Lai, Q.; Christian, M.; Aguey-Zinsou, K.-F. Nanoconfinement of borohydrides in CuS hollow nanospheres: A new strategy compared to carbon nanotubes. Int. J. Hydrogen Energy 2014, 39, 9339–9349. [Google Scholar] [CrossRef]
- Clémençon, D.; Davoisne, C.; Chotard, J.-N.; Janot, R. Enhancement of the hydrogen release of Mg(BH4)2 by concomitant effects of nano-confinement and catalysis. Int. J. Hydrogen Energy 2019, 44, 4253–4262. [Google Scholar] [CrossRef]
- Christian, M.L. Core-Shell Borohydrides for Reversible Hydrogen Storage. Ph.D. Thesis, University of New South Wales, Sydney, Australia, 2013. [Google Scholar]
- Lai, Q.W.; Paskevicius, M.; Sheppard, D.A.; Buckley, C.E.; Thornton, A.W.; Hill, M.R.; Gu, Q.F.; Mao, J.F.; Huang, Z.G.; Liu, H.K.; et al. Hydrogen storage materials for mobile and stationary applications: Current state of the art. ChemSusChem 2015, 8, 2789–2825. [Google Scholar] [CrossRef]
- Lai, Q.; Milanese, C.; Aguey-Zinsou, K.-F. Stabilization of nanosized borohydrides for hydrogen storage: Suppressing the melting with TiCl3 doping. ACS Appl. Energy Mater. 2018, 1, 421–430. [Google Scholar] [CrossRef]
- Mangold, H.; Ettlinger, M.; Kerner, D.; Kleinschmit, P. Boron Oxide-Silicon Dioxide Mixed Oxide. U.S. Patent US6242373B1, 5 June 2001. [Google Scholar]
- Murphy, R.J.; Dickinson, D.J.; Turner, P. Treatment of Wood and Wood-Based Materials. U.S. patent 5330847, 19 July 1994. [Google Scholar]
- Lewis, R.J., Sr.; Lewis, R.A.; Hawley, G.G. Hawley’s Condensed Chemical Dictionary, 16th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Richardson, M.L.; Gangolli, S. The Dictionary of Substances and Their Effects, 2nd ed.; Royal Society of Chemistry: Cambridge, UK, 1992; Volume 2. [Google Scholar]
- Kanth, J.V.; Brown, H.C. Improved procedures for the generation of diborane from sodium borohydride and boron trifluoride. Inorg. Chem. 2000, 39, 1795–1802. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, X.; Zhang, F.; Wang, W.; Liang, Y.; Tang, Y. Uniform ultrasmall manganese monoxide nanoparticle/carbon nanocomposite as a high-performance anode for lithium storage. Electrochim. Acta 2016, 196, 634–641. [Google Scholar] [CrossRef]
- Sun, B.; Chen, Z.; Kim, H.-S.; Ahn, H.; Wang, G. MnO/C core–shell nanorods as high capacity anode materials for lithium-ion batteries. J. Power Sour. 2011, 196, 3346–3349. [Google Scholar] [CrossRef]
- Huang, J.; Ouyang, L.; Gu, Q.; Yu, X.; Zhu, M. Metal-Borohydride-Modified Zr(BH4)4·8NH3: Low-Temperature dehydrogenation yielding highly pure hydrogen. Chem. Eur. J. 2015, 21, 14931–14936. [Google Scholar] [CrossRef]
- Zheng, J.; Xiao, X.; Zhang, L.; Li, S.; Ge, H.; Chen, L. Facile synthesis of bowl-like 3D Mg (BH4)2–NaBH4–fluorographene composite with unexpected superior dehydrogenation performances. J. Mater. Chem. A 2017, 5, 9723–9732. [Google Scholar] [CrossRef]
- Xue, Z. Raman Spectroscopy of Carboxylic Acid and Water Aggregates; Logos Verlag: Berlin, Germany; GmbH: Berlin, Germany, 2011. [Google Scholar]
- Ramesh, S.; Leen, K.H.; Kumutha, K.; Arof, A.K. FTIR studies of PVC/PMMA blend based polymer electrolytes. Spectrochim. Acta Part A 2007, 66, 1237–1242. [Google Scholar] [CrossRef]
- Dai, H.-L. Spectroscopy, Structure, and Energy Transfer of Transient Radicals in Combustion; University of Pennsylvania: Philadelphia, PA, USA, 2007. [Google Scholar]
- Gómez-Ordóñez, E.; Rupérez, P. FTIR-ATR spectroscopy as a tool for polysaccharide identification in edible brown and red seaweeds. Food Hydrocolloids 2011, 25, 1514–1520. [Google Scholar] [CrossRef]
- Cornelius, C.J.; Marand, E. Hybrid inorganic–organic materials based on a 6FDA–6FpDA–DABA polyimide and silica: Physical characterization studies. Polymer 2002, 43, 2385–2400. [Google Scholar] [CrossRef]
- Gautam, C.; Yadav, A.K.; Mishra, V.K.; Vikram, K. Synthesis, IR and Raman spectroscopic studies of (Ba, Sr) TiO3 borosilicate glasses with addition of La2O3. Open J. Inorg. Non Met. Mater. 2012, 2, 47–54. [Google Scholar]
- Lopes, J.d.O.; Garcia, R.A.; Souza, N.D.D. Infrared spectroscopy of the surface of thermally-modified teak juvenile wood. Maderas. Ciencia Tecnología 2018, 20, 737–746. [Google Scholar] [CrossRef]
- Mahmood, Z.; Azam, M.; Mushtaq, A.; Kausar, R.; Kausar, S.; Gilani, S.R. Comparative vapour phase FTIR spectra and vibrational assignment of manganese pentacarbonyls derivatives of the type XMn (CO)5: (where X = Br, Cl, I, H, D, CH3, CD3, CF3). Spectrochim. Acta Part A 2006, 65, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.-Z.; Jackson, M.; Sowa, M.G.; Ju, H.; Dixon, I.M.; Mantsch, H.H. Modification of the extracellular matrix following myocardial infarction monitored by FTIR spectroscopy. BBA Mol. Basis Dis. 1996, 1315, 73–77. [Google Scholar] [CrossRef]
- Genady, A.R. Labeled undecahydro-closo-dodecaborates based on azo dyes for boron neutron capture therapy: Synthesis, characterization, and visualization in cells. Acta Chim. Slov. 2012, 59, 1. [Google Scholar]
- Nelson, M.A.; Kodama, G. Deuterated sodium octahydrotriborate (1-). Inorg. Chem. 1979, 18, 3276–3278. [Google Scholar] [CrossRef]
- Gaines, D.F.; Schaeffer, R.; Tebbe, F. Convenient preparations of solutions containing the triborohydride ion. Inorg. Chem. 1963, 2, 526–528. [Google Scholar] [CrossRef]
- Chen, W.; Wu, G.; He, T.; Li, Z.; Guo, Z.; Liu, H.; Huang, Z.; Chen, P. An improved synthesis of unsolvated NaB3H8 and its application in preparing Na2B12H12. Int. J. Hydrogen Energy 2016, 41, 15471–15476. [Google Scholar] [CrossRef]
- Mirghani, M.; Man, Y.C.; Jinap, S.; Baharin, B.; Bakar, J. FTIR spectroscopic determination of soap in refined vegetable oils. J. Am. Oil Chem. Soc. 2002, 79, 111–116. [Google Scholar] [CrossRef]
- Ramalingam, M.; Sundaraganesan, N.; Saleem, H.; Swaminathan, J. Experimental (FTIR and FT-raman) and ab initio and DFT study of vibrational frequencies of 5-amino-2-nitrobenzoic acid. Spectrochim. Acta Part A 2008, 71, 23–30. [Google Scholar] [CrossRef]
- Gibson, N.; Shenderova, O.; Luo, T.; Moseenkov, S.; Bondar, V.; Puzyr, A.; Purtov, K.; Fitzgerald, Z.; Brenner, D. Colloidal stability of modified nanodiamond particles. Diam. Relat. Mater. 2009, 18, 620–626. [Google Scholar] [CrossRef]
- Caputo, R.; Garroni, S.; Olid, D.; Teixidor, F.; Suriñach, S.; Baro, M.D. Can Na2[B12H12] be a decomposition product of NaBH4? Phys. Chem. Chem. Phys. 2010, 12, 15093–15100. [Google Scholar] [CrossRef]
- Purkayastha, D.; Sarma, B.; Bhattacharjee, C. Surfactant-assisted low-temperature synthesis of monodispersed phase pure cubic CoO solid nanoparallelepipeds via thermal decomposition of cobalt (II) acetylacetonate. Mater Lett. 2013, 107, 71–74. [Google Scholar] [CrossRef]
- Züttel, A.; Rentsch, S.; Fischer, P.; Wenger, P.; Sudan, P.; Mauron, P.; Emmenegger, C. Hydrogen storage properties of LiBH4. J. Alloys Compd. 2003, 356, 515–520. [Google Scholar] [CrossRef]
- Fan, B.; Wei, G.; Zhang, Z.; Qiao, N. Characterization of a supramolecular complex based on octadecylamine and β-cyclodextrin and its corrosion inhibition properties in condensate water. Corros. Sci. 2014, 83, 75–85. [Google Scholar] [CrossRef]
- Jiang, H.; Moon, K.-S.; Dong, H.; Hua, F.; Wong, C. Size-Dependent melting properties of tin nanoparticles. Chem. Phys. Lett. 2006, 429, 492–496. [Google Scholar] [CrossRef]
- Nanda, K. Size-Dependent melting of nanoparticles: Hundred years of thermodynamic model. Pramana 2009, 72, 617–628. [Google Scholar] [CrossRef]
- Olson, E.; Efremov, M.Y.; Zhang, M.; Zhang, Z.; Allen, L. Size-Dependent melting of Bi nanoparticles. J. Appl. Phys. 2005, 97, 034304. [Google Scholar] [CrossRef]
- Honig, R.E. Vapor Pressure Data for the More Common Elements; David Sarnoff Research Center: Princeton, NJ, USA, 1957. [Google Scholar]
- Ngene, P.; van den Berg, R.; Verkuijlen, M.H.; de Jong, K.P.; de Jongh, P.E. Reversibility of the hydrogen desorption from NaBH4 by confinement in nanoporous carbon. Energy Environ. Sci. 2011, 4, 4108–4115. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).