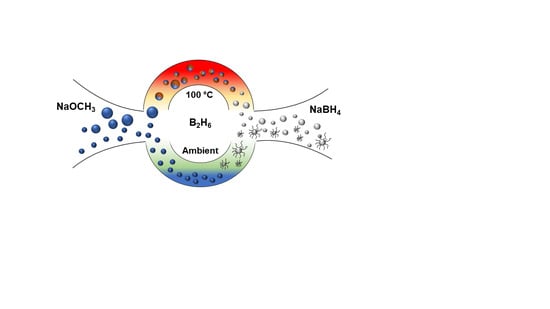
Direct Synthesis of NaBH4 Nanoparticles from NaOCH3 for Hydrogen Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Diborane (B2H6) Precursor
2.3. Synthesis of the NaBH4 Nanoparticles via Solid-Gas Reaction
2.4. Synthesis of the NaBH4 Nanoparticles via Suspension in Hexane and Reaction with B2H6
2.5. Characterization
3. Results and Discussion
3.1. NaBH4 Nanoparticles Synthesized via Solid-Gas Interaction
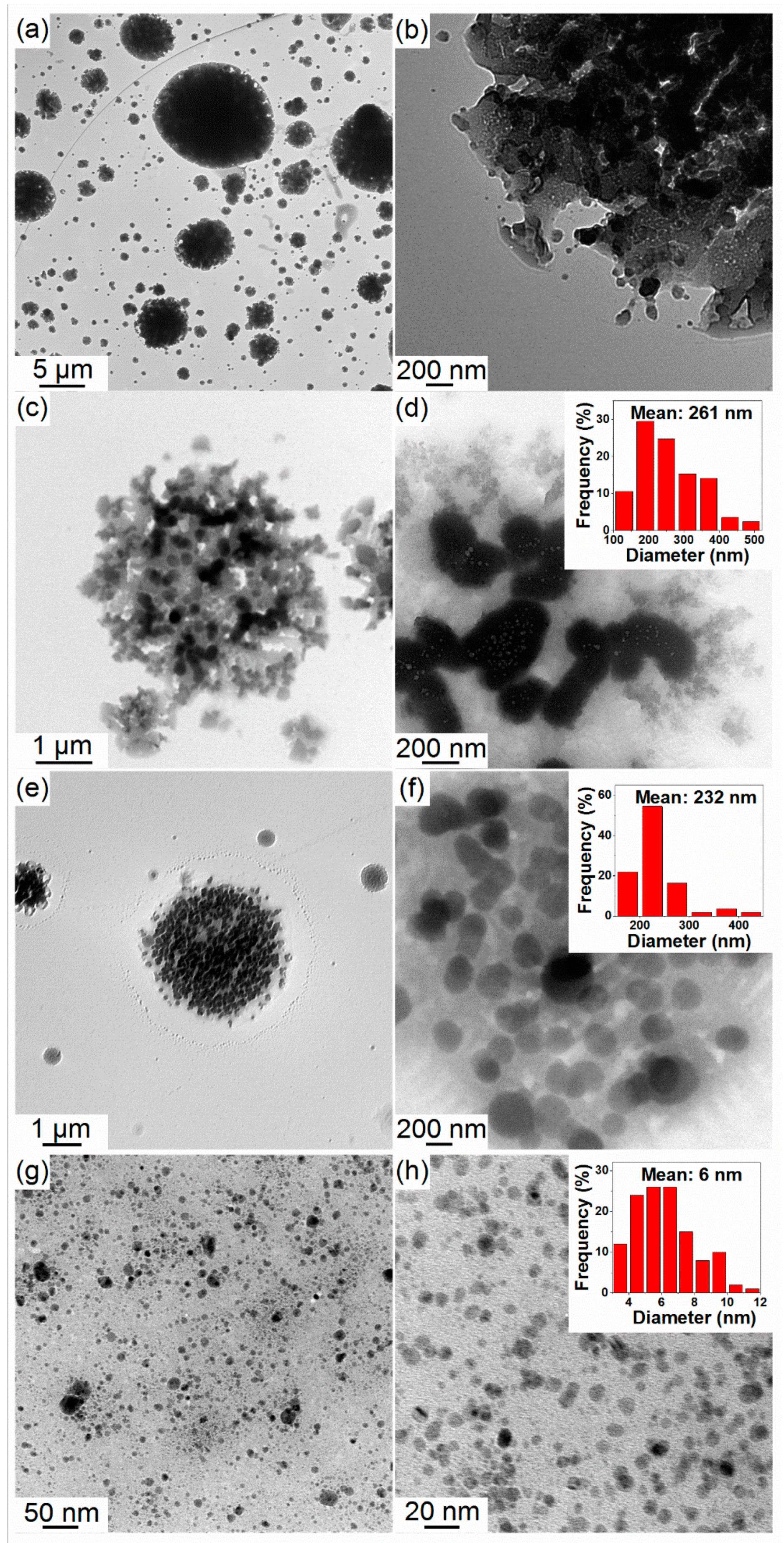
3.1.1. Morphology of the As-Synthesized Materials
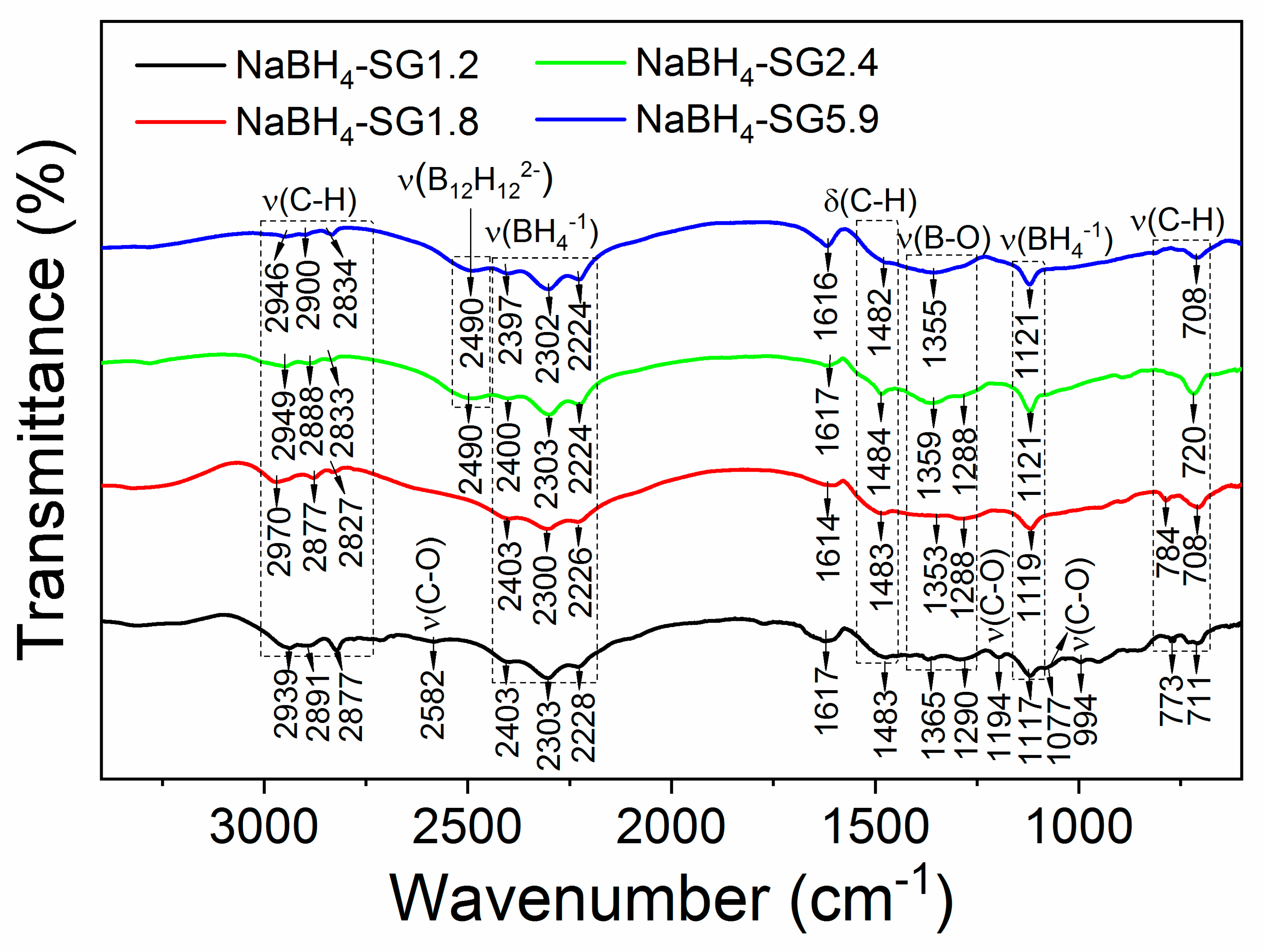
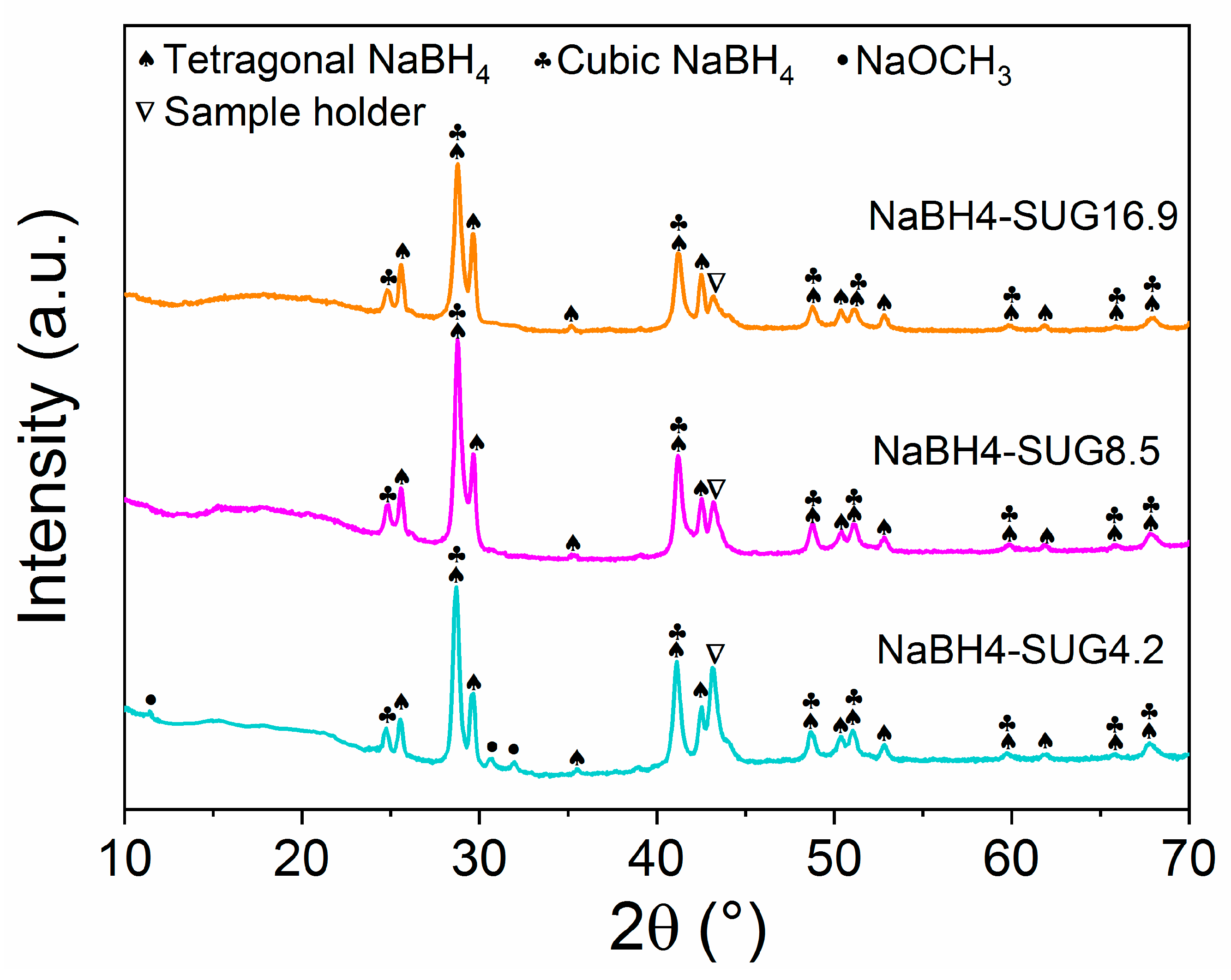
3.1.2. Composition of the As-Synthesized Materials
3.2. NaBH4 Nanoparticles Synthesized from NaOCH3 Nanoparticles Reacted in Suspension
3.2.1. Morphology of the As-Synthesized NaBH4
3.2.2. Composition of the As-Synthesized Materials
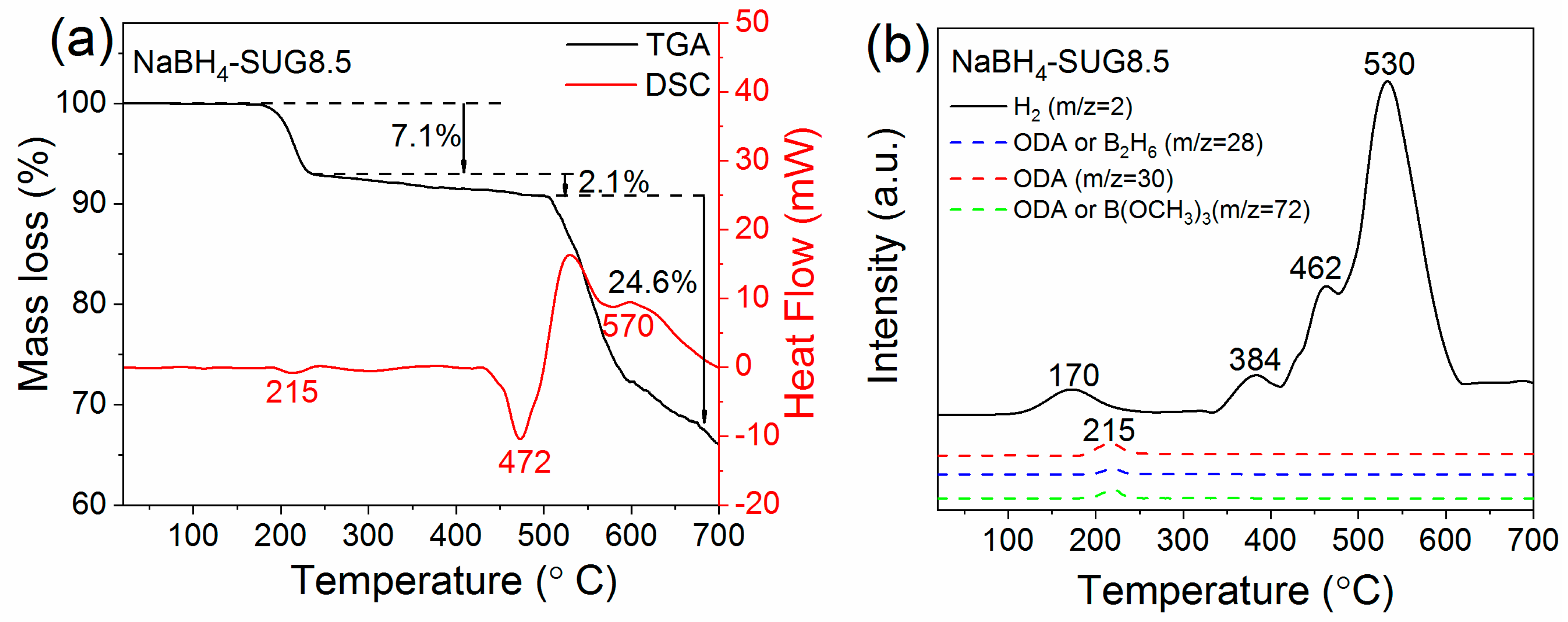
3.3. Hydrogen Desorption Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Christian, M.L.; Aguey-Zinsou, K.-F. Core–Shell strategy leading to high reversible hydrogen storage capacity for NaBH4. ACS Nano 2012, 6, 7739–7751. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Sun, Y.; Wang, T.; Modi, P.; Cazorla, C.; Demirci, U.B.; Ares Fernandez, J.R.; Leardini, F.; Aguey-Zinsou, K.F. How to design hydrogen storage materials? Fundamentals, synthesis, and storage tanks. Adv. Sust. Sys. 2019, 3, 1900043. [Google Scholar] [CrossRef]
- Lai, Q.; Wang, T.; Sun, Y.; Aguey-Zinsou, K.F. Rational design of nanosized light elements for hydrogen storage: Classes, synthesis, characterization, and properties. Adv. Mater. Technol. 2018, 3, 1700298. [Google Scholar] [CrossRef]
- De Jongh, P.E.; Adelhelm, P. Nanosizing and nanoconfinement: New strategies towards meeting hydrogen storage goals. ChemSusChem 2010, 3, 1332–1348. [Google Scholar] [CrossRef]
- Wang, T.; Aguey-Zinsou, K.F. Controlling the growth of LiBH4 nanoparticles for hydrogen storage. Energy Technol. 2019, 7, 1801159. [Google Scholar] [CrossRef]
- Lai, Q.; Yang, Y.; Aguey-Zinsou, K.-F. Nanoconfinement of borohydrides in hollow carbon spheres: Melt infiltration versus solvent impregnation for enhanced hydrogen storage. Int. J. Hydrogen Energy 2019, 44, 23225–23238. [Google Scholar] [CrossRef]
- Lai, Q.; Christian, M.; Aguey-Zinsou, K.-F. Nanoconfinement of borohydrides in CuS hollow nanospheres: A new strategy compared to carbon nanotubes. Int. J. Hydrogen Energy 2014, 39, 9339–9349. [Google Scholar] [CrossRef]
- Clémençon, D.; Davoisne, C.; Chotard, J.-N.; Janot, R. Enhancement of the hydrogen release of Mg(BH4)2 by concomitant effects of nano-confinement and catalysis. Int. J. Hydrogen Energy 2019, 44, 4253–4262. [Google Scholar] [CrossRef]
- Christian, M.L. Core-Shell Borohydrides for Reversible Hydrogen Storage. Ph.D. Thesis, University of New South Wales, Sydney, Australia, 2013. [Google Scholar]
- Lai, Q.W.; Paskevicius, M.; Sheppard, D.A.; Buckley, C.E.; Thornton, A.W.; Hill, M.R.; Gu, Q.F.; Mao, J.F.; Huang, Z.G.; Liu, H.K.; et al. Hydrogen storage materials for mobile and stationary applications: Current state of the art. ChemSusChem 2015, 8, 2789–2825. [Google Scholar] [CrossRef]
- Lai, Q.; Milanese, C.; Aguey-Zinsou, K.-F. Stabilization of nanosized borohydrides for hydrogen storage: Suppressing the melting with TiCl3 doping. ACS Appl. Energy Mater. 2018, 1, 421–430. [Google Scholar] [CrossRef]
- Mangold, H.; Ettlinger, M.; Kerner, D.; Kleinschmit, P. Boron Oxide-Silicon Dioxide Mixed Oxide. U.S. Patent US6242373B1, 5 June 2001. [Google Scholar]
- Murphy, R.J.; Dickinson, D.J.; Turner, P. Treatment of Wood and Wood-Based Materials. U.S. patent 5330847, 19 July 1994. [Google Scholar]
- Lewis, R.J., Sr.; Lewis, R.A.; Hawley, G.G. Hawley’s Condensed Chemical Dictionary, 16th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Richardson, M.L.; Gangolli, S. The Dictionary of Substances and Their Effects, 2nd ed.; Royal Society of Chemistry: Cambridge, UK, 1992; Volume 2. [Google Scholar]
- Kanth, J.V.; Brown, H.C. Improved procedures for the generation of diborane from sodium borohydride and boron trifluoride. Inorg. Chem. 2000, 39, 1795–1802. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, X.; Zhang, F.; Wang, W.; Liang, Y.; Tang, Y. Uniform ultrasmall manganese monoxide nanoparticle/carbon nanocomposite as a high-performance anode for lithium storage. Electrochim. Acta 2016, 196, 634–641. [Google Scholar] [CrossRef]
- Sun, B.; Chen, Z.; Kim, H.-S.; Ahn, H.; Wang, G. MnO/C core–shell nanorods as high capacity anode materials for lithium-ion batteries. J. Power Sour. 2011, 196, 3346–3349. [Google Scholar] [CrossRef]
- Huang, J.; Ouyang, L.; Gu, Q.; Yu, X.; Zhu, M. Metal-Borohydride-Modified Zr(BH4)4·8NH3: Low-Temperature dehydrogenation yielding highly pure hydrogen. Chem. Eur. J. 2015, 21, 14931–14936. [Google Scholar] [CrossRef]
- Zheng, J.; Xiao, X.; Zhang, L.; Li, S.; Ge, H.; Chen, L. Facile synthesis of bowl-like 3D Mg (BH4)2–NaBH4–fluorographene composite with unexpected superior dehydrogenation performances. J. Mater. Chem. A 2017, 5, 9723–9732. [Google Scholar] [CrossRef]
- Xue, Z. Raman Spectroscopy of Carboxylic Acid and Water Aggregates; Logos Verlag: Berlin, Germany; GmbH: Berlin, Germany, 2011. [Google Scholar]
- Ramesh, S.; Leen, K.H.; Kumutha, K.; Arof, A.K. FTIR studies of PVC/PMMA blend based polymer electrolytes. Spectrochim. Acta Part A 2007, 66, 1237–1242. [Google Scholar] [CrossRef]
- Dai, H.-L. Spectroscopy, Structure, and Energy Transfer of Transient Radicals in Combustion; University of Pennsylvania: Philadelphia, PA, USA, 2007. [Google Scholar]
- Gómez-Ordóñez, E.; Rupérez, P. FTIR-ATR spectroscopy as a tool for polysaccharide identification in edible brown and red seaweeds. Food Hydrocolloids 2011, 25, 1514–1520. [Google Scholar] [CrossRef]
- Cornelius, C.J.; Marand, E. Hybrid inorganic–organic materials based on a 6FDA–6FpDA–DABA polyimide and silica: Physical characterization studies. Polymer 2002, 43, 2385–2400. [Google Scholar] [CrossRef]
- Gautam, C.; Yadav, A.K.; Mishra, V.K.; Vikram, K. Synthesis, IR and Raman spectroscopic studies of (Ba, Sr) TiO3 borosilicate glasses with addition of La2O3. Open J. Inorg. Non Met. Mater. 2012, 2, 47–54. [Google Scholar]
- Lopes, J.d.O.; Garcia, R.A.; Souza, N.D.D. Infrared spectroscopy of the surface of thermally-modified teak juvenile wood. Maderas. Ciencia Tecnología 2018, 20, 737–746. [Google Scholar] [CrossRef]
- Mahmood, Z.; Azam, M.; Mushtaq, A.; Kausar, R.; Kausar, S.; Gilani, S.R. Comparative vapour phase FTIR spectra and vibrational assignment of manganese pentacarbonyls derivatives of the type XMn (CO)5: (where X = Br, Cl, I, H, D, CH3, CD3, CF3). Spectrochim. Acta Part A 2006, 65, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.-Z.; Jackson, M.; Sowa, M.G.; Ju, H.; Dixon, I.M.; Mantsch, H.H. Modification of the extracellular matrix following myocardial infarction monitored by FTIR spectroscopy. BBA Mol. Basis Dis. 1996, 1315, 73–77. [Google Scholar] [CrossRef]
- Genady, A.R. Labeled undecahydro-closo-dodecaborates based on azo dyes for boron neutron capture therapy: Synthesis, characterization, and visualization in cells. Acta Chim. Slov. 2012, 59, 1. [Google Scholar]
- Nelson, M.A.; Kodama, G. Deuterated sodium octahydrotriborate (1-). Inorg. Chem. 1979, 18, 3276–3278. [Google Scholar] [CrossRef]
- Gaines, D.F.; Schaeffer, R.; Tebbe, F. Convenient preparations of solutions containing the triborohydride ion. Inorg. Chem. 1963, 2, 526–528. [Google Scholar] [CrossRef]
- Chen, W.; Wu, G.; He, T.; Li, Z.; Guo, Z.; Liu, H.; Huang, Z.; Chen, P. An improved synthesis of unsolvated NaB3H8 and its application in preparing Na2B12H12. Int. J. Hydrogen Energy 2016, 41, 15471–15476. [Google Scholar] [CrossRef]
- Mirghani, M.; Man, Y.C.; Jinap, S.; Baharin, B.; Bakar, J. FTIR spectroscopic determination of soap in refined vegetable oils. J. Am. Oil Chem. Soc. 2002, 79, 111–116. [Google Scholar] [CrossRef]
- Ramalingam, M.; Sundaraganesan, N.; Saleem, H.; Swaminathan, J. Experimental (FTIR and FT-raman) and ab initio and DFT study of vibrational frequencies of 5-amino-2-nitrobenzoic acid. Spectrochim. Acta Part A 2008, 71, 23–30. [Google Scholar] [CrossRef]
- Gibson, N.; Shenderova, O.; Luo, T.; Moseenkov, S.; Bondar, V.; Puzyr, A.; Purtov, K.; Fitzgerald, Z.; Brenner, D. Colloidal stability of modified nanodiamond particles. Diam. Relat. Mater. 2009, 18, 620–626. [Google Scholar] [CrossRef]
- Caputo, R.; Garroni, S.; Olid, D.; Teixidor, F.; Suriñach, S.; Baro, M.D. Can Na2[B12H12] be a decomposition product of NaBH4? Phys. Chem. Chem. Phys. 2010, 12, 15093–15100. [Google Scholar] [CrossRef]
- Purkayastha, D.; Sarma, B.; Bhattacharjee, C. Surfactant-assisted low-temperature synthesis of monodispersed phase pure cubic CoO solid nanoparallelepipeds via thermal decomposition of cobalt (II) acetylacetonate. Mater Lett. 2013, 107, 71–74. [Google Scholar] [CrossRef]
- Züttel, A.; Rentsch, S.; Fischer, P.; Wenger, P.; Sudan, P.; Mauron, P.; Emmenegger, C. Hydrogen storage properties of LiBH4. J. Alloys Compd. 2003, 356, 515–520. [Google Scholar] [CrossRef]
- Fan, B.; Wei, G.; Zhang, Z.; Qiao, N. Characterization of a supramolecular complex based on octadecylamine and β-cyclodextrin and its corrosion inhibition properties in condensate water. Corros. Sci. 2014, 83, 75–85. [Google Scholar] [CrossRef]
- Jiang, H.; Moon, K.-S.; Dong, H.; Hua, F.; Wong, C. Size-Dependent melting properties of tin nanoparticles. Chem. Phys. Lett. 2006, 429, 492–496. [Google Scholar] [CrossRef]
- Nanda, K. Size-Dependent melting of nanoparticles: Hundred years of thermodynamic model. Pramana 2009, 72, 617–628. [Google Scholar] [CrossRef]
- Olson, E.; Efremov, M.Y.; Zhang, M.; Zhang, Z.; Allen, L. Size-Dependent melting of Bi nanoparticles. J. Appl. Phys. 2005, 97, 034304. [Google Scholar] [CrossRef]
- Honig, R.E. Vapor Pressure Data for the More Common Elements; David Sarnoff Research Center: Princeton, NJ, USA, 1957. [Google Scholar]
- Ngene, P.; van den Berg, R.; Verkuijlen, M.H.; de Jong, K.P.; de Jongh, P.E. Reversibility of the hydrogen desorption from NaBH4 by confinement in nanoporous carbon. Energy Environ. Sci. 2011, 4, 4108–4115. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Aguey-Zinsou, K.-F. Direct Synthesis of NaBH4 Nanoparticles from NaOCH3 for Hydrogen Storage. Energies 2019, 12, 4428. https://doi.org/10.3390/en12234428
Wang T, Aguey-Zinsou K-F. Direct Synthesis of NaBH4 Nanoparticles from NaOCH3 for Hydrogen Storage. Energies. 2019; 12(23):4428. https://doi.org/10.3390/en12234428
Chicago/Turabian StyleWang, Ting, and Kondo-Francois Aguey-Zinsou. 2019. "Direct Synthesis of NaBH4 Nanoparticles from NaOCH3 for Hydrogen Storage" Energies 12, no. 23: 4428. https://doi.org/10.3390/en12234428
APA StyleWang, T., & Aguey-Zinsou, K.-F. (2019). Direct Synthesis of NaBH4 Nanoparticles from NaOCH3 for Hydrogen Storage. Energies, 12(23), 4428. https://doi.org/10.3390/en12234428