Abstract
Grass from landscape management or from agricultural practices is currently destined mainly for composting, with the production of a valuable product; however, this process demands energy. Anaerobic digestion, instead, represents an energy-positive process that results in the production of fuel, biogas, and a fertilizer, namely digestate. Previous tests for the evaluation of biogas yield from freshly harvested grass gave promising results. However, for a practical exploitation of this resource, appropriate conservation is necessary in order to enable the daily load of digesters while reducing the loss of organic matter. The present work is focused on the evaluation of biogas and methane yield from dried and ensiled grass (without conditioning) in order to assess eventual biogas potential losses in comparison to digested fresh grass. Tests were performed with grass collected from riverbanks (Veneto, Northern Italy) in batch, lab scale digesters. Dry and ensiled grass showed a good potential for exploitation in the anaerobic digestion process, reaching biogas yields of 565.9 and 573.4 NL∙kgVS−1, respectively. Compared to the biogas yield of 639.7 NL∙kgVS−1 of the fresh grass, the conservation treatment determined yield reductions of 11.5% and 10.4% for dried and ensiled grass, respectively. However, considering the methane yields, conservation treatments showed lower reductions, amounting to 4.8% for dry grass and 0.5% for ensiled grass; presumably the higher concentration of organic acids in ensiled grass determined a higher methane content in biogas and the consequently lower reduction of methane yield.
1. Introduction
The primary sector can play a significant role to reach the goals of sustainable development and circular economy by the exploitation of wastes or by-products for the production of renewable energy [1,2,3,4,5,6].
One of the most promising technologies for the management of fermentable organic wastes is represented by anaerobic digestion (AD) [7]: This energy-positive process provides high values products, such as biogas and digestate [8,9], but it gives a significant contribution to the reduction of the emissions of greenhouse gasses, methane (CH4) and carbon dioxide (CO2) in particular, and of odors deriving from uncontrolled fermentations [10,11].
Agricultural practices and landscape management produce relevant quantities of biomasses that could be successfully treated by the AD process but are not completely exploited [12,13]. Composting still represents the main destination of these feedstocks: This mature process determines the production of a valuable soil amendment, compost, but also requires energy [14]. Lignin-rich biomasses (e.g., branches and wood) can be considered to be best suitable for the composting process or thermal treatment (e.g., gasification and incineration). Grass from landscape management, instead, could represent a promising product to enhance the sustainability of the biogas field [15,16,17,18,19]. As matter of fact, grass does not compete with food production, and it is available in different areas worldwide, including the Italian territory [20] and uncultivated areas of the Veneto territory [21]. Studies focused on the definition of quantity and composition of grass are still missing, but these areas are characterized by a significant quantity of biomass [22,23]. Botanical composition is normally represented by Poaceae spp., Fabaceae spp., or Asteracee spp., but other species of grasses can be found depending on the area [24,25].
The physical–chemical characteristics of grass make this feedstock suitable for exploitation in the AD process. Scientific studies have reported a net energy production from grass—determined as the MWh of renewable CH4 per MWh of fossil energy invested—of 7.4–15.5 (Poaceae spp.) compared to 8–25 (Maize, Zea mays L), depending on the agricultural option considered [8,9].
Laboratory tests allow for the assessment of the degradability of fermentable products and their consequent potential biogas/biomethane yield [26,27]. Ahn et al. [28] determined the biogas yield from switchgrass in a dry AD codigestion process with manures from different animals, while the specific yield from grass was assessed in a batch leach bed by Lehtomäki et al. [29] in a two-phase system [30].
The present research is focused on the evaluation of biogas and biomethane yield from grass collected from riverbanks of Veneto, Northern Italy. In a previous study, the biogas/biomethane yield of fresh spring and summer grass was assessed [31], showing a yield quite similar to the yield obtained from energy crops, with biomethane potentials (BMP) of 340.2 and of 307.7 NL∙kg−1VS, respectively, for spring and summer grass. Despite the promising results obtained from fresh grass, for a practical exploitation of this resource, appropriate conservation is necessary, i.e., drying and ensiling, in order to enable the daily load of digesters while reducing the loss of organic matter. These techniques can potentially determine a loss of organic matter: Drying essentially removes moisture from the feedstock, reducing and eventually stopping the respiration processes that determine the loss of energetic content. Ensiling, instead, is a chemical/biological process that induces the formation of acids in anoxic conditions, with a reduction of pH and consequent stability of the feedstock. The present work, representing a second stage of the previous work, is focused on the evaluation of biogas and methane yield from grass after drying and ensiling in order to assess eventual biogas potential losses in comparison to biogas yields from fresh grass.
2. Material and Methods
2.1. Experimental Site and Field Operations
The AD experiments were conducted on grass collected in early spring from riverbanks of the Adige river in the vicinity of Boara Pisani (Rovigo, Northern Italy). Grass was subjected to visual analysis for the determination of the different species.
Grass was cut by means of scissors, achieving a final size of 40 mm, in order to simulate the operation of forage harvesting machines (i.e., cut and size reduction). Part of the fresh grass was immediately loaded in the digesters for the first test run, another part was air dried for two days, and a final part was ensiled.
The experimental silos are represented by airtight cylinders with a sliding cover to achieve appropriate compression of the feedstock (Figure 1). The ensiling process was performed without the conditioning of the feedstock (i.e., moisture correction) and without additives (i.e., bacteria or enzymes).

Figure 1.
View of the harvesting area (A), detail of the cutting (B), and of the small-scale silo (C).
2.2. Lab Scale Anaerobic Digestion System and Experimental Setup
The laboratory anaerobic digestion system consisted of 6 reactors (4 l each) equipped with a propeller-type mixer (Figure 2).

Figure 2.
Detail of the experimental anaerobic digestion system.
Temperature was controlled by thermostatic baths with electric resistance controlled by digital thermostat, with a water recirculation pump to achieve homogeneous conditions in the tank. Biogas from the digesters was conveyed to condensation traps and biogas meters (Milligascounter, Ritter, Germany) that continuously registered the volume of produced biogas. Biogas quality (CH4 and CO2) was determined by Siemens Ultramat IR analyzer (Siemens Automation Group, Karlsruhe, Germany), and H2S quality was determined by ProTec gas pump with (Komyo Kitagawa, Japan) detection tubes.
As inoculum digestate from a full-scale biogas plant—a 700 m3 single stage system with hydraulic retention time (HRT) of 30 days—was used. The plant operated at mesophilic temperatures (38–40 °C) and was fed with a single feedstock represented by cow manure. The constant characteristics of manure allowed us to achieve a digestate with relatively constant characteristics, essential for the present tests.
Three test runs were conducted in mesophilic conditions (38 °C) for 40 days. In each test run, the reactors—1, 2 and 3—were dedicated to the inoculum (2.5 kg) to assess the residual production from this substrate; reactors 4, 5 and 6 were filled with a mixture of inoculum and grass, dosed to reach an inoculum: Substrate vs inoculum ratio of 2:1, as required by standard biological methane production (BMP tests) [32]:
- Test 1: 2.5 kg of inoculum, 0.5 kg of water, 0.3 kg of fresh grass;
- Test 2: 2.5 kg of inoculum, 0.7 kg of water, 0.1 kg of dry grass;
- Test 3: 2.5 kg of inoculum, 0.5 kg of water, 0.3 kg of ensiled grass.
Net biogas production was calculated by subtracting the production of reactors 4, 5 and 6, and the residual production from the inoculum was measured in digesters 1, 2 and 3. BMP, referred to as the mass unit of volatile solids (VS) contained in input grass (l CH4∙ kgVS−1 or ml CH4∙ gVS−1), was calculated from the combination of net production of biogas and of CH4 concentration.
Input and output were subject to the determination of total solids (TS), volatile solids (VS), pH, fibers (cellulose, lignin, hemicellulose), total Kjeldahl nitrogen (TKN), NH4+ [33]. Redox potential was measured by portable pH/redox probe (Steiel, Padova, Italy). Volatile fatty acids (VFA), alkalinity, and acidity vs. alkalinity ratio were determined by a Biogas Titration Manager (Hach Lange, Düsseldorf, Germany) [34].
In each test, the removal efficiency (R, expressed in %) of TS, VS, TKN, and NH4+ was calculated with the Equation (1):
where CA in and CA out are the concentrations of the chemical parameter A in the input mix and in the digestate, respectively.
3. Results and Discussion
3.1. Characteristics of the Feedstocks
Grass was mainly composed by Poaceae spp, with prevalence of Poa spp. and Festuca spp. Minor percentages of other species, such as Asteraceae spp., Equisetaceae spp. and Polygonaceae spp. were also detected [31,35]. Table 1 reports the characteristics of the inoculum of fresh spring grass, dry grass, and ensiled grass.

Table 1.
Characteristics of the inoculum of fresh, dry and ensiled grass (n.d. indicates not determined).
Fresh grass presented a TS content of 32.43% and a VS content of 79.21%TS. Dry grass presented a higher content of TS, 89.08%, and a VS content of 87.73%TS. Ensiled grass was characterized by a TS content of 30.83% and a VS content of 69.17%TS. The ensiled grass was characterized by a pH of 5.23 as a consequence of the formation of acids typical of the ensiling process.
The inoculum resulted in a mesophilic, complete mix digester operating wet fermentation (quite typical for an agricultural digestate). Furthermore, the characteristics of the samples of the different test results were similar, as expected considering that the biogas plant was fed with a single feedstock (manure from dairy cows). The inoculum was characterized by a TS content of 7.85%–8.61%, typical concentration of solids for a wet AD process [31,36], and a VS content ranging from 79.77%TS to 81.19%TS. TKN varied from 4.56 to 5.58 g∙L−1, while NH4+ ranged from 2.35 to 3.22 g∙L−1, quite constant and typical for a biogas plant fed with dairy manure. A redox potential between 395 and −400 mV indicates good anaerobic conditions, and acidity alkalinity values between 0.22 and 0.24, along with limited concentrations of VFA (Table 2) indicate a good efficiency of the AD process in the full-scale plant [31,34,37].

Table 2.
Characteristics of digestate from the tests (n.d. indicates not determined).
The input mix for the fresh spring grass presented a TS concentration of 8.90%, the input mix with dry grass presented a TS concentration of 8.62%, and the input mix with ensiled grass presented a TS concentration of 9.32% (calculated values)—all typical of a wet AD process and adequate to achieve an efficient mixing/stirring of the substrates [31]. The input mix presented VS concentrations of 80.27%TS, 78.98%TS, and 81.45%TS for fresh, dry, and ensiled grass mix, respectively. The TKN concentrations were 4.10, 4.04, and 3.63 g∙kg−1 for fresh, dry, and ensiled grass mix, respectively.
Digestates collected at the end of the tests presented reductions of TS and VS, as an effect of the degradation of the organic matter by anaerobic microorganisms (Table 2). In particular, the removal efficiency of TS was 29.4%, 29.7% and 31.2%, while the removal efficiency for VS was 37.0%, 32.2% and 39.7%, respectively, for fresh, dry, and ensiled grass. Thus, the highest removal efficiencies were reached by the AD process of the ensiled grass, probably due to the partial acidification of the organic substances during ensiling.
The TKN concentrations in the digestate were 3.99, 3.30 and 3.34 g∙kg−1, respectively, for fresh, dry, and ensiled grass; NH4+ concentrations were 2.29, 1.88 and 1.88 g∙kg−1, respectively, for fresh, dry, and ensiled grass. The appropriate mixing of the inoculum with grasses did not cause a relevant increase of nitrogen concentration in the mixture subject to digestion, maintaining NH4 concentration below toxicity values [10].
The redox potential represents a valid indication of the reductive or oxidative conditions in a substrate [31]: Digestates presented values of −397, −348, and −384 mV for fresh, dry, and ensiled grass, respectively, to indicate appropriate anaerobic conditions [10].
The acidity/alkalinity ratios of the three digestates were similar, with values of 0.15, 0.17, and 0.19 for fresh, dry, and ensiled grass, respectively—not far from the lower value of the optimal range of 0.20–0.50 [10,34]: This indicates that the process was successful in achieving a good degradation of VFA by AD populations, but it also underlines that the organic load was relatively low. This parameter, in fact, is usually used in continuous plants to estimate the correct load of the digesters and the efficiency of the response of bacteria in terms of degradation of VFA. The highlighted range is a reference, and, in specific cases, the performance of the full-scale digester could be optimal even in a different range of values. Furthermore, in the specific case of a discontinuous process, the same parameter underlines a good degradation of grass with conversion of acids into methane.
In detail (Table 3), ensiled grass was characterized by a concentration of lactic acid of 0.70 g∙L−1, acetic acid of 1.64 g∙L−1, propionic acid of 2.75 g∙L−1, iso-butyric acid of 0.33 g∙L−1, n-butyric acid of 0.37 g∙L−1, iso-valerianic of 0.19 g∙L−1, n-valerianic 0.10 g∙L−1 and negligible caproic acid.

Table 3.
Concentration of volatile fatty acids in the ensiled grass and in digestate from the tests.
3.2. Biogas Quality
For each test run, the biogas quality in terms of concentration of CH4 and CO2, appeared to be relatively constant after the first week until the end of the test, after 40 days from the start (Figure 3). In fact, for fresh spring grass CH4 concentration ranged from a minimum of 46%, measured in the initial phase of the process (in correspondence of Day 3) to a maximum of 55.2% recorded at Day 6; after, the concentration remained relatively steady and relatively close to the mean value of 51.5% ± 1.4%—quite typical for co-digestion processes.
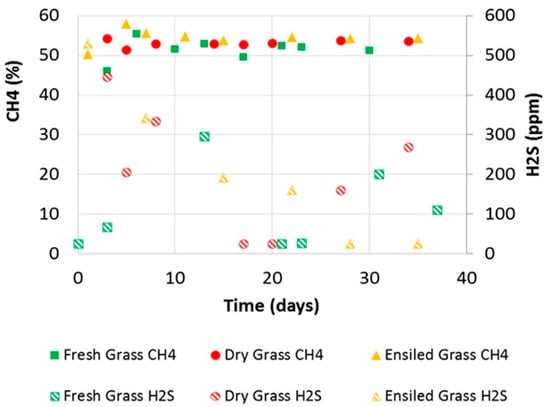
Figure 3.
Biogas quality in terms of CH4, CO2, and H2S concentration, for fresh, dry and ensiled grass.
CO2 concentration in biogas from fresh spring grass resulted in an average value of 46.3% ± 1.4%, with a peak value of 53% measured at Day 3, in correspondence with the minimum concentration of CH4 when the methanogenic activity was not fully developed; the minimum CO2 concentration was 43.6%.
The concentration of H2S was subject to larger fluctuations, presenting a peak of 295 ppm registered in Day 13, after the production peak of Day 6 (Figure 3), and a minimum below the threshold of detection (25 ppm) and average concentration resulted in 93.8 ppm, with a standard deviation 123.7 ppm to highlight these fluctuations.
Biogas from the anaerobic digestion of dry spring grass showed an average CH4 concentration of 53.2% ± 0.5% with a maximum of 54.4% recorded at Days 3 and 41 and a minimum at Day 5. CO2 showed a complementary evolution, with a minimum of 40.7% recorded at Day 3 and a maximum of 47.8% recorded at Day 5; the average CO2 concentration was 45% ±1.3%. The test with dry grass, hence, showed slight better results in terms of biogas quality (i.e., CH4 concentration), with a more rapid achievement of steady methanogenic production. Additionally, in the case of dry-grass, the concentration of H2S was subject to large fluctuations, with a peak of 446 ppm, higher than the peak of fresh grass, corresponding to Day 3 after initial production peak of Day 2 (Figure 3) and minimum below the threshold of detection (25 ppm). The average concentration was also higher, at 278 ppm.
Biogas from the anaerobic digestion of ensiled grass showed an average CH4 concentration of 54.5% ± 1.1%, higher than the average obtained in the previous two tests of 3.0 and 1.3 points, respectively. The minimum was recorded at the beginning of the test and was 50.3%; the maximum value was 57.9%, higher than 53% and 54.4% recorded for fresh and dry grass, respectively, but with a slight delay in time recorded at Day 5 after the initial peak of production of Day 4 (Figure 3). CO2 showed a minimum of 42.6% recorded at Day 5 and a maximum of 45.9% recorded at Day 35; the average CO2 concentration was 44.6% ± 0.9%. The test with ensiled grass showed steady concentrations of CH4 at higher levels than the fresh and dried grass. H2S concentration from the ensiled grass test was subject to large fluctuations, with a peak of 530 ppm, higher than the peak of fresh and dry grass, recorded in correspondence with Day 1—in this case before the production peak—and a minimum below the threshold of detection (25 ppm). The average concentration was 212 ppm, with a standard deviation of 123 ppm also highlighting these fluctuations; this result was higher than 98 ppm recorded for fresh grass but lower than the 278 ppm of dry grass. The average concentrations resulting from all the three tests, and even the peak, were lower than the 300 ppm referred to by manufacturers as a safe threshold for combined heat and power units: Potentially, H2S removal systems may not be necessary, but they are still highly suggested.
A treatment option could be represented by biological removal by Thiobacillus in micro aeration conditions case: Mulbry and colleagues [38] reported an H2S reduction from 74% to >99% on biogases containing H2S from 800 to 7500 mg∙m−3.
In general, biogas quality was appropriate with both drying and ensiling, but it was higher for ensiled grass: This result could be explained by considering that the ensiling process determines the instauration of acid fermentations, leading to the production of VFAs that enhance the methanogenic process.
Yu and colleagues obtained a higher CH4 concentration (average 71%) in biogas from a two-phase AD process treating grass [30], more comparable to a dry AD process with the recirculation of leachate [28] but simulating the degradation times of a landfill: This difference could have been influenced by the chemical composition of grass and different type of processes. The fact that different methane concentrations could be achieved from grass by implementing different anaerobic digestion processes was highlighted by various researches, reporting 71% for upflow anaerobic sludge blanket and 70% for large BMP units, 51%–54% for smaller scale BMP units, and 52% for CSTR digesters [39].
3.3. Biogas and Biomethane Yield
The residual biogas production from inoculum was 12.2, 10.8 and 10.5 L∙kg−1 (on digestate wet basis) for fresh, dry, and ensiled grass test, respectively. As highlighted in previous works [31,35], it is interesting to underline how the inoculum from the full-scale digester (HRT 30 days) still showed biogas potential after 40 days of the AD process, indicating that the farm could have achieved more gas from manure with a bigger digester (higher HRT). A further indication could be that a week could not be sufficient to obtain complete degasification of the inoculum from standard BMP test practices, with a consequent risk of overestimating the actual yield from the feedstock under evaluation.
Figure 4 depicts the cumulated and daily biogas and CH4 yields compared to VS. Each curve represents the net yield of each feedstock (i.e., fresh spring grass, dry grass, and ensiled grass) obtained as average of the three digesters treating grass in each run after subtracting the production of the inoculum.
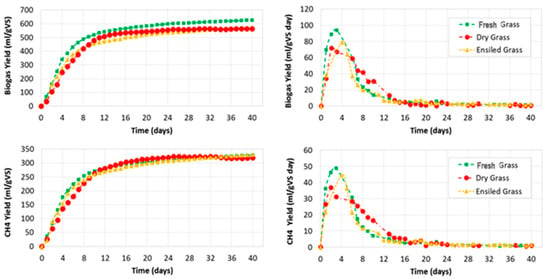
Figure 4.
Cumulated and daily biogas yield and CH4 yield referred to as volatile solids (VS).
Figure 5 shows biogas yield and methane yield of fresh, dry, and ensiled spring grass compared to wet mass, total solids, and volatile solids. Fresh grass showed a rapid increase of production, reaching a peak of 94.0 ± 11.0 mL∙gVS−1 at Day 3, corresponding to a cumulated production of 252.5 ± 30.7 mL∙gVS−1. From Day 3 to Day 7, daily biogas production presented a rapid drop, reaching 33.3 ± 2.6 mL∙gVS−1, followed by a slow but constant reduction to reach values below 2.0 mL∙gVS−1 from Day 14. On Day 7, the cumulated production was 463.6 ± 75.3 mL∙gVS−1; on Day 14, the cumulated production was 556.2 mL∙gVS−1, and, finally, on Day 40, the cumulated production was 627.7 ± 95.4 mL∙gVS−1. While the analyses of the daily biogas yield showed that the main production was concentrated in the first 14 days of the process, the analysis of the cumulated productions showed that from Day 14 to Day 40, the yield still increased 11%.
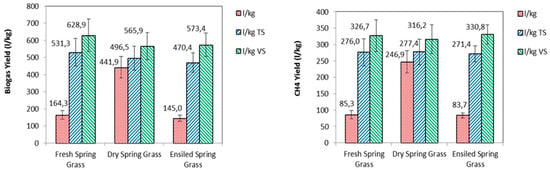
Figure 5.
Biogas yield and methane yield of fresh, dry, and ensiled spring grass compared to wet mass, total solids and volatile solids.
This result shows that grass was sufficiently degraded in relatively short times, but the optimization of the process was achieved within 40 days: This indicates that an HRT of 45 days normally adopted in co-digestion plants can guarantee a complete exploitation of these feedstocks [31].
4. Conclusions
The present study shows that grass could be successfully employed as feedstock in the AD process for the production of biogas or biomethane, with an HRT comparable to that adopted for conventional feedstocks. The AD tests were performed for 40 days, but most of biogas production took place within the first 12 days of process.
The biogas yield of fresh grass was 628.9 NL∙kgVS−1, similar to the yield from energy crops [18,32]. This result, higher than expected, can be explained by considering that the feedstock was loaded in the reactors briefly after being harvested with the highest availability of organic matter. For a practical exploitation of this resource, however, appropriate conservation methods are necessary (e.g., drying or ensiling) in order to enable the daily load of digesters while reducing the losses of fermentable organic matter (i.e., biogas yield).
The lab scale AD tests indicated that grass can be successfully used to produce biogas or biomethane even after drying or ensiling: Dry and ensiled grass showed a biogas yield of 565.9 and 573.4 NL∙kgVS−1, respectively. However, the conservation determined a yield reduction of 11.5% and 10.4%, respectively, which could be considered acceptable in most cases. Dry grass showed a methane yield of 316.2 NL∙kgVS−1, 4.8% lower than the 332.3 NL∙kgVS−1 obtained from fresh grass; for ensiled grass, the higher concentration of organic acids in ensiled grass determined a higher methane content in biogas and a consequent lower reduction of methane yield, which resulted in 330.8 NL∙kgVS−1, with a 0.5% decrease compared to fresh grass.
This result opens the perspective of effective energetic exploitation of grass from parks, riverbanks, public areas, or other green areas.
Author Contributions
Conceptualization, A.C., A.P., and F.d.B.; Methodology, A.C., A.P., and F.d.B.; Software, A.C., A.P., and D.B.; Validation, A.C., A.P., F.d.B., and D.B.; Formal Analysis, A.C., A.P., F.d.B., and D.B.; Investigation, A.C., A.P., F.d.B., and D.B.; Resources, A.C., A.P., and F.d.B.; Data Curation, A.C., A.P., F.d.B., and D.B.; Writing-Original Draft Preparation, A.C., A.P., and F.d.B.; Writing-Review & Editing, A.C., A.P., and F.d.B.; Visualization, A.C., A.P., and F.d.B.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank Penny Lazo for the professional and kind support for the proof reading and English language editing of the paper and Amedeo Bizzotto for the precious assistance in the tests.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Amon, T.; Amon, B.; Kryvoruchko, V.; Machmüller, A.; Hopfner-Sixt, K.; Bodiroza, V.; Hrbek, R.; Friedel, J.; Pötsch, E.; Wagentristl, H.; et al. Methane production through anaerobic digestion of various energy crops grown in sustainable crop rotations. Bioresour. Technol. 2007, 98, 3204–3212. [Google Scholar] [CrossRef] [PubMed]
- Appels, L.; Lauwers, J.; Degrève, J.; Helsen, L.; Lievens, B.; Willems, K.; Van Impe, J.; Dewil, R. Anaerobic digestion in global bio-energy production: Potential and research challenges. Renew. Sustain. Energy Rev. 2011, 15, 4295–4301. [Google Scholar] [CrossRef]
- Coppolecchia, D.; Gardoni, D.; Baldini, C.; Borgonovo, F.; Guarino, M. The influence on biogas production of three slurry-handling systems in dairy farms. J. Agric. Eng. 2015, 46, 30–35. [Google Scholar] [CrossRef]
- Chiumenti, A.; Boscaro, D.; da Borso, F.; Sartori, L.; Pezzuolo, A. Anaerobic digestion of grass: Effect of the harvesting period on biogas yield. In ASABE Annual International Meeting 2017; Spokane: Washington, DC, USA, 2017. [Google Scholar]
- Da Borso, F.; Chiumenti, A.; Sigura, M.; Pezzuolo, A. Influence of automatic feeding systems on design and management of dairy farms. J. Agric. Eng. 2017, 48, 48–52. [Google Scholar] [CrossRef]
- Chiumenti, A.; da Borso, F.; Limina, S. Dry anaerobic digestion of cow manure and agricultural products in a full-scale plant: Efficiency and comparison with wet fermentation. Waste Manag. 2018, 71, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.F.; Chiumenti, A.; Savage, G.M.; Eggerth, L.L. Managing the Organic fraction of Municipal Solid Waste. BioCycle 2006, 47, 50–54. [Google Scholar]
- Gerin, P.A.; Vliegen, F.; Jossart, J.M. Energy and CO2 balance of maize and grass as energy crops for anaerobic digestion. Bioresour. Technol. 2008, 99, 2620–2627. [Google Scholar] [CrossRef]
- Pöschl, M.; Ward, S.; Owende, P. Evaluation of energy efficiency of various biogas production and utilization pathways. Appl. Energy 2010, 87, 3305–3321. [Google Scholar] [CrossRef]
- Chiumenti, R.; Chiumenti, A.; da Borso, F.; Limina, S.; Landa, A. Anaerobic Digestion of Swine Manure in Conventional and Hybrid Pilot Scale Plants: Performance and Gaseous Emissions Reduction. In Proceedings of the International Syposium ASABE 2009, Reno, NV, USA, 21–24 June 2009. [Google Scholar]
- Dinuccio, E.; Balsari, P.; Gioelli, F.; Menardo, S. Evaluation of the biogas productivity potential of some Italian agro-industrial biomasses. Bioresour. Technol. 2010, 101, 3780–3783. [Google Scholar] [CrossRef]
- Boscaro, D.; Pezzuolo, A.; Grigolato, S.; Cavalli, R.; Marinello, F.; Sartori, L. Preliminary analysis on mowing and harvesting grass along riverbanks for the supply of anaerobic digestion plants in north-eastern Italy. J. Agric. Eng. 2015, 46, 100–104. [Google Scholar] [CrossRef]
- Valenti, F.; Porto, S.M.; Cascone, G.; Arcidiacono, C. Potential biogas production from agricultural by-products in Sicily. A case study of citrus pulp and olive pomace. J. Agric. Eng. 2017, 48, 196–202. [Google Scholar] [CrossRef]
- Eggerth, L.L.; Diaz, L.F.; Chang, M.T.F.; Iseppi, L. Marketing of composts. Waste Manag. 2007, 8, 325–355. [Google Scholar]
- Blokhina, Y.N.; Prochnow, A.; Plöchl, M.; Luckhaus, C.; Heiermann, M. Concepts and profitability of biogas production from landscape management grass. Bioresour. Technol. 2011, 102, 2086–2092. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.; Zhong, J.; Hansen, J. Anaerobic digestion of dairy processing waste, algae, and grass in pilot and full scale. Trans. ASABE 2014, 57, 609–614. [Google Scholar]
- Tsapekos, P.; Kougias, P.G.; Egelund, H.; Larsen, U.; Pedersen, J.; Trénel, P.; Angelidaki, I. Improving the energy balance of grass-based anaerobic digestion through combined harvesting and pretreatment. Anaerobe 2017, 46, 131–137. [Google Scholar] [CrossRef]
- Wahid, R.; Feng, L.; Cong, W.; Ward, A.J.; Møller, H.B.; Eriksen, J. Anaerobic mono-digestion of lucerne, grass and forbs—Influence of species and cutting frequency. Biomass Bioenergy 2018, 109, 199–208. [Google Scholar] [CrossRef]
- Bedoić, R.; Čuček, L.; Ćosić, B.; Krajnc, D.; Smoljanić, G.; Kravanja, Z.; Ljubas, D.; Pukšec, T.; Duić, N. Green biomass to biogas—A study on anaerobic digestion of residue grass. J. Clean. Prod. 2019, 213, 700–709. [Google Scholar] [CrossRef]
- Boscaro, D.; Pezzuolo, A.; Sartori, L.; Marinello, F.; Mattioli, A.; Bolzonella, D.; Grigolato, S. Evaluation of the energy and greenhouse gases impacts of grass harvested on riverbanks for feeding anaerobic digestion plants. J. Clean. Prod. 2018, 172, 4099–4109. [Google Scholar] [CrossRef]
- Mattioli, A.; Boscaro, D.; Dalla Venezia, F.; Santacroce, F.C.; Pezzuolo, A.; Sartori, L.; Bolzonella, D. Biogas from residual grass: A territorial approach for sustainable bioenergy production. Waste Biomass Valorization 2017, 8, 2747–2756. [Google Scholar] [CrossRef]
- Pappalardo, S.; Prosdocimi, M.; Tarolli, P.; Borin, M. Assessment of Energy Potential from Wetland Plants along the Minor Channel Network on an Agricultural Floodplain. Environ. Sci. Pollut. Res. 2014, 22, 2479–2490. [Google Scholar] [CrossRef]
- Colantoni, A.; Delfanti, L.; Recanatesi, F.; Tolli, M.; Lord, R. Land use planning for utilizing biomass residues in Tuscia Romana (central Italy): Preliminary results of a multi criteria analysis to create an agro-energy district. Land Use Policy 2016, 50, 125–133. [Google Scholar] [CrossRef]
- Tsapekos, P.; Kougias, P.G.; Egelund, H.; Larsen, U.; Pedersen, J.; Trénel, P.; Angelidaki, I. Mechanical pretreatment at harvesting increases the bioenergy output from marginal land grasses. Renew. Energy 2017, 111, 914–921. [Google Scholar] [CrossRef]
- Hensgen, F.; Richter, F.; Wachendorf, M. Integrated generation of solid fuel and biogas from green cut material from landscape conservation and private households. Bioresour. Technol. 2011, 102, 10441–10450. [Google Scholar] [CrossRef] [PubMed]
- Bishop, G.C.; Burns, R.T.; Shepherd, T.A.; Moody, L.B.; Gooch, C.A.; Spajic, R.; Pronto, J. Evaluation of laboratory biochemical methane potentials as a predictor of anaerobic dairy manure digester biogas and methane production. In Proceedings of the American Society of Agricultural and Biological Engineers Annual International Meeting, Reno, NV, USA, 21–24 June 2009; pp. 5254–5265. [Google Scholar]
- Safferman, S.I.; Kirk, D.M.; Faivor, L.L.; Haan, W.W. Bioremediation and Sustainability: Research and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 103–136. [Google Scholar]
- Ahn, H.K.; Smith, M.C.; Konrad, S.L.; White, J.W. Evaluation of biogas production potential by dry anaerobic digestion of switchgrass-animal manure mixtures. Appl. Biochem. Biotechnol. 2010, 160, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Lehtomäki, A.; Huttunen, S.; Lehtinen, T.M.; Rintala, J.A. Anaerobic digestion of grass silage in batch leach bed processes for methane production. Bioresour. Technol. 2008, 99, 3267–3278. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.W.; Samani, Z.; Hanson, A.; Smith, G. Energy recovery from grass using two-phase anaerobic digestion. Waste Manag. 2002, 22, 1–5. [Google Scholar] [CrossRef]
- Chiumenti, A.; Boscaro, D.; Da Borso, F.; Sartori, L.; Pezzuolo, A. Biogas from fresh spring and summer grass: Effect of the harvesting period. Energies 2018, 11, 1466. [Google Scholar] [CrossRef]
- Baldini, M.; da Borso, F.; Ferfuia, C.; Danuso, F. Ensilage suitability and bio-methane yield of Arundo donax and Miscanthus giganteus. Ind. Crops Prod. 2017, 95, 264–275. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 21th ed.; APHA: Washington, DC, USA, 2005; pp. 5–41. [Google Scholar]
- Lossie, U.; Pütz, P. Targeted control of biogas plants with the help of FOS/TAC. In Practice Report; Hach-Lange: Salford, UK, 2015. [Google Scholar]
- Chiumenti, A.; Pezzuolo, A.; Sartori, L.; Boscaro, D.; da Borso, F. Anaerobic digestion of grass: Effect of drying and ensiling on biogas yield. In 2018 ASABE Annual International Meeting; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2018. [Google Scholar]
- Jantrania, A.R.; White, R.K. High-solids anaerobic fermentation of poultry manure. In Proceedings of the Fifth International Symposium on Agricultural Waste 1985. ASAE, St. Joseph, MI, USA, 16–17 December 1985; pp. 73–80. [Google Scholar]
- Chiumenti, A. Complete nitrification-denitrification of swine manure in a full-scale, non-conventional composting system. Waste Manag. 2015, 46, 577–587. [Google Scholar] [CrossRef]
- Mulbry, W.; Selmer, K.; Lansing, S. Effect of liquid surface area on hydrogen sulfide oxidation during micro-aeration in dairy manure digesters. PLoS ONE 2017, 12, 0185738. [Google Scholar] [CrossRef]
- Nizami, A.S.; Orozco, A.; Groom, E.; Dieterich, B.; Murphy, J.D. How much gas can we get from grass? Appl. Energy 2012, 92, 783–790. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).