Effects of SiO2/Al2O3 Ratios on Sintering Characteristics of Synthetic Coal Ash
Abstract
:1. Introduction
2. Experimental Apparatus and Procedures
2.1. Synthetic Coal Ash Samples Preparation
2.2. Volume Shrinkage Ratio (VSR) Test
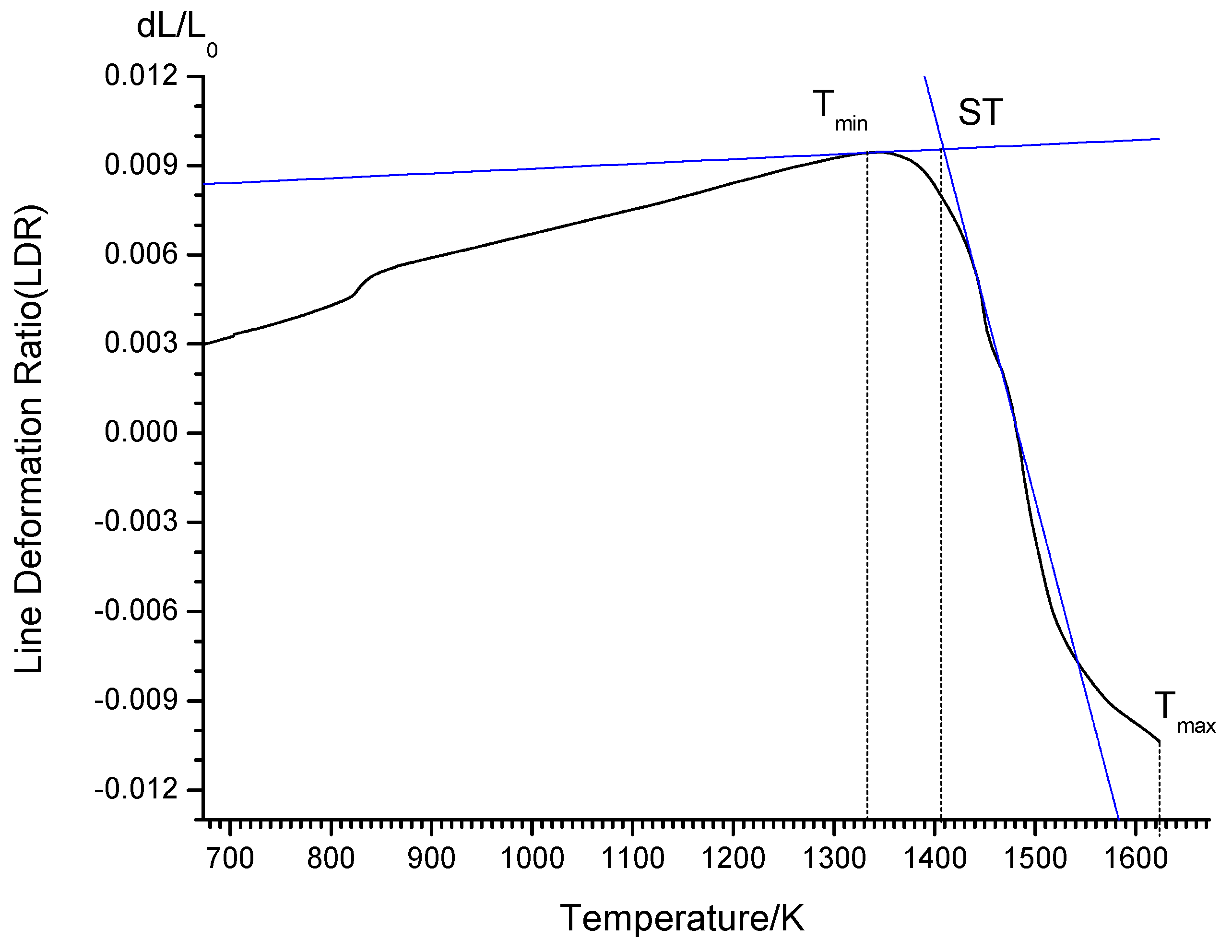
2.3. Sintering Temperature Test
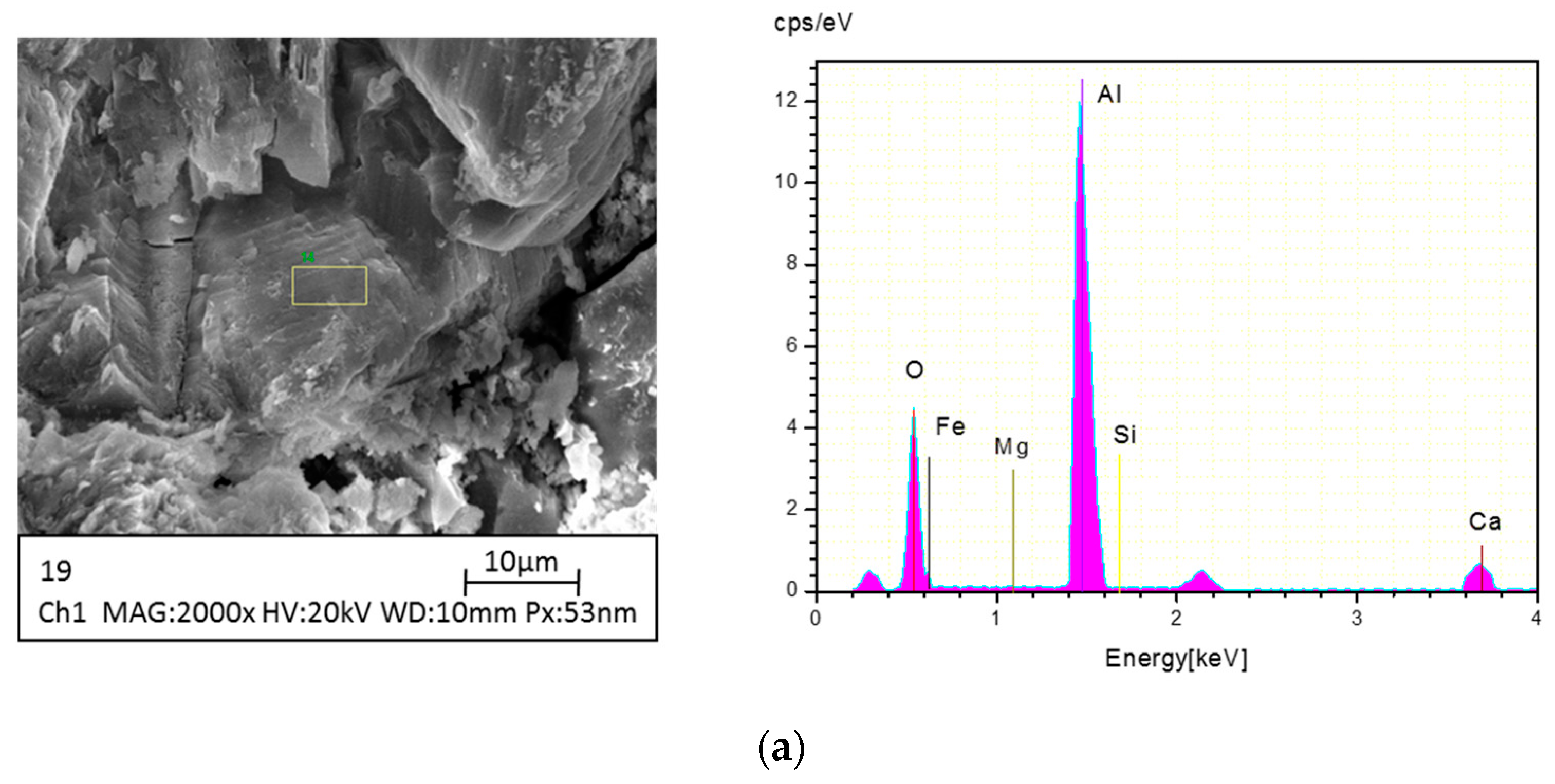
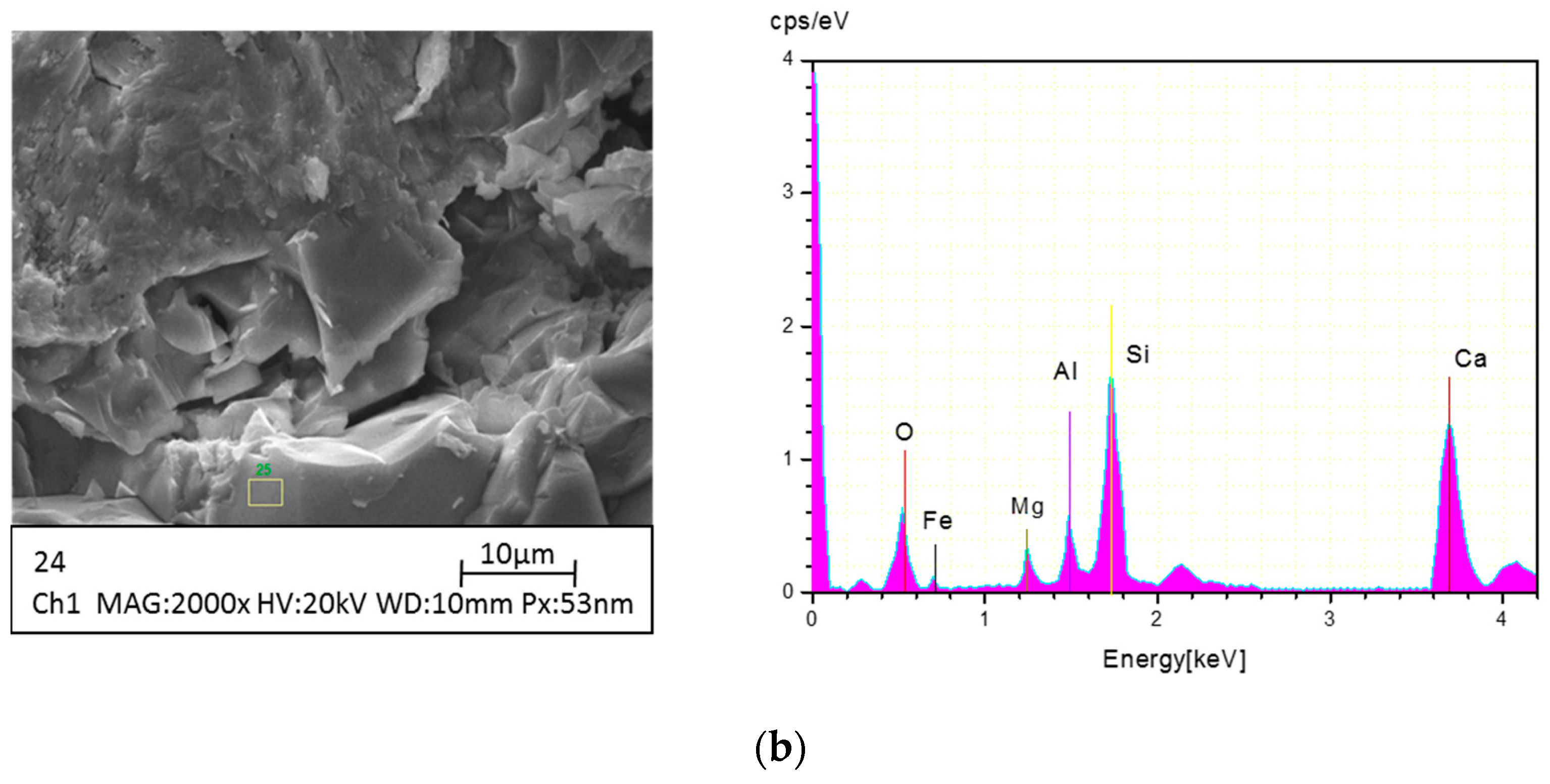
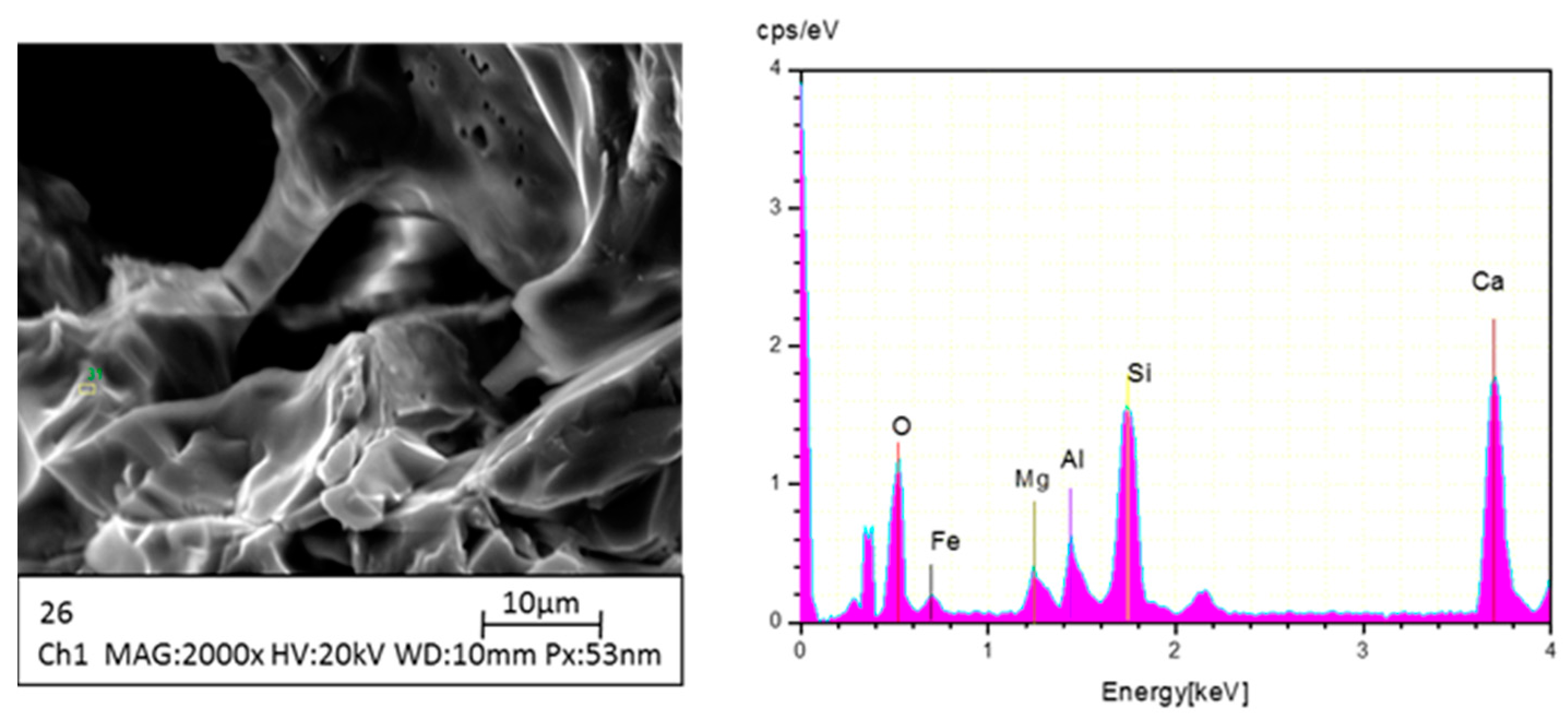
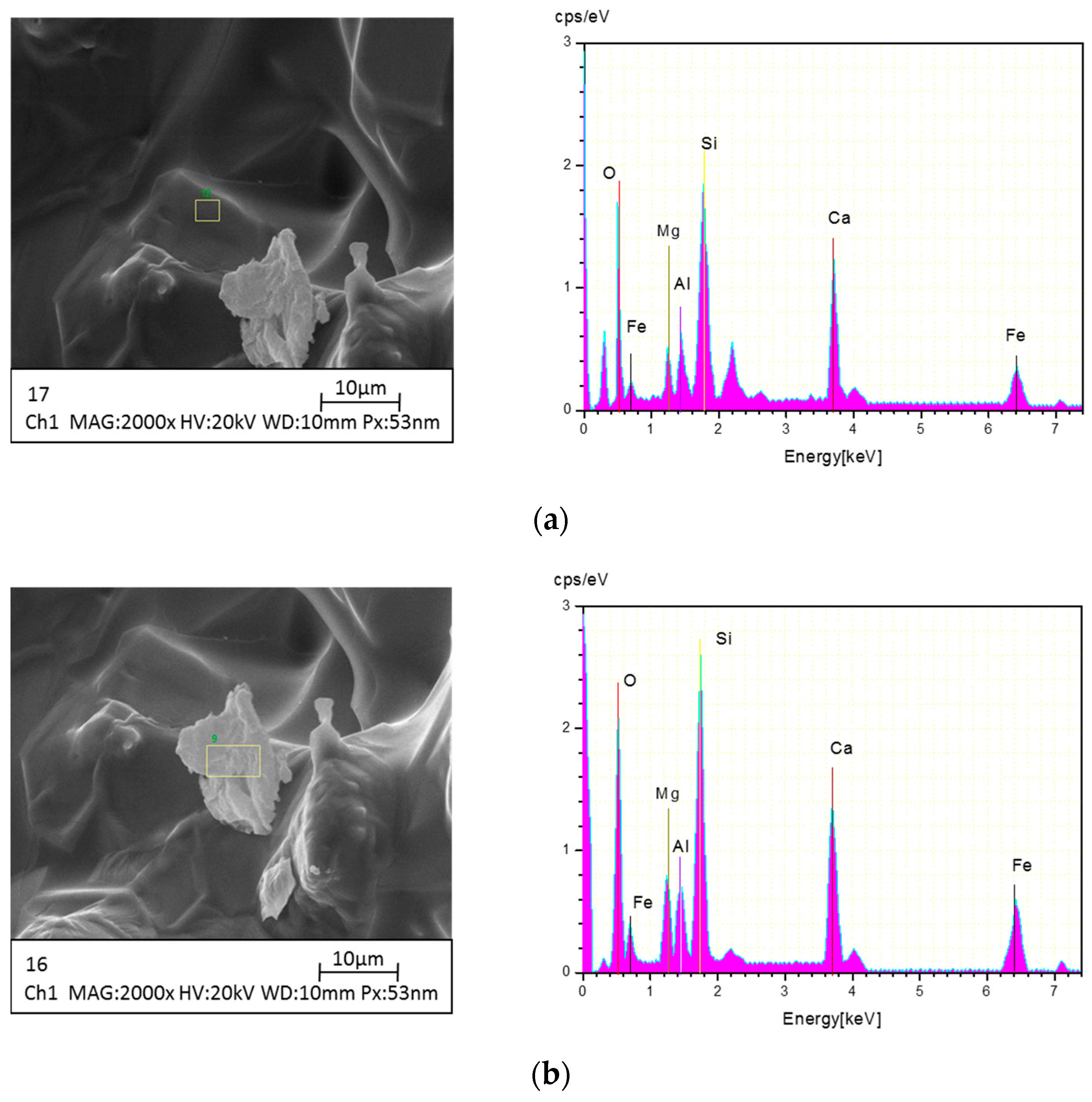
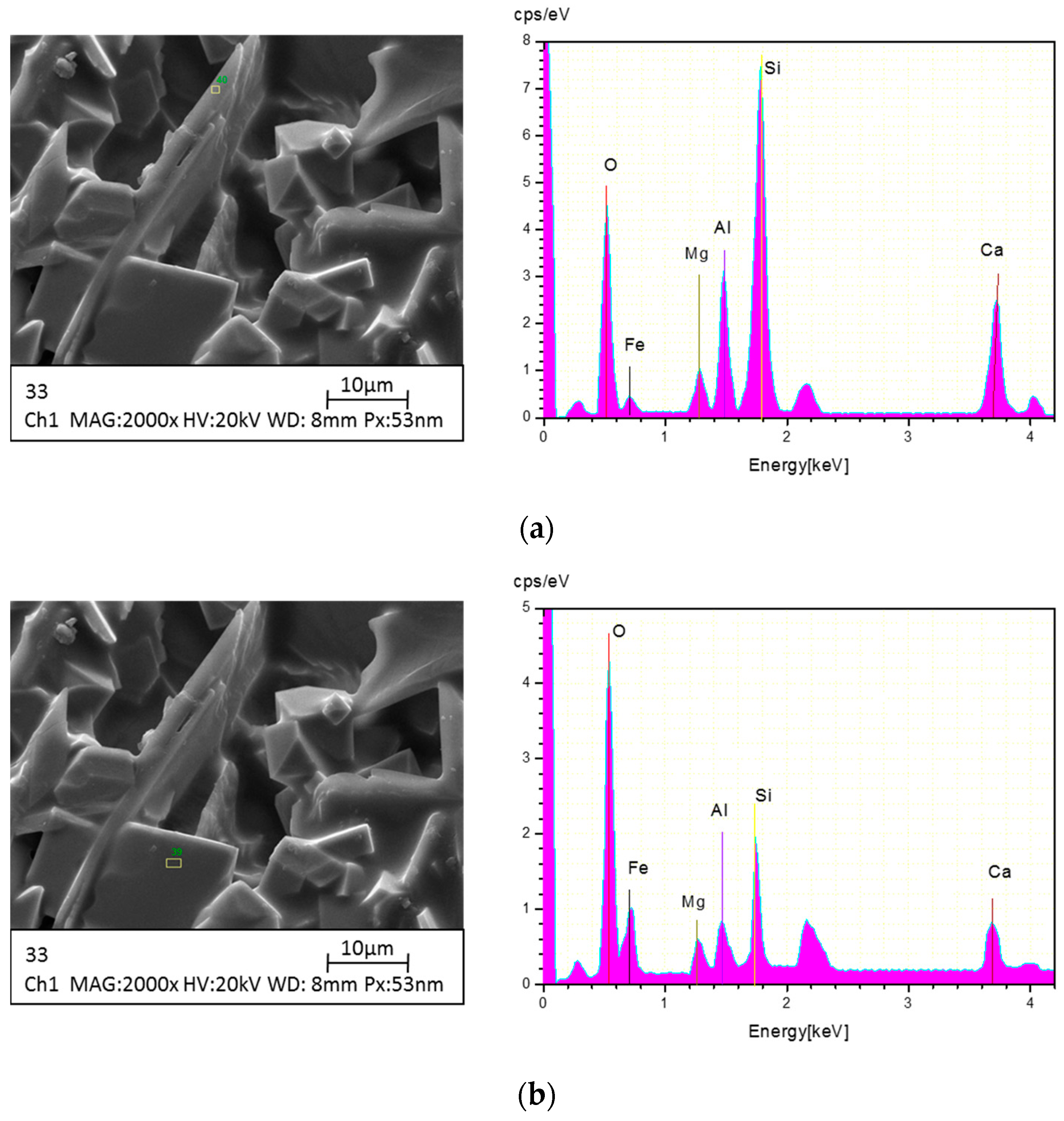
2.4. Instrumental Analysis
3. Results and Discussion
3.1. Sintering Characteristics at Various Temperatures
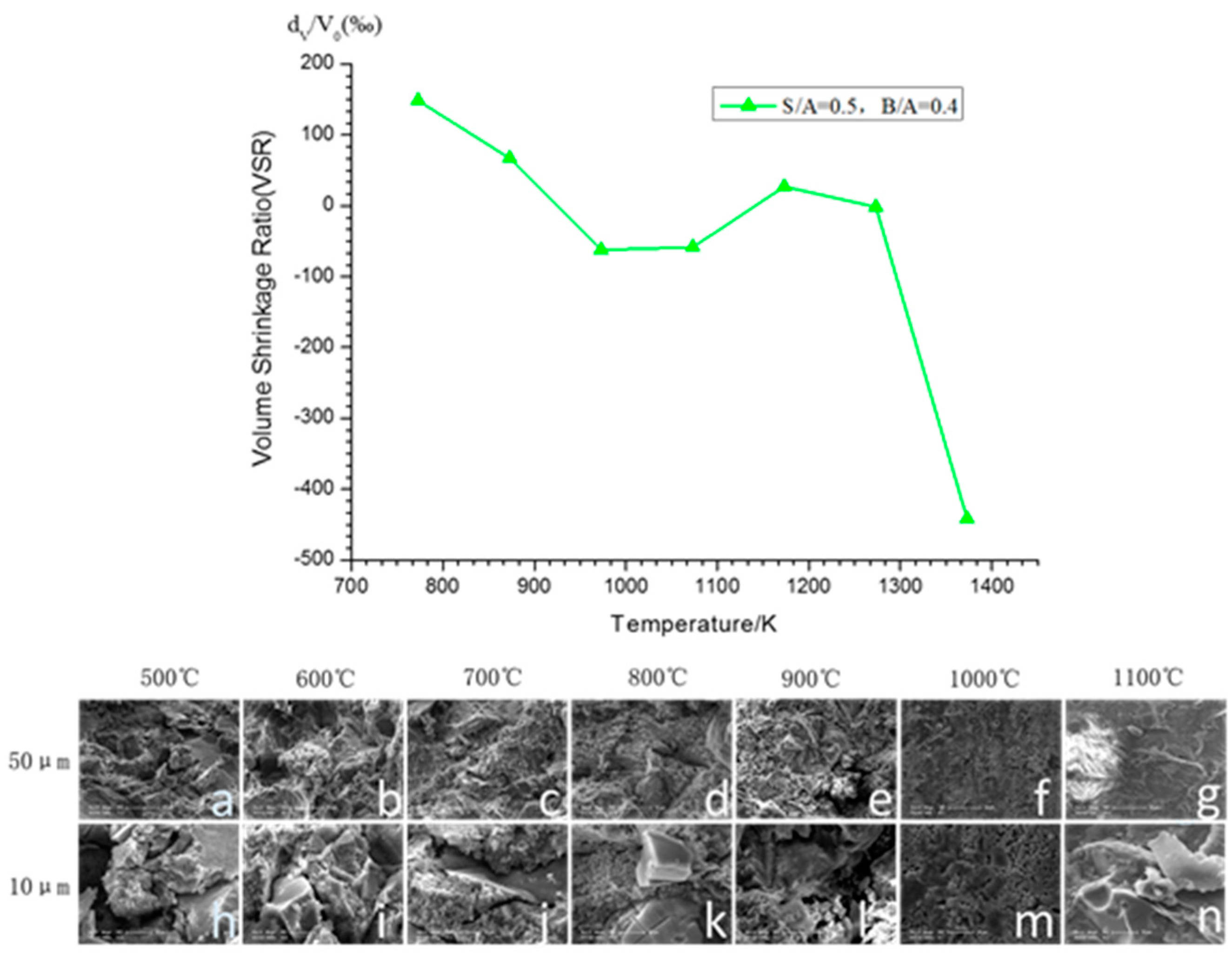
3.1.1. Volume Shrinkage Ratio (VSR) Variations at Various Temperatures
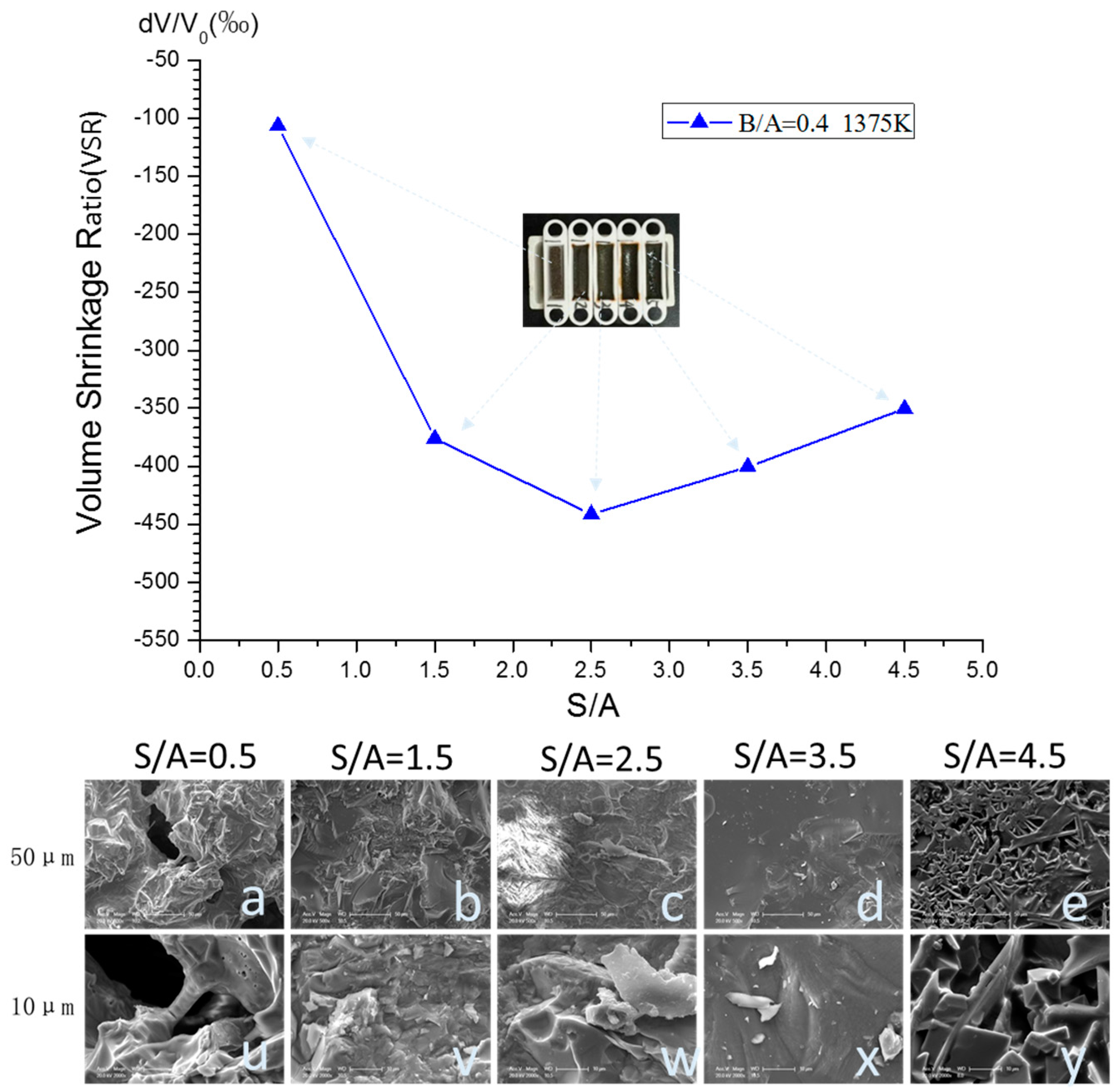
3.1.2. Morphology Variations versus Different Temperatures
- Physical reaction dominant stage (temperature below 973 K). During this period, the volume of the samples shrinks as the temperature increases, following the disappearance of the porous structure. Further, physical reactions, such as dissociative water evaporation and particle transition, play a key role in the volume of the cuboid samples.
- Expanding stage (temperature ranges from 973 K to 1273 K). During this period, the volume of the samples expands as the temperature elevates, accompanied by the reappearance of the porous structure. This stage has also been referred to as the beginning stage of sintering by Wenjia Song [16]. At the same time, eutectics and oxide react with large agglomerations, lancinating large agglomerations into discrete particles.
- Sintering stage (temperature above 1273 K). During this period, the volume of the samples shrinks dramatically, with the production of the amorphous compact structure. Meanwhile, chemical compositions and intermediate products react violently with each other.
3.2. Sintering Characterization versus Different S/As
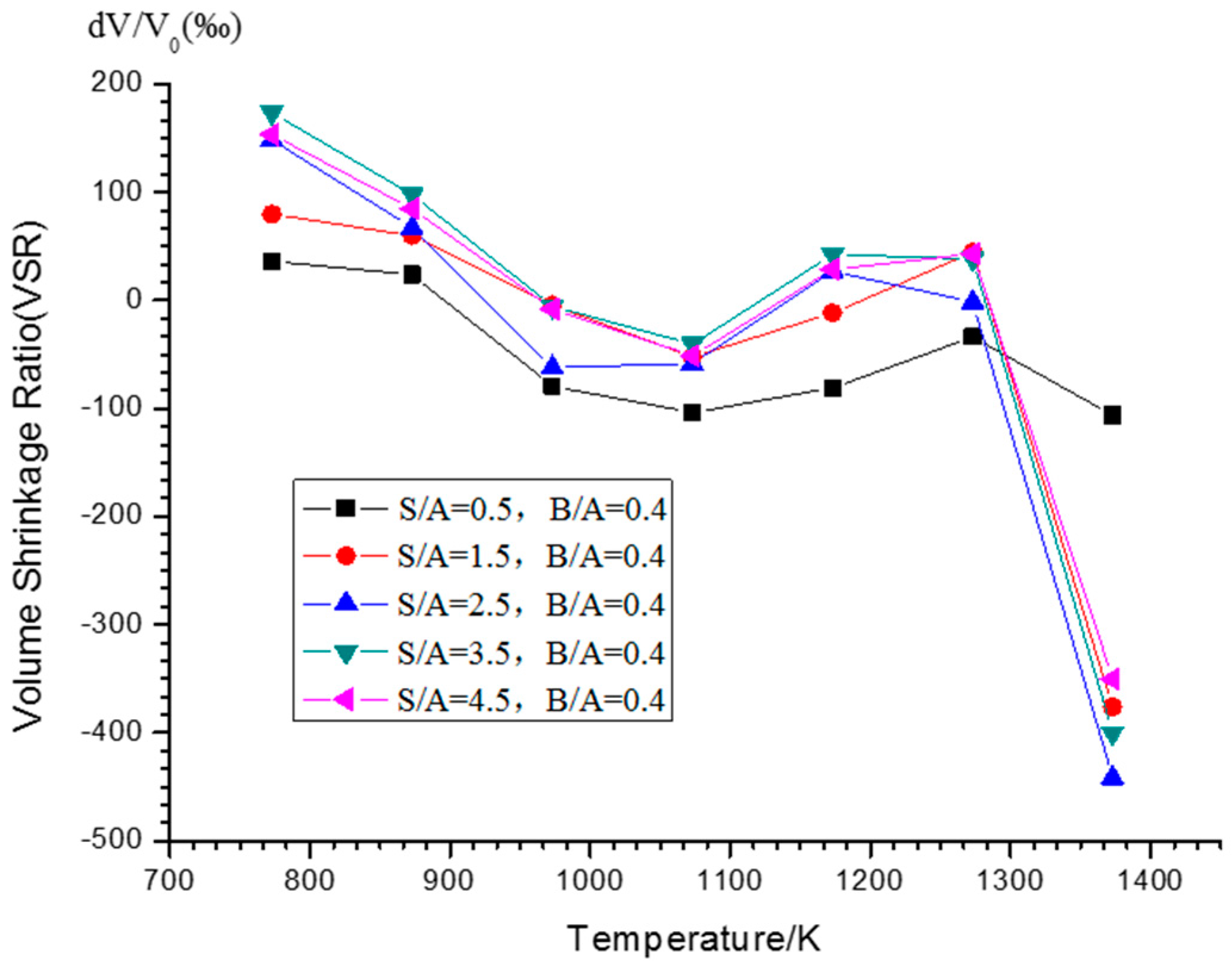
3.2.1. Volume Shrinkage Ratio (VSR) Variations versus Different S/As
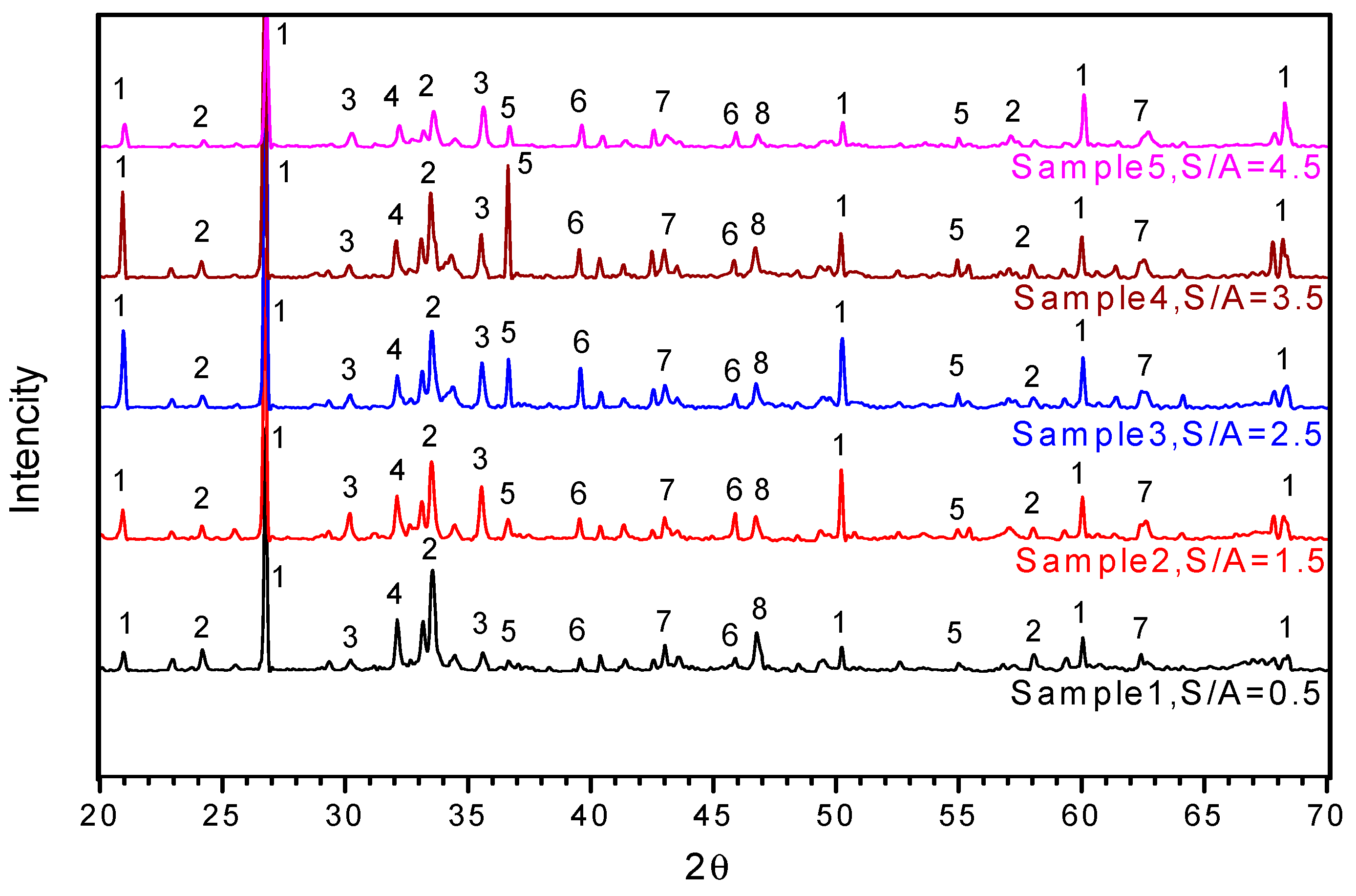
3.2.2. XRD Graph of SCA at Various Temperatures
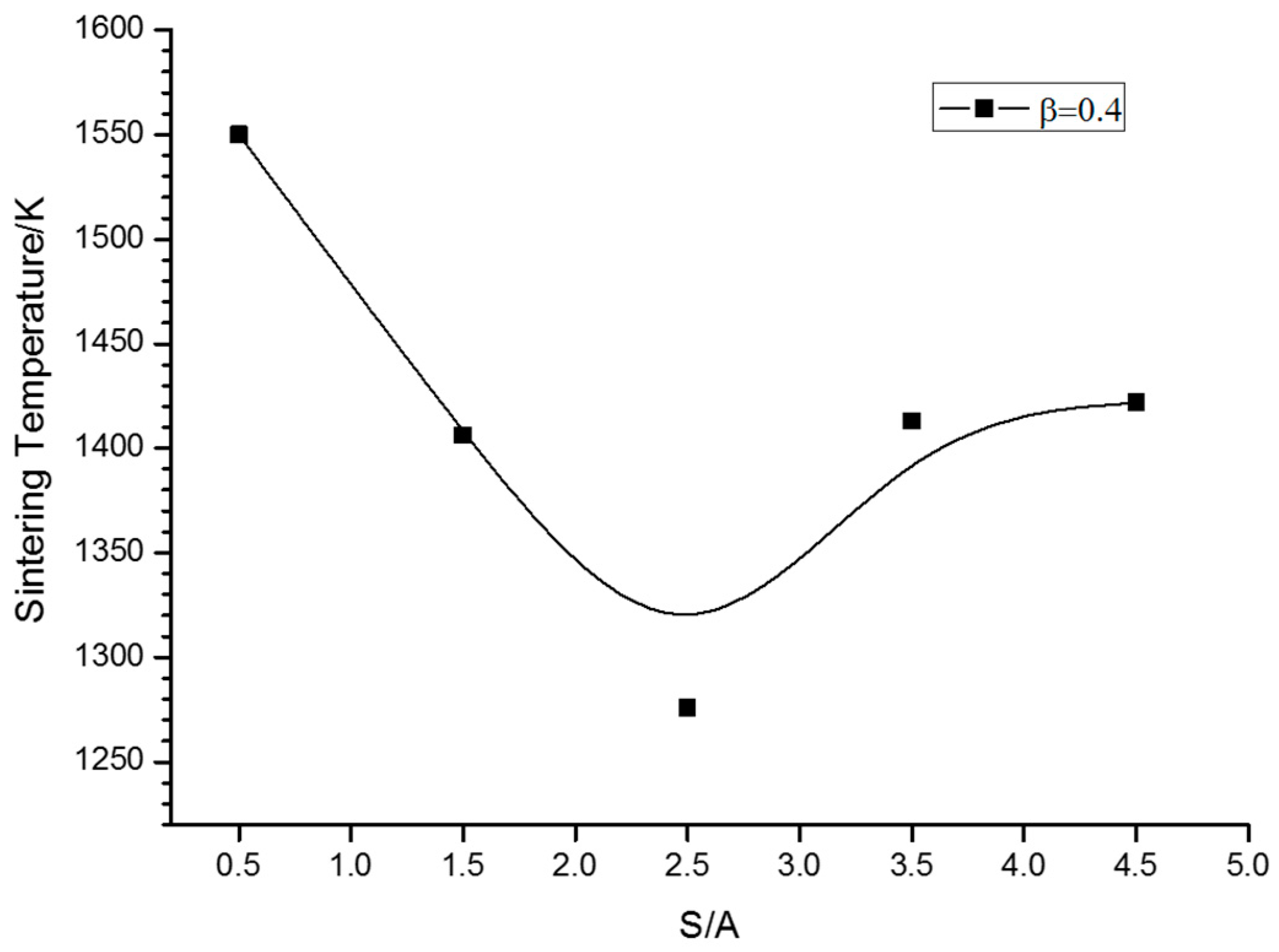
3.2.3. Sintering Temperature of SCA Samples versus Different S/As
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tekin, I. Properties of NaOH activated geopolymer with marble, travertine and volcanic tuff wastes. Constr. Build. Mater. 2016, 127, 607–617. [Google Scholar] [CrossRef]
- Messina, F.; Ferone, C.; Colangelo, F.; Cioffi, R. Low temperature alkaline activation of weathered fly ash: Influence of mineral admixtures on early age performance. Constr. Build. Mater. 2015, 86, 169–177. [Google Scholar] [CrossRef]
- Ferone, C.; Liguori, B.; Capasso, I.; Colangelo, F.; Cioffi, R.; Cappelletto, E.; Di Maggio, R. Thermally treated clay sediments as geopolymer source material. Appl. Clay Sci. 2015, 107, 195–204. [Google Scholar] [CrossRef]
- Ni, J.; Zhou, Z.; Yu, G.; Liang, Q.; Wang, F. Molten slag flow and phase transformation behaviors in a slagging entrained-flow coal gasifier. Ind. Eng. Chem. Res. 2010, 49, 12302–12310. [Google Scholar] [CrossRef]
- Wang, L.; Skreiberg, Ø.; Becidan, M.; Li, H. Investigation of rye straw ash sintering characteristics and the effect of additives. Appl. Energy 2016, 162, 1195–1204. [Google Scholar] [CrossRef]
- Wang, L.; Skreiberg, Ø.; Becidan, M. Investigation of additives for preventing ash fouling and sintering during barley straw combustion. Appl. Therm. Eng. 2014, 70, 1262–1269. [Google Scholar] [CrossRef]
- Rushdi, A.; Sharma, A.; Gupta, R. An experimental study of the effect of coal blending on ash deposition. Fuel 2004, 83, 495–506. [Google Scholar] [CrossRef]
- Shen, M.; Qiu, K.; Zhang, L.; Huang, Z.; Wang, Z.; Liu, J. Influence of coal blending on ash fusibility in reducing atmosphere. Energies 2015, 8, 4735–4754. [Google Scholar] [CrossRef]
- Selvakumaran, P.; Lawerence, A.; Bakthavatsalam, A.K. Effect of additives on sintering of lignites during CFB combustion. Appl. Therm. Eng. 2014, 67, 480–488. [Google Scholar] [CrossRef]
- Dawoud, B.; Amer, E.; Gross, D. Experimental investigation of an adsorptive thermal energy storage. Int. J. Energy Res. 2007, 31, 135–147. [Google Scholar] [CrossRef]
- Luan, C.; You, C.; Zhang, D. An experimental investigation into the characteristics and deposition mechanism of high-viscosity coal ash. Fuel 2014, 119, 14–20. [Google Scholar] [CrossRef]
- Namkung, H.; Xu, L.H.; Shin, W.C.; Kang, T.J.; Kim, H.T. Study on deposition tendency of coal ash under various gasification environments through DTF. Fuel 2014, 117, 1274–1280. [Google Scholar] [CrossRef]
- Magdziarz, A.; Dalai, A.K.; Koziński, J.A. Chemical composition, character and reactivity of renewable fuel ashes. Fuel 2016, 176, 135–145. [Google Scholar] [CrossRef]
- Wang, L.; Becidan, M.; Skreiberg, Ø. Sintering behavior of agricultural residues ashes and effects of additives. Energy Fuels 2012, 26, 5917–5929. [Google Scholar] [CrossRef]
- Pang, C.H.; Hewakandamby, B.; Wu, T.; Lester, E. An automated ash fusion test for characterisation of the behaviour of ashes from biomass and coal at elevated temperatures. Fuel 2013, 103, 454–466. [Google Scholar] [CrossRef]
- Song, W.; Hess, K.U.; Damby, D.E.; Wadsworth, F.B.; Lavallée, Y.; Cimarelli, C.; Dingwell, D.B. Fusion characteristics of volcanic ash relevant to aviation hazards. Geophys. Res. Lett. 2014, 41, 2326–2333. [Google Scholar] [CrossRef]
- LI, H.; Yoshihiko, N.; Dong, Z.; Zhang, M. Application of the FactSage to predict the ash melting behavior in reducing conditions. Chin. J. Chem. Eng. 2006, 14, 784–789. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Z.; Chen, Y.; Zhou, T.; Fan, J.; Piao, G.; Kobayashi, N.; Mori, S.; Itaya, Y. Main mineral melting behavior and mineral reaction mechanism at molecular level of blended coal ash under gasification condition. Fuel Process. Technol. 2010, 91, 1591–1600. [Google Scholar] [CrossRef]
- Matjie, R.H.; Li, Z.; Ward, C.R.; French, D. Chemical composition of glass and crystalline phases in coarse coal gasification ash. Fuel 2008, 87, 857–869. [Google Scholar] [CrossRef]
- Ilyushechkin, A.Y.; Hla, S.S.; Roberts, D.G.; Kinaev, N.N. The effect of solids and phase compositions on viscosity behaviour and TCV of slags from Australian bituminous coals. J. Non-Cryst. Solids 2011, 357, 893–902. [Google Scholar] [CrossRef]
- Song, W.J.; Tang, L.H.; Zhu, X.D.; Wu, Y.Q.; Zhu, Z.B.; Koyama, S. Effect of coal ash composition on ash fusion temperatures. Energy Fuels 2010, 24, 182–189. [Google Scholar] [CrossRef]
- Hurst, H.J.; Novak, F.; Patterson, J.H. Viscosity measurements and empirical predictions for fluxed Australian bituminous coal ashes. Fuel 1999, 78, 1831–1840. [Google Scholar] [CrossRef]
- Patterson, J.H.; Hurst, H.J. Ash and slag qualities of Australian bituminous coals for use in slagging gasifiers. Fuel 2000, 79, 1671–1678. [Google Scholar] [CrossRef]
- Jing, N.; Wang, Q.; Cheng, L.; Luo, Z.; Cen, K. The sintering behavior of coal ash under pressurized conditions. Fuel 2013, 103, 87–93. [Google Scholar] [CrossRef]
- Ji, S.; Li, F.; Wang, T.; Li, Z.; Fang, H.; Huang, J.; Fang, Y. Investigation on the sintering behaviors of low-temperature lignite ashes. J. Therm. Anal. Calorim. 2014, 1311–1320. [Google Scholar] [CrossRef]
- Liu, B.; He, Q.; Jiang, Z.; Xu, R.; Hu, B. Relationship between coal ash composition and ash fusion temperatures. Fuel 2013, 105, 293–300. [Google Scholar] [CrossRef]
- Xuan, W.; Whitty, K.J.; Guan, Q.; Bi, D.; Zhang, J. Influence of isothermal temperature and cooling rates on crystallization characteristics of a synthetic coal slag. Fuel 2014, 137, 193–199. [Google Scholar] [CrossRef]
- Selvakumaran, P.; Bakthavatsalam, A.K. Effect of chemical composition of ash on sintering of lignites in circulating fluid bed combustion and successful operation of large CFBC boilers. Appl. Therm. Eng. 2015, 85, 135–147. [Google Scholar] [CrossRef]
- Liang, W.; Geir, S.; Johan, E.H.; Morten, G.G. Sintering characteristics of sewage sludge ashes at elevated temperatures. Fuel Process. Technol. 2012, 96, 88–97. [Google Scholar]
- Zhao, B.; Zhang, Z.; Wu, X. Prediction of coal ash fusion temperature by least-squares support vector machine model. Energy Fuels 2010, 24, 3066–3071. [Google Scholar] [CrossRef]
| Sample | Chmical Compositions of SCA, m% | B/A | |||||
|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | S/A | ||
| 0 | 51.00 | 20.60 | 12.10 | 13.36 | 2.84 | 2.47 | 0.4 |
| 1 | 23.81 | 47.62 | 12.22 | 13.50 | 2.85 | 0.5 | |
| 2 | 42.86 | 25.57 | 12.22 | 13.50 | 2.85 | 1.5 | |
| 3 | 51.02 | 20.41 | 12.22 | 13.50 | 2.85 | 2.5 | |
| 4 | 55.56 | 15.87 | 12.22 | 13.50 | 2.85 | 3.5 | |
| 5 | 58.44 | 12.99 | 12.22 | 13.50 | 2.85 | 4.5 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, H.; Zhou, K.; Meng, K.; Song, L.; Lin, Q. Effects of SiO2/Al2O3 Ratios on Sintering Characteristics of Synthetic Coal Ash. Energies 2017, 10, 242. https://doi.org/10.3390/en10020242
Hu H, Zhou K, Meng K, Song L, Lin Q. Effects of SiO2/Al2O3 Ratios on Sintering Characteristics of Synthetic Coal Ash. Energies. 2017; 10(2):242. https://doi.org/10.3390/en10020242
Chicago/Turabian StyleHu, Hongwei, Kun Zhou, Kesheng Meng, Lanbo Song, and Qizhao Lin. 2017. "Effects of SiO2/Al2O3 Ratios on Sintering Characteristics of Synthetic Coal Ash" Energies 10, no. 2: 242. https://doi.org/10.3390/en10020242
APA StyleHu, H., Zhou, K., Meng, K., Song, L., & Lin, Q. (2017). Effects of SiO2/Al2O3 Ratios on Sintering Characteristics of Synthetic Coal Ash. Energies, 10(2), 242. https://doi.org/10.3390/en10020242





