Abstract
Heme oxygenase-1 (HO-1) is a stress-inducible enzyme with antioxidant, anti-inflammatory, and anti-apoptotic effects. In this study, the HO-1 gene from Litopenaeus vannamei (Lv-HO-1) was identified. The open reading frame of Lv-HO-1 is 747 bp, encoding a peptide of 248 amino acids as well as a conserved HemO structural domain. Lv-HO-1 is 70–90% homological to crustaceans and about 50% homological to arthropods. The transcript levels of Lv-HO-1 were highest in the hepatopancreas and lower in other tissues. Knockdown of Lv-HO-1 led to structural destruction of the hepatopancreas. After ammonia exposure, Lv-HO-1 was significantly induced. Knockdown of Lv-HO-1 during ammonia exposure resulted in a significant decrease in antioxidant capacity and cellular autophagy levels compared to the control and increased apoptosis. The transcriptional levels of SOD and GSH-Px were considerably reduced (p < 0.05), as were the transcriptional levels of Atg3, Atg4, Atg5, and Atg10. The results indicated that Lv-HO-1 from L. vannamei can be induced by oxidative stress and may have important roles in regulating the host antioxidant system, reducing cell apoptosis.
1. Introduction
The rate-limiting enzyme, heme oxygenase (HO), catalyzes the conversion of heme and generates the antioxidant products ferrous ion, carbon monoxide, and biliverdin [1]. The non-rate-limiting enzyme biliverdin reductase reduces the reaction product biliverdin to bilirubin [2,3]. HO has been found in insects, fish, and mammals, among other organisms [4]. There are three isoforms, HO-1, HO-2, and HO-3 [5]. HO-2 is a constitutively produced protein that has a role in a variety of physiological processes [6,7]. Functional investigations on HO-3 are still restricted and it was discovered to be an HO-2 pseudogene [8]. HO-1 is an inducible isoform [9] that is abundant in the liver and spleen, whereas lower levels were detected in the other tissues. As a stress-response protein [10], HO-1 can be highly induced by a variety of substances that cause oxidative stress. Hypoxia, inflammatory conditions, and endotoxins all stimulate HO-1, which protects cells from oxidation and cellular stresses while also reducing inflammation, apoptosis, and regulating cell proliferation [1,11,12].
In mammals, the function and regulation of HO-1 have been studied using in vitro and in vivo models of oxidant-mediated cell and tissue injury [13]. The relevance of HO-1 has been shown in numerous investigations. HO-1 inhibits glucose-induced apoptosis in human microvascular endothelial cells [14]. Under endotoxic circumstances, HO-1 (−/−) mice were more likely to die or develop hepatic necrosis [15]. Studies have shown that HO-1 is significantly induced in animal models of ischemia/reperfusion and nephrotoxin-induced acute kidney injury (AKI) [16,17]. Cisplatin medication of genetically deficient HO-1 mice results in worse kidney function and tubular damage [18]. In fish, hypoxia increased the activity of HO-1 in the gills of fish living in 7 °C. While HO-1 was inhibited, the ventilation frequency response to acute hypoxia was enhanced [19]. Recent research on Drosophila melanogaster suggests that HO is involved in the regulation of essential apoptosis and autophagy processes in protecting the brain from oxidative damage [20]. However, the physiological control of HO-1 in crustaceans has received less attention than in mammals, leaving a gap in our knowledge of the roles of HO-1 in shrimp.
L. vannamei is an internationally known economic shrimp, widely farmed worldwide, especially in China. However, ammonia is the most common pollutant in aquaculture waters and is highly toxic to crustaceans, leading to oxidative stress [21] and an immune response [22]. High ammonia levels can seriously damage the hepatopancreas, induce apoptosis, and even lead to the death of shrimp [23]. We speculated that Lv-HO-1 participates in the mediation of stress responses and cytoprotective effects in L. vannamei. Therefore, Lv-HO-1 was identified and its properties and functions were investigated. These data may deepen our comprehension of the function of HO-1 in shrimp and the regulatory mechanism of the invertebrate antioxidant system.
2. Materials and Methods
2.1. Shrimp Preparation, Challenge, and Sample Collection
L. vannamei (8.0 ± 0.5 g) were obtained and acclimated in seawater at 28.0 ± 0.5 °C and salinity of 30.0 ± 0.5‰ for 7 days. These shrimps were then randomly selected for the experiment. Three healthy shrimps were sampled to clone the Lv-HO-1 gene and track its distribution in the brain, intestines, gills, epidermis, hepatopancreas, muscle, eyestalk, and hemolymph. Total RNA was extracted immediately.
The ammonia stress experiment was conducted as previously described [24]. Ammonium chloride reagent (Sigma, Shanghai, China) was used with a final concentration of 20 mg/L of ammonia-N added to seawater. After ammonia exposure, tissues of three shrimps were collected as described above at two time points (0, 48 h). Total RNA was extracted immediately.
2.2. RNA Isolation and cDNA Synthesis
RNAiso Plus (TaKaRa, Dalian, China) was used to isolate and extract total RNA from shrimp. The RNA was then extracted with chloroform, the supernatant was centrifuged and precipitated with isopropyl alcohol to obtain the RNA, and finally the RNA was dissolved with 50 μL of RNase-free water. RNA concentration was measured using NANODROP 2000 (Thermo Scientific) and RNA integrity was detected using 1.2% agarose gel. PrimeScript™ RT reagent Kit with gDNA Eraser (TaKaRa, Dalian, China) was used to reverse-transcribe the RNA following the kit instructions. The reverse-transcribed cDNA was diluted at a ratio of 1:50 for subsequent experiments [25,26].
2.3. Cloning and Sequence Analysis of Lv-HO-1
The predicted gene sequence of Lv-HO-1 was obtained from NCBI and partial sequence primers of Lv-HO-1 (HO-1-S and HO-1-A) were designed. The first strand of cDNA was then synthesized from the total mRNA as a polymerase chain reaction (PCR) template, and the HO-1 fragment was amplified and sequenced. Using sequenced sequences, primers were designed for amplification of 5′ terminal sequences (HO-1-5′ RACE and HO-1-5′ RACE nested) and 3′ terminal sequences (HO-1-3′ RACE and HO-1-3′ RACE nested), respectively. Sequence-cloning methods were referenced to the SMARTer RACE 5′/3′ kit instructions (Code No. 634858; Clontech, Mountain View, CA, USA). Eventually, the 3′ RACE and 5′ RACE sequences were spliced via contigExpress application assembly software to derive the full length of the HO-1 gene. Multiple sequence alignment of HO-1 protein was performed via DNAMAN software (version 7.0, LynnonBiosoft, San Ramon, CA, USA). Neighbor-joining (NJ) phylogenetic trees with 1000 bootstrap replications were constructed using MEGA software (version 6.0, Philadelphia, PA, USA).
2.4. RNAi Assay
A set of primers with the T7 promoter sequence was generated based on the HO-1 cDNA sequence and amplification of a fragment of about 500 bp. An exogenous control dsRNA (dsEGFP) fragment of 497 bp was amplified using primers for the enhanced green fluorescent protein (EGFP). The PCR products were in vitro transcribed using T7 RNAi Transcription Kit (Takara, China) to synthesize dsRNA. Double-stranded RNA was precipitated with ethanol after being extracted with chloroform/phenol. The quality and amount of dsRNA were assessed via 1.2% agarose gel electrophoresis and spectrophotometry. The abdomen intramuscular dsRNA was injected as shown below. The double-stranded RNA of Lv-HO-1 was injected into each experimental shrimp at a rate of 0.50 µg/g. An identical amount of EGFP was injected into the control group. The hepatopancreas and muscle were sampled with three replicate samples at three different intervals (3, 5, and 7 days) after injection. Total RNA extraction was performed following the steps described in this section. The efficiency of RNAi was determined via qRT-PCR.
2.5. Ammonia Exposure Assay
The healthy shrimps were selected and randomly divided into 4 groups with 30 shrimps and raised in 100 L of seawater as mentioned above continuously for 3 weeks. For the blank group, each shrimp was injected with 30 μL of PBS. For dsRNA-EGFP and dsRNA-Lv-HO-1 groups, 120 μg of dsRNA-EGFP and dsRNA-Lv-HO-1 were each dissolved in 900 μL of PBS, and then injected into each shrimp separately. All three groups, excluding the blank group, were subjected to ammonia-N stress 3 days after the test and the final concentration of ammonia-N was 20 mg/L [24]. At 48 h after the exposure, hepatopancreas tissues from 3 shrimp were collected for total RNA extraction and histological analysis, respectively.
2.6. Quantitative Real-Time PCR (qRT-PCR)
Tissue distribution and relative expression of Lv-HO-1 were examined by qRT-PCR to detect transcript levels in healthy shrimp. qRT-PCR was carried out utilizing TB Green® Premix Ex Taq™ II (Tli RNaseH Plus) (TaKaRa, Dalian, China) and QuantStudio 6 and 7 Flex Real-Time PCR Systems (Thermo Fisher Scientific, Waltham, USA).PCR (triplicated) consisted of 30 s at 95 °C followed by 40 cycles at 95 °C for 15 s, 60 °C for 30 s, and a melting curve assay at the end. A 184 bp fragment of the Lv-HO-1 gene was amplified with EF1α as the internal reference gene. All primers used in this study are shown in Table S1 (Supplementary Materials). The relevant levels of HO-1 were calculated via the 2−ΔΔCt method [27].
2.7. Histologic Analysis
Hepatopancreas was fixed in a mixture of ethanol, chloroform, and glacial acetic acid with a volume ratio of 6:3:1 for more than 24 h in ethanol dehydrated using a gradient of ethanol (70%, 85%, 95%, and 100%), vitrified in xylene, and then embedded in paraffin. Hematoxylin and eosin staining kit (Beyotime, Shanghai, China) was used on serial 9 μm thick dewaxed and rehydrated sections. Histological analyses were stained using a hematoxylin and eosin staining kit (Beyotime, Shanghai, China) following the manufacturer’s protocols. A Nikon DS-Ri2 camera was used for microscopic observation and photography (Nikon, Tokyo, Japan).
2.8. TUNEL Assay
One-step TUNEL Assay Kit (Green, FITC; Elabscience, Wuhan, China) was used to detect apoptosis. Hepatopancreatic cell apoptosis was detected by paraffin biopsy, according to the manufacturer’s instructions. The apoptotic cells were labeled with fluorescent dUTP and the cells were counter-stained with DAPI (blue) following the manufacturer’s protocols. The section was examined by fluorescence microscopy.
2.9. Enzyme Activities Assays
The excised hepatopancreas was homogenized at 4 °C and separated at 5000× g for 30 min. The antioxidant parameters were measured directly from the supernatant fluids following the protocols of the kits (Nanjing Jiancheng Bioengineering Institute).
2.10. Drawings and Statistical Analysis
The data were presented using mean ± standard deviation (SD). One-way ANOVA and Student’s t-test were used to analyze the significance of differences with Prism software (version 8.0). Homogeneity of variance test was performed prior to one-way ANOVA. Duncan’s post-hoc was used when all variances were equal. Different lowercase letters denote significant differences between groups, (p < 0.05).
3. Results
3.1. Cloning and Bioinformatics Analysis of Lv-HO-1
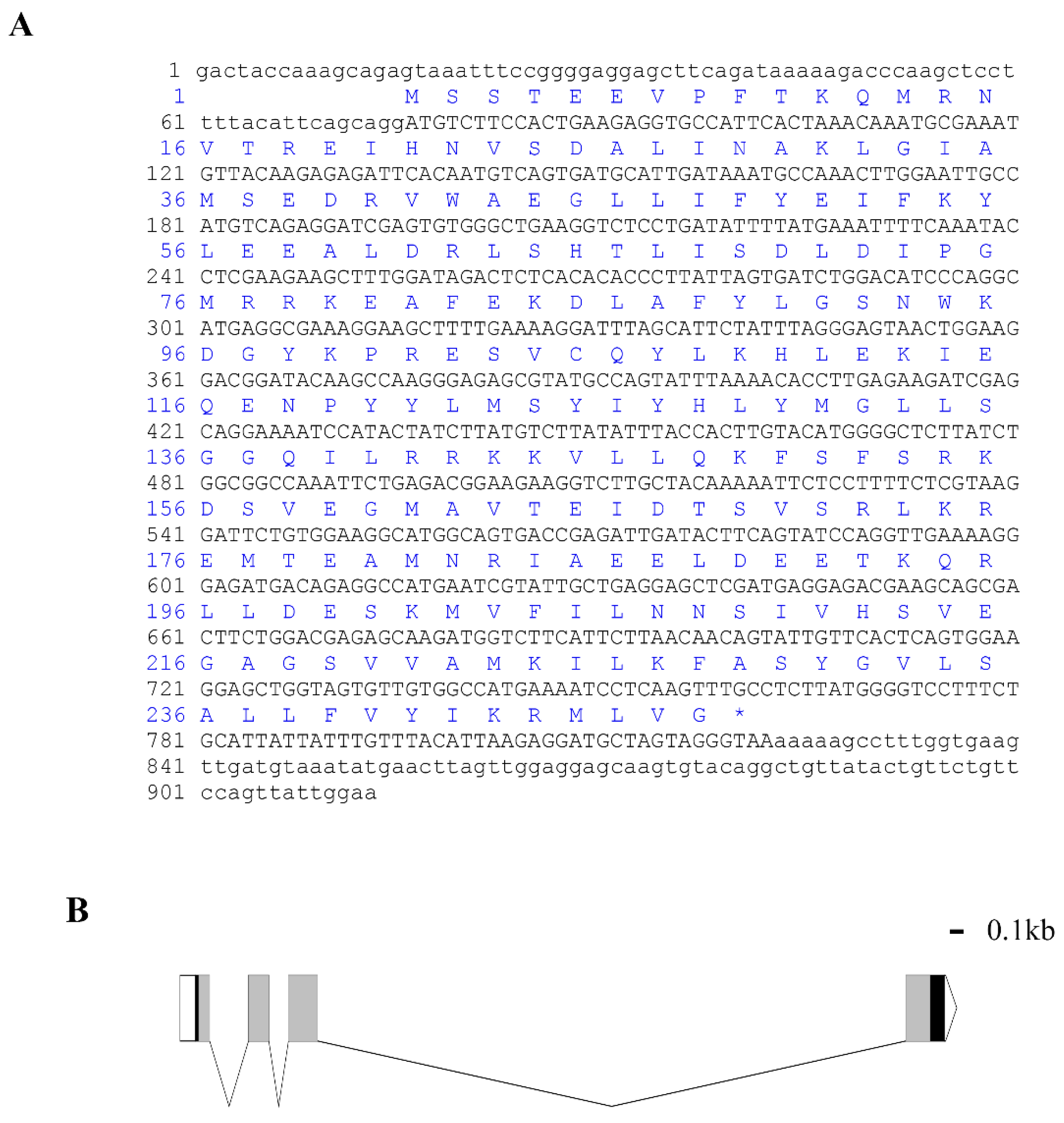
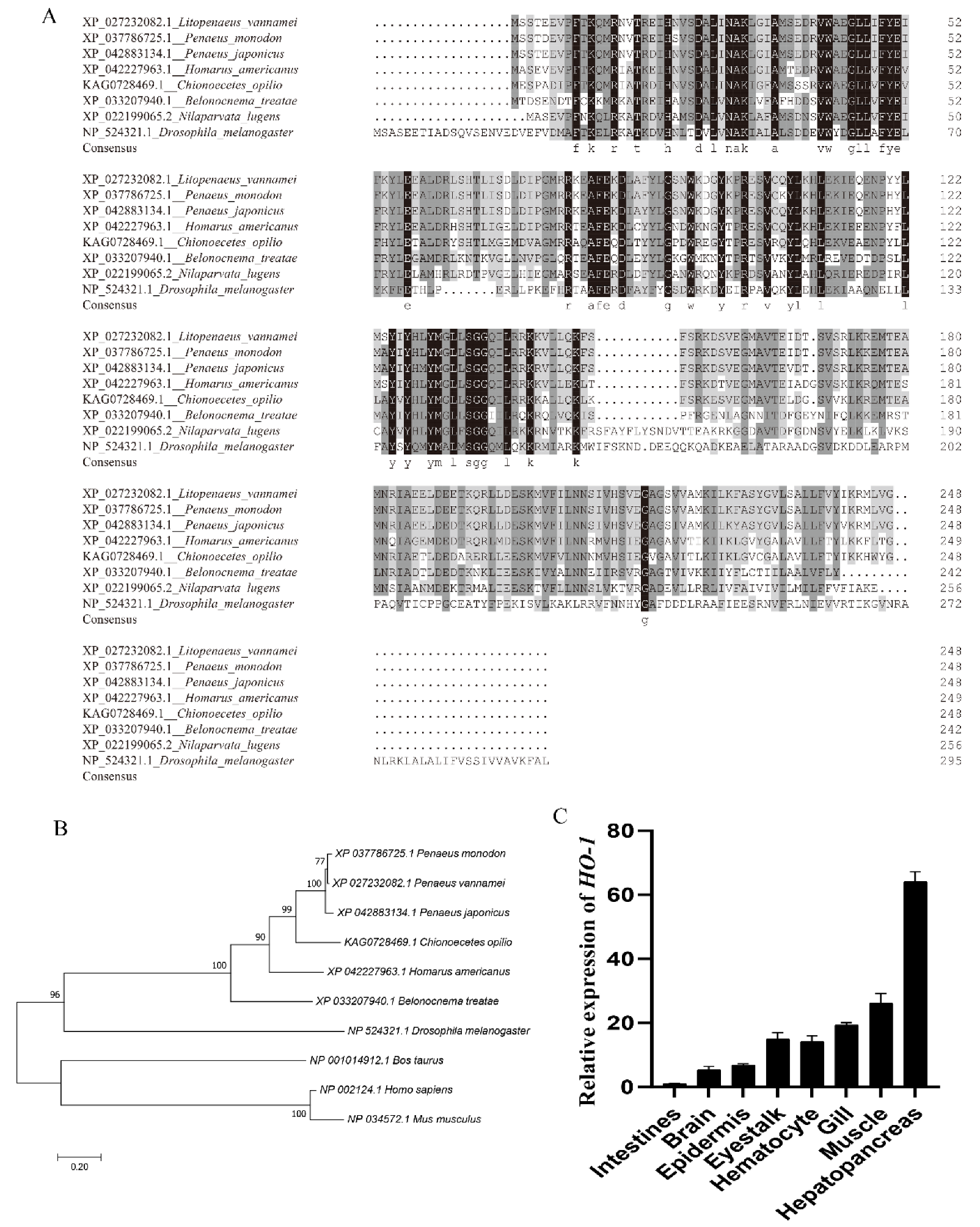
The full length of Lv-HO-1 cDNA is 913 bp with a 747 bp open reading frame (ORF) encoding 248 amino acids (Figure 1A). The predicted protein molecular weight of Lv-HO-1 is 28.6 kDa with a theoretical pI of 6.55. Comparison of genomic sequences showed that Lv-HO-1 consists of four exons separated by three introns (Figure 1B). Multiple sequence alignment revealed that the amino acid sequence of Lv-HO-1 contains a conserved HemO structural domain. The inferred amino acid sequence of Lv-HO-1 has similarities with Lv-HO-1 from other species, according to Basic Local Alignment Search Tool (BLAST, NCBI) analysis. There was a 68–97% match with other shrimp species and a 10% match with vertebrates (Figure 2A). Phylogenetic analysis showed that Lv-HO-1 originally clustered with Penaeus monodon and then with other crustaceans. Finally, Lv-HO-1 clustered with vertebrates (Figure 2B).
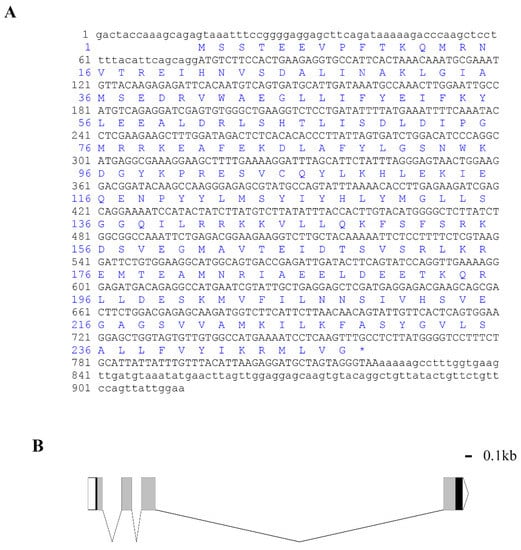
Figure 1.
(A) cDNA sequences and deduced amino acid sequences of Lv-HO-1. The open reading frame (ORF) is capitalized. An asterisk indicates the stop codon. (B) Genomic structure of Lv-HO-1 contains three introns and four exons. Untranslated sections are marked with a white box (UTR).
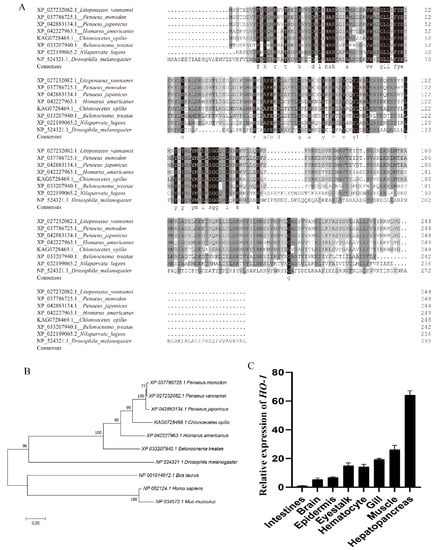
Figure 2.
(A) Multiple alignments of the HO-1 sequence from various species. (B) Phylogenetic tree of the Lv-HO-1 family built via neighbor joining. (C) Lv-HO-1 levels in different tissues in L. vannamei were evaluated via qRT-PCR. All values are the mean ± SD; n = 3.
3.2. Expression Characteristics of Lv-HO-1 in Various Tissues
The expression of Lv-HO-1 in various tissues was detected by qPCR. Among all the analyzed tissues, the Lv-HO-1 mRNA level was highest in hepatopancreas then decreasingly in muscle, gill, epithelium, hemocytes, intestine, and brain (Figure 2C).
3.3. Effects of Ammonia Exposure on Expression of Lv-HO-1
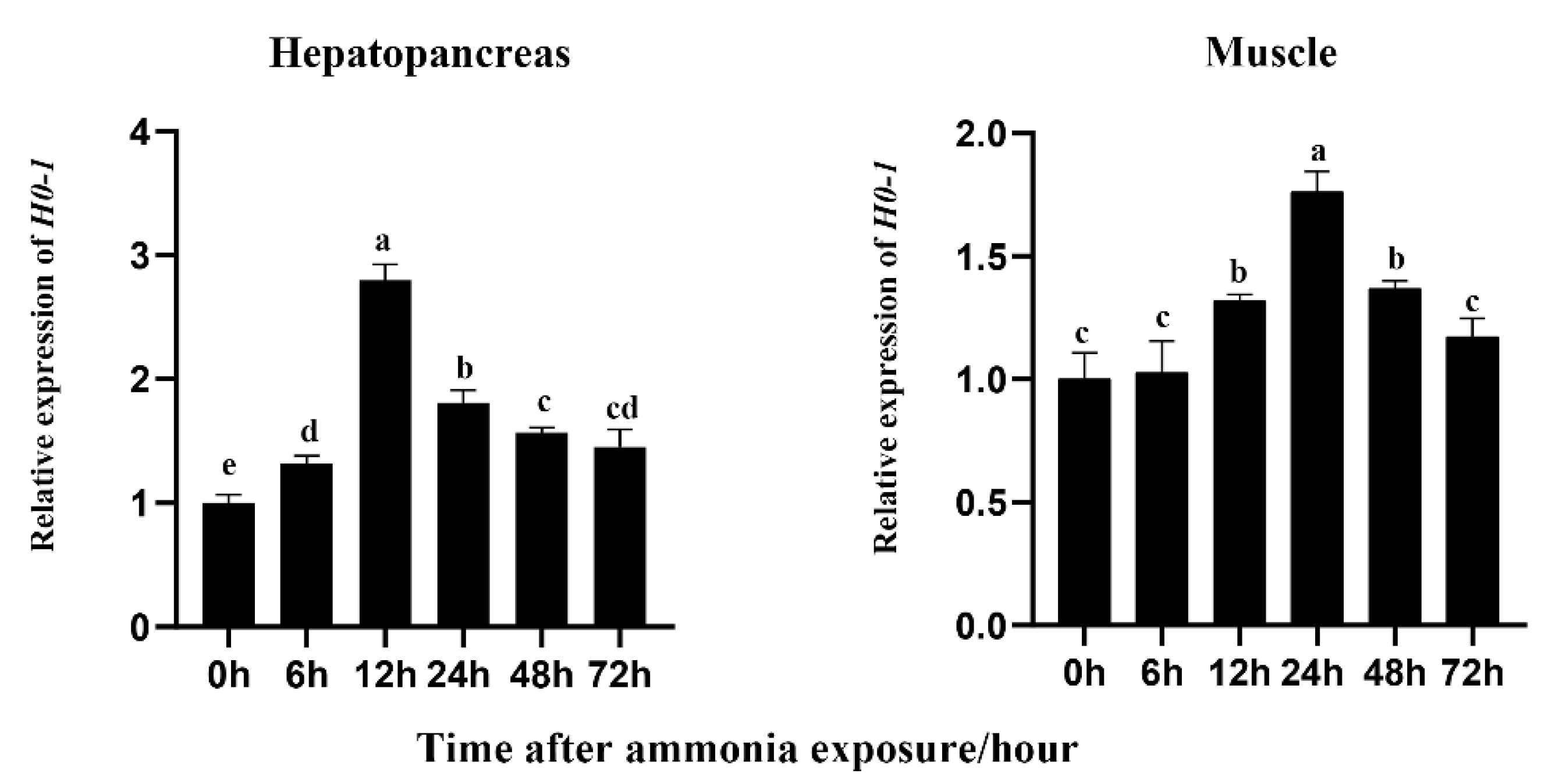
The expression of Lv-HO-1 was abundant in the hepatopancreas and muscle. The hepatopancreas is an important immune and metabolism organ, and muscle is the maximal tissue in L. vannamei. Therefore, the hepatopancreas and muscle were selected to detect the expression levels of Lv-HO-1 during oxidative stress. After ammonia exposure, Lv-HO-1 expression levels were considerably raised in hepatopancreas and muscle (p < 0.05). In addition, after stress, the Lv-HO-1 transcript level peaked at 12 h and folded at 24 h in the hepatopancreas, while it peaked at 24 h and folded at 48 h in the muscle (Figure 3). These findings suggested that Lv-HO-1 might play a role in antioxidants.
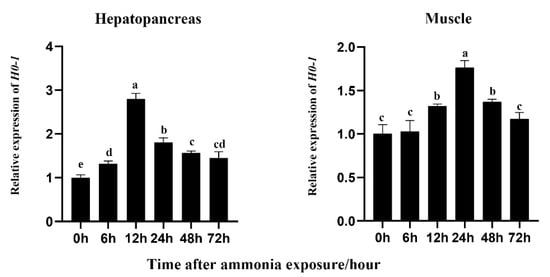
Figure 3.
Transcription patterns of Lv-HO-1 in hepatopancreas and muscle at different time points after ammonia-N stress. To determine the relative expression of the other time point, the Lv-HO-1 expression level at 0 hwas set to 1.00. All values are the mean ± SD, n = 3. Different letters denote a significant difference (p < 0.05).
3.4. Lv-HO-1 Silencing via RNAi
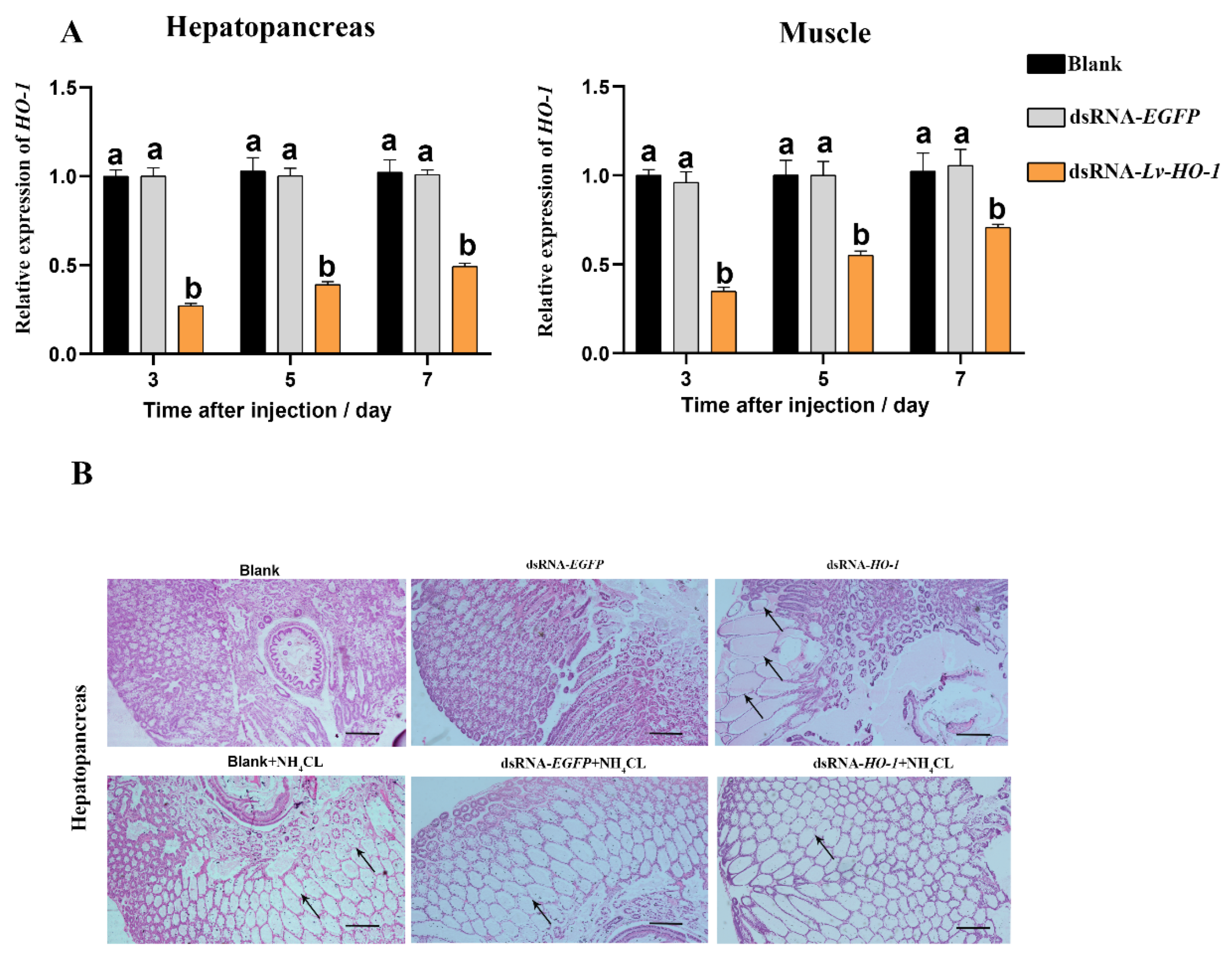
RNAi knockdown efficiency of Lv-HO-1 was assessed using qPCR. The expression levels of Lv-HO-1 in hepatopancreas and muscle were examined 3, 5, and 7 d after dsRNA injection. The expression level of Lv-HO-1 was significantly decreased (p < 0.05) in the dsRNA-Lv-HO-1 group compared to the PBS group, while the dsRNA-EGFP (Figure 4A) was not significantly different. RNAi in hepatopancreas had a higher silencing effectiveness than muscle, reaching a silencing efficiency of 70%. Therefore, RNAi is an effective way to reduce the expression of the Lv-HO-1 gene. Hepatopancreas structures in the dsRNA-EGFP control and dsRNA-LvHO-1 groups were different, as showed by sections stained with H&E. Thinning and dilatation of the hepatic tubule wall were observed in the hepatopancreas of the dsRNA-Lv-HO-1 group (Figure 4B). Meanwhile, the sections showed different levels of pathological changes under ammonia stress. Severe dilatation of the stellate tubular lumen and thinning were observed in the dsRNA-Lv-HO-1 group but occurred only mildly in the dsRNA-EGFP group (Figure 4B).
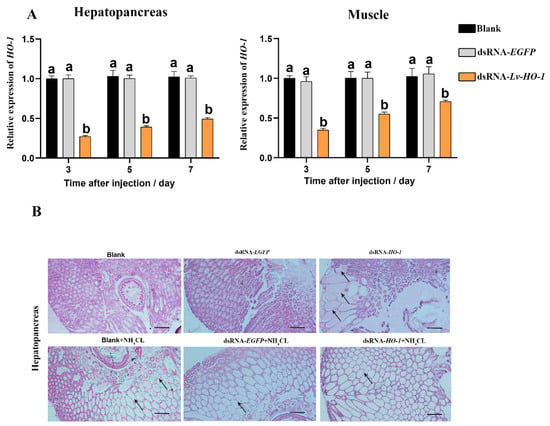
Figure 4.
(A) Levels of Lv-HO-1 mRNA in hepatopancreas and muscle after RNAi injection. Significant difference (p < 0.05) in the dsRNA-Lv-HO-1 group compared with the Blank or PBS group is denoted by different letters. (B) Histological examination of hepatopancreas after RNAi. The sections were stained with H&E (50× magnification, Scale plate: 20 μm). Arrows indicate thinning and dilatation of the hepatic tubule wall.
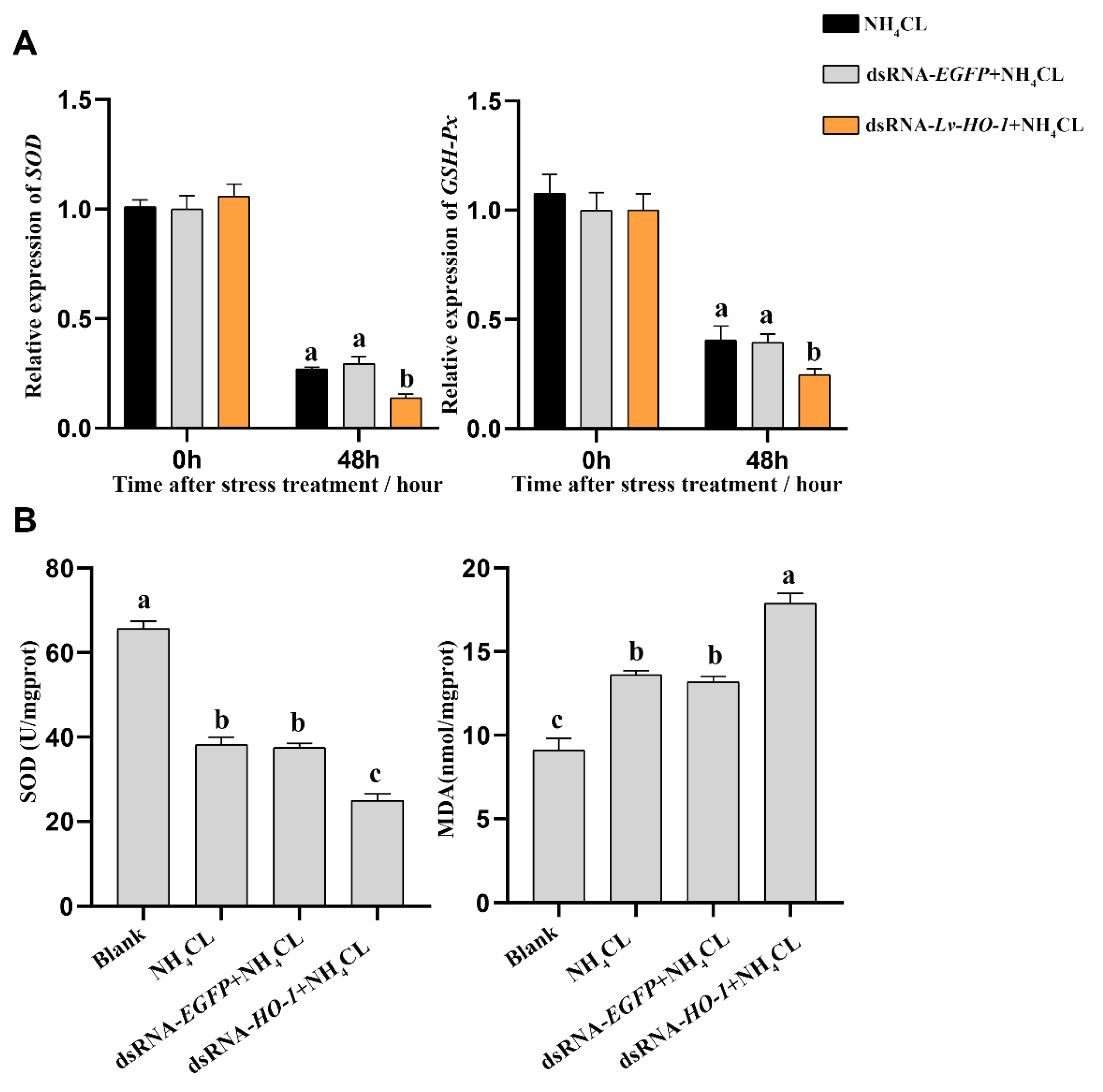
3.5. Effect of Silencing Lv-HO-1 on Antioxidant Capacity
The expression levels of antioxidant-related genes and enzyme activities were also analyzed. The expression levels of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) were significantly decreased (p < 0.05) in each group after ammonia exposure. In the dsRNA-Lv-HO-1 group, the transcription levels of SOD and GSH-Px were significantly decreased compared with the PBS group (Figure 5A). Meanwhile, the enzyme activity of SOD in the dsRNA-Lv-HO-1 group was also significantly decreased (p < 0.05) compared to the PBS group or dsRNA-EGFP group. The MDA level in the dsRNA-Lv-HO-1 group was essentially increased (p < 0.05) compared with the PBS group or dsRNA-EGFP group. The activity of SOD in the dsRNA-Lv-HO-1 group decreased by 35% while the MDA increased by 28% in comparison to the PBS group or dsRNA-EGFP group (Figure 5B).
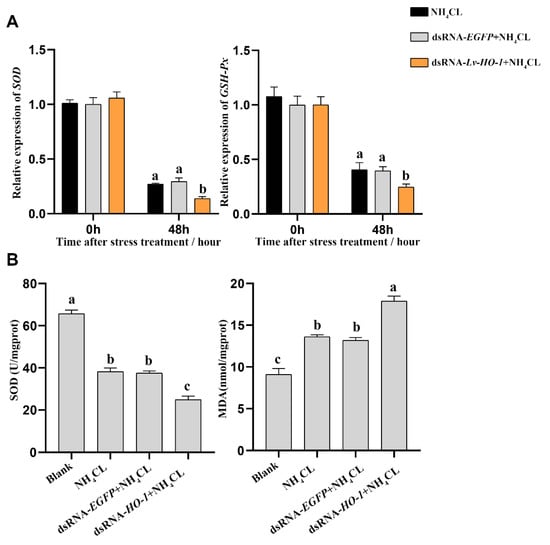
Figure 5.
(A) Mn-SOD and GSH-Px in the hepatopancreas post-RNAi detected by qRT-PCR after ammonia-N stress. All values are the mean ± SD; n = 3. (B) The activities of SOD and the content of MDA in the hepatopancreas after ammonia exposure. Different letters indicate a significant difference (p < 0.05).
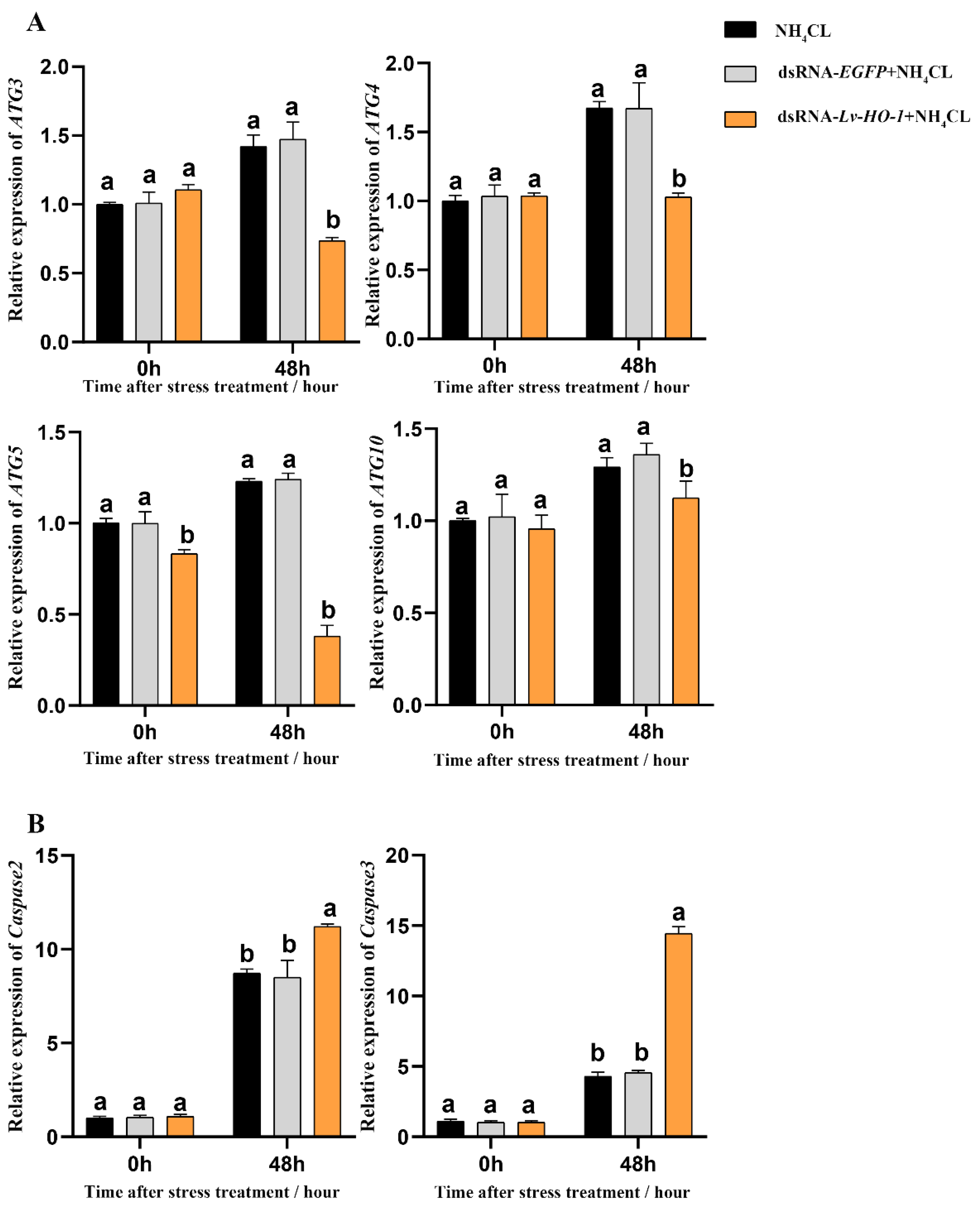
3.6. Effect of Silencing Lv-HO-1 on Autophagy and Apoptosis
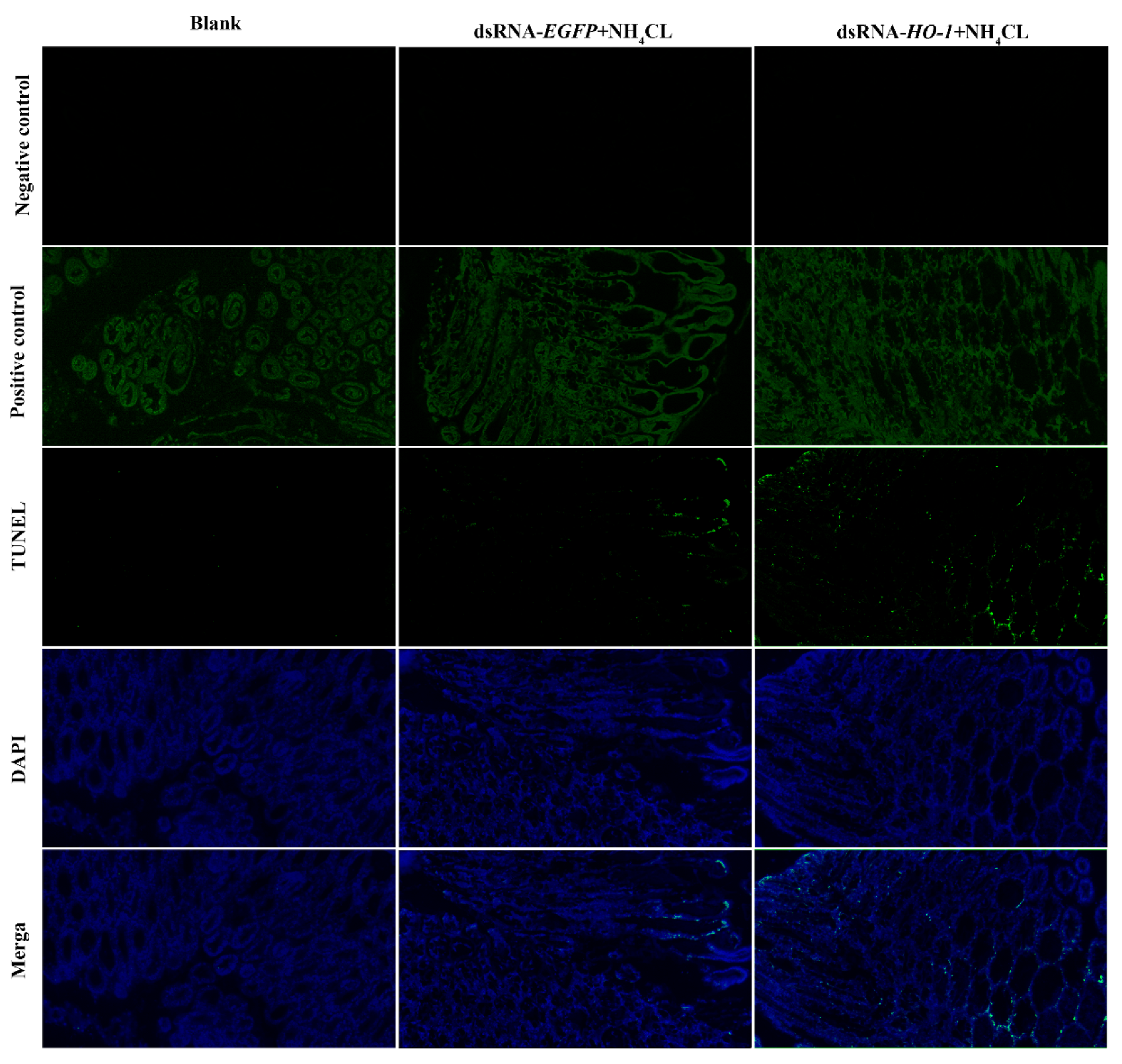
Expression levels of autophagy genes including Atg3, Atg4, Atg5, and Atg10 in the PBS and dsRNA-EGFP groups all increased after ammonia exposure. However, the expression levels of autophagy genes in the dsRNA-Lv-HO-1 group were significantly decreased (p < 0.05) compared with the PBS or dsRNA-EGFP group after stress (Figure 6A). Similarly, the expression levels of apoptosis genes (caspase 2 and caspase 3) significantly increased (p < 0.05). Remarkably, the expression levels of caspase 2 and caspase 3 in the dsRNA-Lv-HO-1 group significantly increased (p < 0.05) compared with the PBS or dsRNA-EGFP group at 48 h (Figure 6B). The apoptosis signals are green and the apoptotic signal in the dsRNA-Lv-HO-1 group was substantially higher than in the dsRNA-EGFP group while the blank group had no positive signal (Figure 7).
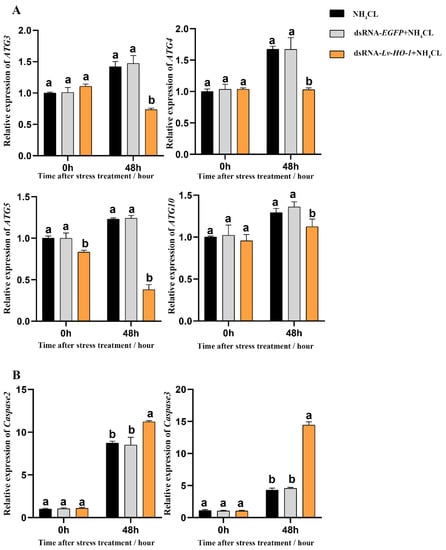
Figure 6.
(A) Expression patterns of autophagy factors Atg3, Atg 4, Atg 5, and Atg 10 as detected by qRT-PCR at different time points after ammonia exposure. (B) Expression patterns of apoptosis factors caspase-2 and caspase-3 as assessed via qRT-PCR at different time points after ammonia exposure. All values are the mean ± SD; n = 3. Different letters indicate a significant difference (p < 0.05).
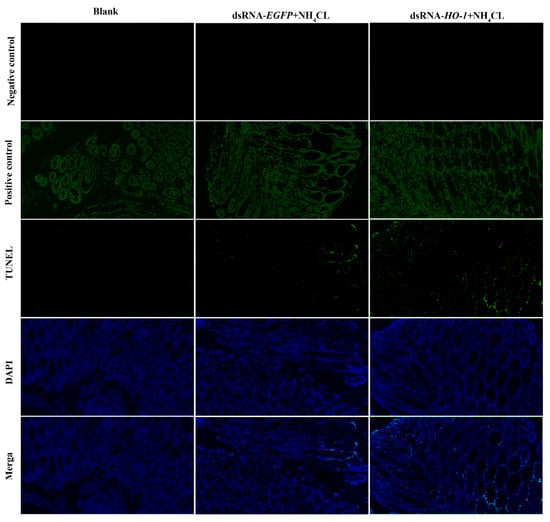
Figure 7.
Apoptosis detection of intestine tissues of PBS group, dsRNA-EGFP+NH4CL group, and dsRNA-HO-1+NH4CL group at 48 h post-challenge via TUNEL assay. Apoptosis signals are marked in green. Nuclei are marked in blue. All sections were observed at 50× magnification.
4. Discussion
In this work, Lv-HO-1 from L. vannamei was identified and characterized. The deduced Lv-HO-1 is a membrane protein that contains a HemO structural domain. The HemO domain is the main activity domain for HO-1 and it is similar to HO-1 found in eukaryotes and some bacteria [28,29,30,31]. More than 80% of other shrimp species and roughly 50% of insects share similarities with the deduced amino acid of Lv-HO-1. The result indicated that Lv-HO-1 is preserved in a wide range of species. Phylogenetic tree analysis showed that Lv-HO-1 is closely evolutionarily related to P. monodon and clustered with vertebrates.
In sea bass and swamp eel, the highest transcription level of HO-1 was observed in the liver and was lower in other tissues [32,33]. Similarly, in golden pompano, the transcriptional level of HO-1 was considerably high in the liver [34]. Furthermore, HO-1 has also been shown to exhibit high levels of activity and gene expression in rat liver [35]. In this study, the expression levels of Lv-HO-1 were mainly detected in the hepatopancreas, muscle, epidermis, and gill. Studies demonstrate that overexpressing HO-1 reduces the replication of the hepatitis C virus (HCV) and oxidative liver damage [36]. In both mice and humans, deficiencies in HO-1 expression can lead to chronic inflammatory states [37]. This observation is comprehensible because the hepatopancreas is the most vital detoxification and immune organ in an organism [38,39]. This finding suggested that Lv-HO-1 may be involved in the immunological response to external stimuli.
Studies have shown that a high concentration of ammonia can induce oxidative stress by increasing the reactive oxygen species (ROS) in aquaculture animals [40]. Overproduction of ROS can harm lipids, proteins, and DNA [41]. Previous studies have proved that the juvenile liver of Nile tilapia suffered oxidative stress after being exposed to NH3 for 72 days [42], and the antioxidant enzymes of Penaeus vannamei were dramatically increased first and then decreased, showing obvious cell fragmentation and vacuoles in hepatic tubules after ammonia exposure [21]. In the present study, Lv-HO-1 expression considerably increased after ammonia-N stress. Furthermore, the expression level of Lv-HO-1 peaked at 12 h in the hepatopancreas but peaked at 24 h in the muscle. In rats, HO-1 was strongly expressed in the spleen and the liver [43]. Additionally, HO-1 can be significantly increased in response to a variety of pro-oxidant and cellular stress-related stimuli, including ROS, nitric oxide, growth factors, free heme, and heavy metals, etc. [44]. In light of the fact that HO-1 lessens tissue oxidative damage [45], it makes sense that HO-1 increases rapidly as a stress protein in hepatopancreas and muscle under ammonia stress. Furthermore, the RNAi of Lv-HO-1 showed that the expression levels of Lv-HO-1 in hepatopancreas and muscle considerably decreased (p < 0.05) with a decreasing expression of SOD and GSH-Px genes and a decreasing enzyme activity of SOD compared with the control group after ammonia exposure. This result was consistent with the results from cattle ovarian granulosa cells, in that inhibition of HO-1 expression by siRNA exacerbates oxidative stress after heat stress [46], which suggested that HO-1 could regulate the antioxidant defense system of L. vannamei against oxidative stress.
In mammals, ROS accumulation contributes to abamectin-induced apoptosis and autophagy [47]. Autophagy can reduce the damage caused by oxidative stress in order to protect cells and increase lifespan [48]. Previous studies showed that after 42 days of ammonia treatment, chicken heart cells showed a clear autophagy phenomenon [49], and miR-252-mediated-ammonia-N-induced autophagy in P. vannamei [21].
Recent research has revealed that HO-1 controls autophagy. In a model of RIPC, the inhibition of HO-1 by Znpp decreased autophagy, aggravating liver damage [50]. HO-1−/− mice also demonstrated that the autophagy genes Atg5 and Atg7 were inhibited, whereas caspase-3 was significantly increased during cisplatin injury [51]. Another study reported that the knockdown of HO-1 significantly increased cellular apoptosis in RAPA-treated cells [52]. A similar study in mice indicated that HO-1 siRNA enhanced apoptosis via increased caspase 3 activity [53]. Our results showed that in an ammonia-nitrogen model, autophagy genes (Atg3, Atg4, Atg5, and Atg10) and apoptosis genes (caspase-2 and caspase-3) can be significantly induced. The knockdown of HO-1 shows a decrease in autophagy and instead exhibits higher levels of caspase-2 and caspase-3 and undergoes apoptosis more readily compared with the PBS group. Similar apoptosis results can also be derived from our TUNEL experiments. Moreover, MDA, an oxidative stress marker, exhibited higher content in the HO-1-RNAi group than in the control group. Additionally, stellate ductal dilatation and cellular atrophy were observed in the HO-1-RNAi group. Similar studies in rats indicated that the MDA content was regulated by HO-1 during septic shock [54]. In previous studies, we demonstrated that when oxidative stress occurred, the oxidative damage of the body was increased due to the inhibition of the autophagy process, which led to an increase in apoptosis. These results indicated that HO-1 can act as an antioxidant and autophagy regulator to protect cells from apoptosis [16,55].
5. Conclusions
In summary, shrimp Heme Oxygenase-1 (Lv-HO-1) was identified and its involvement in oxidative stress was determined. In addition, the knockdown of Lv-HO-1 is associated with a failure to further induce autophagy and leads to increased apoptosis after ammonia exposure. Furthermore, Lv-HO-1 can be induced by oxidative stress and may have important roles in regulating the host antioxidant system, reducing cell apoptosis. This research offers a theoretical framework for further research on the mechanism of HO-1 in shrimp.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes7060356/s1, Table S1: The primers used in this research.
Author Contributions
Y.H.: Conceptualization, Data Curation, Writing—Original Draft; Q.L.: Conceptualization, Methodology; J.Z.: Resources; Z.Z.: Validation; Y.Y.: Resources; B.J.: Resources; J.L.: Resources; S.Y.: Project administration, Writing—Review and Editing; J.J.: Writing—Review and Editing, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. U20A2065, 32073006, 32002426), National Key R&D Program of China (No. 2018YFD0900501), Project of the Agricultural Sci-tech Commissioners of Guangdong Province (KTP20210292), Fund of Southern Marine Science and Engineering Guangdong Laboratory (Zhanjiang) (No. ZJW-2019-06), Modern Seed Industry Park for Whiteleg Shrimp of Guangdong Province (K22219).
Institutional Review Board Statement
The animal study was reviewed and approved by Guangdong Province Laboratory Animal Management Regulations.
Data Availability Statement
Data is contained within the article and Supplementary Materials.
Acknowledgments
Thanks for the support of the Guangdong Provincial Key Laboratory of Aquatic Animal Disease Control and Healthy Culture & Key Laboratory of Control for Diseases of Aquatic Economic Animals of Guangdong Higher Education Institutes.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Agarwal, A.; Nick, H.S. Renal response to tissue injury: Lessons from heme oxygenase-1 GeneAblation and expression. J. Am. Soc. Nephrol. 2000, 11, 965–973. [Google Scholar] [CrossRef]
- Maines, M.D.; Trakshel, G.M.; Kutty, R.K. Characterization of two constitutive forms of rat liver microsomal heme oxygenase. Only one molecular species of the enzyme is inducible. J. Biol. Chem. 1986, 261, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Motterlini, R.; Foresti, R. Heme Oxygenase-1 As a Target for Drug Discovery. Antioxid. Redox Signal. 2014, 20, 1810–1826. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Stocker, R. Heme oxygenase and iron: From bacteria to humans. Redox Rep. 2009, 14, 95–101. [Google Scholar] [CrossRef]
- Czibik, G.; Derumeaux, G.; Sawaki, D.; Valen, G.; Motterlini, R. Heme oxygenase-1: An emerging therapeutic target to curb cardiac pathology. Basic Res. Cardiol. 2014, 109, 450. [Google Scholar] [CrossRef]
- Fang, X.; Wang, H.; Han, D.; Xie, E.; Yang, X.; Wei, J.; Gu, S.; Gao, F.; Zhu, N.; Yin, X.; et al. Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 2672–2680. [Google Scholar] [CrossRef]
- Maines, M.D. The Heme Oxygenase System: A Regulator of Second Messenger Gases. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 517–554. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Omata, Y.; Sakamoto, H.; Higashimoto, Y.; Hara, T.; Sagara, Y.; Noguchi, M. Characterization of rat heme oxygenase-3 gene. Implication of processed pseudogenes derived from heme oxygenase-2 gene. Gene 2004, 336, 241–250. [Google Scholar] [CrossRef]
- Silva, R.C.M.C.; Travassos, L.H.; Paiva, C.N.; Bozza, M.T. Heme oxygenase-1 in protozoan infections: A tale of resistance and disease tolerance. PLoS Pathog. 2020, 16, e1008599. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Li, T.; Zhang, L.; Yang, R.; Li, Y.; Zhang, M.; Dong, Y.; Zhou, Y.; Xu, C.; Yang, B.; et al. Heme oxygenase-1 prevents heart against myocardial infarction by attenuating ischemic injury-induced cardiomyocytes senescence. eBioMedicine 2019, 39, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Dulak, J.; Deshane, J.; Jozkowicz, A.; Agarwal, A. Heme Oxygenase-1 and Carbon Monoxide in Vascular Pathobiology. Circulation 2008, 117, 231–241. [Google Scholar] [CrossRef]
- Nath, K. Heme oxygenase-1: A provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int. 2006, 70, 432–443. [Google Scholar] [CrossRef]
- Choi, A.M.; Alam, J. Heme oxygenase-1: Function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am. J. Respir. Cell Mol. Biol. 1996, 15, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Castilho, F.; Aveleira, C.A.; Leal, E.C.; Simões, N.F.; Fernandes, C.R.; Meirinhos, R.I.; Baptista, F.I.; Ambrósio, A.F. Heme Oxygenase-1 Protects Retinal Endothelial Cells against High Glucose- and Oxidative/Nitrosative Stress-Induced Toxicity. PLoS ONE 2012, 7, e42428. [Google Scholar] [CrossRef]
- Wiesel, P.; Patel, A.P.; DiFonzo, N.; Marria, P.B.; Sim, C.U.; Pellacani, A.; Maemura, K.; LeBlanc, B.W.; Marino, K.; Doerschuk, C.M.; et al. Endotoxin-Induced Mortality Is Related to Increased Oxidative Stress and End-Organ Dysfunction, Not Refractory Hypotension, in Heme Oxygenase-1–Deficient Mice. Circulation 2000, 102, 3015–3022. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Balla, J.; Alam, J.; Croatt, A.J.; Nath, K.A. Induction of heme oxygenase in toxic renal injury: A protective role in cisplatin nephrotoxicity in the rat. Kidney Int. 1995, 48, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Nath, K.A.; Haggard, J.J.; Croatt, A.J.; Grande, J.P.; Poss, K.D.; Alam, J. The Indispensability of Heme Oxygenase-1 in Protecting against Acute Heme Protein-Induced Toxicity in Vivo. Am. J. Pathol. 2000, 156, 1527–1535. [Google Scholar] [CrossRef]
- Shiraishi, F.; Curtis, L.M.; Truong, L.; Poss, K.; Visner, G.A.; Madsen, K.; Nick, H.S.; Agarwal, A. Heme oxygenase-1 gene ablation or expression modulates cisplatin-induced renal tubular apoptosis. Am. J. Physiol. Physiol. 2000, 278, F726–F736. [Google Scholar] [CrossRef] [PubMed]
- Tzaneva, V.; Perry, S.F. Heme Oxygenase-1 (Ho-1) Mediated Respiratory Responses to Hypoxia in the Goldfish. Carassius Auratus. Respir. Physiol. Neurobiol. 2014, 199, 1–8. [Google Scholar] [CrossRef]
- Abaquita, T.A.L.; Damulewicz, M.; Bhattacharya, D.; Pyza, E. Regulation of Heme Oxygenase and Its Cross-Talks with Apoptosis and Autophagy under Different Conditions in Drosophila. Antioxidants 2021, 10, 1716. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Huang, L.; Liao, M.; Dong, W.; Liu, C.; Zhuang, X.; Liu, Y.; Yin, X.; Liang, Q.; Wang, W. Pva-miR-252 participates in ammonia nitrogen-induced oxidative stress by modulating autophagy in Penaeus vannamei. Ecotoxicol. Environ. Saf. 2021, 225, 112774. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, L.; Xu, L.; Si, L. Effects of ammonia-N exposure on the concentrations of neurotransmitters, hemocyte intracellular signaling pathways and immune responses in white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2018, 75, 48–57. [Google Scholar] [CrossRef]
- Si, L.; Pan, L.; Wang, H.; Zhang, X. Ammonia-N exposure alters neurohormone levels in the hemolymph and mRNA abundance of neurohormone receptors and associated downstream factors in the gills of Litopenaeus vannamei. J. Exp. Biol. 2019, 222, jeb.200204. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Huang, Y.; Chen, B.; Liu, H.; Huang, Y.; Cai, S.; Jian, J. Protective effects of sulphoraphane on oxidative damage caused by ammonia in Litopenaeus vannamei. Aquac. Res. 2022, 53, 1197–1204. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Z.; Fan, W.; Huang, Y.; Niu, J.; Luo, G.; Liu, X.; Huang, Y.; Jian, J. LECT2 Protects Nile Tilapia (Oreochromis niloticus) Against Streptococcus agalatiae Infection. Front. Immunol. 2021, 12, 667781. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, B.; Zhang, Z.; Huang, Y.; Xu, Z.; Chen, X.; Huang, Y.; Jian, J. SP protects Nile tilapia (Oreochromis niloticus) against acute Streptococcus agalatiae infection. Fish Shellfish Immunol. 2022, 123, 218–228. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Shibahara, S.; Müller, R.; Taguchi, H.; Yoshida, T. Cloning and expression of cDNA for rat heme oxygenase. Proc. Natl. Acad. Sci. USA 1985, 82, 7865–7869. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.O.; Healey, J.F.; Greene, Y.; Bonkovsky, H.L. Cloning, sequencing and expression of cDNA for chick liver haem oxygenase. Comparison of avian and mammalian cDNAs and deduced proteins. Biochem. J. 1991, 273, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Rashid, I.; Baisvar, V.S.; Singh, M.; Kumar, R.; Srivastava, P.; Kushwaha, B.; Pathak, A.K. Isolation and characterization of hypoxia inducible heme oxygenase 1 (HMOX1) gene in Labeo rohita. Genomics 2020, 112, 2327–2333. [Google Scholar] [CrossRef]
- Wilks, A.; Ikeda-Saito, M. Heme Utilization by Pathogenic Bacteria: Not All Pathways Lead to Biliverdin. Acc. Chem. Res. 2014, 47, 2291–2298. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Zheng, S.; Tang, F.; Yang, L.; Wei, X.; Kong, D.; Sun, W.; Li, W. Heme oxygenase 1 plays a crucial role in swamp eel response to oxidative stress induced by cadmium exposure or Aeromonas hydrophila infection. Fish Physiol. Biochem. 2020, 46, 1947–1963. [Google Scholar] [CrossRef] [PubMed]
- Prevot-D’Alvise, N.; Pierre, S.; Gaillard, S.; Gouze, E.; Gouze, J.-N.; Aubert, J.; Richard, S.; Grillasca, J.-P. cDNA sequencing and expression analysis of Dicentrarchus labrax heme oxygenase-1. Cell Mol. Biol. 2008, 54, Ol1046–O11054. [Google Scholar]
- Xie, J.; He, X.; Fang, H.; Liao, S.; Liu, Y.; Tian, L.; Niu, J. Identification of heme oxygenase-1 from golden pompano (Trachinotus ovatus) and response of Nrf2/HO-1 signaling pathway to copper-induced oxidative stress. Chemosphere 2020, 253, 126654. [Google Scholar] [CrossRef] [PubMed]
- Bauer, I.; Wanner, G.A.; Rensing, H.; Alte, C.; Miescher, E.A.; Wolf, B.; Pannen, B.H.J.; Clemens, M.G.; Bauer, M. Expression pattern of heme oxygenase isoenzymes 1 and 2 in normal and stress-exposed rat liver. Hepatology 1998, 27, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wilson, A.T.; Mathahs, M.M.; Wen, F.; Brown, K.E.; Luxon, B.A.; Schmidt, W.N. Heme oxygenase-1 suppresses hepatitis C virus replication and increases resistance of hepatocytes to oxidant injury. Hepatology 2008, 48, 1430–1439. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.A.; González, P.A.; Kalergis, A.M. Modulation of Antiviral Immunity by Heme Oxygenase-1. Am. J. Pathol. 2017, 187, 487–493. [Google Scholar] [CrossRef]
- Rőszer, T. The invertebrate midintestinal gland (“hepatopancreas”) is an evolutionary forerunner in the integration of immunity and metabolism. Cell Tissue Res. 2014, 358, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Díaz, A.C.; Gimenez, A.V.F.; Mendiara, S.N.; Fenucci, J.L. Antioxidant Activity in Hepatopancreas of the Shrimp (Pleoticus muelleri) by Electron Paramagnetic Spin Resonance Spectrometry. J. Agric. Food Chem. 2004, 52, 3189–3193. [Google Scholar] [CrossRef]
- Liu, M.-J.; Guo, H.-Y.; Liu, B.; Zhu, K.-C.; Guo, L.; Liu, B.-S.; Zhang, N.; Yang, J.-W.; Jiang, S.-G.; Zhang, D.-C. Gill oxidative damage caused by acute ammonia stress was reduced through the HIF-1α/NF-κb signaling pathway in golden pompano (Trachinotus ovatus). Ecotoxicol. Environ. Saf. 2021, 222, 112504. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Hegazi, M.M.; Attia, Z.I.; Ashour, O.A. Oxidative stress and antioxidant enzymes in liver and white muscle of Nile tilapia juveniles in chronic ammonia exposure. Aquat. Toxicol. 2010, 99, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Braggins, P.; Trakshel, G.; Kutty, R.; Maines, M. Characterization of two heme oxygenase isoforms in rat spleen: Comparison with the hematin-induced and constitutive isoforms of the liver. Biochem. Biophys. Res. Commun. 1986, 141, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Fredenburgh, L.E.; Merz, A.A.; Cheng, S. Haeme oxygenase signalling pathway: Implications for cardiovascular disease. Eur. Heart J. 2015, 36, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, B.H.; Chen, L.; An, W. Overexpression of heme oxygenase-1 protects smooth muscle cells against oxidative injury and inhibits cell proliferation. Cell Res. 2002, 12, 123–132. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Elsheikh, N.A.H.; Li, C.; Yang, F.; Wang, G.; Li, L. HO-1 reduces heat stress-induced apoptosis in bovine granulosa cells by suppressing oxidative stress. Aging 2019, 11, 5535–5547. [Google Scholar] [CrossRef]
- Gur, C.; Kandemir, O.; Kandemir, F.M. Investigation of the effects of hesperidin administration on abamectin-induced testicular toxicity in rats through oxidative stress, endoplasmic reticulum stress, inflammation, apoptosis, autophagy, and JAK2/STAT3 pathways. Environ. Toxicol. 2022, 37, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, Z.; Zhang, S.; Zhang, H.; Teng, X. miR-187-5p/apaf-1 axis was involved in oxidative stress-mediated apoptosis caused by ammonia via mitochondrial pathway in chicken livers. Toxicol. Appl. Pharmacol. 2019, 388, 114869. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, J.; Xiong, X.; Xu, Y.; Zhang, H.; Huang, C.; Tian, Y.; Jiao, C.; Wang, X.; Li, X. Remote Ischemic Preconditioning Protects against Liver Ischemia-Reperfusion Injury via Heme Oxygenase-1-Induced Autophagy. PLoS ONE 2014, 9, e98834. [Google Scholar] [CrossRef] [PubMed]
- Bolisetty, S.; Traylor, A.M.; Kim, J.; Joseph, R.; Ricart, K.; Landar, A.; Agarwal, A. Heme Oxygenase-1 Inhibits Renal Tubular Macroautophagy in Acute Kidney Injury. J. Am. Soc. Nephrol. 2010, 21, 1702–1712. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Basu, A.; Wegiel, B.; Otterbein, L.E.; Mizumura, K.; Gasser, M.; Waaga-Gasser, A.M.; Choi, A.M.; Pal, S. Heme Oxygenase-1 Promotes Survival of Renal Cancer Cells through Modulation of Apoptosis- and Autophagy-regulating Molecules. J. Biol. Chem. 2012, 287, 32113–32123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shan, P.; Jiang, D.; Noble, P.W.; Abraham, N.G.; Kappas, A.; Lee, P.J. Small Interfering RNA Targeting Heme Oxygenase-1 Enhances Ischemia-Reperfusion-induced Lung Apoptosis. J. Biol. Chem. 2004, 279, 10677–10684. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-B.; Zhou, F.; Yao, S.-L.; Tang, Z.-H.; Wang, M.; Chen, H.-R. Effect of heme oxygenase-1 on the kidney during septic shock in rats. Transl. Res. 2009, 153, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.P.; Wang, X.; Lee, S.-J.; Huang, M.-H.; Wan, Y.; Ryter, S.W.; Choi, A.M. Autophagic proteins regulate cigarette smoke induced apoptosis: Protective role of heme oxygenase-1. Autophagy 2008, 4, 887–895. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).