Polyculture Affects the Growth, Antioxidant Status, Nutrient Content, and Flavor of Chinese Mitten Crabs (Eriocheir sinensis) and Largemouth Bass (Micropterus salmoides)
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Design
2.3. Sampling Protocol
2.4. Enzyme Activity Assays
2.5. Biochemical Analysis
2.6. Statistical Analysis
3. Results
3.1. Growth Performance
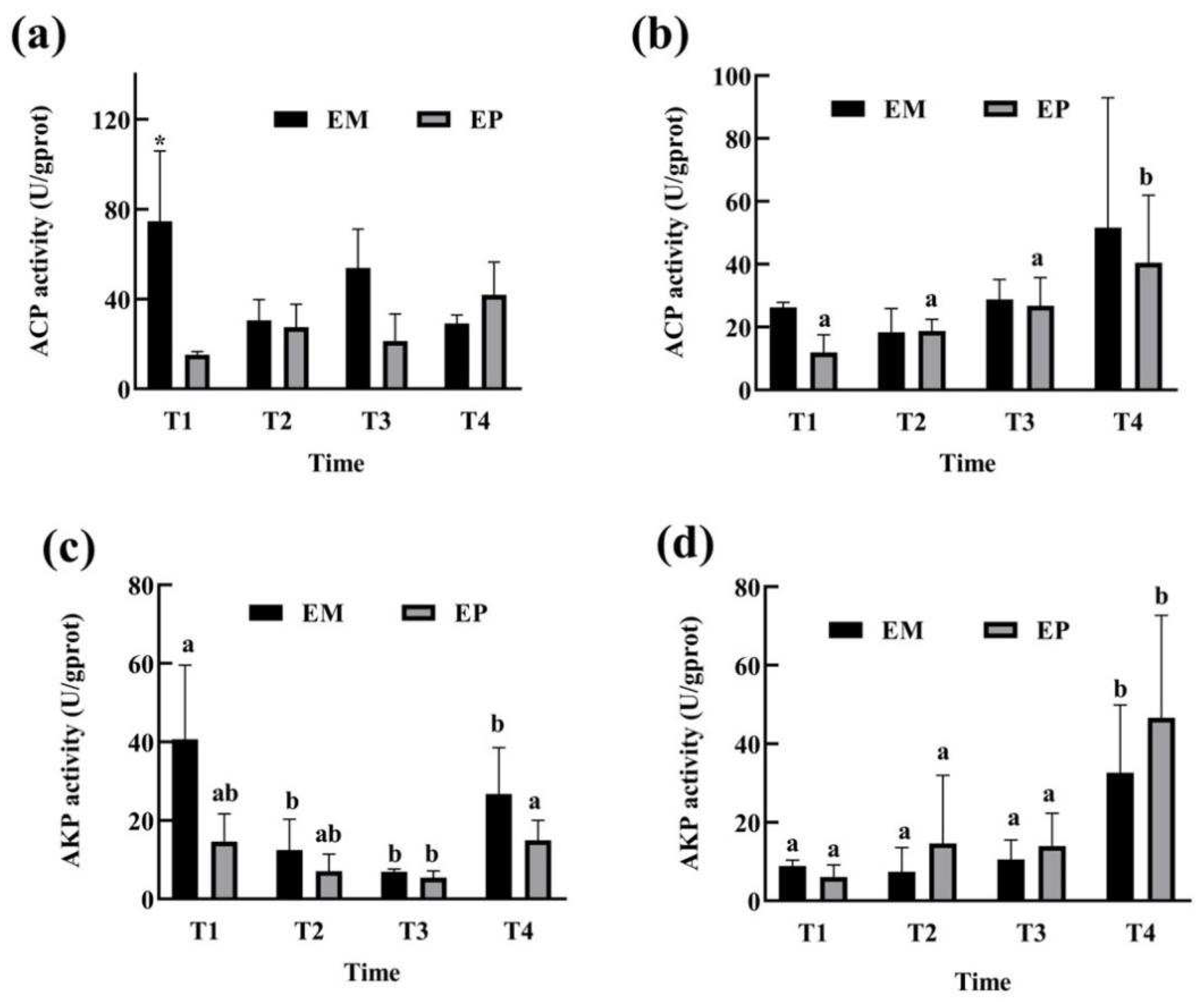
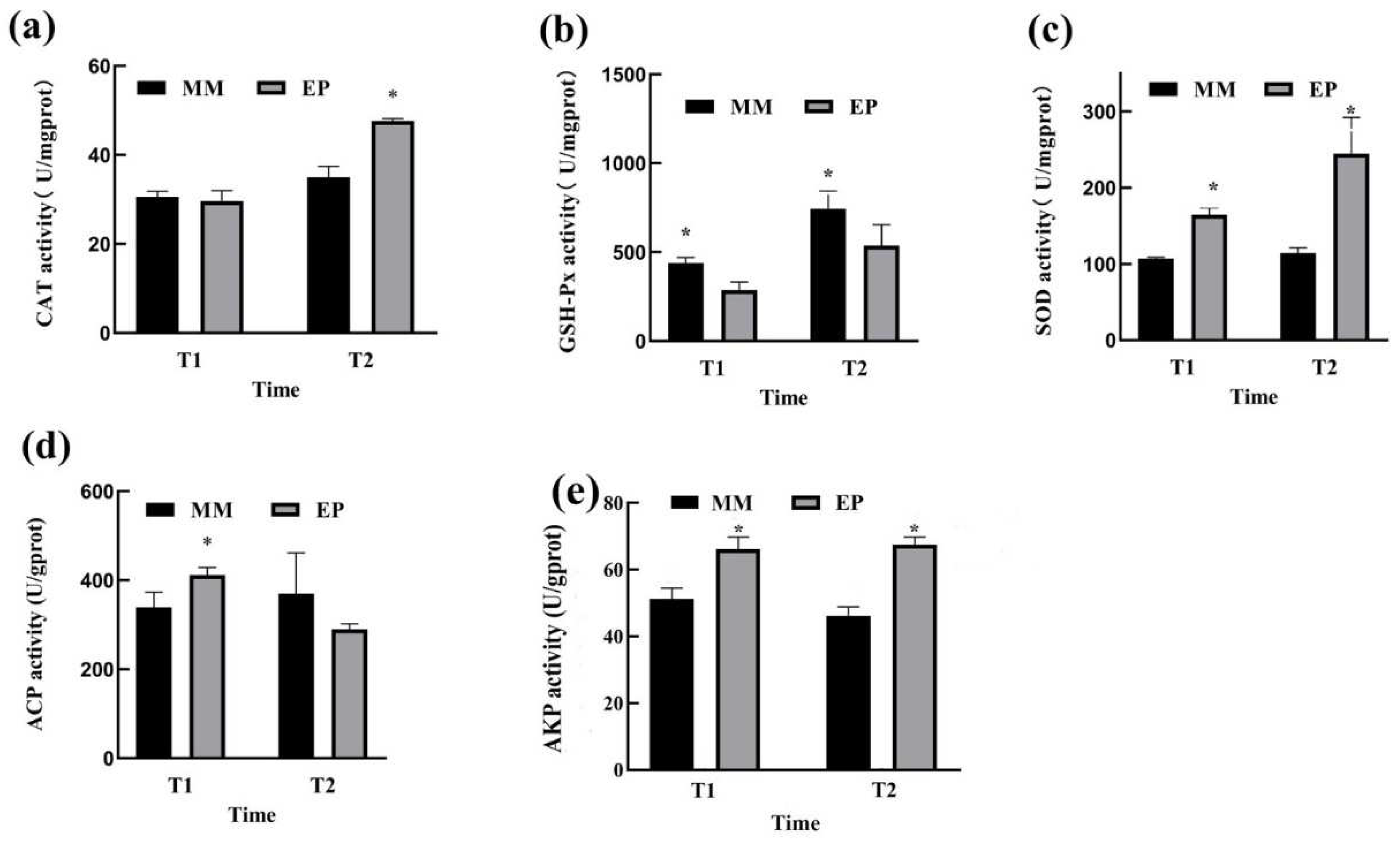
3.2. Antioxidant Status
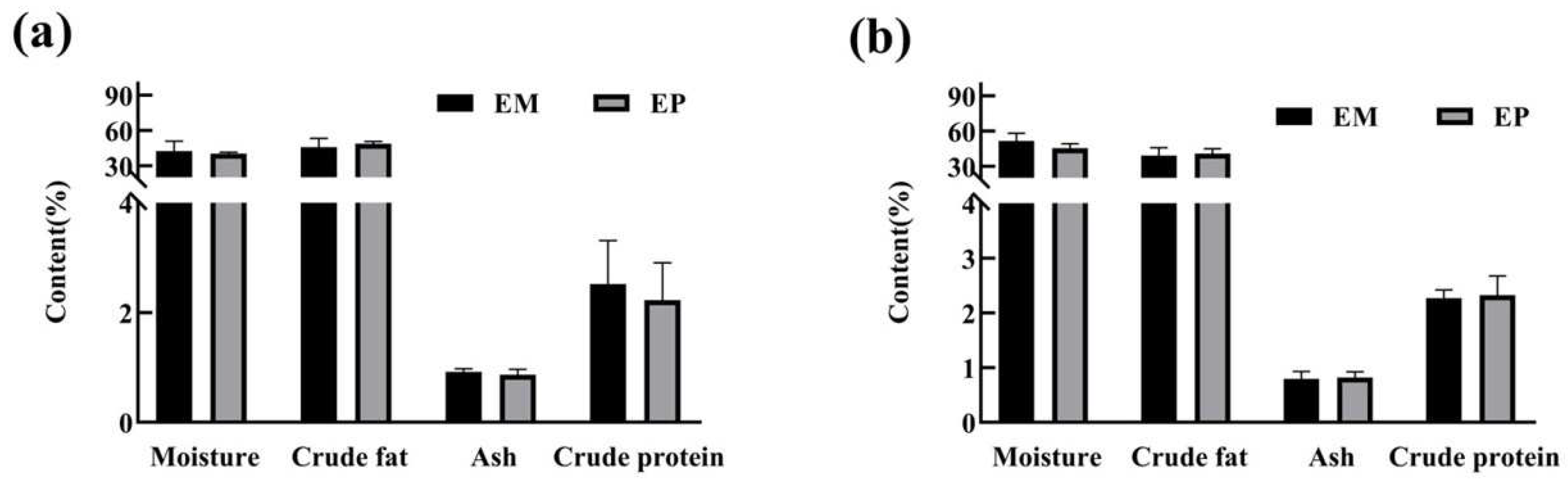
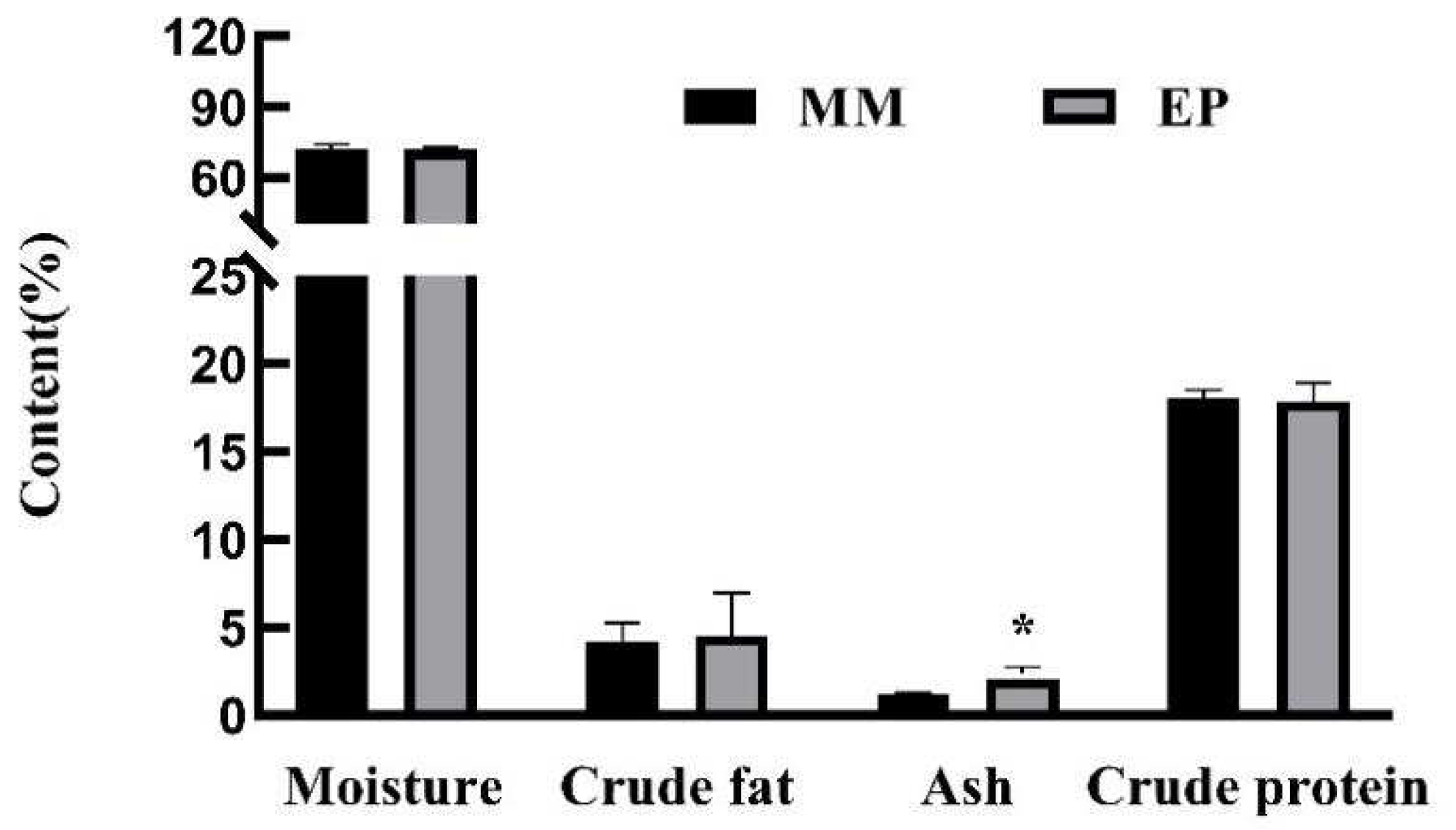
3.3. Proximate Compositions of Edible Tissues
3.4. Composition of Fatty Acids in Edible Tissues
3.5. Amino Acid Composition
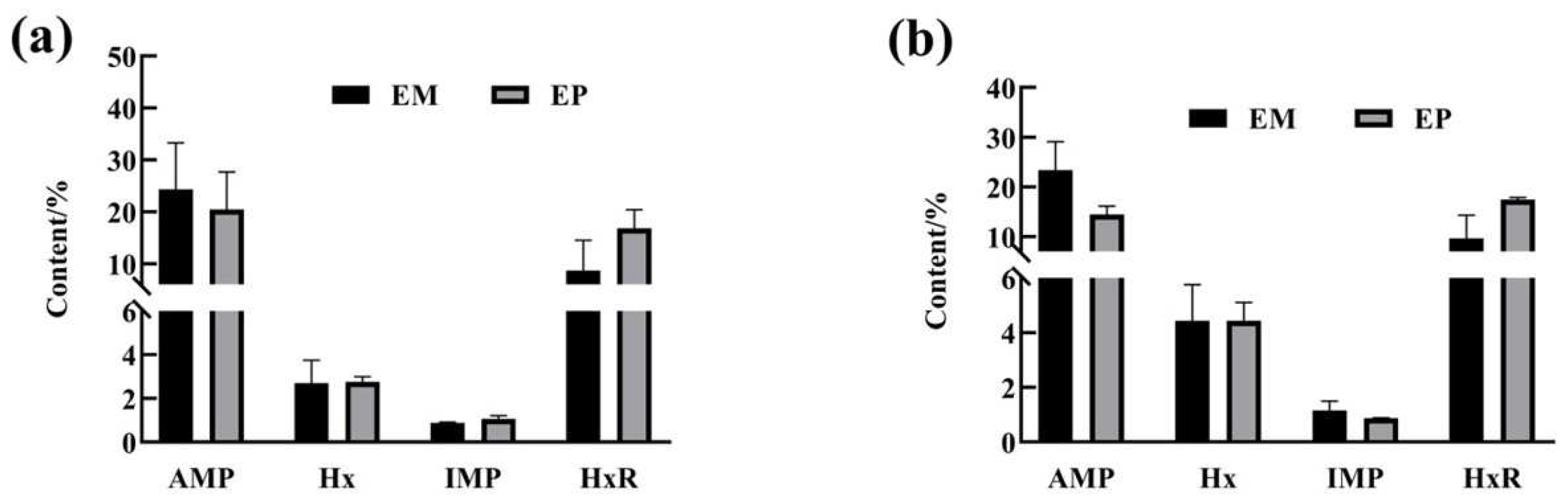
3.6. Comparison of Nucleotide Content in Edible Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Shen, Z.; Wang, C.; Li, E.; Qin, J.G.; Chen, L. Dietary supplementation of selenium yeast enhances the antioxidant capacity and immune response of juvenile Eriocheir Sinensis under nitrite stress. Fish Shellfish Immunol. 2019, 87, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Q.; Wu, X.; Liu, Q.; Cheng, Y. Genetic diversity and genetic structure of farmed and wild Chinese mitten crab (Eriocheir sinensis) populations from three major basins by mitochondrial DNA COI and Cyt b gene sequences. Mitochondrial DNA Part A 2018, 29, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.-M.; Huang, F.; Shen, J.; Yu, H.-M. Using Sr isotopes to trace the geographic origins of Chinese mitten crabs. Acta Geochim. 2020, 39, 326–336. [Google Scholar] [CrossRef]
- Cheng, Y.; Wu, X.; Yang, X.; Hines, A.H. Current trends in hatchery techniques and stock enhancement for Chinese mitten crab, Eriocheir japonica sinensis. Rev. Fish. Sci. 2008, 16, 377–384. [Google Scholar] [CrossRef]
- Fishery Administration Bureau of the Ministry of Agriculture and Villages; National Aquatic Products Technology Extension Station; China Society of Fisheries. In 2022 China Fishery Statistical Yearbook; Chinese Agricultural Press: Beijing, China, 2022.
- Wu, C.; Shan, J.F.; Feng, J.C.; Jiang, A.L.; Nie, G.X.; Wang, X.H. Changes in the growth, nutritional quality and gut microbiota of the adult Chinese mitten crab, Eriocheir sinensis, following overwinter rearing. Aquac. Res. 2022, 53, 1348–1362. [Google Scholar] [CrossRef]
- Habte-Tsion, H.-M.; Kolimadu, G.D.; Rossi, W.; Filer, K.; Kumar, V. Effects of Schizochytrium and micro-minerals on immune, antioxidant, inflammatory and lipid-metabolism status of Micropterus salmoides fed high- and low-fishmeal diets. Sci. Rep. 2020, 10, 7457. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, H.; Fan, J.; Zhou, H.; Zhang, Y.; Cao, Y.; Jiang, W.; Zhang, W.; Deng, J.; Tan, B. Effects of dietary non-starch polysaccharides level on the growth, intestinal flora and intestinal health of juvenile largemouth bass Micropterus salmoides. Aquaculture 2022, 557, 738343. [Google Scholar] [CrossRef]
- Hu, B.; Song, L.; Mao, S.; Xu, P. Effects of four Chinese herbal preparations on growth performance and antioxidant activity in juvenile Micropterus Salmoides. J. Guangdong Ocean Univ. 2019, 39, 101–107. [Google Scholar]
- Guo, Z.-R.; Zhao, Z.; Zhang, C.; Jia, Y.-J.; Qiu, D.-K.; Zhu, B.; Wang, G.-X. Carbon nanotubes-loaded subunit vaccine can increase protective immunity against rhabdovirus infections of largemouth bass (Micropterus Salmoides). Fish Shellfish Immunol. 2020, 99, 548–554. [Google Scholar] [CrossRef]
- Xie, S.; Yin, P.; Tian, L.; Yu, Y.; Liu, Y.; Niu, J. Dietary Supplementation of Astaxanthin Improved the Growth Performance, Antioxidant Ability and Immune Response of Juvenile Largemouth Bass (Micropterus salmoides) Fed High-Fat Diet. Mar. Drugs 2020, 18, 642. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, R.-X.; Zhang, D.-M.; Lei, X.-Y.; Wang, S.; Wan, J.-W.; Liu, H.-J.; Chen, Y.-K.; Zhao, Y.-L.; Wang, G.-Q.; et al. Effects of different stocking densities on growth performance, nutritional quality and economic benefit of juvenile female Chinese mitten crab (Eriocheir sinensis) in rice-crab culture systems. Aquaculture 2022, 553, 738111. [Google Scholar] [CrossRef]
- Zeng, Q.; Xu, Y.; Jeppesen, E.; Gu, X.; Mao, Z.; Chen, H. Farming practices affect the amino acid profiles of the aquaculture Chinese mitten crab. PeerJ 2021, 9, e11605. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Liu, M.; Lou, J.; Mi, G.; Yuan, J.; Gu, Z. Stocking density alters growth performance, serum biochemistry, digestive enzymes, immune response, and muscle quality of largemouth bass (Micropterus salmoides) in in-pond raceway system. Fish Physiol. Biochem. 2021, 47, 1243–1255. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ge, M.; Chen, H.; Jiang, S.; Lin, L.; Lu, J. Comparison between the nutritional qualities of wild-caught and rice-field male Chinese mitten crabs (Eriocheir sinensis). LWT 2020, 117, 108663. [Google Scholar] [CrossRef]
- Zeng, S.; Wei, D.; Hou, D.; Wang, H.; Liu, J.; Weng, S.; He, J.; Huang, Z. Sediment microbiota in polyculture of shrimp and fish pattern is distinctive from those in monoculture intensive shrimp or fish ponds. Sci. Total Environ. 2021, 787, 147594. [Google Scholar] [CrossRef]
- Martinez-Porchas, M.; Martinez-Cordova, L.R.; Porchas-Cornejo, M.; López-Elías, J. Shrimp polyculture: A potentially profitable, sustainable, but uncommon aquacultural practice. Rev. Aquac. 2010, 2, 73–85. [Google Scholar] [CrossRef]
- N’souvi, K.; Sun, C.; Che, B. Aquaculture technology adoption and profitability of the polyculture system practiced by prawn and crab farmers: Case study of Anhui province in China. Aquac. Rep. 2021, 21, 100896. [Google Scholar] [CrossRef]
- Erwiantono, E.; Darmansyah, O.; Saleha, Q.; Sulistianto, E.; Fahrizal, W.; Maryanto, F.; Susilo, H. Impact of shrimp-fish polyculture practices on small-scale farmers’ income in Indonesia. AACL Bioflux 2020, 13, 3407–3419. [Google Scholar]
- Lalramchhani, C.; Balasubramanian, C.P.; Panigrahi, A.; Ghoshal, T.K.; Das, S.; Anand, P.S.S.; Vijayan, K.K. Polyculture of Indian White Shrimp (Penaeus indicus) with Milkfish (Chanos chanos) and its Effect on Growth Performances, Water Quality and Microbial Load in Brackishwater Pond. J. Coast. Res. 2019, 86, 43–48. [Google Scholar] [CrossRef]
- Wang, X.; Yao, Q.; Lei, X.-Y.; Zhang, D.-M.; Wang, S.; Wan, J.-W.; Liu, H.-J.; Chen, Y.-K.; Zhao, Y.-L.; Wang, G.-Q.; et al. Effects of different stocking densities on the growth performance and antioxidant capacity of Chinese mitten crab (Eriocheir sinensis) in rice crab culture system. Aquac. Int. 2022, 30, 883–898. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, L.; Brown, P.; Feng, Y.; Zhu, Z.; Lei, A.; Zhang, Y.; Ding, X.; Bai, Y.; Jia, F.; et al. Effect of replacement of fish meal with cricket meal on growth performance, proximate composition, digestive enzyme activities, serum biochemical indices, and antioxidant capacity in largemouth bass (Micropterus salmoides). Aquac. Res. 2022, 53, 5354–5364. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Q.; Pang, Y.; Song, X.; Zhou, N.; Wang, J.; He, L.; Lv, J.; Song, Y.; Cheng, Y.; et al. The protective effects of melatonin on oxidative damage and the immune system of the Chinese mitten crab (Eriocheir sinensis) exposed to deltamethrin. Sci. Total Environ. 2019, 653, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis International; Association of Analytical Communities: Gaithersburg, MD, USA, 1999. [Google Scholar]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Arlington, VA, USA, 1984. [Google Scholar]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Yi, X.; Gao, J.; Li, L.; Du, J.; Nie, Z.; Zhang, X.; Xu, G. Effects of fattening diets on the nutritional quality and flavor of the adult female Chinese mitten crab (Eriocheir sinensis). Aquac. Rep. 2022, 25, 101223. [Google Scholar] [CrossRef]
- Sun, C.; Zou, X.; Yao, Y.; Jin, J.; Xia, Y.; Huang, J.; Jin, Q.; Wang, X. Evaluation of fatty acid composition in commercial infant formulas on the Chinese market: A comparative study based on fat source and stage. Int. Dairy J. 2016, 63, 42–51. [Google Scholar] [CrossRef]
- Dai, F.; Song, L.; Gao, J.; Tai, X.; Chu, L.; Zhuang, H.; Shao, N.; Hu, J.; Nei, Z.; Wang, Y.; et al. Effect of stocking density on mortality rate, physiological status and nutrient contents of Chinese mitten crab Eriocheir sinensis during overwintering cultivation. Aquac. Rep. 2020, 16, 100241. [Google Scholar] [CrossRef]
- Costa, L.C.D.O.; Xavier, J.A.A.; Neves, L.F.D.M.; Azambuja, A.M.V.D.; Junior, W.W.; Figueiredo, M.R.C. Polyculture of Litopenaeus vannamei shrimp and Mugil platanus mullet in earthen ponds. Rev. Bras. Zootec. 2013, 42, 605–611. [Google Scholar] [CrossRef]
- Ali, M.; Islam, M.; Begum, N.; Suravi, I.; Mia, M.R.; Kashem, M. Effect of Monoculture and Polyculture Systems on Growth and Production of Fishes in Seasonal Waterbodies of Haor Villages, Sunamganj District. J. Sci. Res. 2017, 9, 307. [Google Scholar] [CrossRef][Green Version]
- Banh, S.; Wiens, L.; Sotiri, E.; Treberg, J.R. Mitochondrial reactive oxygen species production by fish muscle mitochondria: Potential role in acute heat-induced oxidative stress. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2016, 191, 99–107. [Google Scholar] [CrossRef]
- Kanak, E.G.; Dogan, Z.; Eroglu, A.; Atli, G.; Canli, M. Effects of fish size on the response of antioxidant systems of Oreochromis niloticus following metal exposures. Fish Physiol. Biochem. 2014, 40, 1083–1091. [Google Scholar] [CrossRef]
- Li, M.-Y.; Liu, X.-Y.; Xia, C.-G.; Wang, G.-Q.; Zhang, D.-M. Astaxanthin enhances hematology, antioxidant and immunological parameters, immune-related gene expression, and disease resistance against in Channa argus. Aquac. Int. 2019, 27, 735–746. [Google Scholar] [CrossRef]
- Dong, X.-Q.; Zhang, D.-M.; Chen, Y.-K.; Wang, Q.-J.; Yang, Y.-Y. Effects of antimicrobial peptides (AMPs) on blood biochemical parameters, antioxidase activity, and immune function in the common carp (Cyprinus carpio). Fish Shellfish Immunol. 2015, 47, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Zhang, P.; Yue, X.; Du, X.; Li, W.; Yin, Y.; Yi, C.; Li, Y. Effect of sub-chronic exposure to lead (Pb) and Bacillus subtilis on Carassius auratus gibelio: Bioaccumulation, antioxidant responses and immune responses. Ecotoxicol. Environ. Saf. 2018, 161, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Ercan, N.; Kockaya, M. Determination of Malondialdehyde (MDA), Superoxide Dismutase (SOD) and Glutathione Peroxidase (GPx) Levels in Kangal Dogs with Maternal Cannibalism. Turk. J. Agric. Food Sci. Technol. 2017, 5, 1493. [Google Scholar] [CrossRef]
- Wang, X.; Yao, Q.; Zhang, D.-M.; Lei, X.-Y.; Wang, S.; Wan, J.-W.; Liu, H.-J.; Chen, Y.-K.; Zhao, Y.-L.; Wang, G.-Q.; et al. Effects of acute salinity stress on osmoregulation, antioxidant capacity and physiological metabolism of female Chinese mitten crabs (Eriocheir sinensis). Aquaculture 2022, 552, 737989. [Google Scholar] [CrossRef]
- Sun, C.-B.; Wang, G.; Chan, S.F. Effects of artificial infection of Litopenaeus vannamei by Micrococcus lysodeikticus and WSSV on the activity of immunity related enzymes. Fish Shellfish Immunol. 2015, 46, 778–786. [Google Scholar] [CrossRef]
- Yin, F.; Gong, H.; Ke, Q.; Li, A. Stress, antioxidant defence and mucosal immune responses of the large yellow croaker Pseudosciaena crocea challenged with Cryptocaryon irritans. Fish Shellfish Immunol. 2015, 47, 344–351. [Google Scholar] [CrossRef]
- Gao, Y.; He, J.; He, Z.; Li, Z.; Zhao, B.; Mu, Y.; Lee, J.-Y.; Chu, Z. Effects of fulvic acid on growth performance and intestinal health of juvenile loach Paramisgurnus dabryanus (Sauvage). Fish Shellfish Immunol. 2017, 62, 47–56. [Google Scholar] [CrossRef]
- Yan, M.; Liu, J.; Li, Y.; Wang, X.; Jiang, H.; Fang, H.; Guo, Z.; Sun, Y. Different concentrations of Edwardsiella tarda ghost vaccine induces immune responses in vivo and protects Sparus macrocephalus against a homologous challenge. Fish Shellfish Immunol. 2018, 80, 467–472. [Google Scholar] [CrossRef]
- Brichacek, A.L.; Brown, C.M. Alkaline phosphatase: A potential biomarker for stroke and implications for treatment. Metab. Brain Dis. 2019, 34, 3–19. [Google Scholar] [CrossRef]
- Jiang, Q.; Ao, S.; Ji, P.; Zhou, Y.; Tang, H.; Zhou, L.; Zhang, X. Assessment of deltamethrin toxicity in Macrobrachium nipponense based on histopathology, oxidative stress and immunity damage. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 246, 109040. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, X.; Yang, Q.; Liu, H.; Nie, Q.; Hu, J.; Chi, S.; Tan, B. Effect of yeast hydrolysate supplementation on the resistance of Pacific white shrimp, Litopenaeus vannamei, challenged with low salinity stress. Aquac. Res. 2021, 53, 04. [Google Scholar] [CrossRef]
- Ivon, E.; Oscar, E. Proximate Composition and Mineral Contents of Edible Part of Four Species of Shellfishes from the Calabar River, Nigeria. Annu. Res. Rev. Biol. 2018, 26, 1–10. [Google Scholar] [CrossRef]
- Ding, A.; Zhu, M.; Qian, X.; Shi, L.; Huang, H.; Xiong, G.; Wang, J.; Wang, L. Effect of fatty acids on the flavor formation of fish sauce. LWT 2020, 134, 110259. [Google Scholar] [CrossRef]
- Jiang, W.-D.; Wu, P.; Tang, R.-J.; Liu, Y.; Kuang, S.-Y.; Jiang, J.; Tang, L.; Tang, W.-N.; Zhang, Y.-A.; Zhou, X.-Q.; et al. Nutritive values, flavor amino acids, healthcare fatty acids and flesh quality improved by manganese referring to up-regulating the antioxidant capacity and signaling molecules TOR and Nrf2 in the muscle of fish. Food Res. Int. 2016, 89, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.-P.; Wang, L.; Zhang, J.-M.; Zhang, L.; Ma, F.-R.; Huang, M.-L.; Liu, S.-S.; Gong, J.-H.; Zhang, M.; Yu, M.; et al. Comparative study on the morphological characteristics and nutritional quality of largemouth bass (Micropterus salmoides) cultured in an aquaculture system using land-based container with recycling water and a traditional pond system. Aquaculture 2022, 549, 737721. [Google Scholar] [CrossRef]
- Rasmussen, R.S.; Heinrich, M.T.; Hyldig, G.; Jacobsen, C.; Jokumsen, A. Moderate exercise of rainbow trout induces only minor differences in fatty acid profile, texture, white muscle fibres and proximate chemical composition of fillets. Aquaculture 2011, 314, 159–164. [Google Scholar] [CrossRef]
- Lv, J.; Wei, L.; Yang, Y.; Wang, B.; Liang, W.; Gao, Y.; Xia, X.; Gao, L.; Cai, Y.; Hou, P.; et al. Amino acid substitutions in the neuraminidase protein of an H9N2 avian influenza virus affect its airborne transmission in chickens. Vet. Res. 2015, 46, 44. [Google Scholar] [CrossRef]
- Schmidt, C.V.; Olsen, K.; Mouritsen, O.G. Umami synergy as the scientific principle behind taste-pairing champagne and oysters. Sci. Rep. 2020, 10, 20077. [Google Scholar] [CrossRef]
- Pedroso, J.A.; Nishimura, L.S.; de Matos-Neto, E.M.; Donato, J., Jr.; Tirapegui, J. Leucine improves protein nutritional status and regulates hepatic lipid metabolism in calorie-restricted rats. Cell Biochem. Funct. 2014, 32, 326–332. [Google Scholar] [CrossRef]
- Gómez-Limia, L.; Franco, I.; Martínez-Suárez, S. Effects of processing step, filling medium and storage on amino acid profiles and protein quality in canned European eels. J. Food Compos. Anal. 2021, 96, 103710. [Google Scholar] [CrossRef]
- Harimana, Y.; Tang, X.; Le, G.; Xing, X.; Zhang, K.; Sun, Y.; Li, Y.; Ma, S.; Karangwa, E.; Tuyishimire, M.A. Quality parameters of black carp (Mylopharyngodon piceus) raised in lotic and lentic freshwater systems. LWT 2018, 90, 45–52. [Google Scholar] [CrossRef]
- Wang, L.; Hong, K.; Agbaka, J.I.; Zhu, G.; Lv, C.; Ma, C. Application of UHPLC-Q/TOF-MS-based metabolomics analysis for the evaluation of bitter-tasting Krausen metabolites during beer fermentation. J. Food Compos. Anal. 2021, 99, 103850. [Google Scholar] [CrossRef]
- Liu, J.; Liu, M.; He, C.; Song, H.; Chen, F. Effect of thermal treatment on the flavor generation from Maillard reaction of xylose and chicken peptide. LWT Food Sci. Technol. 2015, 64, 316–325. [Google Scholar] [CrossRef]
- Zhuang, K.; Wu, N.; Wang, X.; Wu, X.; Wang, S.; Long, X.; Wei, X. Effects of 3 Feeding Modes on the Volatile and Nonvolatile Compounds in the Edible Tissues of Female Chinese Mitten Crab (Eriocheir sinensis). J. Food Sci. 2016, 81, S968–S981. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Capillas, C.; Moral, A. Changes in free amino acids during chilled storage of hake (Merluccius merluccius L.) in controlled atmospheres and their use as a quality control index. Eur. Food Res. Technol. 2001, 212, 302–307. [Google Scholar] [CrossRef]
- Phat, C.; Moon, B.; Lee, C. Evaluation of umami taste in mushroom extracts by chemical analysis, sensory evaluation, and an electronic tongue system. Food Chem. 2016, 192, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-H.; Qiao, K.-N.; Ding, Q.; Zhang, Y.-Y.; Sun, B.-G.; Chen, H.-T. Effects of two cooking methods on the taste components of Sanhuang chicken and Black-bone silky fowl meat. J. Food Process. Preserv. 2018, 42, e13772. [Google Scholar] [CrossRef]
- Yin, C.; Fan, X.; Fan, Z.; Shi, D.; Yao, F.; Gao, H. Comparison of non-volatile and volatile flavor compounds in six Pleurotus mushrooms. J. Sci. Food Agric. 2018, 99, 1691–1699. [Google Scholar] [CrossRef]
- Sato, T.; Ohgami, S.-I.; Kaneniwa, M. Differences in compositions of free amino acids, nucleotide-related compounds, and fatty acids between sexes of the coconut crab Birgus latro in Okinawa, Southwest Japan. Fish. Sci. 2015, 81, 569–579. [Google Scholar] [CrossRef]


| Composition | Crabs Feed | Largemouth Bass Feed |
|---|---|---|
| Moisture (%) | 12.02 ± 0.11 | 8.94 ± 2.15 |
| Crude protein (%) | 37.09 ± 2.33 | 51.32 ± 2.41 |
| Crude lipid (%) | 5.12 ± 0.18 | 8.65 ± 0.72 |
| Ash (%) | 10.66 ± 1.24 | 9.82 ± 0.62 |
| Growth Parameters | Female Crabs | Male Crabs | ||
|---|---|---|---|---|
| EM | EP | EM | EP | |
| Body weight (g) | 173.86 ± 32.89 | 204.57 ± 33.07 | 217.09 ± 29.57 | 283.21 ± 17.00 * |
| Carapace length (mm) | 70.46 ± 4.72 | 74.14 ± 3.10 | 72.95 ± 2.26 | 77.76 ± 1.24 * |
| Carapace width (mm) | 74.78 ± 4.76 | 77.70 ± 3.96 | 78.41 ± 2.38 | 83.98 ± 2.02 * |
| Carapace height (mm) | 38.48 ± 3.08 | 41.91 ± 2.62 | 38.29 ± 0.65 | 40.66 ± 1.67 * |
| Gonadosomatic index (%) | 5.41 ± 2.73 | 5.65 ± 0.82 | 1.26 ± 0.41 | 1.44 ± 0.33 |
| Hepatosomatic index (%) | 10.15 ± 1.29 | 10.31 ± 1.62 | 8.44 ± 0.58 | 8.89 ± 1.63 |
| Meat yield (%) | 18.97 ± 1.84 | 18.05 ± 4.68 | 18.00 ± 2.00 | 18.83 ± 2.85 |
| Total edible yield (%) | 34.53 ± 3.33 | 34.01 ± 5.57 | 27.70 ± 2.49 | 29.16 ± 4.17 |
| Condition factor × 100 (g/cm3) | 49.31 ± 2.84 | 49.89 ± 3.58 | 55.75 ± 4.75 | 60.22 ± 2.78 |
| Growth Parameters | Largemouth Bass | |
|---|---|---|
| MM | EP | |
| Final body weight (g) | 560.51 ± 40.70 | 632.29 ± 35.01 * |
| Weight gain (%) | 204.63 ± 22.12 | 243.63 ± 19.03 * |
| Condition factor × 100 (g/cm3) | 2.54 ± 0.26 | 2.53 ± 0.13 |
| Hepatosomatic index (%) | 2.66 ± 0.44 * | 1.93 ± 0.45 |
| Viscerosomatic index (%) | 7.95 ± 1.02 | 7.33 ± 0.88 |
| Fatty Acids | Female Crabs | Male Crabs | Largemouth Bass | |||
|---|---|---|---|---|---|---|
| EM | EP | EM | EP | MM | EP | |
| C12:0 | 0.13 ± 0.03 | 0.17 ± 0.01 | 0.11 ± 0.04 | 0.18 ± 0.06 | 0.04 ± 0.02 | 0.02 ± 0.00 |
| C14:0 | 1.50 ± 0.09 | 1.57 ± 0.18 | 1.47 ± 0.04 | 1.98 ± 0.77 | 1.32 ± 0.07 * | 1.19 ± 0.06 |
| C15:0 | 0.32 ± 0.05 | 0.27 ± 0.02 | 0.32 ± 0.06 | 0.33 ± 0.03 | 0.20 ± 0.01 | 0.20 ± 0.01 |
| C16:0 | 21.77 ± 0.24 | 23.11 ± 1.39 | 21.85 ± 1.54 | 22.58 ± 0.24 | 18.68 ± 0.42 | 18.66 ± 0.61 |
| C16:1 | 12.68 ± 0.78 | 12.15 ± 0.71 | 9.88 ± 2.48 | 14.91 ± 0.48 * | 3.13 ± 0.20 | 3.04 ± 0.23 |
| C17:0 | 0.27 ± 0.01 | 1.61 ± 0.28 * | 0.32 ± 0.03 | 1.36 ± 0.16 * | 0.20 ± 0.01 | 0.20 ± 0.01 |
| C18:0 | 2.87 ± 0.18 | 3.09 ± 0.49 | 3.15 ± 0.35 * | 2.34 ± 0.30 | 6.85 ± 0.05 | 6.64 ± 0.30 |
| C18:1 | 34.93 ± 0.13 | 32.70 ± 0.23 | 34.87 ± 1.03 * | 29.78 ± 1.70 | 20.74 ± 0.78 | 20.36 ± 0.72 |
| C18:2 | 15.04 ± 1.47 | 14.51 ± 1.52 | 17.53 ± 3.26 | 15.96 ± 1.15 | 22.64 ± 0.35 | 23.83 ± 0.08 * |
| C18:3 | 2.48 ± 0.34 | 3.81 ± 0.50 * | 2.25 ± 0.64 | 3.61 ± 0.15 * | 1.52 ± 0.03 | 1.63 ± 0.11 |
| C20:0 | 0.16 ± 0.03 | 0.17 ± 0.02 | 0.11 ± 0.04 | 0.18 ± 0.10 | 0.23 ± 0.05 | 0.18 ± 0.03 |
| C20:1 | 1.45 ± 0.41 | 1.52 ± 0.22 | 1.43 ± 0.15 | 1.21 ± 0.28 | 0.82 ± 0.07 | 0.88 ± 0.13 |
| C20:2 | 1.00 ± 0.12 | 1.14 ± 0.06 | 0.98 ± 0.21 | 0.81 ± 0.16 | 0.80 ± 0.07 | 0.93 ± 0.06 * |
| C20:3 | 0.28 ± 0.02 * | 0.14 ± 0.01 | 0.36 ± 0.17 | 0.14 ± 0.01 | 0.23 ± 0.04 | 0.28 ± 0.07 |
| C20:4 | 1.12 ± 0.30 | 1.18 ± 0.09 | 1.09 ± 0.07 | 1.34 ± 0.15 | 1.65 ± 0.12 | 1.58 ± 0.08 |
| C20:5 | 0.98 ± 0.27 | 0.92 ± 0.15 | 1.08 ± 0.55 | 1.26 ± 0.18 | 2.26 ± 0.14 | 2.01 ± 0.18 |
| C22:0 | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.00 | 0.05 ± 0.02 | 0.17 ± 0.06 | 0.19 ± 0.04 |
| C22:1 | 0.10 ± 0.03 | 0.07 ± 0.02 | 0.08 ± 0.03 | 0.08 ± 0.03 | 0.17 ± 0.04 | 0.30 ± 0.08 |
| C22:4 | 0.25 ± 0.02 | 0.17 ± 0.18 | 0.28 ± 0.15 | 0.04 ± 0.01 | 0.52 ± 0.04 | 0.56 ± 0.04 |
| C22:5 | 0.38 ± 0.05 * | 0.15 ± 0.04 | 0.47 ± 0.10 * | 0.14 ± 0.02 | 1.68 ± 0.20 | 1.49 ± 0.15 |
| C22:6 | 1.24 ± 0.16 * | 0.53 ± 0.26 | 1.45 ± 0.39 | 0.58 ± 0.07 | 16.15 ± 0.80 | 15.85 ± 0.69 |
| ∑PUFA | 22.78 ± 0.34 | 22.56 ± 0.94 | 25.49 ± 2.89 | 23.88 ± 1.22 | 47.45 ± 0.66 | 48.16 ± 1.20 |
| ∑SFA | 27.06 ± 0.28 | 30.05 ± 1.47 * | 27.38 ± 1.30 | 29.01 ± 0.61 | 27.70 ± 0.31 | 27.28 ± 0.36 |
| ∑PUFA/∑SFA | 0.84 ± 0.01 | 0.75 ± 0.07 | 0.94 ± 0.15 | 0.82 ± 0.04 | 1.71 ± 0.01 | 1.77 ± 0.07 |
| Hydrolyzed Amino Acids | Female Crabs | Male Crabs | Largemouth Bass | |||
|---|---|---|---|---|---|---|
| EM | EP | EM | EP | MM | EP | |
| Asp | 1.70 ± 0.17 | 1.66 ± 0.14 | 1.48 ± 0.26 | 1.36 ± 0.10 | 8.44 ± 0.41 | 8.44 ± 0.20 |
| Glu | 2.24 ± 0.23 | 2.22 ± 0.18 | 2.06 ± 0.34 | 1.87 ± 0.13 | 13.15 ± 0.58 | 13.20 ± 0.25 |
| Ser | 0.62 ± 0.04 | 0.67 ± 0.07 | 0.57 ± 0.10 | 0.53 ± 0.06 | 2.65 ± 0.06 | 2.69 ± 0.04 |
| His | 0.49 ± 0.04 | 0.49 ± 0.03 | 0.41 ± 0.07 | 0.41 ± 0.03 | 2.23 ± 0.11 | 2.25 ± 0.06 |
| Gly | 0.85 ± 0.07 | 0.86 ± 0.08 | 0.76 ± 0.11 | 0.71 ± 0.11 | 3.95 ± 0.22 | 4.04 ± 0.18 |
| Arg | 1.05 ± 0.18 | 1.10 ± 0.06 | 1.08 ± 0.28 | 0.82 ± 0.10 | 4.65 ± 0.22 | 4.60 ± 0.07 |
| Ala | 0.97 ± 0.11 | 1.00 ± 0.06 | 0.97 ± 0.21 | 0.85 ± 0.06 | 4.74 ± 0.21 | 4.73 ± 0.08 |
| Tyr | 0.57 ± 0.14 | 0.45 ± 0.04 | 0.62 ± 0.25 | 0.38 ± 0.08 | 2.48 ± 0.13 | 2.77 ± 0.52 |
| Pro | 0.78 ± 0.13 | 0.76 ± 0.32 | 0.78 ± 0.29 | 0.63 ± 0.03 | 2.56 ± 0.23 | 2.32 ± 0.18 |
| Cys-s | 0.08 ± 0.00 | 0.09 ± 0.01 | 0.07 ± 0.07 | 0.06 ± 0.01 | 0.37 ± 0.08 | 0.40 ± 0.01 |
| Thr | 0.86 ± 0.07 | 0.89 ± 0.09 | 0.77 ± 0.11 | 0.72 ± 0.07 | 3.27 ± 0.13 | 3.26 ± 0.04 |
| Val | 0.96 ± 0.12 | 0.99 ± 0.07 | 0.92 ± 0.19 | 0.76 ± 0.06 | 4.34 ± 0.24 | 4.34 ± 0.11 |
| Met | 0.48 ± 0.12 | 0.37 ± 0.09 | 0.39 ± 0.11 | 0.30 ± 0.09 | 2.48 ± 0.11 | 2.46 ± 0.05 |
| Phe | 0.81 ± 0.10 | 0.79 ± 0.06 | 0.71 ± 0.15 | 0.62 ± 0.06 | 3.42 ± 0.16 | 3.43 ± 0.06 |
| Ile | 0.75 ± 0.10 | 0.74 ± 0.05 | 0.67 ± 0.13 | 0.58 ± 0.05 | 4.10 ± 0.21 | 4.09 ± 0.09 |
| Leu | 1.21 ± 0.16 | 1.27 ± 0.09 | 1.19 ± 0.19 | 0.99 ± 0.07 | 6.39 ± 0.29 | 6.42 ± 0.13 |
| Lys | 1.00 ± 0.16 | 0.95 ± 0.19 | 0.99 ± 0.21 | 0.80 ± 0.07 | 8.37 ± 0.36 | 8.47 ± 0.16 |
| E | 6.07 ± 0.83 | 6.01 ± 0.52 | 5.64 ± 1.09 | 4.77 ± 0.45 | 32.38 ± 1.48 | 32.48 ± 0.65 |
| N | 9.33 ± 0.97 | 9.28 ± 0.67 | 8.81 ± 1.82 | 7.64 ± 0.66 | 45.24 ± 1.95 | 45.44 ± 1.17 |
| T | 15.40 ± 1.80 | 15.29 ± 1.19 | 14.45 ± 2.90 | 12.41 ± 1.11 | 77.62 ± 3.43 | 77.93 ± 1.73 |
| E/T | 0.39 ± 0.01 | 0.39 ± 0.00 | 0.39 ± 0.00 * | 0.38 ± 0.00 | 0.42 ± 0.00 | 0.42 ± 0.00 |
| N/T | 0.61 ± 0.01 | 0.61 ± 0.00 | 0.61 ± 0.00 | 0.62 ± 0.00 * | 0.58 ± 0.00 | 0.58 ± 0.00 |
| Free Amino Acids | Female Crabs | Male Crabs | Largemouth Bass | |||
|---|---|---|---|---|---|---|
| EM | EP | EM | EP | MM | EP | |
| Asp | 0.05 ± 0.01 | 0.06 ± 0.00 | 0.04 ± 0.02 | 0.05 ± 0.02 | 0.17 ± 0.01 * | 0.16 ± 0.00 |
| Glu | 0.17 ± 0.02 | 0.15 ± 0.02 | 0.19 ± 0.04 | 0.19 ± 0.04 | 0.78 ± 0.14 * | 0.20 ± 0.03 |
| Ser | 0.05 ± 0.00 | 0.05 ± 0.01 | 0.04 ± 0.02 | 0.04 ± 0.02 | 0.06 ± 0.01 | 0.06 ± 0.01 |
| His | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.04 ± 0.02 | 0.03 ± 0.02 | 2.88 ± 0.25 | 3.02 ± 0.38 |
| Gly | 0.13 ± 0.01 | 0.12 ± 0.02 | 0.13 ± 0.02 | 0.13 ± 0.06 | 2.35 ± 0.42 | 2.63 ± 0.27 |
| Arg | 0.30 ± 0.04 | 0.38 ± 0.02 * | 0.30 ± 0.09 | 0.26 ± 0.11 | 0.07 ± 0.00 | 0.08 ± 0.01 |
| Ala | 0.23 ± 0.03 | 0.23 ± 0.00 | 0.32 ± 0.11 | 0.25 ± 0.02 | 1.14 ± 0.21 | 0.89 ± 0.04 |
| Tyr | 0.10 ± 0.02 | 0.11 ± 0.01 | 0.09 ± 0.04 | 0.07 ± 0.04 | 0.08 ± 0.00 | 0.10 ± 0.02 |
| Cys-s | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| Pro | 0.21 ± 0.06 | 0.19 ± 0.02 | 0.22 ± 0.17 | 0.17 ± 0.04 | 2.50 ± 1.10 | 1.65 ± 0.40 |
| Thr | 0.12 ± 0.00 | 0.10 ± 0.01 | 0.09 ± 0.04 | 0.08 ± 0.03 | 1.01 ± 0.05 | 0.86 ± 0.04 |
| Val | 0.12 ± 0.00 | 0.11 ± 0.01 | 0.09 ± 0.05 | 0.08 ± 0.04 | 0.15 ± 0.03 * | 0.11 ± 0.01 |
| Met | 0.06 ± 0.00 | 0.06 ± 0.00 | 0.06 ± 0.03 | 0.04 ± 0.02 | 0.06 ± 0.00 * | 0.04 ± 0.00 |
| Phe | 0.10 ± 0.02 | 0.10 ± 0.01 | 0.08 ± 0.05 | 0.07 ± 0.04 | 0.09 ± 0.01 | 0.08 ± 0.01 |
| Ile | 0.09 ± 0.00 | 0.09 ± 0.01 | 0.07 ± 0.04 | 0.06 ± 0.04 | 0.10 ± 0.02 * | 0.06 ± 0.01 |
| Leu | 0.18 ± 0.03 | 0.18 ± 0.02 | 0.15 ± 0.09 | 0.13 ± 0.07 | 0.20 ± 0.04 | 0.13 ± 0.01 |
| Lys | 0.17 ± 0.04 | 0.20 ± 0.02 | 0.16 ± 0.07 | 0.14 ± 0.08 | 0.59 ± 0.18 | 0.87 ± 0.32 |
| E | 0.84 ± 0.10 | 0.85 ± 0.07 | 0.70 ± 0.36 | 0.61 ± 0.32 | 2.20 ± 0.29 | 2.15 ± 0.35 |
| N | 1.32 ± 0.16 | 1.36 ± 0.05 | 1.39 ± 0.40 | 1.19 ± 0.32 | 10.09 ± 1.26 | 8.78 ± 0.20 |
| T | 2.16 ± 0.26 | 2.20 ± 0.12 | 2.10 ± 0.76 | 1.80 ± 0.65 | 12.29 ± 1.46 | 10.39 ± 0.35 |
| E/T | 0.39 ± 0.00 | 0.38 ± 0.01 | 0.32 ± 0.05 | 0.32 ± 0.06 | 0.18 ± 0.02 | 0.20 ± 0.03 |
| N/T | 0.61 ± 0.00 | 0.62 ± 0.01 | 0.68 ± 0.05 | 0.68 ± 0.06 | 0.82 ± 0.02 | 0.80 ± 0.03 |
| E/N | 0.64 ± 0.00 | 0.63 ± 0.03 | 0.48 ± 0.11 | 0.49 ± 0.13 | 0.22 ± 0.03 | 0.25 ± 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Che, S.; Li, S.; Li, Q.; Sun, Y.; Zheng, Z.; Nie, Z.; Tang, Z.; Wang, P.; Gao, J.; Xu, G. Polyculture Affects the Growth, Antioxidant Status, Nutrient Content, and Flavor of Chinese Mitten Crabs (Eriocheir sinensis) and Largemouth Bass (Micropterus salmoides). Fishes 2022, 7, 355. https://doi.org/10.3390/fishes7060355
Che S, Li S, Li Q, Sun Y, Zheng Z, Nie Z, Tang Z, Wang P, Gao J, Xu G. Polyculture Affects the Growth, Antioxidant Status, Nutrient Content, and Flavor of Chinese Mitten Crabs (Eriocheir sinensis) and Largemouth Bass (Micropterus salmoides). Fishes. 2022; 7(6):355. https://doi.org/10.3390/fishes7060355
Chicago/Turabian StyleChe, Silu, Shiheng Li, Quanjie Li, Yi Sun, Zhaowei Zheng, Zhijuan Nie, Zhonglin Tang, Peipei Wang, Jiancao Gao, and Gangchun Xu. 2022. "Polyculture Affects the Growth, Antioxidant Status, Nutrient Content, and Flavor of Chinese Mitten Crabs (Eriocheir sinensis) and Largemouth Bass (Micropterus salmoides)" Fishes 7, no. 6: 355. https://doi.org/10.3390/fishes7060355
APA StyleChe, S., Li, S., Li, Q., Sun, Y., Zheng, Z., Nie, Z., Tang, Z., Wang, P., Gao, J., & Xu, G. (2022). Polyculture Affects the Growth, Antioxidant Status, Nutrient Content, and Flavor of Chinese Mitten Crabs (Eriocheir sinensis) and Largemouth Bass (Micropterus salmoides). Fishes, 7(6), 355. https://doi.org/10.3390/fishes7060355






