Abstract
The aim of this article is to describe the results of the use of demineralized bone matrix putty in alveolar cleft of patients with cleft lip and palate. We performed a prospective, descriptive case series study, in which we evaluated the results of the management of alveolar clefts with demineralized bone matrix. Surgery was performed in 10 patients aged between 7 and 26 years (mean 13 years), involving a total of 13 clefts in the 10 patients. A preoperative cone beam computed tomography (CBCT) was taken to the patients in whom the width of the cleft was measured from each edge of the cleft reporting values between 5.76 and 16.93 mm (average, 11.18 mm). The densities of the clefts weremeasured with a CBCT, 6 months postoperative to assess bone formation. The results showed a register of gray values of 1,148 to 1,396 (mean, 1,270). The follow-up was conducted for 15 to 33 months (mean, 28.2 months). The results did not show satisfactory bone formation in the cleft of patients with the use of demineralized bone matrix.
Alveolar bone grafts are an integral part of the treatment of patients with cleft palate [1]. The objectives of the grafts at the alveolar level are as follows: to fill the cleft with bone, to stabilize the dental arches [2], to support the teeth adjacent to the cleft and promote its eruption [3], to give a proper gingival contour, to generate adequate nasal base support, and to close oronasal fistulae or fissures [4]. Autogenous bone grafts harvested from selected sites in the skeleton such as the iliac crest continue to be the gold standard for these procedures. Despite being highly effective, these techniques subject patients to a second surgical site, which may increase morbidity (8–10%), complications, hospital stay, recovery, and cost. There is also a greater risk for wound infection, more blood loss, and a slower return to normal function.
Allogeneic (human cadaver) bone is the most commonly used alternative to the autogenous harvest, but these bioim-plants offer the potential risk of disease transmission, potential rejection, and resorption. The most common bone’s donor site is the iliac crest (iliac crest grafts are used in 83% of the cases); however, other options have been described as donor sites such as skull, rib, tibia, and mandible [1]. Although it is a common procedure, it is not exempted from the donor site morbidity. Because of the donor site morbidities, other alternatives have been studied. One of this alternatives is the allograft [5,6].
Demineralized bone matrix (DBM) is a biomaterial derived from cadaver bone and is an alternative to avoid autografts. Some of its benefits are as follows: it is an inexhaustible source of grafts, it has the ability to be mixed with autologous bone [6], and it may also be mixed with growth factors such as bone morphogenic protein 2 and fibroblast growth factor [7]. Besides it also reduces surgery time and prevents possible donor site morbidity [5]. The limitations for using DBM are high costs, possible immune adverse reactions, and disease transmission [6]. Urist demonstrated8–10 that implantation of demineralized bone tissue in other tissues produced outbreaks of bone development; this phenomenon has been known as osteoinduction. This is the ability of a biomaterial to stimulate local undifferentiated mesenchymal cells into becoming osteoprogenitor cells, which eventually will form new bone [9]. Recently, it accepted that the osteoinductive potential of the DBM is caused by endogenous morphogens that induce bone formation in the form of morphogenetic proteins (bone morphogenetic proteins) [6].
The final product of the process of demineralization is a mod powder that can be difficult to manage clinically; therefore, different vehicles have been used to incorporate high mass fractions of DBM. The Synthes (Synthes, West Chester, PA) DBX vehicle is sodium hyaluronate, and it comes in different presentations such as paste, putty mix, and strip. In several clinical studies, the osteoconductive and osteoinductive properties have been shown, as well as its bioabsorbable property, with satisfactory results at 6 months after its application [11,12,13]. The main objective of this study is to describe the outcomes when using DBM, DBX putty as a bone substitute to treat the alveolar clefts of patients with cleft lip and palate.
Materials and Methods
This is a prospective case series study. Ten patients taken from the FISULAB Institution, a rehabilitation center for patients with cleft lip and palate, were studied. This study recruited 10 consecutive patients with alveolar clefts who agreed to undergo surgery for closure of the alveolar cleft with DBM DBX putty. An additional group of 10 patients with alveolar clefts taken from the FISULAB Institution during the same period of time, who underwent surgery with autologous iliac crest bone grafts, was recruited to assess the results between these two surgical techniques.
The FISULAB Ethics Committee approved the study protocol, and all surrogates gave informed consent. All the patients and their parents (if the patient was a child) signed the written consent for the procedure. Before the surgical procedure, the patients underwent orthodontic management to align the maxillary arch and to prevent dental germs to be in the area of the cleft grafting. Patients with history of previous alveolar grafts were excluded. A cone beam computed tomography (CBCT) was taken for the patients who underwent surgery for closure of the alveolar cleft with DBM DBX putty before the surgical procedure and 6 months after the surgical procedure. A periapical X-ray was taken for all the patients: the patients who underwent surgery for reconstruction of the alveolar cleft with DBM and the patients who had autologous grafts. The amount of bone formation was evaluated with the CBCT through the measurement of the highest gray values, while the Enemark grading system for evaluation of alveolar bone graft is used in periapical X-ray; this classify the amount of bone adjacent to the tooth from apex to root. According to this scale, grade 1 corresponds to 100 to 75% of bone forma-tion; grade 2 corresponds to 75 to 50% of bone formation; grade 3 corresponds to 50 to 25% of bone formation, and grade 4 corresponds to 25 to 0% of bone formation [1].
The patients were operated under general anesthesia and by the senior author (J.R.P.M.).
Surgical Technique
Alveolar bone grafting was performed under general endotracheal anesthesia. Incisions were made and gingival mucoperiosteal flaps were elevated in the standard fashion for alveolar bone grafting to create a suitable pocket (►Figure 1).
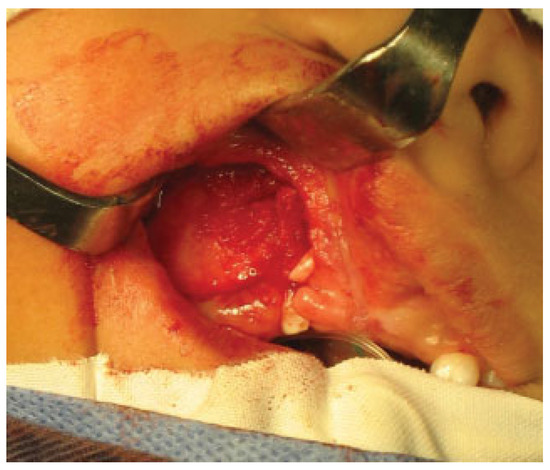
Figure 1.
Elevation of gingival mucoperiosteal flaps and dissection of the pocket.
On the vestibular side, the incision is made along the gingival border to ensure that the gingival flap contains the required width of attached gingiva. Posteriorly, the incision is extended to the first permanent molar where it is angled up into the sulcus. To provide sufficient mobility, it is often necessary to cut through the periosteum at the base of the flap. Anteriorly, the incision is extended to the mesial aspect of the cleft. Vertical incisions are made along the edges of the cleft. Wide exposure of the cleft area is achieved through these incisions. On the palatal side, mucoperiosteum is lifted off the bone in the cleft region and off the frontal and lateral segments of the maxilla. All soft tissues are carefully stripped off the residual alveolar cleft. The nasal mucoperiosteum is pushed upward, and if a fistula is present, the nasal floor is reconstructed. On the palatal side, mucoperiosteal flaps are raised along the edges of the cleft, and usually the margins are trimmed to fit together before suturing with everting mattress sutures. The alveolar cleft is now delimited by denuded bone medially and laterally and by the raw surface of mucoperiosteum on the nasal and the palatal side.
The putty or the iliac crest bone graft was taken and packed into the alveolar cleft between the cortical bone edges (►Figure 2) and then the lateral gingivoperiosteal flap is advanced to cover the cleft and is sutured to the smaller medial (anterior) flap and to the palatal flaps (►Figure 3). In patients with bilateral clefts, the same flap design was used. During the surgical procedure, a dose of cephazolin was administered and once the surgical procedure was finished, the patients were discharged with oral cephalexin for 7 days and liquid diet for 4 weeks.
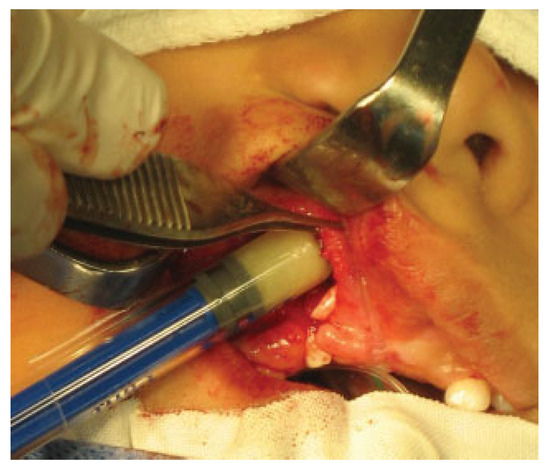
Figure 2.
Introduction of the DBM DBX putty in the alveolar cleft.
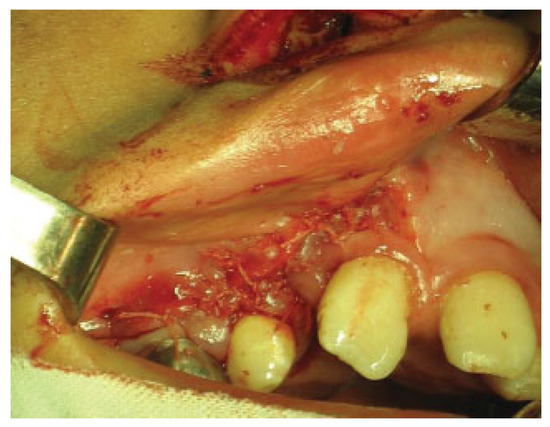
Figure 3.
Closure of gingival mucoperiosteal flaps.
The CBCT was taken by the same team and machine, and studied in the Sirona Galileos software (Sirona Dental Systems, Long Island, NY); in the axial slides, a measurement was performed at the upper edge of the cleft; and during the follow-up (6 months postoperative), a new CBCT was taken to observe bone formation by the evaluation of the densities in the axial, coronal, and sagittal views of the cleft. The highest gray values in the cleft were recorded, and all patients continued their follow-up by plastic surgery and orthodontics. A CBCTwas not taken in the patients who underwent autologous grafts; these patients were followed up by periapical radiographs. Periapical radiographs were taken after at least 1 year of the surgery and were evaluated by Enemark scale, to calculate the percentage of ossification in the cleft area. Among other variables evaluated, we studied the eruption of the canine and the need for a second surgical procedure.
Results
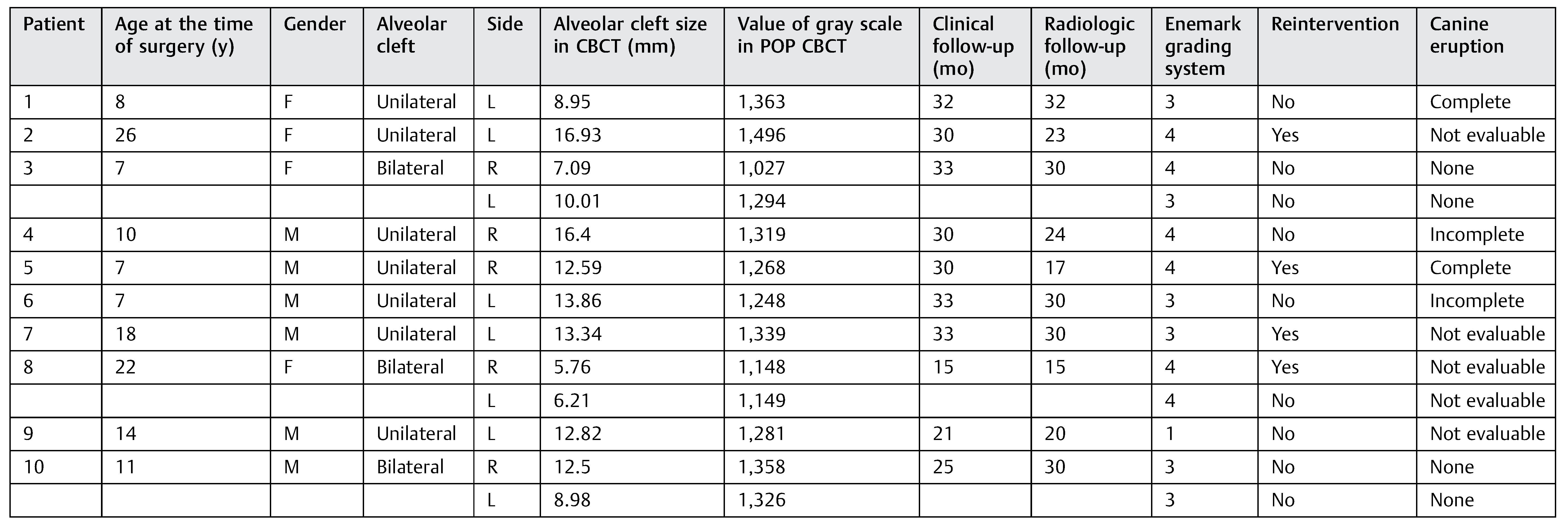
In the DBM group, 10 patients were evaluated (►Table 1) who were between the ages of 7 and 26 years (mean, 13): 3 patients had permanent teeth and 7 patients had mixed dentition, 3 patients (30%) had bilateral alveolar clefts and 7 patients (70%) had unilateral alveolar cleft, and a total of 13 clefts were operated.

Table 1.
Patients DBM group database.
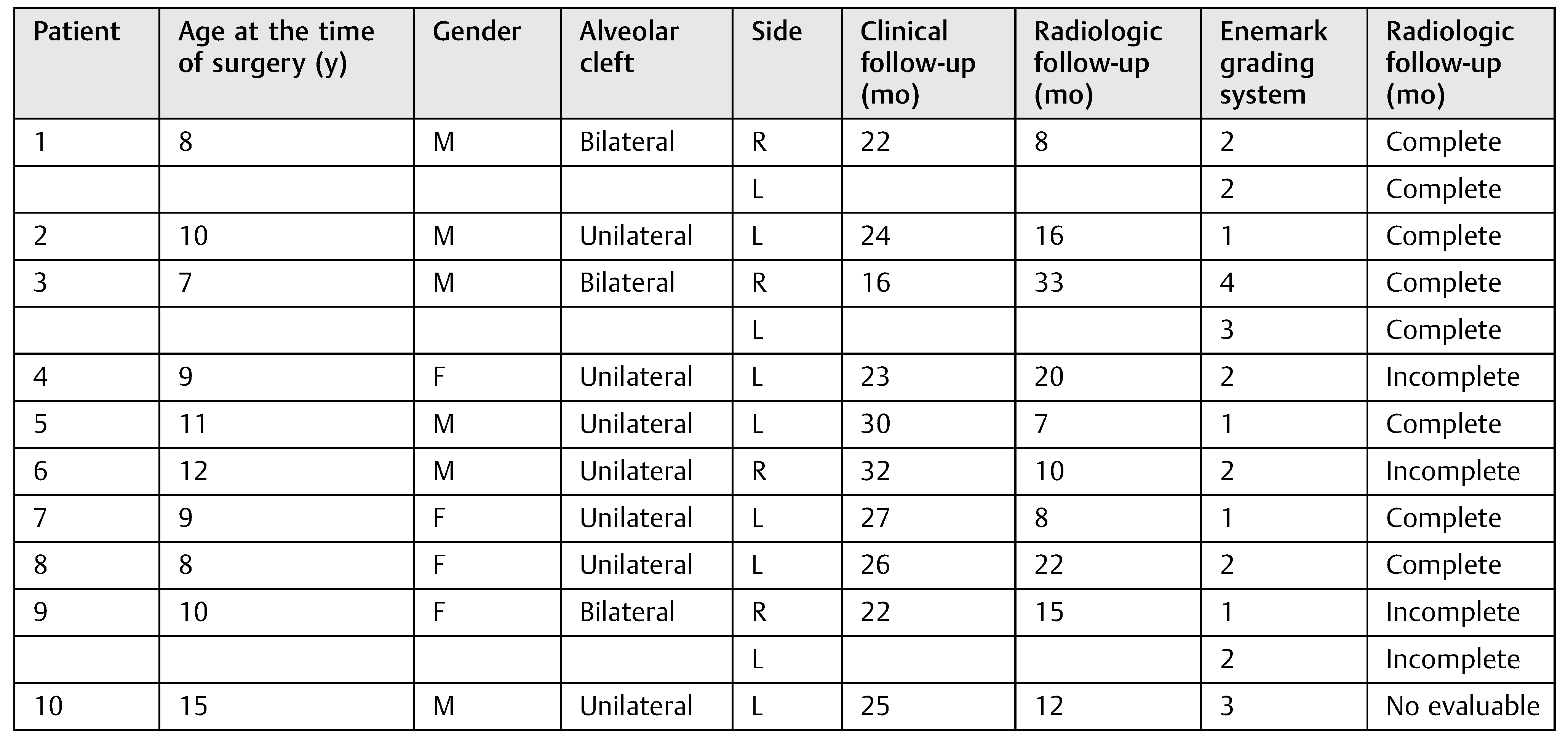
In the autologous graft group, 10 random patients aged between 7 and 15 years (mean 9) were evaluated (►Table 2): 1 patient had permanent teeth and 9 patients had mixed dentition, 3 patients (30%) had bilateral alveolar clefts and 7 patients (70%) had unilateral cleft, and a total of 13 clefts were operated.

Table 2.
Patients autologous iliac crest bone graft group data base.
From the patients with mixed dentition (autologous graft group), four patients with unilateral cleft had complete canine eruption after surgery and two patients had an incomplete canine eruption, while two patients with bilateral cleft had complete canine eruption and one patient had incomplete canine eruption. In one patient with permanent teeth and unilateral alveolar cleft, the canine eruption was not evaluable (►Table 2).
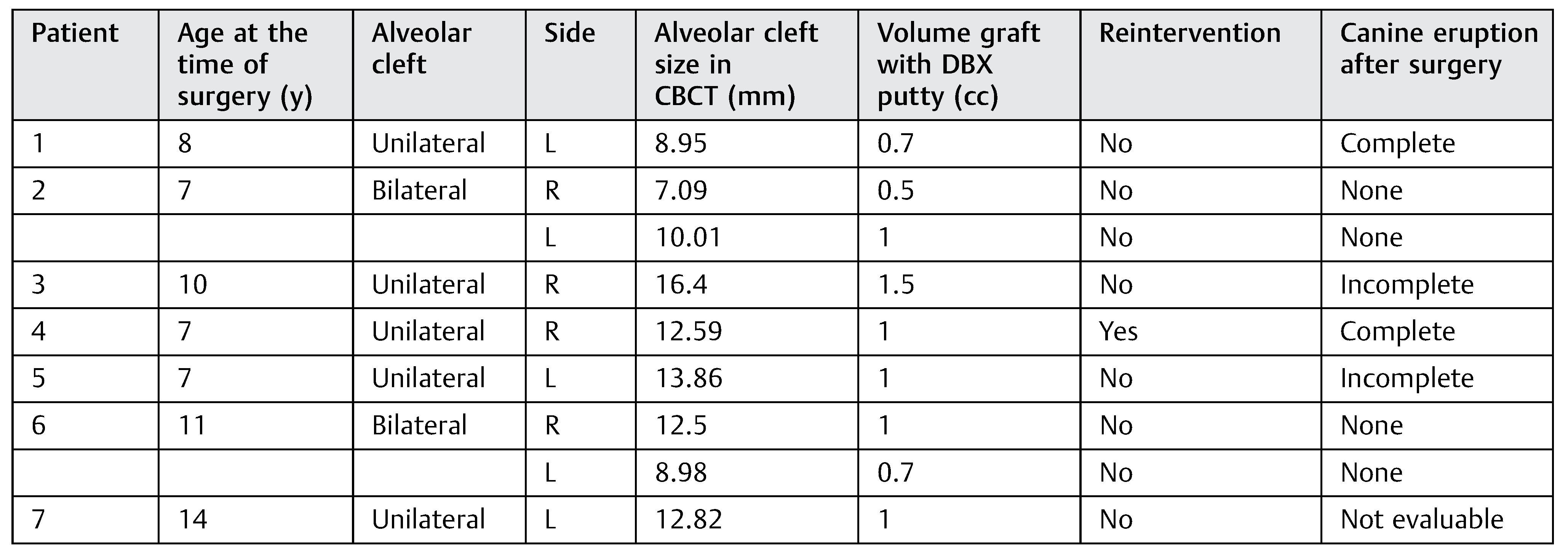
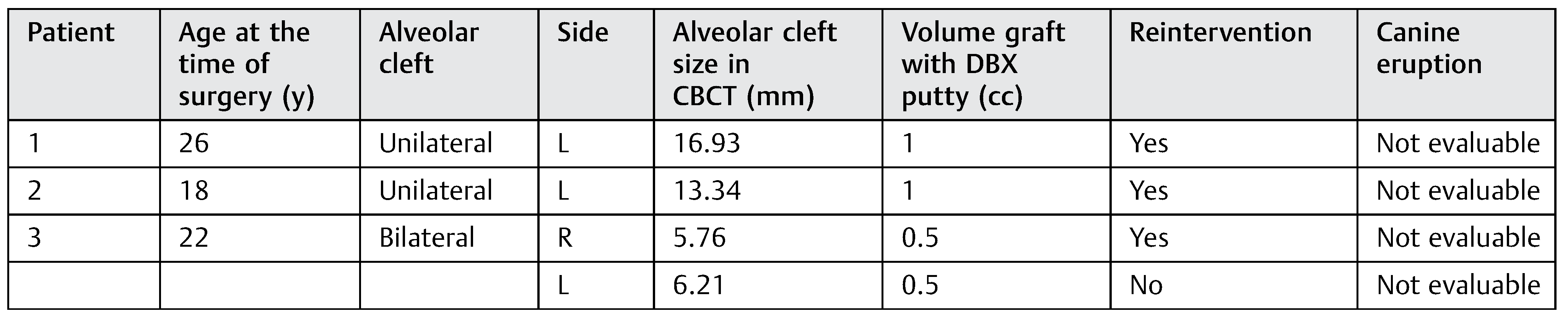
From the patients with mixed dentition (DBM group), the cleft’s width in the upper edge ranged from 7.09 to 16.4 mm. We used in these patients between 0.5 and 1.5 cc of DBM to fill the cleft according to the cleft width. From the group of patients with mixed dentition, two patients with unilateral cleft had a complete canine eruption after the surgery and two patients had an incomplete canine eruption, and in one patient the canine eruption was not evaluable. The canine eruption was absent in two patients with bilateral cleft in the mixed dentition group (►Table 3). In the permanent teeth group (►Table 4), three patients required a second surgical procedure (autologous alveolar grafts), during which there was no evidence of bone formation after at least 6 months of follow-up.

Table 3.
Patients with mixed dentition from the DBM group.

Table 4.
Patients with permanent teeth dentition from the DBM group.
There were no intraoperative or postoperative complications. The fissure length in the upper edge when measured with a rule tool in the CBCT with Sirona Galileos software range from 5.76 to 16.93 mm (mean, 11.18 mm) (►Figure 4). All patients in the DBM group underwent a second CBCT assessment after 6 months of the surgical procedure. In the axial, coronal, and sagittal views and the three-dimensional reconstruction, there was no bone formation in none of the patients. The highest grayscale value in the area of the cleft was registered (►Figure 5), finding gray values between 1,148 and 1,496 (mean, 1,278.15). In our patients, the gray values corresponding to the bone adjacent to the cleft were higher than 1,504. During the follow-up performed 15 to 33 months postoperatively (mean, 28.2 months), occlusal and periapical X-ray were performed and analyzed by applying the Enemark scale; satisfying bone formation of 75% was observed only in just one patient. Four patients were reoperated with conventional surgery, using iliac crest grafts, because they need orthognathic surgery with Le Fort I osteotomy. At the time of reoperation, no bone formation was observed, although a crystallized material was seen in the pocket (►Figure 6) and was removed.
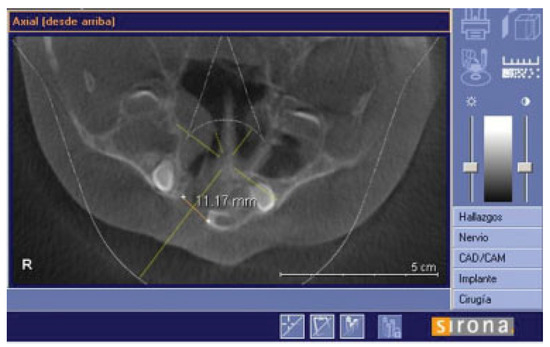
Figure 4.
Axial slide to show how the fissure was measured in the upper edge.
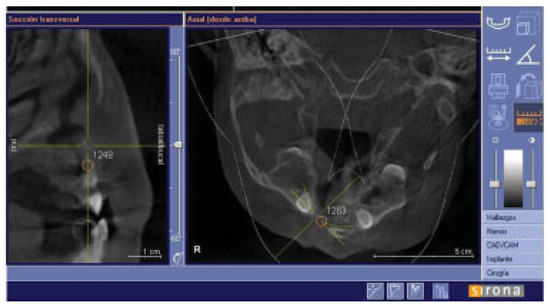
Figure 5.
Sagittal and axial slides to show gray values at the alveolar cleft.
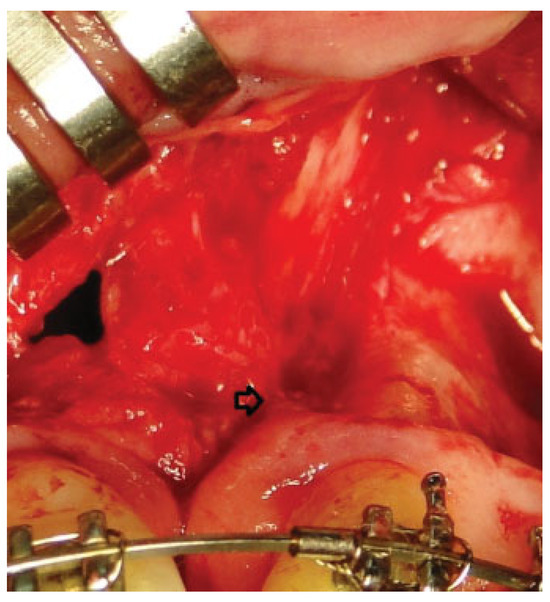
Figure 6.
The arrow shows crystallized material that was found in reoperations.
When applying the Enemark scale in the autologous graft group, satisfying bone formation of 75% was observed in eight patients. There were no complications during surgery; or in the POP, none of the patients required hospitalization, or had recurrent fistulae or material or bone graft extrusions.
Discussion
Kraut was the first to demonstrate in 1987 successful results using frozen and dry allogenic bone graft in the treatment of alveolar clefts [14]. The results of this study may be due to the isolated use of the DBM and the anatomy of the alveolar cleft. It has been shown that the results using DBM depend on the characteristics of defects. The alveolar cleft is geometrically considered as a cone with a superior base; the reconstruction of this defect should be with a three-dimensional block to generate a favorable bed for osteogenesis [6].
In our service, we use iliac crest bone as a donor site for alveolar auto grafts, because it has a proper integration and allows a sufficient volume of graft; however, this donor site is not exempted from complications [2,4]. We avoided a donor site to decrease morbidities. However, in several studies, the mixture of autologous bone grafts with DBM has proven to have good results [15]. Recent reports using DBM and autologous grafts with minimal incisions, reducing the volume of autologous graft and surgical time, have shown excellent results [16]. The authors have also used DBX mixed with cortical chips to help resist the collapse of the alveolar space because the concept of protected bone regeneration suggests that the DBX alone will not keep the space open due to its limited mechanical properties [5].
In our study, most of the patients were in the mixed dentition group which allows a better graft intake, according to the international literature. The amount of retained alveolar bone height appears to be related to teeth erupt through the graft site. When teeth are present and erupt into the grafted alveolus, usually with the aid of surgical exposure and orthodontic assistance, alveolar bone height is retained. When teeth are not present to erupt through the graft, partial resorption of the graft occurs.
Another important fact that could have influence our results is the width of the cleft. It is generally agreed that a successful bone graft needs an environment with minimum of mechanical stress and function. This also seems to apply to alveolar bone grafts. The functional element which determines the complete formation and maintenance of the interdental septum may be related to a close approximation of the teeth. If the distance between the teeth on either side of the cleft is too large, the alveolar bone graft will not maintain its original level.
Other studies have used the DBM enriched with growth factors such as bone morphogenetic protein-2 [17], vascular endothelial growth factor, fibroblast growth factor, and transforming growth factor-β1, also obtaining adequate results [18]. The CBCT has become a useful diagnostic tool with the following advantages: low cost, lower radiation exposure compared with computed tomography [19], and better resolution [20]. Regarding alveolar clefts, the CBCT allows accurate and proper evaluation of the quality of the graft as well as threedimensional measurements of the clefts [20]. However, the ability to assess bone density is limited, and gray values are highly variable depending on the equipment because the tubes can operate with different potentials and filtration [21]. To reduce these biases in this study, all patients took the CBCT in the same machine and had a continuous radiological monitoring after a year with periapical and occlusal X-rays [1]. The gray values were extrapolated to the Hounsfield units, based on a study reported by Mah et al. [22] in which they described and reported that the values corresponding to bone should be greater than 1,504. The units reported by our patients corresponded to soft tissue values which corroborates that there was no bone formation.
Limitations of the current study are its relatively small patient cohort and the heterogeneous groups to compare mixed dentition versus permanent teeth dentition results.
Our follow-up was long enough to detect early complications as infection and possible early bone extrusion and was sufficient to assess the canine tooth eruption.
Conclusion
The DBX DBM when used to treat alveolar clefts in our study is not effective by itself to induce bone formation as we observed in the 10 patients.
References
- Murthy, A.S.; Lehman, J.A. Evaluation of alveolar bone grafting: a survey of ACPA teams. Cleft Palate Craniofac J 2005, 42, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Arrington, E.D.; Smith, W.J.; Chambers, H.G.; Bucknell, A.L.; Davino, N.A. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res 1996, 300–309. [Google Scholar] [CrossRef]
- Boyne, P.J. Use of marrow-cancellous bone grafts in maxillary alveolar and palatal clefts. J Dent Res 1974, 53, 821–824. [Google Scholar] [CrossRef]
- Heary, R.F.; Schlenk, R.P.; Sacchieri, T.A.; Barone, D.; Brotea, C. Persistent iliac crest donor site pain: independent outcome assessment. Neurosurgery 2002, 50, 510–516, discussion 516. [Google Scholar]
- Macisaac, Z.M.; Rottgers, S.A.; Davit, A.J., III; Ford, M.; Losee, J.E.; Kumar, A.R. Alveolar reconstruction in cleft patients: decreased morbidity and improved outcomes with supplemental demineralized bone matrix and cancellous allograft. Plast Reconstr Surg 2012, 130, 625–632. [Google Scholar] [CrossRef]
- Gruskin, E.; Doll, B.A.; Futrell, F.W.; Schmitz, J.P.; Hollinger, J.O. Demineralized bone matrix in bone repair: history and use. Adv Drug Deliv Rev 2012, 64, 1063–1077. [Google Scholar] [CrossRef]
- Francis, C.S.; Mobin, S.S.; Lypka, M.A.; et al. rhBMP-2 with a demineralized bone matrix scaffold versus autologous iliac crest bone graft for alveolar cleft reconstruction. Plast Reconstr Surg 2013, 131, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Urist, M.R. The substratum for bone morphogenesis. Symp Soc Dev Biol 1970, 29, 125–163. [Google Scholar] [PubMed]
- Urist, M.R.; Strates, B.S. Bone morphogenetic protein. J Dent Res 1971, 50, 1392–1406. [Google Scholar] [CrossRef]
- Urist, M.R. Bone: formation by autoinduction. 1965. Clin Orthop Relat Res 2002, 395, 4–10. [Google Scholar] [CrossRef]
- Oakes, D.A.; Lee, C.C.; Lieberman, J.R. An evaluation of human demineralized bone matrices in a rat femoral defect model. Clin Orthop Relat Res 2003, 423, 281–290. [Google Scholar]
- Peterson, B.; Whang, P.G.; Iglesias, R.; Wang, J.C.; Lieberman, J.R. Osteoinductivity of commercially available demineralized bone matrix. Preparations in a spine fusion model. J Bone Joint Surg Am 2004, 86-A, 2243–2250. [Google Scholar]
- Acarturk, T.O.; Hollinger, J.O. Commercially available demineralized bone matrix compositions to regenerate calvarial critical-sized bone defects. Plast Reconstr Surg 2006, 118, 862–873. [Google Scholar] [CrossRef]
- Kraut, R.A. The use of allogeneic bone for alveolar cleft grafting. Oral Surg Oral Med Oral Pathol 1987, 64, 278–282. [Google Scholar]
- Burwell, R.G. Studies in the transplantation of bone. VII. The fresh composite homograft-autografts of cancellous bone: an analysis of inductive mechanisms in bone transplantation. J Bone Joint Surg 1964, 46-B, 110–140. [Google Scholar]
- Davies, S.; Ochs, M. Bone morphogenetic proteins in craniomaxillofacial surgery. Oral and Maxillofacial Surgery Clinics of North America 2010, 22, 17–31. [Google Scholar]
- Chung, V.H.; Chen, A.Y.; Jeng, L.B.; Kwan, C.C.; Cheng, S.H.; Chang, S.C. Engineered autologous bone marrow mesenchymal stem cells: alternative to cleft alveolar bone graft surgery. J Craniofac Surg 2012, 23, 1558–1563. [Google Scholar] [CrossRef]
- Pieske, O.; Wittmann, A.; Zaspel, J.; et al. Autologous bone graft versus demineralized bone matrix in internal fixation of ununited long bones. J Trauma Manag Outcomes 2009, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Reeves, T.E.; Mah, P.; McDavid, W.D. Deriving Hounsfield units using grey levels in cone beam CT: a clinical application. Dentomaxillofac Radiol 2012, 41, 500–508. [Google Scholar]
- Zhang, W.; Shen, G.; Wang, X.; Yu, H.; Fan, L. Evaluation of alveolar bone grafting using limited cone beam computed tomography. Oral Surg Oral Med Oral Pathol Oral Radiol 2012, 113, 542–548. [Google Scholar] [CrossRef]
- Bryant, J.A.; Drage, N.A.; Richmond, S. Study of the scan uniformity from an i-CAT cone beam computed tomography dental imaging system. Dentomaxillofac Radiol 2008, 37, 365–374. [Google Scholar] [PubMed]
- Mah, P.; Reeves, T.E.; McDavid, W.D. Deriving Hounsfield units using grey levels in cone beam computed tomography. Dentomaxillofac Radiol 2010, 39, 323–335. [Google Scholar] [PubMed]
© 2012 by the author. The Author(s) 2014.