Abstract
We introduce a novel computer-based method to digitally fixate midfacial fractures to facilitate more efficient intraoperative fixation. This article aims to describe a novel computer-based algorithm that can be utilized to model midface fracture reduction and fixation and to evaluate the algorithm’s ability to produce images similar to true postoperative images. This is a retrospective review combined with cross-sectional survey from 1 January 2010, to 31 December 2015. This study was performed at a single tertiary care, level-I trauma center. Ten patients presenting with acute midfacial traumatic fractures were evaluated. Thirty-five physicians were surveyed regarding the accuracy of the images obtained using the algorithm. A computer algorithm utilizing AquariusNet (TeraRecon, Inc., Foster City, CA, USA) and Adobe Photoshop (Adobe Systems Inc., San Jose, CA, USA) was developed to model midface fracture repair. Preoperative three-dimensional computed tomographic (CT) images were processed using the algorithm. Fractures were virtually reduced and fixated to generate a virtual postoperative image. A survey comparing the virtual postoperative and the actual postoperative images was produced. A Likert-type scale rating system of 0 to 10 (0 being completely different and 10 being identical) was utilized. Survey participants evaluated the similarity of fracture reduction and fixation plate appearance. The algorithm’s capacity for future clinical utility was also assessed. Survey response results from 35 physicians were collected and analyzed to determine the accuracy of the algorithm. Ten patients were evaluated. Fracture types included zygomaticomaxillary complex, LeFort, and naso-orbito-ethmoidal complex. Thirty-four images were assessed by a group of 35 physicians from the fields of otolaryngology, oral and maxillofacial surgery, and radiology. Mean response for fracture reduction similarity was 7.8 ± 2.5 and fixation plate similarity was 8.3 ± 1.9. All respondents reported interest in the tool for clinical use. This computer-based algorithm is able to produce virtual images that resemble actual post-operative images. It has the ability to model midface fracture repair and hardware placement.
Midface or maxillofacial fractures are frequently encountered in the trauma setting and pose a challenge to facial plastic and reconstructive surgeons. They necessitate thorough patient evaluation due to the potential involvement of the essential neurovascular and structural components of the face as well as proximity to the airway. Midface fractures are treated by buttressing fractured segments to stable skeletal elements through rigid fixation plates.[1] Surgeons routinely choose the location, size, and shape of rigid or semi-rigid fixation plates during fracture repair. This may require alteration of the fixation plates to fit the fractured area. Given the complex nature of the bones of the midface and that fixation involves a thorough understanding of the anatomy of this region, the bony positioningand plate selection are often time consuming.
Computed tomographic (CT) imaging with multiplanar reformation and three-dimensional (3D) reconstruction has become an integral part of the evaluation of midface fractures. This imaging modality allows the thin bones and sinus spaces of the midface to be easily visualized even in the presence of soft-tissue injuries and edema.[2] To preoperatively plan for midface fractures with complex anatomic involvement, virtual surgical planning (VSP) and computer-aided design (CAD) allow for precise 3D reconstruction of the face. These modalities allow the surgeon to preoperatively assess and model the upcoming surgery and to be knowledgeable of structures that will be encountered. Life-size fixation plates may also be placed using VSP.[3] Benefits of VSP include increased accuracy of reconstruction and decreased intraoperative time which lead to better clinical outcomes.[4,5,6] Current limitations of VSP include cost, which may be up to several thousand dollars, the need for a third-party provider, and the need to coordinate planning sessions. Planning may take several hours and may not be feasible for patients who necessitate emergent surgery.[7] Due to these limitations of time and expense, preoperative VSP with 3DCT imaging is not consistently used in the management of patients with midface fractures.
In this article, we introduce a computer-based algorithm that utilizes two software packages that are both affordable and commercially available to reconstruct plate midface fractures. Using the algorithm developed with this software, the surgeon has the ability to plan operative strategy for rigid fixation of bony injuries of the midface without the extensive time and cost associated with third-party VSP. Before assessing integration into clinical practice, it is necessary to test the validity of the algorithm to accurately model actual 3D CT images. To do this, we created a survey comparing the algorithm-created virtual images with the actual postoperative images to (1) examine the algorithm’s ability to create realistic postoperative images and (2) compare these images to actual postoperative images. The algorithm’s ability to approximate fractures and simulate the size, shape, and placement of fixation plates was evaluated by administration of a comparison survey to practicing physicians.
Methods
Retrospective Review
This pilot study consisted of a retrospective review of patients who underwent midface fracture fixation from 1 January 2010, to 31 December 2015, at a single tertiary care center. Approval for this study was obtained from the Human Research Protections Office and Institutional Review Board, University of Maryland, Baltimore, MD. Demographic and clinical data were collected, including age, sex, and type of midface fracture.
Inclusion and Exclusion Criteria
Patients were included in the study who underwent midface fracture fixation and had both preoperative and postoperative CT imaging of adequate resolution and quality. Patients who did not have preoperative or postoperative imaging were excluded. If either CT image was of poor quality or did not adequately capture the fracture or plating, the patient was excluded. Two eligible patients were excluded due to the similarity of fracture and plating pattern to other patients in the study. This was done to provide variability in the fracture patterns evaluated in this pilot study.
Image Acquisition
AquariusNet (TeraRecon Inc., Foster City, CA, USA) is a program that transforms 2D CT imaging data into 3D form, allowing for visualization and manipulation of imaging at any angle. Different filters can be applied that emphasize bone, soft tissue, vasculature, or metal hardware. Multiple images were captured fromeach patient at varying angles to best represent theviewof the bony fracture and plating directly and to limit image distortion based on perspective. The postoperative images were captured so that each would have a corresponding preoperative image at an identical angle. Between one and five images were obtained for each case. The images were produced with a ruler to allow for the user to accurately scale images for use. They were saved to JPEG format for further processing.
The Computer-Based Algorithm
The preoperative and postoperative images exported from AquariusNET were then imported into Photoshop CS6 (Adobe Systems Inc., San Jose, CA, USA). Photographs were taken of all individual Stryker (Kalamazoo, MI, USA) Leibinger Midface module plates next to a ruler. The digital photographs were then imported into Photoshop (Adobe Systems, Inc.). The fixation plates were sized to real-life scale with the “Free Transform” tool, using the photographed ruler as a reference. The ruler markers on the 3D CT image and fixation plate images were printed and compared with an actual ruler for confirmation of proportion. Once the images were sized accurately, the preoperative image was then manipulated to resemble the actual postoperative image. Using the “Polygonal Lasso” tool to select the specific displaced bone part, the individual fracture segments were selected and manipulated into reduction. Each selected fracture piece was shifted into alignment, thereby virtually reducing the fracture. After the bony fracture was virtually reduced, the fixation plates were overlaid onto the image to resemble the plates that were used on the real postoperative image. If necessary, the plates could be manipulated similarly to how they are altered in the operating room, including using the “Cut” tool to shorten the plate, or the “Puppet Warp” tool for bending if necessary. This process of fracture reduction and plating was performed for each captured image only on the most direct view of that fracture and plating. These steps are shown in Figure 1.
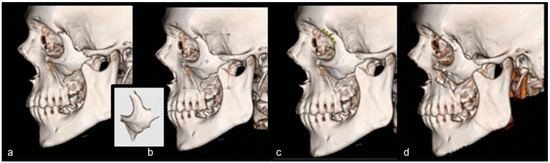
Figure 1.
(a) Preoperative CT scan; (b) fractured piece is selected and rotated (bony piece in inset); (c) fixation plates are added to the reduced fracture; (d) actual postoperative picture. The algorithm-produced picture (c) now resembles the actual postoperative picture (d).
Cross-Sectional Survey
A survey comparing the virtual postoperative images created by the algorithm and the true postoperative images was produced. Given the lack of validated surveys to assess this comparison, a practical survey was developed. A Likert-type scale rating system of 0 to 10 was utilized (0 = completely different; 5 = somewhat similar; 10 = identical). The real postoperative 3D CT image and its corresponding virtual image were displayed side by side. For each pair of images, survey participants were asked to (1) rate the similarity of fracture reduction between the two images and (2) rate the similarity of plate appearance between the two images. Emphasis was placed on rating only the regions that are immediately adjacent to the plate in terms of the reduction of the bony fracture. Seventeen pairs of images were displayed on the survey, with 34 total images assessed on a scale from 0 to 10. Figure 2 displays an example of a survey comparison. In addition, respondents were asked to overlay an actual fixation plate on top ofa scaled and printed plate to assess if it was true to size. A final question inquired if the respondent would utilize a similar program to determine the shape, position, and size of a plate and fracture reduction for preoperative planning. Respondents completed the survey anonymously and only indicated their medical specialty and level of training. Respondents included resident and attending physicians with experience in facial trauma from the fields of otolaryngology, oral and maxillofacial surgery (OMFS), and radiology.
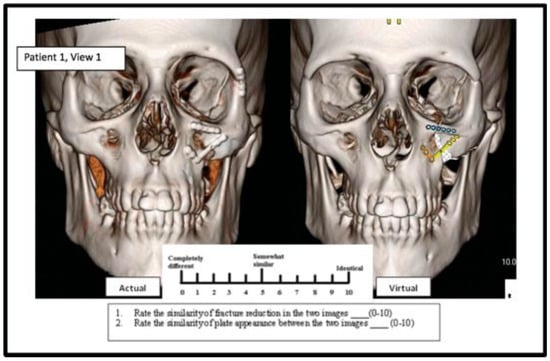
Figure 2.
The actual postoperative picture (left) is compared with the algorithm-created image (right) on a Likert scale of 0 to 10.
Statistical Analysis
Mean and standard deviation values for the questions in each set of images were calculated. A series of unpaired t-tests was used to compare resident and attending responses to all three questions. Respondents were divided into two groups. The surgeon group consisted of otolaryngologists and OMFS respondents. The radiology group consisted of responses by surveyed radiologists. The analysis for similarity of fracture reduction and similarity of plate was calculated. Inter-rater reliability was then calculated to evaluate the overall degree of agreement among individual respondents. This was done by calculating the inter-class correlation coefficient for all pooled data. All statistical analyses were performed using MedCalc for Windows, version 15.0 (MedCalc Software, Ostend, Belgium).
Results
In total, 35 physicians completed the survey, including 18 attendings and 17 residents. Respondents included were as follows: 14 from radiology, 10 from otolaryngology, and 11 from OMFS. A total of 34 images (17 pairs of virtual and actual preoperative) were assessed from 10 cases. Different views of each fracture were evaluated separately. Fracture types included zygomaticomaxillary complex (n = 8), LeFort type I (n = 1), and naso-orbito-ethmoid fracture (n = 1).
When asked to rate the similarity of fracture reduction on a Likert scale of 0 to 10, radiology physicians rated an average of 8.29 1.25 (95% confidence interval [CI]: 8.13–8.45). Surgeons rated the images a mean of 6.82 2.37 (95% CI: 6.56–7.08). Unpaired t-testing showed a statistically significant difference between the surgeon and radiology cohorts. In rating the similarity of plate appearance, the radiology group scored a mean of 8.82 1.09 (95% CI: 8.59–9.06) versus 7.45 2.14 (95% CI: 7.23–7.68) for the surgeon group. These data are depicted in Table 1. The responses between the two groups were statistically significant in unpaired t-testing. There was no significant difference in mean between resident and attending responses. There was also no statistical difference in the rating based on the type of fracture (zygomaticomaxillary vs. LeFort vs. naso-orbito-ethmoid). The distribution of responses across specialties is depicted in Figure 3. On analysis of inter-rater reliability, the inter-class correlation coefficient was 0.778 (95% CI: 0.6328–0.8843) indicating excellent correlation among raters.

Table 1.
Surgeon and radiologist survey response data.
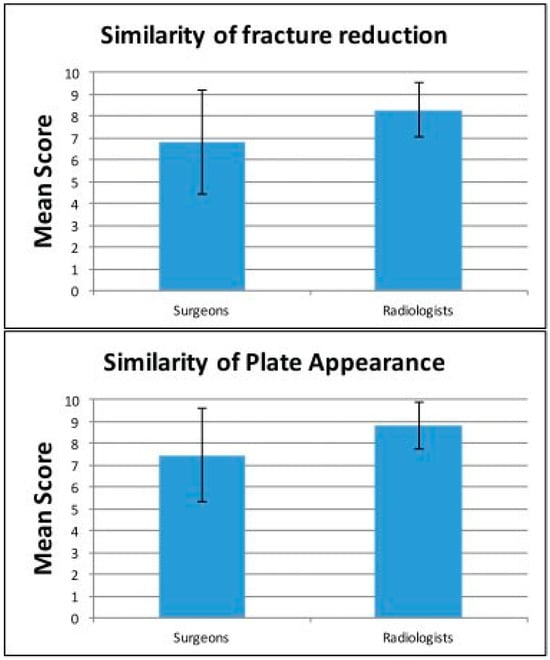
Figure 3.
Graphically depicted responses by both surgeons and radiologists on the similarity of fracture reduction and plate appearance.
To assess future clinical utility, respondents were asked additional questions about the algorithm. All of the survey respondents (35/35) answered that the plate shownwas trueto sizeto its printedversion. All otolaryngology respondents and 7 out of 11 (64%) OMFS respondents indicated that they would use this computer program to preoperatively plan midfacial fracture reduction if available. This question was not applicable to the radiology group. These data are shown in Table 2.

Table 2.
Survey response data assessing clinical utility.
Discussion
The data from this pilot study indicate that this computer algorithm is able to produce virtual images that are comparable to real postoperative 3D CT images of repaired midface fractures. Scores rating fracture reduction were slightly lower than the scores of plate appearance. This is likely due to thefact that it is sometimes difficult to retrospectively match the fracture reduction pattern that was achieved in the operating room using the program. The high rating of plate appearance reinforces the realistic nature of fracture reduction using the program. The actual 3D recreations do not process in color, and the images of plates are occasionally skewed due to scanning artifact as well as compromised resolution from volume averaging. Even given the difference in plate appearance, the scaled and printed algorithm-generated plate was shown to be identical to the real plate 100% of the time when users were asked to overlay an actual Stryker plate on top of a scaled printout of the algorithm plate.
We found that all specialists determined that there was a strong resemblance between the virtual pictures and actual postoperative recreations, as all three specialties rated the images well above “somewhat similar.” Radiologists rated the images significantly higher than the surgeons, which may be due to the different perspectives through which clinicians evaluate images.
A similar algorithm was initially described for mandibular fracture plating, and was adopted and amended for our use in the midface in our study.[8] The major difference of surgical planning of midface fractures compared with mandibular fractures is the multidimensionality and 3D complexity inherent to the reconstruction of the midface. This may add to the time spent in the operating room analyzing the fracture sites, deciding which plates to utilize, and sizing and shaping the places for optimal fit.
VSP is becoming more frequent in its use, and has been reported to be used in many surgical procedures such as fibular free flap reconstruction of the mandible,[9,10,11] orthognathic surgery,[12] orbital floor repair,[13] and orthopedic procedures and repair.[14] VSP has been demonstrated to reduce total operative time in craniofacial surgery, leading to greater operative efficiency in craniofacial surgery, specifically in fibular free flap reconstruction of the mandible.[9,10] While VSP has been demonstrated to be effective in reducing operativetime, thecost maybe prohibitive. Utilizing VSP for a fibular reconstruction is reported to cost anywhere from $1200 to 6890,[15] and planning for orthognathic surgery may cost anywhere from $800 to 1200.[16] In contrast, the only monetary costs of the algorithm are that of the two computer programs utilized. These include the 3D imaging software and the Adobe Photoshop program. In addition, the algorithm described has no monetary cost to the consumer.
Adding to the expenses necessary for VSP and CAD is the need for a third-party provider, and the need to schedule meetings for the planning. This extra step in the preparation for reconstruction is likely to be unreasonable in the setting of facial fracture reconstruction where a timely operation may be necessary, which may explain why it has not been extensively used in facial fracture fixation. Due to these limitations, we have found that it would be valuable to have an accessible program where the surgeon and his or her team may plan for the reconstruction without the increased cost or third-party coordination innate to VSP. Although the algorithm proposed does avoid the time required for reconstruction by VSP techniques, there is time expenditure of the surgeon associated with completing the algorithm. In our experience, it takes approximately 30 to 90 min to complete one virtual midfacial fracture repair. This varies dependent on different fracture patterns of injury. The time commitment required to virtually reduce and fixate fractures using the algorithm is a potential area of further study.
This algorithm also has the potential to be an educational tool for medical students, residents, and fellows to better understand the anatomy and process of reconstructing the midface. It allows users to prepare for an operation by reducing the fractures in its respective planes and deciding on the size, shape, and bend of fixation plates. The user must also learn to visualize the midface fracture three-dimensionally, and this acts as an exercise in teaching users to understand the multidirectional movement that is necessary to repair even a single fracture. Users need to take time to understand this three-dimensionality, and in doing so, they learn to visualize the fracture preoperatively and enter the operating room with better comprehension of the planned procedure. As an extremely accessible tool, it has the potential to be used in routine preoperative design and analysis.
This algorithm presents a new educational opportunity; however, it does pose a challenge, as the user must respect the three-dimensionality of the midface. The movement of one fracture shifts other articulating structures, and this movement may distort spatial relationships of the other fracture segments. Each fracture must be reduced individually in its respective plane while using this algorithm. If the user of the algorithm does not keep this framework in mind, the image should not be representative of the true changes required for bony alignment.
Another limitation of this study is its retrospective nature. As a pilot study, the goal of this study was to describe and assess the ability of this algorithm to produce reliable images before assessing its clinical utility and integration into clinical practice. It would be necessary to perform a prospective study to assess thefeasibilityand true clinical utilityof this algorithm in the setting of real-time preoperative planning. It is evident from these data that this algorithm is able to produce images that resemble actual postoperative images. However, it is necessary to compare preoperative planning images created with actual postoperative images to evaluate whether the algorithm allows for accurate prediction of fracture repair. A prospective study could also be performed to assess differences in operating time and facial fracture fixation outcomes with use of the algorithm. This algorithm does have a learning curve associated with its use. Many practicing physicians may have previous experience with the programs utilized in this algorithm, making integration of the algorithm more feasible. In addition, it remains to be seen whether clinical application of this virtual fixation technique is able to decrease cost, improve outcomes, and have a positive impact on trainee experience. Although answering these questions falls out of the scope of this pilot study, a prospective study should be designed with these goals in mind.
Conclusion
The novel algorithm described has the ability to accurately model midface fracture repair and hardware placement. Although VSP plays a role in preoperative planning, an algorithm using commercially available programs allows the surgeon to personally prepare for the procedure by visualizing the necessary fracture reduction and plating patterns. The algorithm may also serve as an educational tool for learning how to approach the anatomy of a fracture of the midface region. We need to prospectively study the algorithm to determine whether it reduces operative times when the surgeon uses it for preoperative planning.
References
- Vrinceanu, D.; Banica, B. Principles of surgical treatment in the midface trauma—Theory and practice. Maedica 2014, 9, 361–366. [Google Scholar] [PubMed]
- Kaur, J.; Chopra, R. Three dimensional CT reconstruction for the evaluation and surgical planning of mid face fractures: A 100 case study. J Maxillofac Oral Surg 2010, 9, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Suenaga, H.; Taniguchi, A.; Yonenaga, K.; Hoshi, K.; Takato, T. Computer-assisted preoperative simulation for positioning of plate fixation in Lefort I osteotomy: A case report. J Formos Med Assoc 2016, 115, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Roser, S.M.; Ramachandra, S.; Blair, H.; et al. The accuracy of virtual surgical planning in free fibula mandibular reconstruction: Comparison of planned and final results. J Oral Maxillofac Surg 2010, 68, 2824–2832. [Google Scholar] [CrossRef] [PubMed]
- Rodby, K.A.; Turin, S.; Jacobs, R.J.; et al. Advances in oncologic head and neck reconstruction: Systematic review and future considerations of virtual surgical planning and computer aided design/computer aided modeling. J Plast Reconstr Aesthet Surg 2014, 67, 1171–1185. [Google Scholar] [CrossRef] [PubMed]
- Resnick, C.M.; Inverso, G.; Wrzosek, M.; Padwa, B.L.; Kaban, L.B.; Peacock, Z.S. Is there a difference in cost between standard and virtual surgical planning for orthognathic surgery? J Oral Maxillofac Surg 2016, 74, 1827–1833. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, C.J.; Ortlip, T.; Greywoode, J.D.; Vakharia, K.T.; Vakharia, K.T. A novel computer algorithm for modeling and treating mandibular fractures: A pilot study. Laryngoscope 2017, 127, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Kääriäinen, M.; Kuuskeri, M.; Gremoutis, G.; Kuokkanen, H.; Miettinen, A.; Laranne, J. Utilization of three-dimensional computer-aided preoperative virtual planning and manufacturing in maxillary and mandibular reconstruction with a microvascular fibula flap. J Reconstr Microsurg 2016, 32, 137–141. [Google Scholar] [PubMed]
- Wang, Y.Y.; Fan, S.; Zhang, H.Q.; Lin, Z.Y.; Ye, J.T.; Li, J.S. Virtual surgical planning in precise maxillary reconstruction with vascularized fibular graft after tumor ablation. J Oral Maxillofac Surg 2016, 74, 1255–1264. [Google Scholar] [CrossRef]
- Chang, E.I.; Jenkins, M.P.; Patel, S.A.; Topham, N.S. Long-term operative outcomes of preoperative computed tomography-guided virtual surgical planning for osteocutaneous free flap mandible reconstruction. Plast Reconstr Surg 2016, 137, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Wrzosek, M.K.; Peacock, Z.S.; Laviv, A.; et al. Comparison of time required for traditional versus virtual orthognathic surgery treatment planning. Int J Oral Maxillofac Surg 2016, 45, 1065–1069. [Google Scholar] [CrossRef]
- Vehmeijer, M.; van Eijnatten, M.; Liberton, N.; Wolff, J. A novel method of orbital floor reconstruction using virtual planning, 3-dimensional printing, and autologous bone. J Oral Maxillofac Surg 2016, 74, 1608–1612. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Wang, X.; Fu, B.; Lu, Y.; Wang, M. Application of computer-assisted surgical planning in surgical treatment of ankle fractures. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2015, 29, 1469–1473. (In Chinese) [Google Scholar] [PubMed]
- Xia, S.; Zhang, Y.; Wang, X.; et al. Computerized virtual surgery planning for ORIF of proximal humeral fractures. Orthopedics 2015, 38, e428–e433. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zweifel, D.F.; Simon, C.; Hoarau, R.; Pasche, P.; Broome, M. Are virtual planning and guided surgery for head and neck reconstruction economically viable? J Oral Maxillofac Surg 2015, 73, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Hammoudeh, J.A.; Howell, L.K.; Boutros, S.; Scott, M.A.; Urata, M.M. Current status of surgical planning for orthognathic surgery: Traditional methods versus 3D surgical planning. Plast Reconstr Surg Glob Open 2015, 3, e307. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the author. The Author(s) 2017.