Abstract
Attention Deficit Hyperactivity Disorder is a neurodevelopmental disorder with three presentations: inattentive, hyperactive/impulsive and combined. These may represent an independent disease entity. Therefore, the therapeutic approach must be focused on their neurobiological, psychological and social characteristics. To date, there is no comprehensive analysis of the efficacy of different treatments for each presentation of ADHD and each stage of development. This is as narrative overview of scientific papers that summarize the most recent findings and identify the most effective pharmacological and psychosocial treatments by ADHD presentation and age range. Evidence suggests that methylphenidate is the safest and most effective drug for the clinical management of children, adolescents and adults. Atomoxetine is effective in preschoolers and maintains similar efficacy to methylphenidate in adults, whereas guanfacine has proven to be an effective monotherapy for adults and is a worthy adjuvant for the management of cognitive symptoms. The psychosocial treatments with the best results in preschoolers are behavioral interventions that include training of primary caregivers. In adolescents, the combination of cognitive and cognitive-behavioral therapies has shown the best results, whereas cognitive-behavioral interventions are the most effective in adults. Pharmacological and psychosocial treatments must be adjusted to the ADHD presentation and its neurocognitive characteristics through the patient’s development.
1. Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a neurodevelopmental disorder characterized by persistent inattention, hyperactivity and impulsivity behaviors [1]. ADHD is commonly diagnosed in childhood and, given its high incidence and cost of treatment, represents a public health problem throughout the world [2]. ADHD etiology is unknown, but it has a strong hereditary component (~81%) [3] and, unfortunately, 60% of the cases diagnosed during childhood will prevail into adulthood.
The Diagnostic and Statistical Manual of Mental Disorders (DSM-5) distinguishes three clinical presentations of ADHD: predominantly inattentive, predominantly hyperactive/impulsive and combined [1].
The inattentive ADHD presentation is the most commonly diagnosed (53.7%), followed by the combined type (26.8%), while the least diagnosed is the hyperactive/impulsive (19.5%) [4] (see Table 1).

Table 1.
Behavioral Expression of ADHD Subtypes.
Sex and age of patients are associated with ADHD presentation. In women, inattentive presentation is more frequent, while in men the combined type predominates. In contrast, the hyperactive/impulsive presentation shows similar incidences between men and women [4]. According to the stage of development, the most commonly diagnosed presentation in preschool children is the hyperactive-impulsive (2.8%) [5], followed by the combined (2.1%) and the inattentive (0.1%) [6]. In adults, the inattentive type is the most common clinical presentation of ADHD, regardless of gender [7].
The diagnosis of ADHD presentations may vary throughout the life of the patient [5]. Preschool children who meet the diagnostic criteria for hyperactive-impulsive ADHD may progress to combined-type ADHD during their elementary school, whereas children with combined-type symptoms in elementary school may shift to inattentive ADHD and their hyperactivity-impulsivity symptoms progressively decrease [5] To date, there is not enough evidence to establish whether these variations are related to the type of treatment, but it is clear that some ADHD presentations tend to be more stable than others. Lahey et al. found that children who met diagnostic criteria for the combined type maintained this presentation in subsequent evaluations, unlike children diagnosed with the hyperactive-impulsive and inattentive types, who manifested diagnostic criteria for a different presentation on at least two occasions throughout their life [8]. This variability in the diagnosis of ADHD presentation has been associated with hormonal and brain changes that occur throughout body development [3].
The first clinical manifestations of ADHD can appear from the third year of life [6]. However, the diagnosis is usually established between six and nine years of age [9]. Regardless of the ADHD presentation, the most effective treatment is multimodal, a combination of pharmacological and psychosocial approaches. Drug treatment is more effective for managing the core symptoms of ADHD (inattention, hyperactivity and impulsivity), while the psychosocial approach is more effective for treating comorbid symptoms [10]. Unfortunately, 42.2% of children diagnosed with ADHD receive a single treatment that is usually abandoned when parents or caregivers do not perceive the expected results to counteract all the academic, social and family problems associated with ADHD [11]. Therefore, it is crucial to know the most effective therapeutic approaches for each presentation and promote better adherence to treat ADHD patients. The aim of this review is to summarize the current findings of effective treatments for handling ADHD, which can help professionals to establish their clinical management. To this end, we revised the most effective pharmacological and psychosocial treatments used for ADHD in accordance with the predominant symptoms, age of patients and comorbidity. This evidence indicates that the choice of treatment should be based on the ADHD presentation, age, gender and comorbid disorder, which helps improve therapeutic adherence in these patients.
2. Materials and Methods
The present systematic review examines the efficacy reported in pharmacological, neuropsychological, or psychosocial treatments in ADHD according to age or presentation. We searched for articles from the National Library of Medicine by using the PubMed search engine and the terms: efficacy of neuropsychological treatments, OR pharmacological treatments, OR novel psychological treatments OR psychosocial treatments combined with the terms ADHD, ADHD-presentations, OR inattention, OR impulsivity, OR hyperactivity.
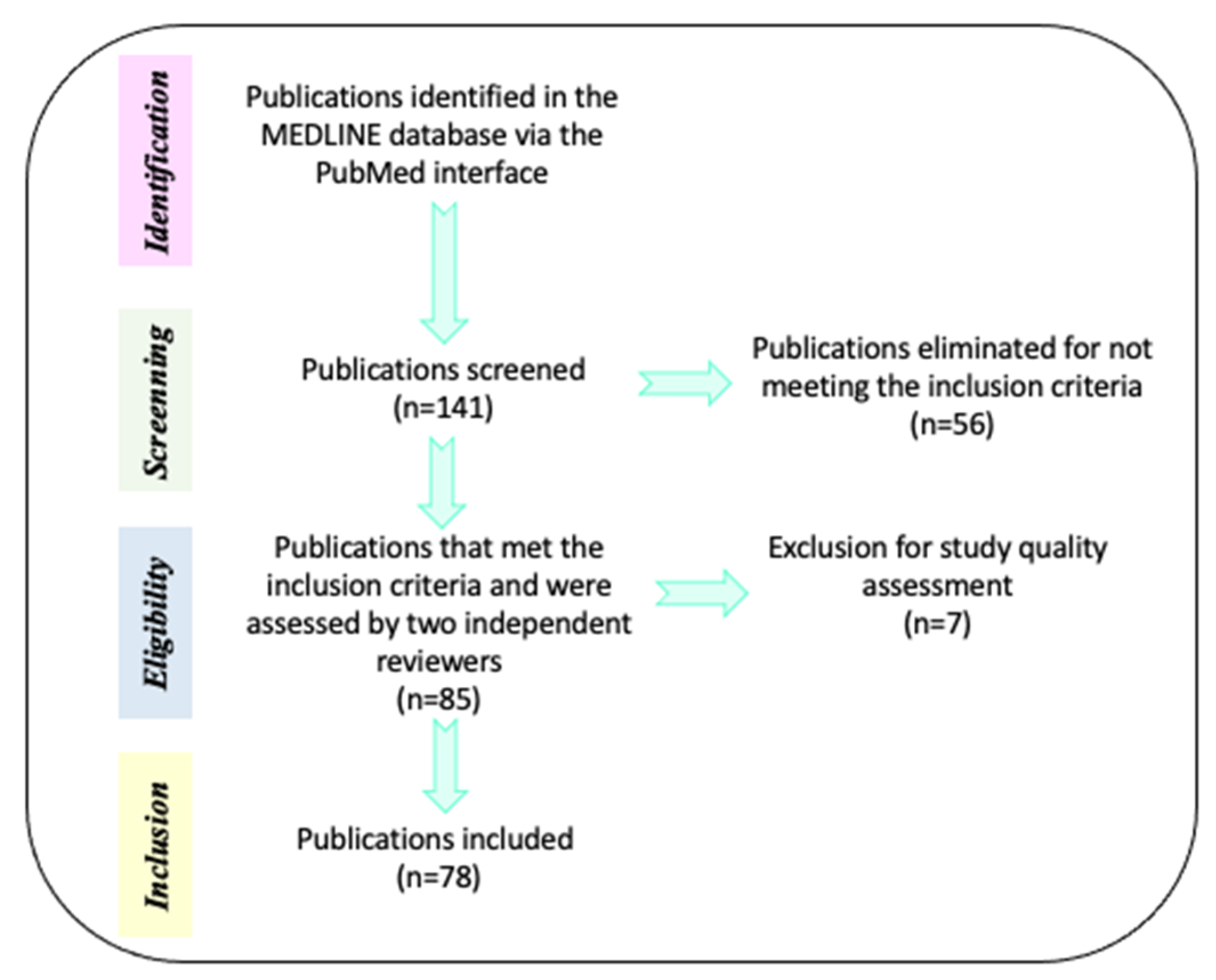
The initial search in the MEDLINE database comprised 141 papers. Of these, 56 references were eliminated because: a) they compared alternative or unregulated therapies (homeopathy, transcranial direct current stimulation, omega 3/6, fatty acids, phosphatidylserine, neurofeedback, or exercise) with pharmacological interventions and b) the efficacy of treatments (pharmacological, neuropsychological, or psychosocial) was evaluated when AHDH was comorbid with diverse neurodevelopmental disorders. Only 85 references were selected because they met inclusion criteria: a) Type of paper (clinical trials, meta-analysis, reviews, systematic reviews, randomized controlled trial, case reports, committee and government reports) and b) Efficacy of pharmacological treatments (methylphenidate—MPH-, Atomoxetine—ATM- and Guanfacine) combined or not with neuropsychological treatments (cognitive training) or psychosocial treatments (behavioral management, stress management, organization training, parental training, social skills training, cognitive therapy and metacognitive therapy) in preschoolers, children, adolescents and adults with a diagnosis of ADHD. For description of the mechanism of action for drug medication in ADHD, we also included animal models. The current review included English- and Spanish-language reports published between the years 1991 and 2021. Then, the pre-selected papers (85 documents) were subjected to methodological quality assessment by two independent reviewers. After this process, 7 reports were eliminated because they contained confirmative information, or their evidence did not approach the aim of the review. Finally, only 78 references were utilized for this review and all the selections followed PRISMA guidelines (Figure 1 and Table 2).
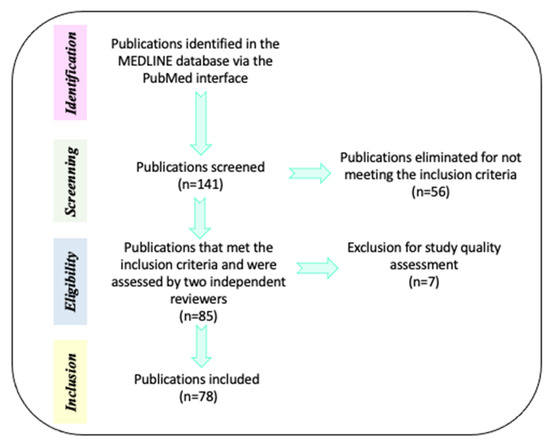
Figure 1.
Methodological process for references selection. Description of the steps performed for the search and selection of references used for this review.

Table 2.
A summary of the main findings of each reference examined for this review.
3. Results
3.1. Efficacy of Pharmacological Treatment According to Patients’ Development
Of the 79 selected references, 34 were used for analysis of the current pharmacological treatments. The conventional treatment for ADHD is based on the use of nervous system stimulants (amphetamines and methylphenidate) [12] and non-stimulants such as guanfacine, atomoxetine, or clonidine [13]. ADHD is the only nondegenerative mental condition where stimulant drugs alleviate the core symptoms [14]. A meta-analysis of double-blind, randomized and controlled trials reported that the combination of amphetamine and methylphenidate produces a good response in 41% of ADHD patients [15]. Nevertheless, 28% of patients show clinical improvements with the monotherapy of amphetamines and 16% with methylphenidate [15]. In children and adolescents, MPH has a higher size effect (measured through risk ratio, RR = 1.14) when compared to other drugs [16], whereas in adults the size of the effect is moderate (Standardized Mean Difference—SMD—0.50) [17]. Despite this evidence, MPH is still the first-line drug of use [18], because it has fewer adverse effects [19] and prolonged therapeutic effect (up to 24 months) after drug withdrawal [20]. However, the average use for stimulant-drug medication is low, 136 days for children and 230 days for adults, and its discontinuation is associated with a stigma about the use of medication, perception of low efficacy, multiple side effects and others [15].
After MPH oral administration, MPH reaches sufficient plasma levels in one hour [21] and blocks the dopamine and norepinephrine reuptake transporters [22] and, in turn, increases dopamine and norepinephrine synaptic levels [23], modifies dendrite length and complexity [24], promotes catecholamine-associated neural stimulation and inhibition [21] and improves neural connectivity between frontal lobe and striatum [25,26]
The long-term therapeutic effect of MPH depends on the dose, route of administration and treatment duration [27]. Seven- to eleven-year-old children diagnosed with ADHD and treated with MPH can show a significant reduction in impulsivity and clinical symptoms from the 6th to 8th week of treatment [27]. Despite that MPH is considered as a safe drug, high doses (270 mg/kg) have been associated with neurotoxicity, suicidal attempts [28,29] and paradoxical effects, such as agitation, hyperactivity and poor cognitive performance [30]. Preschool-age children (<6 years) seem to be more susceptible than older children to develop side effects, such as repetitive behaviors, recurrent thoughts, weight loss, insomnia and appetite loss [21]. Sadness and social withdrawal are additional symptoms that have also been reported in preschool-age children and are more common in the combined ADHD than inattentive presentation [6]. According to the patient’s age, in children and adolescents, MPH had the lowest rate of discontinuation in comparison to amphetamines or placebo [15]. This suggests that the ADHD presentation and the development stage of each patient seem to influence the incidence of side effects after MPH therapy. Thus, some authors recommend gradual increases in MPH doses, starting with 0.15 mg/kg followed by 0.3 to 0.60 mg/kg in children and starting with 40 mg followed by 50 to 80 mg for adults [31].
In most cases, MPH recovers cognitive performance in patients with ADHD, regardless of whether they have comorbid psychiatric disorders or not [30]. In healthy subjects, MPH improves memory performance and induces greater interest in and motivation to perform activities such as mathematics [32]. This effect of MPH on math skills has also been reported in ADHD patients (range 4 to 16 years), which is associated with clinical progression in a dose-independent matter [33], suggesting that MPH efficiently improves motivation. The pharmaceutical presentation of MPH appears to have a considerable influence on biological effects and cognitive performance. In children and adolescents, the osmotic release of MPH improves verbal fluency, selective attention, inhibitory control, spatial intelligence and working memory [27]. The long-lasting release of MPH is very effective for improving academic performance as compared to a short-term-release drug [33]. Therefore, long-lasting presentations are recommended for the management of inattentive ADHD, which is the presentation most frequently associated with academic problems.
MPH is effective for the management of oppositional defiant disorder (ODD) and reduces the incidence and severity of assaults [34]. ODD is a typical comorbid disorder of the combined ADHD presentation. Therefore, MPH is an elective drug for handling this presentation [35]. However, 25% to 35% of preschool infants (3 to 6 years old) and 25% to 78% of adults do not show clinical improvement after using MPH [19]. This evidence suggests that MPH could be more effective for the treatment of school-age children (6 to 13 years) and adolescents (14–19 years). Therefore, other non-stimulant pharmacological alternatives, such as atomoxetine or guanfacine can be recommended for the treatment of preschool and adult patients.
ATM is a norepinephrine reuptake inhibitor that increases neurotransmitter levels in the synaptic space [36]. In preschool children, ATM starting dose is 40 mg/kg and it can be increased to 80–100 mg/kg distributed over 24 h [37]. In adults, the recommended dose of ATM is 80 mg/kg, because lower doses (<60 mg/kg) have shown unfavorable therapeutic adherence and poor symptomatic control [37]. From the second week of treatment, ATM helps significantly reduce inattention, hyperactivity and impulsivity scores, but its clinical effect is more evident between the fourth and sixth week of treatment [37].
ATM is highly recommended for controlling the hyperactive/impulsive presentation in pre-escolar patients. Pharmacological presentation seems to be a significant factor that modifies the efficacy of every drug, for instance, ATM shows a similar size effect when compared to immediate-release vehicles of MPH (SMD −0.05), but a poor size effect when compared to the osmotic-release vehicle of MPH (SMD 0.031) [38]. However, some of these children may present emotional lability with this drug [6]. A physical deficit has also been reported with ATM treatment; in children (6 to 7 years) and a two-year treatment with ATM was associated with a 2.7 cm reduction in the height of the patients [15]. Therefore, the clinicians must be alert and initiate an early intervention when necessary.
Guanfacine was initially used against hypertension, but recent studies demonstrated its efficacy as an adjuvant drug for ADHD management in children and adolescents. The ideal dose of guanfacine to control ADHD symptoms is between 4 and 7 mg/kg [39]. In adults, monotherapy with guanfacine is very effective and shows a higher effect size (0.52) than placebo [40]. Guanfacine is rapidly absorbed and reaches optimal plasma concentrations in the first 4 h after oral intake. The action mechanism lies in its ability to stimulate α2a adrenergic receptors in the prefrontal cortex, which facilitates neural signaling in this region [41] and reduces impulsivity [42]. Guanfacine effects are not as evident as ATM or MPH, but it significantly improves cognitive performance, attention deficit and working memory [43]. For these reasons, this drug could be prescribed as an adjuvant treatment for hyperactive/impulsive presentation, comorbid learning disabilities or ODD.
Recent studies show that the efficacy of MPH, ATM and guanfacine varies according to the stage of development of the patient. MPH has better results in the management of central symptoms in late childhood, adolescence and adulthood. ATM is less effective for the management of symptoms in late childhood and adolescence but has similar efficacy to MPH in adults [44]. Although MPH is considered an effective medication for adults, the high comorbidity of ADHD with the abuse of addictive substances in this population means that some authors recommend pharmacological treatments with non-stimulant drugs, such as ATM or guanfacine [45]. In this regard, non-stimulant drugs might be a better option for adolescents and adults with both ADHD and abuse of drugs (Table 3).

Table 3.
Clinical efficacy of the pharmacology therapy for controlling ADHD symptoms throughout development.
3.2. Efficacy of the Neuropsychological Treatment According to Patients’ Development
To summarize the findings of neuropsychological treatments, we analyzed 10 of the 79 selected references. Recently, a systematic review that compared 34 meta-analyses reported that children and adolescent patients with ADHD present a worsening in neurocognitive performance when compared with typically developing subjects [46]. Several studies have been carried out to identify the neuropsychological profile of ADHD patients and important differences have been found among presentations. These differences largely determine the type of treatment provided, which highlights the importance of understanding the neurocognitive profiles of each presentation in order to establish specific neuropsychological rehabilitation strategies for each subtype. ADHD patients showed deficits in the standardized mean difference (SMD) of cognitive functions associated with the default model network, including intelligence/achievement (SMD = 0.60), reaction time variability (SMD = 0.66), vigilance (SMD = 0.56), response inhibition (SMD = 0.52) and working memory (SMD = 0.54) [46]. In most patients, difficulties in executive functions underly the core symptoms of ADHD [47,48,49,50]. These executive impairments are associated with learning disorders and low academic achievements in 20–30% of pediatric patients [51,52]. In this regard, in children with inattentive, combined, or hyperactive-impulsive type of ADHD, the treatment based on executive-function training improves their executive skills that positively affect their routines in real daily life [53].
Dysfunction in executive functions varies for each ADHD presentation. Children and adolescents with combined or inattentive subtypes show severe deficits in the control of inhibitory responses, whereas those with the hyperactive-impulsive subtype show less executive impairment [5]. Patients with inattentive presentation also show inappropriate working memory that enhances problems in intrinsic motivation, which means that these patients get bored easily in their day-to-day tasks [50]. Hence, this evidence suggests that patients with inattentive and combined types require neuropsychological strategies of rehabilitation that promote executive skills training in behavioral inhibition, set-shifting, verbal fluency, working memory, cognitive flexibility and planning [54]. However, a meta-analysis of cognitive training reported that these treatments have a greater impact when used as an adjunctive therapy that improves some neuropsychological impairments, such as deficits in visual and verbal working memory, but they do not improve core symptoms such as inattention [55].
Other cognitive variables also present differences among ADHD presentations. Inattentive and hyperactive-impulsive types are inversely correlated to intelligence, alertness, response variability, processing speed and delay in the aversive stimulus so it is important to incorporate the evaluation and treatment of these cognitive tasks in both presentations. Notably, the predominance of inattention symptoms seems to be strongly associated with cognitive deterioration [5]. Consequently, neuropsychological treatment should be focused on correcting these symptoms and with special emphasis on women, who have the highest incidence of inattentive presentation [4] (see Table 4).

Table 4.
Neuropsychological Treatments Evidence in ADHD.
3.3. Efficacy of Psychosocial Treatment According to Patients’ Development
To evaluate the efficacy of psychosocial treatment in ADHD, we analyzed 21 of the 79 selected references. In this regard, the efficacy of psychosocial treatment in ADHD has been a discussion subject in the scientific literature [56]. Methodological issues such as diversity in inclusion and exclusion criteria, number of cases, or evaluation approaches can modify results and meta-analyzes, making it difficult to determine the efficacy of psychosocial treatment [57]. Despite that conflicting or contradictory results have been obtained, several psychosocial strategies have shown certain effectiveness [57,58,59,60]. Psychosocial treatments consist of cognitive intervention, social skill training, problem-solving, emotion management, academic skills [61,62] and behavioral intervention in parents and patients [63], aimed at reducing the presence of disruptive behaviors and preventing them. These treatments are mainly based on:
- Behavioral techniques, based on rewards or punishments, that seek to generate more adaptive behaviors.
- Cognitive techniques that identify maladaptive beliefs and replace them with others that generate better adaptation, i.e., cognitive restructuring or rehabilitation.
- Combination of cognitive and behavioral techniques at a group or individual level.
- Training in organizational and/or social skills.
The psychosocial intervention in patients with inattentive- or combined-type ADHD that is based on training in improving organization skills regarding school materials showed a moderate improvement in inattention [58]. In early childhood, behavioral parenting training reduces the core symptoms with a large effect size (>1) [59], whereas training in behavioral skills of parents and teachers has a large effect size for the management of ADHD in adolescents with hyperactive and impulsive symptoms [56]. In parallel, training in organizational skills of children and adolescents moderately improves their academic productivity [58]. However, this training also showed a significant effect size in task planning (d = 1.05), homework completion (d = 0.85) and academic problem solutions (d = 1.30) [56].
Cognitive interventions in children and adolescents have a moderate size of effect on improving inattention and a small effect size on controlling impulsivity and hyperactivity [64]. Similar findings have been found in adults when cognitive-behavior interventions are utilized for 2 and 4 months [65]. Interestingly, better results for improving inattention are obtained when therapy in groups is accomplished, but this approach seems not to be very effective for the management of hyperactivity and impulsivity symptoms [66]. In summary, this evidence suggests that cognitive interventions help improve inattention in children and adolescents, whereas the combination of cognitive-behavioral approaches is more effective for adults.
Up to 70% of ADHD patients have comorbid psychiatric disorders. Depression and anxiety are the internalizing comorbid disorders more prevalent in patients with inattentive and combined presentations [67,68,69,70]. Interestingly, the relationship between depression and anxiety persists throughout life in the inattentive type, but not in the combined type [71]. This may be due to the incompetence feelings that inattention usually causes, which is not as evident in the combined type. Various psychosocial treatments have been used for managing depression and anxiety. Behavioral training for parents has shown partial benefit in managing depressive and anxiety symptoms in infantile patients [72]. In contrast, cognitive-behavioral therapy notably reduces symptoms of emotional disorders in adults with ADHD [66,73]. Besides, this type of training also improves self-esteem and organizational skills [66]. Recently, acceptance and commitment therapy (ACT) is emerging as a promising treatment that improves anxiety in adults [74]. Altogether, this evidence indicates that parental behavioral training is the best approach for the treatment of depression and anxiety in children, whereas cognitive-behavioral therapy in groups seems to be the best option for adults (see Table 5).

Table 5.
Psychosocial Treatments Evidence in ADHD.
Externalizing disorders, such as oppositional-defiant disorder, are psychopathologies that occur with a higher incidence in children with the combined presentation [75,76] and are characterized by the exteriorization of disruptive behaviors and behavioral alterations, where aggressiveness, impulsivity mad oppositionist, lack of behavioral–emotional self-control are consistent [77]. Patients with combined type have extensive externalizing symptoms [67,68,70] that provoke inappropriate social functioning [78]. Training parents in behavioral management and school-material organization have showed to improve the symptomatology of oppositional-defiant disorder, but the size of the effect is modest [64]. In 6- to 12-year-old children, training in contingency management and academic interventions are more effective inducing a significant behavioral change as compared to cognitive-behavioral strategies [59]. Therefore, behavioral interventions are recommended to control externalizing symptoms of ADHD or in patients with the combined type where ODD is frequently diagnosed.
4. Conclusions
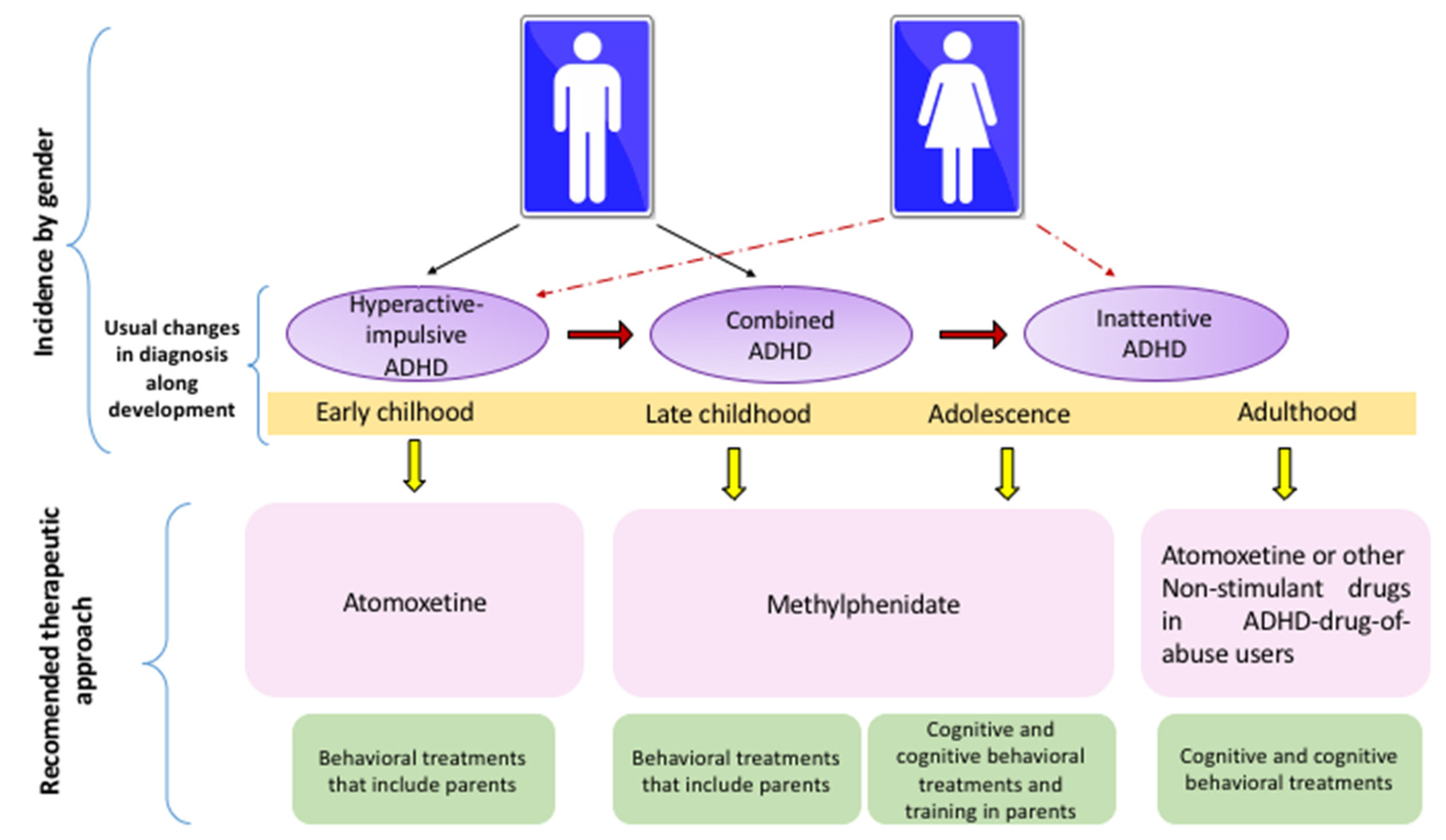
In the preschool stages (3–6 years), the most prevalent presentation of ADHD is the hyperactive/impulsive subtype. In childhood (6–12 years), the combined type predominates in boys, whereas the inattentive type is the most common ADHD presentation in girls. The hyperactive-impulsive ADHD commonly progresses to a combined presentation that, in turn, evolves to the inattentive type in adults (see Table 6).

Table 6.
Recommended Behavioral Therapeutic Approaches for ADHD by Developmental Stage and Clinical Presentation.
Due to cognitive and behavioral comorbidities, the most complex presentations for clinical management are inattentive and combined types. ATM is the drug with better results in preschool children because it has fewer side effects as compared to MPH. ATM is also recommended for the therapeutic management of adolescents and adults with abuse of drugs. MPH is very efficient for clinical management of late childhood and adolescents with inattentive and combined presentations because it improves working memory, intelligence, academic skills and ODD comorbidity. Guanfacine is also recommended as an adjuvant treatment in childhood and adolescents to improve academic symptoms. Guanfacine can also be useful for handling adults. In parallel, neuropsychological treatments act as adjuvant therapy, whereas the most effective psychosocial treatment in early childhood is behavioral intervention that includes training for parents.
Late childhood is benefited by cognitive strategies that improve inattention, whereas cognitive-behavioral approaches reduce hyperactivity and impulsivity. In adolescents and adults, the most effective approach is the combination of cognitive and cognitive-behavioral therapies (see Figure 2).
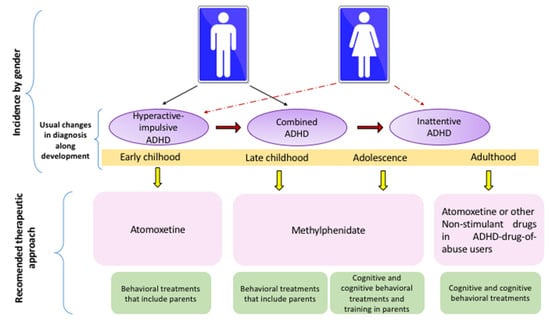
Figure 2.
Recommended Behavioral and Pharmacological Approaches for ADHD by Developmental Stage and Clinical Presentation.
Author Contributions
Conceptualization, methodology, investigation, resources, data curation and writing—original draft preparation: A.Y.G.-C., I.V.-d.l.C. and B.B.-N.; writing—review and editing, visualization and supervision A.Y.G.-C., I.V.-d.l.C., B.B.-N., R.E.G.-C. and O.G.-P.; project administration and funding acquisition, A.Y.G.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by PRO-SNI-Universidad de Guadalajara under grant No. 23706.
Conflicts of Interest
The authors declare no conflict of interest.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; APA: Washington, DC, US, 2013; pp. 31–86. [Google Scholar]
- National Institutes of Health Consensus Development Conference Statement: Diagnosis and treatment of attention-deficit/hyperactivity disorder (ADHD). J. Am. Acad. Child Adolesc. Psychiatry 2000, 39, 182–193. [CrossRef]
- Andersen, S.L. Trajectories of brain development: Point of vulnerability or window of opportunity? Neurosci. Biobehav. Rev. 2003, 27, 3–18. [Google Scholar] [CrossRef]
- Mowlem, F.D.; Rosenqvist, M.A.; Martin, J.; Lichtenstein, P.; Asherson, P.; Larsson, H. Sex differences in predicting ADHD clinical diagnosis and pharmacological treatment. Eur. Child Adolesc. Psychiatry 2018, 28, 481–489. [Google Scholar] [CrossRef]
- Willcutt, E.G. The Prevalence of DSM-IV Attention-Deficit/Hyperactivity Disorder: A Meta-Analytic Review. Neurotherapeutics 2012, 9, 490–499. [Google Scholar] [CrossRef]
- Greenhill, L.L.; Posner, K.; Vaughan, B.S.; Kratochvil, C.J. Attention Deficit Hyperactivity Disorder in Preschool Children. Child. Adolesc. Psychiatry Clin. N. Am. 2008, 17, 347–366. [Google Scholar] [CrossRef]
- Young, S.; Yoon, R.; Ravi, U.; Michael, C. Sleep and daytime function in adults with attention-deficit / hyperactivity disorder: Subtype differences. Sleep Med. Rev. 2013, 14, 648–655. [Google Scholar] [CrossRef]
- Lahey, B.B.; Pelham, W.E.; Loney, J.; Lee, S.S.; Willcutt, E. Instability of the DSM-IV Subtypes of ADHD From Preschool Through Elementary School. Arch. Gen. Psychiatry 2005, 62, 896. [Google Scholar] [CrossRef] [PubMed]
- Charach, A.; Dashti, B.; Carson, P.; Booker, L.; Lim, C.G.; Lillie, E.; Schachar, R. Attention Deficit Hyperactivity Disorder. Attention Deficit Hyperactivity Disorder: Effectiveness of Treatment in At-Risk Preschoolers; Long-Term Effectiveness in All Ages and Variability in Prevalence, Diagnosis, and Treatment; Agency for Healthcare Research and Quality: North Bethesda, MA, US, 2011. Available online: http://www.ncbi.nlm.nih.gov/pubmed/22191110 (accessed on 4 October 2021).
- Chronis, A.M.; Jones, H.A.; Raggi, V.L. Evidence-based psychosocial treatments for children and adolescents with attention-deficit/hyperactivity disorder. Clin. Psychol. Rev. 2006, 26, 486–502. [Google Scholar] [CrossRef]
- Hébert, J.; Cand, A.P.; Joober, R. Adherence to Psychostimulant Medication in Children with. J. Can. Acad. Child Adolesc. Psychiatry 2013, 22, 317–324. [Google Scholar]
- Barbaresi, W.J.; Katusic, S.K.; Colligan, R.C.; Weaver, A.L.; Jacobsen, S.J. Modifiers of Long-Term School Outcomes for Children with Attention-Deficit/Hyperactivity Disorder: Does Treatment with Stimulant Medication Make a Difference? Results from a Population-Based Study. J. Dev. Behav. Pediatr. 2007, 28, 274–287. [Google Scholar] [CrossRef]
- Caye, A.; Swanson, J.M.; Coghill, D.; Rohde, L.A. Treatment strategies for ADHD: An evidence-based guide to select optimal treatment. Mol. Psychiatry 2019, 24, 390–408. [Google Scholar] [CrossRef] [PubMed]
- Minzenberg, M.J. Pharmacotherapy for Attention-Deficit/Hyperactivity Disorder: From Cells to Circuits. Neurotherapeutics 2012, 9, 610–621. [Google Scholar] [CrossRef]
- Cortese, S. Pharmacologic Treatment of Attention Deficit–Hyperactivity Disorder. N. Engl. J. Med. 2020, 383, 1050–1056. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, H.; Fang, Q.; Qin, L. Comparative efficacy and safety of methylphenidate and atomoxetine for attention-deficit hyperactivity disorder in children and adolescents: Meta-analysis based on head-to-head trials. J. Clin. Exp. Neuropsychol. 2017, 39, 854–865. [Google Scholar] [CrossRef]
- Stuhec, M.; Luki, P.; Locatelli, I. Efficacy, Acceptability, and Tolerability of Lisdexamfetamine, Mixed Amphetamine Salts, Methylphenidate, and Modafinil in the Treatment of Attention-Deficit Hyperactivity Disorder in Adults: A Systematic Review and Meta-analysis. Ann. Pharmacother. 2019, 53, 121–133. [Google Scholar] [CrossRef]
- Krogh, H.B.; Storebø, O.J.; Faltinsen, E.; Todorovac, A.; Ydedahl-Jensen, E.; Magnusson, F.L.; Simonsen, E. Methodological advantages and disadvantages of parallel and crossover randomised clinical trials on methylphenidate for attention deficit hyperactivity disorder: A systematic review and meta-analyses. BMJ Open 2019, 9, e026478. [Google Scholar] [CrossRef]
- Wilens, T.E. Effects of Methylphenidate on the Catecholaminergic System in Attention-Deficit/Hyperactivity Disorder. J. Clin. Psychopharmac. 2008, 28, S46–S53. [Google Scholar] [CrossRef]
- Huang, Y.S.; Tsai, M.H. Long-Term Outcomes with Medications for Attention-Deficit Hyperactivity Disorder. CNS Drugs 2011, 25, 539–554. [Google Scholar] [CrossRef]
- Mardomingo-Sanz, M.J. Clinical use of 30:70 controlled-release methylphenidate in the treatment of attention deficit hyperactivity disorder. Rev. Neurol. 2012, 55, 359–369. [Google Scholar] [PubMed]
- Zetterström, T.S.C. Chronic methylphenidate preferentially alters catecholamine protein targets in the parietal cortex and ventral striatum. Neurochem. Int. 2019, 124, 193–199. [Google Scholar] [CrossRef]
- Pietrzak, R.; Mollica, C.; Maruff, P.; Snyder, P. Cognitive effects of immediate-release methylphenidate in children with attention-deficit/hyperactivity disorder. Neurosci. Biobehav. Rev. 2006, 30, 1225–1245. [Google Scholar] [CrossRef] [PubMed]
- Zehle, S.; Bock, J.; Jezierski, G.; Gruss, M.; Braun, K. Methylphenidate treatment recovers stress-induced elevated dendritic spine densities in the rodent dorsal anterior cingulate cortex. Dev. Neurobiol. 2007, 67, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Kodama, T.; Kojima, T.; Honda, Y.; Hosokawa, T.; Tsutsui, K.I.; Watanabe, M. Oral administration of methylphenidate (Ritalin) differentially affects dopamine release between the prefrontal cortex and striatum-a microdialysis study in the monkey. J. Neurosci. 2017, 37, 2116–2155. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.P.; Bédard, A.C.V.; Fan, J.; Hildebrandt, T.B.; Stein, M.A.; Ivanov, I.; Halperin, J.M.; Newcorn, J.H. Striatal Activation Predicts Differential Therapeutic Responses to Methylphenidate and Atomoxetine. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 602–609. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Ota, T.; Iida, J.; Yamamuro, K.; Kishimoto, N.; Okazaki, K.; Kishimoto, T. Differential therapeutic effects of atomoxetine and methylphenidate in childhood attention deficit/hyperactivity disorder as measured by near-infrared spectroscopy. Child Adolesc. Psychiatr. Ment. Health 2017, 11, 26. [Google Scholar] [CrossRef]
- Ozdemir, E.; Karaman, M.G.; Yurteri, N.; Erdogan, A. A case of suicide attempt with long-acting methylphenidate (Concerta). ADHD Atten. Def. Hyp. Disord. 2010, 2, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Krishna, A.S.; Lefevre, C.; Kaagaza, M.; Wittkamp, M. Methylphenidate Overdose Causing Secondary Polydipsia and Severe Hyponatremia in an 8-Year-Old Boy. Pediatr. Emerg. Care 2017, 33, e55–e57. [Google Scholar] [CrossRef][Green Version]
- Cheng, J.; Xiong, Z.; Duffney, L.J.; Wei, J.; Liu, A.; Liu, S.; Chen, G.J.; Yan, Z. Methylphenidate Exerts Dose-Dependent Effects on Glutamate Receptors and Behaviors. Biol. Psychiatry 2014, 76, 953–962. [Google Scholar] [CrossRef]
- Huss, M.; Duhan, P.; Gandhi, P.; Chen, C.W.; Spannhuth, C.; Kumar, V. Methylphenidate dose optimization for ADHD treatment: Review of safety, efficacy, and clinical necessity. Neuropsychiatr. Dis. Treat. 2017, 13, 1741–1751. [Google Scholar] [CrossRef] [PubMed]
- Beyer, C.; Staunton, C.; Moodley, K. The implications of Methylphenidate use by healthy medical students and doctors in South Africa. BMC Medic. Ethics 2014, 15, 1–8. [Google Scholar] [CrossRef]
- Kortekaas-Rijlaarsdam, A.F.; Luman, M.; Sonuga-Barke, E.; Bet, P.M.; Oosterlaan, J. Short-term effects of methylphenidate on math productivity in children with attention-deficit/hyperactivity disorder are mediated by symptom improvements: Evidence from a placebo-controlled trial. J. Clin. Psychopharmacol. 2017, 37, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Masi, G.; Manfredi, A.; Nieri, G.; Muratori, P.; Pfanner, C.; Milone, A. A Naturalistic Comparison of Methylphenidate and Risperidone Monotherapy in Drug-Naive Youth with Attention-Deficit/Hyperactivity Disorder Comorbid with Oppositional Defiant Disorder and Aggression. J. Clin. Psychopharmacol. 2017, 37, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Golubchik, P.; Shalev, L.; Tsamir, D.; Manor, I.; Weizman, A. High pretreatment cognitive impulsivity predicts response of oppositional symptoms to methylphenidate in patients with attention-deficit hyperactivity disorder/oppositional defiant disorder. Int. Clin. Psychopharmacol. 2019, 34, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Reynaud, A.J.; Froesel, M.; Guedj, C.; Ben Hadj Hassen, S.; Cléry, J.; Meunier, M.; Hadj-Bouziane, F. Atomoxetine improves attentional orienting in a predictive context. Neuropharmacology 2019, 150, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Clemow, D.B.; Nyhuis, A.W.; Robinson, R.L. Clinical Impact of Not Achieving Recommended Dose on Duration of Atomoxetine Treatment in Adults with Attention-Deficit/Hyperactivity Disorder. CNS Neurosci. Ther. 2016, 22, 970–978. [Google Scholar] [CrossRef][Green Version]
- Rezaei, J. Best-worst multi-criteria decision-making method: Some properties and a linear model. Omega 2016, 16, 126–130. [Google Scholar] [CrossRef]
- Verplaetse, T.L.; Roberts, W.; Moore, K.E.; Peltier, M.R.; Oberleitner, L.M.; McKee, S.A. Pharmacokinetics and Pharmacodynamics of Immediate-Release Versus Extended-Release Guanfacine in Adult Daily Smokers. J. Clin. Psychopharmacol. 2019, 39, 124–128. [Google Scholar] [CrossRef]
- Iwanami, A.; Saito, K.; Fujiwara, M.; Okotsu, D.; Ichikawa, H. Efficacy and Safety of Guanfacine Extended-Release in the Treatment of Attention-Deficit/Hyperactivity Disorder in Adults: Results of a Randomized, Double-Blind, Placebo-Controlled Study. J. Clin. Psychiatr. 2020, 81, 1–9. [Google Scholar] [CrossRef]
- Okazaki, K.; Yamamuro, K.; Iida, J.; Kishimoto, T. Guanfacine monotherapy for ADHD/ASD comorbid with Tourette syndrome: A case report. Ann. Gen. Psychiatr. 2019, 150, 59–69. [Google Scholar] [CrossRef]
- Okada, M.; Fukuyama, K.; Kawano, Y.; Shiroyama, T.; Suzuki, D.; Ueda, Y. Effects of acute and sub-chronic administrations of guanfacine on catecholaminergic transmissions in the orbitofrontal cortex. Neuropharmacology 2019, 156, 107547. [Google Scholar] [CrossRef]
- Fitzpatrick, C.M.; Andreasen, J.T. Differential effects of ADHD medications on impulsive action in the mouse 5-choice serial reaction time task. Eur. J. Pharmacol. 2019, 847, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Cortese, S.; Adamo, N.; Del Giovane, C.; Mohr-Jensen, C.; Hayes, A.J.; Carucci, S.; Atkinson, L.Z.; Tessari, L.; Banaschewski, T.; Coghill, D.; et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: A systematic review and network meta-analysis. Lancet Psychiatry 2018, 5, 727–738. [Google Scholar] [CrossRef]
- Bastiaens, L.; Scott, O.; Galus, J. Treatment of Adult ADHD without Stimulants: Effectiveness in A Dually Diagnosed Correctional Population. Psychiatr. Q. 2019, 90, 41–46. [Google Scholar] [CrossRef]
- Pievsky, M.A.; McGrath, R.E. The Neurocognitive Profile of Attention-Deficit/Hyperactivity Disorder: A Review of Meta-Analyses. Arch. Clin. Neuropsychol. 2018, 33, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.R.; Araghi, S.M.; Zarafshan, H. Neurocognitive Profile of Children with Attention Deficit Hyperactivity Disorders (ADHD): A comparison between subtypes. Iran. J. Psychiatry 2014, 9, 197–202. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25792987 (accessed on 1 November 2021).
- Barkley, R.A. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychol. Bull. 1997, 121, 65–94. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, F.X.; Tannock, R. Neuroscience of attention-deficit/hyperactivity disorder: The search for endophenotypes. Nat. Rev. Neurosc. 2002, 3, 617–628. [Google Scholar] [CrossRef]
- Diamond, A. Attention-deficit disorder (attention-deficit/ hyperactivity disorder without hyperactivity): A neurobiologically and behaviorally distinct disorder from attention-deficit/hyperactivity disorder (with hyperactivity). Dev. Psychopathol. 2005, 17, 807–825. [Google Scholar] [CrossRef]
- Biederman, J.; Newcorn, J.; Sprich, S. Comorbidity of attention deficit hyperactivity disorder with conduct, depressive, anxiety, and other disorders. Am. J. Psychiatry 1991, 148, 564–577. [Google Scholar] [CrossRef]
- Daley, D.; Birchwood, J. ADHD and academic performance: Why does ADHD impact on academic performance and what can be done to support ADHD children in the classroom? Child Care Health. Dev. 2010, 36, 455–464. [Google Scholar] [CrossRef]
- Shuai, L.; Daley, D.; Wang, Y.F.; Zhang, J.S.; Kong, Y.T.; Tan, X.; Ji, N. Executive function training for children with attention deficit hyperactivity disorder. Chin. Med. J. 2017, 130, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Bahçivan Saydam, R.; Ayvaşik, H.B.; Alyanak, B. Executive Functioning in Subtypes of Attention Deficit Hyperactivity Disorder. Noro Psikiyatr. Ars. 2015, 52, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Cortese, S.; Ferrin, M.; Brandeis, D.; Buitelaar, J.; Daley, D.; Dittmann, R.W.; Holtmann, M.; Santosh, P.; Stevenson, J.; Stringaris, A.; et al. Cognitive training for attention-deficit/hyperactivity disorder: Meta-analysis of clinical and neuropsychological outcomes from randomized controlled trials. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 164–174. [Google Scholar] [CrossRef]
- Chan, E.; Fogler, J.M.; Hammerness, P.G. Treatment of Attention-Deficit/Hyperactivity Disorder in Adolescents. Jama 2016, 315, 1997. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, G.A.; Schatz, N.K.; Aloe, A.M.; Chacko, A.; Chronis-Tuscano, A. A Systematic Review of Meta-Analyses of Psychosocial Treatment for Attention-Deficit/Hyperactivity Disorder. Clin. Child. Fam. Psychol. Rev. 2015, 18, 77–97. [Google Scholar] [CrossRef] [PubMed]
- Bikic, A.; Reichow, B.; McCauley, S.A.; Ibrahim, K.; Sukhodolsky, D.G. Meta-analysis of organizational skills interventions for children and adolescents with Attention-Deficit/Hyperactivity Disorder. Clin. Psychol. Rev. 2017, 52, 108–123. [Google Scholar] [CrossRef]
- Serrano-Troncoso, E.; Guidi, M.; Alda-Díez, J.Á. ¿Es el tratamiento psicológico eficaz para el trastorno por déficit de atención con hiperactividad (TDAH)? Revisión sobre los tratamientos no farmacológicos en niños y adolescentes con TDAH. (Spanish). Actas Esp. Psiquiatry 2013, 41, 44–51. Available online: http://search.ebscohost.com/login.aspx?direct=true&db=fua&AN=87688096&lang=es&site=ehost-live (accessed on 1 November 2021).
- Sibley, M.H.; Kuriyan, A.B.; Evans, S.W.; Waxmonsky, J.G.; Smith, B.H. Pharmacological and psychosocial treatments for adolescents with ADHD: An updated systematic review of the literature. Clin. Psychol. Rev. 2014, 34, 218–232. [Google Scholar] [CrossRef]
- Burke, J.D.; Rolf, L.; Birmaher, B. Oppositional Defiant Disorder and Conduct Disorder: A Review of the Past 10 Years, Part II. J. Am. Acad. Child Adolesc. Psychiatry 2002, 41, 1275–1293. [Google Scholar] [CrossRef]
- Connor, D.F.; Glatt, S.J.; Lopez, I.D.; Jackson, D.; Melloni, R.H. Psychopharmacology and Aggression. I: A Meta-Analysis of Stimulant Effects on Overt/Covert Aggression–Related Behaviors in ADHD. J. Am. Acad. Child Adolesc. Psychiatry 2002, 41, 253–261. [Google Scholar] [CrossRef]
- Pelham, W.E.; Fabiano, G.A. Evidence-Based Psychosocial Treatments for Attention-Deficit/Hyperactivity Disorder. J. Clin. Child Adolesc. Psychol. 2008, 37, 184–214. [Google Scholar] [CrossRef]
- Evans, S.W.; Owens, J.S.; Bunford, N. Evidence-Based Psychosocial Treatments for Children and Adolescents Evidence-Based Psychosocial Treatments for Children and Adolescents with Disruptive Behavior. J. Clin. Child Adolesc. Psychol. 2014, 43, 527–551. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Inada, N.; Tanigawa, Y.; Yamashita, M.; Maeda, E.; Kouguchi, M.; Sarad, Y.; Yano, H.; Ikari, K.; Kuga, H.; et al. Efficacy of Group Cognitive Behavior Therapy Targeting Time Management for Adults with Attention Deficit/Hyperactivity Disorder in Japan: A Randomized Control Pilot Trial. J. Atten. Disord. 2021, 26, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Estrada, R.; Bosch-Munso, R.; Nogueira-Morais, M.; Casas-Brugue, M.; Ramos-Quiroga, J.A. Psychological treatment of attention deficit hyperactivity disorder in adults: A systematic review. Actas Españolas De Psiquiatr. 2012, 40, 147–154. Available online: http://content.ebscohost.com.paloaltou.idm.oclc.org/ContentServer.asp?T=P&P=AN&K=22723133&S=R&D=mnh&EbscoContent=dGJyMNHX8kSep7E4zdnyOLCmr0qepq9Ssaq4SLaWxWXS&ContentCustomer=dGJyMPGut1CzrLZRuePfgeyx44Dt6fIA%5Cnhttps://paloaltou.idm.oclc.org/login?url=http (accessed on 5 November 2021).
- Faraone, S.V.; Biederman, J.; Weber, W.; Russell, R.L. Psychiatric, Neuropsychological, and Psychosocial Features of DSM-IV Subtypes of Attention-Deficit/Hyperactivity Disorder: Results from a Clinically Referred Sample. J. Am. Acad. Child Adolesc. Psychiatry 1998, 37, 185–193. [Google Scholar] [CrossRef]
- Mayes, S.D.; Calhoun, S.L.; Chase, G.A.; Mink, D.M.; Stagg, R.E. ADHD Subtypes and Co-Occurring Anxiety, Depression, and Oppositional-Defiant Disorder. Differences in Gordon Diagnostic System and Wechsler Working Memory and Processing Speed Index Scores. J. Atten. Disord. 2009, 12, 540–550. [Google Scholar] [CrossRef]
- Milich, R. ADHD Combined Type and ADHD Predominantly Inattentive Type Are Distinct and Unrelated Disorders. Clin. Psychl. Sci. Pract. 2001, 8, 463–488. [Google Scholar] [CrossRef]
- Power, T.J.; Costigan, T.E.; Eiraldi, R.B.; Leff, S.S. Variations in Anxiety and Depression as a Function of ADHD Subtypes Defined by DSM-IV: Do Subtype Differences Exist or Not? J. Abnorm. Child Psychol. 2004, 32, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Presentación, M.J.; Siegenthaler, R. Problemática asociada al TDAH subtipo combinado en una muestra escolar. Infanc. Aprendiz. 2005, 28, 261–275. [Google Scholar] [CrossRef]
- van den Hoofdakker, B.J.; van der Veen-Mulders, L.; Sytema, S.; Emmelkamp, P.M.G.; Minderaa, R.B.; Nauta, M.H. Effectiveness of behavioral parent training for children with ADHD in routine clinical practice: A randomized controlled study. J. Am. Acad. Child. Adolesc. Psychiatry 2007, 46, 1263–1271. [Google Scholar] [CrossRef]
- Jensen, C.M.; Amdisen, B.L.; Jørgensen, K.J.; Arnfred, S.M. Cognitive behavioural therapy for ADHD in adults: Systematic review and meta-analyses. ADHD Atten. Deficit Hyperact. Disord. 2016, 8, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Fullen, T.; Galab, N.; Abbott, K.A.; Adamou, M. Acceptance and Commitment Therapy for Adults with ADHD during COVID-19: An Open Trial. Open J. Psychiatry 2020, 10, 205. [Google Scholar] [CrossRef]
- Gadow, K.D.; Drabick, D.A.G.; Loney, J.; Sprafkin, J.; Salisbury, H.; Azizian, A.; Schwartz, J. Comparison of ADHD symptom subtypes as source-specific syndromes. J. Child Psychol. Psychiatry 2004, 45, 1135–1149. [Google Scholar] [CrossRef] [PubMed]
- Nolan, E.E.; Volpe, R.J.; Gadow, K.D.; Sprafkin, J. Developmental, Gender, and Comorbidity Differences in Clinically Referred Children with ADHD. J. Emot. Behav. Disord. 1999, 7, 11–20. [Google Scholar] [CrossRef]
- Díaz Atienza, J. Comorbilidad en el tdah. Rev. Psiquiatr. Psicol. Niño Adolesc. 2006, 6, 44–55. Available online: http://fundacioncadah.org/j289eghfd7511986_uploads/20120606_7mZG5IP3fsJy0YhrYekf_0.pdf (accessed on 5 November 2021).
- Deault, L.C. A Systematic Review of Parenting in Relation to the Development of Comorbidities and Functional Impairments in Children with Attention-Deficit/Hyperactivity Disorder (ADHD). Child Psychiatry Hum. Dev. 2010, 41, 168–192. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).