Abstract
Herein, SnS and Eu-doped SnS QDs have been synthesized by a facile chemical co-precipitation method for efficient photocatalytic degradation of organic dye molecules. The structural, morphological, and optical properties of QDs were investigated by various physiochemical characterization techniques. The photocatalytic degradation of methylene blue (MB) and crystal violet (CV) dyes have been studied under visible light irradiation under direct sunlight using a spectrophotometer. Enhanced photodegradation efficiency of 87% and 94% were attained for SnS and Eu (4%)-doped SnS, respectively. For CV dye, the pure SnS showed only 70.7% however the Eu (4%)-doped SnS achieved 99% efficiency. The rate constant value of the doped SnS was found to be much higher than that of pure SnS for both dyes. The obtained results from various characterization studies provided the reason for the enhancement of the photocatalytic activity of Eu-doped SnS QDs due to the presence of Eu3+ in the SnS lattice, and also smaller crystallite size with high surface area and its morphological features. Moreover, the Eu3+ plays an essential role in reducing the band gap, hampering recombination, and the generation of free radicals, thus the QDs promoted attractive degradation activity and high stability.
1. Introduction
All over the globe, environmental pollutants have been considered one of the major risks that cause adverse effects on human health. Recently, surface water contamination and water evaporation, as well as the shortage of drinking water, are being considered significant problems for the world. The main reason for water contamination is several organic pollutants from the pharmacological, chemical, and textile industries, which carelessly dispose of their waste into the environment and water bodies. These organic toxic effluents cause severe damage to the environment and aquatic species [1]. The various dyes and poisonous organic contaminants in aqueous environments have worsened the universal water source’s condition. Further, the rapid development of the textile industries intensifies the concerns of water pollution due to the perseverance and toxicity of these dyes to the human and aquatic species [2]. As compared to primary effluent treatment methods, which are less efficient and also produce secondary pollutants, semiconductor photocatalysis is very efficient, resulting in the complete degradation of organic pollutants without generating secondary pollution.
Dyes are widely used as coloring agents in textiles, fleece, food, plastics, cosmetics, and other industries [3]. The dyes are complex, water-soluble, degradation-resistant, potentially carcinogenic, and mutagenic organic molecules with limited biodegradability. In general, dyes can be categorized into natural and synthetic/artificial dyes. Natural dyes originate from plant sources, including leaves, wood, roots, berries, bark, fungi, and lichens, whereas synthetic dyes are synthesized via chemicals, minerals, and derivatives of petroleum [4,5], which is used as a colorant and printing actions for papers, textiles, and cosmetic industries [6,7,8]. Synthetic dyes are organic compounds characterized by the existence of chromophores in their molecular structures. They are commonly classified according to their chromophore groups into various classes including azo, anthraquinone, indigoide, phthalocyanine, sulfur, and triphenylmethane derivatives [9,10,11]. Organic dyes such as azo and fluorescein dyes are highly cytotoxic for mammalian tissues and rather difficult to naturally decompose. Dyes are also classified into two basic types, based on the change they possess, which are non-ionic and ionic dyes. Ionic dyes consist of cationic and anionic dyes, which carry positive and negative charges, respectively [12]. Organic dyes have caused severe environmental pollution problems due to their poor biodegradation ability. Hence, the removal of dye molecules from industrial effluent is highly significant for environmental protection [9]. At present, various technologies exist to treat dye industrial wastewater that include carbon adsorption, flocculation, ozonization, and activated sludge processes, but these processes are expensive, and in addition, the degradation kinetics are found to be slow and incomplete [13,14]. A promising method to degrade dye pollutants is advanced oxidation processes (AOPs) as they produce reactive oxygen species (ROS), Hydroxyl (OH), and superoxide (O2−) radicals by using photocatalytic reactions. Photocatalysis is an environmentally friendly and green method, which offers great potential for the total abolition of toxic pollutants in the environment through its effectiveness and broad applicability [15] and has several advantages such as (i) degrading contaminants without the use of secondary chemicals, (ii) operating under ambient conditions, and (iii) mineralizing toxic organic pollutants into non-toxic in-organics such as carbon dioxide and water [16].
Sulfide-based catalysts are extensively used for the decolorization of textile dyes. Tin sulfide is a narrow-bandgap IV–VI group semiconductor. SnS has interesting properties and potential applications, such as photovoltaic [17], photocatalytic [18,19], field emission [20], lithium-ion batteries [21], photodetectors [22,23], and gas sensing [24,25] applications. It is an eco-friendly substance with a band gap of approximately 1.3 eV, with promising optical and electrical features together with a large conversion efficiency of the photoelectric and a large absorption coefficient. Besides, SnS nanomaterial has advantages such as cost-effectiveness and a tunable band gap in the visible region, which can be achieved by the size effect based on the synthesis process and factors such as crystallinity and chemical composition, etc. [18]. In the synthesis process, the phase of SnS can be shared with other materials including SnS2, Sn2S3, and Sn3S4. The most stable phases are SnS and SnS2 with band gaps of approximately 1.3 eV and 2.3 eV, respectively [26]. To further improve the properties of SnS, the strategy of doping rare earth metals is found to be an effective way to tune the bandgap for enhanced photocatalytic reactions [15,27], To prevent the recombination of photo-induced electron-holes and the reduction of the band gap, and shift the absorption band to the visible region in photocatalysis [28]. Past years, researchers reported that rare earth metals (such as Eu, Sm, La, and Nd) can be used as a dopant for enhancing the catalytic properties [29,30,31,32,33,34]. Quantum dots (QDs) have been attracting widespread interest as zero-dimensional nanostructures because of their low harm, outstanding chemical stability, excellent water solubility, noteworthy electrical conductivity, efficient degradation and photostability, and simple functionalization. Particularly, semiconductor quantum dots have favorable optical characteristics so as to be used as a photocatalyst, fluorescent probes in the field of medicine, biosensors, and bioimaging. Furthermore, QDs possess extraordinary catalytic properties owing to their quantum confinement effects, small size, high surface area, efficient charge transportation, etc. In this work, highly uniform Eu-doped SnS quantum dots were synthesized. To the best of our knowledge, Eu-doped SnS quantum dots (QDs) and their photocatalytic behavior have not been reported before. In this manuscript, Eu-doped SnS QDs are prepared by a simple co-precipitation method. The addition of the Eu dopant modifies the properties of SnS QDs. The prepared pure SnS and Eu-doped SnS QDs were used for the degradation of the organic dyes MB and CV under the irradiation of visible light.
2. Results and Discussion
2.1. Structural Analysis
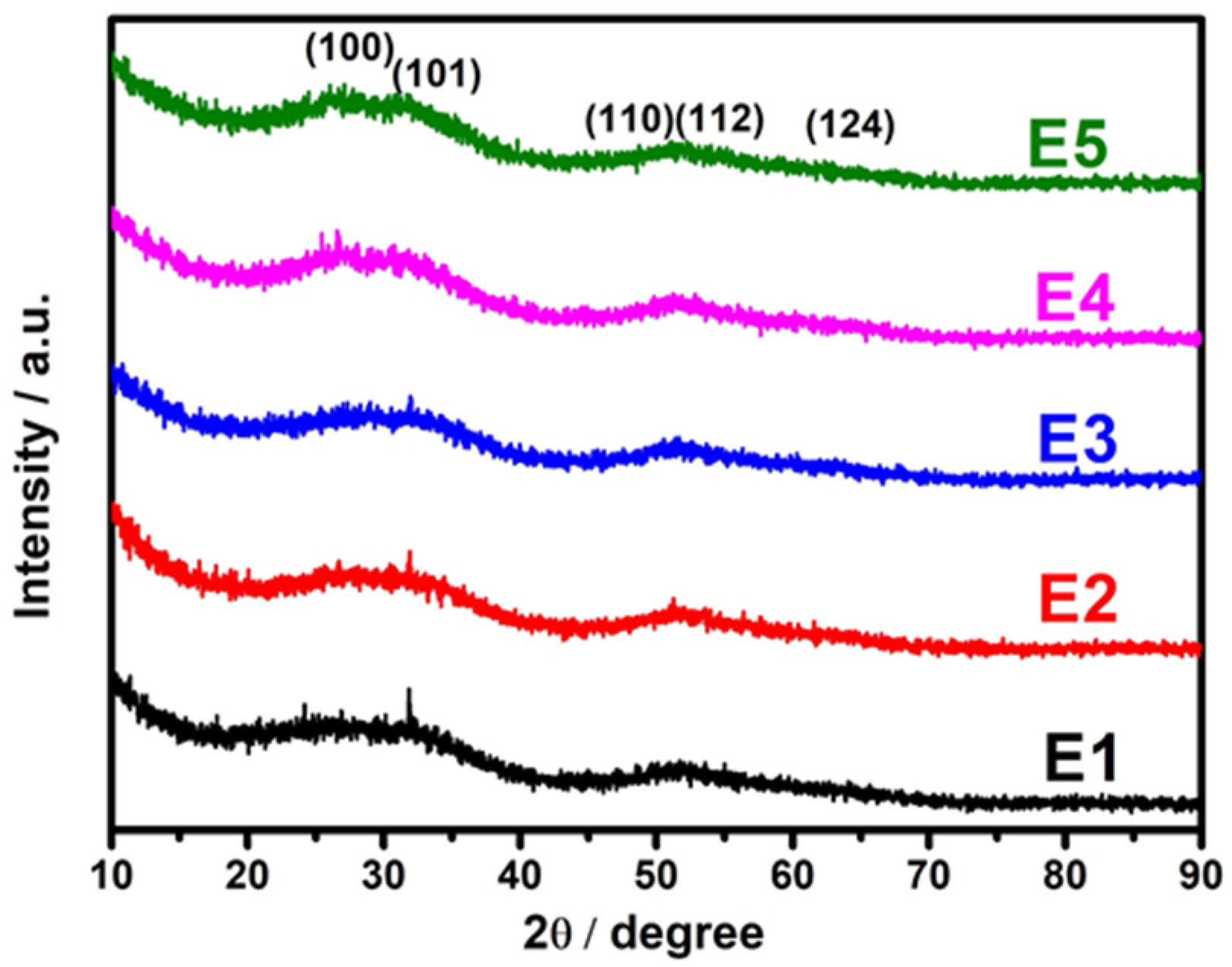
The SnS has two structural phases, namely zinc blende and orthorhombic structures [35]. A weak indirect band gap at ~1.1 eV [36] and a strong direct gap at ~1.43 eV [37] were observed for orthorhombic SnS, and the zinc blende SnS had a bandgap at ~1.2 eV [33]. Figure 1 represents the XRD patterns of pristine SnS and different concentrations of Eu-doped SnS QDs. The diffraction peaks for pristine SnS (2θ) appeared at 27.2°, 31.1°, 51.4°, 54.1°, and 66° corresponding to the planes of (0 2 1), (1 1 1), (1 5 1), (2 3 1), and (1 5 2), which confirms the orthorhombic structure of SnS (JCPDS Card no. 39-0354). Among the diffraction peaks, the peak at 2θ = 31.1° corresponding to the (1 1 1) plane seems to be the dominant one. For Eu-doped samples, there were no analogous diffraction peaks that appeared, which confirms that the Eu ions are well-incorporated into the SnS lattice. As the doping concentration increases, the full width half maximum (FWHM) of the diffraction peaks becomes broader due to the quantum size effect. It is quite common for dopants to act as nucleation sites in nanoparticle syntheses, reducing the energy required for particles to start forming, resulting in a greater number of smaller particles growing. It is mainly due to the dopant atoms exerting a drag force against the crystallite size growth. It eventually leads to a smaller crystallite size. The average grain size of the samples is determined by the Scherrer formula [38].
where D is the average crystallite size, λ is the wavelength of the X-ray, and β and θ are the full width at half maximum and angle of diffraction, respectively. The calculated average grain size of pristine SnS and different concentrations of Eu-doped SnS QDs were measured, expected to be in the 3–5 nm range.
D = 0.9λ/βcosθ
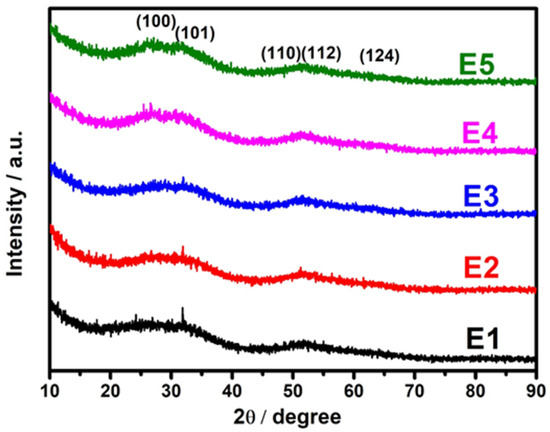
Figure 1.
X-ray diffraction spectra of SnS and Eu-doped SnS QDs.
2.2. Morphological Analysis
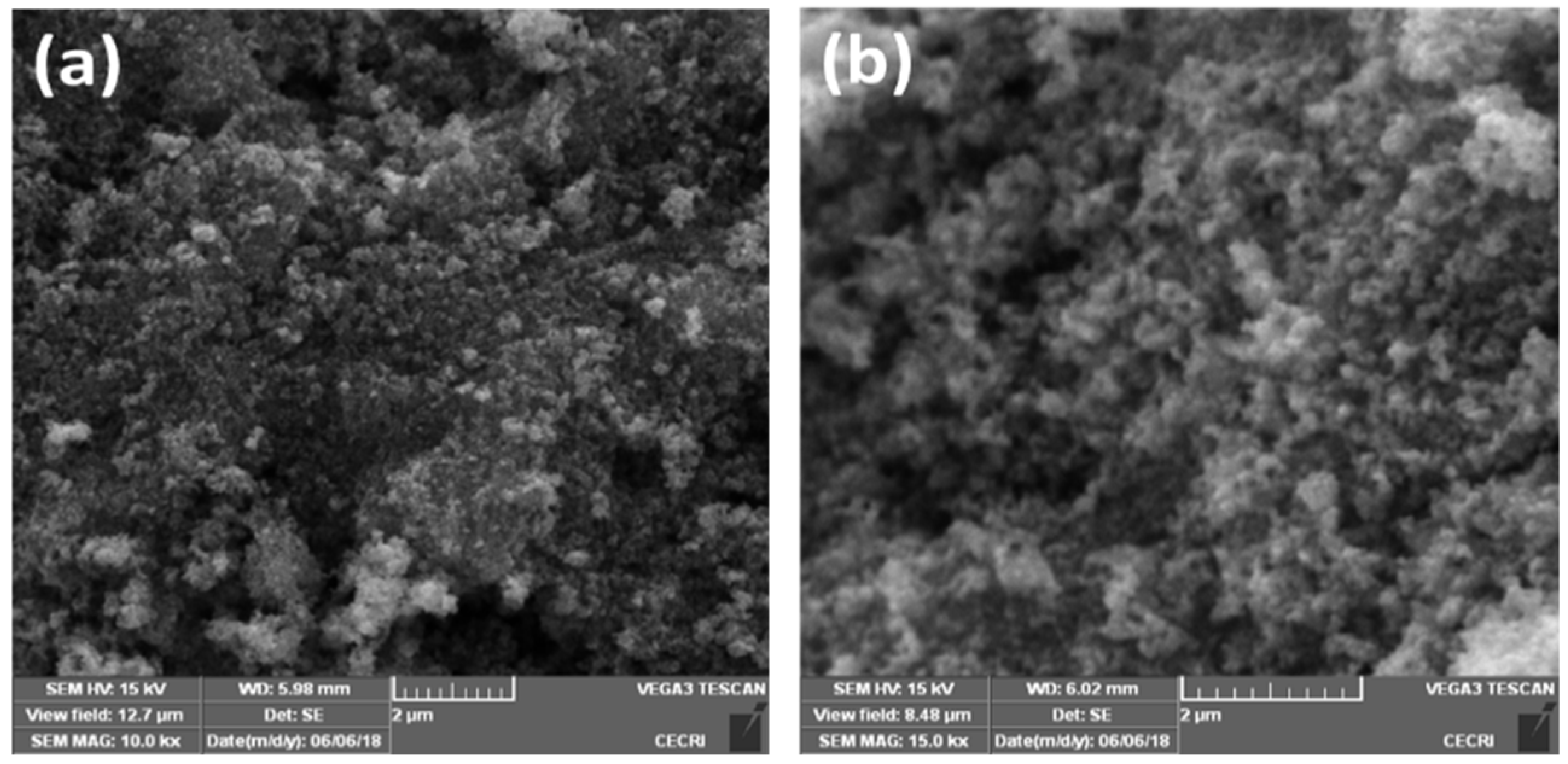
Microstructure and surface morphology play very significant roles in different optoelectronic applications. The size and morphology of the as-prepared Eu-doped SnS QDs were characterized by FESEM as shown in Figure 2. FESEM images with different magnifications of Eu-doped SnS contain very small grains with uniform size and morphology. The sample seems more aggregated, which could be attributed to the high surface energy due to the smaller size of the Eu-doped SnS QDs.
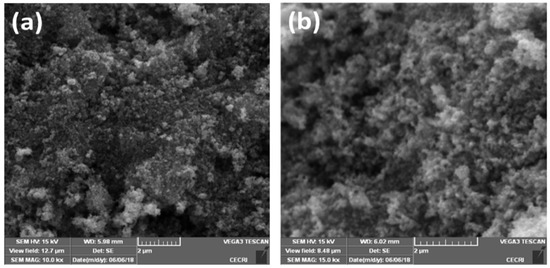
Figure 2.
(a,b) FESEM images of Eu-doped SnS QDs.
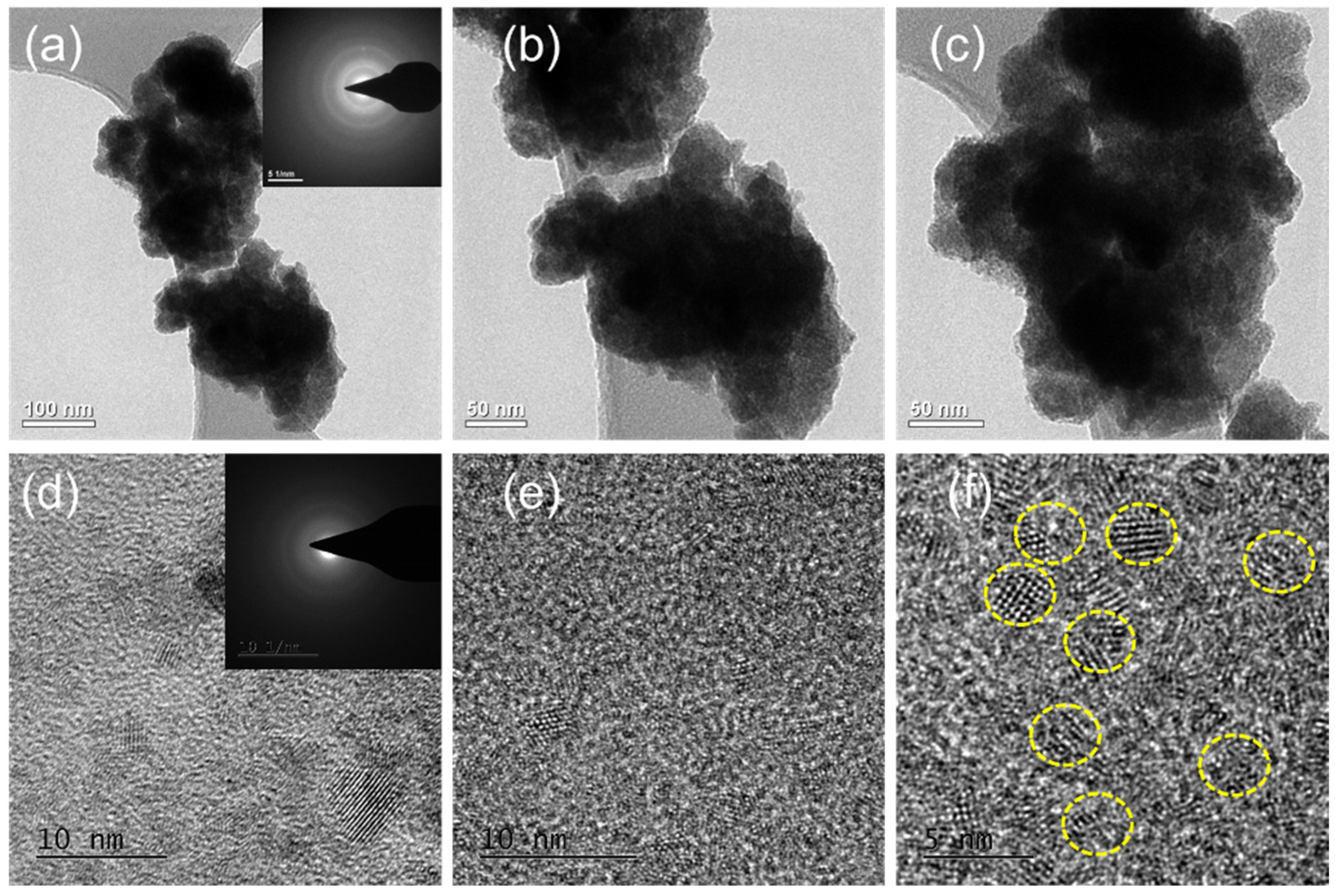
The morphology of pure SnS and Eu-doped SnS QDs was further characterized by TEM analysis with different magnifications. The TEM images shown in Figure 3 reveal the size of pure SnS and Eu-doped SnS QDs in the nanoscale range, which is in good agreement with the XRD and FESEM results. Figure 3a–c show TEM images of the pure SnS with different magnifications. It exhibits a similar morphology to that obtained in FESEM with more aggregates. The insert image in Figure 3c shows the selected area electron diffraction (SAED) pattern from a region of the SnS nanoparticles. Phase identification was performed from scaled SAED images by calculating the lattice spacing and then comparing it with standard JCPDS values (39-0354). The SAED pattern of nanoparticles exhibits diffraction spots that are symmetrically distributed, demonstrating the crystal structure of SnS nanoparticles, in which major three diffraction spots are indexed to the (0 2 1), (1 1 1), and (1 5 1) planes of SnS. Eu-doped SnS is shown in Figure 3d–f with different magnifications. The TEM results clearly showed the obtained samples are of quantum size with a high degree of homogeneity. The Eu-doped SnS QDs play a crucial role in accelerating the separation of the photogenerated charge carriers.
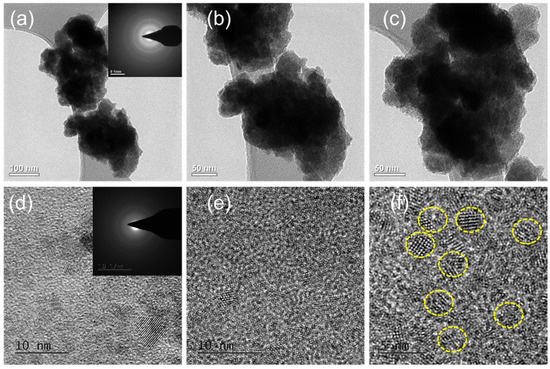
Figure 3.
(a–d) TEM images of SnS and (e,f) Eu-doped SnS QDs with different magnifications.
2.3. Functional Group Analysis
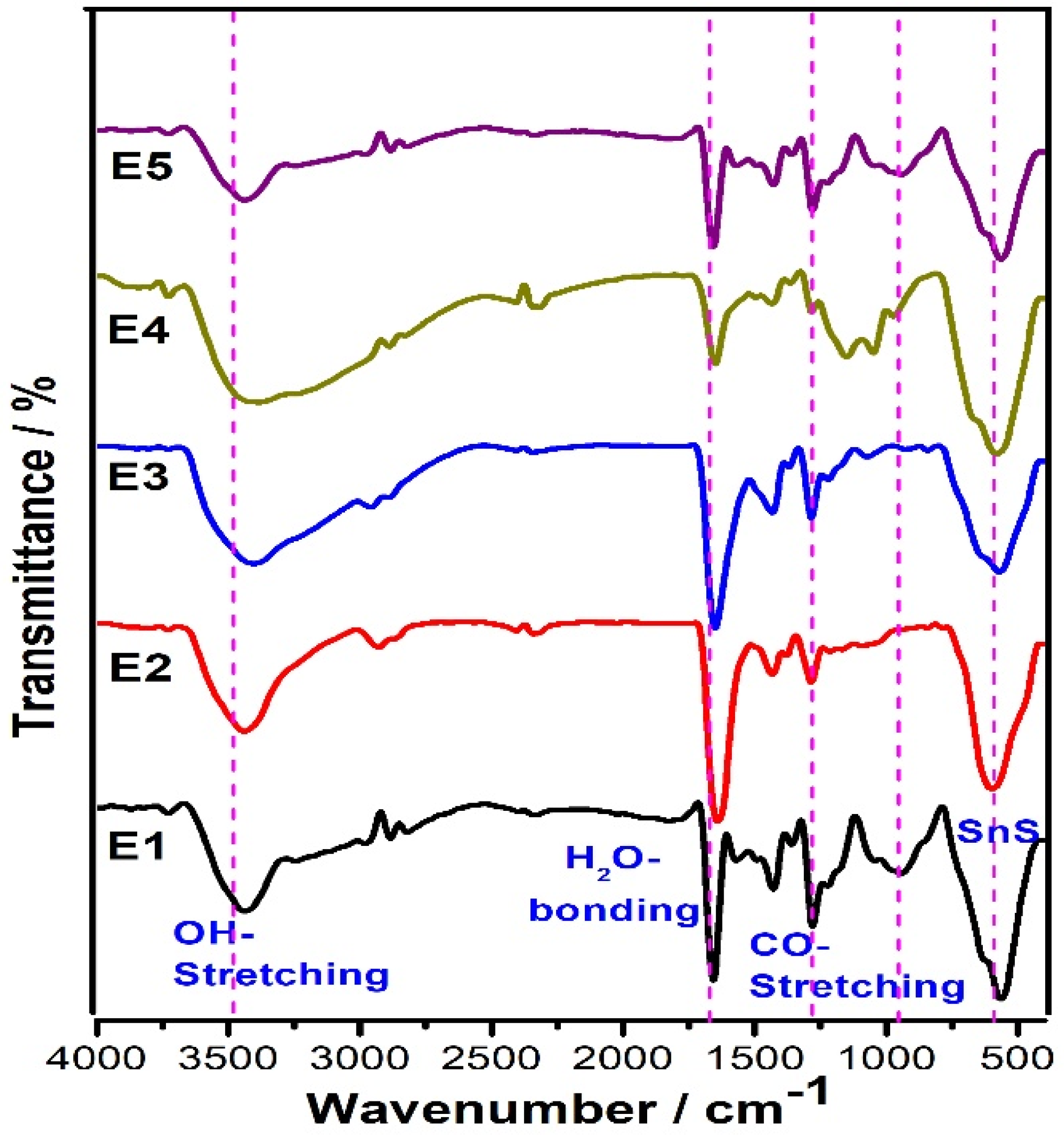
FT-IR analysis is a widely used technique for determining the functional groups or the types of chemical bonds in inorganic compounds. As can be seen from Figure 4, the pure and doped SnS samples have similar peaks. The obtained results evidently show the broad absorption peak at 3400 cm−1 is related to the fundamental vibration of –OH stretching. Furthermore, a weak band (deformation mode) of OH groups was confirmed by the peak at 1658 cm−1. The samples also show a peak forming at approximately 565 cm−1, which can be ascribed to SnS bonding.
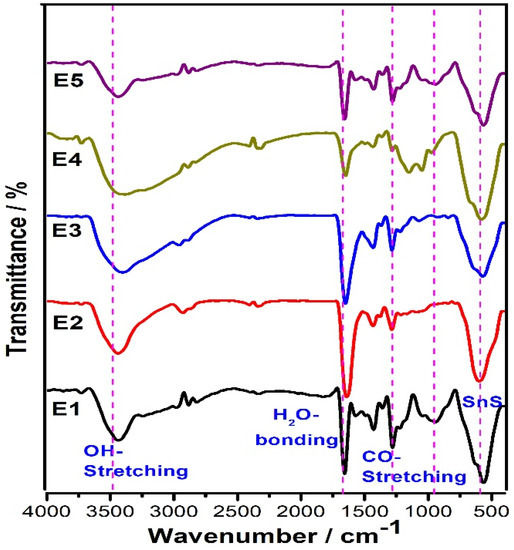
Figure 4.
FT-IR Spectra of SnS and Eu-doped SnS QDs.
2.4. Optical Analysis
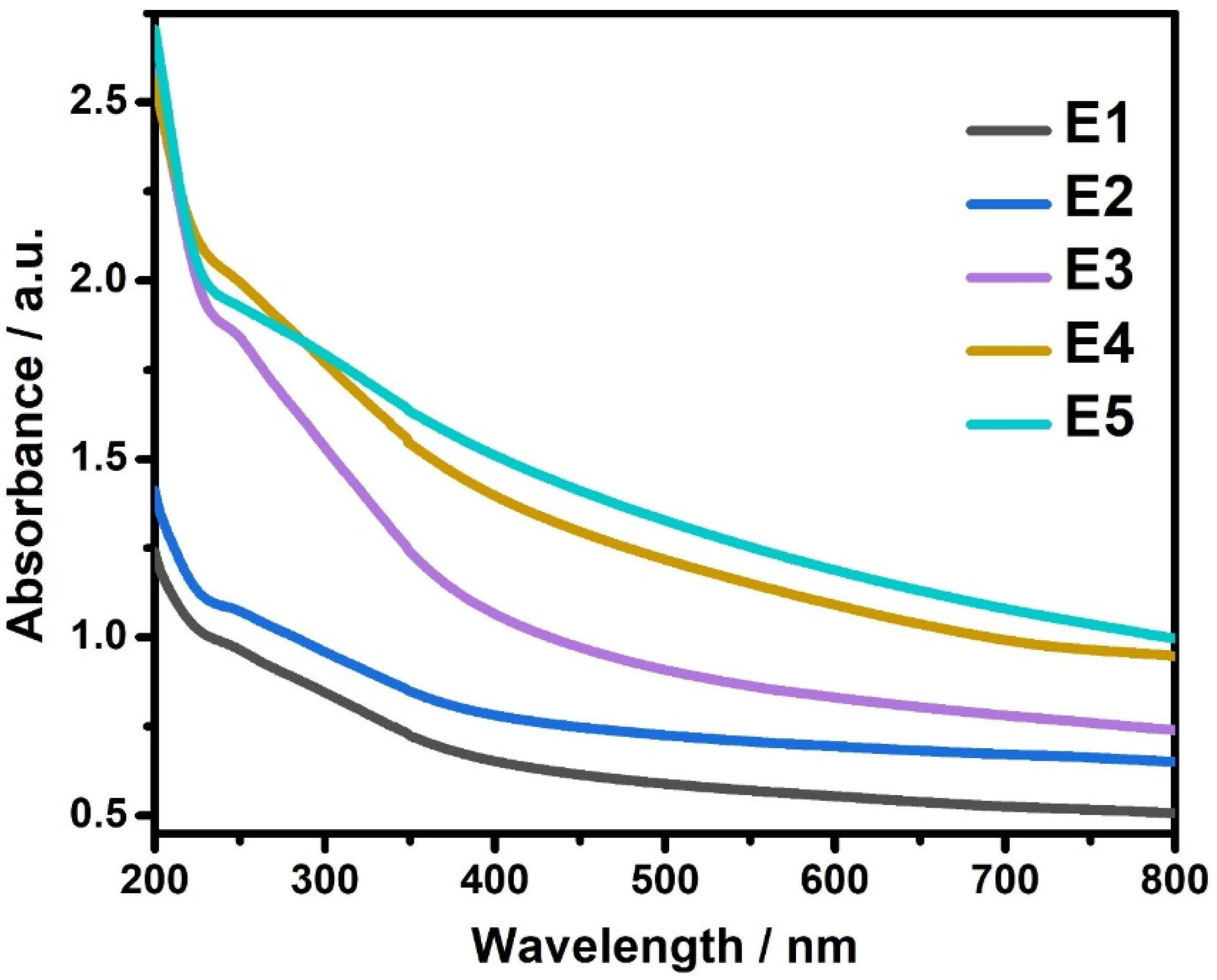
Optical absorption measurements were carried out on pure SnS and Eu-doped SnS nanoparticles. The UV–Visible spectra for pure SnS and Eu-doped SnS QDs are shown in Figure 5. SnS QDs have higher absorption in the UV region, whereas doping with Eu attains a stronger absorption intensity in both ultraviolet and visible regions. By increasing the dopant concentration, the absorption spectrum slightly shifts towards the visible region. This is due to an increased surface-to-volume ratio that promotes more active sites on Eu-doped SnS QDs. The absorption shifting to the visible region could also produce more radicals, which enhances photocatalytic activity for degrading organic pollutants. From the absorption values, the optical band gap is determined using a Tauc plot [35,36]. The obtained band gaps were 3.12, 3.03, 2.89, 2.81, and 2.72 eV for E1, E2, E3, E4, and E5 samples, respectively.
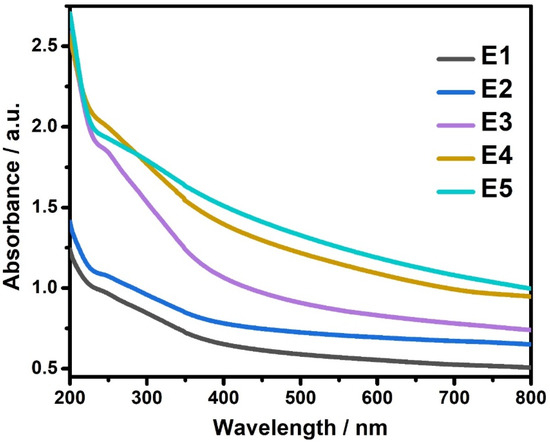
Figure 5.
UV-Vis Spectra of SnS and Eu-doped SnS QDs.
2.5. Photocatalytic Studies of SnS and Eu Doped SnS QDs
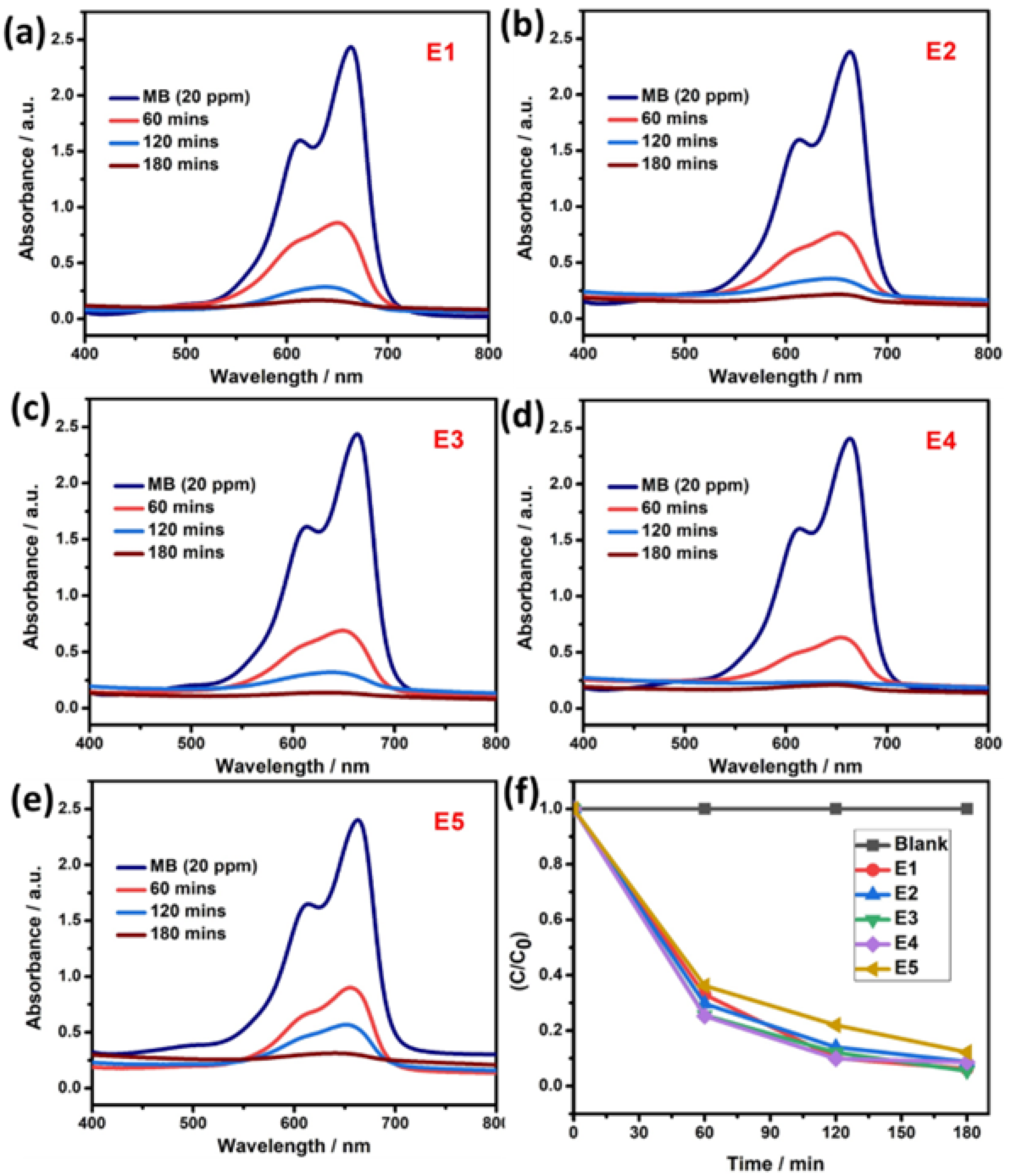
The photocatalytic activity of as-synthesized pure SnS and Eu-doped SnS samples were studied with the degradation of MB and CV as model pollutants by the irradiation of sunlight. In general, photocatalytic activity depends on significant factors such as the crystal structure, size and morphology of catalysts, and dopant concentration. Figure 6a–e represent the UV-visible spectra for the degradation of MB dye with the presence of pure SnS and Eu-doped SnS catalysts, which were sunlight-irradiated for 180 min at a constant time interval. The degradation study was carried out with catalysts such as pure SnS and Eu (1, 3, 5, and 10%)-doped SnS represented as E1, E2, E3, E4, and E5. The degradation efficiency was calculated using Equation (3). For pure SnS samples, (E1) achieved an efficiency of approximately 87% against MB dye under the irradiation of visible light for 180 min. For Eu-doped SnS catalysts (E2, E3, E4, and E5), the degradation was conducted by the same process, but the degradation rate was dramatically increased with the increase in the dopant concentration up to 5% (E4). The efficiency of the rest of the catalysts decreased, such as 93% for the E5 catalyst. The maximum efficiency obtained by catalyst (E4) is ~100% due to the energy level of the dopant, which acts as a trap that slows down the photogenerated carrier recombination. Thus, the photogenerated electrons were separated and traveled to the conduction band where they migrated with the energy levels of the dopant and reduction takes place on the surface of the catalyst, which forms the superoxide radical and then promotes hydroxyl radicals under the irradiation of visible light. For a better perspective, the efficiency of photocatalysts is represented in Figure 6f as E1 < E2 < E3 < E4 > E5. The sample of E4 has a higher efficiency compared to other samples. The degradation efficiency decreased for the higher concentration of dopant (E5) due to the surface sites of the catalyst, which are covered with the dye molecules. Table 1 represents the band gap and degradation efficiency of the catalyst.
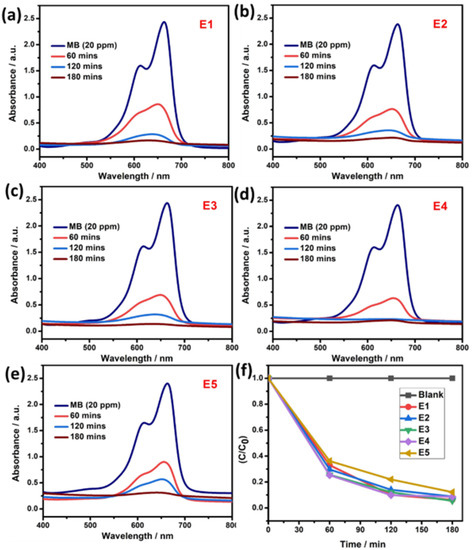
Figure 6.
(a–e) Photocatalytic activity of pure SnS and Eu-doped SnS QDs via degradation of MB dye. (f) Degradation efficiency.

Table 1.
Photocatalytic activity results and band gap of pure and Eu-doped SnS QDs.
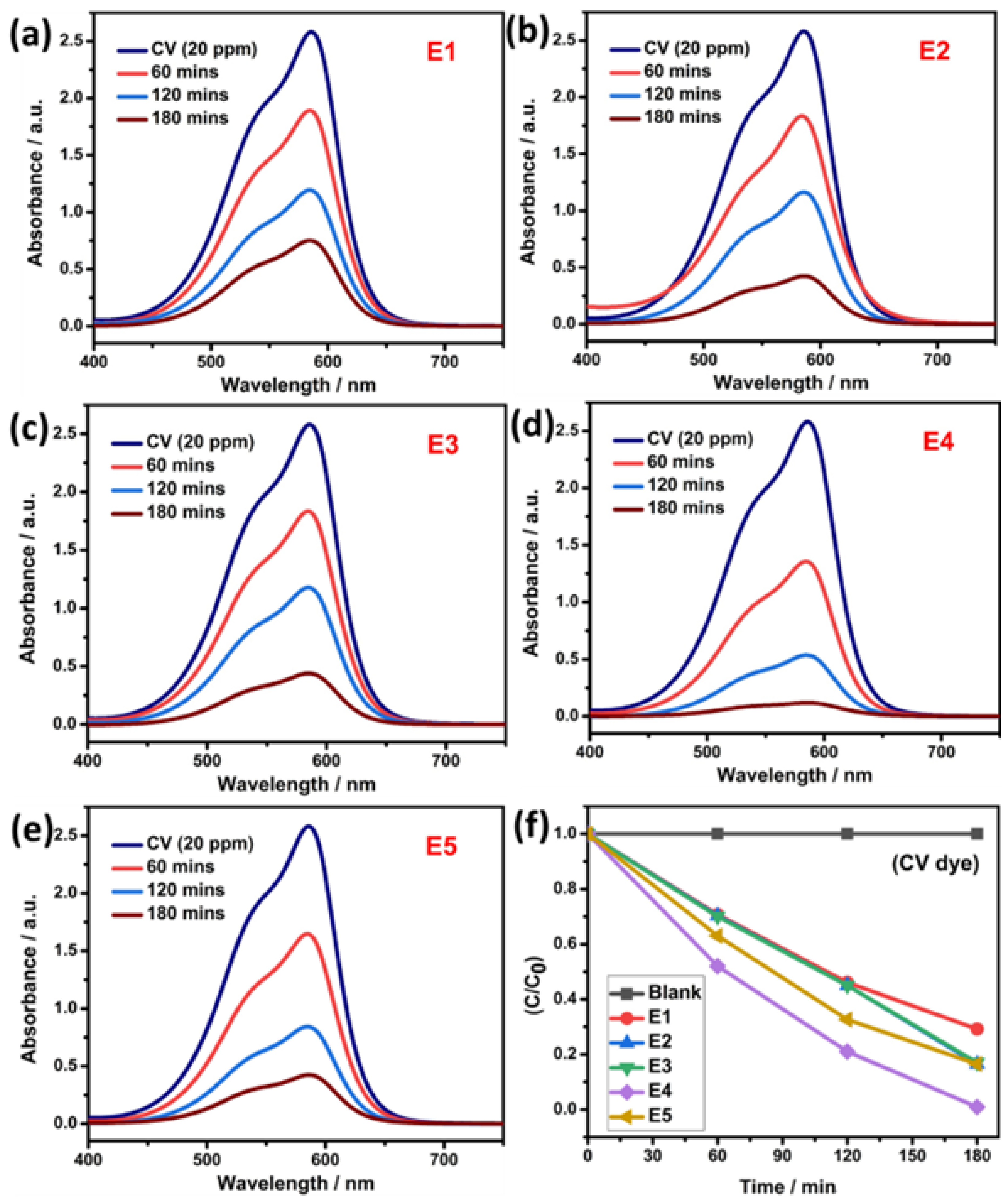
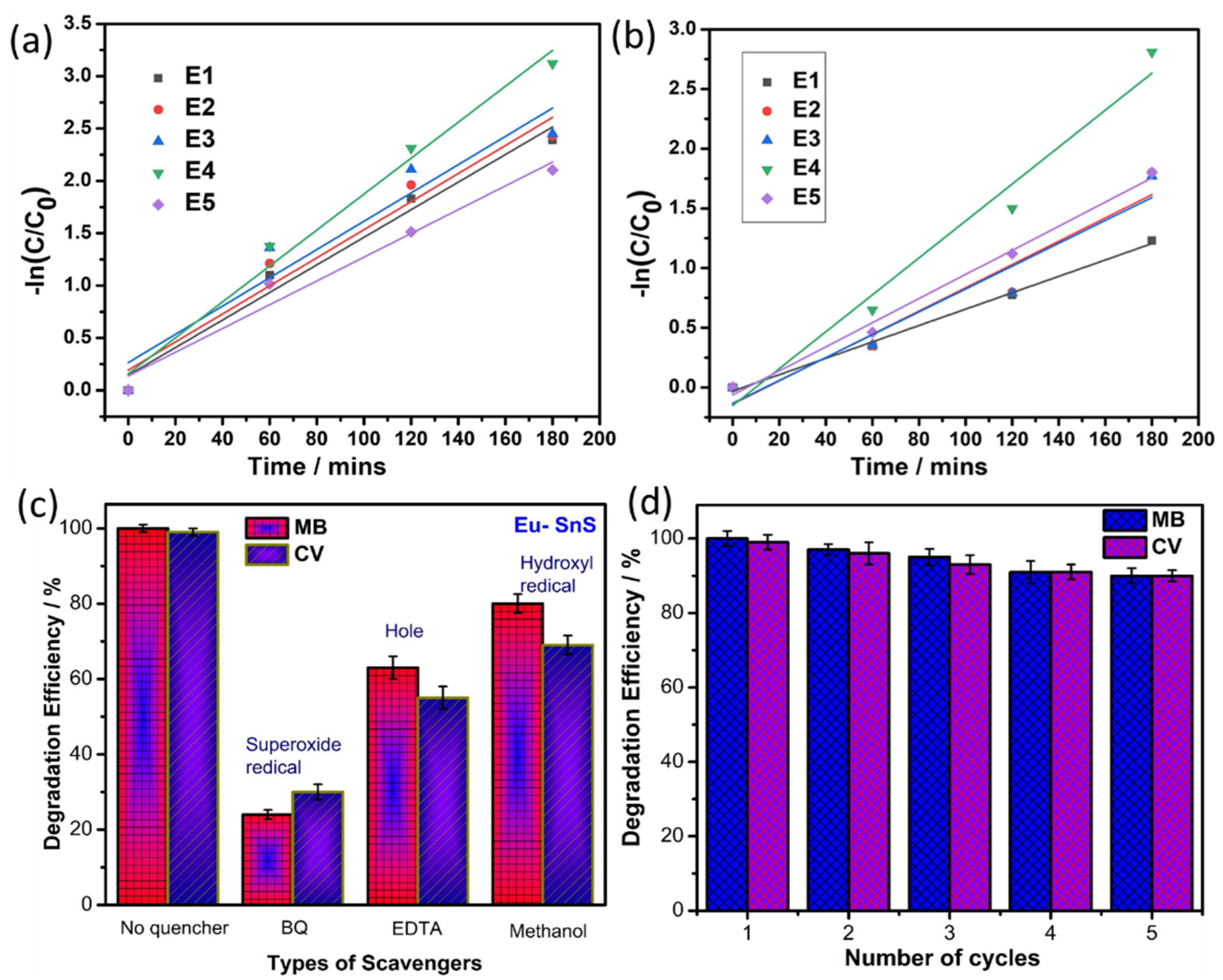
Similarly, the photocatalytic dye degradation study was performed for prepared QD against another model pollutant dye of CV, and the obtained degradation result is shown in Figure 7a–e. For the pure SnS QD, the efficiency reached ~70.7% within 180 min. Under similar conditions, the E4 sample achieved ~99%. Figure 7f depicts the efficiency graph of CV dye degradation. Additional pseudo-first-order kinetics studies are shown in Figure 8a,b. From the studies, the rate constant value and R2 values are listed in Table 1.
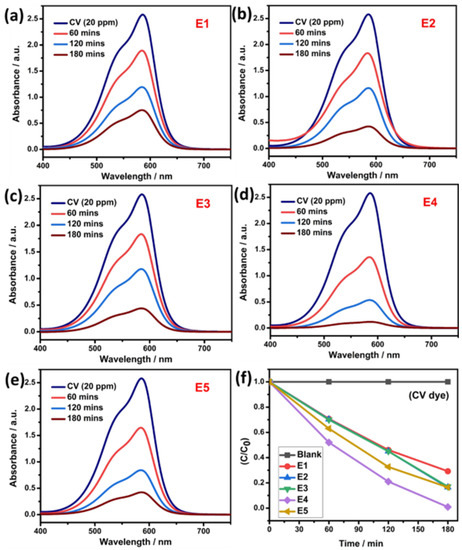
Figure 7.
(a–e) Photocatalytic activity of pure SnS and Eu-doped SnS QDs via degradation of CV dye. (f) Degradation efficiency.
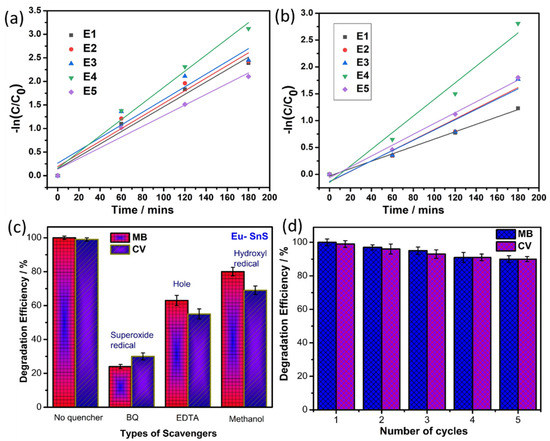
Figure 8.
(a,b) First-order kinetic study graph of MB and CV dye. (c) Scavenger studies and (d) stability of photocatalyst under the degradation of MB and CV dye.
2.6. Reactive Active Species
Generally, the active catalyst generates active species such as H+, OH−, and •O2−, which plays a major role during the degradation of dyes, which is more helpful to undestand the mechanism of the photo-degradation of dyes over catalysts. Hence, the H+, OH-, and •O2− are eliminated by the accumulation of EDTA (h+ scavenger) [39,40], Isopropyl alcohol (OH scavenger), and p-BQ (•O2− scavenger) [38] into the reaction solution. Figure 8c depicts the scavenger’s studies of the E4 sample under MB and CV dye. The addition of p-BQ only shows effectual changes in the photodegradation of MB dye that represents the super oxide radical (•O2−), which plays a critical role in dye degradation.
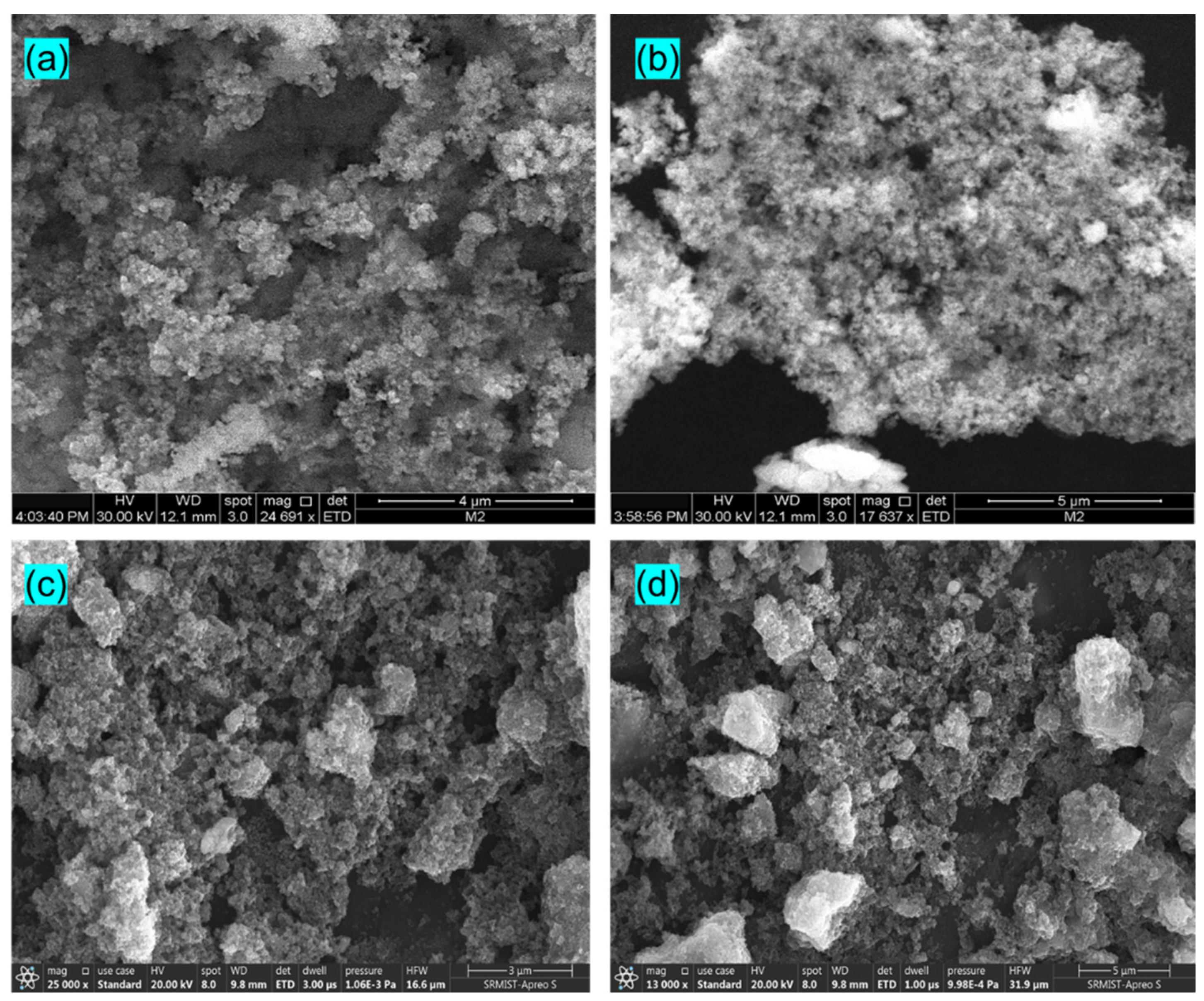
The stability of photocatalysts (E4) was determined by reusing the catalyst five times in the degradation of MB and CV dye, as shown in Figure 8d. The catalyst is used again followed by a centrifuge, a water wash, ethanol treatment, and gentle drying. The reusability results reveal the excellent stability of the catalyst with no significant loss even after five cycles of the photocatalytic process. It clearly shows that the Eu-doped SnS catalyst stands out as an outstanding photocatalyst for practical application. Figure 9a,b shows FESEM images of the Eu-doped SnS QDs after five cycles of degradation of MB and (Figure 9c,d) CV dyes. The similar morphology of the catalyst before (Figure 2a,b) and after five cycles of degradation reveal evidence of the stability of the catalyst.
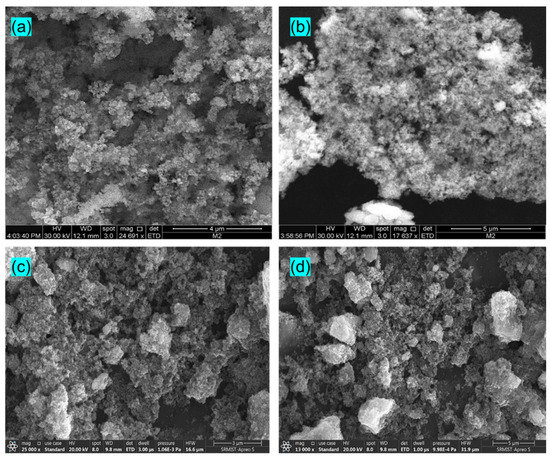
Figure 9.
(a,b) FESEM images of the Eu-doped SnS QDs after 5 cycles of degradation of MB and (c,d) CV dyes.
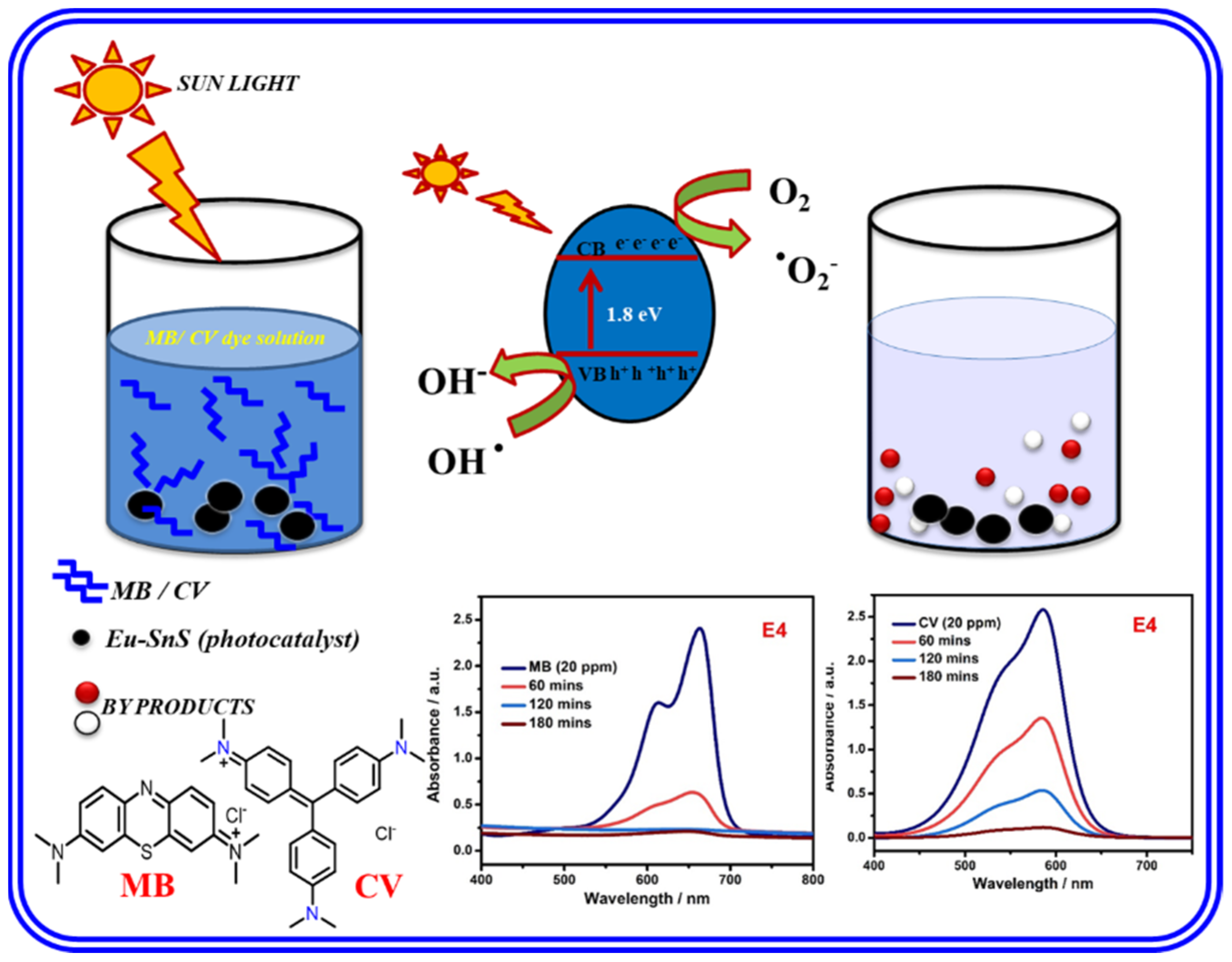
2.7. Photocatalysis Mechanism
The photocatalytic reaction mechanism is useful to determine the activity and generation of reactive oxygen species [41]. The photocatalytic mechanism of pure SnS and Eu-doped SnS QDs is shown in Figure 10. After doping, the absorption wavelength of SnS was slightly blue-shifted to the higher wavelength visible range, which is confirmed by UV spectra. As shown in Table 1, it is clear that the optical band gap markedly decreased with an increasing Eu doping concentration, which confirms the influence of Eu on SnS nanoparticles. The photo-generated electrons in the valance band (VB) of pure SnS move to the impurity of the conduction band (CB) and react with dissolved oxygen on the surface of the catalyst. This produces a huge amount of superoxide radicals (•O2−) under the irradiation of visible light. The superoxide (•O2−) radical is the main radical for the degradation of MB dye [42]. The photo-excited holes react with water to form a highly oxidative hydroxyl radical species (•OH) [43,44]. The mechanism of MB and CV dye degradation is described as follows
Eu/SnS + hν → h + (VB) + e − (CB)
O2 + e− → •O2−
H2O + h+ → •OH
OH− + h+ → •OH
•OH + Dye (MB) → CO2 + H2O
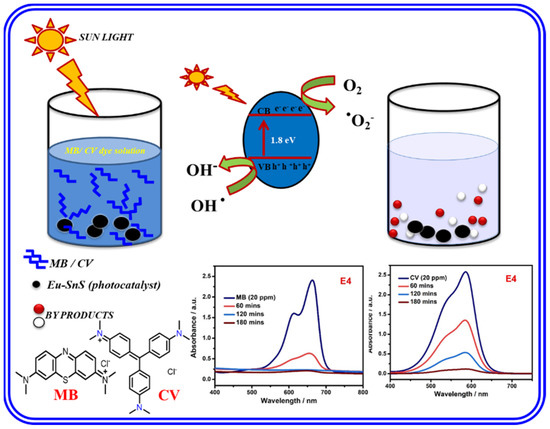
Figure 10.
Mechanism of photocatalytic degradation of MB.
The structure, size, and morphology play an important role in the efficiency of degradation under visible light. Here, the particle size is smaller, which helps the enhancement of the degradation efficiency of the catalyst against the MB dye due to the quantum confinement. Table 2 shows a comparison of the obtained photocatalytic efficiency with reported literature values.

Table 2.
Comparison of the reported studies of various metal sulfide-based nanoparticles and degradation efficiency.
Table 2.
Comparison of the reported studies of various metal sulfide-based nanoparticles and degradation efficiency.
| S. No. | Catalysts | Dye | Dye Conc. | Dose/Volume | Source | Time | Efficiency/% | Reference |
| 1. | SnS/LDPE | MB | 20 ppm | 100 mg/100 mL | Sun light | 180 min | 96.60 | [45] |
| 2. | CdS NPs | MB | 10 ppm | 5 mg/ 100 mL | Sun light | 120 min | 87.12 | [46] |
| 3. | ZnS-Ag | MB | 15 ppm | 100 mg/100 mL | Visible light | 120 min | ~100.00 | [47] |
| 4. | ZnS | MB | 10 ppm | 30 mg/100 mL | Sun light | 180 min | 72.13 | [48] |
| 5. | Ni-ZnS | MB | 10 ppm | 30 mg/100 mL | Sun light | 180 min | 87.38 | [48] |
| 6. | ZnS | MB | 20 ppm | 100 mg/100 mL | Sun light | 180 min | 49.00 | [49] |
| 7. | Sn-ZnS | MB | 20 ppm | 100 mg/100 mL | Sun light | 180 min | 93.00 | [49] |
| 8. | PbS | MB | 1 × 10−5 mol/L−1 (~16 ppm) | 50 mg/50 mL | UV light | 180 min | 25.00 | [50] |
| 9. | Ag-PbS | MB | 1 × 10−5 mol/L−1 (~16 ppm) | 50 mg/50 mL | UV light | 180 min | 68.00 | [51] |
| 10. | Bi2S3 | MB | - | - | Visible light | 140 min | 87.98 | [51] |
| 11. | CuS | MB | 5 ppm | 80 mg/50 mL | Visible light | 120 min | ~100.00 | [52] |
| 12. | CuS | CV | 5 ppm | 80 mg/50 mL | Visible light | 120 min | 84.60 | [52] |
| 13. | CdS | MB | 20 ppm | 25 mg/25 mL | Sun light | 180 min | 91.39 | [53] |
| 14. | Sn/CdS | MB | 20 ppm | 25 mg/ 25 mL | Sun light | 180 min | 97.56 | [53] |
| 15. | Eu-SnS | MB | 20 ppm | 25 mg/ 50 mL | Sun light | 180 min | 100.00 | Present study |
| 16. | Eu-SnS | CV | 20 ppm | 25 mg/ 50 mL | Sun light | 180 min | 97.00 | Present study |
2.8. Effect of pH
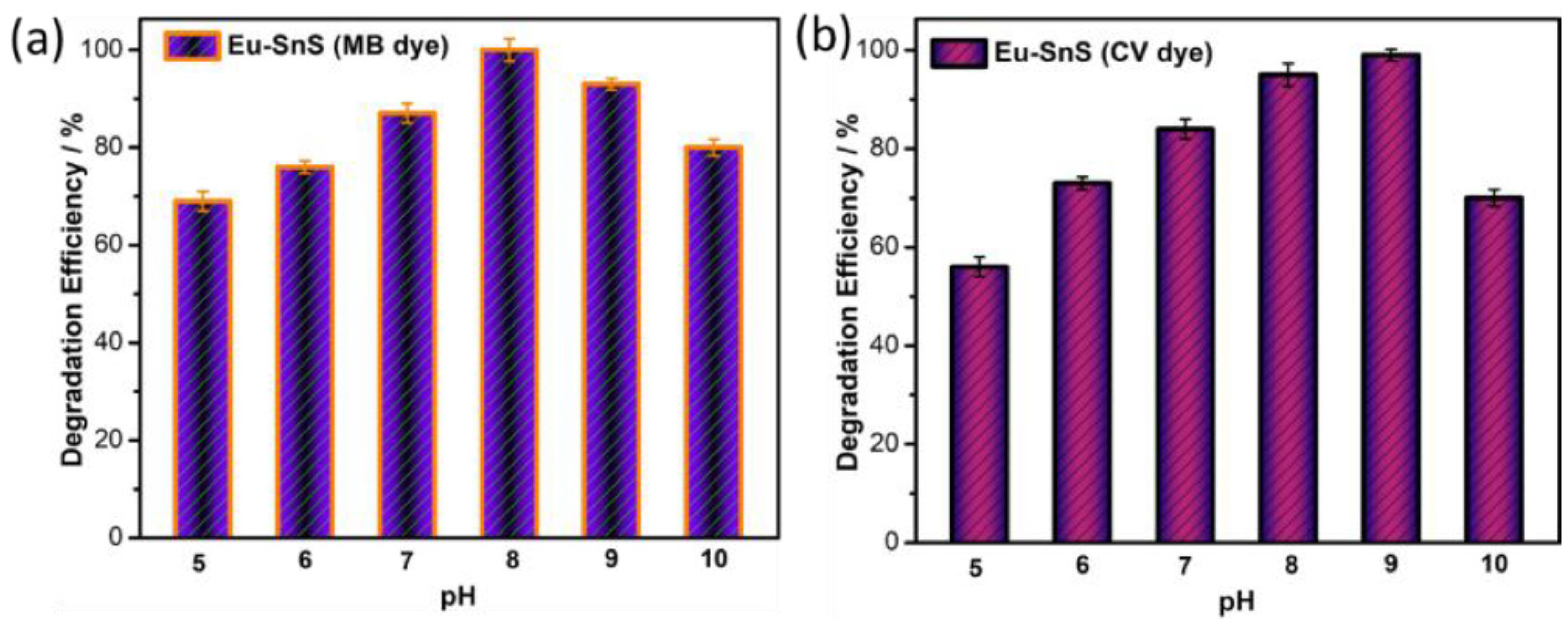
The effect of pH studies is also important to achieve a higher efficiency of photodegradation. Mainly, it influences the surface charge of the catalyst, which changes the efficiency of the photodegradation. Hence, the pH effect was determined to be the catalyst of E4 in the range of pH values from 5 to 11 for both dyes. Figure 10a illustrates the best dye degradation occurred at pH 8 against MB. Similarly, Figure 11b shows the supreme degradation efficiency for CV dye at pH 9. The above pH study clearly proved that the alkaline medium of the dye solution was more appropriate to achieve high degradation efficiency because of the higher adsorption of dye molecules on the surface of the catalyst. In the end, the ultrafast degradation of the Eu-doped SnS proved that it is due to the quantum size effect of the catalyst.
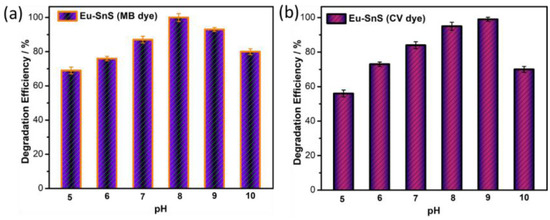
Figure 11.
Photocatalytic dye degradation efficiency as a function of pH for (a) Eu-SnS (MB dye) and (b) Eu-SnS (CV dye).
3. Experimental
3.1. Synthesis of Pure SnS and Eu-Doped SnS Quantum Dots (QDs)
The SnS and Eu-doped SnS were prepared by a facile chemical co-precipitation method. At first, 0.5 M of Tin (II) chloride was dissolved in 50 mL of DI water under constant stirring at 80 °C. Next, 1 g of PVP was directly added to the above solution. Then, 0.2 M of Na2S was dissolved in 50 mL of DI water separately, and then the Na2S solution was added to the above mixture dropwise. A dark brown color precipitate was obtained while adding the Na2S solution into the above reaction mixture. The obtained precipitate was named E1.
For various Eu-doped SnS (E2, E3, E4, and E5) preparations, various weight percentages of europium nitrate (1, 3, 5 and 10 wt%) were dissolved before adding the Na2S solution into the starting solution. After the addition of the Na2S solution, the stirring continued for 2 h. Then, the sample was washed several times with acetone and ethanol and then centrifuged. Finally, the wet sample was dried at 120 °C in a hot air oven for 12 h. The dried SnS and Eu-doped SnS powders were used for further reactions.
3.2. Photocatalytic Dye Degradation
Methylene blue (MB) and Crystal violet (CV) were chosen as the model organic pollutants to investigate the photocatalytic dye degradation studies of the prepared catalysts. Typically, 25 mg of the catalyst was added to the 25 mL (20 mg/L) aqueous dye solution. All the reaction mixtures were kept in the dark to attain the adsorption and desorption equilibrium of dyes on the surface of the catalyst. The suspensions were irradiated under sunlight for an overall time period of 180 min, and the catalysts were separated from the whole reaction mixture using a centrifuge (5000 rpm for 5 min) at a certain time interval (60 min). The collected dye solution was filtered using a 0.2 µm syringe filter. The catalytic degradation efficiency of the catalyst was calculated by the degradation of organic dyes using a UV-Vis spectrophotometer (MB, λmax = 662 nm and CV, λmax = 585 nm). The degradation efficiency is calculated by the formula,
where C0 and C are the initial and variable concentrations of dye molecules. To carry out the stability study, once it had completed the 1st cycle, the catalyst was carefully collected by s centrifuge and then washed with ethanol/water and dried at 120 °C. The regenerated catalyst was used to evaluate the stability and reusability of the catalyst by degrading the dye solution using 180 min of sunlight exposure. For each cycle, the same concentration of dye and catalyst was used.
Degradation efficiency (%) = C0 − C/C0 × 100
The first-order reaction kinetic model was used to determine the correct degradation rate of MB and CV as follows:
where k is the rate of the reaction constant and t is the time. The catalytic activities were performed while the sun was shining between 11:00 AM and 2:00 PM under a clear sky, where the average solar light intensity was measured at approximately 1.00 × 105 lux. Moreover, a pH effect study was performed. For the pH test, the pH of the dye solution was adjusted with the addition of 0.1 M hydrochloric acid solution and 0.1 M of sodium hydroxide solution.
ln(C/C0) = kt
3.3. Characterization
The crystal phase identification of synthesized samples was performed by X-ray diffraction (XRD) analysis (Rigaku–Ultima IV). The morphology of the samples was studied by a Field Emission scanning electron microscope (FESEM; FEI Quanta-250 FEG microscope) and transmission electron microscopy (TEM, JEM 2100 F), operated with the voltage of 200 kV. Then, optical studies were carried out using a UV-Visible spectrophotometer (JASCO V-650 spectrophotometer).
4. Conclusions
In summary, pure and Eu-doped SnS QDs were synthesized by using a facile chemical precipitation method. From various characterization studies, it was deduced that synthesized Eu-doped SnS QDs are aptly crystalline, high quality, and consist of very small particles with a size <5 nm. Compared to the pure SnS catalyst, the Eu-doped SnS catalysts have shown excellent photocatalytic efficiency and high adsorption affinity for the removal of MB and CV textile dyes from water, which was evaluated under visible light irradiation. Compared to the pure SnS catalyst, the 5% doped SnS catalysts show high dye degradation efficiency due to the size reduction and more surface activity. The 5% doped SnS photocatalyst achieved the highest efficiency of 100% and 99% against MB and CV dyes, respectively. Several other parameters affecting the photocatalytic process such as the pH, scavenger, and initial dye concentration were also studied. A similar morphology of the catalyst after five cycles demonstrated that the Eu-doped SnS QDs can be used for a long time. Due to the outstanding features of Eu-doped SnS QDs, they can be used for many applications, especially in wastewater treatment of textile industrial dye waster degradation.
Author Contributions
Conceptualization and methodology, G.M.; validation, J.P.; formal analysis, M.R.K.; writing—original draft preparation, G.M.; writing—review and editing, S.G.P.; project administration, A.A.A. and M.A.H.; funding acquisition, A.A.A. and M.A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by King Saud University grant number RSP-2021/243.
Data Availability Statement
Not applicable.
Acknowledgments
The authors (A.A.A. and M.A.H.) thank King Saud University Researchers Supporting project (RSO 2021/243) for fruitful support. The author (G. Murugadoss) acknowledges the management of Sathyabama Institute of Science and Technology, Chennai, Tamilnadu, India for providing lab facilities and support. One of the authors, Rajesh Kumar Manavalan, thanks contract no. 40/is2.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Elango, G.; Roopan, S.M. Efficacy of SnO2 nanoparticles towards photocatalytic degradation of methylene blue dye. J. Photochem. Photobiol. B Biol. 2016, 155, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Shabani, M.; Haghighi, M.; Kahforoushan, V.; Haghighi, A. Mesoporous-mixed-phase of hierarchical bismuth oxychlorides nanophotocatalyst with enha nced photocatalytic application in treatment of antibiotic effluents. J. Clean. Prod. 2019, 207, 444–457. [Google Scholar] [CrossRef]
- Amini, M.; Ashrafi, M. Photocatalytic degradation of some organic dyes under solar light irradiation using TiO2 and ZnO nanoparticles. Nano. Chem. Res. 2016, 1, 79–86. [Google Scholar]
- Mohamed, H.H. Rationally designed Fe2O3/GO/WO3 Z-Scheme photocatalyst for enhanced solar light photocatalytic water remediation. J. Photochem. Photobiol. A Chem. 2019, 378, 74–84. [Google Scholar] [CrossRef]
- Pavithra, K.G.; Kumar, P.S.; Jaikumar, V.; Rajan, P.S. Removal of colorants from wastewater: A review on sources and treatment strategies. J. Ind. Eng. Chem. 2019, 75, 1–19. [Google Scholar] [CrossRef]
- Lai, X.; Guo, R.; Xiao, H.; Lan, J.; Jiang, S.; Cui, C.; Ren, E. Rapid microwave-assisted bio-synthesized silver/Dandelion catalyst with superior catalytic performance for dyes degradation. J. Hazard. Mater. 2019, 371, 506–512. [Google Scholar] [CrossRef]
- Hussain, W.; Badshah, A.; Imtiaz-ud-Din, R.A.H.; Aleem, M.A.; Bahadur, A.; Iqbal, S.; Farooq, M.U.; Ali, H. Photocatalytic applications of Cr2S3 synthesized from single and multi-source precursors. Mater. Chem. Phys. 2017, 194, 345–355. [Google Scholar] [CrossRef]
- Hussain, W.; Malik, H.; Bahadur, A.; Hussain, R.A.; Shoaib, M.; Iqbal, S.; Hussain, H.; Green, I.R.; Badshah, A.; Li, H. Synthesis and characterization of CdS photocatalyst with different morphologies: Visible light activated dyes degradation study. Kinet. Catal. 2018, 59, 710–719. [Google Scholar] [CrossRef]
- Anwer, H.; Mahmood, A.; Lee, J.; Kim, K.; Park, J.; Yip, A.C.K. Photocatalysts for degradation of dyes in industrial effluents: Opportunities and challenges. Nano Res. 2019, 12, 955–972. [Google Scholar] [CrossRef]
- Das, T.K.; Ganguly, S.; Bhawal, P.; Remanan, S.; Mondal, S.; Das, N.C. Mussel inspired green synthesis of silver nanoparticles-decorated halloysite nanotube using dopamine: Characterization and evaluation of its catalytic activity. Appl. Nanosci. 2018, 8, 173–186. [Google Scholar] [CrossRef]
- Das, T.K.; Remanan, S.; Ghosh, S.; Ghosh, S.K.; Das, N.C. Efficient synthesis of catalytic active silver nanoparticles illuminated cerium oxide nanotube: A mussel inspired approach. Environmental Nanotechnology. Monit. Manag. 2021, 15, 100411. [Google Scholar] [CrossRef]
- Hitam, C.N.C.; Jalil, A.A. A review on exploration of Fe2O3 photocatalyst towards degradation of dyes and organic contaminants. J. Environ. Manag. 2020, 258, 110050. [Google Scholar] [CrossRef] [PubMed]
- Preet, K.; Gupta, G.; Kotal, M.; Kansal, S.K.; Salunke, D.B. Mechanochemical Synthesis of a New Triptycene-Based Imine-Linked Covalent Organic Polymer for Degradation of Organic Dye. Cryst. Growth Des. 2019, 19, 2525–2530. [Google Scholar] [CrossRef]
- Phil, S.; Yong, M.; Chul, H. Photocatalytic activity of SnO2 nanoparticles in methylene blue degradation. Mater. Res. Bull. 2016, 74, 85–89. [Google Scholar]
- Jia, X.; Liu, Y.; Wu, X.; Zhang, Z. A low temperature situ precipitation route to designing Zn-doped SnO2 photocatalyst with enhanced photocatalytic performance. Appl. Surf. Sci. 2014, 311, 609–613. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Abdi, J.; Oveisi, M.; Asli, M.A.; Vossoughi, M. Metal-organic framework (MIL-100 (Fe)): Synthesis, detailed photocatalytic dye degradation ability in colored textile wastewater and recycling. Mater. Res. Bull. 2017, 100, 357–366. [Google Scholar] [CrossRef]
- Ninan, G.G.; Sudha Kartha, C.; Vijayakumar, K.P. On the preparation of n-type SnS:Cu using chemical spray pyrolysis for photovoltaic application: Effect of annealing. Sol. Energy Mater. Sol. Cells 2016, 157, 229–233. [Google Scholar] [CrossRef]
- Jamali-Sheini, F.; Yousefi, R.; Ali Bakr, N.; Cheraghizade, M.; Sookhakian, M.; Huang, N.M. Highly efficient photo-degradation of methyl blue and band gap shift of SnS nanoparticles under different sonication frequencies. Mater. Sci. Semicond. Process. 2015, 32, 172–178. [Google Scholar] [CrossRef]
- Tang, R.; Su, H.; Sun, Y.; Zhang, X.; Li, L.; Liu, C.; Zeng, S.; Sun, D. Enhanced photocatalytic performance in Bi2WO6/SnS heterostructures: Facile synthesis, influencing factors and mechanism of the photocatalytic process. J. Colloid Interface Sci. 2016, 466, 388–399. [Google Scholar] [CrossRef]
- Suryawanshi, S.R.; Warule, S.S.; Patil, S.S.; Patil, K.R.; More, M.A. Vapor-liquid-solid growth of one-dimensional tin sulfide (SnS) nanostructures with promising field emission behavior. ACS Appl. Mater. Interfaces 2014, 6, 2018–2025. [Google Scholar] [CrossRef]
- Vaughn, D.D.; Hentz, O.D.; Chen, S.; Wang, D.; Schaak, R.E. Formation of SnS nanoflowers for lithium-ion batteries. Chem. Commun. 2012, 48, 5608–5610. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Yang, J.; Li, R.; Huo, N.; Li, Y.; Wei, Z.; Li, J. Gas-dependent photoresponse of SnS nanoparticles-based photodetectors. J. Mater. Chem. C 2015, 3, 1397–1402. [Google Scholar] [CrossRef]
- Zhou, X.; Gan, L.; Zhang, Q.; Xiong, X.; Li, H.; Zhong, Z.; Han, J.; Zhai, T. High performance near-infrared photodetectors based on ultrathin SnS nanobelts grown via physical vapor deposition. J. Mater. Chem. C 2016, 4, 2111–2116. [Google Scholar] [CrossRef]
- Wang, J.; Lian, G.; Xu, Z.; Fu, C.; Lin, Z.; Li, L.; Wang, Q.; Cui, D.; Wong, C.P. Growth of Large-Size SnS Thin Crystals Driven by Oriented Attachment and Applications to Gas Sensors and Photodetectors. ACS Appl. Mater. Interfaces 2016, 8, 9545–9551. [Google Scholar] [CrossRef] [PubMed]
- Jamali-sheini, F.; Cheraghizade, M.; Yousefi, R. Ultrasonic synthesis of In-doped SnS nanoparticles and their physical properties. Solid State Sci. 2018, 79, 30–37. [Google Scholar] [CrossRef]
- Cheraghizade, M.; Jamali-sheini, F.; Yousefi, R.; Niknia, F.; Mahmoudian, M.R.; Sokhakian, M. The effect of tin sulfide quantum dots size on photocatalytic and photovoltaic performance. Mater. Chem. Phys. 2017, 195, 187–194. [Google Scholar] [CrossRef]
- Firtina Ertis, I.; Boz, I. Synthesis and Characterization of Metal-Doped (Ni, Co, Ce, Sb) CdS Catalysts and Their Use in Methylene Blue Degradation under Visible Light Irradiation. Mod. Res. Catal. 2017, 6, 1–14. [Google Scholar] [CrossRef]
- Khataee, A.R.; Karimi, A.; Soltani, R.D.C.; Safarpour, M.; Hanifehpour, Y.; Joo, S.W. Europium-doped ZnO as a visible light responsive nanocatalyst: Sonochemical synthesis, characterization and response surface modeling of photocatalytic process. Appl. Catal. A Gen. 2014, 488, 160–170. [Google Scholar] [CrossRef]
- Kumar, A.; Babu, S.; Karakoti, A.S.; Schulte, A.; Seal, S. Luminescence properties of europium-doped cerium oxide nanoparticles: Role of vacancy and oxidation states. Langmuir 2009, 25, 10998–11007. [Google Scholar] [CrossRef]
- Sin, J.C.; Lam, S.M.; Satoshi, I.; Lee, K.T.; Mohamed, A.R. Sunlight photocatalytic activity enhancement and mechanism of novel europium-doped ZnO hierarchical micro/nanospheres for degradation of phenol. Appl. Catal. B Environ. 2014, 148–149, 258–268. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Yayapao, O.; Thongtem, T.; Thongtem, S. Synthesis and characterization of europium-doped zinc oxide photocatalyst. J. Nanomater. 2014, 2014, 367529. [Google Scholar] [CrossRef]
- Khatamian, M.; Khandar, A.A.; Divband, B.; Haghighi, M.; Ebrahimiasl, S. Heterogeneous photocatalytic degradation of 4-nitrophenol in aqueous suspension by Ln (La3+, Nd3+ or Sm3+) doped ZnO nanoparticles. J. Mol. Catal. A Chem. 2012, 365, 120–127. [Google Scholar] [CrossRef]
- Sukriti, P.; Chand, V. Singh, Enhanced visible-light photocatalytic activity of samarium-doped zinc oxide nanostructures. J. Rare Earths. 2020, 38, 29–38. [Google Scholar] [CrossRef]
- Ranjith Kumar, D.; Ranjith, K.S.; Rajendra Kumar, R.T. Structural, optical, photocurrent and solar driven photocatalytic properties of vertically aligned samarium doped ZnO nanorod arrays. Optik 2018, 154, 115–125. [Google Scholar] [CrossRef]
- Greyson, E.C.; Jeremy, E.B.; Teri, W.O. Tetrahedral zinc blende tin sulfide nano- and microcrystals. Small 2016, 2, 368–371. [Google Scholar]
- Johnson, J.B.; Jones, H.; Latham, B.S.; Parker, J.D.; Engelken, R.D.; Barber, C. Optimization of photoconductivity in vacuum-evaporated tin sulfide thin films. Semicond. Sci. Technol. 1999, 14, 501–507. [Google Scholar] [CrossRef]
- Chamberlain, J.M.; Merdan, M. Infrared photoconductivity in p-SnS. J. Phys. C Solid State Phys. 1977, 10, L571–L574. [Google Scholar] [CrossRef]
- Rana, C.; Ranjan, S.; Satyajit, B. Growth of SnS nanoparticles and its ability as ethanol gas sensor. J. Mater. Sci. Mater. Electron. 2019, 30, 2016–2029. [Google Scholar] [CrossRef]
- Sun, H.; Liu, S.; Liu, S.; Wang, S. A comparative study of reduced graphene oxide modified TiO2, ZnO and Ta2O5 in visible light photocatalytic/photochemical oxidation of methylene blue. Appl. Catal. B Environ. 2014, 146, 162–168. [Google Scholar] [CrossRef]
- Xin, L.; Miroslava, E.; Pengwei, H.; Kamila, K. Fabrication of highly stable CdS/g-C3N4 composite for enhanced photocatalytic degradation of RhB and reduction of CO2. J. Mater. Sci. 2020, 55, 3299–3313. [Google Scholar]
- Saravanan, R.; Karthikeyan, S.; Gupta, V.K.; Sekaran, G.; Narayanan, V.; Stephen, A. Enhanced photocatalytic activity of ZnO/CuO nanocomposite for the degradation of textile dye on visible light illumination. Mater. Sci. Eng. C 2013, 33, 91–98. [Google Scholar] [CrossRef]
- Kashinath, V.G.L.; Daniel, D.J. Sol-gel mediated microwave synthesis of pure, La and Zr doped SnS2 nanoflowers an efficient photocatalyst for the degradation of ethylene blue. J. Mater. Sci. Mater. Electron. 2019, 30, 7963–7973. [Google Scholar]
- Ranjith Kumar, D.; Ranjith, K.S.; Nivedita, L.R.; Asokan, K.; Rajendra Kumar, R.T. Swift heavy ion induced effects on structural, optical and photo-catalytic properties of Ag irradiated vertically aligned ZnO nanorod arrays. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms. 2019, 450, 95–99. [Google Scholar] [CrossRef]
- Song, S.; Wu, K.; Wu, H.; Guo, J.; Zhang, L. Effect of Fe/Sn doping on the photocatalytic performance of multi-shelled ZnO microspheres: Experimental and theoretical investigations. Dalton Trans. 2019, 48, 13260–13272. [Google Scholar] [CrossRef]
- Hegde, S.S.; Surendra, B.S.; Priyanka, V.P.; Murahari, P.; Ramesh, K. SnS/LDPE Composite: A reusable floating photocatalyst for solar degradation of organic dyes. Mater. Today Proc. 2021, 47, 4255–4261. [Google Scholar] [CrossRef]
- Ullah, A.; Rasheed, S.; Ali, I.; Ullah, N. Plant mediated synthesis of CdS nanoparticles: Their characterization and application for photocatalytic degradation of toxic organic dye. Chem Rev. Lett. 2021, 4, 98–107. [Google Scholar]
- Sivakumar, P.; Gaurav Kumar, G.K.; Sivakumar, P.; Renganathan, S. Synthesis and characterization of ZnS-Ag nanoballs and its application in photocatalytic dye degradation under visible light. J. Nanostruct. Chem. 2014, 4, 107. [Google Scholar] [CrossRef]
- Jothibas, M.; Manoharan, C.; Johnson Jeyakumar, S.; Praveen, P.; Kartharinal Punithavathy, I.; Prince Richard, J. Synthesis and enhanced photocatalytic property of Ni doped ZnS nanoparticles. Sol. Energy. 2018, 159, 434–443. [Google Scholar]
- Ramki, K.; RajaPriya, A.; Sakthivel, P.; Murugadoss, G.; Thangamuthu, R.; Rajesh Kumar, M. Rapid degradation of organic dyes under sunlight using tin-doped ZnS nanoparticles. J. Mater. Sci. Mater. Electron. 2020, 31, 8750–8760. [Google Scholar] [CrossRef]
- Andrade Neto, N.F.; Oliveira, Y.G.; Paskocimas, C.A.; Bomio, M.R.D.; Motta, F.V. Increase of antimicrobial and photocatalytic properties of silver-doped PbS obtained by sonochemical method. J. Mater. Sci. Mater. Electron. 2018, 29, 19052–19062. [Google Scholar] [CrossRef]
- Arumugam, J.; George, A.; Dhayal Raj, A.; Selvaraj, M.; Albert Irudayaraj, A.; Pazhanivel, T.; Josephine, R.L.; Bhuvaneswari, K. Role of surfactant in tailoring the properties of Bi2S3 nanoparticles for photocatalytic degradation of methylene blue dye. J. Mater. Sci. Mater. Electron. 2022, 33, 8946–8957. [Google Scholar] [CrossRef]
- Ajibade, P.A.; Oluwalana, A.E. Enhanced photocatalytic degradation of ternary dyes by copper sulfide nanoparticles. Nanomaterials. 2021, 11, 2000. [Google Scholar] [CrossRef]
- Venkatesh, N.; Sabarish, K.; Murugadoss, G.; Thangamuthu, R.; Sakthivel, P. Visible light–driven photocatalytic dye degradation under natural sunlight using Sn-doped CdS nanoparticles. Environ. Sci. Pollut. Res. 2020, 27, 43212–43222. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).