Abstract
Catalytic carbon dioxide (CO2) hydrogenation to carbon monoxide (CO) via reverse water-gas shift (RWGS) reaction is of particular interest due to its direct use in various industrial processes as feedstock. However, the competitive CO2 methanation process severely limits the RWGS reaction in a lower temperature range. In this context, we propose a novel nanocatalyst (NC) comprising oxygen vacancy-enriched subnanometer-scale CoPd hybrid cluster (CoOxVPd)-anchored Pd nanoparticles (NPs) on cobalt oxide support underneath (denoted as CP-CoOxVPd) by using a galvanic replacement reaction-assisted wet chemical reduction method. As-developed CP-CoOxVPd NC initiated the RWGS reaction at 423 K temperature while showing an optimum CO production yield of ∼3414 μmol g−1catalyst and a CO selectivity as high as ∼99% at 523 K in the reaction gas of CO2:H2 = 1:3. The results of physical characterizations along with electrochemical and gas chromatography (GC) suggest that abundant oxygen vacancies in the surface-anchored CoOxVPd clusters are vital for CO2 adsorption and subsequent activation, while neighboring Pd domains facilitate the H2 dissociation. The obtained results are expected to provide a feasible design of Co-based NCs for the RWGS reaction.
1. Introduction
The extensive consumption of conventional fossil fuels results in massive CO2 emissions, which later cause alarming energy and environmental issues. To this end, CO2 sequestration is crucial for global sustainability; however, technological and economic bottlenecks are prompting the scientific community to recycle CO2 into value-added fuels or chemicals [1]. CO2 is a potential one-carbon (C1) source for producing commodity fuels such as formic acid (HCOOH), methane (CH4), and carbon monoxide (CO), etc. via CO2 hydrogenation [2]. Nevertheless, CH4 is closest to meeting the commercial demands due to its well-explored industrial application [3]. Multidirectional engineering steps have been demonstrated for direct conversion of CO2 into methane via CO2 methanation (i.e., CO2 to CH4 conversion); however, due to the high activation temperature of CO2 molecules, the production yield is severely limited [4]. Alternatively, the catalytic transformation of CO2 to CO via reverse water–gas shift (RWGS) reaction has gained more attention, because unlike CO2, which is thermodynamically more stable, CO is highly active and its further conversion to value-added commodities is well known [5,6]. It is a well-known fact that the RWGS reaction is endothermic and thus more favored at higher temperatures, while the CO2 methanation is thermodynamically more favored at lower temperatures due to its exothermic nature. Therefore, it can be concluded that the competitive CO2 methanation process suppresses the CO production yield at lower temperatures [7]. Consequently, developing highly efficacious catalysts for RWGS reaction with high CO selectivity and low-temperature operation is needed.
It is an undeniable fact that understanding reaction mechanisms is imperative before designing high-performance catalysts. Therefore, the in-depth study of RWGS reaction carried out in past decades and two possible reaction mechanisms (redox and association) have been proposed [8]. The redox mechanism is two reaction steps, where hydrogen molecules (H2) dissociate in the first step to *H atoms, which further react with surface *O to create oxygen vacancies (OV). In the second step, the CO2 molecule dissociates at the OV sites to produce *CO. The *H-assisted reduction of surface oxygen species is the rate-determining step (RDS) in the redox mechanism [9]. Generally, the reducible oxides follow the redox mechanism [10]. On the other hand, in the associative mechanism, the adsorbed CO2 molecules react with dissociated *H atoms to form intermediate species such as carboxyl (*COOH), and formate (*HCOO), etc., finally decomposing to produce CO and H2O, where, the adsorption and dissociation, respectively, of CO2 and H2 molecules are determined as key elementary steps [11,12]. The association mechanism is mainly followed by the reducible oxide-supported noble metal nanoparticles (NPs) [13]. The aforementioned discussion suggests that catalyst material has a direct influence on the pathway of RWGS reaction. In addition, it can be concluded that two essential reaction sites for CO2 adsorption and H2 dissociation are required in an ideal RWGS reaction catalyst. It is frequently reported in the literature that noble-metal NPs, such as Pd or Pt, facilitate the H2 dissociation, while OV sites favour CO2 adsorption [14,15]. For instance, Arenas et al. elucidated the role of OV in CO2 methanation. They carried out CO2 methanation on two different catalyst systems: one with OV (Ni-CeO2) and the other without OV (Ni-Al2O3), where the OV-enriched Ni/CeO2 catalyst achieved higher methanation yield and selectivity. They demonstrated that CO2 molecules are reduced at the OV sites of CeO2 support, while H2 molecules dissociated at the metallic Ni sites [16]. Bahmanpour et al. also found that OV sites are vital for CO2 adsorption and activation [17]. Our previous works have outlined the role of synergistic collaboration between two or more reaction sites in bimetallic and trimetallic catalyst systems for CO2 methanation and RWGS reactions, respectively. Pd domains promoted CO2 dissociation and late transition metal oxides (Ni, Cu, Co) were reduced by H2 at higher temperature range [18,19].
In line with recent advancements, herein we prepared a heterogeneous nanocatalyst (NC) comprising cobalt oxide-supported Pd NPs with OV-enriched CoPd hybrid cluster (denoted CoOxVPd) decoration (CP-CoOxVPd) using a galvanic replacement reaction-assisted wet chemical reduction method. Such a material delivers a CO production yield of ~3414 μmol g−1catalyst with CO selectivity of ~99% at 523 K in the ambient of the CO2 and H2 mixture. Results of the physical inspections along with the electrochemical analysis ambiguously suggest that the impressive CO production yield and high CO selectivity of CP-CoOxVPd NC can be attributed to the presence of OV reaction sites on the material’s surface, which are vital for CO2 adsorption and subsequent activation. On top of that, adjacent Pd atoms facilitate the H2 dissociation. Consequently, multiple reaction sites in the CP-CoOxVPd simultaneously facilitate all the reaction pathways.
2. Results and Discussion
Physical Characterizations
Figure 1 demonstrates the high-resolution transmission electron microscope (HRTEM) images of Pd/AC and CP-CoOxVPd NCs with the corresponding digital image-processing results (Figure 1a-1,b-1), Forward Fourier transform (FFT) patterns (Figure 1a-2,b-2), the inverse Fourier transform (IFT) images (Figure 1a-3,b-3), and the line histograms of selected fringes (insets) are analyzed for in-depth analysis. The low-magnification TEM images of Pd/AC and CP-CoOxVPd NCs are shown in Figure S1. For easy clarification, an HRTEM image of carbon-supported CoOx NPs (i.e., CoOx/AC) is shown in Figure S2. Accordingly, the CoOx NPs are grown with short-range ordered polycrystalline structure and can be consistently confirmed by the presence of ring-like FFT patterns [20]. Figure 1a shows an HRTEM image of bare Pd NPs (i.e., Pd/AC), where Pd NPs are grown in a drop-like structure with severe surface defects (denoted by the yellow circles in the digital image-processing results in Figure 1a-1) and a typical size range of ~3 to 4 nm. An even closer inspection of Figure 1a reveals that Pd NPs exhibit a long-range ordered atomic structure in three-dimensional space that is consistently confirmed with the hexagonally symmetric FFT pattern (Figure 1a-2). The fuzzy surface (denoted by the red triangles) can be attributed to some extent of surface oxidation in the absence of steric protection. These rationales can be further confirmed by the IFT (Figure 1a-3) derived line histograms (insets) of interplanar spacing of Pd NPs at the surface, as well as subsurface regions. Accordingly, the Pd NPs exhibit random lattice spacing of 0.253 nm and 0.222 nm in the surface and subsurface regions, respectively. These observations integrally indicate a certain extent of lattice expansion at the Pd surface due to possible oxidation. Figure 1b presents the HRTEM image of CoOx-supported Pd NPs with CoPd hybrid cluster decoration (i.e., CP-CoOxVPd NC), where it can be seen that the surface and subsurface atomic arrangements and the particle size of the Pd NPs (denoted by blue circle) are severely changed compared to that of bare Pd NPs. The size of CoOx-supported Pd NPs is increased by 30% (~4–5 nm) compared to that of Pd NPs on the carbon support (Figure 1a), and can be attributed to the limited extent of heteroatomic intermixing (i.e., CoPd alloy formation) at the CoOx-to-Pd interface, as well as surface-anchored CoPd species. These scenarios are consistently confirmed by the increased lattice spacing of 0.246 nm (i.e., lattice relaxation), which is a typical trademark of alloy formation and consistently proved by spectroscopic analysis in later sections. Of utmost importance, the reduced surface defects (Figure 1b-2) along with the FFT pattern exhibiting symmetric bright spots in lateral spaces (Figure 1b-3) implies that the decorated CoPd clusters are accommodated in the defect sites of the Pd crystal without disturbing the local structure ordering.
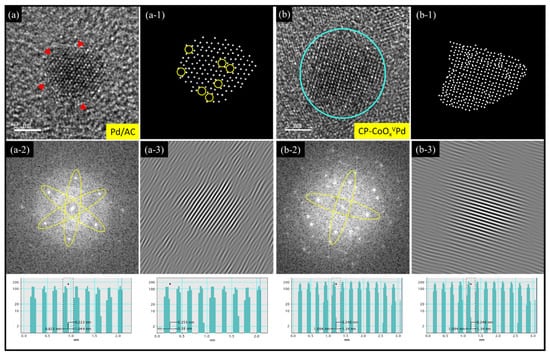
Figure 1.
HRTEM images of as-prepared (a) Pd/AC and (b) CP-CoOxVPd NCs. The digital image-processing results, forward Fourier transformation (FFT) patterns and inverse Fourier transform (IFT) images of the selected areas in HRTEM images are depicted in (a-1,b-1), (a-2,b-2), and (a-3,b-3), respectively, for Pd/AC and CP-CoOxVPd NCs. The d-spacing values of experimental NCs are calculated using IFT images and their corresponding line histograms (insets).
Figure 2a shows the X-ray diffraction (XRD) patterns of CP-CoOxVPd NC and reference samples (AC, CoOx/AC and Pd/AC), where the diffraction peaks “O1,” “O2,” and “O3,” respectively, centered at ~8.45°, ~11.44°, and ~19.18° correspond to the carbon support. The suppressed and broad diffraction (blue lines) confirm the formation of short-range ordered polycrystalline structure, consistent with former HRTEM observations. The Pd/AC and CP-CoOxVPd NCs show three characteristic peak—A, B, and C—centered at about ~17.50°, 20.10°, and 28.80°. These peaks can be assigned to the diffraction signals from (111), (200), and (220) facets of metallic face-centered cubic Pd crystal [21]. Notably, compared to Pd/AC, the diffraction peaks of CP-CoOxVPd NC are significantly broadened, showed higher background (h) and shifted to lower angles (i.e., left side) (Figure 2b), suggesting reduced coherent length (particle size), considerable out-of-phase scattering from its surface with high roughness (due to presence of decorated CoPd species) and higher lattice constant (i.e., expansive strain), respectively. These observations are in good agreement with former HRTEM findings. The presence of peak X (corresponding to the Co3O4 (311)) in the XRD pattern of CP-CoOxVPd NC confirms that the Co is present in form of Co3O4 and exposed to the surface. In addition, the CP-CoOxVPd NC demonstrates a suppressed peak Y, corresponding to the Co2Pd (10–13°) plane, implying the existence of subnanometer Co2Pd alloys.
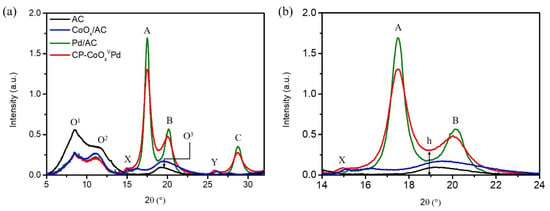
Figure 2.
(a) The comparative XRD patterns of CP-CoOxVPd NC and reference samples (AC, CoOx/AC, and Pd/AC). The enlarged XRD pattern at the 2θ range between 14° and 24° is shown in (b). The wavelength of incident X-ray for XRD measurement is 0.6888 Å (18.0 KeV).
To get more insights into the local atomic and electronic structure of Co atoms in CP-CoOxVPd NC, X-ray absorption spectroscopy (XAS) at Co K-edge was performed. Figure 3a shows the normalized X-ray absorption near edge structure (XANES) spectra of CP-CoOxVPd NC compared with reference samples (CoOx/AC), while the XANES spectra of standard CoO and Co3O4 are also compared. In the Co K-edge XANES spectra, the pre-edge “M” and the absorption edge “N” are corresponding to the 1s-to-3d and 1s-to-4p electron transitions, respectively [22]. Generally, the pre-edge elucidates the local symmetry of target atoms. Accordingly, the suppressed pre-edge intensities of standard samples (i.e., CoO and Co3O4) suggest their octahedral Co-configuration. CoOx/AC and CP-CoOxVPd NCs exhibit intense pre-edge intensity, suggesting tetrahedral coconfiguration (i.e., loss of O-atoms (presence of OV)) in these samples [22]. The inflection point (X) position indicates the oxidation state of the target atoms [23]. Notably, the inflection point of CP-CoOxVPd NC undergoes the lowest energy position among the samples under investigation, implying the lowest oxidation state of Co atoms in CP-CoOxVPd NC and therefore increased OV [24]. With the steric protection of Pd atoms to Co domains underneath, the higher white-line intensity (HM) of CP-CoOxVPd NC compared to CoOx/AC indicates the electron relocation from Co-to-Pd atoms. The Fourier-transformed extended X-ray absorption fine structure (FT-EXAFS) analysis further confirms the existence of OV sites in CP-CoOxVPd NC. Figure 3b shows the FT-EXAFS spectra of experimental samples at Co K-edge, while the model-simulated fitting curves are shown in Figure S3 and corresponding structural parameters summarized in Table 1. Accordingly, compared to the coordination number (CN) of the Co-Co bond pair (CNCo-Co = 1.29), the Co-O bond pair exhibits higher CN (CNCo-O = 2.55). Such a scenario indicates the severe oxidation of Co-atoms in CP-CoOxVPd NC. The CP-CoOxVPd NC shows lower CN for the Co-O bond pair (CNCoL-O+Co-O = 0.32 + 2.23 = 2.55) compared to CoOx/AC (CNCo-O = 2.94). The similar Co content and reduced CN for the Co-O bond pair confirms the presence of OV in CP-CoOxVPd NC [25]. In addition, the existence of a small CN for the Co-Pd bond pair (CNCo-Pd = 0.43) reveals a certain extent of Co-to-Pd heteroatomic intermixing and is consistent with former observations.
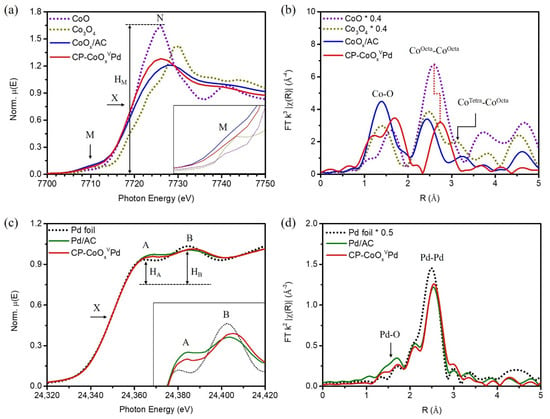
Figure 3.
X-ray absorption spectroscopy of the experimental NCs compared with reference samples. (a) XANES and (b) FT-EXAFS spectra of the experimental NCs at Co K-edge. (c) XANES and (d) FT-EXAFS spectra of the experimental NCs at Pd K-edge. In the FT-EXAFS spectra at Co K-edge, peak CoOcta-CoOcta is the contribution of coordinated Co atoms at octahedral symmetry from the 2nd coordination shell in CoO lattice. The peak of CoTetra-CoOcta is the contribution of coordinated Co atoms (tetrahedral symmetry) in the 2nd coordination shell of CoOx lattice. * The intensity of Pd foil is reduced by 50% for better representation.

Table 1.
Quantitative results of X-ray absorption spectroscopy model analysis at Co and Pd K-edges of experimental and reference NCs.
The results of Pd K-edge XAS analysis complementarily confirm the aforementioned scenarios. Figure 3c shows the normalized XANES spectra of experimental NCs at Pd K-edge, where the similar position of inflection point to Pd foil confirms the metallic state of Pd atoms in Pd/AC and CP-CoOxVPd NC. The two absorption edges “A” and “B” correspond to the electron transition from 1s to unoccupied 5p and 4f orbitals, respectively [25]. Accordingly, compared to Pd/AC, the suppressed intensity of peak “A” implies a slight extent of electron relocation from Co-to-Pd atoms in CP-CoOxVPd NC and is consistent with Co-K-edge XANES analysis. The FT-EXAFS spectra of CP-CoOxVPd NC are shown in Figure 3d, while the corresponding structural parameters are listed in Table 1 and the fitting curves shown in Figure S4. Consistent with XRD and Co K-edge FT-EXAFS results, the presence of a small CN for the Pd-Co bond pair (CNPd-Co = 0.55) confirms the formation of subnanometer CoPd alloys CP-CoOxVPd NC. In addition, the higher CN for the Pd-Pd bond pair compared to Pd-O complementarily proves the metallic characteristic of Pd atoms in CP-CoOxVPd NC. These scenarios are further confirmed by wavelet transformation (WT) patterns of the EXAFS spectrum at Co (Figure S5) and Pd K-edges (Figure S6) and discussed in the supplementary information.
X-ray photoelectron spectroscopy (XPS) was used to elucidate the oxidation states of constituting elements and to prove the presence of OV in CP-CoOxVPd NC. The XPS spectra of CoOx/AC and CP-CoOxVPd NC at Co-2p orbital are, respectively, shown in Figure 4a,b, whereas the corresponding deconvolution results are shown in Table S2. Previously published literature reported that a higher Co2+/Co3+ ratio implies the presence of OV [26]. Accordingly, compared to CoOx/AC (1.11), the higher Co2+/Co3+ (1.29) confirms the abundant OV sites in CP-CoOxVPd NC. Notably, with the similar Co content, a higher Co2+/Co3+ ratio indicates that the majority of OV sites are present in surface-anchored CoOxVPd clusters. The XPS spectra at the O-1s orbital further confirm these observations. Figure 4c,d, respectively, show the XPS spectra at the O-1s orbital of CoOx/AC and CP-CoOxVPd NC. The XPS spectra of the O-1s orbital are further deconvoluted to distinguish the signals from OV and lattice oxygen (denoted by OL), while the corresponding results are summarized in Table S2. Accordingly, the higher area under OV peak suggests the high density of OV sites in CP-CoOxVPd NC. The XPS analysis at Pd-3d orbital confirms these characteristics. Figure S7a,b, respectively, depict the XPS spectra of Pd/AC and CP-CoOxVPd NC at the Pd-3d orbital. Accordingly, the high Pd0/Pd2+ ratio (Table S2) confirms the metallic state of Pd atoms in Pd/AC and CP-CoOxVPd NC, which is in good agreement with XAS results. In contrast to XAS observations, the binding energies of Pd0 and Pd2+ are higher in CP-CoOxVPd NC than CoOx/AC (Table S2); however, this can be attributed to the electron transfer to surface OV sites.
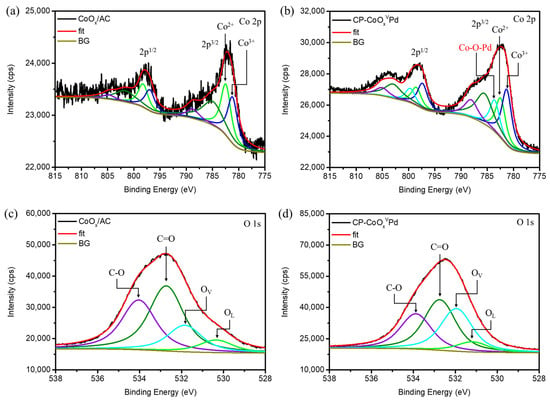
Figure 4.
X-ray photoelectron spectroscopy of (a) CoOx/AC and (b) CP-CoOxVPd NCs at Co-2p orbital. (c) CoOx/AC and (d) CP-CoOxVPd NCs at O-1s orbital.
Cyclic voltammetry (CV) and CO-stripping analysis have been used to confirm the surface chemical identities and geometric configuration of CP-CoOxVPd NC. As shown in Figure 5a, the two characteristic peaks Oads−1 and Oads−2 at the higher potential region in the forward sweep, respectively, correspond to the formation of Co hydroxide and oxide in CoOx/AC, while the presence of the broad Odes−1 peak in the backward sweep can be assigned to the desorption of oxygenated species from these reaction sites. The smeared peak profile (Hdes-Pd) in the forward sweep of the hydrogen underpotential deposition (HUPD) region confirms the high affinity towards proton adsorption for Pd/AC [27]. Notably, the CP-CoOxVPd NC shows a similar (smeared) peak profile to Pd/AC in HUPD region and to CoOx/AC (presence of peaks Oads−1 and Oads−2 in the forward sweep and peaks Odes−1 in the backward sweep) in higher potential regions, which confirms the formation of CoOx-supported Pd NPs and is consistent with our experimental design. In addition, both the Pd/AC and CP-CoOxVPd NC show an obvious oxide reduction peak Odes-Pd in the backward sweep, which corresponds to the oxide reduction from the Pd surface [28].
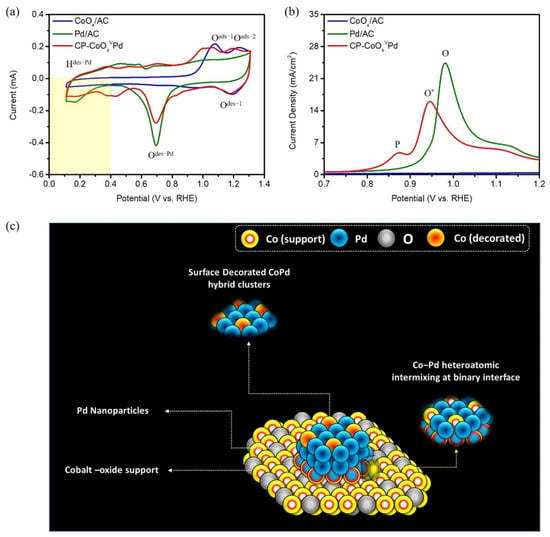
Figure 5.
(a) CV (b) CO-stripping curves CP-CoOxVPd NC compared with reference samples. (c) The schematic representation of the proposed geometric configuration of CP-CoOxVPd NC.
Figure 5b shows the CO-stripping curves of CP-CoOxVPd NC compared with reference samples, where the flat CO-stripping curve of CoOx/AC shows its inert nature towards CO oxidation. The strong CO oxidation peak (O) indicates a high density of reaction sites for CO adsorption and its subsequent reduction on the surface of Pd/AC [29]. For CP-CoOxVPd NC, an offset of the CO oxidation peak O* to lower potential indicates the reduced energy barrier for CO oxidation and can be attributed to the certain extent of charge localization (i.e., electron transfer) from Co-to-Pd atoms, which is consistent with former findings. The presence of a suppressed and broad peak “P” at lower potentials can be assigned to the current response due to the CO oxidation at the low-energy barrier reaction sites of the Co2Pd alloy. Of utmost importance, compared to Pd/AC, the suppression of the main CO oxidation peak “O” indicates the reduced reaction sites for CO oxidation on the surface of CP-CoOxVPd NC and can be attributed to the presence of inert CoOx species in form of CoPd hybrid clusters on the surface of CP-CoOxVPd NC. Based on the aforementioned physical and electrochemical results, the geometric configuration of CP-CoOxVPd NC is proposed and represented in Figure 5c.
Inspired by the potential geometric and electronic properties, the CO2 hydrogenation performance of CP-CoOxVPd NC was evaluated by a previously reported protocol [18,19], where the gaseous products were analyzed at ambient pressure using a gas chromatography (GC) system equipped with a PDHID detector. Figure 6a shows the CO production yield of CP-CoOxVPd NC and reference samples in the reaction gas (CO2:H2 = 1:3), while corresponding performance parameters are listed in Table S3. Accordingly, CoOx/AC, Pd/AC, and the physical mixture of CoOx+Pd are chemically inert toward CO2 below 423 K temperature, while the CO production yield for CP-CoOxVPd NC is 68.2 μmol g−1catalyst at 423 K, which continuously increased with raising the temperature to 573 K, and the highest CO production yield is ~3414 μmol g−1catalyst at 573 K. This value is ~90% and ~57% improved compared to the CoOx/AC (~348 μmol g−1catalyst) and Pd/AC (~1466 μmol g−1catalyst), respectively. In addition, the CP-CoOxVPd NC achieved CO selectivity as high as ~99%. Such high activity and CO selectivity of CP-CoOxVPd NC can be attributed to the presence of OV sites and adjacent Pd domains in subnanometer-scale CoPd clusters. Previously published literature reported that Pd atoms weakly adsorb CO2, while OV sites are favorable for adsorption and subsequent reduction of CO2 molecules [14,15]. These scenarios are consistently proved by assessing the CO2 hydrogenation performance of experimental NCs in the pure CO2 ambient. As shown in Figure S8a, both the CoOx/AC and Pd/AC are inactive toward CO2 in the pure CO2 ambient across the temperature range, while the CP-CoOxVPd NC shows a significant CO production yield in the high-temperature range, confirming that OV sites promote CO2 activation. These observations confirm that the OV and Pd sites synergistically trigger the CO production yield of CP-CoOxVPd NC, where OV sites and adjacent Pd domains, respectively, boost the CO2 activation and H2 dissociation. Finally, the much lower CH4 production yield in pure CO2 ambient (Figure S8b) and the reaction gas (Figure 6b) confirms the suppression of the competitive CO2 methanation process and thus high CO selectivity. The proposed mechanism of CO2 hydrogenation on the CP-CoOxVPd NC is shown in Figure 6c.
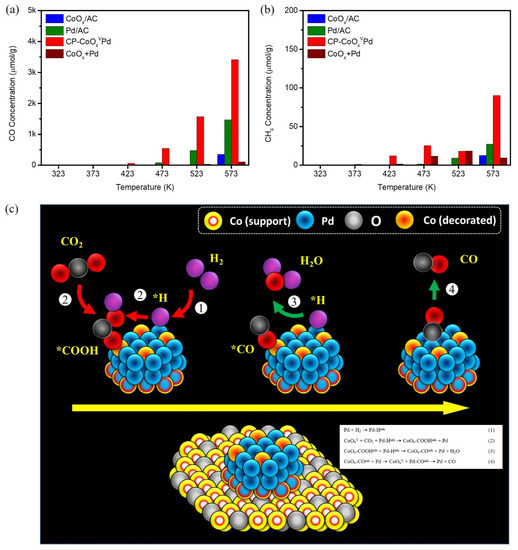
Figure 6.
Gas chromatography (GC)-determined (a) CO and (b) CH4 production yields of CP-CoOxVPd NC compared with CoOx/AC, Pd/AC and physical mixture of CoOx/AC+Pd/AC in the ambient of CO2 and H2 mixture with CO2:H2 ratio of 1:3. (c) The proposed mechanism of CO2 hydrogenation on the CP-CoOxVPd NC.
3. Experimental
Materials and Methods
Cobalt oxide-supported Pd NPs with OV-enriched CoPd hybrid cluster decoration were prepared via a galvanic replacement reaction-assisted wet chemical reduction method. Before synthesis, the catalyst carrier (i.e., carbon black; UR-XC72, UniRegion Bio-Tech, Taipei, Taiwan) was surface-functionalized with an adequate protocol for increasing the metal–support interaction [30]. The surface-functionalized carbon black was denoted as active carbon (AC) in the following of this article. Subsequently, 12 g (0.5 wt.% solution in D.I. water; i.e., the actual amount of AC is 60 mg) of AC was dispersed in 3.06 g of 0.1 M aqueous solution of cobalt (III) chloride (99%, CoCl3, Sigma-Aldrich Co, St. Louis, MO, USA) and stirred at 400 rpm for 4 h at room temperature (solution A) in the 1st step. Solution A (i.e., Co3+ adsorbed AC) contains a 30 wt.% weight ratio of Co to AC. In the 2nd step, 0.12 g of sodium borohydride (NaBH4; 99%, Sigma-Aldrich Co, St. Louis, MO, USA.) dissolved in 20 mL of D.I. water was instantly dropped into solution A and stirred at 400 rpm for 10 s to grow Co NPs on the AC surface (solution B). These Co NPs were partially oxidized in the ambient and resulting in a mixed metallic and oxide phase of Co (i.e., Co/CoOx). After that, in the 3rd step, 3.06 g of 0.1 M Pd precursor solution was mixed into solution B to grow the Pd NPs over CoOx support. In this step, the Pd2+ ions were reduced by the excessive amount of NaBH4 added in the 2nd step. The Pd precursor solution was prepared by dissolving palladium chloride (PdCl2, 99%, Sigma-Aldrich Co, St. Louis, MO, USA) in 1.0 M of HCl(aq). Herein, it is worth noting that the electronegativity of Pd atoms is higher than Co atoms and thus the galvanic replacement reaction between Pd2+ ions and metallic Co atoms is obvious. In this event, subnanometer-scale CoPd hybrid clusters are formed on the surface of Pd NPs due to galvanic replacement between Pd2+ ←→ Co followed by spontaneous deposition of residual Pd2+ and Co3+ ions. Generally, the galvanic replacement reaction is dominated by the electronegativity difference between two atoms. In this case, the electronegativity of Pd is higher than Co and thus, the galvanic replacement reaction between Pd2+ ions and Co0 is obvious. Finally, the as-prepared material was washed with acetone and D.I. water, centrifuged, and dried at 100 °C overnight. In the last step, the OV sites in the as-prepared sample were created by exposure to the ambient environment. Hereafter, the cobalt oxide-supported Pd NPs with OV-enriched CoPd hybrid cluster decoration are denoted as CP-CoOxVPd.
4. Conclusions
We have reported a novel heterogeneous nanocatalyst (NC) consisting of CoOx-supported Pd nanoparticles decorated with an oxygen vacancy-enriched subnanometer CoPd hybrid cluster (CP-CoOxVPd). The prepared material actively suppressed the competitive CO2 methanation reaction and thus achieved CO selectivity as high as ~99%. CP-CoOxVPd NC initiated the CO production at 423 K temperature and achieved an optimum CO production yield of ~3414 μmol g−1catalyst at 573 K in the reaction gas of CO2:H2 = 1:3. The cross-referencing results of physical characterizations, electrochemical analysis, and gas chromatography (GC) indicate that such a high CO2 hydrogenation performance of CP-CoOxVPd NC at low onset temperature originated from the synergistic cooperation between oxygen vacancies and adjacent Pd domains, where oxygen vacancies promoted the CO2 adsorption and neighboring Pd sites favored H2 splitting. In addition, the CoPd alloys at the heterogeneous interface of CoOx-to-Pd offered ideal desorption energy for subsequent reaction steps.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal12101127/s1, Figure S1: Low magnification TEM image of (a) Pd/AC and (b) CP-CoOxVPd NCs; Figure S2: HRTEM images of CoOx/AC. The Forward Fourier Transformed (FFT), Inverse Fourier Transformed (IFT) and the line histogram of are depicted in insets; Figure S3: Model analysis fitting curves compared with experimental FT-EXAFS spectra at Co K-edge of (a) CoO, (b) Co3O4, (c) CoOx/AC and (d) CP-CoOxVPd NCs. Figure S4: Model analysis fitting curves compared with experimental FT-EXAFS spectra at Pd K-edge of (a) Pd foil, (b) Pd/AC and (c) CP-CoOxVPd NCs; Figure S5: The WT for FT-EXAFS spectrum at Co K-edge. (a) Co3O4, (b) CoOx/AC, (c) CoO and (d) CP-CoOxVPd NPs. The intensity of Co3O4 and CoO are normalized by 0.4-fold as in FE-EXAFS; Figure S6: The WT for EXAFS spectrum at Pd K-edge. (a) Pd foil, (b) Pd/AC and (c) CP-CoOxVPd NCs; Figure S7: X-ray photoelectron spectroscopy of (a) Pd/AC and (b) CP-CoOxVPd NCs at Pd-3d orbital; Figure S8: The gas chromatography (GC) determined CO2RR results; Table S1: Benchmark of catalysts for RWGS application. Table S2: Quantitative results of X-ray photoemission spectroscopy model analysis at Co-2p, O-1s and Pd-3d orbitals of experimental and control NCs; Table S3: Calibrated product concentration of CO and CH4 [31,32,33,34,35,36,37,38,39,40,41].
Author Contributions
Conceptualization, T.-Y.C.; methodology, S.-S.Y.; software, Y.-X.C.; validation, D.B., A.B., C.Y. and P.-C.W.; formal analysis, D.B., Y.-C.C., C.-L.C.; investigation, C.Y.; resources, T.-Y.C.; visualization, S.-C.W.; supervision, T.-Y.C.; project administration, T.-Y.C.; funding acquisition, T.-Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Technology, Taiwan (MOST 109-2923-E-007-005-, MOST 109-3116-F-007-001-, and MOST 109-2112-M-007-030-MY3).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors express their gratitude to the staff of the National Synchrotron Radiation Research Center (NSRRC), Hsinchu, Taiwan for the help in various synchrotron-based measurements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Galadima, A.; Muraza, O. Catalytic thermal conversion of CO2 into fuels: Perspective and challenges. Renew. Sustain. Energy Rev. 2019, 115, 109333. [Google Scholar] [CrossRef]
- Deng, L.; Ai, X.; Xie, F.; Zhou, G. Efficient Ni-based catalysts for low-temperature reverse water-gas shift (RWGS) reaction. Chem. Asian J. 2021, 16, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P. Supported Catalysts for CO2 Methanation: A Review. Catalysts 2017, 7, 59. [Google Scholar] [CrossRef]
- Mebrahtu, C.; Krebs, F.; Abate, S.; Perathoner, S.; Centi, G.; Palkovits, R. Chapter 5—CO2 Methanation: Principles and Challenges. In Studies in Surface Science and Catalysis; Albonetti, S., Perathoner, S., Quadrelli, E.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 178, pp. 85–103. [Google Scholar]
- González-Castaño, M.; Dorneanu, B.; Arellano-García, H. The reverse water gas shift reaction: A process systems engineering perspective. React. Chem. Eng. 2021, 6, 954–976. [Google Scholar] [CrossRef]
- Bahmanpour, A.M.; Signorile, M.; Kröcher, O. Recent progress in syngas production via catalytic CO2 hydrogenation reaction. Appl. Catal. B Environ. 2021, 295, 120319. [Google Scholar] [CrossRef]
- Xu, J.; Su, X.; Duan, H.; Hou, B.; Lin, Q.; Liu, X.; Pan, X.; Pei, G.; Geng, H.; Huang, Y.; et al. Influence of pretreatment temperature on catalytic performance of rutile TiO2-supported ruthenium catalyst in CO2 methanation. J. Catal. 2016, 333, 227–237. [Google Scholar] [CrossRef]
- Su, X.; Yang, X.; Zhao, B.; Huang, Y. Designing of highly selective and high-temperature endurable RWGS heterogeneous catalysts: Recent advances and the future directions. J. Energy Chem. 2017, 26, 854–867. [Google Scholar] [CrossRef]
- Jangam, A.; Das, S.; Dewangan, N.; Hongmanorom, P.; Hui, W.M.; Kawi, S. Conversion of CO2 to C1 chemicals: Catalyst design, kinetics and mechanism aspects of the reactions. Catal. Today 2020, 358, 3–29. [Google Scholar] [CrossRef]
- Widmann, D.; Behm, R.J. Active Oxygen on a Au/TiO2 Catalyst: Formation, Stability, and CO Oxidation Activity. Angew. Chem. Int. Ed. 2011, 50, 10241–10245. [Google Scholar] [CrossRef]
- Fujita, S.-I.; Usui, M.; Takezawa, N. Mechanism of the reverse water gas shift reaction over Cu/ZnO catalyst. J. Catal. 1992, 134, 220–225. [Google Scholar] [CrossRef]
- Yu, Y.; Chan, Y.M.; Bian, Z.; Song, F.; Wang, J.; Zhong, Q.; Kawi, S. Enhanced performance and selectivity of CO2 methanation over g-C3N4 assisted synthesis of NiCeO2 catalyst: Kinetics and DRIFTS studies. Int. J. Hydrogen Energy 2018, 43, 15191–15204. [Google Scholar] [CrossRef]
- Goguet, A.; Meunier, F.C.; Tibiletti, D.; Breen, J.P.; Burch, R. Spectrokinetic Investigation of Reverse Water-Gas-Shift Reaction Intermediates over a Pt/CeO2 Catalyst. J. Phys. Chem. B 2004, 108, 20240–20246. [Google Scholar] [CrossRef]
- Zhu, M.; Ge, Q.; Zhu, X. Catalytic Reduction of CO2 to CO via Reverse Water Gas Shift Reaction: Recent Advances in the Design of Active and Selective Supported Metal Catalysts. Trans. Tianjin Univ. 2020, 26, 172–187. [Google Scholar] [CrossRef]
- Mora, J.C.; Nederstigt, Y.C.M.; Hill, J.M.; Ponnurangam, S. Promoting Effect of Supports with Oxygen Vacancies as Extrinsic Defects on the Reduction of Iron Oxide. J. Phys. Chem. C 2021, 125, 14299–14310. [Google Scholar] [CrossRef]
- Cárdenas-Arenas, A.; Quindimil, A.; Davó-Quiñonero, A.; Bailón-García, E.; Lozano-Castelló, D.; De-La-Torre, U.; Pereda-Ayo, B.; González-Marcos, J.A.; González-Velasco, J.R.; Bueno-López, A. Isotopic and in situ DRIFTS study of the CO2 methanation mechanism using Ni/CeO2 and Ni/Al2O3 catalysts. Appl. Catal. B Environ. 2020, 265, 118538. [Google Scholar] [CrossRef]
- Bahmanpour, A.M.; Héroguel, F.; Kılıç, M.; Baranowski, C.J.; Schouwink, P.; Röthlisberger, U.; Luterbacher, J.S.; Kröcher, O. Essential role of oxygen vacancies of Cu-Al and Co-Al spinel oxides in their catalytic activity for the reverse water gas shift reaction. Appl. Catal. B Environ. 2020, 266, 118669. [Google Scholar] [CrossRef]
- Yan, C.; Wang, C.-H.; Lin, M.; Bhalothia, D.; Yang, S.-S.; Fan, G.-J.; Wang, J.-L.; Chan, T.-S.; Wang, Y.-L.; Tu, X.; et al. Local synergetic collaboration between Pd and local tetrahedral symmetric Ni oxide enables ultra-high-performance CO2 thermal methanation. J. Mater. Chem. A 2020, 8, 12744–12756. [Google Scholar] [CrossRef]
- Bhalothia, D.; Hsiung, W.-H.; Yang, S.-S.; Yan, C.; Chen, P.-C.; Lin, T.-H.; Wu, S.-C.; Chen, P.-C.; Wang, K.-W.; Lin, M.-W.; et al. Submillisecond Laser Annealing Induced Surface and Subsurface Restructuring of Cu–Ni–Pd Trimetallic Nanocatalyst Promotes Thermal CO2 Reduction. ACS Appl. Energy Mater. 2021, 4, 14043–14058. [Google Scholar] [CrossRef]
- Bhalothia, D.; Chou, J.-P.; Yan, C.; Hu, A.; Yang, Y.-T.; Chen, T.-Y. Programming ORR Activity of Ni/NiOx@Pd Electrocatalysts via Controlling Depth of Surface-Decorated Atomic Pt Clusters. ACS Omega 2018, 3, 8733–8744. [Google Scholar] [CrossRef]
- Bhalothia, D.; Chen, P.-C.; Yan, C.; Wang, K.-W.; Chen, T.-Y. Heterogeneous NiO2-to-Pd Epitaxial Structure Performs Outstanding Oxygen Reduction Reaction Activity. J. Phys. Chem. C 2020, 124, 2295–2306. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, G.; Tong, H.; Liu, X.; Du, L.; Chen, N.; Wang, J.; Sun, T.; Regier, T.; Sun, S. Cobalt (II) oxide nanosheets with rich oxygen vacancies as highly efficient bifunctional catalysts for ultra-stable rechargeable Zn-air flow battery. Nano Energy 2021, 79, 105409. [Google Scholar] [CrossRef]
- Bhalothia, D.; Lin, C.-Y.; Yan, C.; Yang, Y.-T.; Chen, T.-Y. Effects of Pt metal loading on the atomic restructure and oxygen reduction reaction performance of Pt-cluster decorated Cu@Pd electrocatalysts. Sustain. Energy Fuels 2019, 3, 1668–1681. [Google Scholar] [CrossRef]
- Wang, J.; Lin, L.; He, Y.; Qin, H.; Yan, S.; Yang, K.; Li, A.; Liu, J. Vacancy-assisted oxygen reduction reaction on cobalt-based catalysts in direct borohydride fuel cell revealed by in-situ XAFS and XRD. Electrochim. Acta 2017, 254, 72–78. [Google Scholar] [CrossRef]
- Bhalothia, D.; Shuan, L.; Wu, Y.-J.; Yan, C.; Wang, K.-W.; Chen, T.-Y. A highly mismatched NiO2-to-Pd hetero-structure as an efficient nanocatalyst for the hydrogen evolution reaction. Sustain. Energy Fuels 2020, 4, 2541–2550. [Google Scholar] [CrossRef]
- Yuan, H.; Li, J.; Yang, W.; Zhuang, Z.; Zhao, Y.; He, L.; Xu, L.; Liao, X.; Zhu, R.; Mai, L. Oxygen Vacancy-Determined Highly Efficient Oxygen Reduction in NiCO2O4/Hollow Carbon Spheres. ACS Appl. Mater. Interfaces 2018, 10, 16410–16417. [Google Scholar] [CrossRef]
- Bhalothia, D.; Chen, P.-C.; Yan, C.; Yeh, W.; Tsai, D.-L.; Chan, T.-S.; Wang, K.-W.; Chen, T.-Y. Heterogeneous assembly of Pt-clusters on hierarchically structured CoOx@SnPd2@SnO2 quaternary nanocatalysts manifesting oxygen reduction reaction performance. New J. Chem. 2020, 44, 9712–9724. [Google Scholar] [CrossRef]
- Bhalothia, D.; Lin, C.-Y.; Yan, C.; Yang, Y.-T.; Chen, T.-Y. H2 Reduction Annealing Induced Phase Transition and Improvements on Redox Durability of Pt Cluster-Decorated Cu@Pd Electrocatalysts in Oxygen Reduction Reaction. ACS Omega 2019, 4, 971–982. [Google Scholar] [CrossRef]
- Bhalothia, D.; Huang, T.-H.; Chou, P.-H.; Wang, K.-W.; Chen, T.-Y. Promoting formic acid oxidation performance of Pd nanoparticles via Pt and Ru atom mediated surface engineering. RSC Adv. 2020, 10, 17302–17310. [Google Scholar] [CrossRef]
- Bhalothia, D.; Tsai, D.-L.; Wang, S.-P.; Yan, C.; Chan, T.-S.; Wang, K.-W.; Chen, T.-Y.; Chen, P.-C. Ir-oxide mediated surface restructure and corresponding impacts on durability of bimetallic NiOx@Pd nanocatalysts in oxygen reduction reaction. J. Alloys Compd. 2020, 844, 156160. [Google Scholar] [CrossRef]
- Ai, X.; Xie, H.M.; Chen, S.M.; Zhang, G.Z.; Xu, B.J.; Zhou, G.L. Highly dispersed mesoporous Cu/γ-Al2O3 catalyst for RWGS reaction. Int. J. Hydrogen Energy 2022, 47, 14884–14895. [Google Scholar] [CrossRef]
- Pastor-Pérez, L.; Baibars, F.; Le Sache, E.; Arellano-García, H.; Gu, S.; Reina, T.R. CO2 valorisation via reverse water-gas shift reaction using advanced Cs doped Fe-Cu/Al2O3 catalysts. J. CO2 Util. 2017, 21, 423–428. [Google Scholar] [CrossRef]
- Tarifa, P.; González-Castaño, M.; Cazaña, F.; Monzón, A.; Arellano-García, H. Development of one-pot Cu/cellulose derived carbon catalysts for RWGS reaction. Fuel 2022, 319, 123707. [Google Scholar] [CrossRef]
- Shen, H.D.; Dong, Y.J.; Yang, S.W.; He, Y.; Wang, Q.M.; Cao, Y.L.; Wang, W.B.; Wang, T.S.; Zhang, Q.Y.; Zhang, H.P. Identifying the roles of Ce3+− OH and Ce− H in the reverse water-gas shift reaction over highly active Ni-doped CeO2 catalyst. Nano Res. 2022, 15, 5831–5841. [Google Scholar] [CrossRef]
- Zonetti, P.C.; Letichevsky, S.; Gaspar, A.B.; Sousa-Aguiar, E.F.; Appel, L.G. The NixCe0.75Zr0.25−xO2 solid solution and the RWGS. Appl. Catal. A Gen. 2014, 475, 48–54. [Google Scholar] [CrossRef]
- Yang, L.Q.; Pastor-Perez, L.; Villora-Pico, J.J.; Sepulveda-Escribano, A.; Tian, F.X.; Zhu, M.H.; Han, Y.F.; Reina, T.R. Highly Active and Selective Multicomponent Fe–Cu/CeO2–Al2O3 Catalysts for CO2 Upgrading via RWGS: Impact of Fe/Cu Ratio. ACS Sustain. Chem. Eng. 2021, 9, 12155–12166. [Google Scholar] [CrossRef]
- Nityashree, N.; Price, C.A.H.; Pastor-Perez, L.; Manohara, G.V.; Garcia, S.; Maroto-Valer, M.M.; Reina, T.R. Carbon stabilised saponite supported transition metal-alloy catalysts for chemical CO2 utilisation via reverse water-gas shift reaction. Appl. Catal. B Environ. 2020, 261, 118241. [Google Scholar] [CrossRef]
- Okemoto, A.; Harada, M.R.; Ishizaka, T.; Hiyoshi, N.; Sato, K. Catalytic performance of MoO3/FAU zeolite catalysts modified by Cu for reverse water gas shift reaction. Appl. Catal. A Gen. 2020, 592, 117415. [Google Scholar] [CrossRef]
- Price, C.A.H.; Pastor-Perez, L.; Reina, T.R.; Liu, J. Yolk-Shell structured NiCo@ SiO2 nanoreactor for CO2 upgrading via reverse water-gas shift reaction. Catal. Today 2022, 383, 358–367. [Google Scholar] [CrossRef]
- Ronda-Lloret, M.; Yang, L.Q.Q.; Hammerton, M.; Marakatti, V.S.; Tromp, M.; Sofer, Z.; Sepulveda-Escribano, A.; Ramos-Fernandez, E.V.; Delgado, J.J.; Rothenberg, G.; et al. Molybdenum Oxide Supported on Ti3AlC2 is an Active Reverse Water–Gas Shift Catalyst. ACS Sustain. Chem. Eng. 2021, 9, 4957–4966. [Google Scholar] [CrossRef]
- Dostagir, N.H.M.; Rattanawan, R.; Gao, M.; Ota, J.; Hasegawa, J.Y.; Asakura, K.; Fukouka, A.; Shrotri, A. Co single atoms in ZrO2 with inherent oxygen vacancies for selective hydrogenation of CO2 to CO. ACS Catal. 2021, 11, 9450–9461. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).