A Descriptive Study of Repeated Hospitalizations and Survival of Patients with Metastatic Melanoma in the Northern Italian Region during 2004–2019
Abstract
1. Introduction
2. Material and Methods
2.1. Study Patients
2.2. Study Aims
2.3. Statistical Analysis
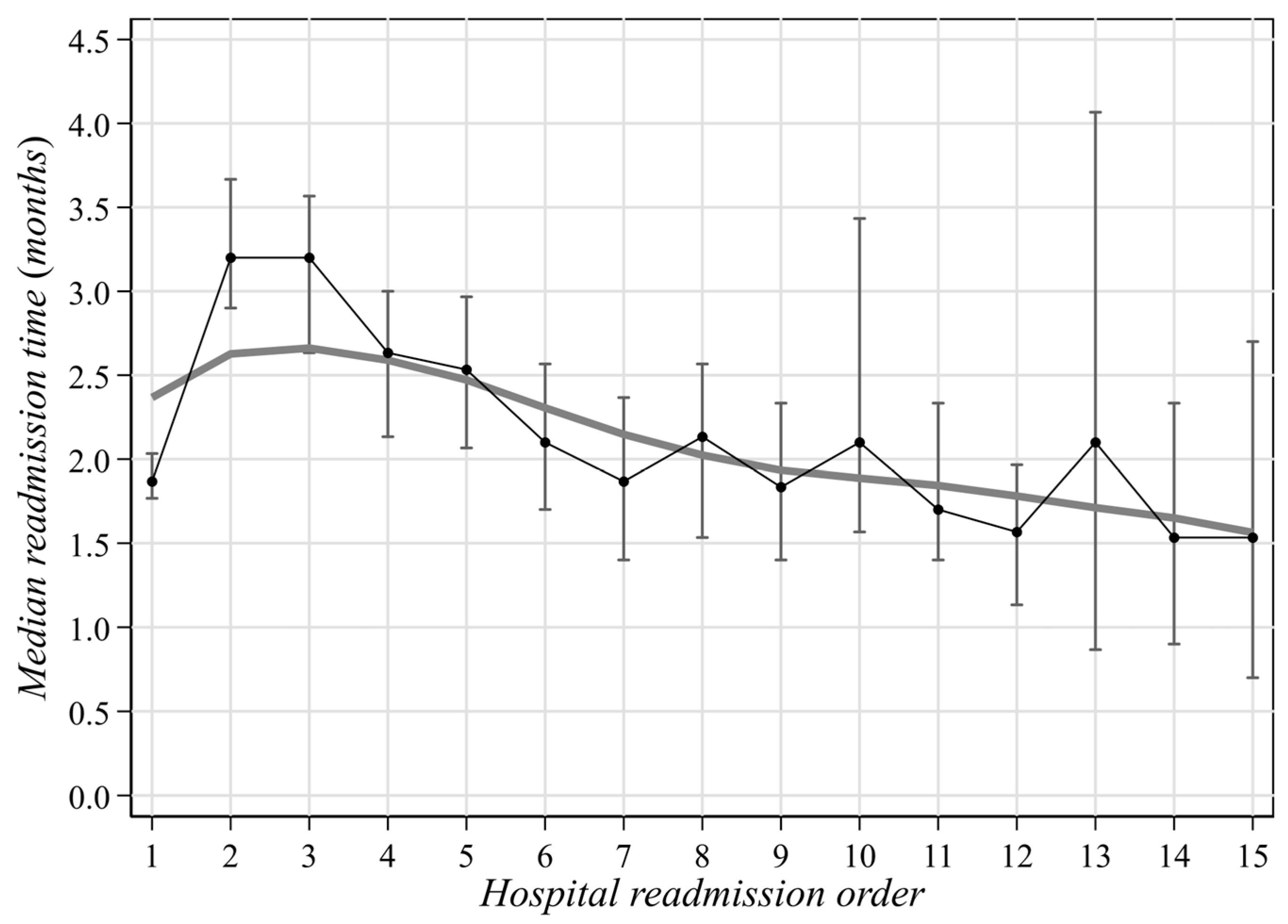
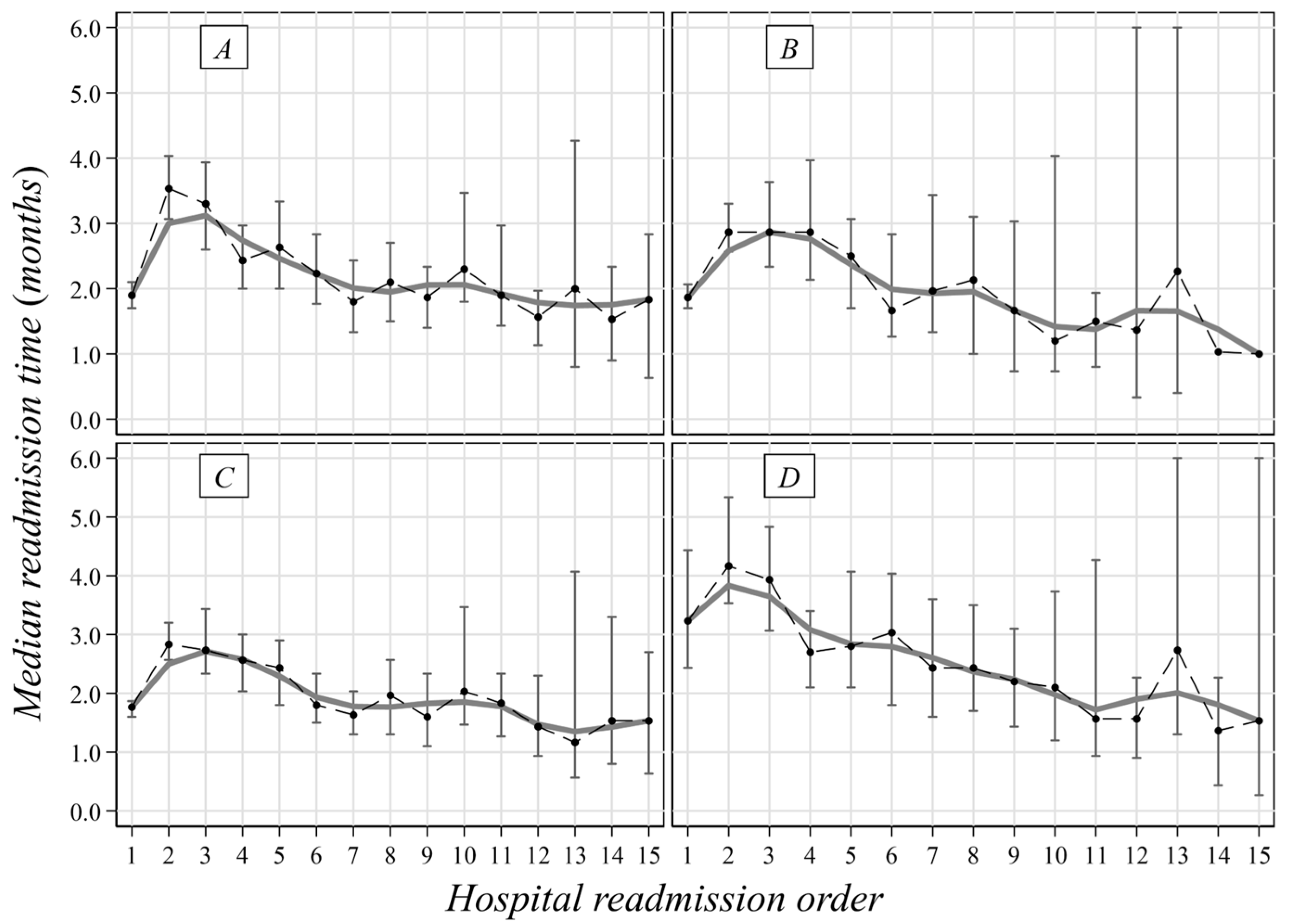
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maio, M.; Ascierto, P.; Testori, A.; Ridolfi, R.; Bajetta, E.; Queirolo, P.; Guida, M.; Romanini, A.; Chiarion-Sileni, V.; Pigozzo, J.; et al. The cost of unresectable stage III or stage IV Melanoma in Italy. J. Exp. Clin. Cancer Res. 2012, 31, 91. [Google Scholar] [CrossRef] [PubMed]
- Apalla, Z.; Lallas, A.; Sotiriou, E.; Lazaridou, E.; Ioannides, D. Epidemiological trends in skin cancer. Dermatol. Pract. Concept. 2017, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- AIOM-AIRTUM I Numeri del Cancro in Italia. 2020. Available online: https://www.registri-tumori.it/cms/sites/default/files/pubblicazioni/new_NDC2020-operatori-web.pdf (accessed on 16 May 2023).
- Bucchi, L.; Mancini, S.; Crocetti, E.; Dal Maso, L.; Baldacchini, F.; Vattiato, R.; Giuliani, O.; Ravaioli, A.; Caldarella, A.; Carrozzi, G.; et al. Mid-term trends and recent birth-cohort-dependent changes in incidence rates of cutaneous malignant melanoma in Italy. Int. J. Cancer 2021, 148, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Keilholz, U.; Ascierto, P.A.; Dummer, R.; Robert, C.; Lorigan, P.; Van Akkooi, A.; Arance, A.; Blank, C.U.; Chiarion Sileni, V.; Donia, M.; et al. ESMO consensus conference recommendations on the management of metastatic melanoma: Under the auspices of the ESMO Guidelines Committee. Ann. Oncol. 2020, 31, 1435–1448. [Google Scholar] [CrossRef]
- Garbe, C.; Peris, K.; Hauschild, A.; Saiag, P.; Middleton, M.; Bastholt, L.; Grob, J.J.; Malvehy, J.; Newton-Bishop, J.; Stratigos, A.J.; et al. Diagnosis and treatment of melanoma. European consensus-based interdisciplinary guideline—Update 2016. Eur. J. Cancer 2016, 63, 201–217. [Google Scholar] [CrossRef]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef]
- Moyers, J.T.; Patel, A.; Shih, W.; Nagaraj, G. Association of Sociodemographic Factors with Immunotherapy Receipt for Metastatic Melanoma in the US. JAMA Netw. Open 2020, 3, e2015656. [Google Scholar] [CrossRef]
- Wehler, E.; Zhao, Z.; Pinar Bilir, S.; Munakata, J.; Barber, B. Economic burden of toxicities associated with treating metastatic melanoma in eight countries. Eur. J. Health Econ. 2015, 18, 49–58. [Google Scholar] [CrossRef]
- Holterhues, C.; Hollestein, L.M.; Nijsten, T.; Koomen, E.R.; Nusselder, W.; De Vries, E. Burden of disease due to cutaneous melanoma has increased in the Netherlands since 1991. Br. J. Dermatol. 2013, 169, 389–397. [Google Scholar] [CrossRef]
- Johnston, K.; Levy, A.R.; Lorigan, P.; Maio, M.; Lebbe, C.; Middleton, M.; Testori, A.; Bédane, C.; Konto, C.; Dueymes, A.; et al. Economic impact of healthcare resource utilisation patterns among patients diagnosed with advanced melanoma in the United Kingdom, Italy, and France: Results from a retrospective, longitudinal survey (MELODY study). Eur. J. Cancer 2012, 48, 2175–2182. [Google Scholar] [CrossRef]
- Orso, M.; Serraino, D.; Abraha, I.; Fusco, M.; Giovannini, G.; Casucci, P.; Cozzolino, F.; Granata, A.; Gobbato, M.; Stracci, F.; et al. Validating malignant melanoma ICD-9-CM codes in Umbria, ASL Napoli 3 Sud and Friuli Venezia Giulia administrative healthcare databases: A diagnostic accuracy study. BMJ Open 2018, 8, e020631. [Google Scholar] [CrossRef] [PubMed]
- Gini, R.; Francesconi, P.; Mazzaglia, G.; Cricelli, I.; Pasqua, A.; Gallina, P.; Brugaletta, S.; Donato, D.; Donatini, A.; Marini, A.; et al. Chronic disease prevalence from Italian administrative databases in the VALORE project: A validation through comparison of population estimates with general practice databases and national survey. BMC Public Health 2013, 13, 15. [Google Scholar] [CrossRef]
- Hilbe, J.M. Negative Binomial Regression, 2nd ed.; CUP: Cambridge, UK, 2011. [Google Scholar]
- Dickman, P.W.; Coviello, E. Estimating and modeling relative survival. Stata J. 2015, 15, 186–215. [Google Scholar] [CrossRef]
- Lyth, M.; Falk, M.; Maroti, H.; Eriksson, C. Ingvar Prognostic risk factors of first recurrence in patients with primary stage I-II cutaneous malignant melanoma-from the population-based Swedish melanoma register. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1468–1474. [Google Scholar] [CrossRef]
- Svedman, F.C.; Pillas, D.; Taylor, A.; Kaur, M.; Linder, R.; Hansson, J. Stage-specific survival and recurrence in patients with cutaneous malignant melanoma in Europe—A systematic review of the literature. Clin. Epidemiol. 2016, 8, 109–122. [Google Scholar] [CrossRef]
- Villani, A.; Scalvenzi, M.; Fabbrocini, G.; Ocampo-Candiani, J.; Ocampo-Garza, S.S. Looking into a Better Future: Novel Therapies for Metastatic Melanoma. Dermatol. Ther. 2021, 11, 751–767. [Google Scholar] [CrossRef] [PubMed]
- Leeneman, B.; Franken, M.G.; Coupé, V.M.H.; Hendriks, M.P.; Kruit, W.; Plaisier, P.W.; Van Ruth, S.; Verstijnen, J.A.M.C.; Wouters, M.W.J.M.; Blommestein, H.M.; et al. Stage-specific disease recurrence and survival in localized and regionally advanced cutaneous melanoma. Eur. J. Surg. Oncol. 2019, 45, 825–831. [Google Scholar] [CrossRef]
- Von Schuckmann, L.A.; Hughes, M.C.B.; Ghiasvand, R.; Malt, M.; Van Der Pols, J.C.; Beesley, V.L.; Khosrotehrani, K.; Smithers, B.M.; Green, A.C. Risk of Melanoma Recurrence After Diagnosis of a High-Risk Primary Tumor. JAMA Dermatol. 2019, 55, 688–693. [Google Scholar] [CrossRef]
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 472–492. [Google Scholar] [CrossRef]
- Corrie, P.; Hategan, M.; Fife, K.; Parkinson, C. Management of melanoma. Br. Med. Bull. 2014, 111, 149–162. [Google Scholar] [CrossRef]
- Schadengarbedorf, D.; Livingstone, E.; Zimmer, L. Treatment in metastatic melanoma—Time to re-think. Ann. Oncol. 2019, 30, 501–503. [Google Scholar] [CrossRef] [PubMed]
- Michielin, O.; Van Akkooi, A.C.J.; Ascierto, P.A.; Dummer, R.; Keilholz, U.; ESMO Guidelines Committee. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1884–1901. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.J.; Flaherty, K.T.; Ribas, A.; Long, G.V. Targeted agents and immunotherapies: Optimizing outcomes in melanoma. Nat. Rev. Clin. Oncol. 2017, 14, 463–482. [Google Scholar] [CrossRef] [PubMed]
- Botta, L.; Dal Maso, L.; Guzzinati, S.; Panato, C.; Gatta, G.; Trama, A.; Rugge, M.; Tagliabue, G.; Casella, C.; Caruso, B.; et al. Changes in life expectancy for cancer patients over time since diagnosis. J. Adv. Res. 2019, 20, 153–159. [Google Scholar] [CrossRef]
- Dal Maso, L.; Panato, C.; Guzzinati, S.; Serraino, D.; Francisci, S.; Botta, L.; Capocaccia, R.; Tavilla, A.; Gigli, A.; Crocetti, E.; et al. Prognosis and cure of long-term cancer survivors: A population-based estimation. Cancer Med. 2019, 8, 4497–4507. [Google Scholar] [CrossRef] [PubMed]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef]
- Kandolf Sekulovic, L.; Peris, K.; Hauschild, A.; Stratigos, A.; Grob, J.J.; Nathan, P.; Dummer, R.; Forsea, A.M.; Hoeller, C.; Gogas, H.; et al. More than 5000 patients with metastatic melanoma in Europe per year do not have access to recommended first-line innovative treatments. Eur. J. Cancer 2017, 75, 313–322. [Google Scholar] [CrossRef]
- Kandel, M.; Dalle, S.; Bardet, A.; Allayous, C.; Mortier, L.; Dutriaux, C.; Guillot, B.; Leccia, M.T.; Dalac, S.; Legoupil, D.; et al. Quality-of-life assessment in French patients with metastatic melanoma in real life. Cancer 2020, 126, 611–618. [Google Scholar] [CrossRef]
- Dobry, A.S.; Zogg, C.K.; Hodi, F.S.; Smith, T.R.; Ott, P.A.; Iorgulescu, J.B. Management of metastatic melanoma: Improved survival in a national cohort following the approvals of checkpoint blockade immunotherapies and targeted therapies. Cancer Immunol. Immunother. 2018, 67, 1833–1844. [Google Scholar] [CrossRef]
- Terheyden, P.; Krackhardt, A.; Eigentler, T. The systemic treatment of melanoma: The place of immune checkpoint inhibitors and the suppression of intracellular signal transduction. Dtsch. Arztebl. Int. 2019, 116, 497–504. [Google Scholar] [CrossRef]
- McArthur, G.A.; Mohr, P.; Ascierto, P.A.; Arance, A.; Banos Hernaez, A.; Kaskel, P.; Weichenthal, M.; Shinde, R.; Stevinson, K. Health Care Resource Utilization and Associated Costs Among Metastatic Cutaneous Melanoma Patients Treated with Ipilimumab (INTUITION Study). Oncologist 2017, 22, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Hauschild, A.; Grob, J.J.; Demidov, L.V.; Jouary, T.; Gutzmer, R.; Millward, M.; Rutkowski, P.; Blank, C.U.; Miller, W.H., Jr.; Kaempgen, E.; et al. Dabrafenib in BRAF-mutated metastatic melanoma: A multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012, 380, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2018, 373, 23–34. [Google Scholar] [CrossRef]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef]
- Ascierto, P.A.; McArthur, G.A.; Dréno, B.; Atkinson, V.; Liszkay, G.; Di Giacomo, A.M.; Mandalà, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): Updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016, 17, 1248–1260. [Google Scholar] [CrossRef]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.; Weber, J.S.; et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann. Oncol. 2019, 30, 582–588. [Google Scholar] [CrossRef]
- Dai, W.F.; Beca, J.M.; Croxford, R.; Isaranawatchai, W.; Menjak, I.B.; Petrella, T.M.; Mittmann, N.; Earle, C.C.; Gavura, S.; Hanna, T.P.; et al. Real-world comparative effectiveness of second-line ipilimumab for metastatic melanoma: A population-based cohort study in Ontario, Canada. BMC Cancer 2020, 20, 304. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- O’Reilly, A.; Hughes, P.; Mann, J.; Lai, Z.; The, J.J.; Mclean, E.; Edmonds, K.; Lingard, K.; Chauhan, D.; Lynch, J.; et al. An immunotherapy survivor population: Health-related quality of life and toxicity in patients with metastatic melanoma treated with immune checkpoint inhibitors. Support Care Cancer 2019, 28, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Bregman, B.; Combemale, P.; Amaz, C.; de Léotoing, L.; Vainchtock, A.; Gaudin, A.F. Hospitalisation costs of metastatic melanoma in France; the MELISSA study (MELanoma In hoSpital coSts Assessment). BMC Health Serv. Res. 2017, 17, 542. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.F.; McDermott, D.F.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016, 17, 1558–1568. [Google Scholar] [CrossRef] [PubMed]
| ICD-9-CM Code a | Description |
|---|---|
| 172 | Malignant melanoma of skin |
| V10.82 | Medical history of melanoma |
| 196–199 | Secondary malignant neoplasm |
| V58.1 | Chemotherapy |
| 99.25 | Injection or infusion of cancer chemotherapeutic substance |
| 99.28 | Immunotherapy agent injection |
| Characteristics and Categories | Patients at H0 a | Readmission | ||||
|---|---|---|---|---|---|---|
| N b | % c | N b | % c | Median | IQR d | |
| Gender | ||||||
| Male | 898 | 57.2 | 3973 | 56.7 | 4 | 2–6 |
| Female | 672 | 42.8 | 3040 | 43.3 | 3 | 1–6 |
| Age at H0 a | ||||||
| 11–52 | 421 | 26.8 | 1870 | 26.7 | 3 | 2–6 |
| 53–64 | 343 | 21.8 | 1697 | 24.2 | 4 | 1–7 |
| 65–73 | 354 | 22.5 | 1795 | 25.6 | 4 | 2–7 |
| 74–96 | 452 | 28.8 | 1651 | 23.5 | 3 | 1–5 |
| Period of H0 a | ||||||
| 2004–2011 | 884 | 56.5 | 4617 | 65.8 | 4 | 2–7 |
| 2012–2019 | 686 | 43.7 | 2396 | 34.2 | 3 | 1–5 |
| Vital status at last discharge | ||||||
| Alive | 689 | 43.9 | 2476 | 35.3 | 3 | 1–6 |
| Dead | 881 | 56.1 | 4537 | 64.7 | 4 | 2–7 |
| Total | 1570 | 100.0 | 7013 | 100.0 | 3 | 1–6 |
| Characteristics and Categories | Days of Stay | Months between Hospitalizations | ||
|---|---|---|---|---|
| Median | IQR b | Median | IQR b | |
| Gender | ||||
| Male | 28 | 13–50 | 20 | 6–45 |
| Female | 30 | 13–52 | 22 | 7–54 |
| Age at H0 a | ||||
| 11–52 | 25 | 8–43 | 22 | 6–54 |
| 53–64 | 33 | 13–58 | 21 | 7–56 |
| 65–73 | 36 | 20–59 | 27 | 9–55 |
| 74–96 | 25 | 13–44 | 14 | 5–36 |
| Period of H0 a | ||||
| 2004–2011 | 32 | 16–56 | 26 | 9–64 |
| 2012–2019 | 24 | 8–45 | 16 | 4–38 |
| Vital status at last discharge | ||||
| Alive | 17 | 6–39 | 21 | 4–61 |
| Dead | 37 | 21–58 | 20 | 8–44 |
| Total | 29 | 13–51 | 20 | 7–49 |
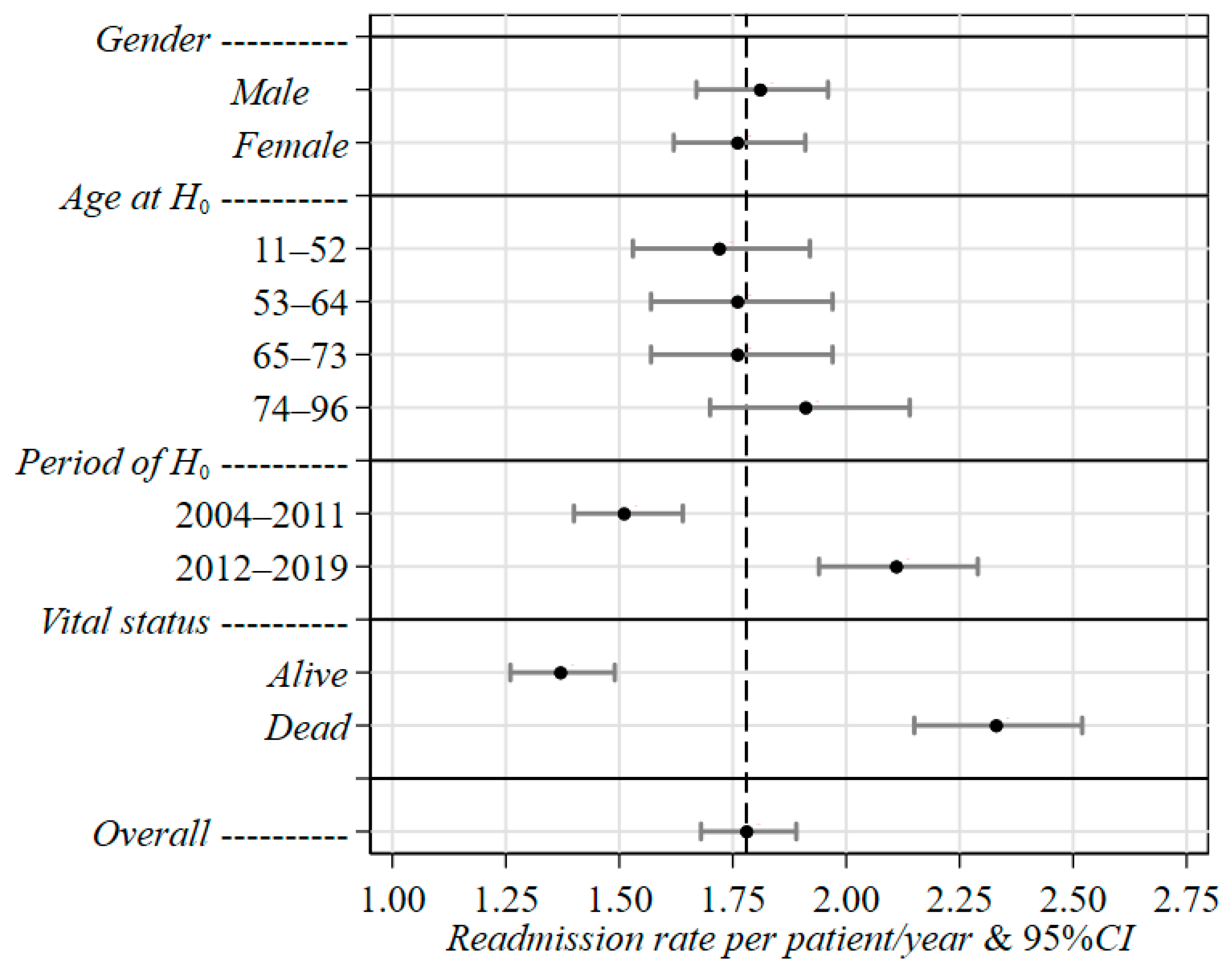
| Characteristics and Categories | Rate c | 95%CI e | RR d | 95%CI e | p-Value f |
|---|---|---|---|---|---|
| Constant a | 1.78 | 1.68–1.89 | – | – | – |
| Gender | 0.579 | ||||
| Male | 1.81 | 1.67–1.96 | 1.00 | (Ref.) g | |
| Female | 1.76 | 1.62–1.91 | 0.97 | 0.86–1.09 | |
| Age at H0 b | 0.584 | ||||
| 11–52 | 1.72 | 1.53–1.92 | 1.00 | (Ref.) g | |
| 53–64 | 1.76 | 1.57–1.97 | 1.02 | 0.87–1.20 | |
| 65–73 | 1.76 | 1.57–1.97 | 1.03 | 0.87–1.20 | |
| 74–96 | 1.91 | 1.70–2.14 | 1.11 | 0.95–1.31 | |
| Period of H0 b | <0.001 | ||||
| 2004–2011 | 1.51 | 1.40–1.64 | 1.00 | (Ref.) g | |
| 2012–2019 | 2.11 | 1.94–2.29 | 1.40 | 1.24–1.57 | |
| Vital status at last discharge | <0.001 | ||||
| Alive | 1.37 | 1.26–1.49 | 1.00 | (Ref.) g | |
| Dead | 2.33 | 2.15–2.51 | 1.70 | 1.52–1.91 |
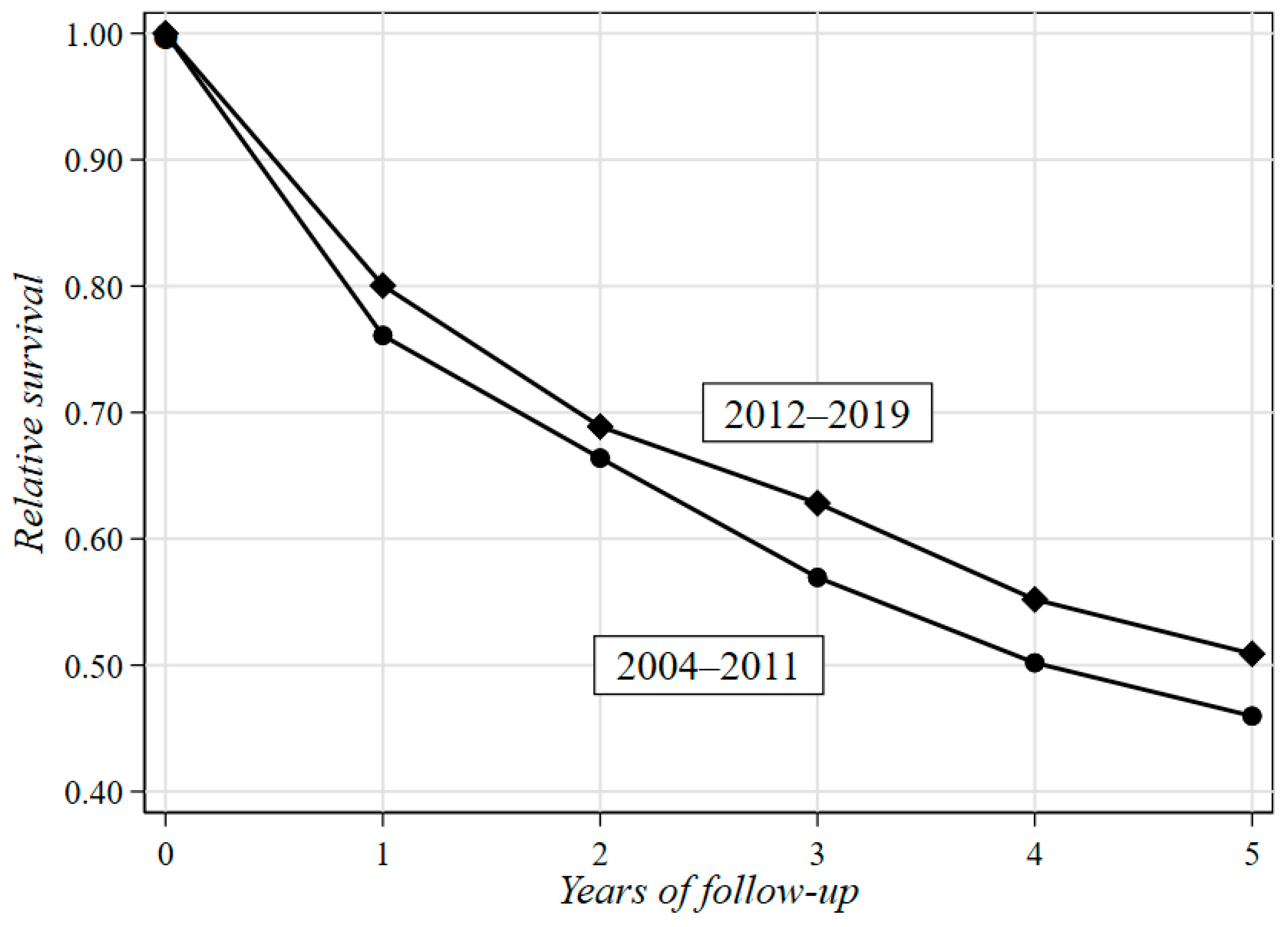
| Gender | Age at H0 a | Period of H0 a | Two-Year Life Expectancy | Five-Year Life Expectancy | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OS b | ES c | RS d | 95%CI e | OS b | ES c | RS d | 95%CI e | |||
| Male | 11–52 | 2004–2011 | 0.68 | 1.00 | 0.69 | 0.59–0.76 | 0.50 | 0.99 | 0.51 | 0.38–0.61 |
| 2012–2019 | 0.81 | 1.00 | 0.82 | 0.71–0.89 | 0.57 | 0.99 | 0.58 | 0.44–0.68 | ||
| 53–64 | 2004–2011 | 0.67 | 0.98 | 0.68 | 0.58–0.77 | 0.44 | 0.95 | 0.46 | 0.34–0.57 | |
| 2012–2019 | 0.73 | 0.99 | 0.74 | 0.60–0.84 | 0.50 | 0.96 | 0.52 | 0.36–0.66 | ||
| 65–73 | 2004–2011 | 0.73 | 0.96 | 0.76 | 0.67–0.83 | 0.36 | 0.88 | 0.41 | 0.29–0.53 | |
| 2012–2019 | 0.74 | 0.96 | 0.77 | 0.65–0.86 | 0.56 | 0.90 | 0.62 | 0.47–0.74 | ||
| 74–96 | 2004–2011 | 0.40 | 0.86 | 0.47 | 0.38–0.57 | 0.21 | 0.66 | 0.32 | 0.20–0.45 | |
| 2012–2019 | 0.40 | 0.87 | 0.46 | 0.34–0.57 | 0.25 | 0.68 | 0.38 | 0.23–0.52 | ||
| Female | 11–52 | 2004–2011 | 0.69 | 1.00 | 0.70 | 0.60–0.77 | 0.53 | 0.99 | 0.53 | 0.41–0.63 |
| 2012–2019 | 0.82 | 1.00 | 0.82 | 0.71–0.90 | 0.58 | 0.99 | 0.59 | 0.43–0.70 | ||
| 53–64 | 2004–2011 | 0.69 | 0.99 | 0.70 | 0.57–0.79 | 0.49 | 0.97 | 0.50 | 0.35–0.63 | |
| 2012–2019 | 0.68 | 0.99 | 0.69 | 0.54–0.79 | 0.45 | 0.98 | 0.45 | 0.28–0.61 | ||
| 65–73 | 2004–2011 | 0.72 | 0.98 | 0.73 | 0.61–0.83 | 0.46 | 0.94 | 0.49 | 0.33–0.63 | |
| 2012–2019 | 0.61 | 0.98 | 0.63 | 0.47–0.75 | 0.46 | 0.94 | 0.49 | 0.32–0.64 | ||
| 74–96 | 2004–2011 | 0.53 | 0.90 | 0.59 | 0.47–0.70 | 0.39 | 0.74 | 0.53 | 0.38–0.67 | |
| 2012–2019 | 0.56 | 0.91 | 0.62 | 0.49–0.73 | 0.37 | 0.78 | 0.47 | 0.31–0.62 | ||
| Overall | 0.64 | 0.96 | 0.67 | 0.64–0.70 | 0.43 | 0.89 | 0.48 | 0.45–0.52 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mannucci, M.; Fontana, V.; Campanella, D.; Filiberti, R.A.; Pronzato, P.; Rosa, A. A Descriptive Study of Repeated Hospitalizations and Survival of Patients with Metastatic Melanoma in the Northern Italian Region during 2004–2019. Curr. Oncol. 2023, 30, 5266-5278. https://doi.org/10.3390/curroncol30060400
Mannucci M, Fontana V, Campanella D, Filiberti RA, Pronzato P, Rosa A. A Descriptive Study of Repeated Hospitalizations and Survival of Patients with Metastatic Melanoma in the Northern Italian Region during 2004–2019. Current Oncology. 2023; 30(6):5266-5278. https://doi.org/10.3390/curroncol30060400
Chicago/Turabian StyleMannucci, Matilde, Vincenzo Fontana, Dalila Campanella, Rosa Angela Filiberti, Paolo Pronzato, and Alessandra Rosa. 2023. "A Descriptive Study of Repeated Hospitalizations and Survival of Patients with Metastatic Melanoma in the Northern Italian Region during 2004–2019" Current Oncology 30, no. 6: 5266-5278. https://doi.org/10.3390/curroncol30060400
APA StyleMannucci, M., Fontana, V., Campanella, D., Filiberti, R. A., Pronzato, P., & Rosa, A. (2023). A Descriptive Study of Repeated Hospitalizations and Survival of Patients with Metastatic Melanoma in the Northern Italian Region during 2004–2019. Current Oncology, 30(6), 5266-5278. https://doi.org/10.3390/curroncol30060400





