Patient Perceived Financial Burden in Haematological Malignancies: A Systematic Review
Abstract
:1. Introduction
- How is financial burden assessed?
- What out-of-pocket costs contribute to financial burden (objective financial burden)?
- What are the impacts of financial burden (subjective financial burden)?
- What is the patient experience of financial burden?
2. Materials and Methods
2.1. Design
2.2. Search Strategy
2.3. Study Selection
2.4. Data Extraction
2.5. Quality and Risk-of-Bias Assessment
2.6. Data Synthesis
3. Results
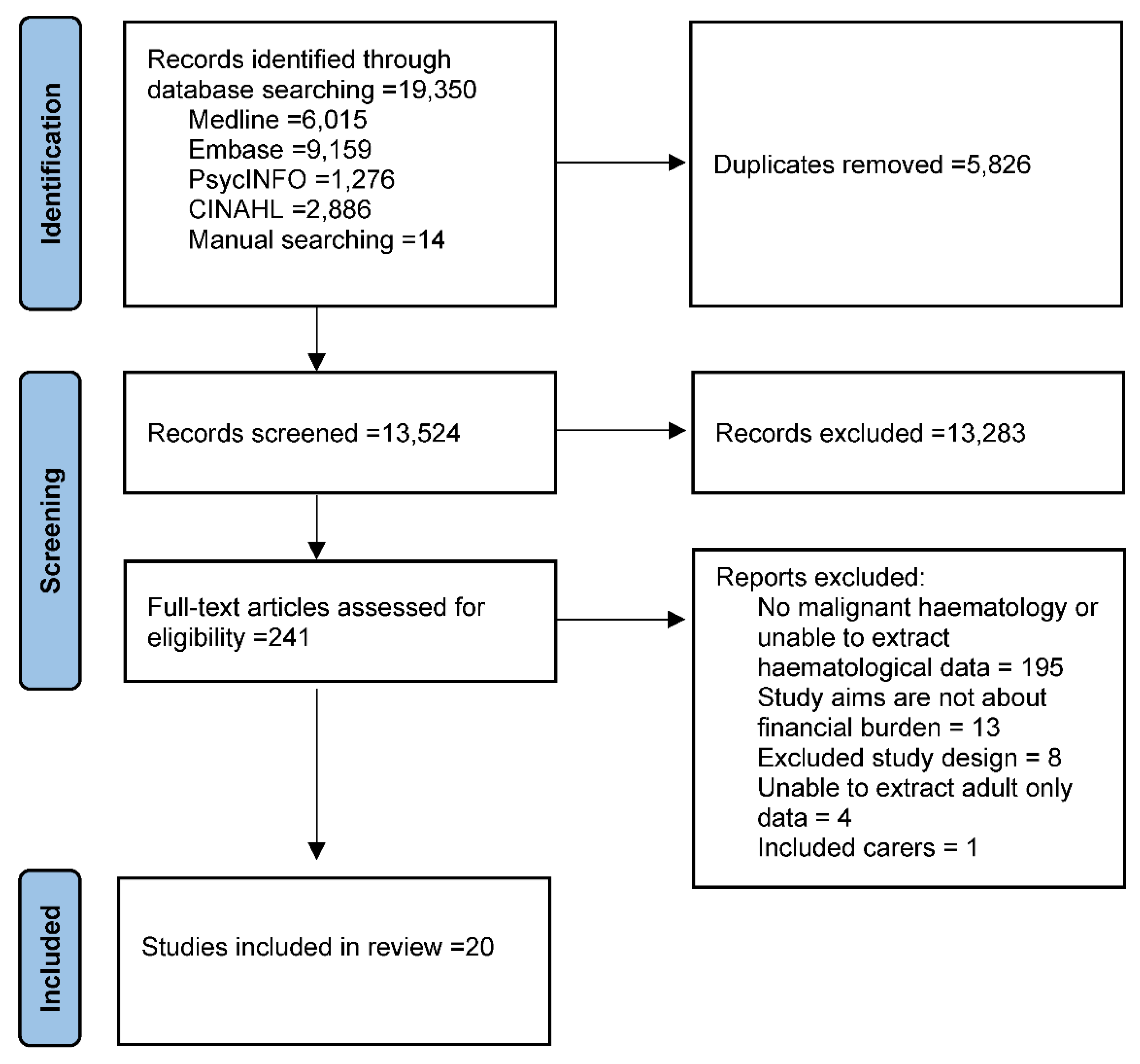
3.1. Study Selection and Inclusion
3.2. Study Characteristics
3.3. RQ1: How Was Financial Burden Assessed?
3.4. RQ2: What Out-of-Pocket Costs Contribute to Financial Burden?
3.5. RQ3: What Are the Reported Impacts of Financial Burden?
3.6. RQ4: What Is the Patient Experience of Financial Burden?
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tietsche de Moraes Hungria, V.; Chiattone, C.; Pavlovsky, M.; Abenoza, L.M.; Agreda, G.P.; Armenta, J.; Arrais, C.; Flores, O.A.; Barroso, F.; Basquiera, A.L.; et al. Epidemiology of Hematologic Malignancies in Real-World Settings: Findings From the Hemato-Oncology Latin America Observational Registry Study. J. Glob. Oncol. 2019, 5, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Dohner, H.; Estey, E.H.; Amadori, S.; Appelbaum, F.R.; Buchner, T.; Burnett, A.K.; Dombret, H.; Fenaux, P.; Grimwade, D.; Larson, R.A.; et al. Diagnosis and management of acute myeloid leukemia in adults: Recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 2010, 115, 453–474. [Google Scholar] [CrossRef] [PubMed]
- Altice, C.K.; Banegas, M.P.; Tucker-Seeley, R.D.; Yabroff, K.R. Financial Hardships Experienced by Cancer Survivors: A Systematic Review. J. Natl. Cancer Inst. 2017, 109, djw205. [Google Scholar] [CrossRef]
- Zafar, S.Y.; Abernethy, A.P. Financial Toxicity, Part I: A New Name for a Growing Problem. Oncology 2013, 27, 80–149. [Google Scholar] [PubMed]
- Gordon, L.; Merollini, K.M.D.; Lowe, A.; Chan, R.J. A Systematic Review of Financial Toxicity Among Cancer Survivors: We Can’t Pay the Co-Pay. Patient—Patient-Cent. Outcomes Res. 2017, 10, 295–309. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.L.; Lopez-Olivo, M.A.; Advani, P.G.; Ning, M.S.; Geng, Y.; Giordano, S.H.; Volk, R.J. Financial Burdens of Cancer Treatment: A Systematic Review of Risk Factors and Outcomes. J. Natl. Compr. Canc. Netw. 2019, 17, 1184–1192. [Google Scholar] [CrossRef]
- Zafar, S.Y.; McNeil, R.B.; Thomas, C.M.; Lathan, C.S.; Ayanian, J.Z.; Provenzale, D. Population-Based Assessment of Cancer Survivors’ Financial Burden and Quality of Life: A Prospective Cohort Study. J. Oncol. Pr. 2015, 11, 145–150. [Google Scholar] [CrossRef]
- Delgado-Guay, M.; Ferrer, J.; Rieber, A.G.; Rhondali, W.; Tayjasanant, S.; Ochoa, J.; Cantu, H.; Chisholm, G.; Williams, J.; Frisbee-Hume, S.; et al. Financial Distress and Its Associations with Physical and Emotional Symptoms and Quality of Life Among Advanced Cancer Patients. Oncologist 2015, 20, 1092–1098. [Google Scholar] [CrossRef] [Green Version]
- Lathan, C.S.; Cronin, A.; Tucker-Seeley, R.; Zafar, S.Y.; Ayanian, J.Z.; Schrag, D. Association of Financial Strain with Symptom Burden and Quality of Life for Patients with Lung or Colorectal Cancer. J. Clin. Oncol. 2016, 34, 1732–1740. [Google Scholar] [CrossRef] [Green Version]
- Chino, F.; Peppercorn, J.; Taylor, D.H.; Lu, Y.; Samsa, G.; Abernethy, A.P.; Zafar, S.Y. Self-Reported Financial Burden and Satisfaction with Care Among Patients with Cancer. Oncologist 2014, 19, 414–420. [Google Scholar] [CrossRef] [Green Version]
- Zafar, S.Y.; Peppercorn, J.M.; Schrag, D.; Taylor, D.H.; Goetzinger, A.M.; Zhong, X.; Abernethy, A.P. The Financial Toxicity of Cancer Treatment: A Pilot Study Assessing Out-of-Pocket Expenses and the Insured Cancer Patient’s Experience. Oncologist 2013, 18, 381–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bestvina, C.M.; Zullig, L.L.; Rushing, C.; Chino, F.; Samsa, G.P.; Altomare, I.; Tulsky, J.; Ubel, P.; Schrag, D.; Nicolla, J.; et al. Patient-Oncologist Cost Communication, Financial Distress, and Medication Adherence. J. Oncol. Pr. 2014, 10, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Kent, E.E.; Forsythe, L.P.; Yabroff, K.R.; Weaver, K.E.; de Moor, J.S.; Rodriguez, J.L.; Rowland, J.H. Are survivors who report cancer-related financial problems more likely to forgo or delay medical care? Cancer 2013, 119, 3710–3717. [Google Scholar] [CrossRef] [PubMed]
- Jagsi, R.; Pottow, J.A.E.; Griffith, K.A.; Bradley, C.; Hamilton, A.S.; Graff, J.; Katz, S.J.; Hawley, S.T. Long-term financial burden of breast cancer: Experiences of a diverse cohort of survivors identified through population-based registries. J. Clin. Oncol. 2014, 32, 1269–1276. [Google Scholar] [CrossRef] [Green Version]
- Ramsey, S.D.; Bansal, A.; Fedorenko, C.R.; Blough, D.K.; Overstreet, K.A.; Shankaran, V.; Newcomb, P. Financial Insolvency as a Risk Factor for Early Mortality Among Patients with Cancer. J. Clin. Oncol. 2016, 34, 980–986. [Google Scholar] [CrossRef] [Green Version]
- Azzani, M.; Roslani, A.C.; Su, T.T. The perceived cancer-related financial hardship among patients and their families: A systematic review. Support. Care Cancer 2015, 23, 889–898. [Google Scholar] [CrossRef]
- Longo, C.J.; Fitch, M.I.; Banfield, L.; Hanly, P.; Yabroff, K.R.; Sharp, L. Financial toxicity associated with a cancer diagnosis in publicly funded healthcare countries: A systematic review. Support. Care Cancer 2020, 28, 4645–4665. [Google Scholar] [CrossRef]
- National Institute for Health Research. PROSPERO: International Prospective Register of Systematic Reviews [Internet]. PROSPERO. Available online: https://www.crd.york.ac.uk/prospero/#aboutpage (accessed on 16 May 2022).
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Joanna Briggs Institute. Critical-Appraisal-Tools—Critical Appraisal Tools | Joanna Briggs Institute [Internet]. Available online: https://jbi.global/critical-appraisal-tools (accessed on 7 June 2021).
- Lizarondo, L.; Stern, C.; Carrier, J.; Godfrey, C.; Rieger, K.; Salmond, S.; Apóstolo, J.L.A.; Kirkpatrick, P.; Loveday, H. Chapter 8: Mixed Methods Systematic Reviews. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; JBI: Adelaide, Australia, 2020; Available online: https://wiki.jbi.global/display/MANUAL/Chapter+8%3A+Mixed+methods+systematic+reviews (accessed on 14 August 2021).
- Popay, J.; Roberts, H.; Sowden, A.; Petticrew, M.; Arai, L.; Rodgers, M.; Britten, N.; Roen, K.; Duffy, S. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews: A Product from the ESRC Methods Programme; Lamcaster University: Lancaster, UK, 2006. [Google Scholar]
- Abel, G.A.; Albelda, R.; Khera, N.; Hahn, T.; Salas Coronado, D.Y.; Odejide, O.O.; Bona, K.; Tucker-Seeley, R.; Soiffer, R. Financial Hardship and Patient-Reported Outcomes after Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2016, 22, 1504–1510. [Google Scholar] [CrossRef] [Green Version]
- Albelda, R.; Wiemers, E.; Hahn, T.; Khera, N.; Salas Coronado, D.Y.; Abel, G.A. Relationship between paid leave, financial burden, and patient-reported outcomes among employed patients who have undergone bone marrow transplantation. Qual. Life Res. 2019, 28, 1835–1847. [Google Scholar] [CrossRef]
- Bala-Hampton, J.E.; Dudkaj, L.; Albrecht, T.; Rosenzweig, M. Perceived economic hardship and distress in acute myelogenous leukemia. J. Oncol. Navig. Surviv. 2017, 8, 258–264. [Google Scholar]
- Buzaglo, J.S.; Miller, M.; Karten, C.; Longacre, M.; Onukwugha, E.; Weiss, E. Medication Adherence among Patients with Chronic Myeloid Leukemia: The Impact of Financial Burden and Psychosocial Distress. J. Oncol. Navig. Surviv. 2017, 8, 168–175. Available online: https://www.jons-online.com/issues/2017/april-2017-vol-9-no-4?view=article&artid=1618:medication-adherence-among-patients-with-chronic-myeloid-leukemia-the-impact-of-financial-burden-and-psychosocial-distress (accessed on 3 August 2021).
- Fenn, K.M.; Evans, S.B.; McCorkle, R.; DiGiovanna, M.P.; Pusztai, L.; Sanft, T.; Hofstatter, E.W.; Killelea, B.K.; Knobf, M.T.; Lannin, D.R.; et al. Impact of Financial Burden of Cancer on Survivors’ Quality of Life. J. Oncol. Pr. 2014, 10, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, J.A.; Coleman, E.A.; Sullivan, E.; Easley, R.; McNatt, P.K.; Chowdhury, N.; Stewart, C.B. Personal Financial Effects of Multiple Myeloma and Its Treatment. Cancer Nurs. 2013, 36, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, J.G.; Wu, L.M.; Austin, J.E.; Valdimarsdottir, H.; Basmajian, K.; Vu, A.; Rowley, S.D.; Isola, L.; Redd, W.H.; Rini, C. Economic survivorship stress is associated with poor health-related quality of life among distressed survivors of hematopoietic stem cell transplantation: Economic survivorship stress and HRQOL. Psycho-Oncol. 2013, 22, 911–921. [Google Scholar] [CrossRef]
- Huntington, S.F.; Weiss, B.M.; Vogl, D.T.; Cohen, A.D.; Garfall, A.L.; Mangan, P.A.; Doshi, J.A.; Stadtmauer, E.A. Financial toxicity in insured patients with multiple myeloma: A cross-sectional pilot study. Lancet Haematol. 2015, 2, e408–e416. [Google Scholar] [CrossRef]
- Khera, N.; Albelda, R.; Hahn, T.; Coronado, D.S.; Odejide, O.O.; Soiffer, R.J.; Abel, G.A. Financial Hardship after Hematopoietic Cell Transplantation: Lack of Impact on Survival. Cancer Epidemiol. Biomark. Prev. 2018, 27, 345–347. [Google Scholar] [CrossRef] [Green Version]
- Head, B.; Harris, L.; Kayser, K.; Martin, A.; Smith, L. As if the disease was not enough: Coping with the financial consequences of cancer. Support. Care Cancer 2018, 26, 975–987. [Google Scholar] [CrossRef]
- Jella, T.K.; Cwalina, T.B.; Treisman, J.; Hamadani, M. Risk Factors for Cost-Related Delays to Medical Care Among Lymphoma Patients: A 22-Year Analysis of a Nationally Representative Sample. Clin. Lymphoma Myeloma Leuk. 2021, 21, e619–e625. [Google Scholar] [CrossRef]
- Gupta, S.; Abouzaid, S.; Liebert, R.; Parikh, K.; Ung, B.; Rosenberg, A.S. Assessing the Effect of Adherence on Patient-reported Outcomes and Out of Pocket Costs Among Patients with Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2018, 18, 210–218. [Google Scholar] [CrossRef] [Green Version]
- McGrath, P. Financial distress during relocation for treatment of a hematological malignancy: Findings for social work. Soc. Work Health Care 2016, 55, 265–279. [Google Scholar] [CrossRef] [PubMed]
- McGrath, P. ‘The bills that were coming in…’: Out of pocket costs during relocation for specialist treatment for haematological malignancies. Support. Care Cancer 2016, 24, 2893–2903. [Google Scholar] [CrossRef] [PubMed]
- McGrath, P. The Use of Credit Cards in Response to the Crisis of Serious Illness. Illn. Crisis Loss 2016, 24, 46–56. [Google Scholar] [CrossRef]
- McGrath, P. Informal financial assistance for patients with a hematological malignancy: Implications for oncology social work practice. Soc. Work Health Care 2015, 54, 892–908. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.L.; Hall, A.E.; Carey, M.L.; Cameron, E.C.; Clinton-McHarg, T. Access to Care and Impacts of Cancer on Daily Life: Do They Differ for Metropolitan Versus Regional Hematological Cancer Survivors? J. Rural. Health 2013, 29, s43–s50. [Google Scholar] [CrossRef]
- Parsons, J.A.; Greenspan, N.R.; Baker, N.A.; McKillop, C.; Hicks, L.K.; Chan, O. Treatment preferences of patients with relapsed and refractory multiple myeloma: A qualitative study. BMC Cancer 2019, 19, 264. [Google Scholar] [CrossRef]
- Wang, J.W.; Shen, Q.; Ding, N.; Zhang, T.R.; Yang, Z.Q.; Liu, C.; Chen, S.J.; Berry, H.L.; Yuan, Z.P.; Yu, J.M. A qualitative exploration of the unmet psychosocial rehabilitation needs of cancer survivors in China: Psychosocial rehabilitation needs. Psycho-Oncol. 2016, 25, 905–912. [Google Scholar] [CrossRef]
- Tan, B.K.; Tan, S.B.; Chen, L.C.; Chang, K.M.; Chua, S.S.; Balashanker, S.; Jaman, H.N.B.K.; Edmund, S.C.; Bee, P.C. Medication-related issues associated with adherence to long-term tyrosine kinase inhibitors for controlling chronic myeloid leukemia: A qualitative study. Patient Prefer. Adherence 2017, 11, 1027–1034. [Google Scholar] [CrossRef] [Green Version]
- Lantz, P.M.; House, J.S.; Mero, R.P.; Williams, D.R. Stress, Life Events, and Socioeconomic Disparities in Health: Results from the Americans’ Changing Lives Study. J. Health Soc. Behav. 2005, 46, 274–288. [Google Scholar] [CrossRef] [Green Version]
- Tucker-Seeley, R.D.; Yabroff, K.R. Minimizing the “Financial Toxicity” Associated with Cancer Care: Advancing the Research Agenda. JNCI J. Natl. Cancer Inst. 2016, 108, djv410. [Google Scholar] [CrossRef] [Green Version]
- Martinez, R.G.; Lewis, C.C.; Weiner, B.J. Instrumentation issues in implementation science. Implement. Sci. 2014, 9, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrera, P.M.; Kantarjian, H.M.; Blinder, V.S. The financial burden and distress of patients with cancer: Understanding and stepping-up action on the financial toxicity of cancer treatment. CA A Cancer J. Clin. 2018, 68, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Witte, J.; Mehlis, K.; Surmann, B.; Lingnau, R.; Damm, O.; Greiner, W.; Winkler, E. Methods for measuring financial toxicity after cancer diagnosis and treatment: A systematic review and its implications. Ann. Oncol. 2019, 30, 1061–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yabroff, K.R.; Zhao, J.; Han, X.; Zheng, Z. Prevalence and Correlates of Medical Financial Hardship in the USA. J. Gen. Intern. Med. 2019, 34, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Jemal, A.; Han, X.; Guy, G.P., Jr.; Li, C.; Davidoff, A.J.; Banegas, M.P.; Ekwueme, D.U.; Yabroff, K.R. Medical financial hardship among cancer survivors in the United States. Cancer 2019, 125, 1737–1747. [Google Scholar] [CrossRef] [PubMed]
- Meneses, K.; Azuero, A.; Hassey, L.; McNees, P.; Pisu, M. Does Economic Burden Influence Quality of Life in Breast Cancer Survivors? Gynecol. Oncol. 2012, 124, 437–443. [Google Scholar] [CrossRef] [Green Version]
- Sharp, L.; Carsin, A.E.; Timmons, A. Associations between cancer-related financial stress and strain and psychological well-being among individuals living with cancer. Psycho-Oncol. 2013, 22, 745–755. [Google Scholar] [CrossRef]
- Lyman, G.H.; Henk, H.J. Association of Generic Imatinib Availability and Pricing with Trends in Tyrosine Kinase Inhibitor Use in Patients with Chronic Myelogenous Leukemia. JAMA Oncol. 2020, 6, 1969–1971. [Google Scholar] [CrossRef]
- Hahn, T.; Paplham, P.; Austin-Ketch, T.; Zhang, Y.; Grimmer, J.; Burns, M.; Balderman, S.; Ross, M.; McCarthy, P.L. Ascertainment of Unmet Needs and Participation in Health Maintenance and Screening of Adult Hematopoietic Cell Transplantation Survivors Followed in a Formal Survivorship Program. Biol. Blood Marrow Transplant. 2017, 23, 1968–1973. [Google Scholar] [CrossRef]
- Khera, N.; Holland, J.C.; Griffin, J.M. Setting the Stage for Universal Financial Distress Screening in Routine Cancer Care. Cancer 2017, 123, 4092–4096. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/cncr.30940 (accessed on 3 July 2019). [CrossRef] [Green Version]
- Moffatt, S.; Noble, E.; Exley, C. ‘Done more for me in a fortnight than anybody done in all me life.’ How welfare rights advice can help people with cancer. BMC Health Serv. Res. 2010, 10, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neill, B.; Prawitz, A.D.; Sorhaindo, B.; Kim, J.; Garman, E.T. Changes in Health, Negative Financial Events, and Financial Distress/Financial Well-Being for Debt Management Program Clients. J. Financ. Couns. Plan. 2006, 17, 46. [Google Scholar]
- Bower, H.; Björkholm, M.; Dickman, P.W.; Höglund, M.; Lambert, P.C.; Andersson, T.M.L. Life Expectancy of Patients with Chronic Myeloid Leukemia Approaches the Life Expectancy of the General Population. J. Clin. Oncol. 2016, 34, 2851–2857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, K.; Bentley, J.P.; Ramachandran, S.; Chang, Y.; Banahan, B.F., III; Shah, R.; Bhakta, N.; Yang, Y. Phase-Specific and Lifetime Costs of Multiple Myeloma among Older Adults in the US. JAMA Netw. Open 2021, 4, e2116357. [Google Scholar] [CrossRef]
| Author, Year, Country | Study Design | Sample Size | Sample Age Reported as Mean, Median or Range (Years) | Percentage Female (%) | Included Haematological Conditions | Timing of Assessment | Main Findings Describing Financial Impact |
|---|---|---|---|---|---|---|---|
| Abel et al., 2016, USA [23] | Cross-sectional | 325 | Median, 61 | 40 | MM, NHL, AML, MDS, HL, ALL, other | 150 days post-HSCT | Unsatisfied with present financial situation = 49% of sample Difficulty meeting monthly payments = 42% of sample Not enough money at months-end = 19% of sample Difficulty paying for HSCT-related costs = 51% of sample Difficultly paying for transportation = 41% of sample Difficulty meeting costs of changed home environment = 19% of sample. Income decline = 46% of sample Multivariate analysis of financial hardship measures with patient-reported outcome measures QOL below median Income decline: OR 1.62 (95% CI: 0.98–2.7, p = 0.06) Hardship_1: OR 2.9 (95% CI:1.7–4.9, p < 0.001) Hardship_2: OR 2.16 (95% CI: 0.99–4.7, p = 0.05) Self-reported health below the median Income decline: OR 1.33 (95% CI: 0.81–2.2, p = 0.26) Hardship_1: OR 2.18 (95% CI: 1.3–3.6, p = 0.003) Hardship_2: OR 1.88 (95% CI: 0.89–3.9, p = 0.10) Perceived stress above median Income decline OR: 2.07 (95% CI: 1.3–3.4, p = 0.004) Hardship_1: OR 2.08 (95% CI: 1.3–3.5, p = 0.005) Hardship_2: OR 3.14 (95% CI: 1.4–6.8, p = 0.004) |
| Albelda et al. *, 2019, USA [24] | Cross-sectional | 171 | Mean, 57 | NR | Any needing BMT, but NR | 6-months post HSCT | Multivariate analysis of financial burden with: “Dissatisfied with financial situation” (OLS coefficients, 95% CI) Health: −0.331, (−0.501, −0.161), p < 0.01 Quality of life: −0.295, (−0.473, −0.118), p < 0.01 Perceived stress: −1.093, (−1.496,−0.689), p < 0.01 “Difficulty paying bills” (OLS coefficients, 95% CI) Health: −0.270 (0.433,−0.108), p < 0.01 Quality of life: −0.177 (−0.348, 0.006), p < 0.05 Perceived stress: −0.720 (−1.118,−0.321), p < 0.01 “Not enough money at the end of the month” (OLS coefficients, 95% CI) Health: 0.404 (−0.680,−0.128), p < 0.01 Quality of life: −0.321 (−0.601, −0.024), p < 0.05 Perceived stress: −0.943 (−1.625, −0.261), p < 0.01 |
| Bala-Hampton et al., 2017, USA [25] | Cross-sectional | 26 | Mean, 58.5 (SD 14.1) | 46.2 | AML | 6 months after diagnosis | Not enough money to cover the cost of treatments = 69.2% of the sample Out-of-pocket expenses greater than expected = 65.4% of the sample Increased financial worry = 77% of the sample No choice in the cost of the care = 85% of the sample Unable to financially contribute to the household = 62% of the sample Dissatisfaction with finances = 73% of the sample Felt financially stressed = 69.2% of the sample Felt not in control of their finances = 85% of the sample |
| Buzaglo et al., 2017, USA [26] | Cross-sectional | 318 | Mean, 56 Range, 18–85 | 68 | CML | Mean of 5.2 years from diagnosis | Out of pocket costs (%of the sample) Spent at least US$100 per month = 49% Spent ≥ US$250 per month = 27% Spent ≥ US$500 per month = 16% Spent ≥ US$1,000 per month = 6% To reduce the cost associated with CML (% of sample): Postponed seeking psychological counselling (sometimes, often, or always) = 23% Missing a dose or oral CML drugs at least monthly = 19% Delayed follow-up on recommendations on complementary treatment = 17% Postponed doctor’s appointments = 16% Postponed filling prescriptions = 14% Skipped doses or CML oral drugs at least sometimes = 10% Because of costs associated with CML (% of sample which varied from 283–287 respondents): Reduced grocery expenditure = 35% Depleted savings = 33% Borrowed against or used money from retirement = 20% of sample Sold personal property = 18% Liquidated assets = 13% Refinanced house = 8% Filed for bankruptcy = 6% Home foreclosed = 4% Multivariate analysis with financial burden Suboptimal treatment adherence p < 0.001 |
| Fenn et al., 2014, USA [27] | Cross-sectional | NR for haematology | NR for haematology | NR for haematology | leukaemia/lymphoma | NR | Multivariate analysis with financial burden and QoL of at least ‘good’ Adjusted OR = 0.91, 95% CI 0.42–1.95, p = 0.799 |
| Goodwin, et al., 2013, USA [28] | Cross-sectional | 762 | Mean, 61 (SD 9.26) | 39 | MM | Received intensive treatment at the site | Out-of-pocket costs as a percentage of income by time since treatment began % income spent during first year of treatment Treatment began < 4years ago = 40% Treatment began ≥ 4 years ago = 33% t = −2.281, p = 0.023, 95%CI −13.658–1.019 % income spent in past 12 months Treatment began < 4years ago = 35% Treatment began ≥ 4 years ago = 23% t = −5.465, p = 0.0005, 95%CI −16.921–7.968 Out-of-pocket costs as a percentage of income by time since treatment ended % income spent during first year of treatment Treatment ended < 4years ago = 37% Treatment began ≥ 4 years ago = 37% t = −0.14, p = 0.998, 95%CI −11.015–10.854 % income spent in past 12 months Treatment ended < 4years ago = 29% Treatment began ≥ 4 years ago = 22% t= −2.143, p = 0.033, 95%CI −13.21–0.564 Other findings Percentage of income used for out-of-pocket costs Mean percentage of income used on treatment-related expenses = 36% during the first 12 months Mean percentage of income used on treatment-related expenses = 28% in the past 12 months Treatment costs are somewhat to very much a burden to themselves or family = 42% of the sample. Income use by treatment modality Percentage of income used for those on chemotherapy vs. not t = 2.03, p = 0.025, 95% CI 0.823–12.443 ingle item from the FACT-BMT regarding burden of treatment costs Financial burden for patients on chemotherapy treatments vs. not t= −3.51, p = 0.000, 95% CI: − 0.57 to − 0.16 |
| Gupta et al., 2018, USA [34] | Cross-sectional | 162 | Mean, 55.9 (SD 13.5) | 49.4 | MM | First line treatment: medicated for ≥8 weeks Second line treatment: ≥6 weeks | Out-of-pocket costs (US$) Cost of clinical appointments = $318.90 (±637.20) Prescription medications = $388 (±1063.40) Over the counter medications = $191.40 (±363.80) Transportation = $67.30 (±114.80) Total out-of-pocket = $709 (±1307.30) Financial burden related to out-of-pocket costs (n, %) None = 48 (29.6) Some = 46 (28.4) Moderate = 50 (30.9) High = 28 (17.3) Extremely high = 7 (4.3) ** MMAS, out-of-pocket costs and financial burden generalised linear modeling (adjusted mean ± SE, 95% CI) Cost of clinical appointments Score ≤ 3 = 147.7 ± 45.7, 80.6–270.6, p > 0.05 Score 4 = 210.3 ± 49.9, 132.1–334.7 Prescription medications Score ≤ 3 = 387.9 ± 168.4, 165.7–908.1, p > 0.05 Score 4 = 220.2 ± 68.4, 119.8–404.8 Over the counter medications Score ≤ 3 = 130.6 ± 34.0, 78.3–217.6, p = 0.006 Score 4 = 46.8 ± 9.1, 32.0–68.4 Transportation Score ≤ 3 = 83.0 ± 18.6, 53.5–128.8, p = 0.03 Score 4 = 43.3 ± 7.6, 30.6–61.2 Total out-of-pocket Score ≤ 3 = 828.3 ± 248.7, 459.9–1491.8, p > 0.05 Score 4 = 395.7 ± 87.2, 256.8–609.5 Financial burden related to out-of-pocket costs by MMAS (adjusted mean ± SE, 95% CI) Score ≤ 3 = 0.7 ± 0.1, 0.6–0.9, p > 0.05 Score 4 = 0.6 ± 0.1, 0.5–0.8 |
| Hamilton et al., 2013, USA [29] | Cross-sectional | 181 | NR | 55.2 | Eligibility: any haematological malignancy requiring HSCT Sample: NR (participants were required to be at least moderately distressed according to standardised measure delivered pre-study) | 9–36 months post HSCT | Perceptions of economic survivorship stressors: Sources of financial stress occurred most frequently as ‘moderately’ or ‘a great deal’ in the past month, including (% of the sample): Reducing or cancelling vacations or leisure activities = 34% Reducing spending on household expenses such as food or clothing = 33% Deciding not to buy something they had planned to purchase = 28% Difficult, very difficult, or extremely difficult to live on their income = 23% Anticipated reducing their standard of living to afford the bare necessities in life ‘at least somewhat’ = 22% Hierarchical regression of financial stress and HRQoL (reported F change, significance) Physical wellbeing −4.05 p < 0.001 Social wellbeing −1.03, p > 0.05 Emotional wellbeing −3.36, p < 0.001 Functional wellbeing −2.83, p < 0.01 |
| Huntington et al., 2015, USA [30] | Cross-sectional | 100 | Mean = 64.1 (SD 9.8) Median = 64.7 (Range: 38.4–90.2) | 53 | Multiple myeloma | 3 months after treatment commenced | 55/100 patients reported reduced spending on basic goods 6/98 patients reported reduced spending on leisure 43/94 patients used savings to pay for treatment 21/98 patients borrowed money 17/100 reported delays in treatment of their multiple myeloma because of cost 36/100 patients applied for financial co-payment assistance 59/100 reported out-of-pocket treatment costs for MM were higher than expected Decreased spending on basic goods (food and clothing): p < 0.0001 Decreased spending on leisure activities: p < 0.0001 Use savings to pay for cancer care: p < 0.0001 Borrow money for cancer care: p < 0.0001 Delay the start of a myeloma treatment: p = 0.0030 Fill only part of myeloma therapy prescription because of cost: p = 0.0077 Stop myeloma therapy prescription because of cost: p = 0.0011 Refuse recommended test because of cost: p = 0.016 Skip clinic visit to save on costs: p = 0.027 Apply for financial assistance: p = 0.14 |
| Jella et al., 2021 USA [33] | Cross-sectional (collected annually between 1997–2018) | 1619 | NR | 47 | Lymphoma | NR | Medical care delayed due to cost, past 12 months? Yes = 161 (10%) No = 1458 (90%) Needed but could not afford medical care, past 12 months? Yes = 105 (7%) No = 1513 (93%) Multivariate analysis of financial stressors (adjusted odds ratio, 95%CI, p value) Medical care delayed due to cost, past 12 months? Age (years) 18–24 = 0.87 (0.15–5.09), p = 0.881 25–44 = 4.63 (2.28–9.41), p < 0.001 45–64 = 5.85 (3.20–10.70), p < 0.001 ≥65 = Reference Race/ethnicity White = Reference Black = 0.89 (0.44–1.84), p = 0.760 Hispanic = 1.63 (0.73–3.65), p = 0.237 Other = 1.08 (0.49–2.36), p = 0.845 Sex Male = Reference Female= 1.62 (1.06–2.48), p = 0.027 Born in the United States Yes = Reference No = 0.27 (0.09–0.83), p = 0.024 Marital Status Married = Reference Single = 1.88 (1.18–3.00), p = 0.009 Self-reported Health status Good to excellent = Reference Poor to fair = 2.47 (1.59–3.83), p < 0.001 Needed but could not afford medical care, past 12 months? Age (years) 18–24 = 0.23 (0.17–1.07), p = 0.172 25–44 = 3.50 (1.13–8.24),p = 0.004 45–64 = 4.87 (2.33–10.17), p ≤ 0.001 ≥65 = Reference Race/ethnicity White = Reference Black = 0.81 (0.35–1.88), p = 0.620 Hispanic = 0.42 (0.17–1.07), p = 0.070 Other = 1.71 (0.69–4.23), p = 0.247 Sex Male = Reference Female = 2.20 (1.28–3.76), p = 0.004 Born in the United States Yes = Reference No = 0.14 (0.02–0.88), p = 0.037 Marital Status Married = Reference Single = 1.63 (0.93–2.85), p= 0.087 Self-reported Health status Good to excellent = Reference Poor to fair = 2.08 (1.23–3.49), p = 0.006 |
| Khera et al. ***, 2018, USA [31] | Cohort | 325 | NR | 40 | MM, NHL, AML, MDS, HL, ALL, other | 1 and 2 years survival, post HSCT | Univariate analysis (Hazard Ratio (95% CI)) Hardship No N = 141 1-year survival HR 0.96 (0.92–0.98) 2-year survival HR 0.91 (0.85–0.95) Yes N = 182 1-year survival HR 0.94 (0.89–0.97) 2-year survival HR 0.87 (0.81–0.91) Extreme Hardship No N =273 1-year survival 0.94 (0.91–0.96) 2-year survival 0.89 (0.84–0.92) Yes N= 50 1-year survival HR 1.00 (-) 2-year survival HR 0.92 (0.79–0.97) |
| Paul, et al., 2013, Australia [39] | Cross-sectional | 268 | Mean = 59.5 (SD 13.4) | 41 | NHL, lymphoma, leukaemia, MM | Diagnosed in the previous 3 years | Difficulty paying bills of other payments (% of the sample by participants residing in metropolitan or non-metropolitan areas) Metropolitan= 24% Non-metropolitan = 16% Χ2 =2.56, p = 0.11 Used up savings (% of the sample by participants residing in metropolitan or non-metropolitan areas) Metropolitan =25% Non-metropolitan = 16% Χ2 = 2.98, p = 0.084 Had trouble with day-to-day expenses (% of the sample by participants residing in metropolitan or non-metropolitan areas) Metropolitan = 15% Non-metropolitan = 8% Χ2 = 3.55, p = 0.06 Other findings (% of the total sample) Cancer-related expenses influenced decision about treatment = 2% Cancer-related out-of-pocket expense = 45% of the sample Percentage of respondents with out of pocket expenses relating to: - parking for medical appointments = 33% - travel costs to appointments = 30% - treatment drugs = 24% - assistance with gardening or housework = 8% - other medical supplies = 4.6% - accommodation while at appointments = 2.3% Difference between metropolitan and non-metropolitan out-of-pocket expenses = F(1,260)= 0.40, p = 0.528 Financial burden from living in a metropolitan city vs. non-metropolitan = χ2 =6.06, p = 0.014 |
| Author, Year, Country | Age Range of Participants (Years) | %Female | Included Haematological Malignancies | Measurement Time-Point | Study Design | Data Collection Technique | Data Analysis Technique |
|---|---|---|---|---|---|---|---|
| Goodwin et al., 2013, USA [28] | 29–77 | 39 | MM | Patients had received intensive therapy (between 0–42 years prior) | Cross-sectional | Open ended survey question | NR |
| Head et al., 2018, USA [32] | 30–67 | 77 | Any | 1–5 years after diagnosis. Participants were experiencing financial hardship as defined by three questions from the COST-PROM | NR | Interviews | Thematic (constructivist grounded-theory approach) |
| McGrath, 2015, Australia * [38] | 18–≥70 | 56 | HL, NHL, AML, ALL, APML, CML, CLL, MM, MDS, MN-ET | NR | Descriptive | Interviews | Thematic |
| McGrath, 2016, Australia * [35] | 18–≥70 | 56 | HL, NHL, AML, ALL, APML, CML, CLL, MM, MDS, MN-ET | NR | Descriptive | Interviews | Thematic |
| McGrath, 2016, Australia * [36] | 18–≥70 | 56 | HL, NHL, AML, ALL, APML, CML, CLL, MM, MDS, MN-ET | NR | Descriptive | Interviews | Thematic |
| McGrath, 2016, Australia * [37] | 18–≥70 | 56 | HL, NHL, AML, ALL, APML, CML, CLL, MM, MDS, MN-ET | NR | Descriptive | Interviews | Thematic |
| Parsons et al., 2019, Canada [40] | 51–83 | 31 | MM | Relapse or refractory disease | Descriptive | Interviews, followed by focus groups | Thematic |
| Tan et al., 2017, Malaysia [42] | 26–67 | 50 | CML | Taking tyrosine kinase inhibitor | NR | Interviews | Thematic |
| Wang et al., 2016, China [41] | 42–78 | 74 | Leukaemia | Cancer survivors | NR | Focus groups | Thematic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parker, C.; Berkovic, D.; Ayton, D.; Zomer, E.; Liew, D.; Wei, A. Patient Perceived Financial Burden in Haematological Malignancies: A Systematic Review. Curr. Oncol. 2022, 29, 3807-3824. https://doi.org/10.3390/curroncol29060305
Parker C, Berkovic D, Ayton D, Zomer E, Liew D, Wei A. Patient Perceived Financial Burden in Haematological Malignancies: A Systematic Review. Current Oncology. 2022; 29(6):3807-3824. https://doi.org/10.3390/curroncol29060305
Chicago/Turabian StyleParker, Catriona, Danielle Berkovic, Darshini Ayton, Ella Zomer, Danny Liew, and Andrew Wei. 2022. "Patient Perceived Financial Burden in Haematological Malignancies: A Systematic Review" Current Oncology 29, no. 6: 3807-3824. https://doi.org/10.3390/curroncol29060305
APA StyleParker, C., Berkovic, D., Ayton, D., Zomer, E., Liew, D., & Wei, A. (2022). Patient Perceived Financial Burden in Haematological Malignancies: A Systematic Review. Current Oncology, 29(6), 3807-3824. https://doi.org/10.3390/curroncol29060305






