Abstract
Selective internal radiation therapy (SIRT) with yttrium-90 (90Y)-loaded microspheres is increasingly used for the treatment of Intrahepatic Cholangiocarcinoma (ICC). Dosimetry verifications post-treatment are required for a valid assessment of any dose-response relationship. We performed a systematic review of the literature to determine how often clinics conducted post-treatment dosimetry verification to measure the actual radiation doses delivered to the tumor and to the normal liver in patients who underwent SIRT for ICC, and also to explore the corresponding dose-response relationship. We also investigated other factors that potentially affect treatment outcomes, including the type of microspheres used and concomitant chemotherapy. Out of the final 47 studies that entered our study, only four papers included post-treatment dosimetry studies after SIRT to quantitatively assess the radiation doses delivered. No study showed that one microsphere type provided a benefit over another, one study demonstrated better imaging-based response rates associated with the use of glass-based TheraSpheres, and two studies found similar toxicity profiles for different types of microspheres. Gemcitabine and cisplatin were the most common chemotherapeutic drugs for concomitant administration with SIRT. Future studies of SIRT for ICC should include dosimetry to optimize treatment planning and post-treatment radiation dosage measurements in order to reliably predict patient responses and liver toxicity.
1. Introduction
Cholangiocarcinoma is the second most common primary hepatic malignancy, and over the past several decades its incidence has increased in the United States and worldwide [1]. Surgical resection and liver transplantation are the only cures for intrahepatic cholangiocarcinoma (ICC); however, most patients are diagnosed with advanced-stage ICC, for which curative surgery is impossible [2]. In addition, more than 50% of patients with early-stage ICC who undergo surgical resection experience disease recurrence after a median of 20 months [3]. In clinical practice, various locoregional therapies are used to treat hepatic tumors; these include thermal liver ablation methods (radiofrequency or microwave), external beam radiation therapy, and trans-arterial therapies such as hepatic artery infusion, chemoembolization, and radioembolization [4,5]. Trans-arterial radio-embolization, or selective internal radiation therapy (SIRT) with yttrium-90 (90Y), was first introduced in 1965 [6] and has evolved to become a treatment option for unresectable primary or metastatic hepatic tumors. SIRT may be used as a first-line treatment for select patients, as an adjunct to systemic chemotherapy, or after the failure of other therapies [7]. Newer generations of systemic therapies, including immunotherapeutic agents, may also be considered to be concomitantly administered with SIRT in future research [8,9].
The liver is a radiosensitive organ, and the radiation dose required to destroy hepatic tumors is greater than the threshold dose of the normal hepatic parenchyma [10]. In view of this, the liver’s dual blood supply, as well as the differential blood flow to the tumor versus the normal liver parenchyma, provided the rationale for SIRT. In SIRT, 90Y microspheres are injected into the hepatic artery, the main supply for liver tumors; the normal liver parenchyma that receives blood from both the portal and systemic circulation is partially spared [11]. In addition to the differential blood supply, the average penetration depth of 2.5 mm for the high-energy beta radiation from 90Y-loaded microspheres further helps to achieve radiation demarcation between the tumor and the liver parenchyma [12].
Most studies on the application of SIRT in ICC presume uniform distribution of 90Y microparticles and perform treatment planning to deliver a mean absorbed dose of 120 ± 20 Gy to the tumor and a threshold dose of no more than 50–70 Gy to the normal liver parenchyma [13]. Historically radioembolization planning employed several dosimetry models, including Body Surface Area Method (activity calculation), the Single Compartment Medical Internal Radiation Dose, and the Partition Model [14], to estimate the absorbed dose to the tumor and normal liver. Models of activity prescription have substantially advanced, with the partition dosimetry model providing the most personalized patient-specific model for predicting radiation uptake in normal and tumoral tissue by using 99mTc-MAA SPECT-CT as a surrogate for 90Y imaging [11] and considering the extrahepatic deposition of radioactivity [15]. Relying only upon pretreatment radiation dose calculations could cause clinicians to overlook the biological parameters affecting treatment outcomes [16]. Moreover, several studies have demonstrated considerable discrepancies between pretreatment predictive planning and dosimetry verification studies after radioembolization [15,17,18]. These discrepancies possibly originate from variations in the flow dynamics, catheter position and the sizes, weights, and densities when comparing 99mTc-MAA particles and 90Y microspheres [18]. Performing verification imaging studies after SIRT is essential for a precise assessment of the activity distribution and the dose delivery, for dose-response and toxicity studies, and for the clinical management of extrahepatic deposition [13]. Moreover, despite nearly three decades of clinical SIRT use, there is no consensus on the optimal dosing for disease control, and few studies have examined the dose-response relationship for SIRT in patients with ICC and it is not clear whether they measured the true, delivered radiation dose through post-treatment dosimetry studies. Therefore, in the present study, we systematically reviewed the literature on the application of SIRT in ICC to investigate how often clinics conducted treatment verification studies after treatment in order to measure the actual radiation doses delivered to the tumor and to the normal liver. It was also investigated whether, in those studies with true post-treatment dosimetry verification, a tumor dose threshold is predictive of tumor response, or a normal liver dose threshold is predictive of hepatic toxicity. We also looked into other factors that can potentially influence treatment outcomes, including the microsphere types and the application of concomitant chemotherapy.
2. Material and Methods
The study was conducted according to the Preferred Reporting Items for Systemic Reviews and Meta-Analyses (PRISMA) guidelines for systematic reviews [19]. A systematic literature review of English-language journal articles and conference abstracts on the application of SIRT in ICC patients was conducted using the PubMed, Embase, and Scopus databases. Articles and abstracts published on or before 10 February 2022, were included. A search query was developed following a review of the search strategies used in published systematic reviews in the same field [20,21]. The following Medical Subject Heading Terms were combined: “yttrium radioisotopes”, “radiopharmaceuticals”, “embolization”, “therapeutic”, “cholangiocarcinoma”, and “bile duct neoplasms”. We also searched for “radioembolization”, “locoregional therapy”, and “liver tumor”.
One reviewer (SHS) screened titles, abstracts, and keywords to exclude irrelevant papers. Two independent reviewers (SHS, PH) then performed full-text assessments against the inclusion and exclusion criteria to identify all journal articles and conference abstracts reporting the use of SIRT in at least one patient with ICC. In the event of a disagreement between two reviewers, a mutual dialogue was convened to resolve the issue. Studies that included ICC patients but reported only unified results for all the examined tumor types were excluded, as were studies that did not have radiation dose information or whose complete results were provided in another published paper. Studies of SIRT in patients with combined hepatocellular carcinoma and ICC histo-pathologies with unified results, as well as case reports, comments, and editorials, were also excluded. Studies with different patient populations were considered to be included in this study when SIRT was used as a first-line treatment or following the failure of other treatments. In addition, in some studies, a mixed population of both treatment-naïve and refractory patients received SIRT, and unified results were reported, which were also included in this review.
Data extracted from the eligible studies were entered into data extraction tables, including the following:
Study characteristics (i.e., authors, year of publication, study design including retrospective versus prospective), sample size, the patient population in terms of previous treatments tried (treatment-naïve and/or refractory); the 90Y-treatment specifications (e.g., type of 90Y microspheres administered, number of treatment courses in case of repeated procedures, concurrent chemotherapy, imaging modality used for post-treatment dosimetry), and treatment outcomes (e.g., imaging-based response assessment, the timing of imaging-based response, overall survival (OS), and progression-free survival (PFS)).
3. Results
A total of 3943 papers were identified for screening. We excluded 3693 irrelevant papers based on their titles, abstracts, and/or keywords, leaving 250 papers that underwent full-text assessment (Figure 1). After implementing our exclusion criteria, 47 papers were eligible for data extraction. Of the final 47 included papers, 40 were journal articles [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61] (Table 1), and seven were conference abstracts [62,63,64,65,66,67,68] (Table 2).
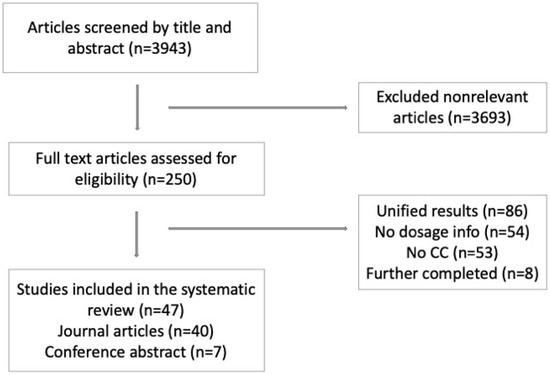
Figure 1.
Identification of included papers. ICC: Intrahepatic Cholangiocarcinoma.

Table 1.
Journal articles included in the study.

Table 2.
Conference abstracts included in the study.
Thirty-two journal articles and five conference abstracts had a retrospective design, and eight journal articles and two conference abstracts were prospective studies. Twenty-eight studies used SIR-Spheres, nine used TheraSpheres, nine used both, and one conference abstract did not specify the type of 90Y microspheres used [64]. In addition, five papers compared SIRT outcomes for patients treated with the two types of microspheres [27,28,34,41,43].
Thirty studies included both treatment-naïve patients and those with the recurrent disease following other treatments, five studies assessed only outcomes following SIRT as a first-line therapy [29,42,43,44,54] and nine studies included only patients in whom prior treatments had failed [22,24,27,34,38,47,55,56,66]. The remaining three studies did not provide details on their patient populations [62,64,68].
The clinical outcome of SIRT was mainly reported as the OS, which was not statistically reached in three studies [29,54,64]. Some studies recorded PFS, liver-specific PFS, and the time to disease progression (Table 1 and Table 2). For tumor response assessment, different sets of imaging response criteria were used in published studies, including the Response Evaluation Criteria in Solid Tumors [RECIST], modified RECIST [mRECIST], Positron Emission Tomography Response Criteria in Solid Tumors [PERCIST], European Association for the Study of Liver Disease [EASL], and World Health Organization (WHO) classification.
In 11 studies, SIRT and chemotherapy were given concomitantly to at least one patient (Table 3). One study was excluded from our review because it provided unified dosimetry results for two different malignancies in their patient population, ICC and pancreatic cancer; however, related information regarding the concomitant chemotherapy was added to Table 3 [69].

Table 3.
Studies in which SIRT and chemotherapy were given concomitantly to at least 1 patient.
Eleven studies described the use of imaging to assess the distribution of 90Y microparticles in the liver after SIRT. Of these, seven used Bremsstrahlung 90Y SPECT-CT [23,24,26,28,41,46,49], and four used 90Y positron emission tomography (PET)-CT [31,32,42,45] to confirm the distribution of 90Y microspheres in the lesions and exclude extrahepatic deposition. True dosimetry verification studies were performed in only four studies (Table 4) [22,25,27,43].

Table 4.
Results of dosimetry verification studies.
Regarding the dose-response association based on true post-treatment dosimetry, Willowson et al. [22] conducted a lesion-based study on 18 patients with ICC treated with resin microspheres, using 90Y PET-CT and 18F-FDG PET-CT for dose and response assessments, respectively. They defined lesion response as total lesion glycolysis reduction of at least 50% and found that there was a trend for a dose-response relationship with a higher tumor average dose in responding lesions, although it did not meet statistical significance (p = 0.29). Cheng et al. [27] considered objective response rate, defined as the pooled, 3-month, mRECIST-based, complete and partial response rates, as the tumor response and measured the tumor delivered radiation dose using 90Y SPECT-CT. Their study demonstrated that a threshold tumor dose of 78.9 and 254.7 Gy for resin and glass microspheres, respectively, can predict tumor response with 80% specificity. In terms of survival analysis, tumor dose cutoff points of ≥75 Gy for resin and ≥150 Gy for glass, were substantially associated with a longer OS. Tumor dose was also demonstrated to positively affect TTP in a study by Depalo et al. [25], using 90Y PET-CT for dosimetry. Based on their calculations, an average dose of 180 Gy in resin-based SIRT, was required to achieve partial tumor response, as defined by RECIST 1.1, at three months after the treatment. The authors of the other article, reporting a retrospective study on a sample size of five patients treated with glass, and five patients with resin microspheres, performed 90Y SPECT-CT–based dosimetry after SIRT and calculated mean tumor doses of 205.7 ± 19.7 Gy and 128.9 ± 10.6 Gy for glass- and resin-based microspheres, respectively (p < 0.001) [43]. However, their study had a very small sample size and lacked a survival-based dose-response analysis and follow-up imaging studies to show responses to treatment.
In terms of toxicity, three studies investigated potential variations based on the administered microsphere and found that glass-based and resin-based microspheres have similar toxicity profiles [27,34,43], of which true post-treatment dosimetry was performed in two studies [27,43]. In one study, the mean absorbed 90Y dose in the normal liver tissue was significantly lower with the use of glass microspheres than the resin (42.4 ± 4.5 Gy and 53.6 ± 4.3 Gy, respectively; p < 0.001), and the tumor-to-normal liver dose ratio was subsequently higher with glass-based spheres (p < 0.001) [43]. Their study results may suggest that glass-based radioembolization can deliver a higher radiation dose to a tumor without a remarkable dose increase to the normal liver parenchyma, lowering the risk of treatment-induced toxicity. However, clinical and laboratory toxicity rates were similar in both groups. In the other study with post-treatment dosimetry, the tumor-to-normal liver ratio was not statistically different between glass and resin-based SIRTs (p = 0.24) as the clinical and laboratory toxicity rates were the same between groups [27]. None of the studies with true post-treatment dosimetry determined a normal liver dose threshold to predict treatment-induced toxicity.
4. Discussion
4.1. Dosimetry and Dose-Response Relationship
Our systematic review showed that most published studies of SIRT in ICC patients reported only a nonspecific mean administered radiation dose based on pretreatment prediction models and did not provide any information on the actual tumor dose based on post-treatment imaging. Most studies that carry out post-treatment verification dosimetry after SIRT include patients with hepatocellular carcinoma because ICC is comparatively rare. As presented in Table 4. we could only identify four published papers conducting a dosimetry study after SIRT. There were two imaging modalities used in verification studies, Bremsstrahlung 90Y SPECT-CT imaging and 90Y PET-CT. The main factor that limits the clinical application of treatment verification using Bremsstrahlung 90Y SPECT-CT stems from the characteristics of 90Y, which lacks a distinct photopeak and thus distorts the image resolution [70]. Several measures have been taken to enhance the quality of these images, such as using certain collimators or applying scatter and attenuation correction methods, but the use of these methods is restricted in practice because of the technical issues involved and the meticulous calibration required [11]. At our institution, 90Y SPECT-CT after SIRT has been standard for years. However, some experts believe that 90Y PET-CT has a better spatial resolution with lower scatter [71], and the most recent international guidelines for SIRT for patients with liver malignancies recommend the use of 90Y PET-CT in dosimetry studies after SIRT [13]. A study is underway at our institution comparing the accuracy of 90Y SPECT-CT versus 90Y PET-CT after SIRT, which will provide more information on this matter.
Out of the three studies that investigated the dose-response relationship based on true post-treatment dosimetry (Table 4), Willowson et al. [22] could not certify any statistically significant dose-response association, although their study showed that metabolically responsive lesions had higher average tumor doses. Depalo et al. [25] proved that a larger mean delivered tumor dose is significantly associated with longer TTP, although no certain dose threshold was calculated. Cheng et al. [27] considered a mean tumor dose of 75 Gy for resin microspheres as a cutoff point predictive of remarkably longer OS. In terms of response assessments based on imaging-based criteria, Depalo et al. [25] found a mean tumor dose threshold of 180 Gy is associated with a better partial response, and Cheng et al. [27] measured a threshold mean tumor dose of 78.9 Gy for a significantly higher rates of complete and/or partial response, both with resin-based treatments. Besides heterogeneities in their patient populations, the application of two different modalities for dosimetry could possibly justify the variations in tumor dose cutoffs for an imaging-based response. Different dose thresholds were measured for glass-based procedures, which are further explained in the following section.
4.2. Types of 90Y Microspheres
Currently, two types of 90Y microspheres have been approved for clinical use in the US: SIR-Spheres (SIRTex Medical, Sydney, Australia), which are 90Y-coated resin microspheres, and TheraSpheres (Therasphere BTG, Ontario, Canada), which are insoluble, glass microspheres embedded with 90Y. The radiation doses of these two microspheres vary because of their different specific activities, specific densities, and particle sizes. The specific activity of glass-based TheraSpheres (~2500 Bq per sphere) is much higher than that of resin-based SIR-Spheres (50 Bq per sphere); therefore, many more resin microspheres are needed to deposit the same level of activity achieved with just a few glass microspheres. This may explain SIR-Spheres’ higher rates of vascular stasis [11]. However, on the basis of our experience, we firmly believe that the tumor response is not only related to the actual delivered dose of radiation but also the number of delivered microspheres per tumor volume and the homogeneity of microsphere distribution in tumor tissue. This viewpoint is justified by the very short penetration depth of high energy beta radiation from the 90Y isotope, as it has been demonstrated that around 90% of the radiation dose is delivered in a radial distance of 300 μm around 90Y microspheres [72].
This review included 28 studies that used resin-based microspheres and nine that used glass-based microspheres for the treatment of patients with ICC. Nine studies used both types of microspheres, and one study did not report the type of microspheres used (Table 1 and Table 2). As expected, in those studies that used both types of microspheres, the mean or median activity or radiation dose administered using glass-based microspheres was higher than that administered using resin. In those publications with post-treatment dosimetry studies, the mean delivered tumor dose was around 200 to 250 Gy for glass-based and 80 to 130 Gy for resin-based treatments (Table 4).
In terms of efficacy, Shaker et al. [41] found a clinically, though not statistically, significant difference in PFS between patients treated with resin-based microspheres and those treated with glass-based microspheres (15.6 months vs. 2.4 months; p = 0.46). Three other studies compared the survival outcome of the SIRT by the type of microsphere used, in none of which a statistically significant difference could be reached in median OS, PFS, or liver-specific PFS [27,28,34]. Imaging-based response assessment carried out by Buettner et al. [34], demonstrated that glass-based microspheres elicited a higher rate of partial response (as determined using RECIST at 6 months after the SIRT) than resin-based microspheres (p = 0.008); Still, their study lacked a true post-treatment dosimetry verification study. There were only two studies that performed a post-SIRT dosimetry study comparing the TheraSpheres and SIR-Spheres, among which only one recent paper by Cheng et al. [27] carried out clinical and radiological outcome analyses, and the other one did not investigate any long-term outcome of the treatment [43]. Cheng et al. [27] found that patients treated with glass-based microspheres have a slightly higher objective response rates, still not statistically significant (p = 0.47), as compared to those receiving resin-based microspheres, using the mRECIST three months after the procedure. In terms of toxicity, three studies investigated potential variations based on the microsphere type and found that glass-based and resin-based microspheres have similar toxicity profiles and no specific normal liver dose threshold was determined to cause clinical or laboratory toxicities [27,34,43]. These results are compatible with the results of a pooled analysis by Zhen et al. [73], which found a comparable outcome of the SIRT employing both microsphere types, with a median overall survival of around 14 months and disease control rate of 77% for both groups. However, it is noteworthy that most of the studies comparing these two groups lack post-treatment dosimetry data and further investigations, including retrospective and prospective studies with larger patient populations and post-treatment verification studies, are required to determine whether either type of microsphere provides more benefit in patients with ICC.
4.3. Concomitant Chemotherapy
The mainstay of treatment for cancers originating in the biliary tract, including ICC, is systemic chemotherapy with gemcitabine-based regimens [74]. Cisplatin has also been established to provide survival benefits when combined with gemcitabine [75]. Although it has been defined variously, concomitant chemotherapy is routinely referred to as the administration of chemotherapy no more than three months prior to SIRT [54], which can potentially provide a more prolonged survival than SIRT alone because of the radio-sensitizing effects of gemcitabine [76]. In a meta-regression study by Cucchetti et al. [7], the pooled median survival duration was 19.5 months for patients who received SIRT and concomitant chemotherapy but only 5.5 months for patients who received SIRT alone, which supports the concomitant use of systemic chemotherapy with SIRT in patients with ICC. The authors also found that the 2-year survival rate of the patients who received the combination treatment (42.5%) was significantly higher than that of the patients who received SIRT alone (<10%; p = 0.04). However, only one of every five studies included in their analysis had patients whose treatment met the aforementioned definition of concomitant chemotherapy [54].
Among the papers included in the present study, 11 reported studies in which at least one patient received concomitant chemotherapy (Table 3). In a study by Manceau et al. [44], treatment-naïve patients received one of the three different chemotherapy regimens: gemcitabine plus cisplatin, cisplatin plus fluorouracil, or gemcitabine plus oxaliplatin. They reported a 3-month response rate of 69% and successful downstaging in 49% of the patients. Edeline et al. [33] assessed the outcome of concomitant chemotherapy with gemcitabine and cisplatin in treatment-naïve patients in a phase II clinical trial. They reported a 3-month objective response rate of 39% and a disease control rate of 98% in 40 of 41 patients, as well as downstaging in 22% of patients. For safety, the gemcitabine dose is usually reduced to 300 mg/m2—the recommended dose for patients with pancreatic cancer undergoing gemcitabine-based chemotherapy concomitantly used with SIRT [77]. However, in a recent phase Ib clinical trial, in which escalating doses of gemcitabine were given concomitantly with SIRT to five treatment-naïve patients with ICC, doses of up to 600 mg/m2 could be administered safely, with only transient liver toxicity [69]. This study was excluded from our review because it provided unified dosimetry results for two different malignancies of ICC and pancreatic cancer in their patient population, however the study’s chemotherapy-related information is included in Table 3. Despite what has been widely accepted on the effectiveness of concomitant chemotherapy, a recent study by Depalo et al. [25] could not prove the radio-sensitizing effects of concomitant chemotherapy; however, their study was limited by the very few numbers of heterogenous patients with a wide standard deviation of means. More studies are warranted to establish optimal chemotherapy doses and to confirm response rates following SIRT with concomitant chemotherapy.
4.4. Treatment Outcome
Many studies investigated the ICC response to the SIRT using different imaging response criteria (Table 1 and Table 2). In a recent, pooled meta-analysis of 14 papers and 608 patients by Mosconi et al. [78], the imaging-based objective response rate (as defined using RECIST at six months), was 19.3%. At least two other meta-analyses have reported imaging-based response assessments. The first, by Boehm et al. [20], reported an objective response rate of 27.4%, found in an analysis of five papers [56,58,59,60,61]. A more complete study by Al-Adra et al. [21], which had three papers in common with the study by Boehm et al., reported a 3-month partial response rate of 28% and a stable disease rate of 54%. Regarding the clinical outcomes of radioembolization in patients with ICC, Mosconi et al. [78] reported a median survival duration of about 13.5 months after treatment. Similar results were obtained in another meta-analysis by Boehm et al. [20] in 2015, which reported a median survival duration of 13.9 months. The publications we included in our study reported a wide range of median OS durations, ranging from 5.7 to 33.6 months [41,52]. The studies’ varied response rates possibly stem from differences in their patient populations and a lack of standardized tumor dosimetry. Moreover, several issues should be taken into consideration when interpreting the results of the survival analysis following radioembolization, including the type of ICC, staging [26], and pathological grading [45]. In 2010, Saxena et al. [60] reported that patients with mass-forming peripheral ICCs have a longer median survival duration than do patients with infiltrative lesions (18.3 months vs. 4.5 months). Similar results were also reported in a recent study by Paz-Fumagalli et al. [26], indicating that patients with mass-forming tumors had a longer survival (p = 0.002). Furthermore, as shown in several studies included in the present review, patient survival varies according to whether SIRT was used as a first-line treatment (for treatment-naïve ICC patients), for chemotherapy-refractory disease, or after the failure of other therapies [23,28,31,35,51]. In a meta-analysis by Cucchetti et al. [7], the median survival duration was about 24 months for treatment-naïve patients but only 11.5 months for patients with the chemotherapy-refractory disease (p = 0.048).
5. Conclusions
This study elucidates the gap in our current knowledge of SIRT in ICC. Post-treatment dosimetry is essential to verify the agreement between the intended and delivered radiation doses and also to investigate dose-response associations which could be carried out using 90Y PET-CT or 90Y Bremsstrahlung SPECT-CT as an alternative. According to our study results, there are very few publications available that have investigated dose-response relationship based on true post-treatment dosimetry, and in these studies, no consistent dose thresholds were established.
It should be noted that studies included in our systematic review had very heterogeneous patient populations in terms of concurrent or prior therapies other than SIRT. Refractory cases of ICC have a different prognosis from treatment-naïve patients, and interpreting the results as a unified matter may be biased. Moreover, studies comparing the efficacy and toxicity of SIRT based on the microsphere’s type were also limited by the shortage of patient populations.
The results of our work warrant further studies to conduct post-treatment dosimetry verification after the SIRT in order to reach a consensus regarding the tumor dose threshold needed to obtain an optimal response and the normal liver dose threshold related to treatment-induced toxicity.
Author Contributions
Conceptualization, P.H. and A.M. and B.C.O.; methodology, R.A.S. and N.N. and M.E.A.; investigation, S.H.S. and P.H.; data extraction, S.H.S. and P.H.; writing—original draft preparation, S.H.S.; writing—review and editing, all authors; visualization, S.H.S. and P.H. and N.N.; supervision, P.H. and A.M.; project administration, P.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank Laura L Russell and MD Anderson Cancer Center Research Medical Library for editing the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rizvi, S.; Khan, S.A.; Hallemeier, C.L.; Kelley, R.K.; Gores, G.J. Cholangiocarcinoma—Evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 2018, 15, 95. [Google Scholar] [CrossRef] [PubMed]
- Bridgewater, J.; Galle, P.R.; Khan, S.A.; Llovet, J.M.; Park, J.-W.; Patel, T.; Pawlik, T.M.; Gores, G.J. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J. Hepatol. 2014, 60, 1268–1289. [Google Scholar] [CrossRef] [PubMed]
- Hyder, O.; Hatzaras, I.; Sotiropoulos, G.C.; Paul, A.; Alexandrescu, S.; Marques, H.; Pulitano, C.; Barroso, E.; Clary, B.M.; Aldrighetti, L. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery 2013, 153, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Sommer, C.M.; Kauczor, H.U.; Pereira, P.L. Locoregional therapies of cholangiocarcinoma. Visc. Med. 2016, 32, 414–420. [Google Scholar] [CrossRef]
- Edeline, J.; Lamarca, A.; McNamara, M.G.; Jacobs, T.; Hubner, R.A.; Palmer, D.; Koerkamp, B.G.; Johnson, P.; Guiu, B.; Valle, J.W. Locoregional therapies in patients with intrahepatic cholangiocarcinoma: A systematic review and pooled analysis. Cancer Treat. Rev. 2021, 99, 102258. [Google Scholar] [CrossRef]
- Ariel, I.M. Treatment of inoperable primary pancreatic and liver cancer by the intra-arterial administration of radioactive isotopes (Y90 radiating microspheres). Ann. Surg. 1965, 162, 267. [Google Scholar] [CrossRef]
- Cucchetti, A.; Cappelli, A.; Mosconi, C.; Zhong, J.H.; Cescon, M.; Pinna, A.D.; Golfieri, R. Improving patient selection for selective internal radiation therapy of intra-hepatic cholangiocarcinoma: A meta-regression study. Liver Int. 2017, 37, 1056–1064. [Google Scholar] [CrossRef]
- Rizzo, A.; Frega, G.; Ricci, A.D.; Palloni, A.; Abbati, F.; De Lorenzo, S.; Deserti, M.; Tavolari, S.; Brandi, G. Anti-EGFR Monoclonal Antibodies in Advanced Biliary Tract Cancer: A Systematic Review and Meta-analysis. In Vivo 2020, 34, 479–488. [Google Scholar] [CrossRef]
- Rizzo, A.; Brandi, G. First-line Chemotherapy in Advanced Biliary Tract Cancer Ten Years after the ABC-02 Trial: “And Yet It Moves!”. Cancer Treat. Res. Commun. 2021, 27, 100335. [Google Scholar] [CrossRef]
- Dawson, L.A.; McGinn, C.J.; Normolle, D.; Ten Haken, R.K.; Walker, S.; Ensminger, W.; Lawrence, T.S. Escalated focal liver radiation and concurrent hepatic artery fluorodeoxyuridine for unresectable intrahepatic malignancies. J. Clin. Oncol. 2000, 18, 2210–2218. [Google Scholar] [CrossRef]
- Cremonesi, M.; Chiesa, C.; Strigari, L.; Ferrari, M.; Botta, F.; Guerriero, F.; De Cicco, C.; Bonomo, G.; Orsi, F.; Bodei, L. Radioembolization of hepatic lesions from a radiobiology and dosimetric perspective. Front. Oncol. 2014, 4, 210. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.L.; Zhang, J.; Tweedle, M.F.; Knopp, M.V.; Hall, N.C. Theranostic imaging of Yttrium-90. BioMed Res. Int. 2015, 2015, 481279. [Google Scholar] [CrossRef] [PubMed]
- Levillain, H.; Bagni, O.; Deroose, C.M.; Dieudonné, A.; Gnesin, S.; Grosser, O.S.; Kappadath, S.C.; Kennedy, A.; Kokabi, N.; Liu, D.M. International recommendations for personalised selective internal radiation therapy of primary and metastatic liver diseases with yttrium-90 resin microspheres. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1570–1584. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.; Lau, W.Y.; Leung, T.W.; Chan, M.; Ngar, Y.K.; Johnson, P.J.; Li, A.K. Partition model for estimating radiation doses from yttrium-90 microspheres in treating hepatic tumours. Eur. J. Nucl. Med. 1996, 23, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Garin, E.; Lenoir, L.; Rolland, Y.; Edeline, J.; Mesbah, H.; Laffont, S.; Porée, P.; Clément, B.; Raoul, J.-L.; Boucher, E. Dosimetry based on 99mTc-macroaggregated albumin SPECT/CT accurately predicts tumor response and survival in hepatocellular carcinoma patients treated with 90Y-loaded glass microspheres: Preliminary results. J. Nucl. Med. 2012, 53, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Toskich, B.B.; Liu, D.M. Y90 radioembolization dosimetry: Concepts for the interventional radiologist. Tech. Vasc. Interv. Radiol. 2019, 22, 100–111. [Google Scholar] [CrossRef]
- Song, Y.S.; Paeng, J.C.; Kim, H.-C.; Chung, J.W.; Cheon, G.J.; Chung, J.-K.; Lee, D.S.; Kang, K.W. PET/CT-based dosimetry in 90Y-microsphere selective internal radiation therapy: Single cohort comparison with pretreatment planning on 99mTc-MAA imaging and correlation with treatment efficacy. Medicine 2015, 94, e945. [Google Scholar] [CrossRef]
- Wondergem, M.; Smits, M.L.; Elschot, M.; de Jong, H.W.; Verkooijen, H.M.; van den Bosch, M.A.; Nijsen, J.F.; Lam, M.G. 99mTc-macroaggregated albumin poorly predicts the intrahepatic distribution of 90Y resin microspheres in hepatic radioembolization. J. Nucl. Med. 2013, 54, 1294–1301. [Google Scholar] [CrossRef]
- Moher, D.; Altman, D.G.; Liberati, A.; Tetzlaff, J. PRISMA statement. Epidemiology 2011, 22, 128. [Google Scholar] [CrossRef]
- Boehm, L.M.; Jayakrishnan, T.T.; Miura, J.T.; Zacharias, A.J.; Johnston, F.M.; Turaga, K.K.; Gamblin, T.C. Comparative effectiveness of hepatic artery based therapies for unresectable intrahepatic cholangiocarcinoma. J. Surg. Oncol. 2015, 111, 213–220. [Google Scholar] [CrossRef]
- Al-Adra, D.; Gill, R.; Axford, S.; Shi, X.; Kneteman, N.; Liau, S.-S. Treatment of unresectable intrahepatic cholangiocarcinoma with yttrium-90 radioembolization: A systematic review and pooled analysis. Eur. J. Surg. Oncol. 2015, 41, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Willowson, K.P.; Eslick, E.M.; Bailey, D.L. Individualised dosimetry and safety of SIRT for intrahepatic cholangiocarcinoma. EJNMMI Phys. 2021, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, A.; Ali, A.; Ljuboja, D.; Weinstein, J.L.; Shenoy-Bhangle, A.S.; Nasser, I.A.; Morrow, M.K.; Faintuch, S.; Curry, M.P.; Bullock, A.J.; et al. Neoadjuvant Yttrium-90 Transarterial Radioembolization with Resin Microspheres Prescribed Using the Medical Internal Radiation Dose Model for Intrahepatic Cholangiocarcinoma. J. Vasc. Interv. Radiol. 2021, 32, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- Paprottka, K.J.; Galiè, F.; Ingrisch, M.; Geith, T.; Ilhan, H.; Todica, A.; Michl, M.; Nadjiri, J.; Paprottka, P.M. Outcome and Safety after 103 Radioembolizations with Yttrium-90 Resin Microspheres in 73 Patients with Unresectable Intrahepatic Cholangiocarcinoma-An Evaluation of Predictors. Cancers 2021, 13, 5399. [Google Scholar] [CrossRef] [PubMed]
- Depalo, T.; Traino, A.C.; Bargellini, I.; Lorenzoni, G.; Bozzi, E.; Vivaldi, C.; Lamastra, R.; Masi, G.; Cioni, R.; Boni, G.; et al. Assessment of radiation sensitivity of unresectable intrahepatic cholangiocarcinoma in a series of patients submitted to radioembolization with yttrium-90 resin microspheres. Sci. Rep. 2021, 11, 19745. [Google Scholar] [CrossRef]
- Paz-Fumagalli, R.; Core, J.; Padula, C.; Montazeri, S.; McKinney, J.; Frey, G.; Devcic, Z.; Lewis, A.; Ritchie, C.; Mody, K.; et al. Safety and initial efficacy of ablative radioembolization for the treatment of unresectable intrahepatic cholangiocarcinoma. Oncotarget 2021, 12, 2075–2088. [Google Scholar] [CrossRef]
- Cheng, B.; Villalobos, A.; Sethi, I.; Wagstaff, W.; Galt, J.; Brandon, D.; Schuster, D.M.; Bercu, Z.; Majdalany, B.; Kokabi, N. Determination of Tumor Dose Response Thresholds in Patients with Chemorefractory Intrahepatic Cholangiocarcinoma Treated with Resin and Glass-based Y90 Radioembolization. Cardiovasc. Interv. Radiol. 2021, 44, 1194–1203. [Google Scholar] [CrossRef]
- Bozkurt, M.; Eldem, G.; Bozbulut, U.B.; Bozkurt, M.F.; Kılıçkap, S.; Peynircioğlu, B.; Çil, B.; Lay Ergün, E.; Volkan-Salanci, B. Factors affecting the response to Y-90 microsphere therapy in the cholangiocarcinoma patients. Radiol. Med. 2021, 126, 323–333. [Google Scholar] [CrossRef]
- Riby, D.; Mazzotta, A.D.; Bergeat, D.; Verdure, L.; Sulpice, L.; Bourien, H.; Lièvre, A.; Rolland, Y.; Garin, E.; Boudjema, K.; et al. Downstaging with Radioembolization or Chemotherapy for Initially Unresectable Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2020, 27, 3729–3737. [Google Scholar] [CrossRef]
- Mosconi, C.; Cucchetti, A.; Bruno, A.; Cappelli, A.; Bargellini, I.; De Benedittis, C.; Lorenzoni, G.; Gramenzi, A.; Tarantino, F.P.; Parini, L.; et al. Radiomics of cholangiocarcinoma on pretreatment CT can identify patients who would best respond to radioembolisation. Eur. Radiol. 2020, 30, 4534–4544. [Google Scholar] [CrossRef]
- Köhler, M.; Harders, F.; Lohöfer, F.; Paprottka, P.M.; Schaarschmidt, B.M.; Theysohn, J.; Herrmann, K.; Heindel, W.; Schmidt, H.H.; Pascher, A.; et al. Prognostic factors for overall survival in advanced intrahepatic cholangiocarcinoma treated with yttrium-90 radioembolization. J. Clin. Med. 2019, 9, 56. [Google Scholar] [CrossRef]
- Filippi, L.; Di Costanzo, G.G.; Tortora, R.; Pelle, G.; Saltarelli, A.; Marsilia, G.M.; Cianni, R.; Schillaci, O.; Bagni, O. Prognostic value of neutrophil-to-lymphocyte ratio and its correlation with fluorine-18-fluorodeoxyglucose metabolic parameters in intrahepatic cholangiocarcinoma submitted to 90Y-radioembolization. Nucl. Med. Commun. 2020, 41, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Edeline, J.; Touchefeu, Y.; Guiu, B.; Farge, O.; Tougeron, D.; Baumgaertner, I.; Ayav, A.; Campillo-Gimenez, B.; Beuzit, L.; Pracht, M.; et al. Radioembolization Plus Chemotherapy for First-line Treatment of Locally Advanced Intrahepatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2020, 6, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Buettner, S.; Braat, A.J.A.T.; Margonis, G.A.; Brown, D.B.; Taylor, K.B.; Borgmann, A.J.; Kappadath, S.C.; Mahvash, A.; Ijzermans, J.N.M.; Weiss, M.J.; et al. Yttrium-90 Radioembolization in Intrahepatic Cholangiocarcinoma: A Multicenter Retrospective Analysis. J. Vasc. Interv. Radiol. 2020, 31, 1035–1043.e2. [Google Scholar] [CrossRef] [PubMed]
- Bargellini, I.; Mosconi, C.; Pizzi, G.; Lorenzoni, G.; Vivaldi, C.; Cappelli, A.; Vallati, G.E.; Boni, G.; Cappelli, F.; Paladini, A.; et al. Yttrium-90 Radioembolization in Unresectable Intrahepatic Cholangiocarcinoma: Results of a Multicenter Retrospective Study. Cardiovasc. Interv. Radiol. 2020, 43, 1305–1314. [Google Scholar] [CrossRef]
- Azar, A.; Devcic, Z.; Paz-Fumagalli, R.; Vidal, L.L.C.; McKinney, J.M.; Frey, G.; Lewis, A.R.; Ritchie, C.; Starr, J.S.; Mody, K.; et al. Albumin-bilirubin grade as a prognostic indicator for patients with non-hepatocellular primary and metastatic liver malignancy undergoing Yttrium-90 radioembolization using resin microspheres. J. Gastrointest. Oncol. 2020, 11, 715–723. [Google Scholar] [CrossRef]
- White, J.; Carolan-Rees, G.; Dale, M.; Patrick, H.E.; See, T.C.; Bell, J.K.; Manas, D.M.; Crellin, A.; Slevin, N.J.; Sharma, R.A. Yttrium-90 Transarterial Radioembolization for Chemotherapy-Refractory Intrahepatic Cholangiocarcinoma: A Prospective, Observational Study. J. Vasc. Interv. Radiol. 2019, 30, 1185–1192. [Google Scholar] [CrossRef]
- Galiè, F.; Paprottka, K.J.; Ingrisch, M.; Todica, A.; Ilhan, H.; Michl, M.; Geith, T.; Fabritius, M.; De Toni, E.; Paprottka, P.M. Impact of Baseline Cholinesterase in Patients with Primary Liver Tumors Undergoing Radioembolization: Impact on Outcome. SN Compr. Clin. Med. 2019, 1, 85–92. [Google Scholar] [CrossRef]
- Bourien, H.; Palard, X.; Rolland, Y.; Le Du, F.; Beuzit, L.; Uguen, T.; Le Sourd, S.; Pracht, M.; Manceau, V.; Lièvre, A.; et al. Yttrium-90 glass microspheres radioembolization (RE) for biliary tract cancer: A large single-center experience. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 669–676. [Google Scholar] [CrossRef]
- Levillain, H.; Derijckere, I.D.; Ameye, L.; Guiot, T.; Braat, A.; Meyer, C.; Vanderlinden, B.; Reynaert, N.; Hendlisz, A.; Lam, M. Personalised radioembolization improves outcomes in refractory intra-hepatic cholangiocarcinoma: A multicenter study. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2270–2279. [Google Scholar] [CrossRef]
- Shaker, T.M.; Chung, C.; Varma, M.K.; Doherty, M.G.; Wolf, A.M.; Chung, M.H.; Assifi, M.M. Is there a role for Ytrrium-90 in the treatment of unresectable and metastatic intrahepatic cholangiocarcinoma? Am. J. Surg. 2018, 215, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Reimer, P.; Virarkar, M.K.; Binnenhei, M.; Justinger, M.; Schön, M.R.; Tatsch, K. Prognostic Factors in Overall Survival of Patients with Unresectable Intrahepatic Cholangiocarcinoma Treated by Means of Yttrium-90 Radioembolization: Results in Therapy-Naïve Patients. Cardiovasc. Interv. Radiol. 2018, 41, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Nezami, N.; Kokabi, N.; Camacho, J.C.; Schuster, D.M.; Xing, M.; Kim, H.S. 90Y radioembolization dosimetry using a simple semi-quantitative method in intrahepatic cholangiocarcinoma: Glass versus resin microspheres. Nucl. Med. Biol. 2018, 59, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Manceau, V.; Palard, X.; Rolland, Y.; Pracht, M.; Le Sourd, S.; Laffont, S.; Boudjema, K.; Lievre, A.; Mesbah, H.; Haumont, L.A.; et al. A MAA-based dosimetric study in patients with intrahepatic cholangiocarcinoma treated with a combination of chemotherapy and 90Y-loaded glass microsphere selective internal radiation therapy. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1731–1741. [Google Scholar] [CrossRef] [PubMed]
- Gangi, A.; Shah, J.; Hatfield, N.; Smith, J.; Sweeney, J.; Choi, J.; El-Haddad, G.; Biebel, B.; Parikh, N.; Arslan, B.; et al. Intrahepatic Cholangiocarcinoma Treated with Transarterial Yttrium-90 Glass Microsphere Radioembolization: Results of a Single Institution Retrospective Study. J. Vasc. Interv. Radiol. 2018, 29, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Swinburne, N.C.; Biederman, D.M.; Besa, C.; Tabori, N.E.; Fischman, A.M.; Patel, R.S.; Nowakowski, F.S.; Gunasekaran, G.; Schwartz, M.E.; Lookstein, R.A.; et al. Radioembolization for Unresectable Intrahepatic Cholangiocarcinoma: Review of Safety, Response Evaluation Criteria in Solid Tumors 1.1 Imaging Response and Survival. Cancer Biother. Radiopharm. 2017, 32, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Paz-Fumagalli, R.; Frey, G.; Sella, D.M.; McKinney, J.M.; Wang, W. Resin-based Yttrium-90 microspheres for unresectable and failed first-line chemotherapy intrahepatic cholangiocarcinoma: Preliminary results. J. Cancer Res. Clin. Oncol. 2017, 143, 481–489. [Google Scholar] [CrossRef]
- Akinwande, O.; Shah, V.; Mills, A.; Noda, C.; Weiner, E.; Foltz, G.; Saad, N. Chemoembolization versus radioembolization for the treatment of unresectable intrahepatic cholangiocarcinoma in a single institution image-based efficacy and comparative toxicity. Hepat. Oncol. 2017, 4, 75–81. [Google Scholar] [CrossRef]
- Soydal, C.; Kucuk, O.N.; Bilgic, S.; Ibis, E. Radioembolization with 90Y resin microspheres for intrahepatic cholangiocellular carcinoma: Prognostic factors. Ann. Nucl. Med. 2016, 30, 29–34. [Google Scholar] [CrossRef]
- Pieper, C.C.; Willinek, W.A.; Thomas, D.; Ahmadzadehfar, H.; Essler, M.; Nadal, J.; Wilhelm, K.E.; Schild, H.H.; Meyer, C. Incidence and risk factors of early arterial blood flow stasis during first radioembolization of primary and secondary liver malignancy using resin microspheres: An initial single-center analysis. Eur. Radiol. 2016, 26, 2779–2789. [Google Scholar] [CrossRef]
- Mosconi, C.; Gramenzi, A.; Ascanio, S.; Cappelli, A.; Renzulli, M.; Pettinato, C.; Brandi, G.; Monari, F.; Cucchetti, A.; Trevisani, F.; et al. Yttrium-90 radioembolization for unresectable/recurrent intrahepatic cholangiocarcinoma: A survival, efficacy and safety study. Br. J. Cancer 2016, 115, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.G.E.H.; Banerjee, A.; Goris, M.L.; Iagaru, A.H.; Mittra, E.S.; Louie, J.D.; Sze, D.Y. Fusion dual-tracer SPECT-based hepatic dosimetry predicts outcome after radioembolization for a wide range of tumour cell types. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Filippi, L.; Pelle, G.; Cianni, R.; Scopinaro, F.; Bagni, O. Change in total lesion glycolysis and clinical outcome after (90)Y radioembolization in intrahepatic cholangiocarcinoma. Nucl. Med. Biol. 2015, 42, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Edeline, J.; Du, F.L.; Rayar, M.; Rolland, Y.; Beuzit, L.; Boudjema, K.; Rohou, T.; Latournerie, M.; Campillo-Gimenez, B.; Garin, E.; et al. Glass microspheres 90Y selective internal radiation therapy and chemotherapy as first-line treatment of intrahepatic cholangiocarcinoma. Clin. Nucl. Med. 2015, 40, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Camacho, J.C.; Kokabi, N.; Xing, M.; Prajapati, H.J.; El-Rayes, B.; Kim, H.S. Modified response evaluation criteria in solid tumors and european association for the study of the liver criteria using delayed-phase imaging at an early time point predict survival in patients with unresectable intrahepatic cholangiocarcinoma following yttrium-90 radioembolization. J. Vasc. Interv. Radiol. 2014, 25, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Rafi, S.; Piduru, S.M.; El-Rayes, B.; Kauh, J.S.; Kooby, D.A.; Sarmiento, J.M.; Kim, H.S. Yttrium-90 radioembolization for unresectable standard-chemorefractory intrahepatic cholangiocarcinoma: Survival, efficacy, and safety study. Cardiovasc. Interv. Radiol. 2013, 36, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Mouli, S.; Memon, K.; Baker, T.; Benson Iii, A.B.; Mulcahy, M.F.; Gupta, R.; Ryu, R.K.; Salem, R.; Lewandowski, R.J. Yttrium-90 radioembolization for intrahepatic cholangiocarcinoma: Safety, response, and survival analysis. J. Vasc. Interv. Radiol. 2013, 24, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R.T.; Paprottka, P.M.; Schön, A.; Bamberg, F.; Haug, A.; Dürr, E.M.; Rauch, B.; Trumm, C.T.; Jakobs, T.F.; Helmberger, T.K.; et al. Transarterial hepatic yttrium-90 radioembolization in patients with unresectable intrahepatic cholangiocarcinoma: Factors associated with prolonged survival. Cardiovasc. Interv. Radiol. 2012, 35, 105–116. [Google Scholar] [CrossRef]
- Haug, A.R.; Heinemann, V.; Bruns, C.J.; Hoffmann, R.; Jakobs, T.; Bartenstein, P.; Hacker, M. 18 F-FDG PET independently predicts survival in patients with cholangiocellular carcinoma treated with 90Y microspheres. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1037–1045. [Google Scholar] [CrossRef]
- Saxena, A.; Bester, L.; Chua, T.C.; Morris, D.L. Yttrium-90 radiotherapy for unresectable intrahepatic cholangiocarcinoma: A preliminary assessment of this novel treatment option. Ann. Surg. Oncol. 2010, 17, S103. [Google Scholar] [CrossRef]
- Ibrahim, S.M.; Mulcahy, M.F.; Lewandowski, R.J.; Sato, K.T.; Ryu, R.K.; Masterson, E.J.; Newman, S.B.; Benson Iii, A.; Omary, R.A.; Salem, R. Treatment of unresectable cholangiocarcinoma using yttrium-90 microspheres: Results from a pilot study. Cancer 2008, 113, 2119–2128. [Google Scholar] [CrossRef] [PubMed]
- Helmberger, T.; Arnold, D.; Balli, T.; Golfieri, R.; Pech, M.; Ronot, M.; De Jong, N.; Sangro, B. Real-world outcomes of patients with intrahepatic cholangiocarcinoma treated with trans-arterial radioembolization: Results from the CIRSE Registry for SIR-Spheres Therapy (CIRT), alarge European prospective multi-center observational study. J. Clin. Oncol. 2021, 39, 308. [Google Scholar] [CrossRef]
- Lorenzoni, A.; Mazzaglia, S.; Spreafico, C.; Scalorbi, F.; Argiroffi, G.; Bhoori, S.; Chiesa, C.; Fuoco, V.; Cascella, T.; Seregni, E.; et al. Transarterial radioembolization of unresectable intrahepatic cholangiocarcinoma with 90Y glass microspheres: Results of a single institution study. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, S278–S279. [Google Scholar] [CrossRef]
- Core, J.; Padula, C.; Elboraey, M.; Devcic, Z.; Ritchie, C.; Lewis, A.; McKinney, J.; Paz-Fumagalli, R.; Frey, G.; Toskich, B. Abstract No. 560 Safety and efficacy of radioembolization for intrahepatic cholangiocarcinoma with ≥150 Gy MIRD: A single-center review. J. Vasc. Interv. Radiol. 2020, 31, S244. [Google Scholar] [CrossRef]
- Pettinato, C.; Mosconi, C.; Cappelli, A.; Tabacchi, E.; Lodi Rizzini, E.; Monari, F.; Civollani, S.; Fanti, S.; Strigari, L. Yttrium-90 radioembolization for unresectable/recurrent intrahepatic cholangiocarcinoma. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, S632–S633. [Google Scholar] [CrossRef]
- Schatka, I.; Jochens, H.; Rogasch, J.; Huang, K.; Wedel, F.; Heimann, U.; Bartel, C.; Furth, C.; Brenner, W.; Gebauer, B.; et al. Radioembolization in patients with intrahepatic cholangiocarcinoma-a prognostic risk stratification model. J. Nucl. Med. 2017, 58, 459. [Google Scholar]
- Boni, G.; Depalo, T.; Bargellini, I.; Vivaldi, C.; Mazzarri, S.; Guidoccio, F.; Bozzi, E.; Caponi, L.; Traino, C.; Manca, G.; et al. Effectiveness and safety of transarterial Y-90 radioembolization for unresectable intrahepatic cholangiocarcinoma. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, S803. [Google Scholar] [CrossRef]
- Peterson, J.; Vallow, L.; Johnson, D.; Heckman, M.; Diehl, N.; Gale, A.; Tzou, K.; Kim, S.; Brown, N.; Paz-Fumagalli, R.; et al. Initial experience with sirspheres Yttrium-90 microsphere radioembolization for cholangiocarcinoma. HPB 2010, 12, 286–287. [Google Scholar] [CrossRef][Green Version]
- Nezami, N.; Camacho, J.C.; Kokabi, N.; El-Rayes, B.F.; Kim, H.S. Phase Ib trial of gemcitabine with yttrium-90 in patients with hepatic metastasis of pancreatobiliary origin. J. Gastrointest. Oncol. 2019, 10, 944. [Google Scholar] [CrossRef]
- Kao, Y.-H.; Steinberg, J.D.; Tay, Y.-S.; Lim, G.K.; Yan, J.; Townsend, D.W.; Takano, A.; Burgmans, M.C.; Irani, F.G.; Teo, T.K. Post-radioembolization yttrium-90 PET/CT-part 1: Diagnostic reporting. EJNMMI Res. 2013, 3, 56. [Google Scholar] [CrossRef]
- Padia, S.A.; Alessio, A.; Kwan, S.W.; Lewis, D.H.; Vaidya, S.; Minoshima, S. Comparison of positron emission tomography and bremsstrahlung imaging to detect particle distribution in patients undergoing yttrium-90 radioembolization for large hepatocellular carcinomas or associated portal vein thrombosis. J. Vasc. Interv. Radiol. 2013, 24, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Gulec, S.A.; Sztejnberg, M.L.; Siegel, J.A.; Jevremovic, T.; Stabin, M. Hepatic structural dosimetry in (90)Y microsphere treatment: A Monte Carlo modeling approach based on lobular microanatomy. J. Nucl Med. 2010, 51, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.; Liu, B.; Chang, Z.; Ren, H.; Liu, Z.; Zheng, J. A pooled analysis of transarterial radioembolization with yttrium-90 microspheres for the treatment of unresectable intrahepatic cholangiocarcinoma. Onco Targets Ther. 2019, 12, 4489–4498. [Google Scholar] [CrossRef] [PubMed]
- Park, J.O.; Oh, D.-Y.; Hsu, C.; Chen, J.-S.; Chen, L.-T.; Orlando, M.; Kim, J.S.; Lim, H.Y. Gemcitabine plus cisplatin for advanced biliary tract cancer: A systematic review. Cancer Res. Treat. 2015, 47, 343–361. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Small, W., Jr.; Berlin, J.; Freedman, G.M.; Lawrence, T.; Talamonti, M.S.; Mulcahy, M.F.; Chakravarthy, A.B.; Konski, A.A.; Zalupski, M.M.; Philip, P.A. Full-dose gemcitabine with concurrent radiation therapy in patients with nonmetastatic pancreatic cancer: A multicenter phase II trial. J. Clin. Oncol. 2008, 26, 942–947. [Google Scholar] [CrossRef]
- Zhu, C.-P.; Shi, J.; Chen, Y.-X.; Xie, W.-F.; Lin, Y. Gemcitabine in the chemoradiotherapy for locally advanced pancreatic cancer: A meta-analysis. Radiother. Oncol. 2011, 99, 108–113. [Google Scholar] [CrossRef]
- Mosconi, C.; Solaini, L.; Vara, G.; Brandi, N.; Cappelli, A.; Modestino, F.; Cucchetti, A.; Golfieri, R. Transarterial Chemoembolization and Radioembolization for Unresectable Intrahepatic Cholangiocarcinoma—A Systemic Review and Meta-Analysis. Cardiovasc. Interv. Radiol. 2021, 44, 728–738. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).