Prognostic Factors Influencing Survival and a Treatment Pattern Analysis of Conventional Palliative Radiotherapy for Patients with Bone Metastases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Data Collection
2.2.1. General Demographics
2.2.2. Treatment Related Information
2.3. Follow-Up and Outcomes
2.4. Statistical Analysis
Analysis of Overall Survival (OS)
2.5. Treatment Pattern Analysis
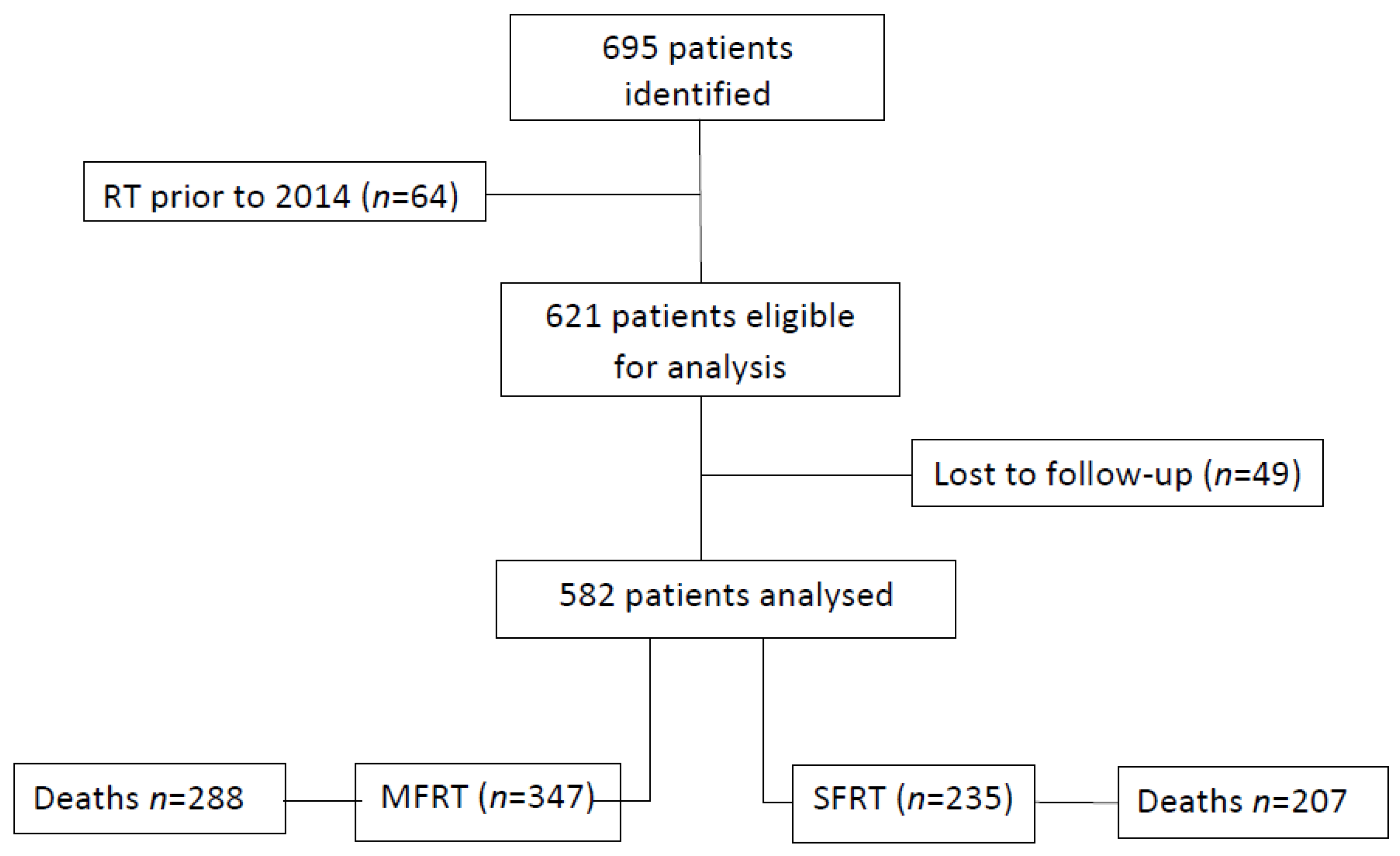
3. Results
3.1. Patient Characteristics
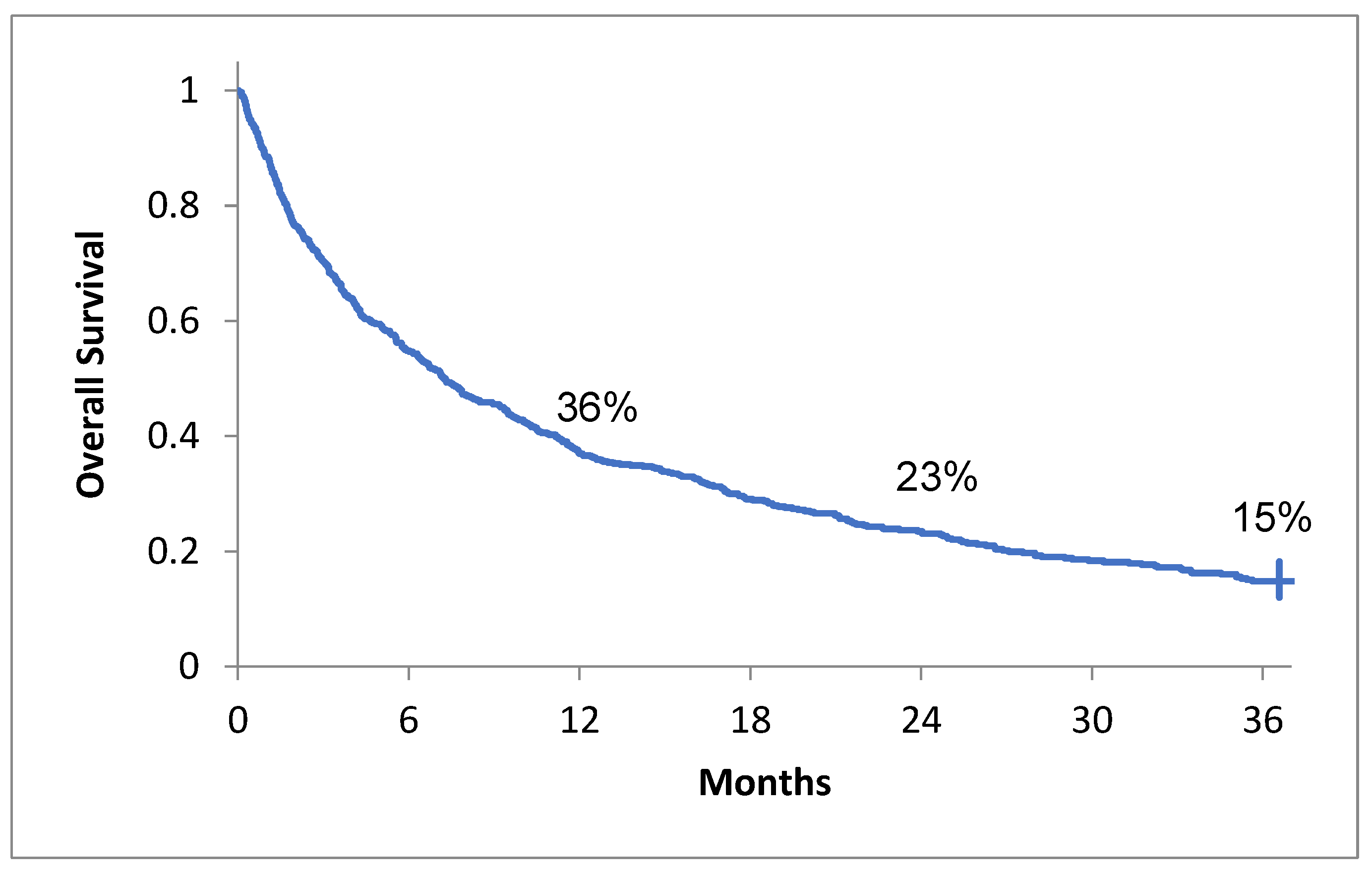
3.2. Overall Survival and Its Association with Clinical Features
3.3. Multivariate Analysis
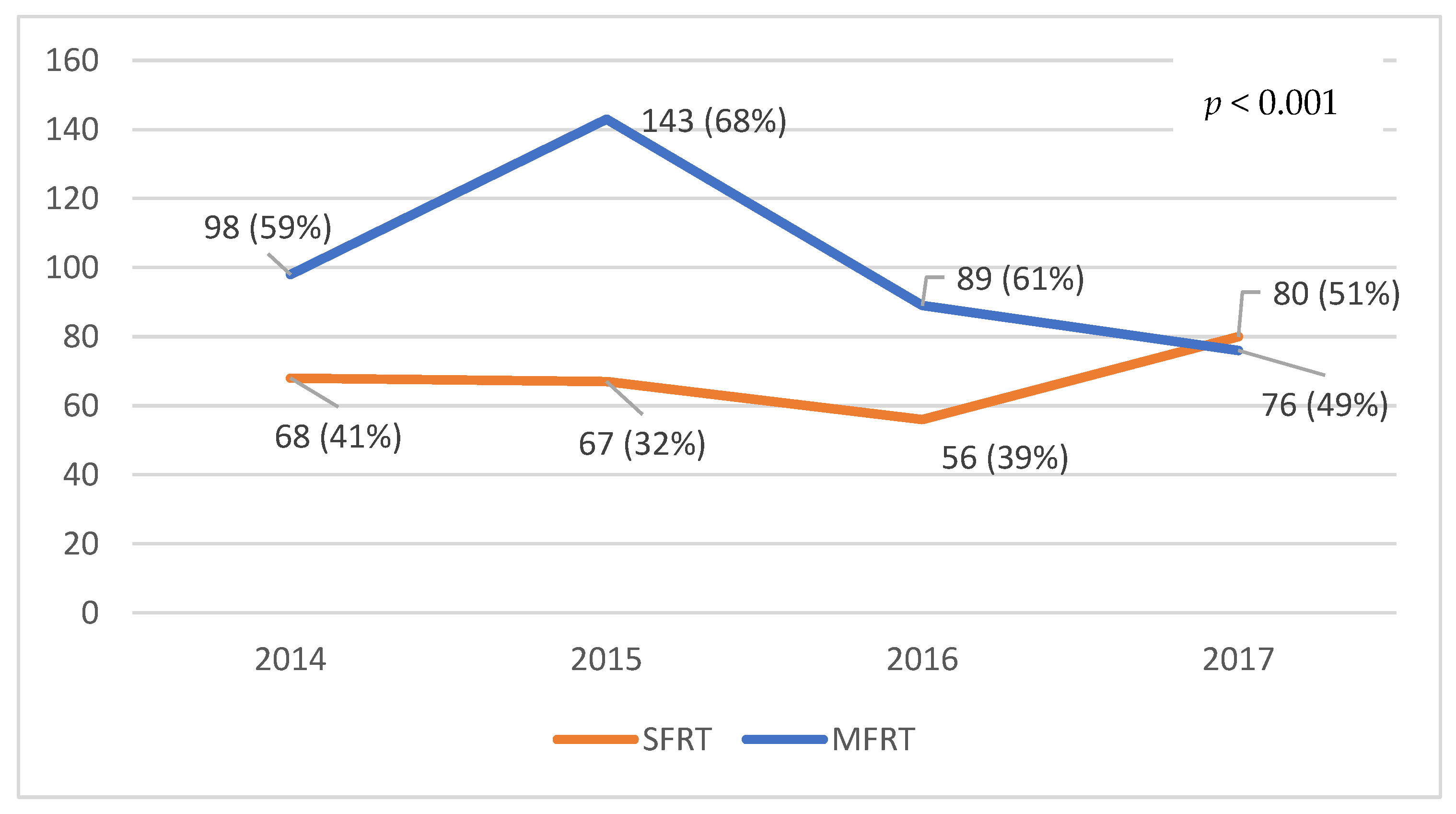
3.4. Treatment Pattern Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coleman, R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 2006, 12, 6243s–6249s. [Google Scholar] [CrossRef] [Green Version]
- Olson, R.A.; Tiwana, M.S.; Barnes, M.; Kiraly, A.; Beecham, K.; Miller, S.; Hoegler, D.; Olivotto, I. Use of single- versus multiple-fraction palliative radiation therapy for bone metastases: Population-based analysis of 16,898 courses in a Canadian province. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 1092–1099. [Google Scholar] [CrossRef]
- Pandit-Taskar, N.; Batraki, M.; Divgi, C.R. Radiopharmaceutical therapy for palliation of bone pain from osseous metastases. J. Nucl. Med. 2004, 45, 1358–1365. [Google Scholar]
- Katagiri, H.; Okada, R.; Takagi, T.; Takahashi, M.; Murata, H.; Harada, H.; Nishimura, T.; Asakura, H.; Ogawa, H. New prognostic factors and scoring system for patients with skeletal metastasis. Cancer Med. 2014, 3, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, J.P.; Swangsilpa, T.; van der Linden, Y.; Rades, D.; Jeremic, B.; Hoskin, P.J. The role of external beam radiotherapy in the management of bone metastases. Clin. Oncol. 2006, 18, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, O.S. Palliative radiotherapy of bone metastases: There is now evidence for the use of single fractions. Radiother. Oncol. 1999, 52, 95. [Google Scholar] [PubMed]
- Lutz, S.; Berk, L.; Chang, E.; Chow, E.; Hahn, C.; Hoskin, P.; Howell, D.; Konski, A.; Kachnic, L.; Lo, S.; et al. Palliative radiotherapy for bone metastases: An ASTRO evidence-based guideline. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, P.J.; Stratford, M.R.L.; Folkes, L.K.; Regan, J.; Yarnold, J.R. Effect of local radiotherapy for bone pain on urinary markers of osteoclast activity. Lancet 2000, 355, 1428–1429. [Google Scholar] [CrossRef]
- Ganesh, V.; Chan, S.; Raman, S.; Chow, R.; Hoskin, P.; Lam, H.; Wan, B.A.; Drost, L.; DeAngelis, C.; Chow, E. A review of patterns of practice and clinical guidelines in the palliative radiation treatment of uncomplicated bone metastases. Radiother. Oncol. 2017, 124, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Anter, A.H. Single fraction versus multiple fraction radiotherapy for treatment of painful bone metastases: A prospective study: Mansoura experience. Forum. Clin. Oncol. 2015, 6, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez Bayard, L.; Sala Buzon, M.D.C.; Angulo Pain, E.; de Ingunza Baron, L. Radiation therapy for the management of painful bone metastases: Results from a randomized trial. Rep. Pract. Oncol. Radiother. 2014, 19, 405–411. [Google Scholar] [CrossRef] [Green Version]
- Majumder, D.; Chatterjee, D.; Bandyopadhyay, A.; Mallick, S.K.; Sarkar, S.K.; Majumdar, A. Single fraction versus multiple fraction radiotherapy for palliation of painful vertebral bone metastases: A prospective study. Indian J. Palliat. Care 2012, 18, 202–206. [Google Scholar] [CrossRef]
- Konski, A.; James, J.; Hartsell, W.; Leibenhaut, M.H.; Janjan, N.; Curran, W.; Roach, M.; Watkins-Bruner, D. Economic analysis of radiation therapy oncology group 97–14: Multiple versus single fraction radiation treatment of patients with bone metastases. Am. J. Clin. Oncol. 2009, 32, 423–428. [Google Scholar] [CrossRef]
- van den Hout, W.B.; van der Linden, Y.M.; Steenland, E.; Wiggenraad, R.G.J.; Kievit, J.; de Haes, H.; Leer, J.W.H. Single- versus multiple-fraction radiotherapy in patients with painful bone metastases: Cost-utility analysis based on a randomized trial. J. Natl. Cancer Inst. 2003, 95, 222–229. [Google Scholar] [CrossRef]
- Chow, R.; Hoskin, P.; Schild, S.E.; Raman, S.; Im, J.; Zhang, D.; Chan, S.; Chiu, N.; Chiu, L.; Lam, H.; et al. Single vs. multiple fraction palliative radiation therapy for bone metastases: Cumulative meta-analysis. Radiother. Oncol. 2019, 141, 56–61. [Google Scholar] [CrossRef] [PubMed]
- James, J.J.; Evans, A.J.; Pinder, S.; Gutteridge, E.; Cheung, K.L.; Chan, S.; Robertson, J.F.R. Bone metastases from breast carcinoma: Histopathological—Radiological correlations and prognostic features. Br. J. Cancer 2003, 89, 660–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomita, K.; Kawahara, N.; Kobayashi, T.; Yoshida, A.; Murakami, H.; Akamaru, T. Surgical strategy for spinal metastases. Spine 2001, 26, 298–306. [Google Scholar] [CrossRef]
- Rich, S.E.; Chow, R.; Raman, S.; Liang Zeng, K.; Lutz, S.; Lam, H.; Silva, M.F.; Chow, E. Update of the systematic review of palliative radiation therapy fractionation for bone metastases. Radiother. Oncol. 2018, 126, 547–557. [Google Scholar] [CrossRef] [PubMed]
- McDonald, R.; Chow, E.; Lam, H.; Rowbottom, L.; Soliman, H. International patterns of practice in radiotherapy for bone metastases: A review of the literature. J. Bone Oncol. 2014, 3, 96–102. [Google Scholar] [CrossRef] [Green Version]
- Tsukamoto, S.; Kido, A.; Tanaka, Y.; Facchini, G.; Peta, G.; Rossi, G.; Mavrogenis, A.F. Current Overview of Treatment for Metastatic Bone Disease. Curr. Oncol. 2021, 28, 3347–3372. [Google Scholar] [CrossRef]
- Willeumier, J.J.; van der Linden, Y.M.; van der Wal, C.W.P.G.; Jutte, P.C.; van der Velden, J.M.; Smolle, M.A.; van der Zwaal, P.; Koper, P.; Bakri, L.; de Pree, I.; et al. An Easy-to-Use Prognostic Model for Survival Estimation for Patients with Symptomatic Long Bone Metastases. J. Bone Joint Surg. Am. 2018, 100, 196–204. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Li, H.F.; Su, M.; Lin, R.F.; Chen, X.X.; Zhang, P.; Zou, C.L. A Simple Scoring System Predicting the Survival Time of Patients with Bone Metastases after RT. PLoS ONE 2016, 11, e0159506. [Google Scholar] [CrossRef]
- Kubota, H.; Soejima, T.; Sulaiman, N.S.; Sekii, S.; Matsumoto, Y.; Ota, Y.; Tsujino, K.; Fujita, I.; Fujimoto, T.; Morishita, M.; et al. Predicting the survival of patients with bone metastases treated with radiation therapy: A validation study of the Katagiri scoring system. Radiat. Oncol. 2019, 14, 1–8. [Google Scholar] [CrossRef]
- Tokuhashi, Y.; Matsuzaki, H.; Oda, H.; Oshima, M.; Ryu, J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine 2005, 30, 2186–2191. [Google Scholar] [CrossRef]
- Mizumoto, M.; Harada, H.; Asakura, H.; Hashimoto, T.; Furutani, K.; Hashii, H.; Takagi, T.; Katagiri, H.; Takahashi, M.; Nishimura, T. Prognostic factors and a scoring system for survival after radiotherapy for metastases to the spinal column: A review of 544 patients at Shizuoka Cancer Center Hospital. Cancer 2008, 113, 2816–2822. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, H.; Takahashi, M.; Wakai, K.; Sugiura, H.; Kataoka, T.; Nakanishi, K. Prognostic factors and a scoring system for patients with skeletal metastasis. J. Bone Jt. Surg. 2005, 87, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, M.S.; Gerds, T.A.; Hindsø, K.; Petersen, M.M. External validation and optimization of the SPRING Model for prediction of survival after surgical treatment of bone metastases of the extremities. Clin. Orthop. Relat. Res. 2018, 476, 1591–1599. [Google Scholar] [CrossRef] [PubMed]
- Janssen, S.J.; van der Heijden, A.S.; van Dijke, M.; Ready, J.E.; Raskin, K.A.; Ferrone, M.L.; Hornicek, F.J.; Schwab, J.H. 2015 Marshall Urist Young Investigator Award: Prognostication in patients with long bone metastases: Does a boosting algorithm improve survival estimates? Clin. Orthop. Relat. Res. 2015, 473, 3112–3121. [Google Scholar] [CrossRef] [Green Version]
- Anderson, A.B.; Wedin, R.; Fabbri, N.; Boland, P.; Healey, J.; Forsberg, J.A. External validation of PATHFx Version 3.0 in patients treated surgically and nonsurgically for symptomatic skeletal metastases. Clin. Orthop. Relat. Res. 2020, 478, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Thio, Q.C.B.S.; Karhade, A.V.; Bindels, B.J.J.; Ogink, P.T.; Bramer, J.A.M.; Ferrone, M.L.; Calderón, S.L.; Raskin, K.A.; Schwab, J.H. Development and internal validation of machine learning algorithms for preoperative survival prediction of extremity metastatic disease. Clin. Orthop. Relat. Res. 2020, 478, 322–333. [Google Scholar] [CrossRef]
- Karhade, A.V.; Thio, Q.C.B.S.; Ogink, P.T.; Bono, C.M.; Ferrone, M.L.; Oh, K.S.; Saylor, P.J.; Schoenfeld, A.J.; Shin, J.H.; Harris, M.B.; et al. Predicting 90-Day and 1-Year Mortality in Spinal Metastatic Disease: Development and Internal Validation. Neurosurgery 2019, 85, E671–E681. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.S.; Xu, H.Y.; Du, Z.S.; Li, X.Y.; Wu, S.X.; Huang, H.C.; Lin, L.X. Impact of Sex on the Prognosis of Patients with Esophageal Squamous Cell Cancer Underwent Definitive Radiotherapy: A Propensity Score-Matched Analysis. Radiat. Oncol. 2019, 14, 74. Available online: https://ro-journal.biomedcentral.com/articles/10.1186/s13014-019-1278-0 (accessed on 23 August 2021). [CrossRef] [PubMed]
- Gasinska, A.; Darasz, Z.; Adamczyk, A.; Biesaga, B.; Niemiec, J.; Reinfuss, M. Gender-related prognostic significance of clinical and biological tumor features in rectal cancer patients receiving short-course preoperative radiotherapy. Rep. Pract. Oncol. Radiother. 2017, 22, 368–377. [Google Scholar] [CrossRef] [PubMed]
- De Courcy, L.; Bezak, E.; Marcu, L.G. Gender-Dependent Radiotherapy: The Next Step in Personalised Medicine? Crit. Rev. Oncol. Hematol. 2020, 147, 102881. Available online: https://www.sciencedirect.com/science/article/abs/pii/S1040842820300196?via%3Dihub (accessed on 23 August 2021). [CrossRef]
- Haupt, S.; Caramia, F.; Klein, S.L.; Rubin, J.B.; Haupt, Y. Sex disparities matter in cancer development and therapy. Nat. Rev. Cancer 2021, 21, 393–407. [Google Scholar] [CrossRef]
- Meeuse, J.J.; van der Linden, Y.M.; van Tienhoven, G.; Gans, R.O.B.; Leer, J.W.H.; Reyners, A.K.L. Efficacy of radiotherapy for painful bone metastases during the last 12 weeks of life: Results from the Dutch Bone Metastasis Study. Cancer 2010, 116, 2716–2725. [Google Scholar] [CrossRef]
- Lutz, S.; Balboni, T.; Jones, J.; Lo, S.; Petit, J.; Rich, S.E.; Wong, R.; Hahn, C. Palliative radiation therapy for bone metastases: Update of an ASTRO Evidence-Based Guideline. Pract. Radiat. Oncol. 2017, 7, 4–12. [Google Scholar] [CrossRef] [Green Version]
- Bekelman, J.E.; Epstein, A.J.; Emanuel, E.J. Single- vs. multiple-fraction radiotherapy for bone metastases from prostate cancer. JAMA 2013, 310, 1501–1502. [Google Scholar] [CrossRef] [Green Version]
- Szostakiewicz, B.; Dziadziuszko, R.; Welnicka-Jaskiewicz, M.; Jassem, J. Palliative irradiation of bone metastases: Patterns of care with focus on single fraction treatment. Rep. Pract. Oncol. Radiother. 2004, 9, 9–12. [Google Scholar] [CrossRef] [Green Version]
- Haddad, P.; Wong, R.K.; Pond, G.R.; Soban, F.; Williams, D.; McLean, M.; Levin, W.; Bezjak, A. Factors influencing the use of single vs. multiple fractions of palliative radiotherapy for bone metastases: A 5-year review. Clin. Oncol. (R. Coll. Radiol.) 2005, 17, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Bradley, N.M.; Husted, J.; Sey, M.S.; Sinclair, E.; Li, K.K.; Husain, A.F.; Danjoux, C.; Barnes, E.A.; Tsao, M.N.; Barbera, L.; et al. Did the pattern of practice in the prescription of palliative radiotherapy for the treatment of uncomplicated bone metastases change between 1999 and 2005 at the rapid response radiotherapy program? Clin. Oncol. (R. Coll. Radiol.) 2008, 20, 327–336. [Google Scholar] [CrossRef]
- Beriwal, S.; Rajagopalan, M.S.; Flickinger, J.C.; Rakfal, S.M.; Rodgers, E.; Heron, D.E. How effective are clinical pathways with and without online peer-review? An analysis of bone metastases pathway in a large, integrated National Cancer Institute designated comprehensive cancer center network. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1246–1251. [Google Scholar] [CrossRef]
- Laugsand, T.S.; Kaasa, S.; Romundstad, P.; Johannesen, T.B.; Lund, J.A. Radiotherapy for bone metastases: Practice in Norway 1997–2007. A national registry-based study. Acta Oncol. 2013, 52, 1129–1136. [Google Scholar] [CrossRef]
- Thavarajah, N.; Zhang, L.; Wong, K.; Bedard, G.; Wong, E.; Tsao, M.; Danjoux, C.; Barnes, E.; Sahgal, A.; Dennis, K.; et al. Patterns of Practice in the Prescription of Palliative Radiotherapy for the Treatment of Bone Metastases at the Rapid Response Radiotherapy Program between 2005 and 2012. Curr. Oncol. 2013, 20, 396–405. [Google Scholar] [CrossRef] [Green Version]
- Ashworth, A.; Kong, W.; Chow, E.; Mackillop, W.J. Fractionation of Palliative Radiation Therapy for Bone Metastases in Ontario: Do Practice Guidelines Guide Practice? Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 31–39. [Google Scholar] [CrossRef]
- Kim, J.O.; Hanumanthappa, N.; Chung, Y.T.; Beck, J.; Koul, R.; Bashir, B.; Cooke, A.; Dubey, A.; Butler, J.; Nashed, M.; et al. Does Dissemination of Guidelines Alone Increase the Use of Palliative Single-Fraction Radiotherapy? Initial Report of a Longitudinal Change Management Campaign at a Provincial Cancer Program. Curr. Oncol. 2020, 27, 190–197. [Google Scholar] [CrossRef]
- Hess, G.; Barlev, A.; Chung, K.; Hill, J.W.; Fonseca, E. Cost of palliative radiation to the bone for patients with bone metastases secondary to breast or prostate cancer. Radiat. Oncol. 2012, 7, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alcorn, S.R.; Hales, R.K.; Smith, T.J.; Rowbottom, L.; Soliman, H. Patterns of fractionation of palliative radiation therapy: A single-institution experience. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 562. [Google Scholar] [CrossRef]
- Loblaw, D.A.; Mitera, G.; Ford, M.; Laperriere, N.J. A 2011 updated systematic review and clinical practice guideline for the management of malignant extradural spinal cord compression. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.; Zeng, L.; Salvo, N.; Dennis, K.; Tzao, M.; Lutz, S. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin. Oncol. 2012, 24, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Howell, D.D.; James, J.L.; Hartsell, W.F.; Suntharalingam, M.; Machtay, M.; Suh, J.H.; Demas, W.F.; Sandler, H.M.; Kachnic, L.A.; Berk, L.B. Single-fraction radiotherapy versus multifraction radiotherapy for palliation of painful vertebral bone metastases-equivalent efficacy, less toxicity, more convenient: A subset analysis of Radiation Therapy Oncology Group trial 97-14. Cancer 2013, 119, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Huisman, M.; van den Bosch, M.A.; Wijlemans, J.W.; van Vulpen, M.; van der Linden, Y.M.; Verkooijen, H.M. Effectiveness of reirradiation for painful bone metastases: A systematic review and meta-analysis. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 8–14. [Google Scholar] [CrossRef]
- Rosenblatt, E.; Zubizarreta, E.; Wondergem, J.; Fidarova, E.; Izewska, J. The International Atomic Energy Agency (IAEA): An active role in the global fight against cancer. Radiother. Oncol. 2012, 104, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Spencer, K.L.; van der Velden, J.M.; Wong, E.; Seravalli, E.; Sahgal, A.; Chow, E.; Verlaan, J.J.; Verkooijen, H.M.; van der Linden, Y.M. Systematic review of the role of stereotactic radiotherapy for bone metastases. J. Natl. Cancer Inst. 2019, 111, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- De la Pinta, C. SBRT in non-spine bone metastases: A literature review. Med. Oncol. 2020, 37, 119. [Google Scholar] [CrossRef]
- Owen, D.; Laack, N.N.; Mayo, C.S.; Garces, Y.I.; Park, S.S.; Bauer, H.J.; Nelson, K.; Miller, R.W.; Brown, P.D.; Olivier, K.R. Outcomes and toxicities of stereotactic body radiation therapy for non-spine bone oligometastases. Pract. Radiat. Oncol. 2014, 4, e143–e149. [Google Scholar] [CrossRef] [Green Version]
- Erler, D.; Brotherston, D.; Sahgal, A.; Cheung, P.; Loblaw, A.; Chu, W.; Soliman, H.; Chung, H.; Kiss, A.; Chow, E.; et al. Local control and fracture risk following stereotactic body radiation therapy for non-spine bone metastases. Radiother. Oncol. 2018, 127, 304–309. [Google Scholar] [CrossRef]
- Sprave, T.; Verma, V.; Förster, R.; Schlampp, I.; Bruckner, T.; Bostel, T.; Welte, S.E.; Tonndorf-Martini, E.; Nicolay, N.H.; Debus, J.; et al. Randomized phase II trial evaluating pain response in patients with spinal metastases following stereotactic body radiotherapy versus three-dimensional conformal radiotherapy. Radiother. Oncol. 2018, 128, 274–282. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, Q.N.; Chun, S.G.; Chow, E.; Komaki, R.; Liao, Z.; Zacharia, R.; Szeto, B.K.; Welsh, J.W.; Hahn, S.M.; Fuller, C.D.; et al. Single-fraction stereotactic vs. conventional multifraction radiotherapy for pain relief in patients with predominantly nonspine bone metastases: A randomized phase 2 trial. JAMA Oncol. 2019, 5, 872–878. [Google Scholar] [CrossRef] [Green Version]
- Cellini, F.; Manfrida, S.; Deodato, F.; Cilla, S.; Maranzano, E.; Pergolizzi, S.; Arcidiacono, F.; Di Franco, R.; Pastore, F.; Muto, M.; et al. Pain REduction with bone metastases STereotactic radiotherapy (PREST): A phase III randomized multicentric trial. Trials 2019, 20, 609. [Google Scholar] [CrossRef] [Green Version]
| Characteristics | No. of Patients | % |
|---|---|---|
| Sex | ||
| female | 286 | 49% |
| male | 296 | 51% |
| Age | ||
| ≤60 years | 298 | 51% |
| >60 years | 284 | 49% |
| ECOG performance status | ||
| 0–1 | 203 | 35% |
| 2 | 196 | 33% |
| 3 | 149 | 26% |
| 4 | 34 | 6% |
| Primary tumor | ||
| lung | 197 | 34% |
| digestive | 47 | 8% |
| gynecologic | 21 | 4% |
| breast | 179 | 31% |
| urogenital | 102 | 17% |
| Other sites | 36 | 6% |
| Control of primary tumor | ||
| Yes | 240 | 41% |
| No | 342 | 59% |
| No. of visceral metastases sites | ||
| No visceral metastases | 297 | 51% |
| 1 | 160 | 27% |
| 2 | 86 | 15% |
| ≥3 | 39 | 7% |
| Lung metastases | ||
| Yes | 115 | 20% |
| No | 467 | 80% |
| Brain metastases | ||
| Yes | 89 | 15% |
| No | 493 | 85% |
| Liver metastases | ||
| Yes | 141 | 24% |
| No | 441 | 76% |
| Complicated bone metastases | ||
| Yes | 172 | 30% |
| No | 410 | 70% |
| Bone metastases surgery | ||
| Yes | 50 | 9% |
| No | 532 | 91% |
| No. of irradiations for bone metastases | ||
| 1 | 498 | 85.5% |
| 2 | 75 | 13% |
| 3 | 7 | 1.20% |
| 4 | 2 | 0.3% |
| Irradiation schedule | ||
| MFRT | 347 | 60% |
| SFRT | 235 | 40% |
| Total | 582 | 100% |
| Variable | 3-Year OS (%) | 95% CI | p-Value | |
|---|---|---|---|---|
| Age | ≤60 years (298) | 17% | 12–22% | 0.02 |
| >60 years (284) | 13% | 9–17% | ||
| Sex | female (286) | 23% | 19–29% | <0.001 |
| male (296) | 6% | 4–10% | ||
| ECOG performance status | 1 (203) | 24% | 18–30% | <0.001 |
| 2 (196) | 13% | 9–19% | ||
| 3 (149) | 9% | 5–15% | ||
| 4 (34) | 0% | - | ||
| Primary tumor | lung (197) | 3% | 1–7% | <0.001 |
| breast (179) | 32% | 25–39% | ||
| urogenital (102) | 14% | 8–23% | ||
| digestive (47) | 6% | 2–17% | ||
| gynecologic (21) | 19% | 8–40% | ||
| other sites (36) | 3% | 0.6–17% | ||
| No. of bone metastases | single (34) | 72% | 55–85% | <0.001 |
| multiple (548) | 11% | 8–14% | ||
| Control of primary tumor | yes (240) | 24% | 19–31% | <0.001 |
| no (342) | 8% | 5–12% | ||
| Visceral metastases | yes (285) | 7% | 5–11% | <0.001 |
| no (297) | 22% | 18–28% | ||
| Brain metastases | yes (89) | 2% | 0.02–8% | <0.001 |
| no (493) | 17% | 14–21% | ||
| Lung metastases | yes (115) | 8% | 4–16% | 0.10 |
| no (467) | 16% | 13–20% | ||
| Liver metastases | yes (141) | 9% | 5–15% | 0.11 |
| no (441) | 17% | 13–21% | ||
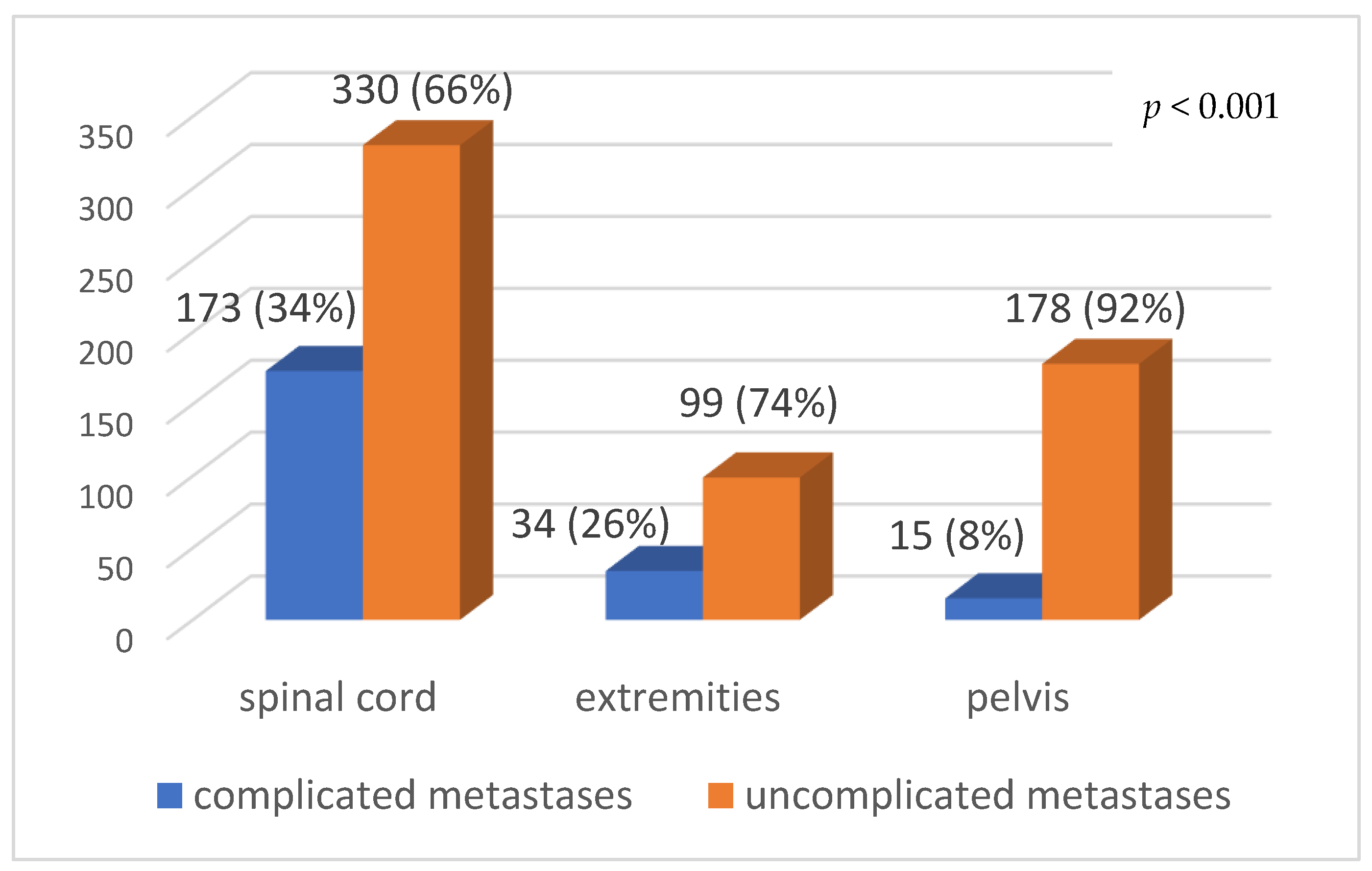
| Complications of bone metastases | yes (222) | 13% | 9–19% | 0.04 |
| no (360) | 16% | 12–20% | ||
| Irradiated anatomical region | Axial skeleton (spine + pelvis) (529) | 16% | 8–29% | 0.57 |
| extremities(53) | 15% | 12–18% | ||
| Irradiation scheme | MFRT(347) | 17% | 12–21% | <0.001 |
| SFRT(235) | 12% | 8–17% | ||
| Variable | HR | 95% CI | p-Value |
|---|---|---|---|
| Age (continuous variable) | 1.00 | 0.99–1.01 | 0.31 |
| Sex (female vs. male) | 1.19 | 0.9–1.49 | 0.11 |
| Primary tumor (breast versus the rest of sites) | 1.24 | 1.05–1.77 | <0.001 |
| ECOG performance status (reference PS = 1) | 1.53 | 1.38–1.69 | <0.001 |
| No. of bone metastases (single vs. multiple) | 5.40 | 2.94–9.91 | <0.001 |
| Complications associated to bone metastases (no vs. yes) | 1.20 | 1.09–1.44 | 0.04 |
| Control of primary tumor (yes vs. no) | 1.26 | 1.04–1.52 | 0.001 |
| Visceral metastases (no vs. yes) | 1.16 | 0.95–1.41 | 0.12 |
| Brain metastases (no vs. yes) | 1.37 | 1.08–1.73 | <0.001 |
| Characteristics | Irradiation Schedule | ||||
|---|---|---|---|---|---|
| MFRT | SFRT | Total | p-Value | ||
| Age | ≤60 years | 227 (63%) | 131 (37%) | 358 | <0.05 |
| >60 years | 179 (56%) | 140 (44%) | 319 | ||
| Sex | female | 227 (66%) | 116 (34%) | 343 | <0.001 |
| male | 179 (54%) | 155 (46%) | 334 | ||
| Primary tumor | lung | 118 (54%) | 99 (46%) | 217 | <0.001 |
| gastrointestinal | 36 (68%) | 17 (32%) | 53 | ||
| gynecologic | 16 (73%) | 6 (27%) | 22 | ||
| breast | 153 (68%) | 72 (32%) | 225 | ||
| urogenital | 59 (49%) | 62 (51%) | 121 | ||
| other sites | 24 (62%) | 15 (38%) | 39 | ||
| ECOG performance index | 1 | 173 (76%) | 55 (24%) | 228 | <0.001 |
| 2 | 138 (57%) | 104 (43%) | 242 | ||
| 3 | 77 (46%) | 89 (54%) | 166 | ||
| 4 | 18 (44%) | 23 (56%) | 41 | ||
| Control of primary tumor | yes | 203 (62%) | 127 (38%) | 330 | 0.42 |
| no | 203 (58%) | 144 (42%) | 347 | ||
| Visceral metastases | yes | 229 (60%) | 150 (40%) | 379 | 0.79 |
| no | 177 (59%) | 121 (41%) | 298 | ||
| Irradiated anatomical site | spine | 258 (68%) | 124 (32%) | 382 | <0.001 |
| pelvis | 41 (57%) | 31 (43%) | 72 | ||
| half-body lower | 50 (41%) | 71 (59%) | 121 | ||
| extremities | 57 (56%) | 45 (44%) | 102 | ||
| Complications associated to metastases | yes | 139 (63%) | 83 (37%) | 222 | 0.33 |
| no | 267 (59%) | 188 (41%) | 455 | ||
| Re-irradiation | yes | 22 (41%) | 32 (59%) | 54 | <0.001 |
| no | 384 (62%) | 239 (38%) | 623 | ||
| Total | 406 (60%) | 271 (40%) | 677 | ||
| Variable | Odds Ratio in Favor of MFRT | 95% CI | p-Value |
|---|---|---|---|
| Sex (female vs. male) | 0.73 | 0.47–1.13 | 0.16 |
| Age (≤60 years vs. >60 years) | 0.83 | 0.59–1.17 | 0.29 |
| Primary site (urogenital vs. other sites) | 0.33 | 0.21–0.53 | <0.001 |
| Primary tumor (lung vs. other sites) | 0.49 | 0.33–0.71 | <0.001 |
| ECOG performance status (reference PS = 1) | 0.55 | 0.45–0.66 | <0.001 |
| Irradiated site (spine vs. other sites) | 2.09 | 1.41–3.12 | <0.001 |
| Irradiated site (half-body lower vs. other sites) | 0.59 | 0.36–0.99 | 0.04 |
| First Author (Year) | Patient Population | Negative Prognostic Factors of Survival | Survival | Reference |
|---|---|---|---|---|
| Katagiri (2005) | 350 patients with bone metastases irradiated and/or operated | Type of primary (lung, stomach, liver, poor PS, visceral/brain metastases, previous chemotherapy, multiple bone metastases | 1-year OS 48% 2-year OS 33% 3-year OS 23% | [26] |
| Mizumoto (2008) | 544 patients with spinal metastases | Increasing age, poor PS, unfavorable primary, visceral metastases, multiple bone metastases, previous chemotherapy, serum calcium, neurologic deficit | Median OS 5.9 months 1-year OS 32% 2-year OS 19% | [25] |
| Janssen (2015) | 927 patients operated for long bone metastases | Age, comorbidities, increased BMI, tumor type with poor prognosis, multiple bone metastases, visceral metastases, low hemoglobin levels | Median OS 8.6 months | [28] |
| Zhang (2016) | 125 patients with bone metastases irradiated and/or operated | Sex, PS, primary tumor (esophagus, colorectal), T stage, differentiation | Median OS 14.1 months | [22] |
| Willeumier (2018) | 1520 irradiated patients with long bone metastases | Poor PS, visceral/brain metastases, unfavorable clinical profile (lung, colon, esophagus, melanoma, stomach, liver) | Median OS 7.4 months | [21] |
| First Author (Year) | Time Period | No. of RT Courses | % of SFRT Prescriptions | Factors Associated with SFRT | Reference |
|---|---|---|---|---|---|
| Szostakiewicz (2004) | 1995–2002 | 1754 | 19% | Lung and breast primaries, irradiation of ribs and long bones | [39] |
| Haddad (2005) | 1998–2002 | 882 | 32% | Increased age, poor PS, greater weight loss | [40] |
| Bradley (2008) | 1999–2005 | 965 | 65% | Increased age, prostate primaries, poor PS, non-spine sites | [41] |
| Beriwal (2012) | 2003–2010 | 7905 | 3.9% | Spine and extremities were more likely to receive MFRT | [42] |
| Bekelman (2013) | 2006–2009 | 3050 | 3.3% | Poor PS | [38] |
| Laugsand (2013) | 1997–2007 | 14380 | 31.3% | Increased age, poor PS, lung and prostate primaries | [43] |
| Thavarajah (2013) | 2005–2012 | 2549 | 65% | Increased age, poor PS, prostate primary, non-spine sites, re-irradiation | [44] |
| Olson (2014) | 2007–2011 | 16898 | 49.2% | Hematologic and prostate primaries, irradiation of ribs and extremities, poor PS | [2] |
| Ashworth (2016) | 1984–2012 | 161835 | 43.9% | Increased age, poor PS, non-spine sites | [45] |
| Kim (2020) | 2016 | 807 | 62% | Prostate primary, uncomplicated metastases, non-spine sites, re-irradiation | [46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ignat, P.; Todor, N.; Ignat, R.-M.; Șuteu, O. Prognostic Factors Influencing Survival and a Treatment Pattern Analysis of Conventional Palliative Radiotherapy for Patients with Bone Metastases. Curr. Oncol. 2021, 28, 3876-3890. https://doi.org/10.3390/curroncol28050331
Ignat P, Todor N, Ignat R-M, Șuteu O. Prognostic Factors Influencing Survival and a Treatment Pattern Analysis of Conventional Palliative Radiotherapy for Patients with Bone Metastases. Current Oncology. 2021; 28(5):3876-3890. https://doi.org/10.3390/curroncol28050331
Chicago/Turabian StyleIgnat, Patricia, Nicolae Todor, Radu-Mihai Ignat, and Ofelia Șuteu. 2021. "Prognostic Factors Influencing Survival and a Treatment Pattern Analysis of Conventional Palliative Radiotherapy for Patients with Bone Metastases" Current Oncology 28, no. 5: 3876-3890. https://doi.org/10.3390/curroncol28050331
APA StyleIgnat, P., Todor, N., Ignat, R.-M., & Șuteu, O. (2021). Prognostic Factors Influencing Survival and a Treatment Pattern Analysis of Conventional Palliative Radiotherapy for Patients with Bone Metastases. Current Oncology, 28(5), 3876-3890. https://doi.org/10.3390/curroncol28050331





