Abstract
Virtual care in cancer care existed in a limited fashion globally before the COVID-19 pandemic, mostly driven by geographic constraints. The pandemic has required dramatic shifts in health care delivery, including cancer care. We conducted a systematic review of comparative studies evaluating virtual versus in-person care in patients with cancer. Embase, APA PsycInfo, Ovid MEDLINE, and the Cochrane Library were searched for literature from January 2015 to 6 August 2020. We adhered to PRISMA guidelines and used the modified GRADE approach to evaluate the data. We included 34 full-text publications of 10 randomized controlled trials, 13 non-randomized comparative studies, and 5 ongoing randomized controlled trials. Evidence was divided into studies that provide psychosocial or genetic counselling and those that provide or assess medical and supportive care. The limited data in this review support that in the general field of psychological counselling, virtual or remote counselling can be equivalent to in-person counselling. In the area of genetic counselling, telephone counselling was more convenient and noninferior to usual care for all outcomes (knowledge, decision conflict, cancer distress, perceived stress, genetic counseling satisfaction). There are few data for clinical outcomes and supportive care. Future research should assess the role of virtual care in these areas. Protocol registration: PROSPERO CRD42020202871.
Keywords:
cancer; phone; systematic review; telehealth; telemedicine; teleoncology; videoconferencing; virtual care 1. Introduction
Virtual care, defined as interaction between a patient and clinician(s) that is not in-person, is also commonly referred to as remote care, telemedicine, or teleoncology, and is a subset of eHealth; the primary modalities are telephone and videoconferencing. Virtual care in patients with cancer existed in a limited fashion globally before the COVID-19 pandemic, mostly driven by geography constraints. While in-person care has been considered the “gold standard” of interaction between patients and physicians, there are several components of in-person care that may be delivered with equivalent effectiveness using non-in-person or virtual platforms. Evidence on virtual care is emerging but there remain numerous unknowns that need to be addressed to guide health care systems and cancer clinicians, as well as patients and caregivers, in understanding the potential for virtual care to substitute for in-person care. There are questions regarding efficacy and quality, as well as the system- and patient-level resources required.
There has been rapid adoption of virtual care due to the COVID-19 pandemic. This transition to virtual care has occurred in the absence of evidence as to its equivalency to traditional care. The objective of this systematic review was to find and evaluate clinical studies of virtual versus in-person care. The full review, including results of reports and publications addressing technical requirements, equity, inter-professional care, and health-care provider compensation for optimal delivery of virtual cancer, is available on the Ontario Health (Cancer Care Ontario) website (https://www.cancercareontario.ca/en/guidelines-advice/types-of-cancer/68836), accessed on 24 August 2021.
2. Materials and Methods
2.1. Systematic Review Planning and Registration
A search of systematic reviews in PROSPERO, evaluation of known reviews and guidelines, and subject area knowledge of the Working Group members suggested that a systematic review specifically on this topic had not been published. We therefore designed and conducted this review, and the review protocol was registered on the International Prospective Register of Systematic Reviews (PROSPERO), CRD42020202871.
2.2. Literature Search Method
Embase, APA PsycInfo, MEDLINE, the Cochrane Library, and CINAHL were searched from 2015 to 1 August 2020 (Supplementary Material, Table S1). Clinicaltrials.gov was searched on 5 November 2020, for ongoing trials (Table S2), as well as systematic reviews and guidelines. While some systematic reviews or guidelines addressed aspects of the topic, they covered narrower topics, did not focus on cancer, missed several of the studies that we found, or were based on non-comparative studies, and are not discussed in this publication.
2.3. Search Strategy and Study Selection
This review included patients diagnosed with cancer who were undergoing treatment or follow-up. The comparison was virtual care versus in-person care between the patient and the same clinician (or team of clinicians).
Studies in which a subset of in-person visits were replaced by virtual visits were included. Studies had to be full-text primary publications in English or French with at least 30 patients per group. Non-comparative studies with more than 100 patients receiving virtual care were also included if they met all other criteria. Those excluded were trials that studied other interventions such as reduction in frequency of appointments; phone or text reminders; use of mobile or online apps, educational materials, or lifestyle adaptation; or replacement of in-person care from one professional or treatment team/unit (e.g., oncologist plus nurse) with virtual or in-person care by a different profession/team (e.g., community nurse or general practitioner). Publications of conference abstracts or other non-full text reports, editorials, opinions, comments or commentaries, notes, or news articles were also excluded.
Title and abstract screening were performed by one reviewer. In cases of uncertainty, the full working group determined inclusion or exclusion. Preferred (critical) outcomes were recurrence, survival, or other long-term objective outcomes. Patient experience outcomes, including acceptance of virtual care, symptoms, and quality of life, were considered important outcomes.
2.4. Data Extraction and Assessment of Risk of Bias
All included primary studies underwent data extraction by one reviewer, with subsequent independent audit of all extracted data. The risk of bias for randomized studies was assessed per outcome and per study using methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions and the RoB2 tool [1,2]. The ROBINS-I tool was used for non-RCTs [3]. The risk of bias was performed by M.F. and G.G.F. independently and discussed with X.Y. to get consensus. The current review considered risk of bias, inconsistency, indirectness, imprecision, and publication bias (based on GRADE approach) in evaluating the quality of evidence [4].
2.5. Synthesizing the Evidence
Due to the large number of different study designs, interventions, comparators, patient populations, follow-up periods, and the outcome reporting time and methods involved, a meta-analysis or network meta-analysis was inappropriate to perform. Instead, the results of each study were presented individually in a descriptive fashion. Ratios, including odds ratios for dichotomous outcomes, were expressed with a ratio of <1.0 indicating a benefit for virtual care compared with in-person care. For continuous outcomes, mean differences or standardized mean differences were used as effect measures, and a two-sided significance level of α = 0.05 was assumed.
3. Results
The literature search resulted in 11,307 citations, of which 216 required full-text review. Thirty-nine publications representing 23 clinical studies and 5 ongoing trials met our pre-planned selection criteria (Figure 1). The trials found have been divided into those that provide psychosocial or genetic counselling, and those that provide or assess medical and supportive care.
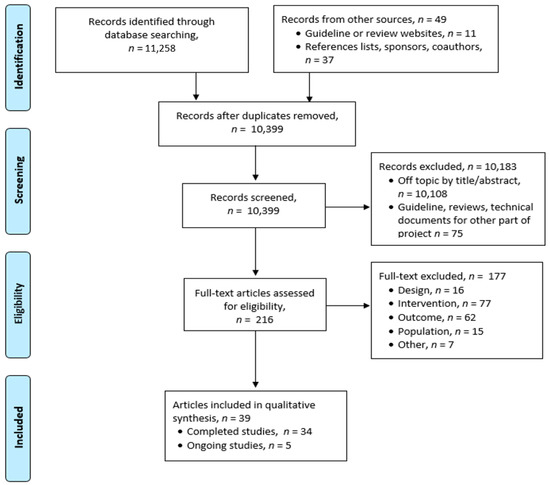
Figure 1.
PRISMA Flow Diagram.
The overall risk of bias for randomized controlled trials (RCTs) was considered low for two studies and high for eight studies (Table S3). The risk of bias for non-RCTs ranged from moderate to critical risk using the ROBINS tool [3]. After also considering other domains (inconsistency, indirectness, imprecision, and publication bias), the overall certainty of evidence per outcome and per study was evaluated as moderate to very low for RCTs and very low for non-RCTs. Thus, we did not provide the risk of bias assessment table for non-RCTs.
3.1. Counselling Studies
Table 1 includes 16 publications of 10 studies, grouped as either general or genetic counselling [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20]. The group psychotherapy RCT studied women with emotional distress after primary oncology treatment [5]. Emotional distress and post-traumatic stress symptoms improved in on-line counselling and in-person counselling groups; there were no significant differences between groups. An RCT of cognitive behaviour in patients with cancer and high psychological needs for mental health compared telephone with face-to-face therapy [6]. Both arms had significant improvement in anxiety and depression, and equivalent improvement in stress and worry. They did not demonstrate full equivalence of the two arms, although both were effective and telephone care was non-inferior. The LEAN study compared telephone weight-loss counselling versus in-person counselling versus usual care in 100 breast cancer survivors [7]. Both groups improved, but there were no significant differences between study groups.

Table 1.
General or genetic counselling.
A survey of 209 cancer patients or their family members receiving psycho-oncology counselling using videoconferencing due to COVID-19 restrictions found that patients were grateful that care could be continued, but that it was more distant due to lack of non-verbal communication and non-recognition of signs of distress [8]. Therapists also missed non-verbal communication and informal physical contact and felt more exhausted; they were willing to continue videoconferencing if patients requested this, but preferred in-person sessions for more complex therapies. Approximately one-half of the patients indicated a preference to use video-consults a portion of the time when in-person care is again allowed.
The studies on genetic counselling included patients with higher risk for hereditary cancers and sometimes close family members. Counselling took place prior to genetic testing (and sometimes after), with the purpose of providing education and emotional support and helping patients to decide whether to undergo testing.
Two RCTs focused on rural communities and the third was conducted through cancer centres in four large cities [9,10,11,12,13,14,15,16,17]. They all found that virtual care via video or telephone was more cost-effective than in-person counselling. For the system, this was driven mainly by lower overhead (office space) and travel time for staff to attend remote clinics, while for the patient, it was due to less travel time and expense, and less time off work. The videoconference trial reported no difference in patient satisfaction [9]. The REACH trial reported that at one-year follow-up, telephone was not inferior to in-person counselling for all psychosocial and informed decision-making outcomes (anxiety, cancer-specific distress, perceived personal control, decisional conflict) [11,12,13]. The Jacobs et al. RCT reported that telephone was more convenient, resulted in no difference in knowledge or stress, but with less perceived support and emotional recognition [14,15,16,17]. There was no overall difference in patient satisfaction.
In the study by Mette et al., patients received in-person care if the genetic counselor or oncologist visited the regional extension centre on the day of their appointment; otherwise, they received counselling by video-teleconferencing at the regional centre [18]. There were no differences in satisfaction, feeling comfortable talking to the counsellor or listened to, having enough time, understanding information, finding the information to be valuable or that it helped to make health decisions, or whether they would recommend the program.
The non-randomized study by Solomons et al. compared videoconferencing for 106 patients in two remote sites versus counselling for 68 patients in an in-person clinic, with the grouping mainly determined by geographic proximity to the site [19]. Groups were unequal regarding age, personal history of cancer and type of cancer, and education. Knowledge of hereditary breast and ovarian cancer improved equally, and anxiety decreased in both groups. While videoconferencing reduced transportation needs and work absence, 32% of the patients indicated a preference for in-person care.
The study by Tutty et al. surveyed patients with high grade serous ovarian cancer who had received telephone genetic counselling [20]. Most patients were satisfied with the timing of the telephone call (97%) and the information provided (94%). Face-to-face counselling would have been preferred by 17% of patients, while 34% had no preference.
3.2. Medical or Supportive Care
As indicated in Table 2, the literature search found four RCTs, with one each for topics of pain management, solid tumour systemic therapy follow-up, endometrial cancer follow-up, and prostate cancer follow-up [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38].

Table 2.
Studies except counselling.
An RCT found that videoconference delivery of psychosocial pain management resulted in higher rates of session completion, was noninferior for pain severity and pain interference, and was more feasible than in-person management [21,22]. Both groups had similar patient burden and engagement, and degree of acceptability. A strength of this study was that the videoconference group was given a tablet (iPad) with a data plan; these were also given to those in the in-person group if needed to access the study website for self-assessment and to enter content preferences.
In the NCT-MOBILE RCT, those having video follow-up after systemic therapy for solid tumours had significantly greater satisfaction with the interaction and more confidence in the physician, and found it had greater efficiency, punctuality, time saving, and lower cost, compared to those having standard in-person visits [23,24]. Difficulties in the first appointment were mostly due to software incompatibility and internet connections that were resolved for the second appointment.
The ENDCAT trial randomized patients to either telephone or traditional hospital follow-up after hysterectomy for endometrial cancer [25,26,27]. Differences in patient satisfaction, quality of life, being able to ask questions, having questions answered, feeling anxious prior to appointments, feeling reassured, and cost were not significant. Telephone appointments were more likely to be on time and thorough. Recurrence was the same in both groups (4%) and all were symptomatic; patients with recurrence were excluded from further follow-up.
The Mayo Clinic trial randomized 70 men after radical prostatectomy with no urologic concerns to either video or in-office follow-up for one visit [28]. Patients reported no difference or similar trust in the provider, education provided, satisfaction, visit confidentiality, and ability to share personal information. There were significantly lower costs to the patients for video visits.
Nine non-randomized studies were small (65–296 patients) and in most cases the comparative group, if any, was not equivalent [29,30,31,32,33,34,35,36,37,38]. Three of the studies had patients attend a local clinic with nurses or non-specialist physicians and remote contact to specialists [29,30,31]. Patients were generally satisfied, although some preferred a mixture of traditional and remote care; transportation costs were reduced and care for some was more accessible. The narrow disease-specific stage-specific studies suggest virtual care may be suitable in well-defined specific situations [32,33,34]. As is generally the case for non-randomized studies, the risk of bias is high and quality of evidence from these trials is considered low to very low. They do suggest, however, that most patients were satisfied with virtual care.
Three recent studies of virtual care implemented due to the COVID-19 pandemic were included. The Smrke et al. study reported higher patient satisfaction with telephone consultations than in-person visits [35]. Most patients (80%) preferred at least some future appointments to be by telemedicine for reasons of travel time (42%), travel expense (20%), or convenience (30%). Clinicians reported phone consultation sometimes (44%) or often (17%) affected the ability to perform examinations, but most favoured this for active surveillance or stable-dose oral medication. The comparison in-person group was not considered equivalent. Two studies involved surveys or questionnaires of patients who received virtual care (no comparison groups). One survey indicated that most patients with advanced genitourinary cancer wanted therapy continued despite COVID-19 risks [36]. They accepted virtual discussion of staging results and therapy decisions, but had lower acceptance of referral to secondary care oncologists for therapy. Another survey showed that video-based telemedicine was rated high for usefulness, effectiveness, and satisfaction, but lower on reliability due to limitations on physical examinations in otolaryngology patients [37,38].
3.3. Ongoing, Unpublished, or Incomplete Studies
Five ongoing studies were found in the literature search and details are summarized in the Supplementary Material (Table S2) [39,40,41,42,43].
4. Discussion
This systematic review determined that oncology studies with direct comparison between virtual and in-person care are limited and generally provide low to very-low quality evidence, with the exception of RCTs that studied very specific situations: genetic counselling and endometrial cancer follow-up. While there is intense interest in understanding where virtual care may be at least equivalent to in-person care during active cancer management (post surgical care, chemotherapy radiation therapy), this review found little published evidence that directly addressed this aspect of cancer care.
In the general field of psychological counselling, virtual or remote counselling has been reported in several reviews to be equivalent to in-person counselling [44,45,46,47,48,49]. While evidence with cancer patients in the current review is limited, studies suggest virtual counselling and in-person counselling may have similar effectiveness in treating anxiety, stress, depression, and adjustment issues [5,6]. A survey of patients and therapists involved in psycho-oncology counselling video-consults found that approximately one-half of the patients expressed preference for video-consults in the future for a portion of sessions [8]. While therapists were willing to provide video counselling if requested, they preferred in-person sessions, especially for more complex issues. These studies indicate that individual situations and patient preferences need to be considered.
In the area of genetic counselling, it was reported that telephone counselling was noninferior to usual care for all outcomes (knowledge, decision conflict, cancer distress, perceived stress, genetic counseling satisfaction) and was more convenient [14,15,16,17]. On the negative side, there was lower perceived support and emotional recognition. Some studies also found lower costs to the patient or system for virtual counselling. It should be noted that genetic counselling is usually limited to a small number of appointments, usually a pre-test appointment and sometimes a follow-up appointment for patients choosing to undergo genetic testing. This model typically involves few interactions between patients and providers with little long-term follow-up, in contrast to many other areas of cancer care. As such, results from this study may not be transferable to other types of clinician-patient interaction. The combined evidence from all the counselling studies suggests that visual cues (body language, expression) available with in-person or video sessions may be more important for in-depth counselling, and therefore, video methods have advantages over telephone use.
Another area where virtual care appears to have extensive use is in long-term follow-up for asymptomatic patients with endometrial cancer. The ENDCAT trial, evaluated as high quality, was conducted in patients with stage I endometrial cancer [25,26,27]. It found high patient satisfaction and noninferior psychological morbidity for telephone versus in-person follow-up. All recurrences were symptomatic and detected between scheduled virtual or in-person visits, suggesting that virtual follow-up in early-stage endometrial cancer does not place patients at increased risk. Most other studies measured outcomes of patient satisfaction, feasibility, and cost, and one studied pain management, but they did not include long-term outcomes. Overall, the results were consistent in showing feasibility and patient acceptance of virtual care. It remains unknown if these findings in the area of endometrial cancer can be generalized to follow up of asymptomatic patients in other cancer disease sites.
Limitations
First, since our literature search period is from 2015, some relevant earlier publications may have been missed. However, virtual care technology has developed rapidly in recent years, and the pace of change has accelerated with the onset of the global pandemic in 2020. Older studies by phone have been supplanted by videoconferencing and new delivery platforms. This changing environment makes a traditional model of data collection and evidence generation very challenging. One option is to consider regional demonstration projects with common data collection elements that map to traditional quality frameworks. Second, although ten RCTs met our pre-planned study selection criteria, eight of them were of low to very low quality. It is realized that the conduct of RCTs testing virtual care strategies is susceptible to high risks for bias for the measurement of patients reported outcomes. Third, only one eligible study reported recurrence rate, which we consider to be a critical outcome. Even in ongoing trials from the clinicaltrial.gov search (Table S2), no trials will report recurrence, survival, or other long-term outcomes.
5. Conclusions
According to current but limited evidence, virtual care does not appear to be inferior to in-person care when considering patient satisfaction. The published literature does support the use of virtual platforms for counselling interventions that include genetics and psychosocial care. It is clear that more work is required to understand the disease, patient, and system factors that a virtual care approach can reliably support.
The COVID pandemic has necessitated rapid adoption of virtual cancer care without a strong evidence base or systematic stakeholder engagement around the perceived benefits and limitations of this approach, especially during active cancer treatment. The current environment offers a unique opportunity for gathering data and evidence at both the patient and cancer system levels. RCTs examining evidence-based evaluation of virtual care are lacking, although the post pandemic phase may provide some opportunity as the pendulum of balance between in-person and virtual care shifts. Given the investment in infrastructure and rapid implementation of virtual care, there is an important opportunity to address some of the critical issues that have not been comprehensively studied to date that include treatment outcomes and patient safety.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/curroncol28050301/s1, Table S1: Literature Search Strategy (Databases); Table S2: Ongoing trials; Table S3: Summary Table for Risk of Bias Assessment for Randomized Controlled Trials.
Author Contributions
Conceptualization, S.S. and J.S.; methodology, S.S., G.G.F., X.Y. and J.S.; investigation, X.Y. and G.G.F.; writing—original draft preparation, all authors; writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research was conducted by the Program in Evidence-Based Care (PEBC) at McMaster University, a provincial initiative of Ontario Health (Cancer Care Ontario) supported by the Ontario Ministry of Health (OMH). All work produced by the PEBC is editorially independent from the OMH.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data used in this study can be found in the included original studies.
Acknowledgments
The authors would like to thank Faith Maelzer for searching the clinicaltrials.gov database of ongoing trials and conducting a data audit and Megan Smyth for assisting in assessing the risk of bias for RCTs.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.1; Updated September 2020; Cochrane: London, UK, 2020; Available online: www.training.cochrane.org/handbook (accessed on 11 March 2021).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [Green Version]
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. (Eds.) Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach (Updated October 2013). GRADE Working Group, 2013. Available online: https://gdt.gradepro.org/app/handbook/handbook.html (accessed on 11 March 2021).
- Lleras de Frutos, M.; Medina, J.C.; Vives, J.; Casellas-Grau, A.; Marzo, J.L.; Borras, J.M.; Ochoa-Arnedo, C. Video conference vs face-to-face group psychotherapy for distressed cancer survivors: A randomized controlled trial. Psychooncology 2020, 29, 1995–2003. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.; White, C.; Lynch, A.; Mohammed, K. Telephone-delivered individual cognitive behavioural therapy for cancer patients: An equivalence randomised trial. Psychooncology 2017, 26, 301–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrigan, M.; Cartmel, B.; Loftfield, E.; Sanft, T.; Chagpar, A.B.; Zhou, Y.; Playdon, M.; Li, F.; Irwin, M.L. Randomized trial comparing telephone versus in-person weight loss counseling on body composition and circulating biomarkers in women treated for breast cancer: The Lifestyle, Exercise, and Nutrition (LEAN) Study. J. Clin. Oncol. 2016, 34, 669–676. [Google Scholar] [CrossRef] [PubMed]
- van der Lee, M.L.; Schellekens, M.P.J. Bridging the distance: Continuing psycho-oncological care via video-consults during the COVID-19 pandemic. Psychooncology 2020, 13, 13. [Google Scholar]
- Buchanan, A.H.; Datta, S.K.; Skinner, C.S.; Hollowell, G.P.; Beresford, H.F.; Freeland, T.; Rogers, B.; Boling, J.; Marcom, P.K.; Adams, M.B. Randomized trial of telegenetics vs. in-person cancer genetic counseling: Cost, patient satisfaction and attendance. J. Genet. Couns. 2015, 24, 961–970. [Google Scholar] [CrossRef] [Green Version]
- Datta, S.K.; Buchanan, A.H.; Hollowell, G.P.; Beresford, H.F.; Marcom, P.K.; Adams, M.B. Telemedicine vs in-person cancer genetic counseling: Measuring satisfaction and conducting economic analysis. Comp. Eff. Res. (Auckl.) 2011, 1, 43–50. [Google Scholar] [CrossRef]
- Steffen, L.E.; Du, R.; Gammon, A.; Kohlmann, W.K.; Lee, J.H.; Buys, S.S.; Stroup, A.M.; Campo, R.A.; Flores, K.G. Genetic testing in a population-based sample of breast and ovarian cancer survivors from the REACH randomized trial: Cost barriers and moderators of counseling mode. Cancer Epidemiol. Biomarkers Prev. 2017, 26, 1772–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinney, A.Y.; Steffen, L.E.; Brumbach, B.H.; Kohlmann, W.; Du, R.; Lee, J.H.; Gammon, A.; Butler, K.; Buys, S.S.; Stroup, A.M.; et al. Randomized noninferiority trial of telephone delivery of BRCA1/2 genetic counseling compared with in-person counseling: 1-year follow-up. J. Clin. Oncol. 2016, 34, 2914–2924. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; Near, A.M.; Butler, K.M.; Hoeffken, A.; Edwards, S.L.; Stroup, A.M.; Kohlmann, W.; Gammon, A.; Buys, S.S.; Schwartz, M.D.; et al. Economic evaluation alongside a clinical trial of telephone versus in-person genetic counseling for BRCA1/2 mutations in geographically underserved areas. J. Oncol. Pract. 2016, 12, e1–e13. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, A.S.; Schwartz, M.D.; Valdimarsdottir, H.; Nusbaum, R.H.; Hooker, G.W.; DeMarco, T.A.; Heinzmann, J.E.; McKinnon, W.; McCormick, S.R.; Davis, C.; et al. Patient and genetic counselor perceptions of in-person versus telephone genetic counseling for hereditary breast/ovarian cancer. Fam. Cancer. 2016, 15, 529–539. [Google Scholar] [CrossRef] [Green Version]
- Peshkin, B.N.; Kelly, S.; Nusbaum, R.H.; Similuk, M.; DeMarco, T.A.; Hooker, G.W.; Valdimarsdottir, H.B.; Forman, A.D.; Joines, J.R.; Davis, C.; et al. Patient perceptions of telephone vs. in-person BRCA1/BRCA2 genetic counseling. J. Genet. Couns. 2016, 25, 472–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, M.D.; Valdimarsdottir, H.B.; Peshkin, B.N.; Mandelblatt, J.; Nusbaum, R.; Huang, A.-T.; Chang, Y.; Graves, K.; Isaacs, C.; Wood, M.; et al. Randomized noninferiority trial of telephone versus in-person genetic counseling for hereditary breast and ovarian cancer. J. Clin. Oncol. 2014, 32, 618–626. [Google Scholar] [CrossRef]
- Butrick, M.; Kelly, S.; Peshkin, B.N.; Luta, G.; Nusbaum, R.; Hooker, G.W.; Graves, K.; Feeley, L.; Isaacs, C.; Valdimarsdottir, H.B.; et al. Disparities in uptake of BRCA1/2 genetic testing in a randomized trial of telephone counseling. Genet Med. 2015, 17, 467–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mette, L.A.; Saldivar, A.M.; Poullard, N.E.; Torres, I.C.; Seth, S.G.; Pollock, B.H.; Tomlinson, G.E. Reaching high-risk underserved individuals for cancer genetic counseling by video-teleconferencing. J. Community Support. Oncol. 2016, 14, 162–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomons, N.M.; Lamb, A.E.; Lucas, F.L.; McDonald, E.F.; Miesfeldt, S. Examination of the patient-focused impact of cancer telegenetics among a rural population: Comparison with traditional in-person services. Telemed. J. e-Health 2018, 24, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Tutty, E.; Petelin, L.; McKinley, J.; Young, M.A.; Meiser, B.; Rasmussen, V.M.; Forbes Shepherd, R.; James, P.A.; Forrest, L.E. Evaluation of telephone genetic counselling to facilitate germline BRCA1/2 testing in women with high-grade serous ovarian cancer. Eur. J. Hum. Genet. 2019, 27, 1186–1196. [Google Scholar] [CrossRef]
- Kelleher, S.A.; Winger, J.G.; Dorfman, C.S.; Ingle, K.K.; Moskovich, A.A.; Abernethy, A.P.; Keefe, F.J.; Samsa, G.P.; Kimmick, G.G.; Somers, T.J. A behavioral cancer pain intervention: A randomized noninferiority trial comparing in-person with videoconference delivery. Psychooncology 2019, 28, 1671–1678. [Google Scholar] [CrossRef]
- Winger, J.G.; Nunez, C.; Kelleher, S.A.; Ingle, K.K.; Gandhi, V.; Keefe, F.J.; Somers, T.J. Predictors of intervention session completion in a randomized clinical trial of a behavioral cancer pain intervention. J. Pain Symptom Manag. 2020, 59, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Walle, T. NCT MOBILE—Smartphone-Based Video Consultations in Medical Oncology, 2018. Available online: http://www.drks.de/DRKS00015788 (accessed on 29 October 2020).
- Walle, T.; Erdal, E.; Mühlsteffen, L.; Singh, H.M.; Gnutzmann, E.; Grün, B.; Hofmann, H.; Ivanova, A.; Köhler, B.; Korell, F.; et al. Completion rate and impact on physician-patient relationship of video consultations in medical oncology: A randomized controlled open label trial. ESMO Open 2020, 5, e000912. [Google Scholar] [CrossRef] [PubMed]
- Beaver, K.; Williamson, S.; Sutton, C.; Hollingworth, W.; Gardner, A.; Allton, B.; Abdel-Aty, M.; Blackwood, K.; Burns, S.; Curwen, D.; et al. Comparing hospital and telephone follow-up for patients treated for stage-I endometrial cancer (ENDCAT trial): A randomised, multicentre, non-inferiority trial. BJOG 2017, 124, 150–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, P.; Beaver, K.; Williamson, S.; Sutton, C.; Martin-Hirsch, P.; Hollingworth, W. Cost-consequence analysis alongside a randomised controlled trial of hospital versus telephone follow-up after treatment for endometrial cancer. Appl. Health Econ. Health Policy 2018, 16, 415–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaver, K.; Williamson, S.; Sutton, C.J.; Gardner, A.; Martin-Hirsch, P. Endometrial cancer patients’ preferences for follow-up after treatment: A cross-sectional survey. Eur. J. Oncol. Nurs. 2020, 45, 101722. [Google Scholar] [CrossRef]
- Viers, B.R.; Lightner, D.J.; Rivera, M.E.; Tollefson, M.K.; Boorjian, S.A.; Karnes, R.J.; Thompson, R.H.; O’Neil, D.A.; Hamilton, R.L.; Gardner, M.R.; et al. Efficiency, satisfaction, and costs for remote video visits following radical prostatectomy: A randomized controlled trial. Eur. Urol. 2015, 68, 729–735. [Google Scholar] [CrossRef]
- Chan, B.A.; Larkins, S.L.; Evans, R.; Watt, K.; Sabesan, S. Do teleoncology models of care enable safe delivery of chemotherapy in rural towns? Med. J. Aust. 2015, 203, 406–406.e6. [Google Scholar] [CrossRef]
- Hamilton, E.; Van Veldhuizen, E.; Brown, A.; Brennan, S.; Sabesan, S. Telehealth in radiation oncology at the Townsville Cancer Centre: Service evaluation and patient satisfaction. Clin. Transl. Radiat. Oncol. 2019, 15, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Jue, J.S.; Spector, S.A.; Spector, S.A. Telemedicine broadening access to care for complex cases. J. Surg. Res. 2017, 220, 164–170. [Google Scholar] [CrossRef]
- Li, M.; Zhang, M.; Wang, H.; Pan, X.; Wu, W.; Zhang, Q.; Liu, Y.; Zhang, H. The efficacy of internet-based intervention on quality of life for patients with chronic post-surgical pain. Iran. J. Public Health 2016, 45, 1604–1609. [Google Scholar] [PubMed]
- Verma, R.; Treasure, P.; Hughes, R. Development and evaluation of radiographer led telephone follow up following radical radiotherapy to the prostate. A report of a Macmillan Cancer Support sponsored pilot project at Mount Vernon Hospital. Radiography 2015, 21, 16–24. [Google Scholar] [CrossRef]
- Patil, V.M.; Pande, N.; Chandrasekharan, A.M.C.; Tonse, R.; Krishnatry, R.; Goda, J.S.; Dsouza, H.; Vallathol, D.H.; Chakraborty, S. Shadow study: Randomized comparison of clinic with video follow-up in glioma undergoing adjuvant temozolomide therapy. CNS Oncol. 2018, 7, CNS14. [Google Scholar] [CrossRef] [Green Version]
- Smrke, A.; Younger, E.; Wilson, R.; Husson, O.; Farag, S.; Merry, E.; Macklin-Doherty, A.; Cojocaru, E.; Arthur, A.; Benson, C.; et al. Telemedicine during the COVID-19 pandemic: Impact on care for rare cancers. JCO Glob. Oncol. 2020, 6, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Rodler, S.; Apfelbeck, M.; Schulz, G.B.; Ivanova, T.; Buchner, A.; Staehler, M.; Heinemann, V.; Stief, C.; Casuscelli, J. Telehealth in uro-oncology beyond the pandemic: Toll or lifesaver? Eur. Urol. Focus 2020, 10, 10. [Google Scholar]
- Layfield, E.; Triantafillou, V.; Prasad, A.; Deng, J.; Shanti, R.M.; Newman, J.G.; Rajasekaran, K. Telemedicine for head and neck ambulatory visits during COVID-19: Evaluating usability and patient satisfaction. Head Neck 2020, 42, 1681–1689. [Google Scholar] [CrossRef] [PubMed]
- Triantafillou, V.; Layfield, E.; Prasad, A.; Deng, J.; Shanti, R.M.; Newman, J.G.; Rajasekaran, K. Patient perceptions of head and neck ambulatory telemedicine visits: A qualitative study. Otolaryngol. Head Neck Surg. 2020, 164, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Chua, I.S.; Zachariah, F.; Dale, W.; Feliciano, J.; Hanson, L.; Blackhall, L.; Quest, T.; Curseen, K.; Grey, C.; Rhodes, R.; et al. Early integrated telehealth versus in-person palliative care for patients with advanced lung cancer: A study protocol. J. Palliat. Med. 2019, 22, 7–19. [Google Scholar] [CrossRef] [Green Version]
- National Library of Medicine (US). Telemedicine Nurse-Led Intervention for Rural Cancer Survivors. 2020. Available online: https://clinicaltrials.gov/show/NCT04267627 (accessed on 7 August 2020).
- Australian and New Zealand Clinical Trials Registry. Telehealth for Palliative Care Patients in Metropolitan and Rural Settings; NHMRC Clinical Trials Centre, University of Sydney: Sydney, Australia, 2018; Available online: https://anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12618001007224 (accessed on 7 August 2020).
- National Library of Medicine (US). Supportive Care Delivered by Telemedicine to Cancer Patients at Home. 2019. Available online: https://clinicaltrials.gov/show/NCT04136340 (accessed on 7 August 2020).
- National Library of Medicine (US). Assessing the System for High-Intensity Evaluation during Radiotherapy during Changes in Response to COVID-19 (CORONA-SHIELD). 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04357574 (accessed on 10 November 2020).
- Luo, C.; Sanger, N.; Singhal, N.; Pattrick, K.; Shams, I.; Shahid, H.; Hoang, P.; Schmidt, J.; Lee, J.; Haber, S.; et al. A comparison of electronically-delivered and face to face cognitive behavioural therapies in depressive disorders: A systematic review and meta-analysis. EClinicalMedicine 2020, 24, 100442. [Google Scholar] [CrossRef]
- Osenbach, J.E.; O’Brien, K.M.; Mishkind, M.; Smolenski, D.J. Synchronous telehealth technologies in psychotherapy for depression: A meta-analysis. Depress Anxiety 2013, 30, 1058–1067. [Google Scholar] [CrossRef]
- Sloan, D.M.; Gallagher, M.W.; Feinstein, B.A.; Lee, D.J.; Pruneau, G.M. Efficacy of telehealth treatments for posttraumatic stress-related symptoms: A meta-analysis. Cogn. Behav. Ther. 2011, 40, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Berryhill, M.B.; Culmer, N.; Williams, N.; Halli-Tierney, A.; Betancourt, A.; Roberts, H.; King, M. Videoconferencing psychotherapy and depression: A systematic review. Telemed J. e-Health 2019, 25, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Varker, T.; Brand, R.M.; Ward, J.; Terhaag, S.; Phelps, A. Efficacy of synchronous telepsychology interventions for people with anxiety, depression, posttraumatic stress disorder, and adjustment disorder: A rapid evidence assessment. Psychol. Serv. 2019, 16, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Drago, A.; Winding, T.N.; Antypa, N. Videoconferencing in psychiatry, a meta-analysis of assessment and treatment. Eur. Psychiatry 2020, 36, 29–37. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).