Abstract
Annually over 200 million adults undergo noncardiac surgery worldwide. Myocardial ischaemia is a frequent cause of perioperative cardiac morbidity and mortality. Approximately 8 million patients will suffer a myocardial injury after noncardiac surgery (MINS) each year. MINS is defined as a prognostically important myocardial injury due to ischaemia that occurs during, or within 30 days after, noncardiac surgery. The diagnostic criterion for MINS is an elevated troponin measurement resulting from myocardial ischaemia. MINS is a strong, independent predictor of 30-day and 1-year mortality. The majority of patients suffering MINS would go undetected without troponin monitoring since >80% of these patients do not experience ischaemic symptoms. Intensification of pharmacotherapy may reduce 30-day mortality in patients who have experienced MINS. This paper will review the epidemiology, prevention, prognosis and treatment of MINS.
Epidemiology
Worldwide over 200 million adults undergo noncardiac surgery annually [,]. Conservative estimates suggest that at least 100 million adults undergoing noncardiac surgery are in an atrisk age group for major perioperative vascular events []. Approximately 8 million of these patients will suffer a myocardial injury aher noncardiac surgery (MINS) [] and, as a result, over 1 million adults will die within 30 days of noncardiac surgery worldwide annually [,]. The magnitude of this problem is predicted to increase owing in part to an aging population, a rise in the incidence of cardiovascular related problems, and a trend toward surgical intervention in elderly patients.
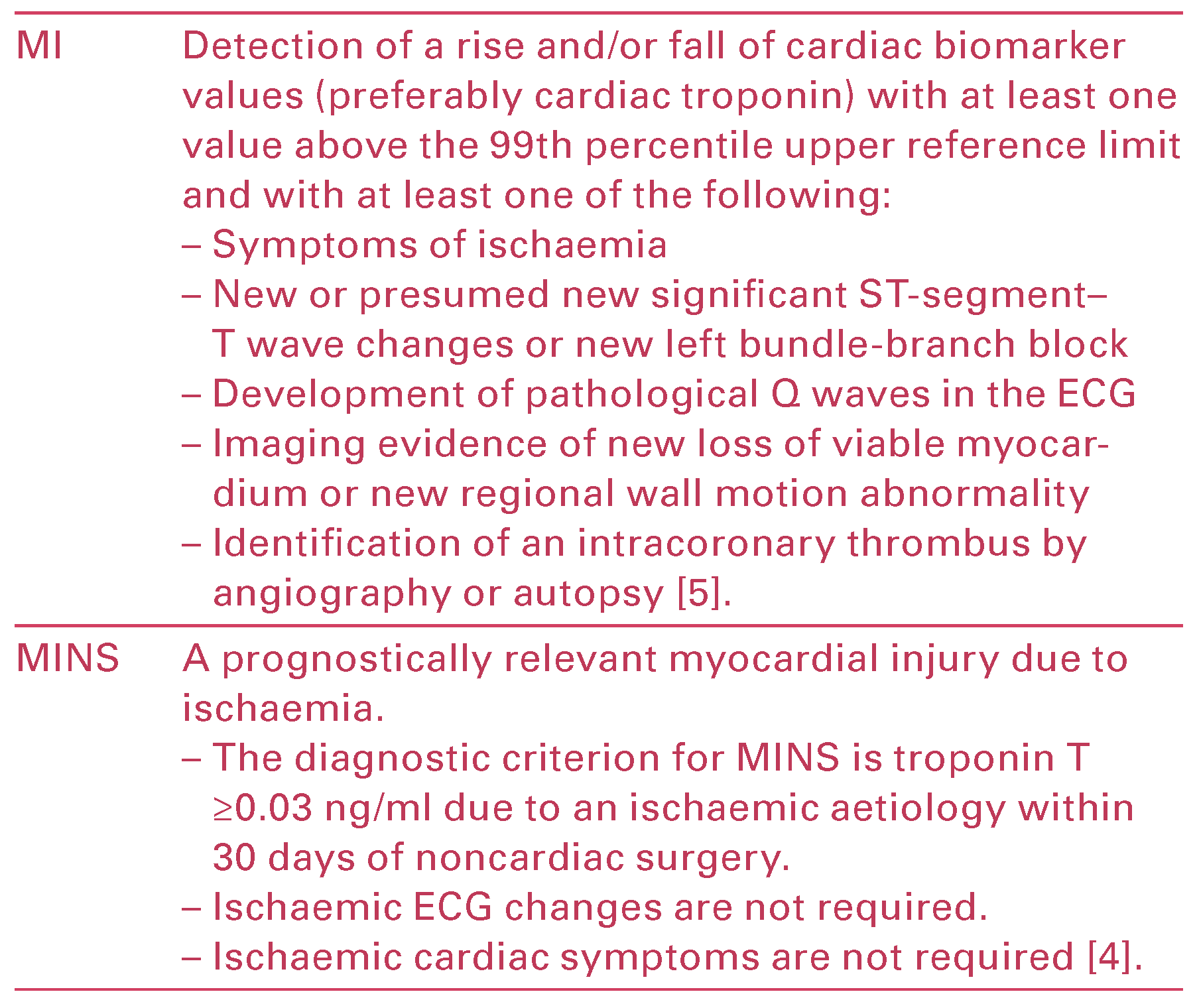
Myocardial infarction (MI) is defined in the Third Uni versal Definition of Myocardial Infarction, an expert consensus document by the global Myocardial Infarc tion Task Force [] (Table 1). While MI is the cardiac end point in many perioperative studies, it is important to differentiate MINS from MI as a multitude of factors limit the ability to diagnose an MI in the perioperative period (Table 1).

Table 1.
Definition of myocardial infarction (MI) and myocardial injury after noncardiac surgery (MINS).
Typically, postoperative patients receive some form of analgesia. This is ohen an opioid or similar agent, which can effectively mask chest pain from myocardial ischaemia []. Furthermore, patients who are sedated or intubated postoperatively are unable to effectively communicate and thus perioperative ischaemia may be overlooked. The majority of troponin measure ments and ECGs are ordered on the basis of ischaemic symptoms [], therefore acute perioperative MI or MINS may be missed in patients receiving analgesia or in patients whose communication is impaired.
Based on the VISION Study (a prospective, internatio nal, cohort study involving 40 000 patients, ≥45 years of age having noncardiac surgery), the majority of patients (87%) with MINS experienced the ischaemic injury within the first 3 days aher surgery []. A mere 15.8% of patients who developed MINS experienced ischaemic cardiac symptoms []. Therefore, without postoperative troponin monitoring, 84.2% of MINS events would go undetected []. Furthermore, in VISION, all patients with an elevated troponin measurement had an ECG. Only 34.9% of patients had ischaemic ECG changes and a minority (41.8%) of patients suffering MINS fulfilled the universal definition of MI (Figure 1) [].
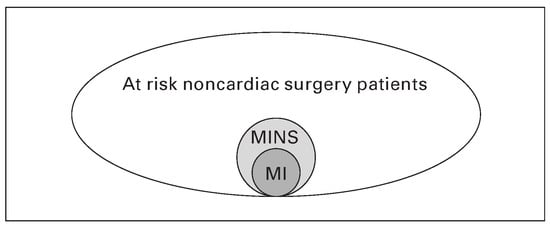
Figure 1.
At least 100 milion patients undergoing noncardiac surgery are at risk for perioperative vascular complications annually. Nearly 10% will suffer from MINS. Of patients with MINS, only 41.8% fulfill the diagnostic criteria for MI [].
In the POISE Trial (an international β blocker trial of 8351 patients who underwent noncardiac surgery), only 34.7% of patients with perioperative MI experienced symptoms of ischaemia []. Therefore, the absence of reported ischaemic cardiac symptoms and imperfect timing of the ECG (e.g., ECG performed in response to a detected elevated troponin on routine screening but aher the acute ischaemic event) may result in a missed diagnosis of perioperative MI or MINS [].
Historical Perspective
In 1967, JAMA published the VA Hypertension Trial in which US veterans with diastolic blood pressure of 115–129 mm Hg were randomised to antihypertensive drugs or placebo []. The follow up period was 18 months. At the time, many experts believed that hypertension was essential for brain perfusion. The primary outcome included death, dissecting/ruptured aortic aneurysm, cerebral haemorrhage / disabling stroke, MI, congestive heart failure (CHF), retinal haemorrhage, papilloedema and rapidly progressive renal failure []. Thirty nine percent of the placebo group versus three percent of the antihypertensive group experienced the primary outcome. The relative risk reduction for antihypertensive drugs was 93%, p = 0.00000003 [].
Historically, physicians did not believe that non valvular atrial fibrillation was a risk factor for stroke, but rather simply a nuisance for patients who experi enced palpitations [,]. Currently, it is recognised that both valvular and nonvalvular atrial fibrillation carry a significant risk of embolic stroke.
This historical perspective suggests that physicians can overlook important diagnoses. Evidence suggests that MINS is prognostically important and overlooked by most physicians. This is primarily due to a lack of perioperative troponin monitoring. In addition, the fragmented nature of perioperative follow up likely facilitates physicians’ underappreciation of the impact of MINS.
Prognosis
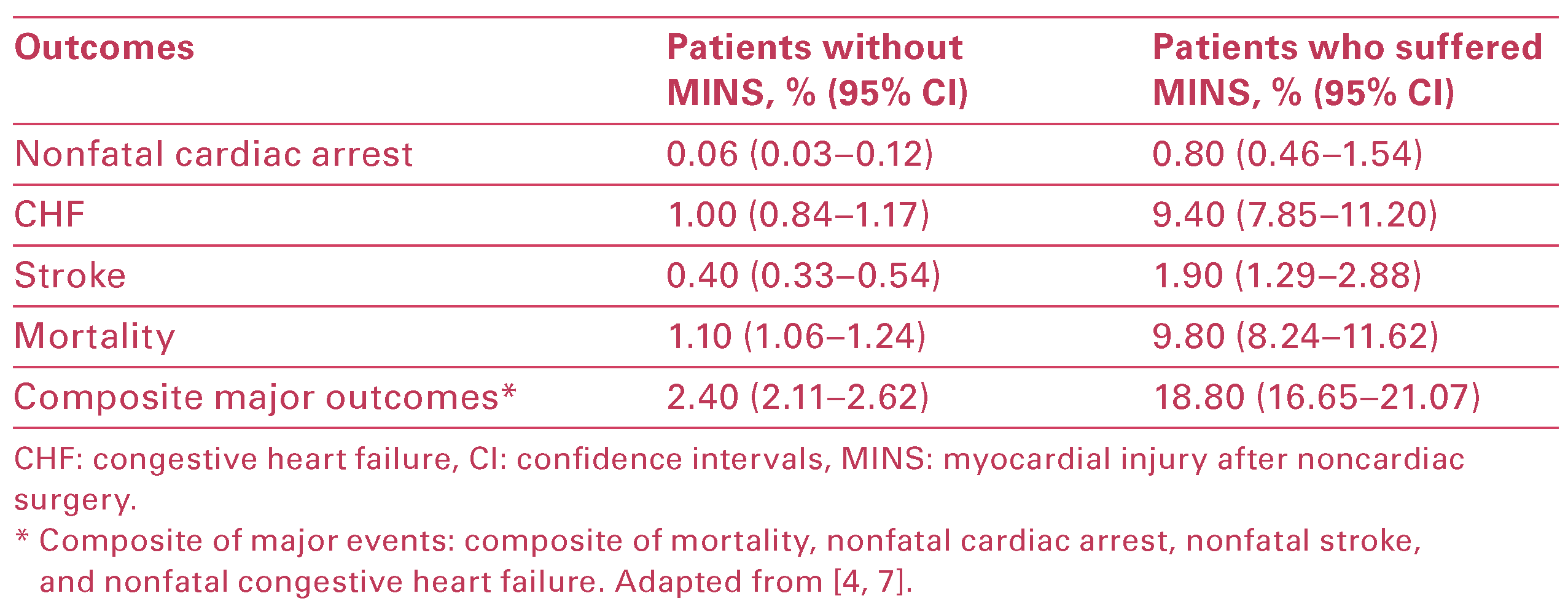
VISION demonstrated that MINS is not a benign entity and it independently predicts major vascular events and mortality at 30 days (Table 2) [,]. Levy et al. per formed a systematic review and meta analysis evaluat ing the intermediate and long term prognostic value of troponin and creatinine kinase MB measurement aher noncardiac surgery. The findings demonstrate that an elevated troponin aher noncardiac surgery strongly predicts mortality at 1 year [odds ratio (OR) 6.7, 95% confidence interval (CI) 4.1–10.9] []. Based on its high prevalence, asymptomatic nature and substantial influence on perioperative mortality, MINS has been classified as a “silent killer” [].

Table 2.
Thirty-day risk of major vascular events after suffering a MINS.
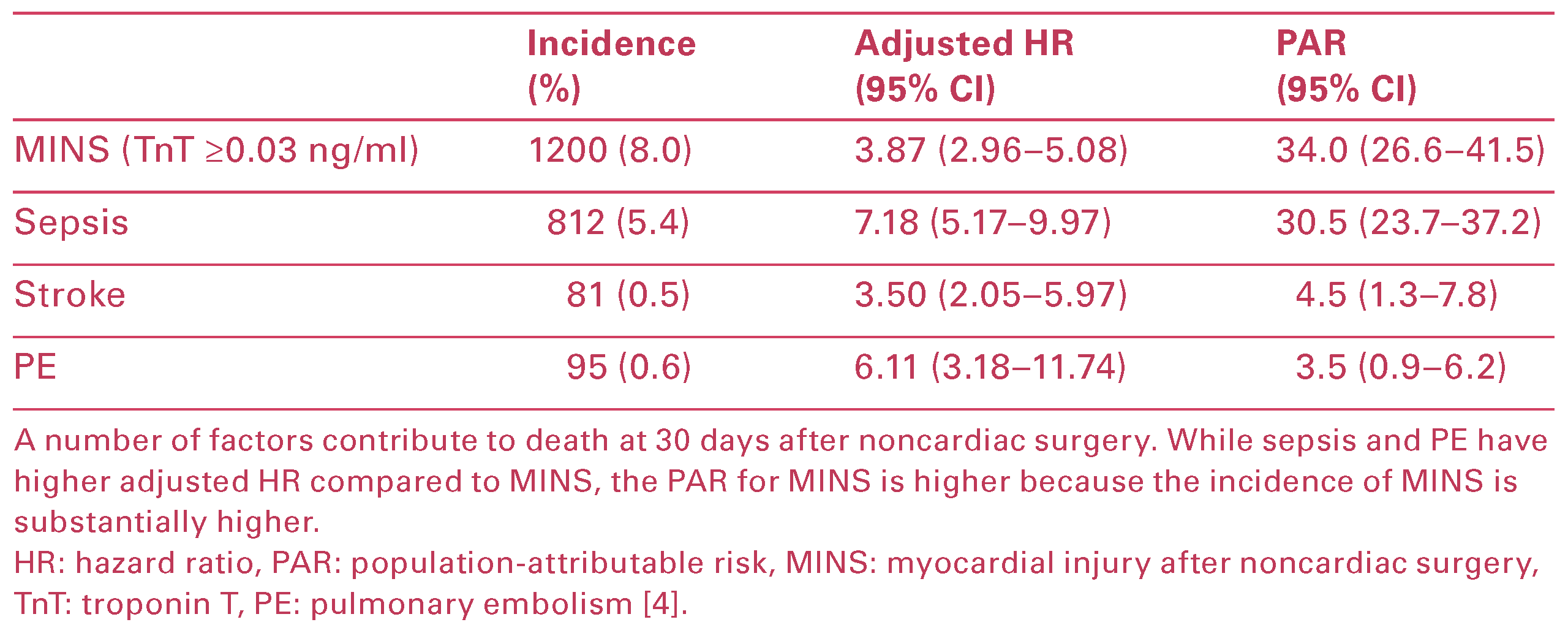
MINS is associated with an adjusted hazard ratio (HR) of 3.87 (95% CI 2.96–5.08) for 30 day mortality and MINS has the highest population attributable risk (PAR) (34%) compared with other postoperative complica tions that predict death at 30 days aher surgery (Table 3) []. The PAR represents the proportion of all deaths potentially attributable to the relevant risk factor (e.g., MINS) if causality was proven [].

Table 3.
Postoperative variables predicting death at 30 days after surgery.
Higher levels of postoperative troponin T elevation correlate with increased risk of mortality. In VISION, the incidence of 30 day mortality was 1.0, 4.0, 9.3 and 16.9% in patients with peak troponin T values of 0.01, 0.02, 0.03–0.29, and ≥0.30 ng/ml, respectively (Figure 2) [].
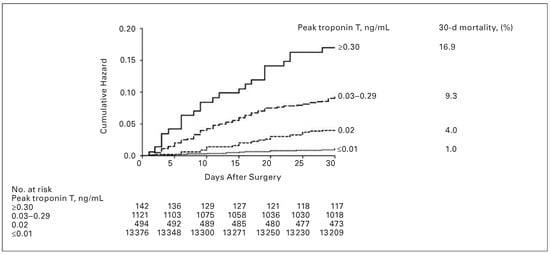
Figure 2.
Kaplan-Meier estimates of 30-day mortality based on peak troponin T values. Copyright © 2012 American Medical Association. All rights reserved. Adapted and reprinted, with permission, from : Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) Study Investigators, Devereaux PJ, Chan MT, Alonso-Coello P, Walsh M, Berwanger O, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012, 307, 2295–304.
Higher levels of postoperative troponin elevation also correlate with lower median days to death. A peak tro ponin T value of 0.03–0.29 ng/ml was associated with median time to death of 9.0 days [interquartile range (IQR) 3.5–16], whereas the median time to death for a peak troponin T value of ≥0.3 ng/ml was 6.5 days (IQR 1.5–15) []. The median time from discharge to death was 11 days (IQR 4–15 days) and 26.6% of the patients who died, did so aher hospital discharge []. Therefore, MINS may serve as a “red flag” or foreshadowing of a more serious vascular event to follow in the next 30 days and up to 1 year aher noncardiac surgery [,].
Pathophysiology of Perioperative MI and MINS
The precise pathophysiology of perioperative MI and MINS has not yet been clearly defined. The two pre dominant theories involve myocardial oxygen supply– demand mismatch [], and coronary artery thrombo sis [,].
Multiple factors may increase myocardial oxygen demand perioperatively including fluid shihs, catecholamine surges, hypotension, anaemia, pain, hypothermia and hypoxia []. In coronary arteries with high grade lesions, the inability to respond adequately to increased myocardial oxygen demand may lead to supply–demand mismatch resulting in myocardial ischaemia []. Thus, preexisting coronary artery disease (CAD) is an intuitive culprit. However, in an angiographic study involving vascular surgery patients, the majority of MINS occurred in myocar dium supplied by arteries without high grade stenosis []. The landmark CARP Trial (510 patients) demon strated no reduction in perioperative MI with pre operative revascularisation for coronary stenosis ≥70% []. The coronary computed tomographic angiography (CTA) VISION Study performed coronary CTA on 955 patients before noncardiac surgery. This study showed that while the majority (72%) of perioperative MIs occurred in patients with obstructive or extensive obstructive CAD, 24% and 4% of the perioperative MIs occurred in patients with nonobstructive disease or a normal preoperative CTA, respectively [].
In contrast to CARP, a recent trial randomised 426 pa tients to preoperative coronary angiography followed by selective percutaneous coronary intervention (PCI) or coronary artery bypass grahing (CABG) versus no preoperative coronary angiogram or revascularisation before elective carotid endarterectomy []. This trial demonstrated a reduction in the risk of MI in the group allocated to preoperative coronary angiography (p = 0.01). Although this represents encouraging data, there were only six perioperative MIs and thus the results require cautious interpretation because of the fragility of this finding [].
Evidence also supports coronary artery thrombus as a potential culprit for perioperative MI. Sympathetic activation in the perioperative period promotes a hypercoaguable state by up regulating platelets and down grading fibrosis [,,]. This hypercoaguable state, coupled with increased sheer wall stress, may lead to plaque fracture and subsequent thrombus formation [,]. Small autopsy studies in <70 patients who suffered a fatal perioperative MI found intra coronary thrombus in one third of patients []. How ever, given the late timing of autopsy relative to the MI, it is possible that resolution of additional intra coronary thrombus occurred prior to the time of examination [].
More recently, a study evaluated 120 consecutive patients who suffered a perioperative acute coronary syndrome (PACS) aher noncardiac surgery and sub sequently underwent coronary angiography []. The angiography results of the PACS patients were com pared with the angiographic results of a group of 120 patients who suffered a nonoperative ACS (recruited from the emergency room on randomly selected days) and 240 patients with stable CAD (who were recruited prior to angiography on randomly selected days). Angiography in the PACS group showed that 45% of patients had Ambrose’s type II lesions (i.e., findings strongly associated with a disrupted plaque) versus 56.7% in the nonoperative ACS group and 16.4% in the stable CAD group (p <0.001) []. Both PACS and non operative ACS patients had more complex lesions (i.e., intraluminal filling defect, plaque ulceration, plaque irregularity/haziness, or TIMI flow <3) than patients in the stable CAD group (56.7 vs 79.2 vs 31.8%, respectively; p <0.001) []. These results suggest that a substantial proportion of patients suffering MINS have angio graphic evidence that it was due to a thrombotic event, and that the frequency of these findings is similar to that in patients suffering a nonoperative MI.
It seems probable that both intrinsic and extrinsic fac tors influence patients’ risk of adverse cardiovascular events. It is possible that the underlying mechanism for MINS may vary among patients with different risk factors. The perioperative period is fraught with a multitude of stressors including increased sym pathetic stimulation, hypercoaguability, bleeding, inflammation, hypotension, tachycardia, hypo thermia, hypoxia and pain [,]. These stressors, super imposed on preexisting chronic conditions such as renal insufficiency, CAD, peripheral vascular disease, cerebrovascular disease, diabetes, CHF, atrial fibrilla tion, hypertension, advanced age, male sex [] and se vere aortic stenosis [], may lead to increased suscep tibility to cardiovascular complications. Patients with recent high risk CAD [,,], recent coronary artery stent [,], recent stroke [], acute trauma (e.g., hip fracture) [] and the need for urgent or emergency surgery [] are at particularly high risk of complica tions including MINS, CHF, nonfatal cardiac arrest, and cardiovascular death [].
Perioperative Prevention of MINS
Clinical Risk Assessment, Noninvasive Risk Stratification and Biomarkers
Accurate preoperative risk assessment serves a number of important purposes for both physicians and patients. Accurate risk estimates provide physi cians with guidance for selection of surgical approach and anaesthetic techniques, as well as the location and intensity of postoperative care []. For patients, accurate risk assessment may assist with informed decision making about the appropriateness or timing of the proposed surgery []. For example, patients may forgo an operation if they deem the risk of a major pe rioperative cardiac complication unacceptable, or they may opt to defer the procedure (e.g., to experience an important life event) []. MINS is an independent pre dictor of death [], and the risk remains elevated up to a year postoperatively []. Therefore, in addition to immediate perioperative complications, patients and clinicians should consider the risk implications for the coming year.
Many surgical patients may have occult cardiac dis ease, but owing to their underlying disease states (e.g., arthritis, cancer, peripheral vascular disease), their activity level may be insufficient to exhibit symptoms []. Thus, for patients undergoing major noncardiac surgery, clinical cardiovascular risk assessment tools have only modest predictive power []. Noninvasive cardiac testing (e.g., dobutamine echocardiography, dipyridamole myocardial perfusion scan) may provide some additional predictive value beyond clinical varia bles []; however, data are limited and these investiga tions are costly and time consuming. Biomarkers, specifically brain natriuretic peptide (BNP) secreted from ventricular cardiomyocytes, may offer a fast, simple and cost effective method to enhance preoperative cardiovascular risk assessment [].
A recent systematic review and meta analysis of nine observational studies (3281 patients) investigated whether preoperative serum concentrations of BNP (a prohormone) or its inactive cleavage product N termi nal fragment (NT proBNP) could serve as an independ ent predictor of adverse cardiovascular outcomes within 30 days of noncardiac surgery []. The preoper ative BNP measurement was an independent predictor of perioperative cardiovascular events (death, cardio vascular death, or MI) (OR 44.2, 95% CI 7.6–257.0, I2 51.6%) []. These results suggest that an elevated preoperative measurement of BNP or NT proBNP is a powerful, inde pendent predictor of cardiovascular events in the first 30 days aher noncardiac surgery []. Given that NT proBNP is more accurate, efficient and less costly than a preoperative noninvasive cardiac stress test suggests that this biomarker is the preferred preoperative cardiac test.
Evidence-Based Perioperative Pharmacology
Increased sympathetic drive increases a patient’s heart rate and hence myocardial demand, which may lead to myocardial oxygen supply–demand mismatch. More over, it can also induce a hypercoaguable state [,,] and catecholamine release that increases shear stress []. This may trigger plaque rupture and acute coro nary syndromes (ACS) perioperatively []. Thus, in an attempt to prevent MINS or major adverse cardiac events (MACE) in the perioperative setting, various agents including β blockers, α2 adrenergic antagonists, statins and aspirin (acetylsalicylic acid, ASA) have been trialed to reduce the sympathetic response [,], stabilise coronary plaque [] or to inhibit platelet function [,].
Beta-Blockers
Beta blockers were proposed as a potential cardiopro tective agent in the perioperative period []. A small trial in the 1990s found that β blockers had a large effect in preventing perioperative MI; however, it had methodological limitations including not performing an intent to treat analysis []. Two later trials of mod erate size with fewer limitations did not show a benefit of perioperative β blocker use [,]. POISE, a large international randomised controlled trial (RCT), com pared metoprolol with placebo initiated on the day of surgery []. The results showed decreased MI (HR 0.73, 95% CI 0.60–0.90, p = 0.002) but a significantly higher risk of stroke (HR 2.17, 95% CI 1.26–3.74, p = 0.005) and death (HR 1.33, 95% CI 1.03–1.74, p = 0.002) []. More patients in the metoprolol group experienced clinically important hypotension (HR 1.55, 95% CI 1.38–1.74). Sub sequently, a meta analysis of high quality trials found that beta blockade resulted in a 27% relative risk (RR) (95% CI 1.01–1.60, p = 0.04) increase in 30 day mortality, increased stroke risk (RR 1.73, 95% CI 1.00–2.99, p = 0.05) and hypotension (RR 1.51, 95% CI 1.37–1.67, p <0.00001) []. Given the association with increased mortality and stroke, current guidelines no longer recommend initiation of β blocker therapy in the perioperative period [,,,].
Aspirin
Noncardiac surgery is associated with platelet activa tion []. ASA inhibits thrombus formation and obser vational data had suggested that discontinuation of ASA prior to surgery would result in increased throm botic risk [,]. A systematic review and two small RCTs showed mixed results but with a potential decreased risk of vascular events in patients on ASA in the perioperative period [,,]. In contrast, the PEP trial, involving 13 356 patients undergoing hip surgery, demonstrated more cardiac ischaemic events (death due to ischaemic heart disease or nonfatal MI) in patients randomised to ASA versus placebo (HR 1.33, 95% CI 1.00–1.78) []. There was also an increased risk of bleeding (6 per 1000 patients) [].
In 2014, POISE 2 (an international, multicentre RCT of 10 010 patients) found a significant increase in major bleeding risk for patients randomised to ASA []. ASA naïve patients were randomised to initiation of ASA or placebo, starting on the morning of surgery and con tinued for 30 days; patients on chronic ASA therapy were randomised to restart ASA or to placebo on the day of surgery and for 30 days thereaher []. Patients who had been taking ASA chronically on average stopped it 7 days before surgery. ASA had no significant effect on the primary outcome of death or MI at 30 days (7% in ASA group vs 7.1% in placebo group; HR 0.99, 95% CI 0.86–1.15, p = 0.92). Major bleeding, how ever, was more common in the ASA group than with placebo (4.6% vs 3.8%; HR 1.23, 95% CI 1.01–1.49, p = 0.04).
Major bleeding was defined as a significant drop in haemoglobin requiring red blood cell transfusion or intervention (i.e., embolization, superficial vascular re pair, nasal packing); or bleeding in a hight risk location (i.e., intraspinal). The authors theorised that while ASA may have prevented some MIs due to coronary artery thrombus, it may have contributed to MI via supply– demand mismatch from bleeding and hypotension, giving rise to an overall neutral MI signal []. In sum mary, the largest trial in this area, with the power to detect changes in outcome, shows that continuing ASA in the perioperative period causes more harm than benefit [].
Statins
There is overwhelming evidence for the benefit of sta tin use in secondary prevention for patients who have suffered an MI. Statins may also prevent perioperative complications through pleiotropic mechanisms like plaque stabilisation, anti inflammatory effects and im proved endothelial function [,]. A Cochrane review of three vascular surgery RCTs (178 patients) found a nonsignificant decrease of death and MI at 30 days []; two recent systematic reviews, predominantly in vas cular noncardiac surgery, found a decrease in MI and all cause mortality in patients taking statins [,]. However, because of the limited number of cardiac events in dedicated RCTs, there remains uncertainty as to the degree of support for recommendations for peri operative statin use [,,,].
The VISION study compared 18.4% of patients on statin therapy with 29% of controls. Preoperative statin use was associated with a lower risk of the composite primary outcome (all cause mortality, MINS or stroke at 30 days) (RR 0.83, 95% CI 0.73–0.95, p = 0.007) [].
This was driven by a statistically significant lower risk of MINS and death []. This relative effect corre sponded to an absolute risk reduction of 2.0% (95% CI 0.5–3.2%, p = 0.005). VISION is the only study to look at the association of statin use with MINS, and the results are hypothesis generating in that preoperative statin use may reduce the risk of adverse perioperative car diac outcomes []. A large RCT is required to evaluate these findings further.
Alpha-2 Adrenergic Agonists
Results of small RCTs initially suggested that clonidine (an α2 adrenergic agonist) may prevent MI [,] by blunting central sympathetic outflow with associated anxiolytic, and anti inflammatory effects [,]. How ever, these trials were small (<300 patients) with few events [,,,]. A meta analysis of 12 RCTs looking at α2 adrenergic agonists in noncardiac surgery showed no difference in overall mortality or MI in the entire study population; however, a decrease in MI and death was found in the vascular surgery subgroup []. These findings were driven largely by a trial from 1999 that used mivazerol, an α2 adrenergic agonist [,].
More recently, POISE 2, found that clonidine did not reduce the rate of death or nonfatal MI at 30 days (HR 1.08, 95% CI 0.93–1.26, p = 0.29) []. It did, however, increase the rate of nonfatal cardiac arrest (HR 3.20, 95% CI 1.17–8.73, p = 0.02) and clinically important bradycardia (HR 1.49, 95% CI 1.32–1.69, p <0.001) and hypotension (HR 1.32, 95% CI 1.24–1.40, p <0.001). While enhanced heart rate control may be protective [], perioperative hypotension is an independent risk fac tor for perioperative MI [,]. Thus, in the largest trial in this area, α2 adrenergic agonists were not pro tective and increased the risk of significant periopera tive hypotension and bradycardia [].
Perioperative Hypotension
POISE demonstrated that clinically significant hypo tension (defined as systolic blood pressure <90 mm Hg requiring intervention) had the largest PAR (37.3%) for perioperative death and the largest PAR for stroke (14.7%) []. In POISE 2, more patients in the clonidine group had clinically important hypotension, brady cardia and an increased risk of nonfatal cardiac arrest []. Prospective observational studies have suggested an association between intraoperative hypotension with myocardial injury [,] and 30 day mortality []. A recent cohort study on perioperative hypo tension assessed adults ≥60 years of age undergoing vascular surgery with routine troponin monitoring on postoperative days 0–3 []. The authors found that intraoperative hypotension (defined as decrease of 40% from preinduction mean blood pressure for >30 minutes) was associated with increased postoperative myocardial injury (RR 1.8, 95% CI 1.2–2.6, p <0.001) []. The association of hypotension with adverse cardiac events has important implications for perioperative management of antihypertensive agents. In POISE 2, clinically important hypotension occurred more ohen aher patients leh the postanaesthetic care unit (PACU). In the clonidine group, the median intraoperative period of hypotension was 15 minutes and on the first postoperative day it was 180 minutes []. This high lights the need for caution regarding the use of anti hypertensives in the perioperative setting, including consideration of omitting some or all antihyperten sive agents on the day of surgery, careful reintroduc tion of antihypertensives postoperatively, and close monitoring of vital signs once the patient has returned to the ward aher surgery. Future studies are required to assess whether close monitoring for postoperative hypotension with rapid, protocol driven intervention may be cardioprotective.
Treatment Options for MINS
Data that informs on the optimal treatment for MINS patients is limited; however, extrapolation from the ACS literature [,] and other recent perioperative work [,,] provides the modern day clinician with a reasonable strategy until future RCTs provide further guidance. Examination of the placebo arms from ACS studies demonstrates that some patients sur vive, and may do well clinically, despite not being on the active agent []. However, at the time of the acute event, it is not possible to predict with precision which patients will benefit from the drug and which patient will not and thus clinicians err on the side of caution by prescribing a standard cocktail of cardiac medica tions to each ACS patient.
Multivariable regression analysis among patients suf fering MINS from the original POISE Trial identified two drugs that were associated with reduced 30 day risk of death: ASA [adjusted odds ratio (aOR) 0.54, 95% CI 0.29–0.99] and statin (aOR 0.26, 95% CI 0.13–0.54) [].
In a propensity matched study on 1 year outcomes (death, MI, coronary revascularisation, or CHF requir ing hospitalisation), 66 MINS patients were compared with 132 matched non MINS patients (controls) []. Among the MINS patients, 43 received therapeutic intensification of ≥1 of four cardiac medications [ASA, statin, β blocker, angiotensin converting enzyme inhibitor (ACE inhibitor)], while 23 patients did not receive therapeutic intensification aher MINS. MINS patients not receiving therapeutic intensification had a hazard ratio of 1.77 (95% CI 1.13–2.42) while MINS pa tients receiving therapeutic intensification had a haz ard ratio of 0.63 (95% CI 0.1–1.19) []. These data suggest that secondary cardiac prevention interventions may benefit MINS patients.
MANAGE (an international, multicentre RCT) is cur rently evaluating the impact of an anticoagulant (dabigatran 110 mg b.i.d) versus placebo on major vascular complications in patients suffering MINS []. INTREPID (an open label, randomised pilot study) is currently evaluating the impact of ticagrelor (anti platelet agent, 90 mg bid) versus ASA (81 mg) on the rate of cardiovascular events in patients with elevated troponin levels aher major, noncardiac surgery []. More treatment focused RCTs are needed, but until these trials are conducted, the evidence in the availa ble literature suggests that pharmacological intensifi cation for MINS patients may prove beneficial and pos sibly even life saving. At the very least, these patients need to be identified and referred to internal medicine or cardiology departments for close outpatient fol low up, preferably within 1 week of discharge given that the median time to death following MINS was found to be 11 days [].
A cost consequence study analysed the cost associated with postoperative troponin monitoring, including the assumption that every patient will have an echo cardiogram and therapeutic cardiac medication intensification []. This study demonstrated that post operative troponin monitoring, which predicts death within 30 days, is profoundly less expensive than can cer screening which typically predicts death within several years.
Conclusions
MINS is common and is associated with poor out comes. One in ten patients suffering from MINS will die in 30 days aher noncardiac surgery []. Failure to monitor troponin aher noncardiac surgery will miss over 80% of MINS events [].
The current model of perioperative patient care lacks continuity of care, and it is easy to assume that pa tients do well postoperatively if they are not followed longitudinally. Clinicians are unlikely to attribute a MI occurring 6 weeks aher surgery to a complex cascade of inflammation and hypercoagulation that was first initiated during the perioperative period. However, there is strong evidence to support the conclusion that MINS is an important and clinically relevant entity with a profound impact on perioperative mortality [,,]. Dismissal of asymptomatic perioperative tro ponin elevation as “troponitis” comes at a risk to pa tients. Clinicians should recognise MINS as a marker of increased risk of perioperative morbidity and mortal ity. Furthermore, clinicians should be proactive in monitoring troponin postoperatively for patients with elevated cardiovascular risk [,], offer MINS patients cardiac medications for secondary prevention (includ ing ASA, statin, plus consideration of an ACE inhibitor and potentially a beta blocker) [,,] and arrange timely patient follow up with internal medicine or cardiology departments aher hospital discharge.
A shared care model that integrates anaesthesia, inter nal medicine, cardiology and surgery would be a step forward in helping to ensure the continuity of peri operative patient care while providing potentially life saving risk stratification and secondary preven tion.
Disclosure Statement
Dr Devereaux is a member of a research group with a policy of not accepting honorariums or other payments from industry for personal financial gain. They do accept honorariums/payments from industry to support research endeavours and to participate in meetings. Based on study questions Dr Devereaux has originated and grants he has written, he has received grants from Abbott Diagnostics, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Covidien, Octa pharma, Philips Healthcare, Roche Diagnostics and Stryker. Dr Dever eaux i has participated in an advisory board meeting for GlaxoSmith Kline and an expert panel meeting with,AstraZeneca and Boehringer Inhgelheim.
References
The full list of references is included in the online version of the article at www.cardiovascmed.ch.
References
- Devereaux, P.J.; Chan, M.; Eikelboom, J. Major vascular complications in patients undergoing noncardiac surgery the magnitude of the problem, risk prediction, surveillance, and prevention. In Evidence-Based Cardiology, 3rd ed.; Yusuf, S., Cairns, J.A., Camm, A.J., Fallen, E.L., Gersh, B.J., Eds.; BMJ Books: London, England, 2009; pp. 47–62. [Google Scholar]
- Weiser, T.G.; Regenbogen, S.E.; Thompson, K.D.; Haynes, A.B.; Lipsitz, S.R.; Berry, W.R.; et al. An estimation of the global volume of surgery: A modelling strategy based on available data. Lancet. 2008, 372, 139–144. [Google Scholar] [CrossRef]
- Mangano, D. Peri-operative cardiovascular morbidity: New developments. Bailliere’s Clin Anaesthesiol. 1999, 13, 335–348. [Google Scholar] [CrossRef]
- Botto, F.; Alonso-Coello, P.; Chan, M.T.; Villar, J.C.; Xavier, D.; Srinathan, S.; et al. Myocardial injury after noncardiac surgery a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014, 120, 564–578. [Google Scholar] [PubMed]
- Thygesen, K.; Aplert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D.; et al. Third universal definition of myocardial infarction. Eur Heart J. 2012, 33, 2551–2567. [Google Scholar] [CrossRef]
- Herlitz, J.; Hjalmarson, A.; Waagstein, F. Treatment of pain in acute myocardial infarction. Brit Heart J. 1989, 61, 1–13. [Google Scholar] [CrossRef]
- Bessissow, A.; Duceppe, E.; Devereaux, P.J. Addressing Perioperative Myocardial Ischemia. Curr Anesthesiol Rep. 2014, 4, 107–112. [Google Scholar] [CrossRef]
- POISEStudy Group; Devereaux, P.J.; Yang, H.; Yusuf, S.; Guyatt, G.; Leslie, K.; et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): A randomised controlled trial. Lancet. 2008, 371, 1839–1847. [Google Scholar] [PubMed]
- Veterans Administrative Cooperative Study Group on Hypertensive Agents. Effects of treatment on morbidity in hypertension. JAMA. 1967, 202, 116–122. [Google Scholar]
- Orgain, E.S.; Wolff, L.; White, P.D. Uncomplicated auricular fibrillation and auricular flutter: Frequent occurrence and good prognosis in patients without other evidence of cardiac disease. Arch Intern Med (Chic). 1936, 57, 493–513. [Google Scholar] [CrossRef]
- Wolf, P.A.; Abbott, R.D.; Kannel, W.B. Atrial fibrillation as an independent risk factor for stroke: The Framingham study. Stroke. 1991, 22, 983–988. [Google Scholar] [CrossRef]
- Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) Study Investigators, Devereaux PJ, Chan MT, Alonso-Coello P, Walsh M, Berwanger O.; et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012, 307, 2295–2304. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Heels-Ansdell, D.; Hiralal, R.; Bhandari, M.; Guyatt, G.; Yusuf, S.; et al. Prognostic value of troponin and creatine kinase muscle and brain isoenzyme measurement after noncardiac surgery a systematic review and meta-analysis. Anesthesiology. 2011, 114, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Galyfos, G.; Sigala, F.; Zografos, K.; Filis, K. Cardiac damage after vascular surgery procedures: A silent killer. OA Surg. 2014, 2, 1–6. [Google Scholar]
- Landesberg, G.; Beattie, W.S.; Mosseri, M.; Jaffe, A.S.; Alpert, J.S. Perioperative myocardial infarction. Circulation. 2009, 119, 2936–2944. [Google Scholar] [CrossRef]
- Gualandro, D.M.; Campos, C.A.; Calderaro, D.; Yu, P.C.; Marques, A.C.; Pastana, A.F.; et al. Coronary plaque rupture in patients with myocardial infarction after noncardiac surgery: Frequent and dangerous. Atherosclerosis. 2012, 222, 191–195. [Google Scholar] [CrossRef]
- Devereaux, P.J.; Goldman, L.; Cook, D.J.; Gilbert, K.; Leslie, K.; Guyatt, G.H. Perioperative cardiac events in patients undergoing noncardiac surgery: A review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. CMAJ. 2005, 173, 627–634. [Google Scholar] [CrossRef]
- Ellis, S.G.; Hertzer, N.R.; Young, J.R.; Brener, S. Angiographic correlates of cardiac death and myocardial infarction complicating major nonthoracic vascular surgery. Am J Cardiol. 1996, 77, 1126–1128. [Google Scholar] [CrossRef]
- McFalls, E.O.; Herbert, W.B.; Thomas, E.M.; Goldman, S.; Krupski, W.C.; Littooy, F.; et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med. 2004, 351, 2795–2804. [Google Scholar] [CrossRef] [PubMed]
- Sheth, T.; Chan, M.; Butler, C.; Chow, B.; Tandon, V.; Nagele, P.; et al. Prognostic capabilities of coronary computed tomographic angiography before non-cardiac surgery: Prospective cohort study. BMJ. 2015, 350, h1907. [Google Scholar] [CrossRef]
- Illuminati, G.; Schneider, F.; Greco, C.; Mangieri, E.; Schiariti, M.; Tanzilli, G.; et al. Long-term results of a randomized controlled trial analyzing the role of systematic pre-operative coronary angiography before elective carotid endarterectomy in patients with asymptomatic coronary artery disease. Eur J Vasc Endovasc Surg. 2015, 49, 366–374. [Google Scholar] [CrossRef]
- Walsh, M.; Srinathan, S.K.; McAuley, D.F.; Mrkobrada, M.; Levine, O.; Ribic, C.; et al. The statistical significance of randomized controlled trial results is frequently fragile: A case for a fragility index. J Clin Epidemiol. 2014, 67, 622–628. [Google Scholar] [CrossRef]
- von Känel, R.; Dimsdale, J.E. Effects of sympathetic activation by adrenergic infusions on hemostasis in vivo. Eur J Haematol. 2000, 65, 357–369. [Google Scholar] [CrossRef]
- von Känel, R.; Mills, P.J.; Ziegler, M.G.; Dimsdale, J.E. Effect of β2-adrenergic receptor functioning and increased norepinephrine on the hypercoagulable state with mental stress. Am Heart J. 2002, 144, 68–72. [Google Scholar] [CrossRef]
- Yun AJLee, P.Y.; Bazar, K.A. Can thromboembolism be the result, rather than the inciting cause, of acute vascular events such as stroke, pulmonary embolism, mesenteric ischemia, and venous thrombosis?: A maladaptation of the prehistoric trauma response. Med Hypotheses. 2005, 64, 706–716. [Google Scholar]
- Cohen, M.C.; Aretz, T.H. Histological analysis of coronary artery lesions in fatal postoperative myocardial infarction. Cardiovasc Pathol. 1999, 8, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Samarendra, P.; Mangione, M.P. Aortic stenosis and perioperative risk with noncardiac surgery. J Am Coll Cardiol. 2015, 65, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Livhits, M.; Ko, C.Y.; Leonardi, M.J.; Zingmond, D.S.; Gibbons, M.M.; de Virgilio, C. Risk of surgery following recent myocardial infarction. Ann Surg. 2011, 253, 857–864. [Google Scholar] [CrossRef]
- Sanders, R.D.; Bottle, A.; Jameson, S.S.; Mozid, A.; Aylin, P.; Edger, L.; et al. Independent preoperative predictors of outcomes in orthopedic and vascular surgery: The influence of time interval between an acute coronary syndrome or stroke and the operation. Ann Surg. 2012, 255, 901–7. [Google Scholar] [CrossRef]
- Brauer, C.A.; Coca-Perraillon, M.; Cutler, D.M.; Rosen, A.B. Incidence and mortality of hip fractures in the United States. JAMA. 2009, 302, 1573–1579. [Google Scholar] [CrossRef]
- Karthikeyan, G.; Moncur, R.A.; Levine, O.; Heels-Ansdell, D.; Chan, M.T.; Alonso-Coello, P.; et al. Is a pre-operative brain natriuretic peptide or N-terminal pro-B-type natriuretic peptide measurement an independent predictor of adverse cardiovascular outcomes within 30 days of noncardiac surgery? A systematic review and meta analysis of observational studies. J Am Coll Cardiol. 2009, 54, 1599–1606. [Google Scholar] [PubMed]
- Vanzetto, G.; Machecourt, J.; Blendea, D.; Fagret, D.; Borrel, E.; Magne, J.L.; et al. Additive value of thallium single photon computed tomography myocardial imaging for prediction of perioperative events in clinically selected high cardiac risk patients having abdominal aortic surgery. Am J Cardiol. 1996, 77, 143–148. [Google Scholar] [CrossRef]
- Priebe, H.J. Triggers of perioperative myocardial ischaemia and infarction. Br J Anaesth. 2004, 93, 9–20. [Google Scholar] [CrossRef]
- Devereaux, P.J.; Sessler, D.I.; Leslie, K.; Kurz, A.; Mrkobrada, M.; Alonso-Coelle, P.; et al. Clonidine in patients undergoing noncardiac surgery. N Engl J Med. 2014, 370, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- de Waal, B.A.; Buise, M.P.; van Zundert, A.A. Perioperative statin therapy in patients at high risk for cardiovascular morbidity undergoing surgery: A review. Br J Anaesth. 2015, 114, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Devereaux, P.J.; Mrkobrada, M.; Sessler, D.I.; Leslie, K.; Alonso Coello, P.; Kurz, A.; et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014, 370, 1494–1503. [Google Scholar] [CrossRef]
- Mangano, D.T.; Layug, E.L.; Wallace, A.; Tateo, I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med. 1996, 335, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Raymer, K.; Butler, R.; Parlow, J.; Roberts, R. The effects of perioperative beta–blockade: Results of the Metoprolol after Vascular Surgery (MaVS) study, a randomized controlled trial. Am Heart J. 2006, 152, 983–990. [Google Scholar] [CrossRef]
- Juul, A.B.; Wetterslev, J.; Gluud, C.; Kofoed-Enevoldsen, A.; Jensen, G.; Callesen, T.; et al. Effect of perioperative beta blockade in patients with diabetes undergoing major non-cardiac surgery randomised placebo controlled, blinded multicentre trial. BMJ. 2006, 332, 1482–1488. [Google Scholar] [CrossRef]
- Bouri, S.; Shun-Shin, M.J.; Cole, G.D.; Mayet, J.; Francis, D.P. Meta analysis of secure randomised controlled trials of beta blockade to prevent perioperative death in non-cardiac surgery. Heart. 2014, 100, 456–464. [Google Scholar] [CrossRef]
- Kristensen, S.D.; Knuuti, J.; Saraste, A.; Anker, S.; Botker, H.E.; Hert, S.D.; et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: Cardiovascular assessment and management. Eur Heart J. 2014, 35, 2383–2431. [Google Scholar] [CrossRef]
- Fleisher, L.A.; Fleischmann, K.E.; Auerbach, A.D.; Barnason, S.A.; Beckman, J.A.; Bozkurt, B.; et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. J Am Coll Cardiol. 2014, 64, e77–137. [Google Scholar] [CrossRef] [PubMed]
- Naesh, O.; Frilis, J.T.; Hindberg, I.; Winther, K. Platelet function in surgical stress. Thromb Haemost. 1985, 54, 849–852. [Google Scholar] [CrossRef]
- Burger, W.; Chemnitius, J.M.; Kneissl, G.D.; Rucker, G. Low-dose aspirin for secondary cardiovascular prevention – cardiovascular risks after its perioperative withdrawal versus bleeding risks with its continuation – review and meta-analysis. J Int Med. 2005, 257, 399–414. [Google Scholar] [CrossRef]
- Gerstein, N.S.; Schulman, P.M.; Gerstein, W.H.; Petersen, T.R.; Tawil, I. Should more patients continue aspirin therapy perioperatively? Clinical impact of aspirin withdrawal syndrome. Ann Surg. 2012, 255, 811–819. [Google Scholar] [CrossRef]
- Mantz, J.; Samama, C.M.; Tubach, F.; Devereaux, P.J.; Collet, J.P.; Albaladejo, P.; et al. Impact of preoperative maintenance or interruption of aspirin on thrombotic and bleeding events after elective non-cardiac surgery: The multicentre, randomized, blinded, placebo-controlled, STRATAGEM trial. Br J Anaesth. 2011, 107, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Oscarsson, A.; Gupta, A.; Fredrikson, M.; Jarhult, J.; Nystrom, M.; Pettersson, E.; et al. To continue or discontinue aspirin in the perioperative period: A randomized, controlled clinical trial. Br J Anaesth. 2010, 104, 305–312. [Google Scholar] [CrossRef]
- Robless, P.; Mikhailidis, D.P.; Stansby, G. Systematic review of antiplatelet therapy for the prevention of myocardial infarction, stroke or vascular death in patients with peripheral vascular disease. Brit J Surg. 2001, 88, 787–800. [Google Scholar] [CrossRef]
- Pulmonary Embolism Prevention (PEP) Trial Collaborative Group. Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet. 2000, 355, 1295–1302. [Google Scholar] [CrossRef]
- Sanders, R.D.; Nicholson, A.; Lewis, S.R.; Smith, A.F.; Alderson, P. Perioperative statin therapy for improving outcomes during and after noncardiac vascular surgery (review). Cochrane Database Syst Rev. 2013, 7. [Google Scholar]
- Antoniou, G.A.; Hajibandeh, S.; Hajibandeh, S.; Vallabhaneni, S.R.; Brennan, J.A.; Torella, F. Meta-analysis of the effects of statins on perioperative outcomes in vascular and endovascular surgery. J Vasc Surg. 2015, 61, 519–532. [Google Scholar] [CrossRef]
- Berwanger, O.; Le Manach, Y.; Suzumura, E.A.; Biccard, B.; Srinathan, S.K.; Szczeklik, W.; et al. Association between preoperative statin use and major cardiovascular complications among patients undergoing non-cardiac surgery: The VISION study. Eur Heart J. 2015, ehv456. [Google Scholar]
- Wallace, A.W.; Galindez, D.; Salahieh, A.; Layug, E.L.; Lazo, E.A.; Haratonik, K.A.; et al. Effect of clonidine on cardiovascular morbidity and mortality after noncardiac surgery. Anesthesiology. 2004, 101, 84–293. [Google Scholar] [CrossRef]
- Ellis, J.E.; Drijvers, G.; Pedlow, S.; Laff, S.P.; Sorrentino, M.J.; Foss, J.F. Premedication with oral and transdermal clonidine provides safe and efficacious postoperative sympatholysis. Anesth Analg. 1994, 79, 1133–1140. [Google Scholar] [CrossRef]
- Wu, C.T.; Jao, S.W.; Borel, C.O.; Yeh, C.C.; Li, C.Y.; Lu, C.H.; et al. The effect of epidural clonidine on perioperative cytokine response, postoperative pain, and bowel function in patients undergoing colorectal surgery. Anesth Analg. 2004, 99, 502–509. [Google Scholar] [CrossRef]
- Oliver, M.F.; Goldman, L.G.; Julian, D.G.; Holme, I. Effect of mivazerol on perioperative cardiac complications during non-cardiac surgery in patients with coronary heart disease-the European Mivazerol Trial (EMIT). Anesthesiology. 1999, 91, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Wijeysundera, D.N.; Naik, J.S.; Beattie, W.S. Alpha-2 adrenergic agonists to prevent perioperative cardiovascular complications. Am J Med. 2003, 114, 742–752. [Google Scholar] [CrossRef]
- Mascha, E.J.; Dongsheng, Y.; Weiss, S.; Sessler, D.I. Intraoperative mean arterial pressure variability and 30-day mortality in patients having noncardiac surgery. Anesthesiology. 2015, 123, 79–91. [Google Scholar] [CrossRef]
- van Waes, J.A.; van Klei, W.A.; Wijeysundera, D.N.; van Wolfswinkel, L.; Lindsay, T.; Beattie, W.S. Association between intraoperative hypotension and myocardial injury after vascular surgery. Anesthesiology. 2016, 124. [Google Scholar] [CrossRef]
- Walsh, M.; Devereaux, P.J.; Garg, A.X.; Kurz, A.; Turan, A.; Roseth, R.N.; et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: Toward an empirical definition of hypotension. Anesthesiology. 2013, 119, 507–515. [Google Scholar] [CrossRef]
- Mukherjee, D.; Fang, J.; Chetcuti, S.; Mosucci, M.; Kline-Rogers, E.; Eagle, K.A. Impact of combination evidence-based medical therapy on mortality in patients with acute coronary syndromes. Circulation. 2004, 109, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Ramanath, V.S.; Eagle, K.A. Evidence-based medical therapy of patients with acute coronary syndromes. Am J Cardiovasc Drugs. 2007, 7, 95–116. [Google Scholar] [CrossRef] [PubMed]
- Devereaux, P.J.; Xavier, D.; Pogue, J.; Guyatt, G.; Sigamai, A.; Garutti, I.; et al. Characteristics and short-term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: Cohort study. Ann Int Med. 2011, 154, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Foucrier, A.; Rodseth, R.; Aissaoui, M.; Ibanes, C.; Goarin, J.P.; Landais, P.; et al. The long-term impact of early cardiovascular therapy intensification for postoperative troponin elevation after major vascular surgery. Anesth Analg. 2014, 119, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S). Lancet. 1994, 344, 1383–9.
- Devereaux, P.J. ClinicalTrials.gov identifier: NCT01661101. MANAGE. [updated Nov 20, 2015, accessed Nov 22, 2015] Available from: https://clinicaltrials.gov/ct2/show/NCT01661101. 2013. [Google Scholar]
- Menon, V. ClinicalTrials.gov identifier: NCT022914110. INTREPID. [updated July 23, 2. https://clinicaltrials.gov/ct2/show/NCT0229141. 2014. [Google Scholar]
- Buse, G. Economic analysis of postoperative troponin T screening. [M.Sc thesis]. Devereaux PJ, editor. Hamilton, Ontario: McMaster University. 2012. [Google Scholar]
© 2016 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.