Antiplatelet Therapy Before Cardiac Surgery
Abstract
Introduction
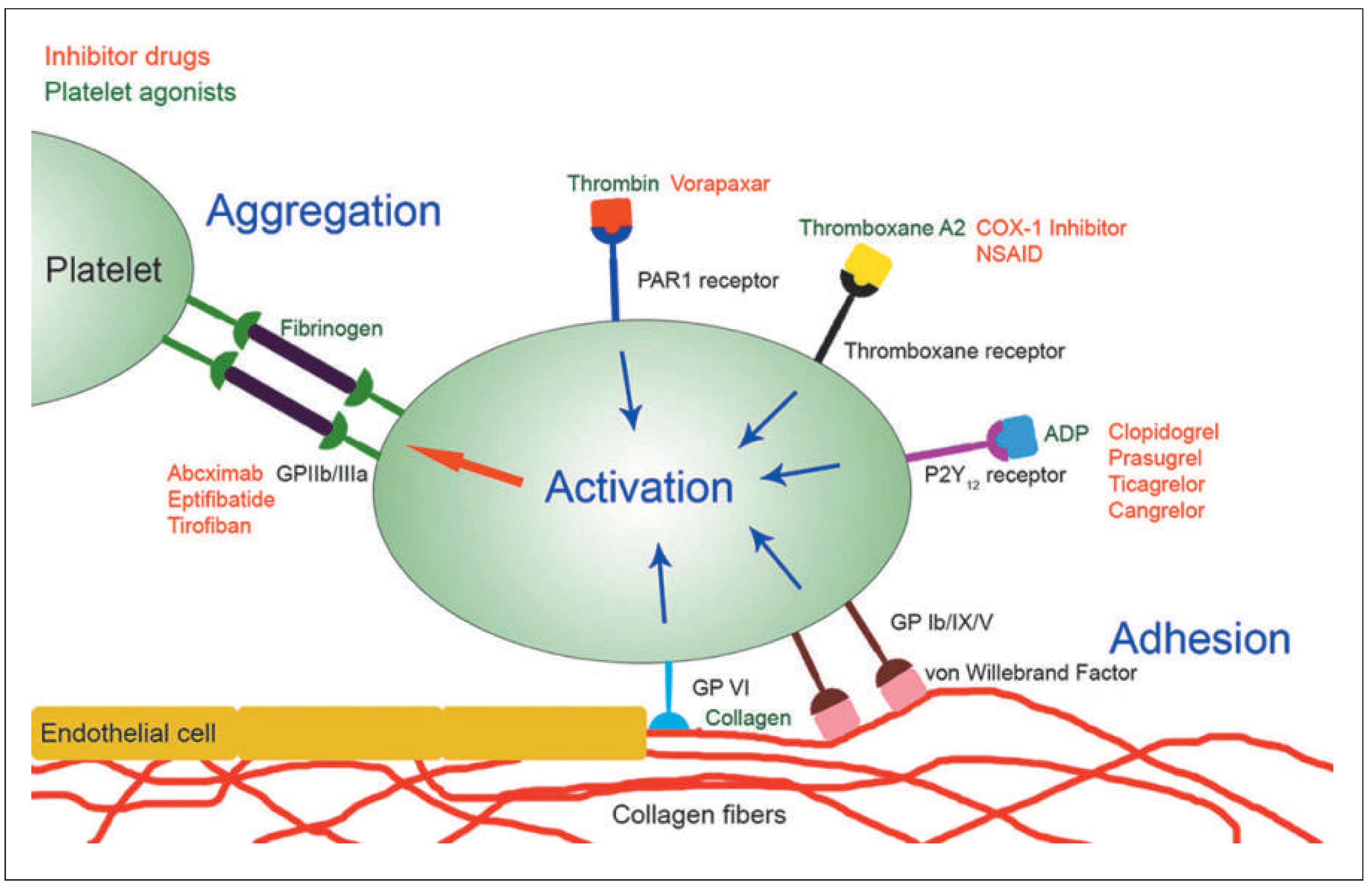
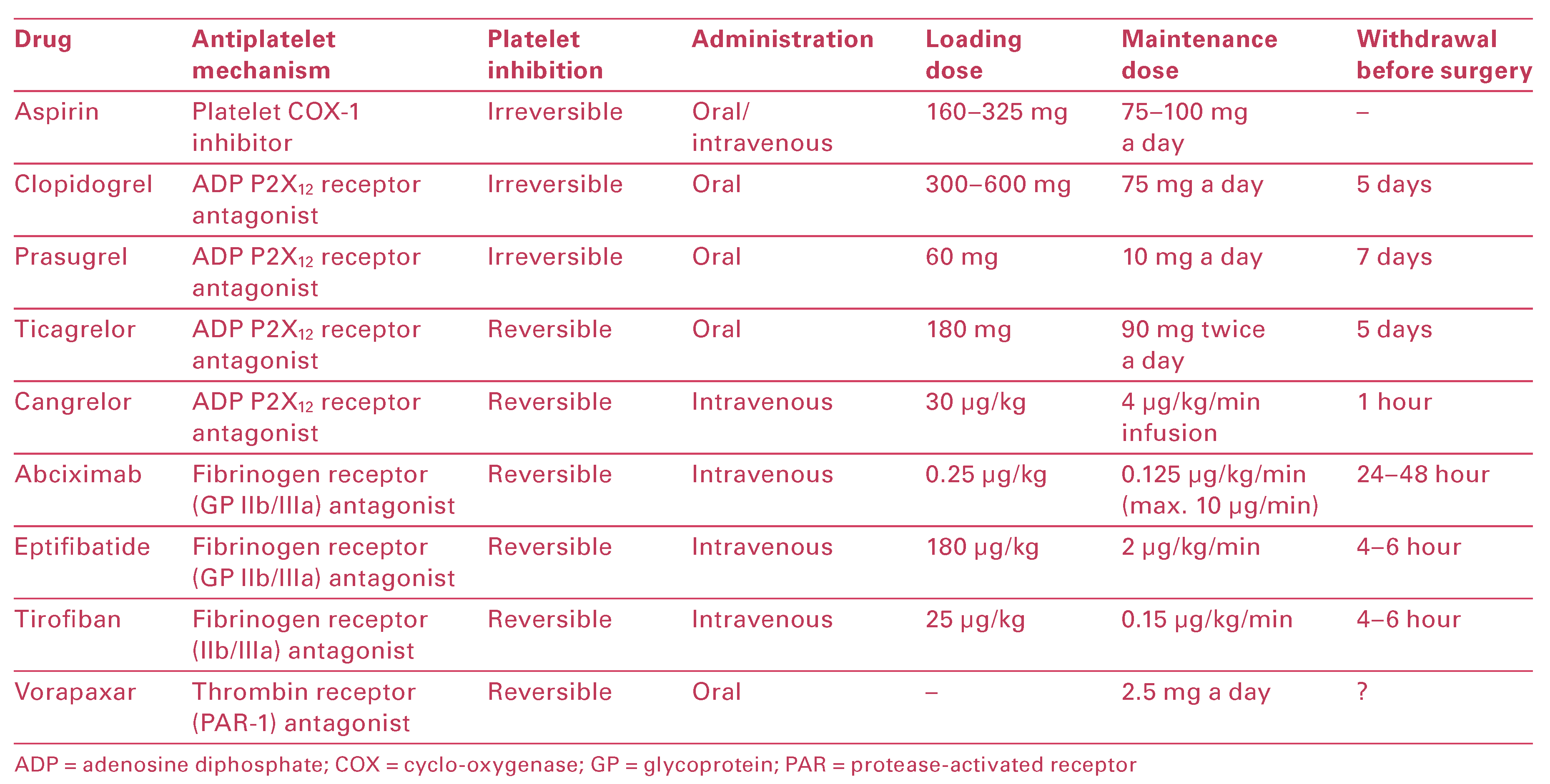
Overview of Antiplatelet Agents and Their Mechanisms of Action
Review of Current Guidelines
- The risks of bleeding and thrombosis, and decisionmaking regarding DAPT and timing of surgery should be assessed by the heart team prior to CABG surgery (Class I, Level C).
- It is reasonable to base timing of surgery on platelet function monitoring rather than arbitrary use of a specified period of delay in patients on DAPT (Class IIa, Level C).
- Bridging with cangrelor, if available, is recommended in high-risk patients (Class I, Level B recommendation).
- Bridging with short-acting intravenous GPIIb/IIIa inhibitors may be considered in patients at high risk for ischaemic events (Class IIb, Level C).
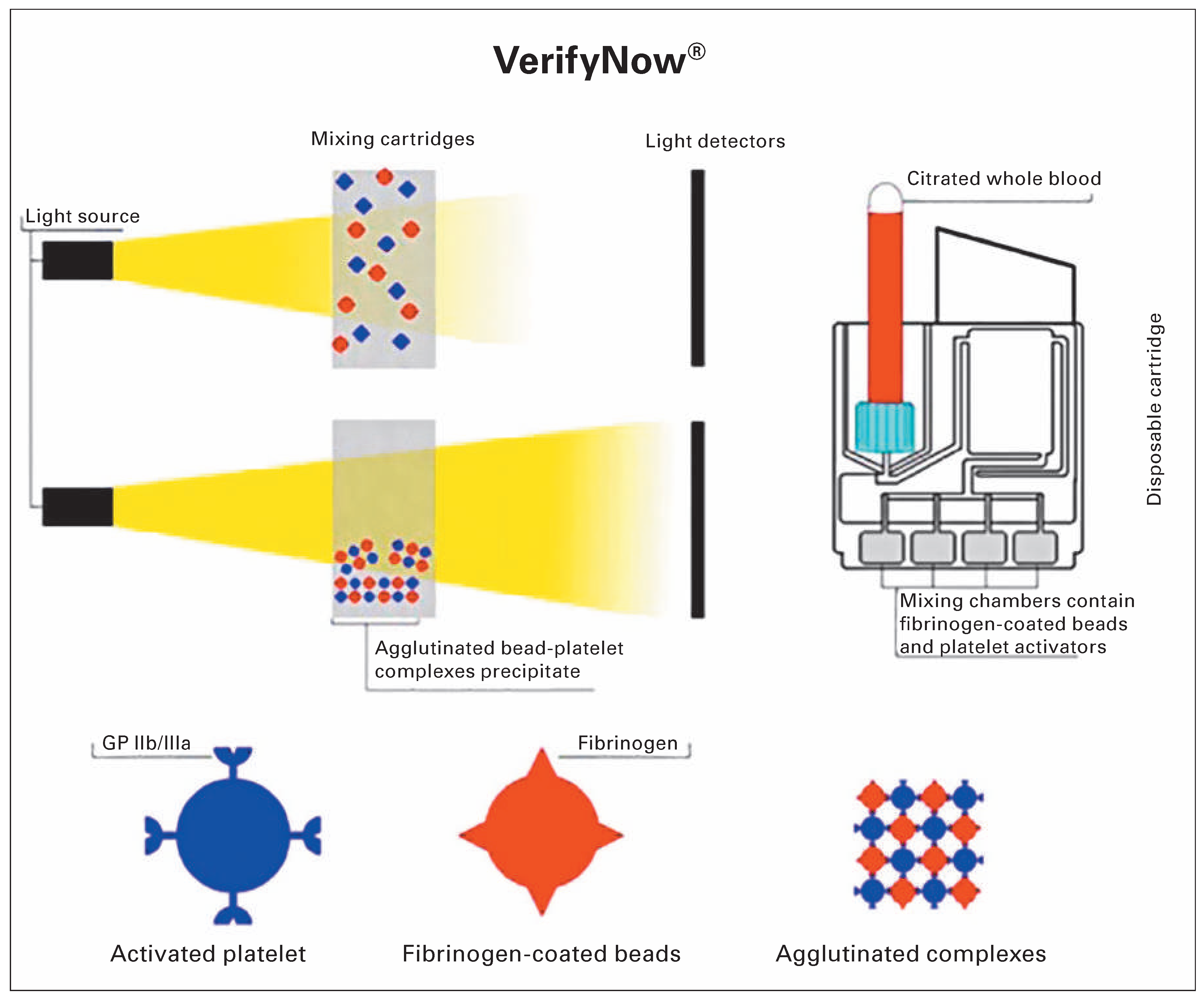
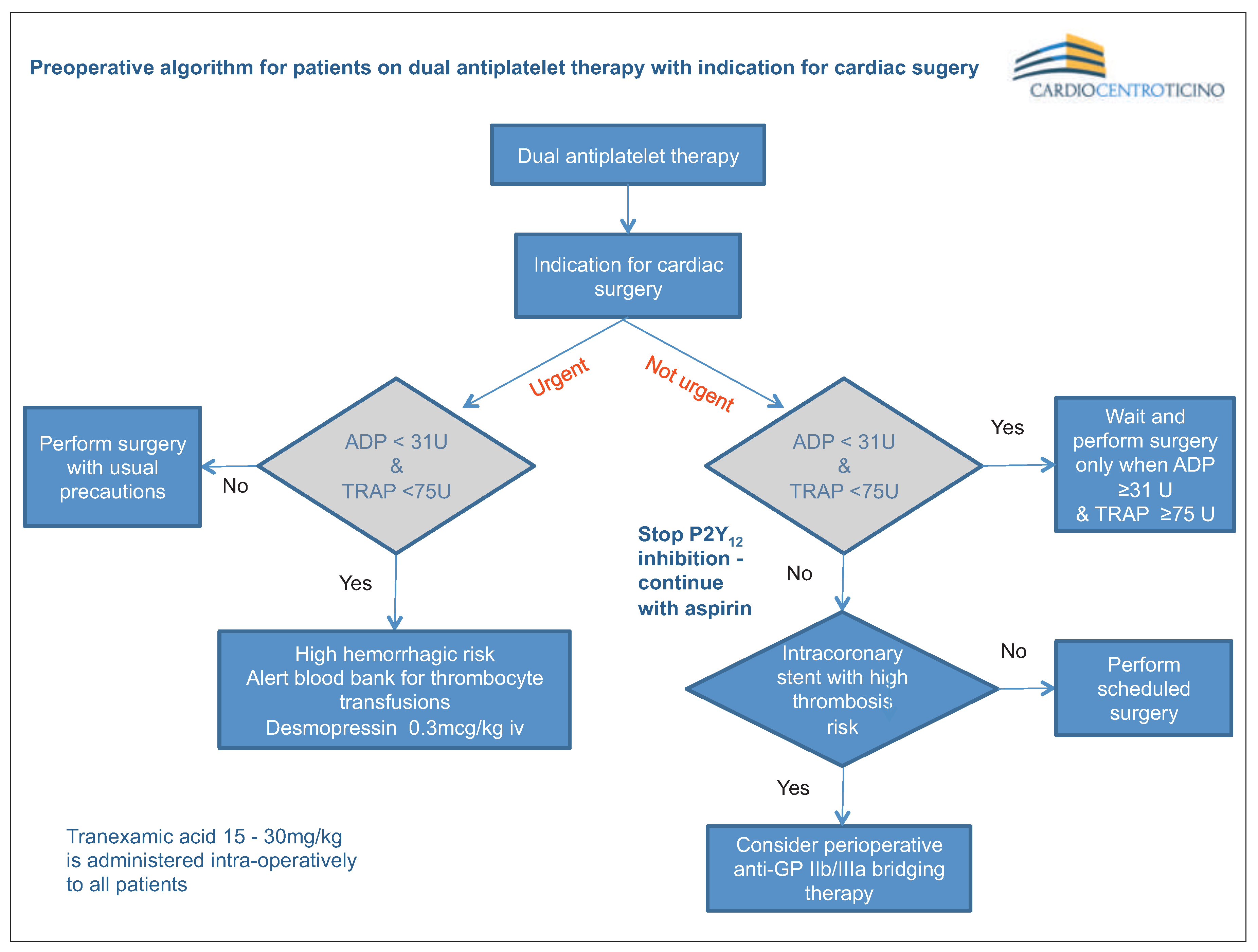
Point-of-Care Platelet Function Analysis Approach: Tailoring of Decision-Making
Discussion
Disclosure statement
References
References
- Koch, C.G.; Li, L.; Duncan, A.I.; Mihaljevic, T.; Cosgrove, D.M.; Loop, F.D.; et al. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006, 34, 1608–1616. [Google Scholar] [CrossRef]
- Ranucci, M.; Baryshnikova, E.; Castelvecchio, S.; Pelissero, G. Surgical and Clinical Outcome Research (SCORE) Group. Major bleeding, transfusions, and anemia: The deadly triad of cardiac surgery. Ann Thorac Surg. 2013, 96, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Gupta, K.; Vacek, J.L. Anticoagulation and antiplatelet therapy in acute coronary syndromes. Cleve Clin J Med 2014, 81, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Roffi, M.; Patrono, C.; Collet, J.-P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent st-segment elevation: Task force for the management of acute coronary syndromes in patients presenting without persistent st-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2015. [Google Scholar]
- Hongo, R.H.; Ley, J.; Dick, S.E.; Yee, R.R. The effect of clopidogrel incombination with aspirin when given before coronary artery bypass grafting. J Am Coll Cardiol. 2002, 40, 231–237. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007, 357, 2001–2015. [Google Scholar] [CrossRef]
- Jacobs, A.K.; Kushner, F.G.; Ettinger, S.M. Practice guideline: Methodology ACCF/AHA clinical practice guideline methodology summit report. A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2013, 127, e310. [Google Scholar] [CrossRef]
- Fitchett, D.; Eikelboom, J.; Fremes, S.; Mazer, D.; Singh, S.; Bittira, B.; et al. Dual antiplatelet therapy in patients requiring urgent coronary artery bypass grafting surgery: A position statement of the canadian cardiovascular society. Can J Cardiol. 2009, 25, 683–689. [Google Scholar] [CrossRef]
- Annich, G.M.; Meinhardt, J.P.; Mowery, K.A.; Ashton, B.A.; Merz, S.I.; Hirschl, R.B.; et al. Reduced platelet activation and thrombosis in extracorporeal circuits coated with nitric oxide release polymers. Crit Care Med. 2000, 28, 915–920. [Google Scholar] [CrossRef]
- De Somer, F.; Francois, K.; Van Oeveren, W.; Poelaert, J.; De Wolf, D.; Ebels, T.; Van Nooten, G. Phosphorylcholine coating of extracorporeal circuits provides natural protection against blood activation by the material surface. Eur J Cardiothorac Surg. 2000, 18, 602–606. [Google Scholar] [CrossRef][Green Version]
- Casati, V.; Bellotti, F.; Gerli, C.; Franco, A.; Oppizzi, M.; Cossolini, M.; et al. Tranexamic acid administration after cardiac surgery: A prospective, randomized, double-blind, placebo-controlled study. Anesthesiology. 2001, 94, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Karkouti, K.; Beattie, W.S.; Dattilo, K.M.; McCluskey, S.A.; Ghannam, M.; Hamdy, A.; et al. A propensity score casecontrol comparison of aprotinin and tranexamic acid in high-transfusion-risk cardiac surgery. Transfusion. 2006, 46, 327–338. [Google Scholar] [CrossRef]
- Ferraris, V.A.; Saha, S.P.; Oestreich, J.H.; Song, H.K.; Rosengart, T.; Reece, T.B.; et al. 2012 update to the society of thoracic surgeons guideline on use of antiplatelet drugs in patients having cardiac and noncardiac operations. Ann Thorac Surg. 2012, 94, 1761–1781. [Google Scholar] [CrossRef]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E.; Ganiats, T.G.; -Holmes, D.R.; et al. 2014 AHA/ACC guideline for the management of patients with non-st-elevation acute coronary syndromes: A report of the american college of cardiology/american heart association task force on practice guidelines. J Am Coll Cardiol. 2014, 64, e139–228. [Google Scholar] [CrossRef]
- Sousa-Uva, M.; Storey, R.; Huber, K.; Falk, V.; Leite-Moreira, A.F.; Amour, J.; et al. Expert position paper on the management of antiplatelet therapy in patients undergoing coronary artery bypass graft surgery. Eur Heart J. 2014, 35, 1510–1514. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P. Platelet function analysis. Blood Rev. 2005, 19, 111–123. [Google Scholar] [CrossRef]
- Pakala, R.; Waksman, R. Currently available methods for platelet function analysis: Advantages and disadvantages. Cardiovasc Revasc Med. 2011, 12, 312–322. [Google Scholar] [CrossRef]
- Michelson, A.D. Methods for the measurement of platelet function. Am J Cardiol. 2009, 103, 20A–26A. [Google Scholar] [CrossRef]
- Paniccia, R.; Priora, R.; Liotta, A.A.; Abbate, R. Platelet function tests: A comparative review. Vasc Health Risk Manag. 2015, 11, 133. [Google Scholar] [CrossRef]
- Enriquez, L.J.; Shore-Lesserson, L. Point-of-care coagulation testing and transfusion algorithms. Br J Anaesth. 2009, 103 (suppl. 1), i14–i22. [Google Scholar] [CrossRef]
- Tóth, O.; Calatzis, A.; Penz, S.; Losonczy, H.; Siess, W. Multiple electrode aggregometry: A new device to measure platelet aggregation in whole blood. Thromb Haemost. 2006, 781–788. [Google Scholar]
- Danese, E.; Fava, C.; Beltrame, F.; Tavella, D.; Calabria, S.; Benati, M.; et al. Relationship between pharmacokinetics and pharmacodynamics of clopidogrel in patients undergoing percutaneous coronary intervention: Comparison between vasodilator-stimulated phosphoprotein phosphorylation assay and multiple electrode aggregometry. J Thromb Haemost. 2015. [Google Scholar] [CrossRef]
- Eppihimer, M.J.; Sushkova, N.; Grimsby, J.L.; Efimova, N.; Kai, W.; Larson, S.; et al. Impact of stent surface on thrombogenicity and vascular healing A comparative analysis of metallic and polymeric surfaces. Circ Cardiovasc Interv. 2013, 6, 370–377. [Google Scholar] [CrossRef]
- Singh, S.; Singh, M.; Grewal, N.; Khosla, S. Comparative efficacy and safety of prasugrel, ticagrelor, and standard-dose and high-dose clopidogrel in patients undergoing percutaneous coronary intervention: A network meta analysis. Am J Ther. 2015. [Google Scholar] [CrossRef][Green Version]
- Rahe-Meyer, N.; Winterhalter, M.; Boden, A.; Froemke, C.; Piepenbrock, S.; Calatzis, A.; Solomon, C. Platelet concentrates transfusion in cardiac surgery and platelet function assessment by multiple electrode aggregometry. Acta Anaesthesiol Scand. 2009, 53, 168–175. [Google Scholar] [CrossRef]
- Ranucci, M.; Baryshnikova, E.; Soro, G.; Ballotta, A.; De Benedetti, D.; Conti, D. Surgical and Clinical Outcome Research (SCORE) Group. Multiple electrode whole-blood aggregometry and bleeding in cardiac surgery patients receiving thienopyridines. Ann Thorac Surg. 2011, 91, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Poston, R.; Gu, J.; Manchio, J.; Lee, A.; Brown, J.; Gammie, J.; et al. Platelet function tests predict bleeding and thrombotic events after off-pump coronary bypass grafting. Eur J Cardiothorac Surg. 2005, 27, 584–591. [Google Scholar] [CrossRef][Green Version]
- Kwak, Y.-L.; Kim, J.-C.; Choi, Y.-S.; Yoo, K.-J.; Song, Y.; Shim, J.-K. Clopidogrel responsiveness regardless of the discontinuation date predicts increased blood loss and transfusion requirement after off-pump coronary artery bypass graft surgery. J Am Coll Cardiol. 2010, 56, 1994–2002. [Google Scholar] [CrossRef]
- Solomon, C.; Hartmann, J.; Osthaus, A.; Schöchl, H.; Raymondos, K.; Koppert, W.; Rahe-Meyer, N. Platelet concentrates transfusion in cardiac surgery in relation to preoperative point-of-care assessment of platelet adhesion and aggregation. Platelets. 2010, 21, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Alström, U.; Granath, F.; Oldgren, J.; Ståhle, E.; Tydén, H.; Siegbahn, A. Platelet inhibition assessed with verifynow, flow cytometry and plateletmapping in patients undergoing heart surgery. Thromb Res. 2009, 124, 572–577. [Google Scholar] [CrossRef]
- Ranucci, M.; Colella, D.; Baryshnikova, E.; Di Dedda, U. Surgical and Clinical Outcome Research (SCORE) Group. Effect of preoperative P2Y12 and thrombin platelet receptor inhibition on bleeding after cardiac surgery. Br J Anaesth. 2014, 113, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Velik-Salchner, C.; Maier, S.; Innerhofer, P.; Streif, W.; Klingler, A.; Kolbitsch, C.; Fries, D. Point-of-care whole blood impedance aggregometry versus classical light transmission aggregometry for detecting aspirin and clopidogrel: The results of a pilot study. Anesth Analg. 2008, 107, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Paniccia, R.; Antonucci, E.; Maggini, N.; Miranda, M.; Gori, A.M.; Marcucci, R.; et al. Comparison of methods for monitoring residual platelet reactivity after clopidogrel by point-of-care tests on whole blood in high-risk patients. Thromb Haemost. 2010, 104, 287–292. [Google Scholar] [PubMed]
- Ko, Y.-G.; Suh, J.-W.; Kim, B.H.; Lee, C.J.; Kim, J.-S.; Choi, D.; et al. Comparison of 2 point-of-care platelet function tests, verifynow assay and multiple electrode platelet aggregometry, for predicting early clinical outcomes in patients undergoing percutaneous coronary intervention. Am Heart J. 2011, 161, 383–390. [Google Scholar] [CrossRef]
- Chen, F.; Maridakis, V.; Oneill, E.A.; Beals, C.; Radziszewski, W.; de Lepeleire, I.; et al. A randomized clinical trial comparing point-of-care platelet function assays and bleeding time in healthy subjects treated with aspirin or clopidogrel. Platelets. 2012, 23, 249–258. [Google Scholar] [CrossRef]
- Velik-Salchner, C.; Maier, S.; Innerhofer, P.; Kolbitsch, C.; Streif, W.; Mittermayr, M.; et al. An assessment of cardiopulmonary bypass-induced changes in platelet function using whole blood and classical light transmission aggregometry: The results of a pilot study. Anesth Analg. 2009, 108, 1747–1754. [Google Scholar] [CrossRef]
- Casso, G.; Lanzi, F.; Marcucci, C.E. Point-of-Care platelet function tests. In Perioperative Hemostasis; Marcucci, C.E., Schoettker, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 45–63. [Google Scholar]

© 2016 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Demertzis, S.; Cassina, T.; Casso, G. Antiplatelet Therapy Before Cardiac Surgery. Cardiovasc. Med. 2016, 19, 110. https://doi.org/10.4414/cvm.2016.00401
Demertzis S, Cassina T, Casso G. Antiplatelet Therapy Before Cardiac Surgery. Cardiovascular Medicine. 2016; 19(4):110. https://doi.org/10.4414/cvm.2016.00401
Chicago/Turabian StyleDemertzis, Stefanos, Tiziano Cassina, and Gabriele Casso. 2016. "Antiplatelet Therapy Before Cardiac Surgery" Cardiovascular Medicine 19, no. 4: 110. https://doi.org/10.4414/cvm.2016.00401
APA StyleDemertzis, S., Cassina, T., & Casso, G. (2016). Antiplatelet Therapy Before Cardiac Surgery. Cardiovascular Medicine, 19(4), 110. https://doi.org/10.4414/cvm.2016.00401




