Summary
Background: Optimal management of the vascular access site is crucial to early ambulation after percutaneous coronary intervention (PCI) and has thus major impact on the costs of the procedure. In this study, we assessed the cost-effectiveness, safety, and patient comfort of the Angio-SealTM Hemostatic Puncture Closing Device in PCI patients. Methods: In a single-centre trial, 43 patients were prospectively randomised to either immediate arterial sheath removal with AngioSeal™ access site closure (Angio-Seal group; n = 21), or sheath removal 4 hours after elective PCI followed by manual compression (MC group; n = 22). In the Angio-Seal group, patients were ambulated 4 hours after PCI if haemostasis was achieved. In the MC group, patients were ambulated the day after PCI. Results: The time to achieve haemostasis was significantly shorter (Angio-SealTM: 2.0 ± 1.0 vs MC: 22.5 ± 4.6 min; p <0.001), and back pain after the intervention was significantly lower (Angio-SealTM: pain score 1.5 ± 1.4 vs MC: 6.0 ± 3.0; p <0.001) in the Angio-Seal group. Haemostasis was successful in all patients and no major complications occurred. The AngioSealTM device allowed earlier ambulation (Angio-SealTM: 4.0 ± 2.0 vs MC: 18.5 ± 2.7 hours; p <0.001). Total direct costs were significantly lower in the Angio-SealTM compared to the MC group (514 Euro saved per patient, representing a reduction of 54%) due to reductions of nursing time (Angio-SealTM: 7.1 ± 2.5 vs MC: 9.4 ± 2.6 hours; p = 0.006) and time of the interventional physician (Angio-SealTM: 5.4 ± 7.9 vs MC: 22.5 ± 4.6 min; p <0.001). Conclusions: Compared to MC the use of the Angio-SealTM is associated with cost saving cost saving due to shorter time to haemostasis and thus earlier ambulation and hospital discharge, as well decreased personnel and infrastructural demands. In addition, the use of Angio-Seal. is safe and increases patient comfort.
Introduction
In recent years, several different approaches have been made to reduce the costs of percutaneous coronary intervention (PCI). As the length of hospital stay is one of the major determinants of costs, attempts have been made to minimise the patient’s time spent in the hospital. Outpatient PCI by the transradial approach has been shown to be safe, and a definite potential to lower the costs of these invasive procedures has been demonstrated [,,]. However, the femoral artery is the access site most frequently used to perform catheterbased vascular interventions. The standard technique for management of the femoral puncture site remains manual compression (MC), which is associated with prolonged bed rest, significant patient discomfort, and significant local complications in up to 10% of patients, leading to transfusion or vascular repair in 1–2% []. In pursuit of new methods to achieve haemostasis, suture-based and collagen-based devices have been developed. These new devices permit faster haemostasis and consequently earlier ambulation compared to MC of the femoral access site [,,], thus providing the possibility of shortening the patient’s hospital stay. In a previous study, the use of a suture-based device was shown to reduce total post-PCI costs by 13% []. We therefore decided to compare the cost-effectiveness of a collagen-based closure device under similar circumstances. The Angio-SealTM Haemostatic Puncture Closure Device (St. Jude Medical, Minnetonka, MN) uses a biodegradable anchor and a collagen plug to facilitate local haemostasis after cardiac catheterisation. This device has been shown to be safe, achieving immediate haemostasis and early ambulation without increasing the risk of bleeding complications [,,,]. The aim of the present prospective study was to evaluate the potential of earlier patient discharge, and to assess the cost-effectiveness of the Angio-Seal™ device compared to MC.
Method
Study Population
Between September and November 2002, we conducted a single-centre, multiple operator prospective study in 43 patients with stable angina pectoris undergoing elective PCI via the femoral approach with 6–8 F sheaths. At the end of PCI, patients were randomised to be treated either with the Angio-Seal™ vascular closure device (Angio-Seal group; n = 21) or conventional MC therapy (MC group; n = 22). To be eligible for the study, patients had to be >18 years of age and had to be willing to give written informed consent. Exclusion criteria were known severe peripheral arterial obstructive disease, known allergy to any of the materials of the Angio-Seal™ device, severe acute non-cardiac systemic disease or terminal illness, and PCI after 4 p.m. (these patients treated late in the day all received the Angio-Seal™ device, but were not randomised to the study). Patients were also excluded if a palpable haematoma had been observed at the end of the PCI. The hospital ethics committee had reviewed and approved the study protocol.
Insertion of the closure device
The Angio-Seal™ Vascular Closure Device consists of three fully absorbable components in a special carrier system: a small rectangular anchor, a small bovine collagen sponge and an absorbable traction suture []. It is also supplied with an insertion sheath, arteriotomy locator and guidewire. The device was deployed as previously described []. In brief, at the end of an interventional procedure, the Angio-Seal™ guidewire is inserted into the procedure sheath, which is then removed, and exchanged for the arteriotomy locator/insertion sheath assembly. The appearance of a steady flow of blood from the arteriotomy locator indicates positioning of the sheath tip within the artery lumen. The locator system is then slowly withdrawn until blood flow slows to a trickle or stops. This indicates that the tip of the insertion sheath has just exited the artery. From this point, the locator system is advanced a distance of 1–2 cm. While holding the insertion sheath steady, the arteriotomy locator and the guidewire are removed and exchanged for the Angio-Seal™ carrier, which is introduced through the insertion sheath. The insertion system places the anchor against the inner arterial wall and is then slowly withdrawn, releasing the contained collagen at the outer arterial wall. Through the tension of the suture thread the anchor and the collagen sponge are drawn together by the pulley action of the suture, thus sandwiching the arteriotomy. The collagen is then tamped securely onto the surface of the artery while tension is maintained on the suture. This results in a secure mechanical seal around the arterial wall defect and thus immediate haemostasis. Finally the suture is cut below the skin level [,]. Ambulation is possible within 2 to 4 hours after PCI, and has been reported to be as soon as immediately in some cases []. The anchor and collagen sponge are fully bio-absorbable and dissolve within 60–90 days after application.
Intervention and follow-up
Patients randomised to the Angio-Seal group underwent treatment with the sealing device immediately after PCI. Three operators trained in Angio-Seal™ deployment performed all of the procedures. Additional MC was allowed if ongoing bleeding was present at the puncture site after Angio-Seal™ deployment. If no bleeding was present, a light bandage was applied and patients were allowed to ambulate 4 hours later. In patients assigned to MC, the procedure sheath was left in the femoral artery up to four hours after PCI. It was then removed, and MC was used to achieve haemostasis followed by additional local compression (done by the interventional cardiologist). Patients of the MC group were ambulated the following morning.
Efficacy assessment was defined as time to haemostasis, time to ambulation and time to discharge. Time to haemostasis in MC patients was measured from sheath removal until the bandage was applied after achieving haemostasis. Safety end points included the occurrence of major (need for surgical intervention, local infection, need of blood transfusion) and minor complications (pseudoaneurysm, visible local haematoma and haematoma assessed by ultrasound). Visible haematomas were classified according to their size as small (<6 cm2), medium (6–12 cm2) or large (>12 cm2). All patients were examined clinically and by Doppler ultrasound prior to discharge.
The patients’ discomfort was evaluated the day following the intervention by the means of a visual analogue scale, graded with numbers from 0 (best) to 10 (worst). Local pain due to Angio-Seal™ deployment, sheath removal and local compression, back pain and problems with urination were assessed.
Cost analysis
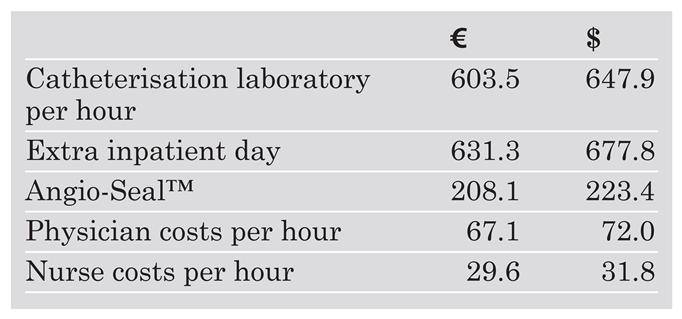
Analysis of costs was confined to those associated with management of the patient following the procedure. Direct medical costs (Angio-Seal™ device, infrastructure and personnel costs) were derived from those applying to the institution for the year 2002. Costs of the catheterisation room, hospitalisation and personnel were derived from an analysis of the accounting department of the Kantonsspital St.Gallen. Unit costs are shown in Table 1. Costs are given in Euro and US Dollars (exchange rate: 1 Euro = 1.461 sFr = 1.064 US Dollars [January 2003]).

Table 1.
Cost elements.
Statistical analysis
Data are expressed either as actual values or means ± standard deviation. P values <0.05 were considered statistically significant. Differences between the Angio-Seal™ device and MC were analysed by student’s t-test or Fishers exact test as appropriate. All data were stored using Microsoft Excel software, and analysed using a commercially available statistical program (SPSS Inc., Version 10.1, Chicago, Illinois).
Results
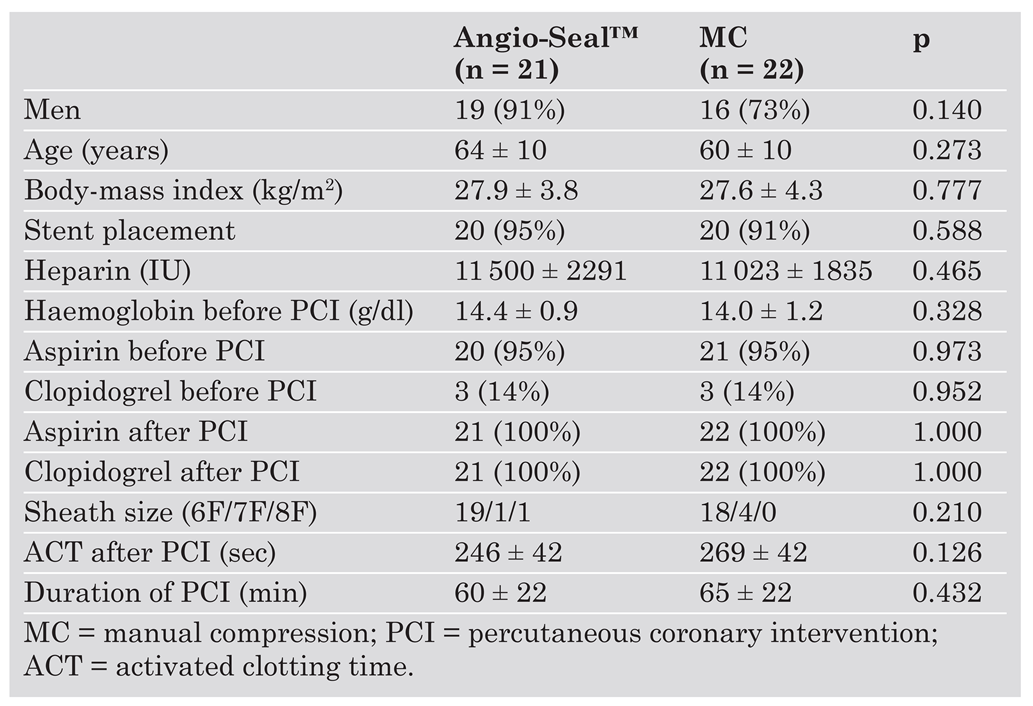
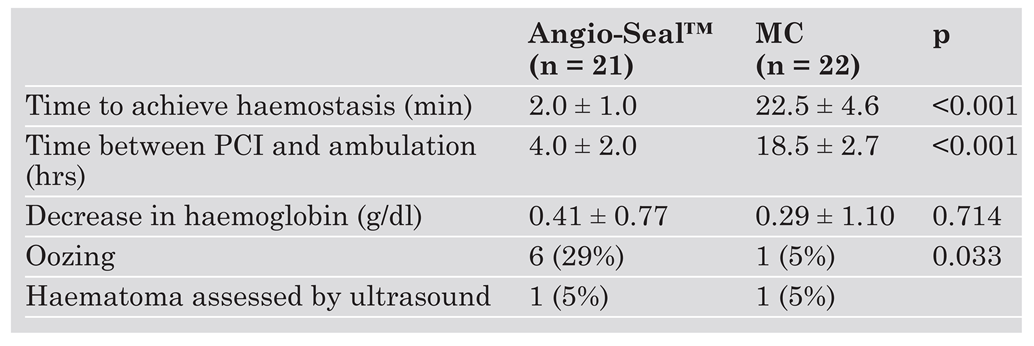
Forty-three patients were enrolled in this study; 22 patients received standard MC, and 21 patients were treated with the Angio-Seal™ device. Table 2 presents baseline characteristics of the two patient groups. There were no significant differences between the Angio-Seal and the MC group. Outcomes with regard to efficacy and safety are shown in Table 3. Successful haemostasis without major complications was achieved in all patients in both groups. In the Angio-Seal group the time to haemostasis was significantly shorter than in the MC group (Angio-Seal™: 2.0 ± 1.0 vs MC: 22.5 ± 4.6 min; p <0.001). All 21 patients treated with the Angio-Seal™ device were successfully ambulated on the day of PCI. Their time to ambulation was significantly shorter compared to patients of the MC group (Angio-Seal™: 4.0 ± 2.0 vs MC: 18.5 ± 2.7 hours; p <0.001). The mean decrease in blood haemoglobin concentration after PCI was similar in both groups (Angio-Seal™: 0.41 ± 0.77 g/dl vs MC: 0.29 ± 1.10 g/dl; p = 0.714) and did not exceed 2 g/dl in any patient. There was a slight difference between the two groups with regard to the incidence of minor complications: Oozing was noted in 6/21 patients (29%) in the Angio-Seal group compared to one of 22 patients (5%) in the MC group (p = 0.033). There was a trend towards an increased rate of oozing with higher heparin doses during PCI (p = 0.09). In patients with oozing a light compressive femoral bandage was applied, but they were still ambulated on the day of PCI without further complications. One patient of each group (5%) was found to have a small haematoma when assessed by ultrasound.

Table 2.
Baseline characteristics.

Table 3.
Efficacy and safety results.
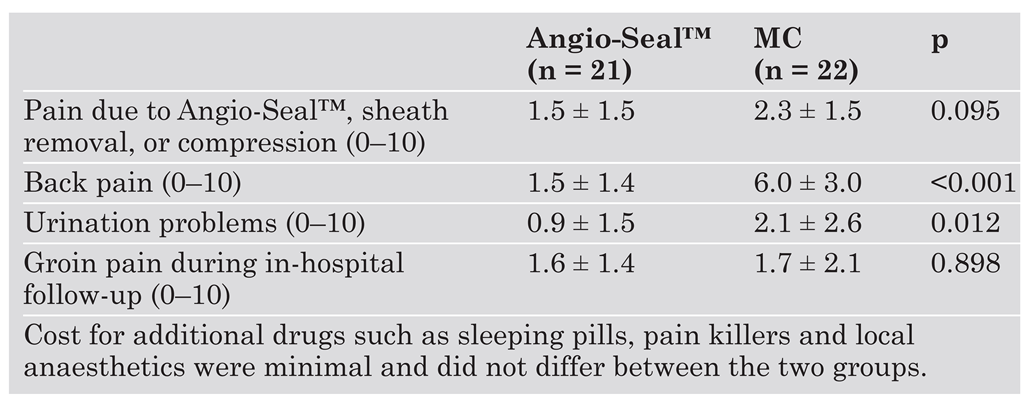
During in-hospital follow-up (Table 4), patients in the Angio-Seal group expressed significantly less discomfort regarding back pain (Angio-Seal™: 1.5 ± 1.4 vs MC: 6.0 ± 3.0; p <0.001) and urination problems (Angio-Seal™: 0.9 ± 1.5 vs MC: 2.1 ± 2.6; p = 0.012) after the procedure compared to the patients in the MC group. With respect to pain due to deployment of the Angio-Seal™ device, sheath removal or compression, we noted a slight but not significant difference in favour of the device (Angio-Seal™: 1.5 ± 1.5 vs MC: 2.3 ± 1.5; p = 0.095). No significant difference in groin pain could be observed during the in-hospital follow-up (Angio-Seal™: 1.6 ± 1.4 vs MC: 1.7 ± 2.1; p = 0.898).

Table 4.
Patient discomfort.
As shown in Figure 1, total post-PCI costs were significantly reduced in the Angio-Seal group as compared to the MC group (AngioSeal™: 425.2 ± 83.4 Euro vs MC: 935.9 ± 82.8 Euro; p <0.001). The additional costs for the Angio-Seal™ device (208.1 Euro) were exceeded by savings of ward costs due to an earlier discharge (Angio-Seal™: 0 Euro vs MC: 631.3 Euro; p <0.001). In addition, cardiologists (Angio-Seal™: 5.4 ± 7.9 vs MC: 22.5 ± 4.6 min; p <0.001) and caring nurses (AngioSeal™: 7.1 ± 2.5 vs MC: 9.4 ± 2.6 hours; p = 0.006) spent significantly less time per PCI patient in the Angio-Seal group compared to the MC group (fig. 2) resulting in reductions of personnel costs for cardiologists (AngioSeal™: 6.1 ± 8.8 Euro vs MC: 25.2 ± 5.2 Euro; p <0.001) as well as nurses (Angio-Seal™: 211.0 ± 74.6 Euro vs MC: 279.4 ± 77.6 Euro; p <0.001). Angio-Seal™ use was therefore found to reduce total costs by 514 Euro (54%).
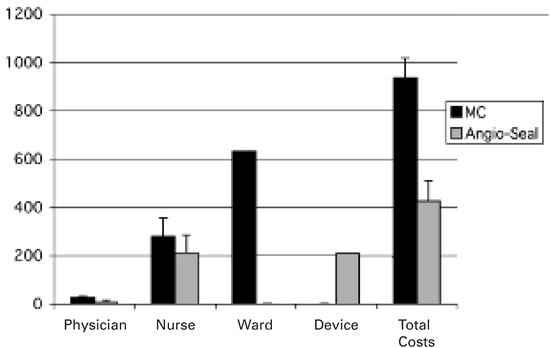
Figure 1.
Total costs in Euro including costs of the interventional physician, nurse, and the device in patients treated with manual compression (MC) or the Angio-SealTM device for closure of the femoral arterial access after elective percutaneous coronary interventions.
Discussion
This present study demonstrates that a significant reduction of the overall costs of post-PCI care is possible when using the Angio-Seal™ device despite its additional cost. This reduction is mainly due to earlier hospital discharge. In addition, use of the Angio-Seal™ device showed time savings for the interventional cardiologist and the nursing staff. In regard to safety and efficacy, our study confirmed previous reports [,,]. Finally, we found all aspects of patient discomfort to be significantly reduced when the Angio-Seal™ device was used.
Conventional management of the femoral access site after PCI by MC can be accomplished safely and effectively []. However, it necessitates delayed sheath removal and prolonged bed rest usually until the day after PCI. In contrast, the Angio-Seal™ device permits immediate sheath removal followed by immediate haemostasis and earlier ambulation compared to MC [,,,]. Consequently, patients treated with the Angio-Seal™ device require a shorter hospital stay than patients treated with conventional MC [,].
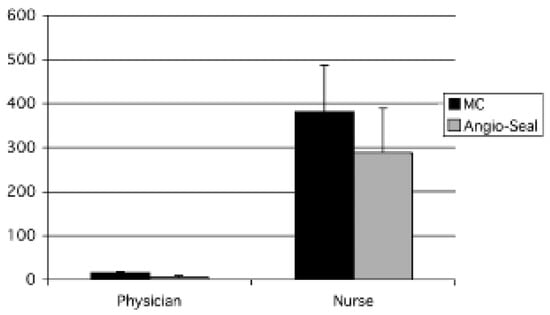
In today’s cost-conscious healthcare environment, there is a profound need to decrease the costs of everyday procedures such as PCI. One of the most powerful paths to decrease the costs of an interventional procedure is to decrease its associated length of stay. However, this should result in an equivalent clinical outcome before evaluating the cost minimisation of these procedures. Faster haemostasis and shorter time to ambulation can only be considered cost-effective or cost-saving if the complication rate is not increased [].
Clinical efficacy and safety of the AngioSeal™ vascular closure device have been documented in several clinical studies [,,,,,]. A recent meta-analysis on the complication rate associated with the use of vascular closure devices found no differences between Angio-Seal™ and MC in the only diagnostic setting, but a non-significant trend a favour of the Angio-Seal™ over MC when analysing randomised trials in the PCI setting []. Interestingly, Resnic and co-workers showed that closure device use, which was predominantly Angio-Seal™, reduced hospital length of stay and complications, particularly in patients receiving glycoprotein IIb/IIIa (GPIIb/IIIa) inhibitor therapy []. These data are confirmed by a recently published trial of Boccolandro and co-workers who assessed safety and efficacy of the Angio-Seal™ in patients undergoing rescue-PCI. Although these patients had received a full dose of a thrombolytic agent within 6 hours before rescue-PCI using GPIIb/IIIa inhibitors, the incidence of access site complications was not higher in the Angio-Seal group as compared to patients treated with MC and even similar as in patients undergoing elective PCI [].
In accordance with these studies we observed only a few minor complications, which did not have a significant impact on the patients’ outcomes. Oozing was more prominent in the Angio-Seal group, but did not have any further consequences for the affected patients. The only factor associated with the incidence of oozing was a higher level of heparin in those patients. A number of studies focusing on bleeding complications after PCI found that high levels of heparin were predictors for bleeding complications at the vascular access site [,,,]. One of the major cost factors of post-procedural care is the hospitalisation time, which can be shortened when using the Angio-Seal™ due to earlier haemostasis, ambulation and thus discharge []. The feasibility of same-day discharge after PCI has been reported for the transradial approach several years before [,], and due to vascular closure devices has become reality also for the femoral approach in a majority of patients [,,]. Cohen et al. showed that the average non-procedural hospital costs were reduced from $900 for transfemoral stenting to just $300 per patient with outpatient transradial stenting []. Another estimate was made by Khatri, who calculated potential savings due to same-day discharge to be 1079 Canadian dollars per patient [].
We have shown time savings for the interventional cardiologist and the nursing staff, which increases efficiency and productivity of the hospital. Our results are in contrast to those of Juergens and co-workers who also found a shorter time to haemostasis and ambulation, but similar resource utilisation (nurses and medical officer) when comparing the Angio-Seal™ device to MC using the femostop device []. The authors argued that regular observation of the access site and the distal pulses is still necessary, and that the higher rate of oozing in the Angio-Seal™ (25.9%; similar to the rate of 29% in our study) requiring bedside time and secondary MC balanced the effect of time saving to due AngioSeal™ deployment instead of MC []. However, in our patients oozing was only minimal and could be managed simply by application of a light bandage and thus did not require much bedside time of the interventional cardiologist. The economic benefit of outpatient elective PCI only becomes apparent when the socioeconomic perspectives and the insurance system of each country are taken into account. Consequently, we calculated the direct institutional costs for MC and the Angio-Seal™ device, showing cost savings for the institution when the device was used. The full economic benefit, eg due to earlier return to work, may be substantially larger however.
In addition to efficacy and cost-effectiveness the patient’s comfort is a major issue to look for. Several studies evaluated patient satisfaction in patients receiving the AngioSeal™ device compared to MC. Duffin et al. reported a significantly higher patient satisfaction and a significantly lower level of discomfort when using the Angio-Seal™ device as compared to MC and the Perclose device, a suture-mediated closure device []. Patients also uniformly reported preference for AngioSeal™ if they had had previous experience with the other two approaches. The reduced patient discomfort with the Angio-Seal™ compared to MC reported in our study is similar to the findings of Juergens et al., who by comparing the Angio-Seal™ to Femostop-assisted MC found a higher pain score 4 and 8 hours after PCI as well as a higher maximal pain level in patients treated with the Angio-Seal™ as compared to MC [].
The collagen-based Angio-Seal™ device has been found to be equivalent to the suture based Perclose system with respect to vascular complications []. The latter device was also subject of a cost-effectiveness study and proved to reduce the post-procedural costs of PCI by 13% compared to MC []. In comparison, we found that use of Angio-Seal™ was associated with a reduction in costs of 54%. In a pilot study, Wilentz et al. were the first to assess the feasibility of outpatient stenting via the femoral approach using mainly the VasoSeal™ device to close the access site []. Even though there was no control group, the procedure was found to be safe and cost savings were estimated to be between $400 and $500. However, the results of a recent meta analysis suggest a higher rate of vascular complications in the setting of PCI when using the VasoSeal™ device as compared to MC []. Therefore, either the Perclose or the AngioSeal™ device seem to preferable since the have been shown to be both safe and cost-effective.
Study Limitations
Although patients were ambulated and encouraged to move around, they were not effectively discharged from the hospital in order to closely assess the safety and efficacy of the device by clinical examination and ultrasound surveillance. We determined that all patients in this study could have been discharged on the day of the procedure, whereas this was not possible in the MC patients due to management of the access site. In the MC group, delayed sheath removal and compression by the interventional cardiologist him-self is a conservative approach, but corresponds to the current practice in many Swiss centres, whereas early sheet removal and MC done by a nurse is obviously frequent in the US. The latter practice could have changed the results, but it would not have been a real setting in Switzerland. We are aware that our study is limited by small numbers, but we feel that our results demonstrate the feasibility and potential cost-savings of same-day discharge with the use of the Angio-Seal™ device in the Swiss setting. Our cost analysis is derived from Swiss treatment patterns and Swiss cost structures. National cost studies are difficult to transfer to other countries with different health care systems. However, separate studies from the United States and Canada determined hospitalisation time to be a major cost factor in percutaneous coronary interventions [,,]. Therefore, it is likely that cost savings due to shortened hospital stay, as reported in this study, are applicable to other first-world countries.
Finally, it should be acknowledged that the size of this study did not allow for the careful evaluation of very rare and potentially severe complications. Treatment of such complications could increase the over-all costs. Thus the results of this study need to be verified with larger, maybe even multicenter studies.
Conclusion
Compared to MC the use of the Angio-Seal™ is associated with cost saving due to shorter time to haemostasis and thus earlier ambulation and hospital discharge, as well de-creased personnel and infrastructural demands. In addition, the use of Angio-Seal™ is safe and increases patient comfort
Acknowledgments
We would like to thank Michael Braendle, MD, MPH, Department of Internal Medicine, Division of Cardiology, Kantonsspital St. Gallen, Switzerland, for critical review of the manuscript.
References
- Cohen, D.J. Outpatient transradial coronary stenting: implications for cost-effectiveness. J Invas Cardiol 1996, (Suppl. D), 36–9D. [Google Scholar]
- Kiemeneij, F.; Laarman, G.J.; Slagboom, T.; Van der Wieken, R. Outpatient coronary stent implantation. J Am Coll Cardiol 1997, 29, 323–7. [Google Scholar] [CrossRef]
- Mann, T.; Cowper, P.A.; Peterson, E.D.; Cubeddu, G.; Bowen, J.; Giron, L.; et al. Transradial coronary stenting: comparison with femoral access closed with an arterial suture device. Catheter Cardiovasc Interv 2000, 49, 150–6. [Google Scholar] [CrossRef]
- Baim, D.S.; Knopf, W.D.; Hinohara, T.; Schwarten, D.E.; Schatz, R.; Pinkterton, C.A.; et al. Suture-mediated closure of the femoral access site after cardiac catheterization: results of the suture to ambulate and discharge (STAND I and STAND II) trials. Am J Cardiol 2000, 85, 864–9. [Google Scholar] [CrossRef] [PubMed]
- Eggebrecht, H.; Haude, M.; Von Birgelen, C.; Woertgen, U.; Schmermund, A.; Baumgart, D.; et al. Early clinical experience with the 6 french Angio-Seal™ device: immediate closure of femoral puncture sites after diagnostic and interventional coronary procedures. Catheter Cardiovasc Interv 2001, 53, 437–42. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, E.K.; Bloch, R.D. Percutaneous arterial closure devices. J Vasc Interv Radiol 2003, 14, 865–85. [Google Scholar] [CrossRef]
- Rickli, H.; Unterweger, M.; Sütsch, G.; Brunner-La Rocca, H.P.; Sagmeister, M.; Ammann, P.; et al. Comparison of costs and safety of a suture-mediated closure device with conventional manual compression after coronary artery interventions. Catheter Cardiovasc Interv 2002, 57, 297–302. [Google Scholar] [CrossRef]
- Kussmaul, W.G.; Buchbinder, M.; Whitlow, P.L.; Aker, U.T.; Heuser, R.R.; King, S.B.; et al. Rapid arterial hemostasis and decreased access site complications after cardiac catheterization and angioplasty: results of a randomized trial of a novel hemostatic device. J Am Coll Cardiol 1995, 25, 1685–92. [Google Scholar] [CrossRef]
- Ward, S.; Casale, P.; Raymond, R.; Kussmaul, W.G.; Simpendorfer, C. Efficacy and safety of a hemostatic puncture closure device with early ambulation after coronary angiography. Am J Cardiol 1998, 81, 569–72. [Google Scholar] [CrossRef]
- Kapadia, S.R.; Raymond, R.; Knopf, W.; Jenkins, S.; Chapekis, A.; Ansel, G.; et al. The 6 Fr Angio-Seal arterial closure Device: results from a multimember prospective registry. Am J Cardiol 2001, 87, 789–91. [Google Scholar] [CrossRef]
- Juergens, C.P.; Leung, D.Y.C.; Crozier, J.A.; Wong, A.M.; Robinson, J.T.; Lo, S.; et al. Patient tolerance and resource utilization associated with an arterial closure versus an external compression device after percutaneous coronary intervention. Catheter Cardiovasc Interv 2004, 63, 166–70. [Google Scholar] [CrossRef]
- Sanborn, T.; Ogilby, J.D.; Ritter, J.M.; Stone, G.W.; Klugherz, B.D.; Fields, R.H.; et al. Reduced vascular complications after percutaneous coronary interventions with a nonmechanical suture device: results from the randomized RACE study. Catheter Cardiovasc Interv 2004, 61, 327–32. [Google Scholar] [CrossRef]
- Sesana, M.; Vaghetti, M.; Albiero, R.; Corvaja, N.; Martini, G.; Sivieri, G.; et al. Effectiveness and complications of vascular access closure devices after interventional procedures. J Invas Cardiol 2000, 12, 395–9. [Google Scholar]
- Cohen, D.J. Understanding and evaluating the cost-effectiveness of new coronary interventions. J Invas Cardiol 1997, (Suppl. D), 24–7D. [Google Scholar]
- Nikolsky, E.; Mehran, R.; Halkin, A.; Aymong, E.D.; Mintz, G.S.; Lasic, Z.; et al. Vascular complications associated with arteriotomy closure devices in patients undergoing percutaneous coronary procedures. J Am Coll Cardiol 2004, 44, 1200–9. [Google Scholar] [CrossRef] [PubMed]
- Resnic, F.S.; Blake, G.J.; Ohno-Machado, L.; Selwyn, A.P.; Popma, J.J.; Rogers, C. Vascular closure devices and the risk of vascular complications after percutaneous coronary intervention in patients receiving glycoprotein IIb/IIIa inhibitors. Am J Cardiol 2001, 88, 493–6. [Google Scholar] [CrossRef] [PubMed]
- Boccalandro, F.; Assali, A.; Fujse, K.; Smalling, R.W.; Sdringola, S. Vascular access site complications with the use of closure devices in patients treated with platelet glycoprotein IIb/IIIa inhibitors during rescue angioplasty. Catheter Cardiovasc Interv 2004, 63, 284–9. [Google Scholar] [CrossRef]
- Lauer, M.A.; Karweit, J.A.; Cascade, E.F.; Lin, N.D.; Topol, E.J. Practice patterns and outcomes of percutaneous coronary interventions in the United States: 1995 to 1997. Am J Cardiol 2002, 89, 924–9. [Google Scholar] [CrossRef]
- Lincoff, A.M.; Tcheng, J.E.; Califf, R.M.; Bass, T.; Popma, J.J.; Teirstein, P.S.; et al. Standard versus low-dose weight-adjusted heparin in patients treated with the platelet glycoprotein IIb/IIIa receptor antibody fragment Abciximab (c7E3Fab) during percutaneous coronary revascularization. PROLOG Investigators. Am J Cardiol 1997, 79, 286–91. [Google Scholar] [CrossRef]
- Mandak, J.S.; Blankenship, J.C.; Gardner, L.H.; Berkowitz, S.D.; Aguirre, F.V.; Sigmon, K.N.; et al. Modifiable risk factors for vascular access site complications in the IMPACT II trial of angioplasty with versus without eptifibatide. Integrilin to Minimize Platelet Aggregation and Coronary Thrombosis. J Am Coll Cardiol 1998, 31, 1518–24. [Google Scholar] [CrossRef]
- Chevalier, B.; Lancelin, B.; Koning, R.; Henry, M.; Gommeaux, M.; Pilliere, R.; et al. HEMOSTASE Trial Investigators. Effect of a closure device on complication rates in high-local-risk patients: results of a randomized multicenter trial. Catheter Cardiovasc Interv 2003, 55, 285–91. [Google Scholar]
- Khatri, S.; Webb, J.G.; Carere, R.G.; Amis, A.; Woolcott, J.; Chugh, S.; et al. Safety and cost benefit of same-day discharge after percutaneous coronary intervention. Am J Cardiol 2002, 90, 425–7. [Google Scholar] [CrossRef]
- Dalby, M.; Davies, J.; Rakhit, R.; Mayet, J.; Foale, R.; Davies, D.W. Feasibility and safety of day-case transfemoral coronary stenting. Catheter Cardiovasc Interv 2003, 60, 18–24. [Google Scholar] [CrossRef]
- Yee, K.M.; Lazzam, C.; Richards, J.; Ross, J.; Seidelin, P.H. Sameday discharge after coronary stenting: a feasibility study using a hemostatic femoral puncture closure device. J Interv Cardiol 2004, 17, 315–320. [Google Scholar] [CrossRef]
- Duffin, D.C.; Muhlestein, J.B.; Allisson, S.B.; Horne, B.D.; Fowles, R.E.; Sorensen, S.G.; et al. Femoral arterial puncture management after percutaneous coronary procedures: a comparison of clinical outcomes and patient satisfaction between manual compression and two different vascular closure devices. J Invas Cardiol 2001, 13, 354–62. [Google Scholar]
- Wilentz, J.R.; Mishkel, G.; McDermott, D.; Ravi, K.; Fox, J.T.; Reimers, S.D.; et al. Outpatient coronary stenting: femoral approach with vascular sealing. Herz 1999, 24, 624–33. [Google Scholar] [CrossRef]
© 2006 by the authors. Attribution - Non-Commercial - NoDerivatives 4.0.