Abstract
Anaemia and iron deficiency are common co-morbidities in chronic heart failure (CHF) patients and are both independently associated with poor outcomes. Their cause in heart failure is multifactorial with contributing factors including proinflammatory cytokines, renal dysfunction, haemodilution, intestinal malabsorption and heart failure medications. Since anaemia in heart failure is considered an anaemia of chronic disease, its treatment remains a clinical challenge. While the long-term safety and risk/benefit ratio of chronic erythropoietin (EPO) substitution is highly debated, correction of iron deficiency in CHF patients with or without anaemia was shown to be safe and efficacious in improving exercise capacity and heart failure symptoms. This review focuses on the pathophysiology, diagnosis and clinical consequences of anaemia and iron deficiency in CHF and discusses what treatment options have been explored in clinical trials.
Keywords:
heart failure, anaemia, iron deficiency Prevalence of anaemia and iron deficiency in chronic heart failure
Chronic heart failure (CHF) is a systemic condition that affects multiple organ systems and metabolic pathways. In patients with CHF, impairment of kidney function, bone marrow depression, inhibition and resistance to EPO as well as changes in iron metabolism frequently result in anaemia [1]. If defined according to the World Health Organization (WHO) criteria as a haemoglobin (Hb) concentration <12 g/dl in women and <13 g/dl in men, the prevalence of anaemia in heart failure is estimated in the range of 25–50%, with lower prevalence in mild CHF and higher prevalence in severe CHF [2,3]. Beside more advanced NYHA class, factors associated with anaemia include older age, chronic kidney disease and diabetes mellitus. Interestingly, the prevalence of anaemia is similar regardless of the underlying condition of heart failure, i.e., with or without preserved systolic function.
In the majority of cases, anaemia in heart failure is associated with iron deficiency, either in the context of anaemia of chronic disease (functional iron deficiency) or as a consequence of depletion of iron stores (absolute iron deficiency). Bone marrow biopsies revealed that iron deficiency was present in 73% of anaemic patients admitted to the hospital because of advanced heart failure (NYHA IV) [4]. In a prospective observational study including less symptomatic CHF patients the prevalence of iron deficiency, defined as ferritin <100 µg/l, or 100–300 µg/l with transferrin saturation <20%, was 57% in anaemic and 32% in non-anaemic patients. In this study, iron deficiency was independently associated with female gender, advanced NYHA class, high plasma NT-proBNP, and high serum hs-C-reactive protein [5].
The economic burden of anaemia in heart failure
There are several studies implicating anaemia in higher health care expenditures in patients with heart failure. Patients with anaemia have longer hospital stays and generate higher costs during their hospitalisation [6]. A retrospective analysis in Medicare patients with heart failure estimated the per-member-per-month costs being 25% higher in anaemic patients after adjustment for baseline demographic factors and comorbidities compared to non-anaemic patients [7].
Mechanisms of anaemia and iron deficiency in CHF
As in other chronic diseases, anaemia in CHF must primarily be considered as an indicator of overall illness. Its pathophysiology is complex and multifactorial (Figure 1. The factors that cause anaemia in CHF patients are subsequently listed by order of their probable contribution.
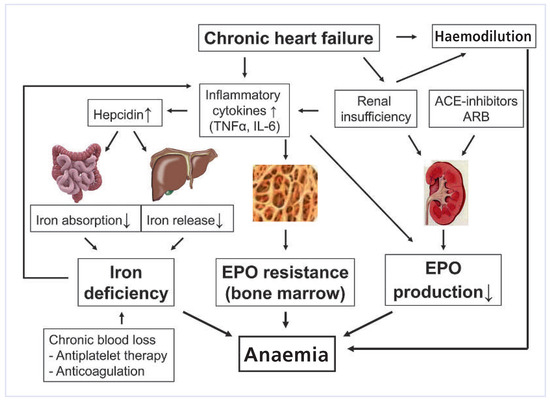
Figure 1.
Pathophysiology of anaemia in CHF.
Inflammatory cytokines
CHF is a chronic inflammatory state with increased levels of tumour necrosis factor (TNF)-α and interleukin (IL)-6. These inflammatory cytokines reduce EPO production in the kidneys and attenuate EPO sensitivity of the bone marrow and therefore are responsible for a blunted EPO production and EPO resistance observed in CHF patients. In addition, IL-6 increases the production and release of hepcidin from the liver. Via inhibition of ferroportin, hepcidin blocks gastrointestinal iron absorption and the release of iron from its storage in macrophages and hepatocytes. Thus, hepcidin reduces the net iron availability to the bone marrow, thereby resulting in iron deficiency anaemia, even in the presence of adequate total iron stores. This type of iron deficiency, in which inflammatory cytokines block the release of iron from its stores, is referred to as “functional iron deficiency” and is typically present in anaemia of chronic disease. There seems to be a vicious circle between inflammatory cytokines and iron levels. Not only do inflammatory cytokines reduce iron levels but reduced iron levels also increase inflammatory cytokines [8]. From the inflammatory perspective, anaemia in CHF is similar to the one seen in other chronic diseases (e.g., inflammatory bowel disease, chronic rheumatoid diseases) that are associated with elevated inflammatory cytokines.
Chronic kidney disease (CKD)
Impaired renal function is a predominant problem in patients with CHF. Besides common cardiovascular risk factors for CHF and CKD (e.g., hypertension, diabetes), CKD in CHF is mainly the result of chronically reduced renal perfusion caused by low cardiac output and enhanced vasoconstriction. Renal hypoperfusion increases EPO production in the kidneys. EPO serum levels are therefore frequently elevated in patients with CHF and are inversely correlated with prognosis [9]. If corrected to the Hb levels, however, EPO levels are inappropriately low in CHF patients (blunted EPO production) and the response of hematopoietic cells to EPO is attenuated (EPO resistance) due to elevated inflammatory cytokines. The prevalence of anaemia begins to rise as glomerular filtration rate falls below 60 ml/min/1.73 m2. This interrelationship between cardiac function, renal function and anaemia has been referred to as the “cardiorenal anaemia syndrome”.
Haemodilution
Haemodilution is a contributing factor to anaemia in most patients with CHF. However, it is of note that despite the presence of haemodilution, a true red cell deficit is found in the majority of anaemic CHF patients [10].
Angiotensin converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARB)
Because angiotensin II is a stimulator of erythropoiesis and EPO, inhibition of the effects of angiotensin II by these agents results in inhibition of EPO synthesis and facilitates the development of anaemia [11]. In general, however, the effects of ACEI and ARB on haematocrit are not clinically significant.
Malabsorption
Patients with CHF often have altered intestinal function including redistribution of blood away from the splanchnic region and an increased bowel wall thickness. Both aspects are likely to negatively influence iron absorption in the gut.
Prognostic impact of anaemia and iron deficiency in CHF
Findings from multiple heart failure trials and cohorts consistently established anaemia as an independent risk factor for reduced exercise tolerance, low quality of life, heart failure hospitalisations and all cause mortality. Data from the COMET trial have shown that the risk of hospitalisation for heart failure was increased by 43% in anaemic patients compared to non-anaemic patients (p <0.0001) [12]. Similarly, analysis of data from the COMET and CHARM trial programmes revealed an inverse and linear association between Hb values and the risk of death [3]. It is of note that even relatively mild degrees of anaemia, that may not be considered significant in clinical practice, confer increased morbidity and mortality. In a study by Horwich et al., significantly impaired survival was already seen in women with Hb <11.6 g/dl and men with Hb <12.6 g/dl [13]. In this study, each 1g/dl decrease in Hb was associated with a 16% increase in mortality. The relationship between Hb and the cause of death was also investigated, revealing that Hb was a significant predictor of progressive heart failure death, but not sudden death.
It has been a subject of debate as to what extent iron deficiency might be a risk factor for morbidity and mortality in CHF patients. As a component of haemoglobin, iron plays a pivotal role in oxygen transport. In addition, iron is a component of oxidative enzymes and respiratory chain proteins and thus plays a role in the oxidative metabolism of skeletal muscle and the myocardium. Given its central role in oxygen generation and delivery, it is tentative to speculate that lack of iron may result in poor exercise tolerance in patients with CHF. Recently, Jankowska et al. provided first evidence that iron deficiency independently of anaemia might exert detrimental effects on the prognosis of patients with CHF. In this study, including patients with systolic CHF the presence of iron deficiency was associated with a 12% increase in mortality within three years of follow up (p = 0.0006) [5].
Diagnosis of anaemia in CHF
Given the impact of anemia on morbidity and mortality, screening for anemia should be performed in all patients with CHF as recommended by the latest guidelines of the European Society of Cardiology (ESC) [14]. If anaemia is present (Hb <12 g/dl in women, Hb <13 g/dl in men) a routine evaluation of classical causes should be performed (Figure 2). Starting from the mean corpuscular volume (MCV) of erythrocytes, differentiation of microcytic, normocytic and macrocytic anaemia is made. The presence of microcytosis or normocytosis should prompt evaluation of iron stores (ferritin). If macrocytosis is present, vitamin B12 and folic acid should be assessed. The reticulocyte count helps to distinguish hyporegenerative anaemia (e.g., renal anaemia, anaemia of chronic disease, myelodysplastic syndrome) from hyperregenerative anaemia (haemolysis, blood loss).
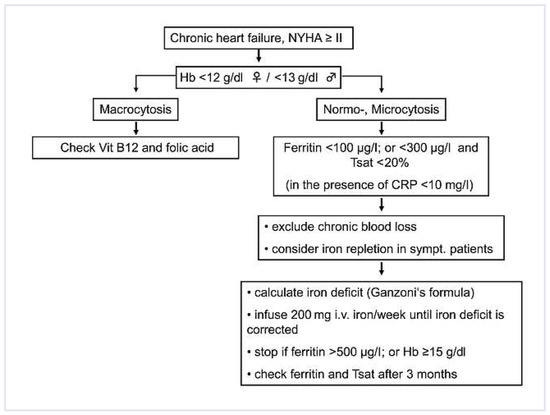
Figure 2.
Management of anaemia and iron deficiency in CHF.
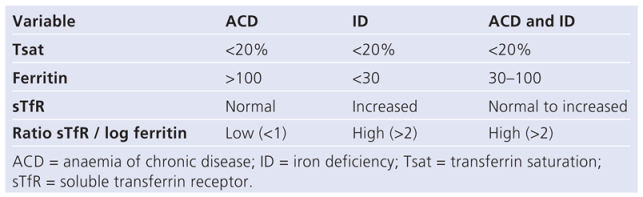
In patients with CHF the predominant form of anaemia is anaemia of chronic disease (60%), followed by iron deficiency anaemia (21%) [15]. Anaemia of chronic disease cannot be diagnosed by direct means, but it is postulated if no other specific underlying cause of anaemia can be identified. The distinction between anaemia of chronic disease with or without iron deficiency versus pure iron deficiency is made using the ratio between the soluble transferrin receptor (sTfR) and log ferritin as shown in Table 1. In general, any unexplained iron deficiency should be regarded as a sign of chronic bleeding and should trigger screening for occult gastrointestinal blood loss. Anaemia due to vitamin B12 or folic acid deficiency is of secondary importance in patients with CHF.

Table 1.
Distinction between ACD und ID (adapted from [33]).
Diagnosis of iron deficiency in CHF
The evaluation of iron stores and availability is made by determination of plasma ferritin, transferrin saturation, and sTfR. Being an acute phase protein, ferritin is increased in the presence of inflammation. Therefore, any inflammatory state, including CHF, will increase ferritin. This has to be taken into account when interpreting ferritin levels in CHF patients. While in non-CHF patients ferritin levels ≤30 µg/l are diagnostic for iron deficiency, in CHF patients ferritin levels <100 µg/l are indicative of iron deficiency [16]. Taking into consideration the diagnostic approaches towards iron deficiency in other chronic diseases (e.g., CKD, inflammatory bowel disease, cancer) iron deficiency in CHF was defined as follows: Ferritin <100 µg/l (absolute iron deficiency) or ferritin 100–300 µg/l and transferrin saturation <20% (functional iron deficiency) (Figure 2) [16]. Given the pivotal role of hepcidin in the pathophysiology of functional iron deficiency, measurement of hepcidin would be of additional value. However, to date, there are no reliable, standardised hepcidin assays available.
Treatment of anaemia in CHF
Although the latest ESC Guidelines recommend screening for anaemia in patients with CHF [14], correction of anaemia has not yet been established as routine therapy in CHF. The fact that anaemia in CHF is mainly caused by inflammation makes its treatment a clinical challenge. Because inflammatory processes interfere both with the production of EPO and the delivery of iron to the bone marrow, protocols for the treatment of anaemia in CHF have focused primarily on the chronic substitution of erythropoiesis-stimulating agents (ESA’s) and the administration of intravenous (IV) iron.
Erythropoiesis-stimulating agents (ESA’s)
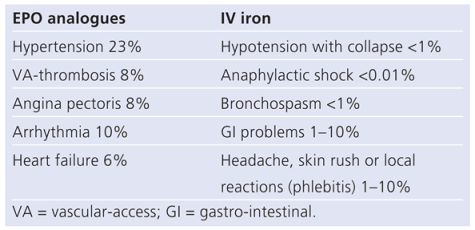
Preliminary, single-centre, open-label studies have shown that treatment of anaemia with erythropoiesis-stimulating agents (ESAs) may improve exercise capacity and reduce the need for hospitalisation in patients with CHF. However, the largest randomised, double-blinded, placebo-controlled study to date, the study of anaemia in heart failure trial (STAMINA-HeFT), failed to reproduce these findings [17]. In STAMINA-HeFT administration of the EPO derivate darbepoietin-alpha every two weeks for one year did not result in any benefit in terms of exercise tolerance, NYHA class or quality of life. There was, however, a trend towards lower risk of all-cause mortality and first HF hospitalisation in the darbepoietin-treated group compared to the placebo group. A recent metaanalysis of randomised controlled trials including a total of 650 patients also pointed towards a reduction in HF hospitalisation (relative risk = 0.59; p = 0.006) in patients treated with ESA’s compared to standard heart failure treatment [18]. In recent years, concerns have emerged regarding the safety of chronic ESA treatment in patients with cardiovascular diseases. Chronic ESA treatment aggravates hypertension and may increase the risk of thromboembolism and acute coronary syndrome (Table 2). Two studies in patients with chronic kidney disease (CHOIR, CREATE), in which EPO was administrated to achieve either a higher (up to 15.0 g/dl) or a lower (up to 11.5 g/dl) target Hb showed that a higher Hb level may be associated with increased risk of morbidity (i.e., cardiovascular events, hypertension, thromboembolism) and mortality [19,20]. Moreover, ESA treatment was associated with worse outcomes in patients with cancer [21]. The results of the recently completed TREAT study, which randomised 4,044 patients with type 2 diabetes, CKD, and anaemia to treatment with darbepoietin-alpha or placebo with a target Hb of 13.0 mg/dl was neutral in terms of overall mortality but revealed an excess rate of stroke in the darbepoietin treated group (101 versus 53) [22]. In TREAT, 1347 participants were reported to have a history of heart failure, many more than randomised in prior trials investigating the effects of ESA’s in patients with CHF. When these patient data are included in a new meta-analysis together with data from the afore-mentioned meta-analysis (total of 2039 patients), the use of ESA’s to manage anaemia in patients with CHF was associated with a neutral effect on both mortality and HF hospitalisations [23]. Because these meta-analyses included very heterogeneous trials with variable definitions of heart failure and variable follow-up duration they have important limitations. Therefore, to date, there is insufficient data to conclude the value of ESA’s in the treatment of anaemia in CHF patients and thus such therapy cannot be recommended in routine clinical practice. Hopefully, the results of the ongoing RED-HF trial that randomises 2600 anaemic CHF patients to darbepoetin or placebo will provide conclusive evidence on the role of ESA’s in anaemia of heart failure [24]. The results of RED-HF are expected to be available in 2012.

Table 2.
Side effects of EPO analogues and IV iron (after: Arzneimittel-Kompendium der Schweiz 2010).
Iron substitution
Oral iron
Although oral iron substitution is common clinical practice for decades, it is not suitable for all patients. For instance, in patients with anaemia of chronic disease, oral iron is poorly absorbed and fails to efficiently replenish iron stores as measured by serum ferritin and transferrin saturation [25]. Further, in patients with heart failure, oedema of the gastrointestinal mucosa aggravates gastrointestinal malabsorption and the frequent occurrence of gastrointestinal side effects may lead to premature discontinuation of the treatment.
Intravenous iron
In contrast, IV iron therapy bypasses the issues of malabsorption and the restrictions of iron release imposed by hepcidin, thereby efficiently providing extra iron to the bone marrow. Compared to oral iron substitution, IV iron therapy proved more effective in improving haematologic and iron parameters, as well as quality of life in patients with iron deficiency syndromes [25]. Common side effects of IV iron include gastrointestinal symptoms and injection side irritations. Serious adverse reactions such as anaphylactic shock or symptomatic hypotension are rare with modern-day dextran-free iron preparations (Table 2).
At present, the safety and efficacy of IV iron administration for the treatment of iron deficient anaemia in CHF patients was evaluated in six studies [16,26,27,28,29,30], two of which were double-blind placebo-controlled by design [16,27]. In these trials, iron deficiency was defined by ferritin levels and transferrin saturation as mentioned above (see diagnosis of iron deficiency) and IV iron was administrated in the form of iron sucrose or iron carboxymaltose. Both formulations were well tolerated and there was no evidence of serious adverse effects of the IV iron treatment compared to placebo. In all except one [28] of these studies, anaemic CHF patients exhibited a significant increase in Hb levels. The magnitude of Hb increase across these studies seems dependent on the total iron dose administered, suggesting a possible dose-response relationship. In addition to Hb correction, IV iron administration resulted in significant improvement in NYHA class [16,26,27,28,29], exercise tolerance and quality of life [16,26,27,28], LVEF [27,29], N-terminal pro BNP [27] and renal function [16]. There was also a trend to less cardiovascular events, including HF hospitalisations in patients treated with IV iron compared to the placebo group [16,27]. Interestingly, clinical improvements occurred rapidly within the first month of treatment. Subgroup analyses demonstrated that not only anaemic patients benefited from IV iron but also iron deficient patients without anaemia, suggesting that part of the treatment efficacy is Hb-independent. Taken together, these studies demonstrate that anaemia in heart failure patients can be improved by IV iron alone if iron deficiency is present. The consistent observation that correction of iron deficiency in CHF results in improvements of symptoms and exercise capacity even in the absence of anaemia defines iron deficiency as a novel therapeutic target in CHF. However, it is worth mentioning that excessive iron may promote free radical formation via the Fenton reaction leading to cell and tissue damage. Moreover, increased iron stores are associated with endothelial dysfunction via inhibition of nitric oxide signalling [31] and related to the risk of carotid atherosclerosis [32]. Therefore, it is crucial to correctly estimate the degree of iron deficiency to avoid overload.
Despite the strong association of anaemia with mortality in heart failure, evidence that correction of anaemia improves survival in these patients is still missing. Besides the lack of adequately powered studies, this might be explained by the complex biology of the heart failure syndrome with anaemia mirroring the severity of the disease rather than directly contributing to mortality.
Recommendations
Recommendations on how to manage anaemia and iron deficiency in patients with CHF are depicted in a flow chart in Figure 2. It is reasonable to monitor Hb levels and to check for iron deficiency in patients with CHF on an annual basis. At present, the use of ESA’s is not recommended to treat anaemia in heart failure patients unless there is a nephrologic indication (moderate to severe CKD with a target Hb level of 11 g/dl). If ESA’s are indicated, they should only be administered with concomitant IV iron substitution. If unexplained iron deficiency is present in CHF patients, occult gastrointestinal blood loss has to be excluded. There are currently no guidelines with respect to the beginning and the end of IV iron substitution in CHF patients with iron deficiency. According to the available trial data, we recommend considering IV iron substitution if ferritin is <100 µg/l or <300 µg/l with concomitant transferrin saturation <20% in symptomatic heart failure patients. In this setting IV iron substitution might improve exercise tolerance and quality of life. In order to prevent potentially deleterious iatrogenic iron overload, it is of utmost importance to calculate the total iron dose required to restore iron stores. The total cumulative iron dose is calculated according to Ganzoni’s formula (Table 3). Except for a history of allergic reaction to components of the iron preparation used and the absence of iron deficiency, there are no contraindications to IV iron therapy. Regarding the frequency of administration, most clinical trials with CHF patients pursued a prudent approach administering only 200 mg iron/week until reaching the total cumulative dose [16,27,28]. There is still a need for further clinical trials showing the safety and efficacy of higher single doses of intravenous iron preparations in patients with CHF. Iron therapy should be stopped if ferritin levels exceed 500 µg/l or Hb levels reach 15 g/dl. Because serum ferritin levels are not representative of total iron stores within the first 3 months after IV iron administration, serum ferritin and transferrin saturation should only be measured at least 3 months after the last iron administration in order to document successful replenishment of iron stores.

Table 3.
Estimation of iron deficit (Ganzoni’s formula).
Conclusion
Anaemia and iron deficiency are prevalent in patients with CHF and are independently associated with higher morbidity and mortality. There is a growing body of evidence that IV iron substitution may have a significant impact on morbidity in CHF patients with absolute or relative iron deficiency. Thus, it will be important for the future to better define the role of iron deficiency in heart failure, both on an epidemiological and mechanistic level. Clearly, long-term, adequately powered, placebo controlled studies of IV iron treatment in CHF using hard endpoints such as HF hospitalisation and survival are needed. In the meantime, given the established safety profile of today’s available IV iron preparations, IV iron substitution can be considered to improve exercise tolerance and symptoms in symptomatic CHF patients with iron deficiency with or without anaemia.
Funding
The authors received consulting fees from Vifor Pharma.
Acknowledgments
We thank Dr. David Thorn for the careful reading of the manuscript.
References
- Kazory, A.; Ross, E.A. Anemia: The point of convergence or divergence for kidney disease and heart failure? J Am Coll Cardiol. 2009, 53, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N.; et al. Anemia as a risk factor for kidney function decline in individuals with heart failure. Am J Cardiol. 2007, 99, 1137–1142. [Google Scholar] [CrossRef]
- O’Meara, E.; et al. Clinical correlates and consequences of anemia in a broad spectrum of patients with heart failure: Results of the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Program. Circulation. 2006, 113, 986–994. [Google Scholar]
- Nanas, J.N.; et al. Etiology of anemia in patients with advanced heart failure. J Am Coll Cardiol. 2006, 48, 2485–2489. [Google Scholar]
- Jankowska, E.A.; et al. Iron deficiency: An ominous sign in patients with systolic chronic heart failure. Eur Heart J. 2012, 31, 1872–1880. [Google Scholar] [CrossRef]
- Nordyke, R.J.; et al. Impact of anemia on hospitalization time, charges, and mortality in patients with heart failure. Value Health. 2004, 7, 464–471. [Google Scholar] [CrossRef]
- Solid, C.A.; et al. Anemia and cost in Medicare patients with congestive heart failure. Congest Heart Fail. 2006, 12, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; et al. Effect of iron treatment on circulating cytokine levels in ESRD patients receiving recombinant human erythropoietin. Kidney Int. 2003, 64, 572–578. [Google Scholar] [CrossRef]
- Belonje, A.M.; et al. Endogenous erythropoietin and outcome in heart failure. Circulation. 2010, 121, 245–251. [Google Scholar] [PubMed]
- Abramov, D.; et al. Comparison of blood volume characteristics in anemic patients with low versus preserved left ventricular ejection fractions. Am J Cardiol. 2008, 102, 1069–1072. [Google Scholar] [CrossRef]
- Sica, D.S. Pharmacotherapy in congestive heart failure: ACE inhibitors and anemia in congestive heart failure. Congest Heart Fail. 2000, 6, 330–332. [Google Scholar] [CrossRef] [PubMed]
- Komajda, M.; et al. The impact of new onset anaemia on morbidity and mortality in chronic heart failure: Results from COMET. Eur Heart J. 2006, 27, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Horwich, T.B.; et al. Anemia is associated with worse symptoms, greater impairment in functional capacity and a significant increase in mortality in patients with advanced heart failure. J Am Coll Cardiol. 2002, 39, 780–786. [Google Scholar]
- Dickstein, K.; et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J. 2008, 29, 2388–2442. [Google Scholar]
- Ezekowitz, J.A.; McAlister, F.A.; Armstrong, P.W. Anemia is common in heart failure and is associated with poor outcomes: Insights from a cohort of 12 065 patients with new-onset heart failure. Circulation. 2003, 107, 223–225. [Google Scholar] [CrossRef]
- Anker, S.D.; et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009, 361, 2436–2448. [Google Scholar] [CrossRef]
- Ghali, J.K.; et al. Randomized double-blind trial of darbepoetin alfa in patients with symptomatic heart failure and anemia. Circulation. 2008, 117, 526–535. [Google Scholar] [CrossRef]
- van der Meer, P.; et al. Erythropoietin treatment in patients with chronic heart failure: A meta-analysis. Heart. 2009, 95, 1309–1314. [Google Scholar]
- Singh, A.K.; et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006, 355, 2085–2098. [Google Scholar]
- Drueke, T.B.; et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006, 355, 2071–2084. [Google Scholar]
- Rizzo, J.D.; et al. American Society of Hematology/American Society of Clinical Oncology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. Blood. 2010, 116, 4045–4059. [Google Scholar] [CrossRef]
- Solomon, S.D.; et al. Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N Engl J Med. 2010, 363, 1146–1155. [Google Scholar] [CrossRef]
- Desai, A.; et al. Impact of erythropoiesis-stimulating agents on morbidity and mortality in patients with heart failure: An updated, post-TREAT meta-analysis. Eur J Heart Fail. 2010, 12, 936–942. [Google Scholar] [CrossRef]
- McMurray, J.J.; et al. Design of the Reduction of Events with Darbepoetin alfa in Heart Failure (RED-HF): A Phase III, anaemia correction, morbidity-mortality trial. Eur J Heart Fail. 2009, 11, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.A.; et al. Meta-analysis of efficacy and safety of intravenous ferric carboxymaltose (Ferinject) from clinical trial reports and published trial data. BMC Blood Disord. 2011, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.P.; et al. Intravenous iron alone for the treatment of anemia in patients with chronic heart failure. J Am Coll Cardiol. 2006, 48, 1225–1227. [Google Scholar] [CrossRef] [PubMed]
- Toblli, J.E.; et al. Intravenous iron reduces NT-pro-brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J Am Coll Cardiol. 2007, 50, 1657–1665. [Google Scholar] [CrossRef]
- Okonko, D.O.; et al. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC-HF: A randomized, controlled, observer-blinded trial. J Am Coll Cardiol. 2008, 51, 103–112. [Google Scholar] [CrossRef]
- Usmanov, R.I.; et al. Intravenous iron without erythropoietin for the treatment of iron deficiency anemia in patients with moderate to severe congestive heart failure and chronic kidney insufficiency. J Nephrol. 2008, 21, 236–242. [Google Scholar]
- Drakos, S.G.; et al. Anemia in chronic heart failure. Congest Heart Fail. 2009, 15, 87–92. [Google Scholar] [CrossRef]
- Naito, Y.; et al. Dietary iron restriction prevents hypertensive cardiovascular remodeling in Dahl salt-sensitive rats. Hypertension. 2011, 57, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Kiechl, S.; et al. Body iron stores and the risk of carotid atherosclerosis: Prospective results from the Bruneck study. Circulation. 1997, 96, 3300–3307. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Goodnough, L.T. Anemia of chronic disease. N Engl J Med. 2005, 352, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
© 2012 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.