Case report
A 66-year-old woman was referred because of suspected acute coronary syndrome (ACS). On admission the patient was complaining of chest pain irradiating into her back, and she also had shortness of breath on minimal exertion and even at rest. Blood pressure was 134/89 mm Hg, pulse was regular (82 bpm). Physical examination revealed evidence of biventricular cardiac decompensation with lower leg edema, congested neck veins, and bibasilar crackles. Chest X-ray confirmed pulmonary venous hypertension. The ECG showed sinus rhythm and extensive pathological Q waves in leads II, III, and aVF as well as the praecordial leads V1–V4 (Figure 1). Plasma concentration of B-type natriuretic peptide was 3287 ng/l (normal <50 ng/l), and cardiac troponin I was mildly elevated (1.92 µg/l, normal <0.5 µg/l). There was evidence of renal failure (estimated glomerular filtration rate 35 ml/min/1.73 m2). The bedside echocardiogram (Figure 2) performed for triage of ACS revealed a normal-sized left ventricle with symmetrically and massively increased wall thickness (left ventricular end-diastolic diameter 43 mm, interventricular septum 28 mm, posterior wall 23 mm) with a speckled appearance of the myocardium, diffusely impaired contractility (left ventricular ejection fraction 35–40%, long axis shortening virtually absent, radial shortening preserved), a restrictive mitral inflow pattern with significantly reduced tissue velocities (peak early diastolic mitral annular velocity [e’] measured at the septal annulus 3 cm/s), biatrial dilatation, and a small pericardial effusion. Ultimately a diagnosis of AL-amyloidosis was obtained based on amyloid deposits in the abdominal fat pad fine needle aspirate and free light chains in serum. Bone marrow biopsy did not show evidence of multiple myeloma. Chemotherapy with alkeran, dexamethason, and thalidomide was initiated but the patient died six weeks after the initial presentation.
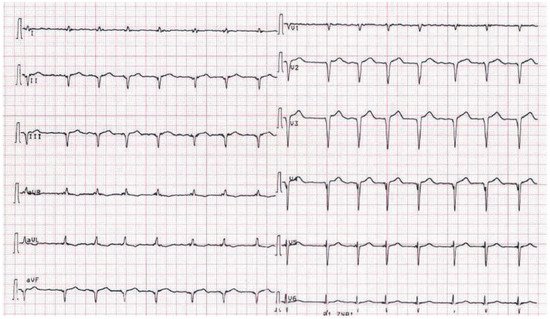
Figure 1.
ECG on admission showing sinus rhythm and Q waves in leads II, II, and aVF as well as V2–V4.
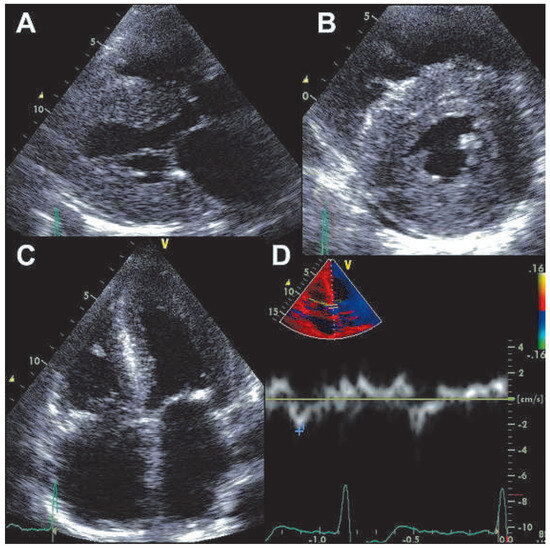
Figure 2.
Transthoracic echocardiogram. Panel A: parasternal long-axis view showing symmetrically increased left ventricular wall thickness and left atrial dilation. Panel B: parasternal short axis view showing symmetrically increased left ventricular wall thickness with concentric left ventricular geometry and small pericardial effusion. Panel C: apical four chamber view showing symmetrically increased left ventricular wall thickness and biatrial dilatation. Panel D: pulsed wave tissue Doppler recording at the septal mitral annulus showing significantly reduced peak early mitral annular velocity.
Discussion
The ECG in this patient showed pathological Q waves suggestive of previous inferior and anterior myocardial infarction. However, everything in the echocardiogram did not fit such a diagnosis but showed many very typical features of cardiac amyloidosis. A so-called “pseudoinfarction pattern”, i.e., the presence of pathological Q waves, typically in leads II, III, and aVF and V1–V4 without echocardiographic evidence of a previous myocardial infarction is a typical ECG feature in cardiac amyloidosis, which can be found in more than 50% of these patients (83% [1] and 60% [2] respectively in two large series). Although a pseudoinfarction pattern is not specific for cardiac amyloidosis [2], a pseudoinfarction pattern combined with a very typical echocardiogram allowed us to reach the diagnosis in the present case. Notably, chest pain is not rare in patients with cardiac amyloidosis [3]. It has been suggested that diffuse narrowing of the small coronary vessels due to amyloid deposition may result in the development of clinically significant myocardial ischaemia [3]. However, elevated cardiac troponin does not necessary indicate ACS in patients with amyloidosis but is a marker of a poor prognosis [4], possibly as an unspecific marker of ischaemic or non-ischaemic (amyloid infiltration) myocyte damage. In the present patient, coronary angiography was not performed due to the high risk of worsening of renal failure.
In summary, this case highlights that the ECG in cardiac amyloidosis may mimic previous myocardial infarction. A low threshold to perform echocardiography in patients with chest pain and possible ACS will usually help to differentiate the two entities without difficulties.
Funding/potential competing interests
No financial support and no other potential conflict of interest relevant to this article was reported.
References
- Roberts, W.C.; Waller, B.F. Cardiac amyloidosis causing cardiac dysfunction: analysis of 54 necropsy patients. Am J Cardiol. 1983, 52, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Rahman, J.E.; Helou, E.F.; Gelzer-Bell, R.; Thompson, R.E.; Kuo, C.; Rodriguez, R.; et al. Noninvasive diagnosis of biopsy-proven cardiac amyloidosis. J Am Coll Cardiol. 2004, 43, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Hongo, M.; Yamamoto, H.; Kohda, T.; Takeda, M.; Kinoshita, O.; Uchikawa, S.; et al. Comparison of electrocardiographic findings in patients with AL (primary) amyloidosis and in familial amyloid polyneuropathy and anginal pain and their relation to histopathologic findings. Am J Cardiol. 2000, 85, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Disperenzi, A.; Kyle, R.A.; Gertz, M.A.; Therneau, T.M.; Miller, W.L.; Chandrasekaran, K.; et al. Survival in patients with primary systemic amyloidosis and raised serum cardiac troponins. Lancet 2003, 361, 1787–1789. [Google Scholar] [CrossRef] [PubMed]
© 2012 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.