1. Case Report
A 79-year-old man was admitted to the hospital for dyspnoea and worsening lower leg edema. Nineteen years earlier, he had undergone aorto-coronary bypass surgery following two myocardial infarctions. Paroxysmal atrial fibrillation was treated with acenocoumaron. Twenty-four months earlier, he suffered from a deep vein thrombosis under oral anticoagulation (haemoglobin 204 g/L, haematocrit 60%, platelets 213 × 109/L, leucocytes 18.3 × 109/L) and thirteen months after that event, a spontaneous acute on chronic left-sided subdural haematoma with right-sided hemiplegia and aphasia (initial INR 1.8 under treatment with acenocoumaron) was diagnosed and neurosurgically evacuated. Oral anticoagulation was resumed thereafter. Prior to the admission he was treated with moxifloxacin for clinically diagnosed pneumonia by his general practitioner. On admission the patient was tachypnoeic with dyspnoea at rest and orthopnoea. Body temperature was normal. Haemoglobin was 173 g/L, haematocrit 60%, MCHC 29 g/dL, MCV 71 fl, erythrocytes 8.5 × 1012/L, platelets 168 × 109/L, and leucocytes 22.9 × 109/L. INR was 4.6, creatinine 95 μmol, urea 6.0 mmol/L and CRP 15 mg/L (<5 mg/L). Creatine kinase and troponin I were normal. BNP was elevated (421 pg/mL, reference ≤ 100 pg/mL). ECG revealed atrial fibrillation (ventricular rate around 120/min), incomplete right fascicular conduction block and no signs of acute myocardial infarction. A chest X-ray showed an enlarged cardiac silhouette, signs of acute decompensated heart failure, a right sided pleural effusion with a possible adjacent pneumonic infiltrate.
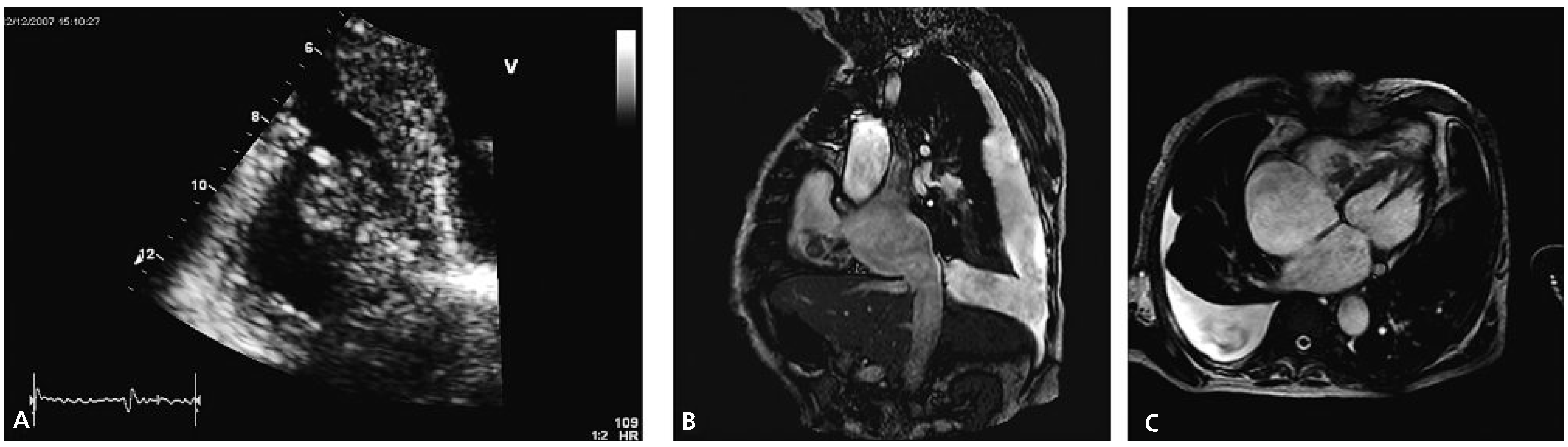
Transthoracic echocardiography revealed concentric left ventricular hypertrophy and a severely reduced global systolic left ventricular function (ejection fraction of 20–25%) due to inferior and posterior akinesia and substantial hypokinesia of the remaining wall segments. Both atria appeared dilated. The right ventricle was visually dilated with marked tricuspid annular dilation (50 mm) and functionally impaired (TAPSE 1.1 cm, TDI 10 cm/s). The aortic and mitral valves were remarkable for severe degenerative signs but besides a slight mitral regurgitation they were functionally normal. Attached to the septal leaflet of the tricuspid valve, an inhomogeneous structure partially prolapsing into the right atrium during systole was found (
Figure 1A), associated with visually mild tricuspid valve regurgitation. The pressure gradient across the tricuspid valve was 65 mm Hg suggesting severely elevated systolic pulmonary arterial pressure. A CT scan of the chest confirmed pulmonary emboli in the right main pulmonary artery and in a segmental artery of the left upper lobe.
Cardiac MRI confirmed cardiac thrombus since there was no uptake of contrast agent and no signs suggestive of a tumour (
Figure 1B,C). Antibiotics were stopped upon admission. Blood cultures remained negative and the CRP value remained low. Real-time PCR was positive for the V617F mutation of the JAK2-gene making the suspected diagnosis of polycythemia vera (PV) very likely. A bone marrow examination was refused by the patient. Phlebotomy (three times 500 mL) was performed and therapy with hydroxyurea (1000 mg/d) was started. Initially peripheral haematological values were checked daily and following discharge from the hospital they were checked weekly. The anticoagulation was changed to phenprocoumon in order to achieve less variable INR values. Additionally low-molecular weight heparins were applied until stable INR values between 2 and 3 were obtained. Due to the spontaneous intracranial bleeding eleven months earlier, therapy with acetylsalicylic acid was not added.
One month later, the patient was hospitalised because of symptomatic thrombocytopenia (haemoglobin 109 g/L, MCHC 32 g/dL, MCV 71 fl, lowest platelet count 18 × 109/L, creatinine 51 µmol) with lower extremity petechial bleedings, epistaxis and anemia, after the patient’s general practitioner had stopped therapy with hydroxyurea four days, and phenprocoumon two days, prior to readmission. INR was 2.1 and the CRP value was 5 mg/L excluding an infectious cause. There were no clinical signs of gastrointestinal bleeding. Phenprocoumon was reinstituted together with low-molecular weight heparins once thrombocyte counts were above 50 × 109/L.
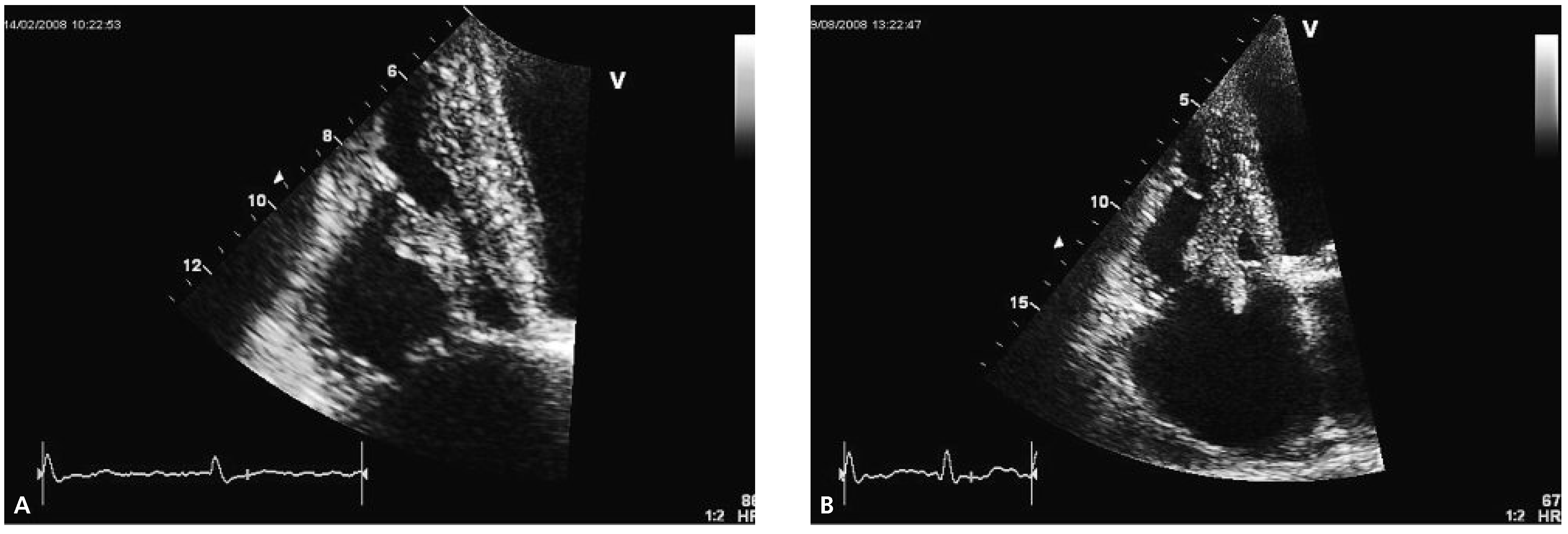
Echocardiography two months later revealed a slightly improved left ventricular ejection fraction (30–35%) and an obvious reduction in size of the thrombus (
Figure 2A).
Follow-up echocardiography was performed again half a year later. At that time, the size of the right ventricle appeared normal whereas the systolic right ventricular function was still reduced (TAPSE 1.8 cm, TDI 9 cm/s). The thrombus had increased in size with new highly mobile parts prolapsing into the right atrium (
Figure 2B). The patient passed away at home seven months later from an unknown reason.
2. Discussion
We report the case of a patient who presented with an intracardiac thrombus despite oral anticoagulation and was subsequently diagnosed to suffer from a myeloproliferative neoplasm, most likely PV characterised by proliferation of one or more of the myeloid lineages due to a clonal disorder of haematopoetic stem cells. According to the 2008 WHO diagnostic criteria for PV, our patient met the two major criteria (haemoglobin >185 g/L, presence of JAK2V617F). The abnormal elevation of haemoglobin and leucocyte counts retrospectively presented over many years make the diagnosis of PV extremely likely even in the absence of bone marrow histology, an examination that the patient refused, and without testing for subnormal Epo levels [
1]. Treatment of PV is primarily based on stratification of the thrombotic risk of a single patient (for review see [
2,
3]). High risk patients are those older than 60 years and those with a history of thrombosis. Extreme thrombocytosis in turn (platelet count > 1500 × 10
9/L) constitutes a risk factor for bleeding. Additionally an increased leucocyte count is regarded to be an independent risk factor for thrombosis.
Phlebotomy to maintain haematocrit below 0.45, together with low-dose acetylsalicylic acid in those without highly elevated risk for bleeding complications, is the mainstay of therapy in all patients with PV. In addition, for patients with a high risk of thrombosis or severe symptomatic iron deficiency due to phlebotomy, cytoreductive therapy with hydroxyurea should be considered. In selected patients, therapy with Interferon-α to suppress the proliferation of haematopoietic progenitors, or in the future specific inhibition of JAK2 tyrosine kinase, could be other treatment options. Evidence for the treatment of specific cardiovascular complications in PV is scarce. In general for bleeding complications, withdrawal of antithrombotic therapy and anticoagulants and correction of thrombocytosis usually with hydroxyurea is suggested. In venous thromboembolism, patients should be treated with low molecular weight heparins followed by oral anticoagulation with vitamin K antagonists targeted to an INR value of 2 to 3. Acute arterial vascular events are managed according to published current guidelines of respective associations and societies. Low dose acetylsalicylic acid is recommended unless there is a contraindication for anti-platelet therapy. Regarding the treatment of an intracardiac thrombus, long-term anticoagulation and cytoreductive therapy seem logical, although intracardiac thrombus in the scenario of PV is extremely rare and only a few case reports are published regarding this topic [
4]. Successful surgical removal of a right ventricular thrombus has been reported [
5]. Another possible approach is intravenous thrombolysis, reported once several years ago [
6].
In our patient, possible therapeutic options regarding the thromboembolic disease were limited since an increased risk for major bleeding was obvious based on the patient’s history of chronic subdural haematoma and because the patient refused any possible surgical approach. Lacking therapeutic alternatives, oral anticoagulation with a vitamin K antagonist was continued and a cytoreductive therapy with hydroxyurea installed resulting in a reduction in thrombus size and a subjective improvement of dyspnoea and physical activity. Therapy with hydroxyurea had to be stopped because of symptomatic thrombopenia. It is not known whether oral anticoagulation in the face of appropriate cytoreductive therapy in patients with PV can consistently result in resolution of intracardiac thrombi.