Abstract
Numerous native Australian plants are widely used as traditional medicines by the Australian Aboriginal and Torres Strait Islander peoples. Among the native plants, Pittosporum angustifolium Lodd. (Gumby Gumby) is claimed to be a promising medicinal plant in the treatment of a wide range of diseases that includes viral symptoms (colds and coughs), eczema, cancer, muscle aches, varicose veins, and many more. Various extraction techniques are used to extract the bioactive compounds of P. angustifolium, which are formulated into nutraceuticals. The present paper will provide an overview of the recent development in the extraction of bioactive ingredients from P. angustifolium, as well as the findings on the phytochemicals and antimicrobial activity of P. angustifolium extracts.
1. Introduction
Nutraceutical is a term referring to both nutrition and pharmaceuticals. In addition to nourishing the body, a nutraceutical is anticipated to help in the treatment and prevention of diseases [1]. Nutraceuticals are considered to be safer and more acceptable, to have fewer undesired symptoms, and to have higher bioavailability than drugs or medication [2,3,4]. Generally, nutraceuticals use food phytochemicals in pharmaceutical forms as the active principles [4]. Examples of phytochemicals that bring health benefits and can be found in food include the sulphur-containing ingredients of the Alliaceac, glucosinolates, terpenoids (carotenoids, monoterpenes, and phytosterols), and polyphenols (anthocyanins, proanthocyanidins, hydroxycinnamates, flavones, flavan-3-ols, isoflavones, stilbenoids, coumarins, ellagic acid and ellagitannins, lignans, etc.) [4].
A widely used nutraceutical in traditional Aboriginal medicine is derived from Pittosporum angustifolium Lodd. (family Pittosporaceae, previously known as Pittosporum phylliraeoides), commonly known as the Gumby Gumby plant. P. angustifolium is an indigenous plant in Australia and was first discovered by Robert Brown in 1814 when he established the family Pittosporaceae. One of the first P. angustifolium samples was collected by William Dampier from Shark Bay (in remote, north-western Australia) in 1699 [5]. A medium tree of P. angustifolium generally reaches 10 m in height, consisting of pendulous branches, falcate, and glabrous leaves. P. angustifolium flowers are yellow, and the plant bears fragrant, orange fruit [6]. Its flowering with mature fruit period is in winter and spring. It commonly appear in areas near inland lakes and drainage lines in sandy soils [5].
P. angustifolium has been used as a treatment for a plethora of ailments. This includes the treatment of rheumatoid arthritis, inflammatory diseases, skin issues, spasmodic symptoms, and microbial infections [7]. Various parts of the Gumby Gumby plant, such as the leaf, flower, root, bark, seed, and fruit pulp, are used [7]. Treatments include infusion of leaf for the treatment of colds, coughs, and cramps or fruit extracts in the form of a poultice or decoction to treat eczema, muscle aches, and other forms of pruritus [8]. While P. angustifolium has not been approved by the Therapeutic Goods Administration (TGA) for therapeutic use in humans, research is ongoing and interest in the Gumby Gumby plant is mounting due to its potential medicinal values.
The increasing interest in P. angustifolium lies in the nutritional values of its bioactive compounds and the application of its nutraceuticals. Several bioactive compounds are found in most parts of P. angustifolium, from the leaves and seeds to the bark and root. The aerial components, which are exposed to air, including leaves, flowers, fruits, and stems, are utilised in traditional medicine to treat numerous different conditions [9]. A number of the bioactive compounds found in P. angustifolium are tabulated in Table 1. These include phytochemicals, such as saponins and terpenoids, that contribute to its antifungal and medicinal properties [10].

Table 1.
Bioactive compounds in Pittosporum angustifolium.
Several initiatives to investigate the phytochemical and therapeutic potential of P. angustifolium have been conducted. The extracts of various species of Pittosporum were shown to possess anticancer activity [16,17,18,19]. Literature reported the presence of pentacyclic triterpenoid sapogenins in P. angustifolium [13], which have been found to exhibit anti-HIV, anti-tumour (CC50 values of 10–48 µg/mL against human oral squamous cell carcinoma, human salivary gland tumour, and human normal gingival fibroblasts), and antioxidant effects [10,20]. The validity of various circumstantial proclamations of successful cancer treatment via the intake of P. angustifolium leaves was also examined by Lindquist [21]. Triterpenoid saponins, terpenoids, phenols, and coumarin compounds were found and were reportedly shown to have cytotoxicity in varying potencies against assorted cancerous tumours. These findings were supported in a review by Errington and Jefferies, where a mixture of triterpenoid sapogenins was extracted via solvent extraction [13]. P. angustifolium was also found to exhibit antiviral activities where leaf extracts were capable of inhibiting more than 25% of Ross-River-virus-induced cytopathicity. This finding supports the traditional Aboriginal actions of using Gumby Gumby extracts to treat viral infections [10]. Current research in the works includes investigations on the applications of P. angustifolium for treatments such as eczema, cancers, psoriasis, varicose veins, muscle aches, sprains, cramps, and induction of lactation in mothers of the newborn [15,21,22].
The literature search for the present paper included the keywords “Pittosporum angustifolium” and “Pittosporum phillyraeoides”. From Scopus, a search of “P. angustifolium” resulted in 20 papers dated between the years 2000 and 2021, which included 16 articles, 2 conference papers, 1 letter, and 1 review, while “P. phillyraeoides” resulted in two article papers dated between the years 1965 and 1988. Based on a search in SciFinder, “P. angustifolium” resulted in 29 papers dated between 1955 and 2021 that included 27 articles, 2 reviews, 1 conference paper, and 1 dissertation, while “P. phylliraeoides” resulted in 24 papers in which most of the results were found to overlap with “P. angustifolium”.
The aim of this mini review is to consolidate the existing extraction techniques of P. angustifolium. It provides a comparison of the efficiency of several extraction methods and the phytochemical and therapeutic values of P. angustifolium extracts.
2. Extraction of Bioactive Compounds from the Plant Matrices of P. angustifolium
Recent advances in extractive techniques, coupled with the development in chromatographic and spectrometric techniques, have allowed for targeted extraction and identification of bioactive compounds from plant matrices. The utilisation of each extractive technique generally influences the quality and the quantity of the targeted compounds extracted. As the number of bioactive compounds in a plant matrix can be large, it is vital to identify the extractive method that is more selective towards the targeted compound. Hence, comprehensive extraction studies are imperative in order to distinguish the technique that works best for the targeted extraction of a particular group of bioactive compounds from various plant species.
Recent literature provides an overview of the extractive methods that have been applied to the leaf, fruit, and seed of the Gumby Gumby plant. These techniques of extraction include the Soxhlet extractive method, hydrodistillation, solvent extraction, and extractive fermentation. The most recent literature on the extraction of P. angustifolium and the corresponding bioactive compounds extracted is summarised in Table 2.

Table 2.
Summary of the most recent literature on extraction techniques used for P. angustifolium and the corresponding extracted bioactive compounds.
2.1. Soxhlet Extractive Method
The Soxhlet extractive technique is a conventional method of extraction. This technique is usually the standard for reference to which newly developed extractive methods are compared [26]. The basis for the use of the Soxhlet method is founded upon the solvating power of the extractive solvents, with the addition of heat for an increased extracting power.
The use of the Soxhlet apparatus to extract the leaves and seeds of P. angustifolium was reported by several papers [11,14,23,24]. The extractive method is briefly explained here. Pulverised and dried and/or ground leaves are defatted with dichloromethane via Soxhlet for 10 to 24 h and then extracted three times with 80% (v/v) ethanol under reflux. The extract obtained is subjected to open column chromatography using Sephadex LH-20 (Sigma-Aldrich), eluting with methanol for analyses.
In the studies conducted by Bäcker et al. [11,23], the authors were able to extract and identify eight pittangretosides-barrigenol glycosides and five polyphenolic compounds (quercetin glycosides rutin, isoquercitrin, 600-(3-hydroxy-3-methylglutaroyl)-isoquercitrin (as well as 3,4-), and 4,5-dicaffeoylquinic acid) from the Gumby Gumby leaf after 10 h of extraction with the Soxhlet apparatus. In a 24 h extraction, the authors were able to isolate and characterise 10 triterpene saponins, including nine novel compounds named pittangretosides A-I and a known glycoside (22α-angeloyloxy-3β-[β-d-glucopyranosyl-(1→2)]-[α-l-arabinopyranosyl-(1→3)]-β-d-glucoronopyranosyloxyolean-12-ene-15α,16α,28-triol) [23,27]. The authors further extracted the seed of the Gumby Gumby plant using Soxhlet, resulting in the finding of seven acrylated triterpene glycosides [24].
The compounds isolated by Bäcker et al. (2013) were assessed by the authors for in vitro cytotoxic characteristics against human urinary bladder carcinoma cells [23]. However, only saponins containing angeloyl residue at C-22 of the aglycone were shown to exhibit antiproliferative effects on human urinary bladder carcinoma cells while others did not display cytotoxic effects [23]. The cytotoxicity of saponins from the seeds of P. angustifolium was also studied against non-tumourigenic cell lines (human keratinocyte), and three tumourigenic cell lines, including human urinary bladder carcinoma, human breast cancer, and human glioblastoma, were found to contain antiproliferative effects. The half-maximal inhibitory concentration (IC50) values of 1.7 ± 0.1 µM, 3.9 ± 0.1 µM, 8.9 ± 0.8 µM, and 2.2 ± 0.5 µM were achieved in urinary bladder carcinoma, breast cancer, glioblastoma, and keratinocyte, respectively [24].
Bäcker et al. [9] further screened the isolated compounds (triterpene saponins) extracted from the Gumby Gumby plant for their cytotoxic potential against tumourigenic cell lines. The authors found evidence of triterpene saponins causing the inhibition of human topoisomerase I in a DNA relaxation assay. IC50 values were found to range between 2.8 and 46.5 µM, with most compounds having comparative or higher activities (2.8–8.6 µM) than the positive control camptothecin (7.4 µM). The study revealed the cytotoxic effect was caused only by the inhibition of topoisomerase I [9]. In a blind docking study, all triterpene saponins investigated were expected to display a similar binding mode to camptothecin. Saponins were determined by Wang et al. [28] to have no inhibition effect on topoisomerase II.
2.2. Hydrodistillation
Hydrodistillation is traditionally used to extract essential oils from plant matrices. Similar to the concept of a standard distillation technique, hydrodistillation involves the separation of volatile compounds via the heating of the product. The uniqueness of the hydrodistillation technique (in comparison to other distillation methods) is the non-involvement of organic solvents during distillation, with hot water or steam being the only solvent involved [26]. The advantage of using a hydrodistillation technique is that plant materials do not need to be dried prior to the extraction process. However, hydrodistillation typically poses limitations during the extraction of natural products due to the required high temperature and the thermal instability of plant matter. As some natural compounds are thermally labile, the solutes of interest may be destroyed or damaged by high temperatures in the hydrodistillation process. In addition, hydrodistillation typically results in low yield and poor selectivity; thus, further research and technological developments are much sought after [29].
Essential oils were hydrodistilled from the fruit and leaf of P. angustifolium with a modified approach [13]. Briefly, the methodology included diced fragmented leaf or fruit of P. angustifolium being placed in water in a round bottom flask. A layer of pumice or perlite obsidian was added to the mixture to crush suds produced from saponins during the process of boiling. The hydrodistillation process commenced with essential oil collected after 3 to 4 h. The essential oil was analysed by gas chromatography–mass spectrometry after drying with anhydrous sodium sulphate and being dissolved in dichloromethane [15]. The extracted essential oil was subsequently tested for its antimicrobial activities.
The authors found that the predominant components in the essential oils of P. angustifolium were mostly n-alkane, n-cycloalkanes, alkene esters, alkane, alkane and alkene aldehydes, monoterpene acetates, sesquiterpenes, and sesquiterpenols [13]. In addition, higher quantities of esters and sesquiterpenols were found in the leaf compared to the fruits of the Gumby Gumby plant. While the composition of the essential oils from the leaf was distinctly different from that of the fruit, the essential oils from both leaf and fruit showed similar antimicrobial activities. The authors also determined that the fruit-extracted essential oil from P. angustifolium had major constituents that varied with geographical location. This observation pointed to the existence of at least two chemotypes, of which, one has an abundance of acetic acid decyl ester that is commensurate with higher antimicrobial activity. Sadgrove and Jones [6] hypothesised that this explains the use of P. angustifolium by some Aboriginal tribes but not others; centrally located groups, such as the Pitjantjatjara tribe, were seen to not utilise the fruit at all, whilst other accounts describe frequent use of P. angustifolium.
2.3. Solvent Extraction (Methanol, Water, Ethyl Acetate, Chloroform, Hexane, Dichloromethane)
Solvent-based extraction is conducted based on the solubility differences between desired and undesirable constituents in a solvent. A solvent is generally selected based on its higher selectivity towards the desired solutes and its lower preference towards the undesired materials. In a solvent-based extraction, the plant matrix is usually soaked in the liquid solvent with the possible addition of heat and/or agitation. The desired solutes diffuse and dissolve into the solvent and then are removed together with the solvent. The solutes are subsequently separated from the solvent. Although typically resulting in an adequate final yield, there are concerns over the residual organic solvents left in the extract if the extract is later consumed, such as rosemary oil [30].
The following paragraphs elucidate recent, solvent-based extraction studies on the Gumby Gumby plant [6,7,31]. In general, the solvent-based extraction method comprises of the following steps: The plant material is first dried and ground. Solvents are then added to the ground plant material and extracted for a duration of between 15 min and 30 h at 4°C or room temperature and may be accompanied by gentle shaking. The extracts obtained are filtered through filter paper under vacuum followed by drying by rotary evaporation. The dry extract is weighed and redissolved in deionised water for analyses [10,25] or dissolved in methanol with boron trifluoride for the subsequent analyses [6] or dissolved in the extraction solvents for high-performance thin-layer chromatography (HPTLC) analysis [12].
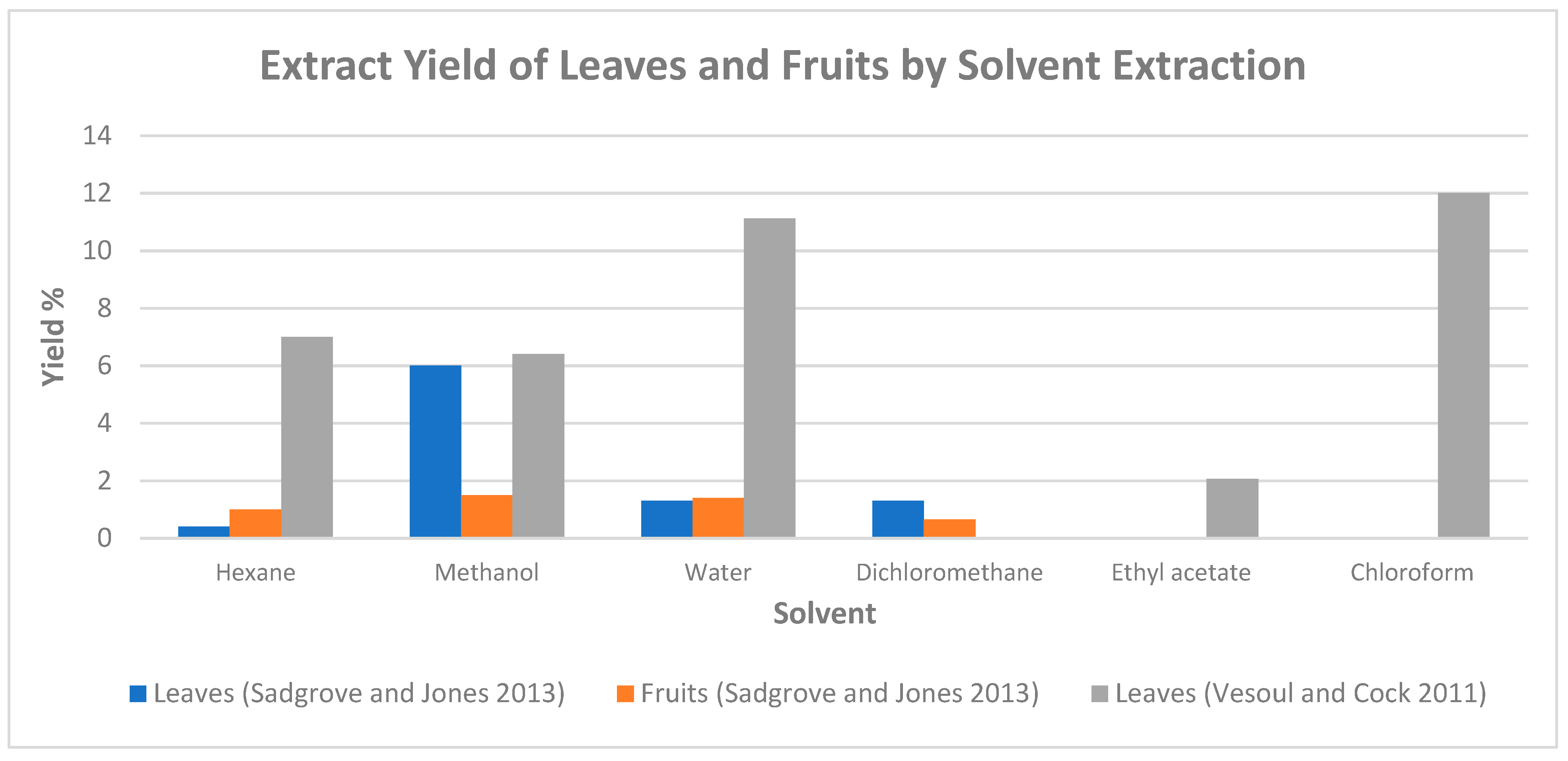
In the study conducted by Sadgrove and Jones [15], water, methanol, hexane, and dichloromethane were used as the extractive solvents. The extracts obtained with solvent water were frozen at three concentrations: (i) immediately after the extraction; (ii) after the concentration was reduced to 10% of its original volume using a rotor vacuum pump on a water bath at 40 °C; and (iii) after evaporation for complete dryness. Figure 1 illustrates the yields of leaf and fruit extracts using various solvents. Of the four solvents used, the highest extract yield from both leaves and fruits was obtained from methanol [15]. Saponins, phenols, and flavonoids were found to be present predominantly in the hexane and methanol extracts, while the presence of polysteroids, cardiac glycosides or alkaloids, or anthraquinones was not detected.
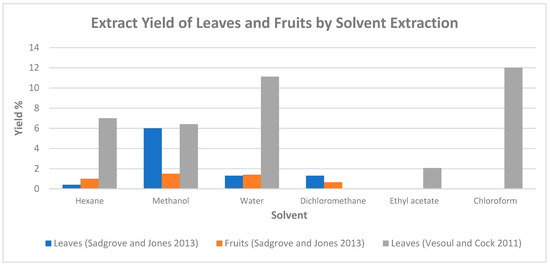
Figure 1.
Extract yield of leaves and fruits of P. angustifolium by solvent extraction (chart plotted based on data from [10,15]).
The authors further evaluated the antimicrobial activity of the extracts in which both leaf and fruit extracts were found to exhibit antimicrobial activity against Staphylococcus aureus, Staphylococcus epidermidis, and Candida albicans, especially the fruit extracts [15]. Minimum inhibitory concentrations (MIC) of the antimicrobial activity were found to range from 1 mg/mL to 250 mg/mL. In this work, the authors found that stronger antimicrobial activity was obtained from water-leaf extracts that were concentrated using a rotary evaporator. The exception that did not exhibit an increase in activity of the water-leaf extract was C. albicans. The correlation between antimicrobial activity and concentration was not observed for the fruit extract.
Solvent-based extraction on the Gumby Gumby leaf was also conducted by Vesoul and Cock [10] with methanol, hexane, water, ethyl acetate, and chloroform as the extractive solvents. Among the solvents used, chloroform and water achieved the highest extraction yields of 12.01% and 11.12%, respectively (Figure 1). The results of the phytochemicals analysis on each solvent extract are summarised in Table 3. Both methanol and water samples extracted the widest range of phytochemicals, with water being the only sample that extracted tannins.

Table 3.
Phytochemicals of P. angustifolium by the solvent extraction method (data retrieved from [10,15]).
Vesoul and Cock (2011) further examined the antimicrobial activity of the extracts, which are tabulated in Table 4. Each extract demonstrated antimicrobial activity, with the methanol extract also showing slight antifungal capacities against Aspergillus niger at a minimum inhibitory concentration of 40.4 μL/mL [10]. The methanolic extract was also found to be potent against Proteus mirabilis, with 10 μL of the extract displaying a zone inhibition of 16.3 ± 0.3 mm in a disc diffusion assay. It was also determined that the effectiveness of the methanolic extract against P. mirabilis was higher than the controls of ampicillin and chloramphenicol. The methanol and hexane extracts were found to be able to inhibit the growth of Gram-positive bacteria, with the methanolic extract inhibiting Staphylococcus pyogenes (6.7 ± 0.3 mm) while the hexane extract inhibited Staphylococcus aureus (7.3 ± 0.6 mm). Water, ethyl acetate, and chloroform extracts were not capable of inhibiting the four Gram-positive bacteria tested. The water extract was found to be the least effective against bacteria growth, inhibiting only 2 of the 14 bacteria types tested, yet showed potency against P. mirabilis with a zone inhibition of 16.3 ± 0.3 mm. It is noted here that the observations made in the literature [15,25] were contradictory and warrant further investigations.

Table 4.
Inhibition of various bacteria types by the extracts from solvent extraction (data retrieved from [10,15,25]). Yellow highlighted cells indicated contradicting data presented by literature.
Blonk and Cock [25] demonstrated that methanol and water were the most efficient solvents, having the highest extract yield and the broadest diversity of phytochemical classes. Inhibition of clinical P. mirabilis bacteria was observed to be more susceptible in aqueous (water) and methanol extracts. The zone of inhibition was determined to be approximately 15 mm and 14 mm for the aqueous and methanolic extracts, respectively. The methanolic extract had a disc diffusion minimum inhibitory concentration (DD MIC) of 48 μg/mL and a liquid dilution minimum inhibitory concentration (LD MIC) of 38 μg/mL. For the aqueous extract, DD MIC was recorded at 625 μg/mL while LD MIC was at 570 μg/mL. On the other hand, chloroform extract was the strongest inhibitor for reference bacterial strains of P. mirabilis among all the other extracts, as well as ampicillin and chloramphenicol. The hexane extract was found to exhibit the lowest inhibition in both clinical and reference bacterial strains of P. mirabilis. Methanol extract showed the strongest inhibition of Klebsiella pneumonia while the inhibition of Proteus vulgaris was also notable with ZOI < 8 mm. Aqueous extract presented the strongest inhibition of Acinetobacter baylyi and clinical Pseudomonas aeruginosa strain while ethyl acetate extract demonstrated the highest inhibition of the reference bacteria strain of P. aeruginosa.
In terms of inhibiting Streptococcus pyogenes, methanol extract exhibited the highest inhibition, followed by the water and chloroform extracts. Hexane extract exhibited the lowest to no inhibition activity of all bacteria strains in the study by Blonk and Cock [25]. The authors also verified that all extracts were nontoxic after testing in the Artemia lethality assay and MTS cell viability assay.
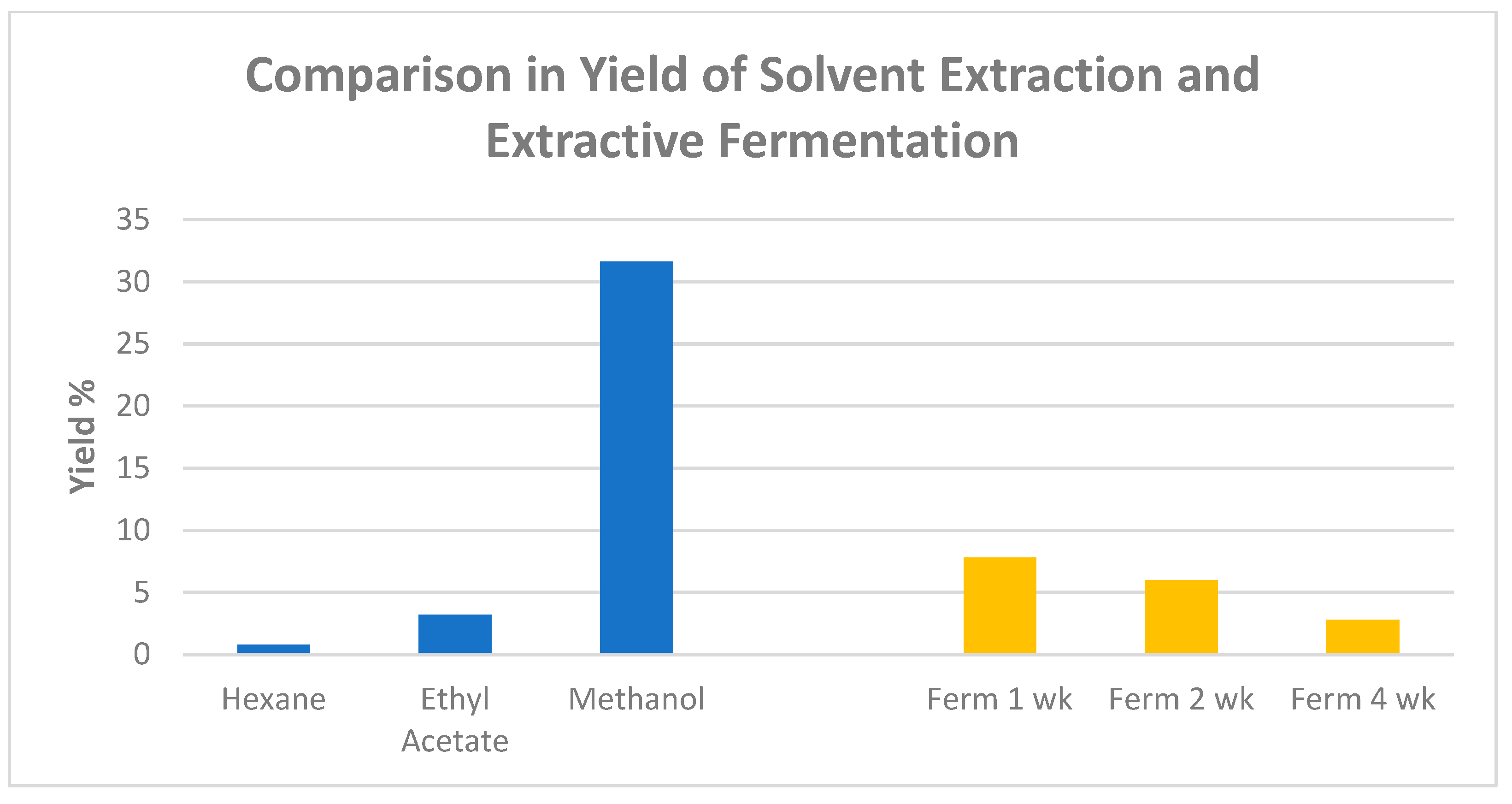
In a recent study conducted by Agatonovic-Kustrin and coauthors [12], methanol, ethyl acetate, and n-hexane were used as solvents to extract Gumby Gumby leaf. Comparisons of the yields obtained through the three solvents are illustrated in Figure 2. The highest extraction yield (31.6%) was achieved with the methanol as the extracting solvent. However, ethyl acetate was determined by the authors to be the most efficient in extracting a wide range of bioactive ingredients that are rich in phytosterols due to their solubility.
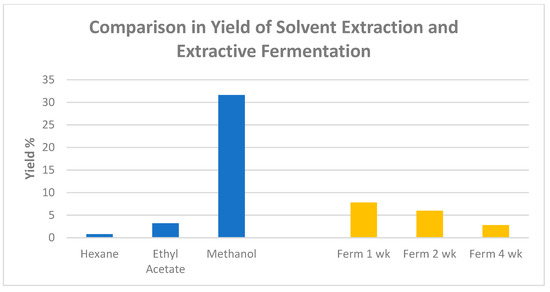
Figure 2.
Comparison of extract yield between solvent extraction and extractive fermentation techniques for P. angustifolium (chart plotted based on published data [12]).
2.4. Extractive Fermentation
Fermentation on plant materials has been investigated with an aim to reduce adverse effects and to enhance biological properties such as antioxidant activity, anti-diarrhoeal effect, anti-inflammatory effect, anti-allergic effect, anti-obesity effect, anti-diabetic effect, and hepatoprotective effect. Fermentation is a biocatalytic process that involves the decomposition and biotransformation of complex substrates to compatible bioactive components by the action of microbial enzymes [31].
Agatonovic-Kustrin and coauthors conducted fermentation on ground Gumby Gumby plant material before undertaking a solvent extraction using ethyl acetate [12]. The fermentation of the plant material was initiated by soaking ground plant material in 2.5% w/v sodium chloride solution (salt environment) to generate Lactobacillus that produces lactic acid with an aim to avoid the growth of other bacterial cultures. Acid fermentation occurred once the material was brined, with the appearance of CO2 bubbles. The sample was incubated in the dark at room temperature for a duration of 1 to 4 weeks. Subsequently, the sample was extracted from the fermented broth through the addition of ethyl acetate (EA). EA was chosen due to its wide-ranging solvating power that allows for the efficient extraction of phenolic compounds and phytosterols. The extract of the plant material was obtained after the separation and evaporation of solvents [12].
After fermentation for 1 week, the extraction yield was the highest, at 7.8%, compared to the fermentation duration of 2 weeks and 4 weeks (Figure 2). The fermented extracts were demonstrated to be rich in soluble lignin oligomers that enhanced antioxidant activity as compared to non-fermented extracts. Agatonovic-Kustrin et al. (2021) also verified that the fermentation process did not affect or reduce the health benefits and bioactivities of P. angustifolium extracts [12]. The comparison between fermentation-based extraction and solvent-based extraction is also shown in Figure 2.
3. Conclusions
The extracts of P. angustifolium were demonstrated to have high total phenolic content, antioxidative capacity, and prominent bioactivity. Among the extraction methods in the literature, the extractive fermentation technique revealed the possibility of enhancing antioxidant activity by increasing the total phenolic content while maintaining α-amylase inhibitory activity. Nevertheless, the extraction yield of P. angustifolium was higher for the solvent extraction technique than extractive fermentation.
Leaf extracts obtained from the solvent extraction method showed low to no toxicity. The leaf extracts obtained from various solvents also exhibited inhibition against various bacteria strains including A. hydrophila, E. coli, P. mirabilis, P. vulgaris, K. pneumonia, S. marcescens, S. aureus, A. niger, A. baylti, P. aeruginosa, and S. pyogenes.
The current work on extracting bioactive ingredients from the Gumby Gumby plant is still in its infancy. The literature review has established that a number of active ingredients obtained from the extracts of the Gumby Gumby plant are potentially of medicinal value. In fact, recent extraction studies also isolated and characterised newly identified bioactive compounds. However, further investigative works are required to determine economically viable extraction processes that are able to target the respective bioactive ingredients. In addition, further studies are required to establish the available bioactive compounds in the Gumby Gumby plant and the corresponding ethnopharmacological significance.
Author Contributions
Conceptualization, C.C.B.; methodology, C.C.B.; validation, C.C.B. and W.H.T.; formal analysis, C.C.B.; investigation, C.C.B. and W.H.T.; resources, C.C.B. and W.H.T.; data curation, C.C.B.; writing—original draft preparation, C.C.B.; writing—review and editing, C.C.B. and W.H.T.; visualization, C.C.B. and W.H.T.; supervision, C.C.B. and W.H.T.; project administration, C.C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Andrew, R.; Izzo, A.A. Principles of pharmacological research of nutraceuticals. Br. J. Pharmacol. 2017, 174, 1177–1194. [Google Scholar] [CrossRef] [PubMed]
- Ozdal, T.; Tomas, M.; Toydemir, G.; Kamiloglu, S.; Capanoglu, E. Chapter 1-Introduction to nutraceuticals, medicinal foods, and herbs. In Aromatic Herbs in Food; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 1–34. [Google Scholar]
- Santini, A.; Tenore, G.C.; Novellino, E. Nutraceuticals: A paradigm of proactive medicine. Eur. J. Pharm. Sci. 2017, 96, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Espín, J.C.; García-Conesa, M.T.; Tomás-Barberán, F.A. Nutraceuticals: Facts and fiction. Phytochemistry 2007, 68, 2986–3008. [Google Scholar] [CrossRef]
- Cayzer, L.W.; Crisp, M.D.; Telford, I.R.H. Revision of Pittosporum (Pittosporaceae) in Australia. Aust. Syst. Bot. 2000, 13, 845–902. [Google Scholar] [CrossRef]
- Sadgrove, N.; Jones, G.L. Phytochemical variability of Pittosporum angustifolium Lodd.(Pittosporaceae): A traditional and contemporary Aboriginal Australian medicine. In XXIX International Horticultural Congress on Horticulture: Sustaining Lives, Livelihoods and Landscapes (IHC2014): V World 1125; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2014; pp. 303–308. [Google Scholar]
- Phan, A.D.T.; Chaliha, M.; Hong, H.T.; Tinggi, U.; Netzel, M.E.; Sultanbawa, Y. Nutritional Value and Antimicrobial Activity of Pittosporum angustifolium (Gumby Gumby), an Australian Indigenous Plant. Foods 2020, 9, 887. [Google Scholar] [CrossRef]
- Mani, J.S.; Johnson, J.B.; Hosking, H.; Ashwath, N.; Walsh, K.B.; Neilsen, P.M.; Broszczak, D.A.; Naiker, M. Antioxidative and therapeutic potential of selected Australian plants: A review. J. Ethnopharmacol. 2021, 268, 113580. [Google Scholar] [CrossRef]
- Bäcker, C.; Drwal, M.N.; Preissner, R.; Lindequist, U. Inhibition of DNA–Topoisomerase I by Acylated Triterpene Saponins from Pittosporum angustifolium Lodd. Nat. Prod. Bioprospecting 2016, 6, 141–147. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vesoul, J.; Cock, I.E. An Examination of the Medicinal Potential of Pittosporum phylliraeoides: Toxicity, Antibacterial and Antifungal Activities. Pharmacogn. Commun. 2011, 1, 8–17. [Google Scholar] [CrossRef]
- Bäcker, C.; Jenett-Siems, K.; Bodtke, A.; Lindequist, U. Polyphenolic compounds from the leaves of Pittosporum angustifolium. Biochem. Syst. Ecol. 2014, 55, 101–103. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Gegechkori, V.; Morton, D.W. The effect of extractive lacto-fermentation on the bioactivity and natural products content of Pittosporum angustifolium (gumbi gumbi) extracts. J. Chromatography. A 2021, 1647, 462153. [Google Scholar] [CrossRef]
- Errington, S.G.; Jefferies, P.R. Triterpenoid sapogenins of Pittosporum phillyraeoides. Phytochemistry 1988, 27, 543–545. [Google Scholar] [CrossRef]
- Bäcker, C.; Jenett-Siems, K.; Siems, K.; Wurster, M.; Bodtke, A.; Niedermeyer, T.H.; Lindequist, U. New Mono-and Bisdesmosidic Triterpene Glycosides from Pittosporum angustifolium Lodd. Z. Für Nat. B 2014, 69, 1026–1044. [Google Scholar] [CrossRef]
- Sadgrove, N.J.; Jones, G.L. Chemical and biological characterisation of solvent extracts and essential oils from leaves and fruit of two Australian species of Pittosporum (Pittosporaceae) used in aboriginal medicinal practice. J. Ethnopharmacol. 2013, 145, 813–821. [Google Scholar] [CrossRef]
- Seo, Y.; Berger, J.M.; Hoch, J.; Neddermann, K.M.; Bursuker, I.; Mamber, S.W.; Kingston, D.G. A new triterpene saponin from Pittosporum viridiflorum from the Madagascar rainforest. J. Nat. Prod. 2002, 65, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Koduru, S.; Grierson, D.S.; Afolayan, A.J. Ethnobotanical information of medicinal plants used for treatment of cancer in the Eastern Cape Province, South Africa. Curr. Sci. 2007, 92, 906–908. [Google Scholar]
- Eparvier, V.; Thoison, O.; Bousserouel, H.; Guéritte, F.; Sévenet, T.; Litaudon, M. Cytotoxic farnesyl glycosides from Pittosporum pancheri. Phytochemistry 2007, 68, 604–608. [Google Scholar] [CrossRef] [PubMed]
- D’Acquarica, I.; Di Giovanni, M.C.; Gasparrini, F.; Misiti, D.; D’Arrigo, C.; Fagnano, N.; Guarnieri, D.; Iacono, G.; Bifulco, G.; Riccio, R. Isolation and structure elucidation of four new triterpenoid estersaponins from fruits of Pittosporum tobira ait. Tetrahedron 2002, 58, 10127–10136. [Google Scholar] [CrossRef]
- Taniguchi, S.; Imayoshi, Y.; Kobayashi, E.; Takamatsu, Y.; Ito, H.; Hatano, T.; Sakagami, H.; Tokuda, H.; Nishino, H.; Sugita, D. Production of bioactive triterpenes by Eriobotrya japonica calli. Phytochemistry 2002, 59, 315–323. [Google Scholar] [CrossRef]
- Lindquist, U. Biological and chemical analysis of Pittosporum phillyraeoides. Ph.D Thesis, University of Greifswald, Greifswald, Germany, 2007. [Google Scholar]
- Lassak, E.V. Australian Medicinal Plants; Lassak, E.V., McCarthy, T., Eds.; Methuen Australia: Sydney, Australia, 1983. [Google Scholar]
- Bäcker, C.; Jenett-Siems, K.; Siems, K.; Wurster, M.; Bodtke, A.; Chamseddin, C.; Crüsemann, M.; Lindequist, U. Triterpene Glycosides from the Leaves of Pittosporum angustifolium. Planta Med. 2013, 79, 1461–1469. [Google Scholar] [CrossRef]
- Bäcker, C.; Jenett-Siems, K.; Siems, K.; Wurster, M.; Bodtke, A.; Lindequist, U. Cytotoxic saponins from the seeds of Pittosporum angustifolium. Z. Für Nat. C 2014, 69, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Blonk, B.; Cock, I.E. Interactive antimicrobial and toxicity profiles of Pittosporum angustifolium Lodd. extracts with conventional antimicrobials. J. Integr. Med. 2019, 17, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, S.; Ownby, S.; Wang, P.; Yuan, W.; Zhang, W.; Scott Beasley, R. Phenolic compounds and rare polyhydroxylated triterpenoid saponins from Eryngium yuccifolium. Phytochemistry 2008, 69, 2070–2080. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ownby, S.; Zhang, Z.; Yuan, W.; Li, S. Cytotoxicity and inhibition of DNA topoisomerase I of polyhydroxylated triterpenoids and triterpenoid glycosides. Bioorganic Med. Chem. Lett. 2010, 20, 2790–2796. [Google Scholar] [CrossRef]
- Ghasemi, E.; Raofie, F.; Najafi, N.M. Application of response surface methodology and central composite design for the optimisation of supercritical fluid extraction of essential oils from Myrtus communis L. leaves. Food Chem. 2011, 126, 1449–1453. [Google Scholar] [CrossRef]
- Conde-Hernández, L.A.; Espinosa-Victoria, J.R.; Trejo, A.; Guerrero-Beltrán, J.Á. CO2-supercritical extraction, hydrodistillation and steam distillation of essential oil of rosemary (Rosmarinus officinalis). J. Food Eng. 2017, 200, 81–86. [Google Scholar] [CrossRef]
- Hussain, A.; Bose, S.; Wang, J.H.; Yadav, M.K.; Mahajan, G.B.; Kim, H. Fermentation, a feasible strategy for enhancing bioactivity of herbal medicines. Food Res. Int. 2016, 81, 1–16. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).