Goji Berry: Health Promoting Properties
Abstract
1. Introduction
Search Strategy
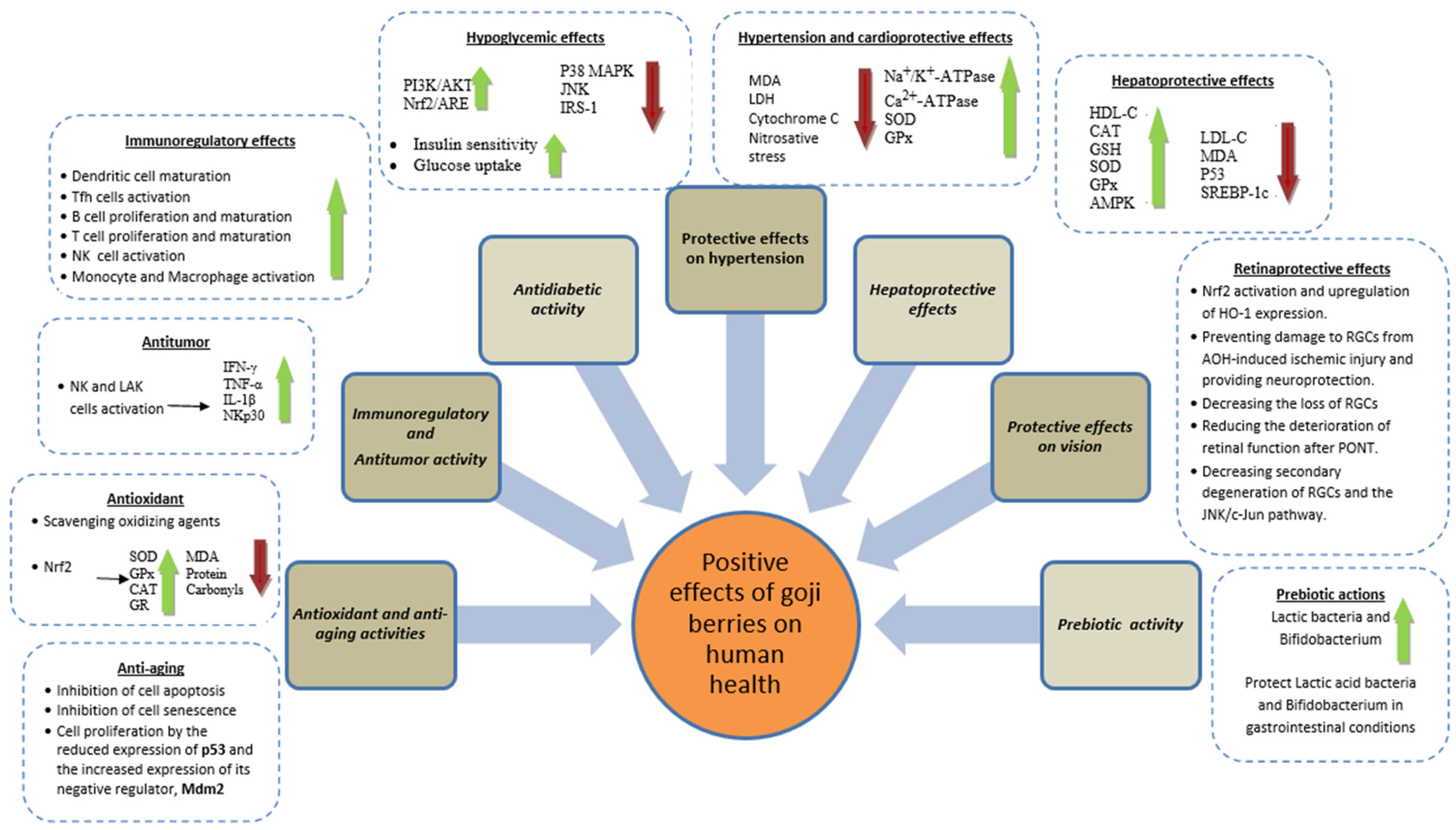
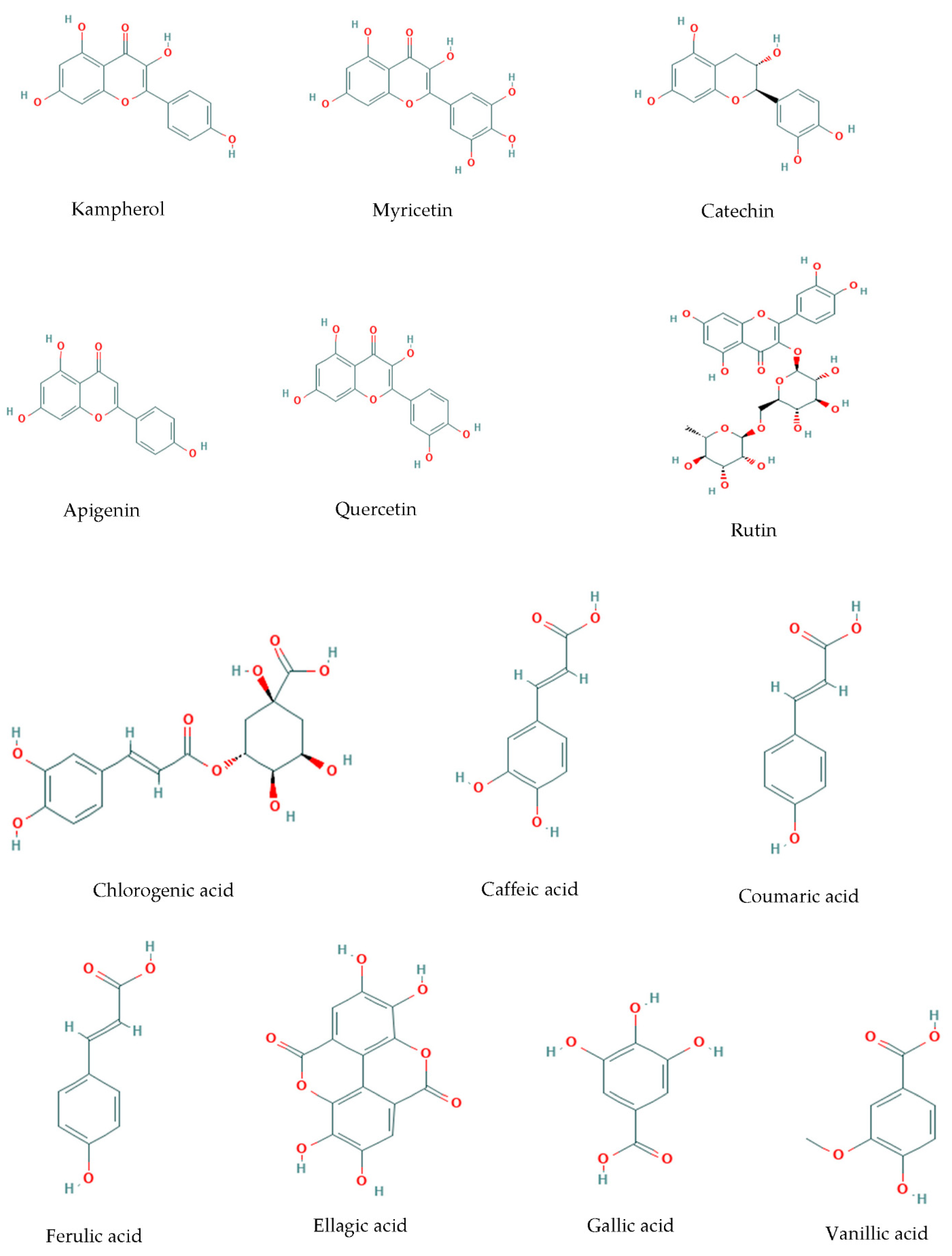
2. Bioactivities
2.1. Antioxidant Activity
2.2. Antiaging Activity
2.3. Antitumor and Immunoregulatory Activity
2.4. Antidiabetic Activity
2.5. Hypertension and Heart Protective Effects
2.6. Hepatoprotective Activity
2.7. Eye and Vision Activity
2.8. Pre-Biotic Activity
2.9. Other Bioactivities
3. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knight, K.; Badamgarav, E.; Henning, J.M.; Hasselblad, V.; Anacleto, P.; Gano, P.; Ofman, J.J.; Weingarten, S.R. A Systematic review of diabetes disease management programs. Am. J. Manag. Care 2005, 11, 242–250. [Google Scholar] [PubMed]
- Kannel, W.B. Diabetes and cardiovascular disease. The Framingham study. JAMA 1979, 241, 2035–2038. [Google Scholar] [CrossRef] [PubMed]
- Sowers, J.R.; Epstein, M.; Frohlich, E.D. Diabetes, hypertension, and cardiovascular disease. Hypertension 2001, 37, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar] [CrossRef] [PubMed]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Leontopoulos, S.; Skenderidis, P.; Kalorizou, H.; Petrotos, K. Bioactivity potential of polyphenolic compounds in human health and their effectiveness against various food borne and plant pathogens. A review. J. Food Biosyst. Eng. 2017, 7, 1–19. [Google Scholar]
- Lampakis, D.; Skenderidis, P.; Leontopoulos, S. Technologies and extraction methods of polyphenolic compounds derived from pomegranate (Punica granatum) peels. A mini review. Processes 2021, 9, 236. [Google Scholar] [CrossRef]
- Leontopoulos, S.; Skenderidis, P.; Vagelas, I.K. Potential use of polyphenolic compounds obtained from olive mill waste waters on plant pathogens and plant parasitic nematodes. In Plant Defence: Biological Control; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 137–177. ISBN 978-3-030-51034-3. [Google Scholar]
- Greathead, H. Plants and plant extracts for improving animal productivity. Proc. Nutr. Soc. 2003, 62, 279–290. [Google Scholar] [CrossRef]
- Skenderidis, P.; Mitsagga, C.; Giavasis, I.; Petrotos, K.; Lampakis, D.; Leontopoulos, S.; Hadjichristodoulou, C.; Tsakalof, A. The in vitro antimicrobial activity assessment of ultrasound assisted Lycium barbarum fruit extracts and pomegranate fruit peels. J. Food Meas. Charact. 2019, 13, 2017–2031. [Google Scholar] [CrossRef]
- Leontopoulos, S.; Skenderidis, P.; Petrotos, K.; Giavasis, I. Corn silage supplemented with pomegranate (Punica granatum) and avocado (Persea americana) pulp and seed wastes for improvement of meat characteristics in poultry production. Molecules 2021, 26, 5901. [Google Scholar] [CrossRef]
- Zengin, Z.B.; Meza, L.; Pal, S.K.; Grivas, P. Chemoimmunotherapy in urothelial cancer: Concurrent or sequential? Lancet Oncol. 2021, 22, 894–896. [Google Scholar] [CrossRef]
- Negi, G.; Kumar, A.; Joshi, R.P.; Sharma, S.S. Oxidative stress and Nrf2 in the pathophysiology of diabetic neuropathy: Old perspective with a new angle. Biochem. Biophys. Res. Commun. 2011, 408, 1–5. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Vivekananthan, D.P.; Penn, M.S.; Sapp, S.K.; Hsu, A.; Topol, E. Use of antioxidant vitamins for the prevention of cardiovascular disease: Meta-analysis of randomised trials. Lancet 2003, 361, 2017–2023. [Google Scholar] [CrossRef]
- Rocchetti, G.; Senizza, B.; Putnik, P.; Kovačević, D.B.; Barba, F.J.; Trevisan, M.; Lucini, L. Untargeted screening of the bound/free phenolic composition in tomato cultivars for industrial transformation. J. Sci. Food Agric. 2019, 99, 6173–6181. [Google Scholar] [CrossRef]
- Rocchetti, G.; Lucini, L.; Corrado, G.; Colla, G.; Cardarelli, M.; De Pascale, S.; Rouphael, Y. Phytochemical profile, mineral content, and bioactive compounds in leaves of seed-propagated artichoke hybrid cultivars. Molecules 2020, 25, 3795. [Google Scholar] [CrossRef]
- Skenderidis, P.; Mitsagga, C.; Lampakis, D.; Petrotos, K.; Giavasis, I. The effect of encapsulated powder of goji berry (Lycium barbarum) on growth and survival of probiotic bacteria. Microorganisms 2020, 8, 57. [Google Scholar] [CrossRef]
- Skenderidis, P.; Leontopoulos, S.; Petrotos, K.; Giavasis, I. Vacuum microwave-assisted aqueous extraction of polyphenolic compounds from avocado (Persea Americana) solid waste. Sustainability 2021, 13, 2166. [Google Scholar] [CrossRef]
- Amagase, H.; Sun, B.; Borek, C. Lycium barbarum (goji) juice improves in vivo antioxidant biomarkers in serum of healthy adults. Nutr. Res. 2009, 29, 19–25. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Nance, D.M.; Amagase, H. A meta-analysis of clinical improvements of general well-being by a standardized Lycium barbarum. J. Med. Food 2012, 15, 1006–1014. [Google Scholar] [CrossRef]
- Yao, R.; Heinrich, M.; Zou, Y.; Reich, E.; Zhang, X.; Chen, Y.; Weckerle, C. Quality variation of goji (Fruits of Lycium spp.) in China: A comparative morphological and metabolomic analysis. Front. Pharmacol. 2018, 9, 151. [Google Scholar] [CrossRef] [PubMed]
- Skenderidis, P.; Lampakis, D.; Giavasis, I.; Leontopoulos, S.; Petrotos, K.; Hadjichristodoulou, C.; Tsakalof, A. Chemical properties, fatty-acid composition, and antioxidant activity of goji berry (Lycium barbarum L. and Lycium chinense Mill.) Fruits. Antioxidants 2019, 8, 60. [Google Scholar] [CrossRef]
- Cao, S.; Du, J.; Hei, Q. Lycium barbarum polysaccharide protects against neurotoxicity via the Nrf2-HO-1 pathway. Exp. Ther. Med. 2017, 14, 4919–4927. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Wang, M.; Shu, S. Effect of commercial Bacillus thuringiensis toxins on Tyrophagus putrescentiae (Schrank) fed on wolfberry (Lycium barbarum L.). Int. J. Acarol. 2016, 42, 1–6. [Google Scholar] [CrossRef]
- Cui, B.; Liu, S.; Lin, X.; Wang, J.; Li, S.; Wang, Q.; Li, S. Effects of Lycium barbarum aqueous and ethanol extracts on high-fat-diet induced oxidative stress in rat liver tissue. Molecules 2011, 16, 9116–9128. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Protective effects of caffeic acid and the Alzheimer’s brain: An update. Mini-Rev. Med. Chem. 2017, 17, 667–674. [Google Scholar] [CrossRef]
- Zhang, R.; Kang, K.A.; Piao, M.J.; Kim, K.C.; Kim, A.D.; Chae, S.; Park, J.S.; Youn, U.J.; Hyun, J.W. Cytoprotective effect of the fruits of Lycium chinense Miller against oxidative stress-induced hepatotoxicity. J. Ethnopharmacol. 2010, 130, 299–306. [Google Scholar] [CrossRef]
- Changbo, D. Supplementation of Lycium barbarum polysaccharides protection of skeletal muscle from exercise-induced oxidant stress in mice. Afr. J. Pharm. Pharmacol. 2012, 6, 643–647. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Zhang, X.; Liu, J.; Hao, Y.; Yang, X.; Wang, Y. Comparative evaluation of the antioxidant effects of the natural vitamin C analog 2-O-β-D-glucopyranosyl-L-ascorbic acid isolated from Goji berry fruit. Arch. Pharmacal. Res. 2011, 34, 801–810. [Google Scholar] [CrossRef]
- Li, H.; Li, Z.; Peng, L.; Jiang, N.; Liu, Q.; Zhang, E.; Liang, B.; Li, R.; Zhu, H. Lycium barbarum polysaccharide protects human keratinocytes against UVB-induced photo-damage. Free Radic. Res. 2017, 51, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Skenderidis, P.; Kerasioti, E.; Karkanta, E.; Stagos, D.; Kouretas, D.; Konstantinos, P.; Hadjichristodoulou, C. Assessment of the antioxidant and antimutagenic activity of extracts from goji berry of Greek cultivation. Toxicol. Rep. 2018, 5, 251–257. [Google Scholar] [CrossRef]
- Bucheli, P.; Gao, Q.; Redgwell, R.; Karine, V.; Wang, J.; Zhang, W.; Nong, S.; Cao, B. Chapter 14 Wolfberry biomolecular and clinical aspects of Chinese. In Herbal Medicine: Biomolecular and Clinical Aspects; CRC Press: Boca Raton, FL, USA, 2013; pp. 1–17. [Google Scholar]
- Kaur, D.; Rasane, P.; Singh, J.; Kaur, S.; Kumar, V.; Mahato, D.K.; Dey, A.; Dhawan, K.; Kumar, S. Nutritional interventions for elderly and considerations for the development of geriatric Foods. Curr. Aging Sci. 2019, 12, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, A. Evaluation of antioxidant activity of the polysaccharides extracted from Lycium barbarum fruits in vitro. Eur. Polym. J. 2007, 43, 488–497. [Google Scholar] [CrossRef]
- Yi, R.; Liu, X.-M.; Dong, Q. A study of Lycium barbarum polysaccharides (LBP) extraction technology and its anti-aging effect. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 171–174. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xia, G.; Xin, N.; Liu, W.; Yao, H.; Hou, Y.; Qi, J. Inhibitory effect of Lycium barbarum polysaccharides on cell apoptosis and senescence is potentially mediated by the p53 signaling pathway. Mol. Med. Rep. 2014, 9, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.-C.; Liu, J.-H.; Wu, R.-Y. Establishment of the mimetic aging effect in mice caused by D-galactose. Biogerontology 2003, 4, 15–18. [Google Scholar] [CrossRef]
- Deng, H.-B.; Cui, D.-P.; Jiang, J.-M.; Feng, Y.-C.; Cai, N.-S.; Li, D.-D. Inhibiting effects of Achyranthes bidentata polysaccharide and Lycium barbarum polysaccharide on non-enzyme glycation in D-galactose induced mouse aging model. Biomed. Environ. Sci. 2003, 16, 267–275. [Google Scholar]
- Wang, Y.; Zhao, H.; Sheng, X.; Gambino, P.E.; Costello, B.; Bojanowski, K. Protective effect of Fructus lycii polysaccharides against time and hyperthermia-induced damage in cultured seminiferous epithelium. J. Ethnopharmacol. 2002, 82, 169–175. [Google Scholar] [CrossRef]
- Ji, L.L. Antioxidant signaling in skeletal muscle: A brief review. Exp. Gerontol. 2007, 42, 582–593. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- David, J.A.; Rifkin, W.J.; Rabbani, P.S.; Ceradini, D.J. The Nrf2/Keap1/ARE Pathway and oxidative stress as a therapeutic target in type II diabetes mellitus. J. Diabetes Res. 2017, 2017, 4826724. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Li, D.; Jin, S.; Ding, J.; Guo, J.; Shi, W.; Wang, C. Stimuli-responsive biodegradable poly (methacrylic acid) based nano-capsules for ultrasound traced and triggered drug delivery system. Biomaterials 2014, 35, 2079–2088. [Google Scholar] [CrossRef]
- Gao, Y.; Wei, Y.; Wang, Y.; Gao, F.; Chen, Z. Lycium barbarum: A traditional Chinese herb and a promising anti-aging agent. Aging Dis. 2017, 8, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Ming, M.; Guanhua, L.; Zhanhai, Y.; Guang, C.; Xuan, Z. Effect of the Lycium barbarum polysaccharides administration on blood lipid metabolism and oxidative stress of mice fed high-fat diet in vivo. Food Chem. 2009, 113, 872–877. [Google Scholar] [CrossRef]
- Luo, Q.; Cai, Y.; Yan, J.; Sun, M.; Corke, H. Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sci. 2004, 76, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Im, A.-R.; Lee, H.J.; Youn, U.J.; Hyun, J.W.; Chae, S. Orally administered betaine reduces photodamage caused by UVB irradiation through the regulation of matrix metalloproteinase-9 activity in hairless mice. Mol. Med. Rep. 2016, 13, 823–828. [Google Scholar] [CrossRef]
- Cao, G.; Yang, W.; Du, P. Observation of the effects of LAK/IL-2 Therapy combining with Lycium barbarium polysaccharides in the treatment of 75 cancer patients. Chin. J. Oncol. 1994, 16, 428–431. [Google Scholar]
- Gan, L.; Zhang, S.H.; Yang, X.L.; Xu, H.B. Immunomodulation and antitumor activity by a polysaccharide À protein complex from Lycium barbarum. Int. Immunopharmacol. 2004, 4, 563. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, H.; Huang, J.; Li, Z.; Zhu, C.; Zhang, S. Effect of Lycium barbarum polysaccharide on human hepatoma QGY7703 cells: Inhibition of proliferation and induction of apoptosis. Life Sci. 2005, 76, 2115–2124. [Google Scholar] [CrossRef]
- Su, C.-X.; Duan, X.-G.; Liang, L.-J.; Wang, F.; Zheng, J.; Fu, X.-Y.; Yan, Y.-M.; Huang, L.; Wang, N.-P. Lycium barbarum polysaccharides as an adjuvant for recombinant vaccine through enhancement of humoral immunity by activating Tfh cells. Veter-Immunol. Immunopathol. 2014, 158, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.-F.; Zhao, C.; Chen, X.; Chan, S.-W.; Wu, J.-Y. Chemical properties and bioactivities of Goji (Lycium barbarum) polysaccharides extracted by different methods. J. Funct. Foods 2015, 17, 903–909. [Google Scholar] [CrossRef]
- He, N.; Yang, X.; Jiao, Y.; Tian, L.; Zhao, Y. Characterisation of antioxidant and antiproliferative acidic polysaccharides from Chinese wolfberry fruits. Food Chem. 2012, 133, 978–989. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Q.-Y.; Jiang, Q.-Y.; Kang, X.-M.; Zhao, L. Polysaccharides derived from Lycium barbarum suppress IGF-1-induced angiogenesis via PI3K/HIF-1α/VEGF signalling pathways in MCF-7 cells. Food Chem. 2012, 131, 1479–1484. [Google Scholar] [CrossRef]
- Deng, X.; Luo, S.; Luo, X.; Hu, M.; Ma, F.; Wang, Y.; Zhou, L.; Huang, R. Fraction From Lycium barbarum Polysaccharides Reduces Immunotoxicity and Enhances Antitumor Activity of Doxorubicin in Mice. Integr. Cancer Ther. 2018, 17, 860–866. [Google Scholar] [CrossRef]
- Kim, N.H.; Sung, B.; Kang, Y.J.; Jang, J.Y.; Hwang, S.Y.; Lee, Y.; Kim, M.; Im, E.; Yoon, J.-H.; Kim, C.M.; et al. Anti-inflammatory effects of betaine on AOM/DSS-induced colon tumorigenesis in ICR male mice. Int. J. Oncol. 2014, 45, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Rasane, P.; Kaur, S.; Kumar, V.; Dhawan, K.; Mahato, D.K.; Malhotra, S.; Sarma, C.; Kaur, D.; Bhattacharya, J. Nutritional interventions and considerations for the development of low calorie or sugar free foods. Curr. Diabetes Rev. 2020, 16, 301–312. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the Year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef]
- Silva, C.; Alves, B.; Azzalis, L.; Junqueira, V.; Fonseca, R.; Fonseca, A.; Fonseca, F. Goji Berry (Lycium Barbarum) in the treatment of diabetes melitus: A systematic review. Food Res. 2017, 1, 221–224. [Google Scholar] [CrossRef]
- Wu, H.; Guo, H.; Zhao, R. Effect of Lycium barbarum Polysaccharide on the improvement of antioxidant ability and DNA damage in NIDDM Rats. Yakugaku Zasshi 2006, 126, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Tang, X.; Wang, F.; Zhang, Q.; Zhang, Z. Characterization of Lycium barbarum polysaccharide and its effect on human hepatoma cells. Int. J. Biol. Macromol. 2013, 61, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Bagul, P.K.; Middela, H.; Matapally, S.; Padiya, R.; Bastia, T.; Madhusudana, K.; Reddy, B.R.; Chakravarty, S.; Banerjee, S.K. Attenuation of insulin resistance, metabolic syndrome and hepatic oxidative stress by resveratrol in fructose-fed rats. Pharmacol. Res. 2012, 66, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, Y.; Kaneto, H.; Kawamori, D.; Hatazaki, M.; Miyatsuka, T.; Matsuoka, T.-A.; Kajimoto, Y.; Matsuhisa, M.; Yamasaki, Y.; Hori, M. Modulation of the JNK pathway in liver affects insulin resistance status. J. Biol. Chem. 2004, 279, 45803–45809. [Google Scholar] [CrossRef] [PubMed]
- Kaneto, H. The JNK pathway as a therapeutic target for diabetes. Expert Opin. Ther. Targets 2005, 9, 581–592. [Google Scholar] [CrossRef]
- Kaneto, H.; Matsuoka, T.-A.; Nakatani, Y.; Kawamori, D.; Miyatsuka, T.; Matsuhisa, M.; Yamasaki, Y. Oxidative stress, ER stress, and the JNK pathway in type 2 diabetes. Klin. Wochenschr. 2005, 83, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, W.; Li, Y.; Wang, Q.; Gao, L.; Zhao, J. Dietary Lycium barbarum Polysaccharide induces Nrf2/ARE pathway and ameliorates insulin resistance induced by high-fat via activation of PI3K/AKT signaling. Oxidative Med. Cell. Longev. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Cai, H.; Liu, F.; Zuo, P.; Huang, G.; Song, Z.; Wang, T.; Lu, H.; Guo, F.; Han, C.; Sun, G. Practical application of antidiabetic efficacy of Lycium barbarum polysaccharide in patients with Type 2 diabetes. Med. Chem. 2015, 11, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Gao, X.; Zhang, T.; Li, X. Effects of Lycium barbarum. polysaccharide on type 2 diabetes mellitus rats by regulating biological rhythms. Iran. J. Basic Med. Sci. 2016, 19, 1024–1030. [Google Scholar] [CrossRef]
- Finegold, J.A.; Asaria, P.; Francis, D.P. Mortality from ischaemic heart disease by country, region, and age: Statistics from World Health Organisation and United Nations. Int. J. Cardiol. 2013, 168, 934–945. [Google Scholar] [CrossRef]
- Levy, D.; Ehret, G.B.; Rice, K.; Verwoert, G.C.; Launer, L.J.; Dehghan, A.; Glazer, N.L.; Morrison, A.C.; Johnson, A.D.; Aspelund, T.; et al. Genome-wide association study of blood pressure and hypertension. Nat. Genet. 2009, 41, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.-P.; Zhao, P.-T. Chemical characterization of Lycium barbarum polysaccharides and their reducing myocardial injury in ischemia/reperfusion of rat heart. Int. J. Biol. Macromol. 2010, 47, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.-F.; Zhou, G.-L.; Deng, Z.-Y.; Chen, Y.-X.; Wu, Y.-G.; Xu, P.-S.; Xuan, Y.-X. Protective effect of Lycium barbarum on doxorubicin-induced cardiotoxicity. Phytother. Res. 2007, 21, 1020–1024. [Google Scholar] [CrossRef]
- Xin, Y.-F.; Wan, L.-L.; Peng, J.-L.; Guo, C. Alleviation of the acute doxorubicin-induced cardiotoxicity by Lycium barbarum polysaccharides through the suppression of oxidative stress. Food Chem. Toxicol. 2011, 49, 259–264. [Google Scholar] [CrossRef]
- Xin, Y.; Zhang, S.; Gu, L.; Liu, S.; Gao, H.; You, Z.; Zhou, G.; Wen, L.; Yu, J.; Xuan, Y. Electrocardiographic and Biochemical evidence for the cardioprotective effect of antioxidants in acute doxorubicin-induced cardiotoxicity in the beagle dogs. Biol. Pharm. Bull. 2011, 34, 1523–1526. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, X.; Lin, Y.; Suo, M.; Gong, L.; Chen, J.; Hui, R. Anti-hypertensive effect of Lycium barbarum L. with down-regulated expression of renal endothelial lncRNA sONE in a rat model of salt-sensitive hypertension. Int. J. Clin. Exp. Pathol. 2015, 8, 6981–6987. [Google Scholar]
- Guo, X.F.; Li, Z.H.; Cai, H.; Li, D. The Effects of Lycium Barbarum L. (L. Barbarum) on cardiometabolic risk factors: A me-ta-analysis of randomized controlled trials. Food Funct. 2017, 8, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Demori, I.; Voci, A.; Fugassa, E.; Burlando, B. Combined effects of high-fat diet and ethanol induce oxidative stress in rat liver. Alcohol 2006, 40, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Orman, E.S.; Odena, G.; Bataller, R. Alcoholic liver disease: Pathogenesis, management, and novel targets for therapy. J. Gastroenterol. Hepatol. (Aust.) 2013, 28, 77–84. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, J.; Xing, F.; Han, T.; Jiao, R.; Liong, E.C.; Fung, M.-L.; So, K.-F.; Tipoe, G.L. Zeaxanthin Dipalmitate therapeutically improves hepatic functions in an alcoholic fatty liver disease model through modulating MAPK pathway. PLoS ONE 2014, 9, e95214. [Google Scholar] [CrossRef]
- Xiao, J.; Zhu, Y.; Liu, Y.; Tipoe, G.L.; Xing, F.; So, K.-F. Lycium barbarum polysaccharide attenuates alcoholic cellular injury through TXNIP-NLRP3 inflammasome pathway. Int. J. Biol. Macromol. 2014, 69, 73–78. [Google Scholar] [CrossRef]
- Assy, N.; Kaita, K.; Mymin, D.; Levy, C.; Rosser, B.; Minuk, G. Fatty infiltration of liver in hyperlipidemic patients. Am. J. Dig. Dis. Sci. 2000, 45, 1929–1934. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Choi, J.S.; Kim, M.H.; Jung, M.H.; Lee, Y.S.; Song, J. Effects of dietary genistein on hepatic lipid metabolism and mitochondrial function in mice fed high-fat diets. Nutrition 2006, 22, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Lee, E.J.; Kim, H.P.; Kim, Y.C.; Moon, A. A novel cerebroside from Lycii fructus preserves the hepatic glutathione redox system in primary cultures of rat hepatocytes. Biol. Pharm. Bull. 1999, 22, 873–875. [Google Scholar] [CrossRef]
- Xiao, J.; Liong, E.C.; Ching, Y.P.; Chang, R.C.C.; So, K.F.; Fung, M.L.; Tipoe, G.L. Lycium barbarum polysaccharides protect mice liver from carbon tetrachloride-induced oxidative stress and necroinflammation. J. Ethnopharmacol. 2012, 139, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, N.I.; Landrum, J.T.; Bone, R.A. Biologic mechanisms of the protective role of Iutein and Zeaxanthin in the eye. Annu. Rev. Nutr. 2003, 23, 171. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.R.; West, S.; Muñoz, B.; Rosenthal, F.S.; Bressler, S.B.; Bressler, N.M. The Long-term effects of visible light on the eye. Arch. Ophthalmol. 1992, 110, 99–104. [Google Scholar] [CrossRef]
- Tham, Y.-C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.-Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Quigley, H.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef]
- Prasanna, G.; Hulet, C.; Desai, D.; Krishnamoorthy, R.R.; Narayan, S.; Brun, A.-M.; Suburo, A.M.; Yorio, T. Effect of elevated intraocular pressure on endothelin-1 in a rat model of glaucoma. Pharmacol. Res. 2005, 51, 41–50. [Google Scholar] [CrossRef]
- Leung, I.; Tso, M.; Li, W.; Lam, T. Absorption and tissue distribution of Zeaxanthin and Iutein in rhesus monkeys after taking Fructus lycii (Gou Qi Zi) extract. Investig. Ophthalmol. Vis. Sci. 2001, 42, 466. [Google Scholar]
- Chan, H.H.-L.; Lam, C.H.-I.; Choi, K.-Y.; Li, S.Z.-C.; Lakshmanan, Y.; Yu, W.-Y.; Chang, R.C.-C.; Lai, J.S.-M.; So, K.-F. Delay of cone degeneration in retinitis pigmentosa using a 12-month treatment with Lycium barbarum supplement. J. Ethnopharmacol. 2019, 236, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.H.W.; Li, H.-Y.; Chin, M.-P.; So, K.-F.; Chan, H.H.L. Effect of Lycium barbarum (Wolfberry) polysaccharides on preserving retinal function after partial optic nerve transection. PLoS ONE 2013, 8, e81339. [Google Scholar] [CrossRef]
- Li, H.-Y.; Ruan, Y.-W.; Kau, P.W.-F.; Chiu, K.; Chang, R.C.-C.; Chan, H.H.L.; So, K.-F. Effect of Lycium barbarum (Wolfberry) on alleviating axonal degeneration after partial optic nerve transection. Cell Transplant. 2015, 24, 403–417. [Google Scholar] [CrossRef]
- Mi, X.-S.; Feng, Q.; Lo, A.C.Y.; Chang, R.C.-C.; Lin, B.; Chung, S.K.; So, K.-F. Protection of retinal ganglion cells and retinal vasculature by Lycium barbarum polysaccharides in a mouse model of acute ocular hypertension. PLoS ONE 2012, 7, e45469. [Google Scholar] [CrossRef]
- Mi, X.S.; Chiu, K.; Van, G.; Leung, J.W.; Lo, A.C.; Chung, S.K.; Chang, R.C.; So, K.F. Effect of Lycium barbarum polysaccharides on the expression of endothelin-1 and its receptors in an ocular hypertension model of rat glaucoma. Neural Regen. Res. 2012, 7, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Y.; Yang, D.; Yeung, C.-M.; Yu, W.Y.; Chang, R.C.-C.; So, K.-F.; Wong, D.; Lo, A.C.Y. Lycium barbarum polysaccharides reduce neuronal damage, blood-retinal barrier disruption and oxidative stress in retinal ischemia/reperfusion injury. PLoS ONE 2011, 6, e16380. [Google Scholar] [CrossRef]
- He, M.; Pan, H.; Chang, R.C.-C.; So, K.-F.; Brecha, N.C.; Pu, M. Activation of the Nrf2/HO-1 antioxidant pathway contributes to the protective effects of Lycium barbarum polysaccharides in the rodent retina after ischemia-reperfusion-induced damage. PLoS ONE 2014, 9, e84800. [Google Scholar] [CrossRef]
- Flammer, J.; Mozaffarieh, M. What is the present pathogenetic concept of glaucomatous optic neuropathy? Surv. Ophthalmol. 2007, 52, S162–S173. [Google Scholar] [CrossRef] [PubMed]
- Bucheli, P.; Vidal, K.; Shen, L.; Gu, Z.; Zhang, C.; Miller, L.; Wang, J. Goji berry effects on macular characteristics and plasma antioxidant levels. Optom. Vis. Sci. 2011, 88, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, A.; Ishiko, S.; Akiba, J.; Kitaya, N.; Nagaoka, T. Radiating retinal folds detected by scanning laser ophthalmoscopy using a diode laser in a dark-field mode in idiopathic macular holes. Graefe’s Arch. Clin. Exp. Ophthalmol. 1998, 236, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Prasad, T.; Zhu, P.; Verma, A.; Chakrabarty, P.; Rosario, A.M.; Golde, T.E.; Li, Q. Amyloid β peptides overexpression in retinal pigment epithelial cells via AAV-mediated gene transfer mimics AMD-like pathology in mice. Sci. Rep. 2017, 7, 3222. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.-S.; Yu, M.-S.; Lai, C.S.-W.; So, K.-F.; Yuen, W.-H.; Chang, R.C.-C. Characterizing the neuroprotective effects of alkaline extract of Lycium barbarum on β-amyloid peptide neurotoxicity. Brain Res. 2007, 1158, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Chien, K.; Horng, C.; Huang, Y.; Hsieh, Y.; Wang, C.; Yang, J.; Lu, C.; Chen, F. Effects of Lycium barbarum (goji berry) on dry eye disease in rats. Mol. Med. Rep. 2018, 17, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Lilly, D.M.; Stillwell, R.H. Probiotics: Growth-promoting factors produced by microorganisms. Science 1965, 147, 747–748. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Probiotics in Food Health and Nutritional Properties and Guidelines for Evaluation; Food and Nutrition Paper; FAO: Rome, Italy, 2001; Volume 85. [Google Scholar]
- Guo, L.; Li, T.; Tang, Y.; Yang, L.; Huo, G. Probiotic properties of Enterococcus strains isolated from traditional naturally fermented cream in China. Microb. Biotechnol. 2015, 9, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Ohimain, E.I.; Ofongo, R.T.S. The Effect of probiotic and prebiotic feed supplementation on chicken health and gut microflora: A Review. Int. J. Anim. Vet. Adv. 2012, 4, 135–143. [Google Scholar]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Gibson, G.R.; Scott, K.P.; Rastall, R.A.; Tuohy, K.M.; Hotchkiss, A.; Dubert-Ferrandon, A.; Gareau, M.; Murphy, E.F.; Saulnier, D.; Loh, G.; et al. Dietary prebiotics: Current status and new definition. Food Sci. Technol. Bull. Funct. Foods 2010, 7, 1–19. [Google Scholar] [CrossRef]
- Trowell, H.; Southgate, D.A.T.; Wolever, T.M.S.; Leeds, A.R.; Gassull, M.A.; Jenkins, D.J.A. Dietary fibre redefined. Lancet 1976, 307, 967. [Google Scholar] [CrossRef]
- Schweizer, T.F.; Würsch, P. The physiological and nutritional importance of dietary fibre. Experientia 1991, 47, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Baba, A.S.; Najarian, A.; Shori, A.B.; Lit, K.W.; Keng, G.A. Viability of lactic acid bacteria, Antioxidant activity and in vitro inhibition of angiotensin-I-converting enzyme of Lycium barbarum yogurt. Arab. J. Sci. Eng. 2014, 39, 5355–5362. [Google Scholar] [CrossRef]
- Liao, M.; Wu, Z.Y.; Yu, G.H.; Zhang, W.X. Improving the quality of Sichuan pickle by adding a traditional Chinese medicinal herb Lycium barbarumin its fermentation. Int. J. Food Sci. Technol. 2017, 52, 936–943. [Google Scholar] [CrossRef]
- Zhou, F.; Jiang, X.; Wang, T.; Zhang, B.; Zhao, H. Lycium barbarum polysaccharide (LBP): A novel prebiotics candidate for Bifidobacterium and Lactobacillus. Front. Microbiol. 2018, 9, 1034. [Google Scholar] [CrossRef]
- González-Rodríguez, I.; Sánchez, B.; Ruiz, L.; Turroni, F.; Ventura, M.; Ruas-Madiedo, P.; Gueimonde, M.; Margolles, A. Role of extracellular transaldolase from Bifidobacterium bifidum in mucin adhesion and aggregation. Appl. Environ. Microbiol. 2012, 78, 3992–3998. [Google Scholar] [CrossRef]
- Nami, Y.; Abdullah, N.; Haghshenas, B.; Radiah, D.; Rosli, R.; Khosroushahi, A.Y. Probiotic potential and biotherapeutic effects of newly isolated vaginal Lactobacillus acidophilus 36YL strain on cancer cells. Anaerobe 2014, 28, 29–36. [Google Scholar] [CrossRef]
- Alp, G.; Aslim, B.; Suludere, Z.; Akca, G. The role of hemagglutination and effect of exopolysaccharide production on bifidobacteria adhesion to Caco-2 cells in vitro. Microbiol. Immunol. 2010, 54, 658–665. [Google Scholar] [CrossRef]
- Al-Sheraji, S.H.; Ismail, A.; Manap, M.Y.; Mustafa, S.; Yusof, R.M. Viability and activity of Bifidobacteria during refrigerated storage of yoghurt containing Mangifera pajang fibrous polysaccharides. J. Food. Sci. 2012, 77, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Redgwell, R.J.; Curti, D.; Wang, J.; Dobruchowska, J.M.; Gerwig, G.J.; Kamerling, J.P.; Bucheli, P. Cell wall polysaccharides of Chinese Wolfberry (Lycium barbarum): Part Characterisation of arabinogalactan-proteins. Carbohydr. Polym. 2011, 84, 1075–1083. [Google Scholar] [CrossRef]
- Redgwell, R.J.; Curti, D.; Wang, J.; Dobruchowska, J.M.; Gerwig, G.J.; Kamerling, J.P.; Bucheli, P. Cell wall polysaccharides of Chinese Wolfberry (Lycium barbarum): Part Characterization of soluble and insoluble polymer fractions. Carbohydr. Polym. 2011, 84, 1344–1349. [Google Scholar] [CrossRef]
- Reeve, V.E.; Allanson, M.; Arun, S.J.; Domanski, D.; Painter, N. Mice drinking goji berry juice (Lycium barbarum) are protected from UV radiation-induced skin damage via antioxidant pathways. Photochem. Photobiol. Sci. 2010, 9, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.-J.; Zheng, J.; Wu, J.; Qiao, H.-Q.; Chang, Q.; Niu, Y.; Sun, T.; Li, Y.-X.; Yu, J.-Q. Beneficial effects of Lycium barbarum polysaccharide on spermatogenesis by improving antioxidant activity and inhibiting apoptosis in streptozotocin-induced diabetic male mice. Food Funct. 2017, 8, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Vidović, B.B.; Milinčić, D.D.; Marčetić, M.D.; Djuriš, J.D.; Ilić, T.D.; Kostić, A.Ž.; Pešić, M.B. Health benefits and applications of goji berries in functional food products development: A review. Antioxidants 2022, 11, 248. [Google Scholar] [CrossRef] [PubMed]
- Mocan, A.; Vlase, L.; Vodnar, D.C.; Bischin, C.; Hanganu, D.; Gheldiu, A.-M.; Oprean, R.; Silaghi-Dumitrescu, R.; Crișan, G. Polyphenolic content, antioxidant and antimicrobial activities of Lycium barbarum L. and Lycium chinense Mill. leaves. Molecules 2014, 19, 10056–10073. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skenderidis, P.; Leontopoulos, S.; Lampakis, D. Goji Berry: Health Promoting Properties. Nutraceuticals 2022, 2, 32-48. https://doi.org/10.3390/nutraceuticals2010003
Skenderidis P, Leontopoulos S, Lampakis D. Goji Berry: Health Promoting Properties. Nutraceuticals. 2022; 2(1):32-48. https://doi.org/10.3390/nutraceuticals2010003
Chicago/Turabian StyleSkenderidis, Prodromos, Stefanos Leontopoulos, and Dimitrios Lampakis. 2022. "Goji Berry: Health Promoting Properties" Nutraceuticals 2, no. 1: 32-48. https://doi.org/10.3390/nutraceuticals2010003
APA StyleSkenderidis, P., Leontopoulos, S., & Lampakis, D. (2022). Goji Berry: Health Promoting Properties. Nutraceuticals, 2(1), 32-48. https://doi.org/10.3390/nutraceuticals2010003






