Abstract
Voluntary HIV testing and counselling (VCT) in the workplace could reach population groups who may be at risk for HIV but may not readily seek out testing from other services. We conducted a scoping review to understand (a) the nature of evidence related to initiatives and interventions for vocationally active adults on VCT in occupational settings, and (b) any facilitators and barriers to the delivery of and/or engagement with VCT initiatives/interventions in the workplace. JBI scoping review methodology was followed. The protocol was pre-registered. Included studies focused on vocationally active adults (population), VCT interventions or initiatives (concept), and workplaces in any sector or country (context). The review included studies published after 2000, in English, and of any research design. Studies relating to mandatory workplace HIV screening were excluded. MEDLINE, CINAHL, Scopus, PsycINFO, and the Cochrane Central Register of Control Trials were searched. Sources of grey literature included Google Scholar and governmental and organisational websites. One reviewer screened titles and abstracts; a second reviewer independently screened 10%. Data extraction utilised a modified JBI data extraction tool. We identified 17 studies reporting on 12 workplace VCT interventions (20,985 participants, 15–70 years). Studies were conducted in eight countries between 2001 and 2022. Interventions were delivered in organisations of different types, sizes and sectors. Testing included rapid blood tests and oral fluid self-tests. Where reported, the average on-site HIV testing uptake rate was 63%, and the average linkage to care rate was 86.85%. Views of workers, employers and service providers were largely positive. Barriers included being male, masculinity-driven workplace culture, HIV-related stigma, poor knowledge, low risk perceptions, lack of time and low support. Facilitators included on-site testing for convenience and accessibility, rapid and free tests, organisational, managerial and peer support, and embedding HIV tests within general health checks. Evaluation methods varied, although randomised trial designs were uncommon. Despite the limited number of studies, the workplace appears to be a viable route to the delivery of community-based VCT, albeit barriers should be addressed. Reporting quality of interventions and associated evaluations is variable and could be improved with the use of appropriate checklists.
1. Introduction
The Human Immunodeficiency Virus (HIV) specifically targets and damages the body’s immune system, gradually deteriorating its function and leaving the infected individual vulnerable to life-threatening infections and diseases [1]. If left untreated, HIV can progress to acquired immunodeficiency syndrome (AIDS), which ultimately leads to death [2]. HIV primarily spreads through unprotected sexual intercourse, sharing needles or syringes, and from mother to child during childbirth or breastfeeding. The global impact of HIV/AIDS is significant; in 2023, there were an estimated 39.9 million people living with HIV, with 1.3 million new infections and 630,000 AIDS-related deaths reported in the same year [3]. These statistics highlight the pressing need for prevention, treatment, and support programmes on a global scale.
HIV testing plays a crucial role in the prevention of the spread of the virus. Global data from 2023 show that 14% of people living with HIV are not aware of their HIV status [3], which highlights the need for increased testing efforts. Early detection of HIV is, therefore, a life-saving step as it allows for prompt initiation of treatment, which not only improves individual health outcomes but also reduces the risk of transmission to others [4]. Although there is currently no cure for HIV, antiretroviral therapy (ART) effectively suppresses viral replication, leading to lower viral loads in the blood and reduced transmission risk [5]. The concept of ‘Undetectable = Untransmittable’ (U = U) is relevant here because it highlights that people living with HIV who are on treatment and have a fully suppressed viral load have a zero risk of transmitting the virus to their sexual partners. Efforts to maximise early detection through screening are, therefore, critical.
Based on employment-to-population ratios worldwide, around 58% of the global working-age population is employed [6]. Given the high proportion of time adults spend at work, the workplace is increasingly seen as an important avenue for promoting health and wellbeing [7,8]. Health screening is becoming a common feature in workplace health promotion programmes, such as for diabetes [9], cardiovascular disease risk [10], mental health [11], and general health checks [12]. However, workplace interventions rarely include HIV testing and counselling [13]. The inclusion of voluntary HIV testing and counselling (VCT) in the context of workplace health promotion may help to reduce HIV-related stigma and increase access to testing. Further, certain occupational settings pose a higher risk for HIV transmission among vocationally active adults. The risk for HIV transmission in workplace settings is increased by exposure to blood, body fluids, or tissues while undertaking job-related tasks. Examples include health workers, police officers, fire fighters, and correctional facility personnel. Also, settings that typically encompass male-dominated workplaces, such as the construction industry [14], may be linked to locations where there may be high levels of sex work. Risk is also high in work environments involving direct exposure to blood or other infectious substances, such as through unsafe injection equipment, like needles or syringes [15].
Existing knowledge in this area is limited, and there is a need to map out research on initiatives and interventions for VCT in the workplace in terms of ‘how’ and ‘where’ they have been implemented and evaluated. There is also a need to identify the facilitators and barriers that exist in relation to the delivery of, and participation in, VCT in workplace settings. Exploring these factors can provide a better understanding of the current efforts and challenges in promoting VCT among vocationally active adults in occupational settings. This knowledge could contribute to the development of targeted strategies and interventions to improve HIV prevention, awareness, and support in community contexts.
A preliminary search of MEDLINE, the Cochrane Database of Systematic Reviews, and JBI Evidence Synthesis was conducted in October 2023, and no current or underway systematic reviews or scoping reviews on the topic were identified. There were a limited number of reviews available focusing on preventing risky sexual behaviours [16], community-based approaches [17], and barriers to workplace HIV testing in South Africa [18], but they did not cover a global perspective or provide insights from an occupational standpoint on applied VCT. Therefore, we aimed to conduct a scoping review to describe and understand what research has been undertaken on initiatives and interventions about VCT in occupational settings, in a global context.
The review questions were:
1. What is the evidence related to initiatives and interventions for vocationally active adults on VCT in occupational settings?
2. What are the facilitators and barriers to the delivery of and/or engagement with VCT initiatives/interventions in the workplace?
2. Materials and Methods
The scoping review was conducted in accordance with the JBI methodology for scoping reviews [19] and reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) [20] (Table S1). The protocol was pre-registered: https://doi.org/10.17605/OSF.IO/BAMJ7 (accessed on 10 Feb 2025). A scoping review was appropriate as the aim was to describe, map and characterise the evidence rather than synthesise data to answer a focused clinical question [21].
2.1. Inclusion Criteria
The inclusion and exclusion criteria follow the participant, concept, and context (PCC) framework [22].
Participants: This review included studies involving vocationally active adults. Vocationally active adults are defined as people who are currently engaged in the workforce, pursuing a career, or engaging in vocational activities such as seasonal working. This term was deliberately chosen since it includes all types of working status involving being employed, self-employed, or actively seeking employment while utilising their skills, knowledge, and expertise in a specific field or occupation. The review focus intended to be on adult populations as defined in the United Kingdom (UK) (18 years of age or over), but studies with an age range of 15 years or over were included as it was not possible to separate the results. This decision was made because the initial search identified some studies that presented age ranges from 15 to 24 years.
Concept: This review included any study that explores or evaluates opt-in VCT initiatives or interventions. Mandatory HIV screening via occupational health surveillance programmes was excluded.
Context: Any type of occupational setting without geographical limitation was included in the review. However, studies relating to sex work were excluded due to the highly variable context and occupational/legal status of sex work settings worldwide (i.e., ranging from highly criminalised and not considered an ‘occupation’ nor governed by labour laws in some settings to a more formalised legal status in other settings) [23]. Due to the severe stigma and ambiguous legal/occupational status of sex work globally, it was felt that this context was not comparable to other potential settings and would require a separate review that could take these factors into account.
2.2. Types of Sources
This scoping review included any type of study design.
2.3. Search Strategy
The search strategy aimed to locate both published and unpublished studies. An initial limited search of MEDLINE, the Cochrane Library, and JBI Evidence Synthesis was undertaken by one reviewer (MY) to identify articles on the topic. The text words contained in the titles and abstracts of relevant articles, and the index terms used to describe the articles were used to develop a full search strategy (Text S1). The search strategy was developed with support from a librarian. The databases searched included CINAHL, Embase, Scopus, the Cochrane Register of Control Trials, and Epistemonicus. The search strategy, including all identified keywords and index terms, was adapted for each included database and/or information source. The reference list of all included sources of evidence was screened for additional studies, and forward citation searching was undertaken. Sources of unpublished studies/grey literature included well-known HIV/AIDS-related websites such as WHO, UNAIDS, and PERFAR. Searches were conducted in November 2023. We focused on literature published since 2000 because HIV rapid tests [24] and combined HIV drug therapies [25] began to become widely available in the 2000s. The context and consequences of testing, therefore, altered significantly from the 2000 period onwards.
2.4. Study/Source of Evidence Selection
Following the search, all identified citations were collated and uploaded into Covidence (Veritas Health Innovation, Melbourne, Australia), and all duplicates were removed. Following a pilot phase where 10% of titles and abstracts were screened against the inclusion criteria by two independent reviewers (MY, SL), the remainder were screened by one reviewer (MY) for assessment in the review. The full text of selected citations was assessed in detail against the inclusion criteria by MY. Reasons for the exclusion of sources of evidence at the full-text stage that did not meet the inclusion criteria were recorded and reported in the scoping review. The results of the search and the study inclusion process were reported in full in the final scoping review and presented in a PRISMA-ScR [26] flow diagram. Excluded studies can be found in Supplementary Files (Text S2).
2.5. Data Extraction
Data were extracted from studies included in the scoping review by MY. Data extraction was guided by a modified JBI data extraction tool (Table S2). The tool was modified during the pilot phase and modifications are detailed within the review. The data extracted included specific details about the participants, concept, context, study methods and key findings relevant to the review questions. A 5-item TIDieR-Lite checklist [27] (Table S3) was used to map intervention components (By Whom, What, Where, To What Intensity, How Often) to standardised the way in which interventions in the included studies were characterised.
2.6. Data Analysis and Presentation
The aim of this review was to map and understand the evidence on voluntary HIV testing and counselling in occupational settings. Hence, the data are presented in narrative and tabular formats to facilitate the identification and summarisation of evidence.
3. Results
3.1. Study Inclusion
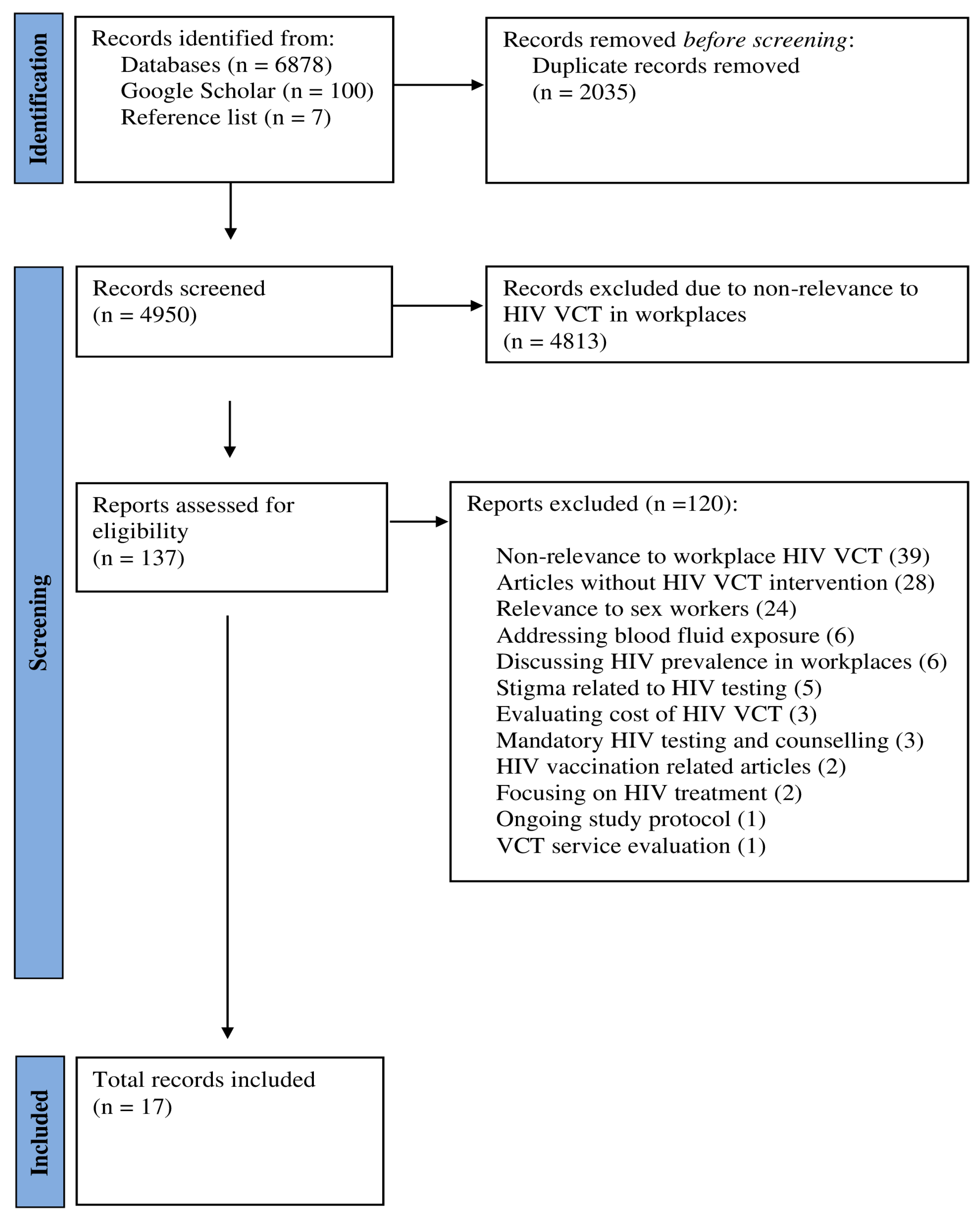
The search identified a total of 6878 records. A further 107 records were obtained from Google Scholar (100 new records) and hand searching reference lists (7 new records), summing to 6985 records. After removing duplicates, 4950 records were screened. During the title and abstract review phase, 4813 records were excluded primarily due to their lack of relevance to HIV testing and counselling in workplaces.
Subsequently, 137 records were selected for full-text review. Of these, 120 articles did not meet the inclusion criteria and were excluded. The reasons for exclusion included non-relevance to workplace VCT (n = 39), no HIV-related VCT intervention (n = 28), focused on sex workers (n = 24), addressed blood fluid exposure (n = 6), focused on HIV prevalence in workplaces (n = 6), or stigma related to HIV testing (n = 5), only evaluated costs of VCT (n = 3), involved mandatory (rather than opt-in) VCT (n = 3), HIV vaccination related articles (n = 2), focused on HIV treatment (n = 2), ongoing study protocol (n = 1), and VCT service evaluation (n = 1).
Finally, 17 articles met inclusion criteria for data charting and summary about HIV VCT in workplace settings. Figure 1 shows the study selection process. A list of excluded studies with reasons can be found in Supplementary Files (Text S2).
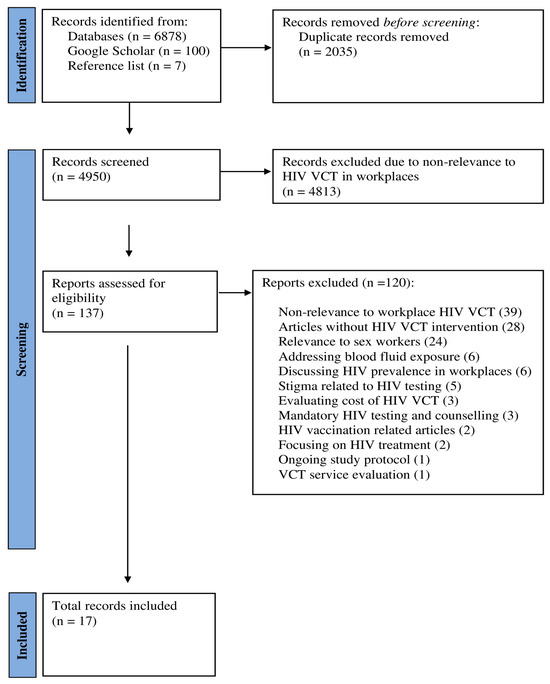
Figure 1.
Study selection process.
3.2. Characteristics of Included Studies
The studies included in this review reflect the global landscape of workplace HIV-related VCT interventions across diverse populations and occupational settings. The 17 identified reports related to 12 distinct VCT interventions. Studies were conducted in various countries across Europe, North America and sub-Saharan Africa. Studies were undertaken in the UK [14,28,29,30,31,32], Italy [33], the Netherlands [34], South Africa [35,36,37,38,39], Uganda [40], Zimbabwe [41], Nigeria [42], and Canada [43]. The studies were published between 2001 and 2022.
Various study designs were applied to evaluate the interventions, but surveys [28,29,30,31,33,42] and interviews [14,28,29,32,39] were predominantly used. Two studies were pilot trials [40,43], and one study employed a cluster randomised control trial design [41]. The other study designs included prospective cohort study [36], case study [35], retrospective analysis [34], and quasi-experimental design [37,38].
The sample sizes for the included workplace HIV testing and counselling interventions ranged from 17 to 9723, with a total of 20,985 participants. Age ranges spanned from 15 to 70 years. Information about gender was not available in some studies [28,35,41]. However, the remaining studies included a total of 9482 male and 6418 female participants.
The settings of the included studies varied widely, reflecting diverse workplace environments across different sectors and regions. In the UK studies, settings included construction [14,31,32] or mixed settings, including leisure, manufacturing, distribution/retail, healthcare, and food production [28,29]. Three studies were conducted in two South African automotive companies [37,38,39]. Settings for the remaining studies included fishing communities [40], manufacturing of various goods (hardware, construction, industrial, clothing, food), telecommunications [41], an industrial company [35], agricultural migrant workers [33], a rural South African factory [30], a sugar mill community [36], service-based industries [42], a brewing company in sub-Saharan Africa [34], and a hospital in Cape Town, South Africa [43]. The characteristics of the included studies are presented in Table 1.

Table 1.
Characteristics of included studies.
3.3. Characteristics of HIV Testing and Counselling Interventions
The interventions were delivered by healthcare professionals, including doctors, nurses, and sexual health specialists. Different HIV testing products were used, mostly rapid blood tests [14,28,29,31,32,33,34,41] and oral fluid self-tests [40,43]. The interventions primarily consisted of one-time workplace events [14,28,29,31,32,33,37,38,39,41,43]. Most interventions linked participants to care after HIV testing [28,29,35,40,41]; however, only a few studies [34,36,40,43] reported a specific linkage rate to care pathways or processes (patient entry into specialist HIV care after diagnosis), with an average rate of 86.85%. The specific features of the interventions were described using the TIDieR-Lite checklist [27]. Table 2 presents intervention-related information.

Table 2.
Characteristics of voluntary HIV testing and counselling interventions based on the TIDieR-Lite Checklist.
The interventions were implemented in organisations of varied sizes, types and sectors. The Healthy Hub Roadshow intervention [28,29] was implemented in 11 organisations, including three small, four medium, and four large-sized companies from various sectors, including leisure, manufacturing, distribution/retail, hospital, local authority, food production, and food industry. Corbett and colleagues [40] implemented intervention in small- and medium-sized businesses (22 in total) involving the manufacturing of hardware, construction, or industrial goods (n = 14), clothing (n = 3), food (n = 3), and telecommunications (n = 2). The Test@Work intervention [14,31,32] was hosted at 16 construction sites across 10 participating organisations (over 21 events in total), including one medium- and nine large-sized companies. Additionally, an HIV testing and counselling campaign with incentives was conducted in two medium-sized automotive companies [37,38,39].
On-site HIV test uptake ranged from 23 to 100%, with an average of 63%. Only one study reported off-site uptake (outside of the workplace), and this rate was considerably lower (4.3%) than that observed in the other included studies [41]. Two studies reported gender differences in participation in HIV testing and counselling interventions, with men being less likely to attend than women [30,34].
3.4. Facilitators and Barriers to HIV Testing and Counselling Interventions
Overall, the evidence suggests a positive perception and acceptance of HIV-related VCT interventions across the workplace settings among intervention participants [14,29,30,31,33,34,36,41,42,43], employers [28,32,35] and service providers [32,40]. Some studies reported that interventions were effective in reaching previously untested populations [29,31,40,41]. These studies reported high proportions of their participants were first-time testers: 75% [29], 1 in 4 [40], 85% on-site and 87.5% off-site [41], and 78% [31].
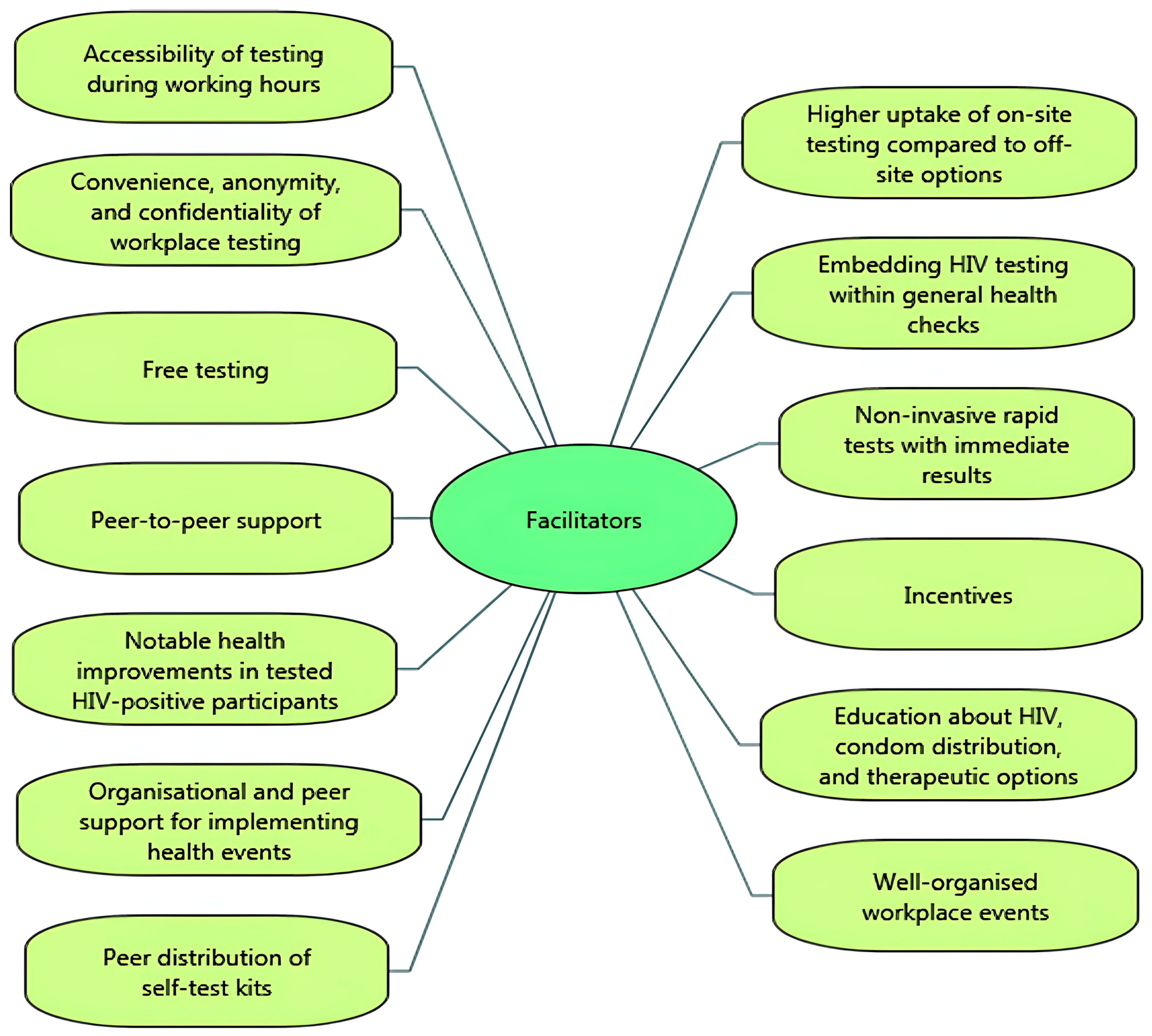
Important facilitators of uptake for testing participants were the accessibility of voluntary HIV testing and counselling testing during working hours (i.e., not needing to take time off work) [14,29], with higher uptake of on-site testing compared to off-site testing options [41]. Participants valued the convenience, anonymity, and confidentiality of workplace testing [30], and the inclusion of VCT by embedding it within a general health check [14,28,29,30,32,33] (i.e., normalising testing by combining HIV-related VCT with other health checks and tests, such as obesity, hypertension, cholesterol, and diabetes). Participants also valued free testing [29], and the availability of non-invasive rapid tests that had immediate results [43]. Uptake of testing was facilitated by peer-to-peer support, creating a sense of social cohesion and collective effort, and the use of incentives (such as lottery incentive schemes, free t-shirts or salary prizes) [38,39]. In the brewing sector, workers in sub-Saharan Africa witnessed improvements in the health of HIV-infected colleagues after testing, which encouraged uptake [34]. One study showed that providing education about HIV, condom distribution, and therapeutic options for those who tested positive increased participation in HIV VCT [36].
From the perspective of employers, managers and VCT service providers, facilitators of successful workplace HIV testing and counselling were at organisational level, management level and worker level. A key facilitator was well-organised workplace events (e.g., in terms of event promotion, planning and facilitating through pre-booking appointments [32]). Uptake of workplace VCT was found to be facilitated by peers (other workers), via ‘peer educators’ (e.g., workers having a role in disseminating information, creating strong communication, decreasing stigma, and encouraging participation across different departments and divisions of the organisation [35]) and ‘peer distributors’ of self-test kits [40].
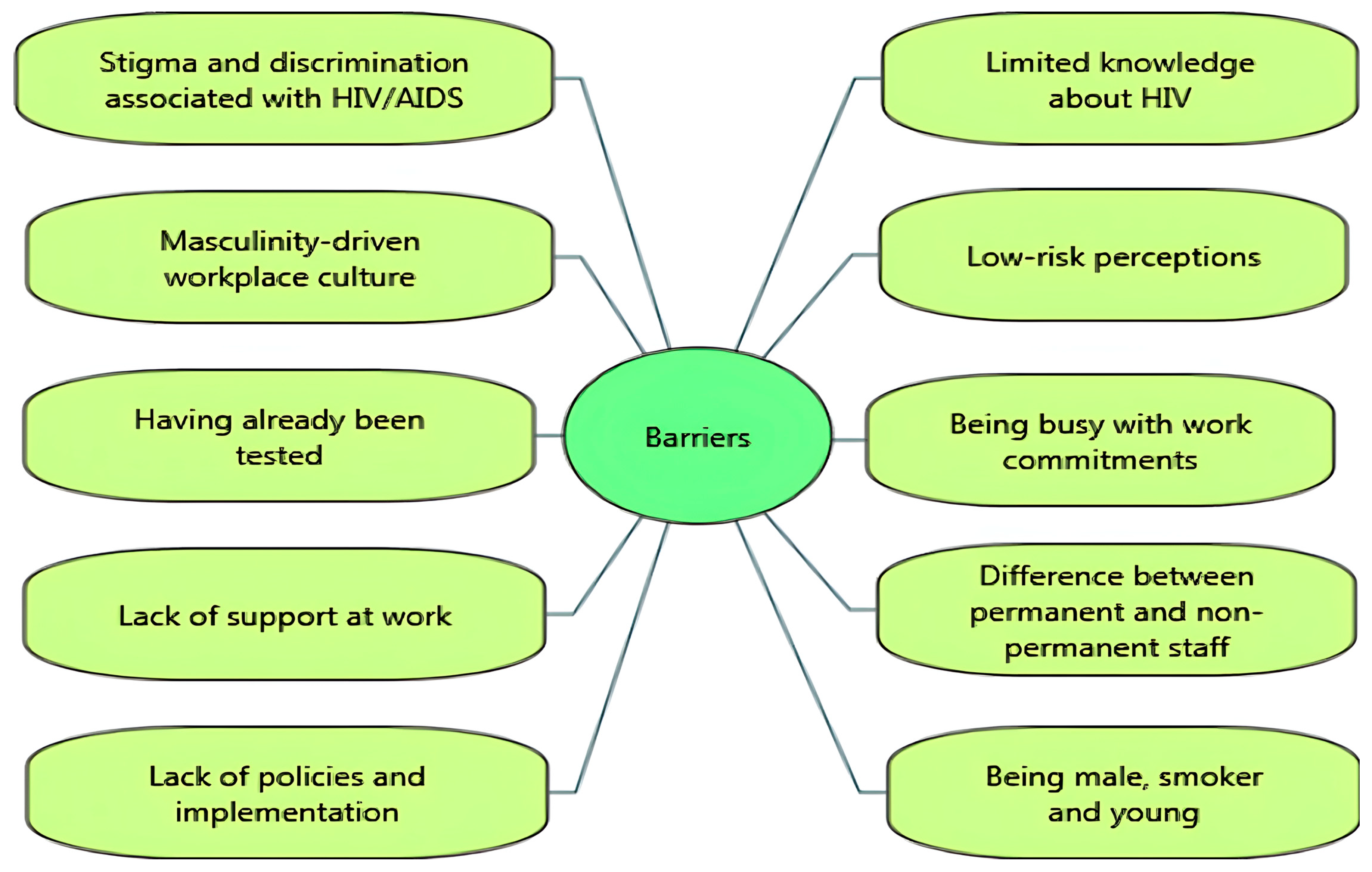
Several barriers were reported to impede the uptake of HIV-related VCT. Stigma and discrimination associated with HIV/AIDS were significant barriers [14,29,35,39]. For example, the UK-based Healthy Hub Roadshow study suggested that limited knowledge and stigma about HIV appeared to be linked to decreased participation in testing [29]. While one study reported attempts to decrease stigma around HIV testing through group discussions at work, it was still highlighted that fear and stigmatisation were barriers [39]. In some settings, employees reported that ‘hyper-masculinity’ in the workplace culture discouraged them from seeking help for sexual and mental health issues [14]. Findings from post-test questionnaire responses reported several barriers to uptake of workplace HIV testing: low perceived risk [29,34,40,42], lack of confidence [29], having already been tested [31,32], and fear of positive test results [14,29]. In the Test@Work studies, both employees and employers reported being busy with work commitments as a barrier to uptake [14,31,32].
A few studies revealed organisational barriers to participation, including perceived lack of support at work [14,35] and differences between groups of workers in terms of awareness or access to health-related support in the workplace. Qualitative data from the Test@Work study suggested that office staff and permanent employees were more likely to be aware of available support or interventions in the workplace than contractors/agency staff, who were more likely to report facing challenges in accessing support from site managers [14]. In a mining company, several challenges were reported, including a lack of policies such as “reasonable accommodations” for HIV-positive workers with inconsistent implementation among departments and the absence of managerial expertise, monitoring, and evaluation [35].
In a ‘Wellness Day’ for factory workers (which included HIV testing), follow-up testing was less well attended by male participants, smokers, and young participants (aged 19–29) [30]. Similarly, Jones and colleagues [31] found that younger participants were more reluctant to participate in health check interventions including HIV testing. Figure 2 and Figure 3 summarise the key facilitators and barriers to HIV testing and counselling in the workplace setting identified in the included studies. Facilitators and barriers to participation in workplace HIV testing and counselling are reported for each study in Table 3.
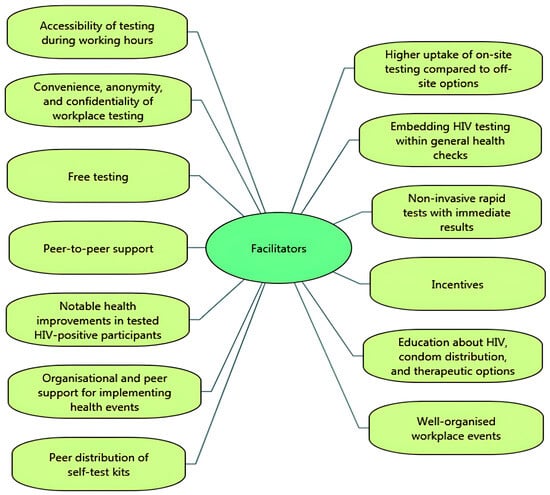
Figure 2.
Facilitators of HIV testing and counselling in the workplace setting.
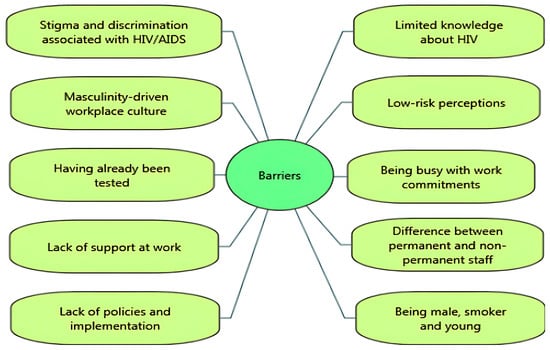
Figure 3.
Barriers to HIV testing and counselling in the workplace setting.

Table 3.
Facilitators and barriers to participation in workplace HIV testing and counselling in included studies.
4. Discussion
This is the first scoping review to map out the nature of the global evidence on initiatives and interventions for vocationally active adults on voluntary HIV testing and counselling (VCT) in occupational settings and to summarise facilitators and barriers to the delivery of and/or engagement with VCT initiatives/interventions in the workplace.
Overall, there were 17 identified articles reporting on 12 workplace VCT interventions. Studies were conducted in eight countries, clustering in the African region and Europe (mostly the UK), with one study in North America. There may be myriad reasons why the focus on workplace VCT may be more common in certain regions. However, it could be partially explained by the fact that, despite substantial variation in HIV prevalence across localities [44], the African region has the highest prevalence of HIV globally (an estimated 25.6 million people in 2022 [45] and, therefore, initiatives to increase access to testing are prevalent. Europe and North America have the highest per capita spending on wellness initiatives than other regions of the world [46], and a burgeoning government-level focus on workplace and health, which includes economic arguments for employers to engage with workplace health initiatives [47].
Interventions were delivered in organisations of different types, sizes and sectors. This demonstrates the potential viability of this health testing approach across occupational settings and diverse worker populations. The successful implementation of workplace VCT in a range of occupational settings concurs with prior survey research in which employers reported positive views towards the concept of workplace HIV testing, with many considering offering HIV testing for their workforce in the future [13]. Although the uptake rate for HIV testing varied across the included studies (ranging from 23% to 100%), on average, two-thirds of participants in the interventions received an HIV test on-site at their workplace. In this review, many of the interventions were delivered in a geographical region with a high prevalence of HIV or were delivered in occupational settings through which disproportionately affected populations could be reached (e.g., in our review, these studies included migrant workers and male-dominated industries such as fishing, agriculture, mining, and construction). It is, therefore, possible that, as a community testing route, workplace HIV-related VCT interventions may contribute to reducing inequalities in testing access. This is particularly important since some of the included studies reported that workplace VCT initiatives reached many first-time testers, further supporting the workplace as a potential venue for community testing. Indeed, data from the UK show that testing people for HIV through community services reaches more first-time testers than national self-sampling schemes [47]. Further, prior studies of general workplace health checks have suggested that delivering health interventions through the workplace setting may help to access groups that are considered hard to reach by other routes (e.g., low-paid workers living in socially and economically deprived areas [48]).
However, a key finding from this scoping review is that the exact reach of these workplace interventions across employment settings and worker populations could not be fully determined since most of the studies did not report the characteristics of the organisations in which they were implemented, and some did not provide details about the participating workers. To be able to fully synthesise the published evidence on intervention reach, there is a clear need for more consistency in the description of settings and populations for workplace VCT.
Although there was heterogeneity in the nature of the interventions delivered, all testing was delivered by healthcare professionals (doctors, nurses or sexual health providers), using rapid blood or oral fluid tests. Where reported, the average linkage to care rate in workplace VCT interventions (86.85%) was satisfactory compared to that found in other community HIV testing initiatives (e.g., 89% [49]; 95%, 95% CI = 87–98% [50]) and higher than rates reported for self-testing in the community (e.g., 56% [40]). Most workplace VCT studies, however, did not report linkage to care.
Most of the studies reported evaluations of one-off health events that were either focused on or included VCT. The Test@Work study [14,31,32], for example, embedded HIV testing within a general health check (one-off health event with a range of health tests and checks), and this approach was perceived by organisations, workers, and testing providers to normalise HIV testing and reduce HIV-related stigma. Although views might vary according to setting, workforce gender balance, or cultural norms (Test@Work was conducted in the UK construction industry), the inclusion of HIV testing within a wider package of opt-in health tests and checks was proposed by employers as the most appropriate, if not the only, way to engage the workers in on-site HIV testing.
In terms of the delivery format, it was unclear why one-off events were more common, and there were not enough interventions to meaningfully explore differences between on-off events and longer/repeated interventions (i.e., in reach, uptake, or implementation enablers or barriers). It is possible that one-off events may have been selected by researchers as the limited time input helps them to persuade employers to sign-up as host organisations. Our suggested reasons are twofold; first, one-off events offer an option for workplace health intervention that can be easily slotted into an ongoing programme of workplace health initiatives already being offered by employing organisations. Second, with one-off events, organisations without existing workplace health programmes can experience a ‘taster’ of how interventions could be implemented within the organisation (and how well they are received) without excessive time and effort. The latter may be important to engage small- to medium-sized enterprises (SMEs) in VCT that may have less structured support and resources available to invest in workplace health [51]. Finding ways to engage SMEs in workplace health promotion initiatives is particularly relevant since SMEs are the backbone of economies worldwide, accounting for over 95% of firms and employing 60–70% of the global workforce [52]. Their broad reach makes increasing access to workplace health promotion vital in these settings.
An important finding of this review was that interventions were not consistently described, which made it difficult to make direct comparisons between interventions (e.g., delivery format and intervention type), draw firm conclusions about the appropriateness of workplace VCT for diverse host organisations and recipients (e.g., workplace types, sizes and sectors, geographical regions, worker populations), and reflect on their findings. In this review, we used the TiDiER-Lite checklist [27] to extract intervention details. We recommend that researchers use this checklist (or the full version [27]) to foster consistency in the reporting of future evaluative studies relating to workplace VCT.
Overall, this review identified more factors enabling the uptake of workplace VCT than barriers. A study of routine health check attendance (not specific to VCT or the workplace setting) showed that those least likely to attend routine health checks were men on low incomes, low socio-economic status, unemployed or less well-educated [53]. Workplace-delivered health testing may, therefore, reach populations who may not otherwise access this through other settings. Findings from this review suggest that the uptake of workplace HIV-related VCT was facilitated by the ‘convenience’ of accessing health tests at work (on-site), the ‘immediacy’ of results using rapid tests, the provision of ‘free’ testing and condoms, ‘incentives’ to participate, and the provision of HIV-related ‘education’. The high value placed on the convenience of accessing HIV tests at work aligns directly with findings from prior evaluations of general health checks in workplace settings [12]. Offering HIV-related education alongside HIV testing seems important since limited knowledge about HIV, low risk perceptions and HIV-related stigma were key barriers to workplace VCT uptake in the included studies. Indeed, studies have found that educational intervention can improve men’s behavioural intentions to engage in health screening [54]. Our review findings show that being male, and a masculinity-driven workplace culture, were barriers to uptake of workplace HIV VCT. This concurs with other research showing that men are less likely to attend health screening (including HIV testing), than women, with male-dominant barriers to testing uptake including a heterosexual self-presentation [54].
Factors relating to the organisational context could also be barriers or facilitators of testing uptake. For example, uptake was enhanced when VCT was delivered within well-organised events (e.g., a health check event) which involved the commitment and support of managers and peers for distributing test kits or helping to organise or implement events. Lack of time due to work commitments was a barrier to testing uptake for some. Importantly, there were differences between ease of access for different occupational groups, with more challenges to access experienced by contract workers and agency staff than permanent and office-based staff. Some workers felt there was a lack of support for engaging in health initiatives at work; this could be at the manager level (perceived lack of manager support) or organisational level (e.g., lack of organisational policy surrounding workplace health and clarity around which staff groups could, or could not, access this during working hours).
4.1. Limitations of the Review
The review is limited to studies published in the English language and the small number of interventions in this field. The searches were conducted up to November 2023 and, therefore, there may be more reviews published from December 2023 onwards. We found that methods for workplace VCT interventions vary considerably, with many of the studies using surveys or qualitative interviews with stakeholder groups. We did not systematically collect information about how interventions were funded (e.g., by employers, external donors, or as part of research grants), which could be explored in future studies to assess the feasibility of scale-up and long-term sustainability of workplace VCT. This review aimed to map out the nature of the evidence, and it was, therefore, beyond the scope of this review to determine VCT intervention ‘effectiveness’ in terms of diagnostic, clinical or health outcomes, which could be investigated in high-quality randomised controlled trials.
4.2. Limitations of Included Studies
Scoping reviews do not include a requirement to assess methodological quality of included studies [19]. Nonetheless, this scoping review highlights that the reporting quality of workplace VCT interventions and their associated evaluations is variable, which means we were unable to meaningfully report comparisons on intervention type, duration, and frequency, or the enablers and barriers to implementation in different occupational settings. This could be examined in future research.
Regarding study designs, we observed that randomised controlled trial (RCT) designs were uncommon among our included studies, with two pilot trials and just one study which reported findings from a full-scale cluster randomised trial. Although RCTs are considered the ’gold standard’ of evaluative health and medical research, it has long been recognised that the RCT may not be the most appropriate design for evaluating occupational health interventions [55]. It has previously been reported that the adoption of RCTs is scarce in the evaluation of workplace occupational health interventions compared to their use in the medical sciences due to the challenges of conducting RCTs in occupational settings [56]. Such challenges may include working with (often changing) gatekeepers, layers of ‘red tape’, competing organisational priorities and workplace policies, data sharing issues, randomisation processes and risk of contamination, and the lack of timeliness of RCTs in generating outcomes of perceived value in uncontrolled, dynamic ‘real-world’ contexts. If conducting an RCT, researchers should ensure that organisational issues are well-considered in the RCT design and consider reporting using a RCT checklist, which takes organisational issues into account [56]. Alternatively, future studies might consider the challenges of undertaking an RCT in employment settings and consider alternative evaluative research designs [55], such as a stepwise approach or a realist evaluation.
4.3. Reflexivity
Due to time and resource constraints, no patient or public involvement was undertaken as part of this review. All team members have previously undertaken and published evidence reviews. The project lead is a female health psychologist with expertise in public health and workplace health promotion. The project collaborator is a registered nurse with expertise in HIV and sexual health. Both these team members are female and conducted the Healthy Hub and Test@Work studies, which are included in this review. This may have influenced their interpretation of the review findings. The two researchers (one male, one female) involved in the review data collection and extraction have backgrounds in health research and were not involved in the design or delivery of any of the included studies.
5. Conclusions
This scoping review is the first to identify the published evidence for workplace HIV counselling and testing interventions in a global context. Despite the limited number of studies, the workplace appears to be a viable and potentially valuable route to the delivery of community VCT. The uptake rates combined with a high number of enabling factors indicate that such interventions are largely acceptable to workers, employers and service providers. Workplace VCT could, therefore, contribute to improving access to HIV testing and early diagnosis of HIV. However, there are several barriers to participation and organisational challenges that need to be considered. In terms of actionable recommendations, this review suggests that, in the delivery of workplace VCT, we should provide education (to address poor knowledge and low risk perceptions) and make testing easy to access, convenient, and confidential/private. Rapid tests for immediate results are valued. Embedding HIV VCT within general health checks helps to normalise testing and reduce HIV-related stigma. Raising health awareness within organisations and ensuring top-level support for health events is critical, particularly in a masculinity-driven workplace culture. Reporting quality of interventions and associated evaluations is highly variable and could be improved with the use of appropriate checklists to enhance the quality and consistency of descriptions of the characteristics of workers, occupational settings and interventions in workplace VCT. There is a clear need to enhance consistency in study outcomes measured and reported. Further research is warranted to explore differences between intervention types (e.g., one-off events versus longer/repeated interventions) on reach, uptake, acceptability and outcomes. There is scope to further examine differences in reach, uptake, acceptability, and outcomes within and between different worker populations, job roles, work patterns, occupational settings (organisation type, size, sector/industry) and locations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph22020263/s1, Text S1: Search strategy, Text S2: Excluded Studies, Table S1: Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist, Table S2: Modified JBI data extraction tool, Table S3: TIDieR-Lite checklist.
Author Contributions
Conceptualisation, H.B.; methodology, H.B., C.E., M.Y., and S.J.L.; formal analysis, M.Y. and S.J.L.; investigation, M.Y.; resources, H.B.; data curation, M.Y.; writing—original draft preparation, H.B. and M.Y.; writing—review and editing, C.E. and S.J.L.; supervision, H.B.; project administration, M.Y.; funding acquisition, H.B. and C.E. All authors have read and agreed to the published version of the manuscript.
Funding
This review was undertaken as part of a wider programme of research called Test@Work funded by Gilead Sciences, Inc. (Grant Reference Number—Blake: INUK276 5347HIVDVE). S.J.L. is funded by a National Institute for Health and Care Research Advanced Fellowship [grant number NIHR300863]. The sponsors were not involved in the study design, the collection, analysis and interpretation of data, or the preparation of the article.The views expressed are those of the author(s) and not necessarily those of the National Institute for Health and Care Research or the Department of Health and Social Care or Gilead Sciences, Inc.
Institutional Review Board Statement
Not applicable. This type of study does not require ethical review.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of the study are available within the article and its Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| VCT | voluntary testing and counselling |
| HIV | Human Immunodeficiency Virus |
References
- Lucas, S.; Nelson, A.M. HIV and the spectrum of human disease. J. Pathol. 2015, 235, 229–241. [Google Scholar] [CrossRef]
- Poorolajal, J.; Hooshmand, E.; Mahjub, H.; Esmailnasab, N.; Jenabi, E. Survival rate of AIDS disease and mortality in HIV-infected patients: A meta-analysis. Public Health 2016, 139, 3–12. [Google Scholar] [CrossRef] [PubMed]
- AIDS by the Numbers. 2023. Available online: https://www.unaids.org/en (accessed on 15 January 2024).
- May, M.T. Better to know: The importance of early HIV diagnosis. Lancet Public Health 2017, 2, e6–e7. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.S.; Chen, Y.Q.; McCauley, M.; Gamble, T.; Hosseinipour, M.C.; Kumarasamy, N.; Hakim, J.G.; Kumwenda, J.; Grinsztejn, B.; Pilotto, J.H.; et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N. Engl. J. Med. 2016, 375, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Dyvik, E.H. Global Employment Figures 2023. Statista 2023. Available online: https://www.statista.com/statistics/1258612/global-employment-figures/ (accessed on 15 January 2024).
- Promoting Healthy, Safe and Resilient Workplaces for All. 2023. Available online: https://www.who.int/activities/promoting-healthy-safe-and-resilient-workplaces-for-all (accessed on 15 January 2024).
- Andersen, L.L.; Proper, K.I.; Punnett, L.; Wynne, R.; Persson, R.; Wiezer, N. Workplace health promotion and wellbeing. Sci. World J. 2015, 2015, 606875. [Google Scholar] [CrossRef]
- Bali, V.; Yermilov, I.; Koyama, A.; Legorreta, A.P. Secondary prevention of diabetes through workplace health screening. Occup. Med. 2018, 68, 610–616. [Google Scholar] [CrossRef]
- Barnes, A. Cardiovascular disease risk screening for commercial drivers examined in occupational practice: Implementing evidence-based practice to champion the health of essential workers. Workplace Health Saf. 2023, 71, 465–475. [Google Scholar] [CrossRef]
- Strudwick, J.; Gayed, A.; Deady, M.; Haffar, S.; Mobbs, S.; Malik, A.; Akhtar, A.; Braund, T.; A Bryant, R.; Harvey, S.B. Workplace mental health screening: A systematic review and meta-analysis. Occup. Environ. Med. 2023, 80, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Blake, H.; Bennett, E.; Batt, M.E. Evaluation of occupational health checks for hospital employees. Int. J. Workplace Health Manag. 2014, 7, 247–266. [Google Scholar] [CrossRef]
- Blake, H.; Banerjee, A.; Evans, C. Employer attitudes towards general health checks and HIV testing in the workplace. Public Health 2018, 156, 34–43. [Google Scholar] [CrossRef]
- Somerset, S.; Evans, C.; Blake, H. Accessing voluntary HIV testing in the construction industry: A qualitative analysis of employee interviews from the test@ work study. Int. J. Environ. Res. Public Health 2021, 18, 4184. [Google Scholar] [CrossRef] [PubMed]
- Pépin, J.; Abou Chakra, C.N.; Pépin, E.; Nault, V.; Valiquette, L. Evolution of the global burden of viral infections from unsafe medical injections, 2000–2010. PLoS ONE 2014, 9, e99677. [Google Scholar] [CrossRef]
- Ojo, O.; Verbeek, J.H.; Rasanen, K.; Heikkinen, J.; Isotalo, L.K.; Mngoma, N.; Ruotsalainen, E. Interventions to reduce risky sexual behaviour for preventing HIV infection in workers in occupational settings. Cochrane Database Syst. Rev. 2011, CD005274. [Google Scholar] [CrossRef]
- Sulat, J.S.; Prabandari, Y.S.; Sanusi, R.; Hapsari, E.D.; Santoso, B. The impacts of community-based HIV testing and counselling on testing uptake: A systematic review. J. Health Res. 2018, 32, 152–163. [Google Scholar] [CrossRef]
- Weihs, M.; Meyer-Weitz, A. Barriers to workplace HIV testing in South Africa: A systematic review of the literature. AIDS Care 2016, 28, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.D.J.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Guidance for conducting systematic scoping reviews. Int. J. Evid. Based Healthc. 2015, 13, 141–146. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for Scoping Reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef]
- Peters, M.D.J.; Godfrey, C.; McInerney, P.; Zachary Munn, A.C.T.; Khalil, H. Chapter 11: Scoping reviews. In JBI Manual for Evidence Synthesis; JBI: 2020. [CrossRef]
- Global Mapping of Sex Work Laws. NSWP n.d. Available online: https://www.nswp.org/sex-work-laws-map (accessed on 17 September 2024).
- Gompels, M.; Michael, S.; Davies, C.; Jones, T.; Macleod, J.; May, M. Trends in HIV testing in the UK primary care setting: A 15-year retrospective cohort study from 2000 to 2015. BMJ Open 2019, 9, e027744. [Google Scholar] [CrossRef] [PubMed]
- Cihlar, T.; Fordyce, M. Current status and prospects of HIV treatment. Curr. Opin. Virol. 2016, 18, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Rev. Esp. Cardiol. 2021, 74, 790–799. [Google Scholar] [CrossRef]
- Hoffmann, T.C.; Glasziou, P.P.; Boutron, I.; Milne, R.; Perera, R.; Moher, D.; Altman, D.G.; Barbour, V.; Macdonald, H.; Johnston, M.; et al. Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014, 348, g1687. [Google Scholar] [CrossRef] [PubMed]
- Blake, H.; Hussain, B.; Hand, J.; Juma, A.; Evans, C. Employers’ views of the ‘Healthy Hub Roadshow’: A workplace HIV testing intervention in England. AIDS Care 2019, 31, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Blake, H.; Hussain, B.; Hand, J.; Rowlands, D.; Juma, A.; Evans, C. Employee perceptions of a workplace HIV testing intervention. Int. J. Workplace Health Manag. 2018, 11, 333–348. [Google Scholar] [CrossRef]
- Houdmont, J.; Munir, F.; Grey, M. Acceptance of repeat worksite HIV voluntary counselling and testing in a rural South African factory. AIDS Care 2013, 25, 1199–1202. [Google Scholar] [CrossRef]
- Jones, W.; Somerset, S.; Evans, C.; Whittingham, K.; Middleton, M.; Blake, H. Test@work: Evaluation of workplace HIV testing for construction workers using the RE-AIM framework. BMC Public Health 2021, 21, 1737. [Google Scholar] [CrossRef]
- Somerset, S.; Jones, W.; Evans, C.; Cirelli, C.; Mbang, D.; Blake, H. Opt-in HIV testing in construction workplaces: An exploration of its suitability, using the socioecological framework. BMC Public Health 2022, 22, 1409. [Google Scholar] [CrossRef]
- Di Gennaro, F.; Lattanzio, R.; Falanga, C.; Negri, S.; Papagni, R.; Novara, R.; Panico, G.G.; Totaro, V.; Poliseno, M.; Bavaro, D.F.; et al. Low-wage agricultural migrant workers in Apulian ghettos, Italy: General health conditions assessment and HIV screening. Trop. Med. Infect. Dis. 2021, 6, 184. [Google Scholar] [CrossRef]
- Van der Borght, S.F.; van der Loeff, M.F.S.; Clevenbergh, P.; Kabarega, J.P.; Kamo, E.; van Cranenburgh, K.; Rijckborst, H.; Lange, J.M.; de Wit, T.F.R. Long-term voluntary counseling and testing (VCT) uptake dynamics in a multicountry HIV workplace program in sub-Saharan Africa. AIDS Care 2010, 22, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, D. Managing HIV/AIDS in the South African workplace: Just another duty? S. Afr. J. Econ. Manag. Sci. 2003, 6, 25–49. [Google Scholar] [CrossRef]
- Morris, C.N.; Cheevers, E.J. A package of care for HIV in the occupational setting in Africa: Results of a pilot intervention. AIDS Patient Care STDS 2001, 15, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Weihs, M.; Meyer-Weitz, A.; Baasner-Weihs, F. The influence of lotteries on employees’ workplace HIV testing behaviour. Afr. J. AIDS Res. 2018, 17, 9–21. [Google Scholar] [CrossRef]
- Weihs, M.; Meyer-Weitz, A. Do employees participate in workplace HIV testing just to win a lottery prize? A quantitative study. SA J. Hum. Resour. Manag. 2016, 14. [Google Scholar] [CrossRef]
- Weihs, M.; Meyer-Weitz, A. A lottery incentive system to facilitate dialogue and social support for workplace HIV counselling and testing: A qualitative inquiry. SAHARA J. 2014, 11, 116–125. [Google Scholar] [CrossRef]
- Choko, A.T.; Nanfuka, M.; Birungi, J.; Taasi, G.; Kisembo, P.; Helleringer, S. A pilot trial of the peer-based distribution of HIV self-test kits among fishermen in Bulisa, Uganda. PLoS ONE 2018, 13, e0208191. [Google Scholar] [CrossRef] [PubMed]
- Corbett, E.L.; Dauya, E.; Matambo, R.; Cheung, Y.B.; Makamure, B.; Bassett, M.T.; Chandiwana, S.; Munyati, S.; Mason, P.R.; E Butterworth, A.; et al. Uptake of workplace HIV counselling and testing: A cluster-randomised trial in Zimbabwe. PLoS Med. 2006, 3, e238. [Google Scholar] [CrossRef]
- Onoja, A.J.; Sanni, F.O.; Abiodun, P.O.; Onoja, S.; Shaibu, J.; Oguche, D.; Adamu, I. Voluntary counselling and testing for HIV among allied workers in rural area of Nigeria: Evaluation of community-based interventions. Int. J. Occup. Saf. Health 2020, 10, 73–81. [Google Scholar] [CrossRef]
- Pai, N.P.; Behlim, T.; Abrahams, L.; Vadnais, C.; Shivkumar, S.; Pillay, S.; Binder, A.; Deli-Houssein, R.; Engel, N.; Joseph, L.; et al. Will an unsupervised self-testing strategy for HIV work in health care workers of South Africa? A cross sectional pilot feasibility study. PLoS ONE 2013, 8, e79772. [Google Scholar] [CrossRef]
- Haeuser, E.; Serfes, A.L.; Cork, M.A.; Yang, M.; Abbastabar, H.; Abhilash, E.S.; Adabi, M.; Adebayo, O.M.; Adekanmbi, V.; Adeyinka, D.A.; et al. Mapping age- and sex-specific HIV prevalence in adults in sub-Saharan Africa, 2000–2018. BMC Med. 2022, 20, 488. [Google Scholar] [CrossRef] [PubMed]
- WHO. HIV Statistics, Globally and by WHO Region, 2023. 2023. Available online: https://cdn.who.int/media/docs/default-source/hq-hiv-hepatitis-and-stis-library/j0294-who-hiv-epi-factsheet-v7.pdf (accessed on 18 September 2024).
- Global Wellness Institute. The Global Wellness Economy: Country Rankings. 2022. Available online: https://globalwellnessinstitute.org/wp-content/uploads/2022/02/GWI2022_GlobalWellnessEconomy_CountryRankings_Final.pdf (accessed on 18 September 2024).
- Gov.UK. Health Matters: Health and Work. 2019. Available online: https://www.gov.uk/government/publications/health-matters-health-and-work/health-matters-health-and-work (accessed on 18 September 2024).
- Abbas, S.Z.; Pollard, T.M.; Wynn, P.; Learmonth, A.; Joyce, K.; Bambra, C. The effectiveness of using the workplace to identify and address modifiable health risk factors in deprived populations. Occup. Environ. Med. 2015, 72, 664–669. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bares, S.; Eavou, R.; Bertozzi-Villa, C.; Taylor, M.; Hyland, H.; McFadden, R.; Shah, S.; Pho, M.T.; Walter, J.; Badlani, S.; et al. Expanded HIV testing and linkage to care: Conventional vs. Point-of-care testing and assignment of patient notification and linkage to care to an HIV care program. Public Health Rep. 2016, 131 (Suppl. S1), 107–120. [Google Scholar] [CrossRef]
- Sharma, M.; Ying, R.; Tarr, G.; Barnabas, R. Systematic review and meta-analysis of community and facility-based HIV testing to address linkage to care gaps in sub-Saharan Africa. Nature 2015, 528, S77–S85. [Google Scholar] [CrossRef] [PubMed]
- Burge, P.; Lu, H.; Smith, P.; Koch, N. Incentivising SME Uptake of Health and Wellbeing Support Schemes: Summary. March 2023, DWP Research Report No. 1024. Available online: https://assets.publishing.service.gov.uk/media/640f0d67d3bf7f02ff3f5744/incentivising-SME-uptake-of-health-and-wellbeing-schemes-report.pdf (accessed on 16 January 2025).
- Rivera, H.C. Small & Medium-Sized Enterprises—National Action Plans on Business and Human Rights 2019? Bus. Hum. Rights J. 2019, 4, 213–237. [Google Scholar]
- Dryden, R.; Williams, B.; McCowan, C.; Themessl-Huber, M. What do we know about who does and does not attend general health checks? Findings from a narrative scoping review. BMC Public Health 2012, 12, 723. [Google Scholar] [CrossRef] [PubMed]
- Teo, C.H.; Ng, C.J.; Booth, A.; White, A. Barriers and facilitators to health screening in men: A systematic review. Soc. Sci. Med. 2016, 165, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Schelvis, R.M.C.; Oude Hengel, K.M.; Burdorf, A.; Blatter, B.M.; Strijk, J.E.; van der Beek, A.J. Evaluation of occupational health interventions using a randomized controlled trial: Challenges and alternative research designs. Scand. J. Work. Environ. Health 2015, 41, 491–503. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, D.; O’ Connell, B.H.; Gallagher, S. Randomised controlled trials in WOHP interventions: A review and guidelines for use. Appl. Psychol. 2016, 65, 190–222. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).