Abstract
Studies examining associations between greenspace and Alzheimer’s disease and related dementia (ADRD) outcomes are rapidly on the rise, yet no known literature reviews have summarized the racialized/ethnic group and geographic variation of those published studies. This is a significant gap given the known disparities in both greenspace access and ADRD risk between racialized/ethnic groups and between developed versus developing countries. In this rapid literature review, we (1) describe the diversity of published greenspace–brain health studies with respect to racialized/ethnic groups and geographic regions; (2) determine the extent to which published studies have investigated racialized/ethnic group differences in associations; and (3) review methodological issues surrounding studies of racialized/ethnic group disparities in greenspace and brain health associations. Of the 57 papers meeting our inclusion criteria as of 4 March 2022, 21% (n = 12) explicitly identified and included individuals who were Black, Hispanic/Latinx, and/or Asian. Twenty-one percent of studies (n = 12) were conducted in developing countries (e.g., China, Dominican Republic, Mexico), and 7% (n = 4) examined racialized/ethnic group differences in greenspace–brain health associations. None of the studies were framed by health disparities, social/structural determinants of health, or related frameworks, despite the known differences in both greenspace availability/quality and dementia risk by racialized/ethnic group and geography. Studies are needed in developing countries and that directly investigate racialized/ethnic group disparities in greenspace—brain health associations to target and promote health equity.
1. Introduction
Alzheimer’s disease (AD) and related dementias (ADRD) are a group of neurodegenerative diseases that significantly impact an individual’s daily life and functional abilities. These conditions typically differ in their clinical presentations depending on etiology. For instance, AD often initially presents with episodic memory deficits (i.e., recollection of personal experience/past events) [1] and Parkinson’s disease with motor decline [2]. ADRD prevalence is projected to increase from the current 6.7 million [3] to over 13 million by 2050 [4]. Concurrently, the population will become more racially/ethnically diverse, with the Black population expected to grow by 34% and the Hispanic/Latinx population expected to grow by 86% in the next three decades [5]. ADRD are responsible for significant physical, social, and economic burdens on patients, families, and the healthcare system [6], and minoritized groups bear the brunt of these burdens as patients and caregivers [7]. Black and Hispanic (versus White) individuals have a higher risk of ADRD (1.5 to 2 times as high) [8]. In addition, health conditions such as cardiovascular disease and diabetes, which are associated with increased ADRD risk, are more prevalent in Black and Hispanic individuals [9,10,11].
ADRD disparities between minoritized, racialized/ethnic groups and between developed and developing countries (e.g., higher versus lower-middle income) are well documented and hypothesized to result from structural and social determinants of health (S/SDOH) [12,13,14,15]. Structural determinants of health are the culture, values, policies, practices, and laws that shape a society (e.g., genderism, racism, capitalism). They are the overarching factors that shape living, working, and educational environments, resources, and opportunities (i.e., social determinants of health) that in turn impact individual-level health. Social determinants of health broadly include healthcare access and quality, educational access and quality, social and community context, neighborhood and built environment, and economic stability [16]. Thus, for example, disparities in S/SDOH, such as fewer educational opportunities, higher rates of poverty, and greater exposure to adversity and discrimination by racialized/ethnic groups, may help explain the greater risk of ADRD in Black and Hispanic communities [17,18].
S/SDOH influence environmental exposures, health behaviors, and quality of life throughout the life course, and these mechanisms are posited to affect brain health. Brain health is “the state of brain functioning across cognitive, sensory, social-emotional, behavioral, and motor domains, allowing a person to realize their full potential over the life course ” [19]. Social determinants are inextricably tied to the modifiable risk factors known to decrease brain health and increase ADRD risk (e.g., social contact, air pollution exposure, physical activity, and depression), and they differ by the racialized/ethnic group [20]. For instance, an analysis reported that worldwide, the highest estimated population-attributable risk (PAR) for ADRD was for low educational attainment (19%), while in the US, the highest estimated PAR was for physical inactivity (21%) [21]. A separate US study found that the modifiable risk factors most prominently associated with ADRDs were midlife obesity, physical inactivity, and low education. The proportion of ADRD cases associated with modifiable risk factors was higher among American Indian and Alaska Native, Black, and Hispanic individuals compared with Asian and White individuals [22].
The social-ecological model posits that community-level and individual-level factors influence health, yet only recently more attention has been directed toward community factors associated with ADRD outcomes [23]. Greenspaces, which are outdoor areas with natural vegetation, including but not limited to parks and gardens, are an integral part of the environment in which people live, learn, work, and age (Figure 1). They can provide physical and mental health benefits, and preliminary evidence suggests that they might help lower the risk of neurodegenerative diseases. A rapid review of 22 studies published as of 13 February 2020, found that 77% of studies demonstrated beneficial greenspace—brain health associations for children through older adults [24], and a meta-analysis of 12 studies on greenness and dementia risk published as of 30 March 2022, found that intermediate levels of greenness were associated with a slightly reduced risk of dementia [25]. Greater greenspace access in childhood, adulthood, and older age has been associated with slower cognitive decline over time in middle- and older-age adults [26,27]. Similarly, greener neighborhoods (more healthy, green vegetation) in childhood, adulthood, and older age have been associated with better cognitive functioning among middle- to older-age adults [26,28,29]. Few studies have focused on ADRD-related biomarkers, such as from magnetic resonance imaging (MRI), but early evidence suggests positive associations between more neighborhood greenspace and better brain imaging outcomes (e.g., amygdala integrity, larger regional brain volumes) in middle- to older-age adults [30,31].
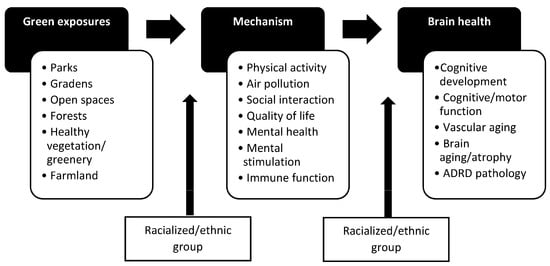
Figure 1.
Conceptual framework for greenspace and brain health associations and effect modification by the racialized/ethnic group.
Increasing the quantity and quality of community greenspaces might provide a novel and feasible public health intervention to reduce ADRD risk [32], particularly for historically disadvantaged neighborhoods [33,34]. For example, in the US, census tracts with a higher proportion of minoritized, racialized/ethnic groups had less greenspace in 2001 and lost more greenspace between 2001 and 2011 compared with census tracts with lower proportions of minoritized groups [33]. In addition, studies of several U.S. cities found that minoritized individuals with lower socioeconomic status (SES) living in redlined neighborhoods [34] (i.e., experienced historic discriminatory mortgage lending practices), have less neighborhood tree canopy [35], greenspace [36,37], and parks [38,39], although this is not universally the case [40].
Studies examining associations between greenspace and ADRD outcomes have been rapidly on the rise since a previous rapid review [24] on this topic, yet no known reviews have summarized the racialized/ethnic group and geographic variation of the published studies. This is a significant gap given the known disparities in both greenspace access and ADRD risk between racialized/ethnic groups and between developed and developing countries [12,13,14,34,41]. Thus, in this study, we aim to determine the extent to which published greenspace—brain health studies have examined different racial/ethnic groups and geographic regions. We conducted a rapid literature review [42] to delineate the diversity of published greenspace—brain health studies with respect to included racialized/ethnic groups and geographic regions, the extent that published studies have investigated racialized/ethnic group differences in associations, and methodological issues surrounding studies of racialized/ethnic group disparities in greenspace and brain health associations. The overarching goal of this review is to provide preliminary evidence and expose gaps in the study of underrepresented racialized/ethnic groups and lower- to middle-income/developing countries among greenspace—brain health studies to inform future systematic reviews and primary research.
2. Materials and Methods
We build upon the work of a previous rapid literature review [24], employing the same inclusion criteria, databases, and search terms. However, in the current review, we include an additional two years of published papers (2020–2022) and, unlike the prior review, provide a unique focus on the racialized/ethnic group and geographic diversity of the studies. Rapid reviews employ methods similar to systematic or scoping literature reviews, allowing an evaluation of existing literature on emerging topics but differ in that they are typically conducted over a shorter time span by fewer individuals when time and resources are limited [42]. The prior review searched PubMed, Web of Science Core Collection, and Embase for papers published through 13 February 2020 that included greenspace as the exposure and ADRD brain health outcomes, and excluded papers that were (1) not in English; (2) not primary research studies; (3) focused on indoor greenspaces/views or virtual reality views; (4) ecological studies; (5) focused on attention restoration/mental fatigue (short-term effects); or (6) focused on greenspace activities (e.g., gardening). For this review, we automatically included the 22 papers published in the prior rapid review and repeated the search methods and inclusion/exclusion criteria to add any new papers published in the approximately 2 years following that review (i.e., 14 February 2020 to 4 March 2022). A single reviewer (LMB) performed searches of titles and abstracts for the following two sets of keywords (the same keywords searched for in the prior review):
- “greenspace or green space or greenness or parks or park or park space or parkspace” AND “cognition or cognitive or memory or brain aging or Alzheimer or Alzheimer’s or dementia or cognitive impairment”
- “neighborhood environment or wilderness or greenery or natural space or natural environment or public garden or recreational resource or normalized difference vegetation index or built environment or open space or woodland” AND “brain volume or brain atrophy or neurodegenerative disease or Alzheimer biomarker or cognition or cognitive or memory or brain aging or Alzheimer or Alzheimer’s or dementia or cognitive impairment”
Papers with titles and abstracts meeting the inclusion criteria moved on to a full-text review by a single reviewer (LMB), and those continuing to meet the criteria contributed to the final sample. Three reviewers (LMB, MPJ, and CR) divided up the final set of papers and charted a predetermined set of fields on (1) basic characteristics of the papers (author, year published, study location, data source, sample size, age groups under study, and statistical methods); (2) details on the specific greenspace and ADRD outcome measures (e.g., objective or self-reported; geographic information systems (GIS) buffers of interest (if applicable); measured at a one-time point or as changes over time); (3) observed associations between greenspace and ADRD outcomes; (4) details regarding racialized/ethnic groups under study, statistical methods for examining racialized/ethnic group differences in associations, and observed associations by the racialized/ethnic group; and (5) authors’ discussions of health disparities framework or related conceptual models, such as social determinants of health or structural racism. The detailed charted data are available in Tables S1–S3.
We created figures to display the number of studies by age group under study, participant racialized/ethnic group, geographic region, greenspace measure, ADRD outcome measure, studied associations (e.g., between greenness and cognition or between park space and ADRD diagnosis), and observed association (positive, negative, or null). The following age groups were chosen based on the importance of different life stages to brain development [43,44] and ADRD risk/pathology development [45]: 0–18 years (hereafter termed childhood for simplicity), 18–44 years (young adulthood), 45–64 years (middle age), and ≥65 years (older age). Only participants who were specifically identified by their racialized/ethnic group in the study were categorized as such in this study, and participants from studies without such specificity were categorized as “not specified”.
For the figure on geographic region, studies from the U.S., Canada, Australia, Western Europe, Lithuania, Bulgaria, and Japan were categorized as developed [46]. The remaining developing country categories included Latin America/Caribbean and East Asia (no studies were from South Africa, the Middle East/North Africa, or South Asia).
3. Results
The final sample included 57 papers published through 4 March 2022 (Figure 2) [26,27,29,30,31,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98]. Papers that met eligibility criteria followed a sequential review of titles, abstracts, and full text; duplicates across the three databases were eliminated; one study with duplicate reporting of results and one study focused only on school-level cognitive test scores (i.e., ecological study) were removed; and three new papers identified from a review of reference lists of the papers meeting our criteria in the rapid review were added.
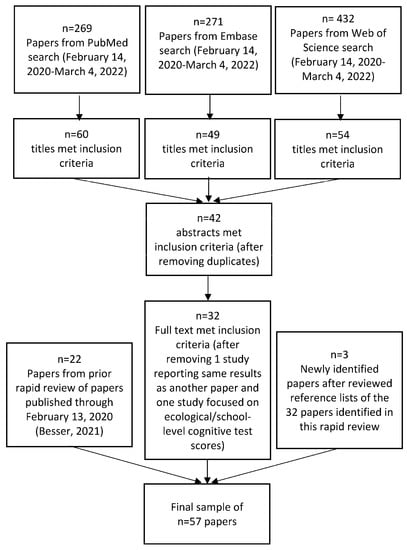
Figure 2.
Sample size flow diagram for rapid literature review.
The sample size, age groups, geographic locations, racialized/ethnic groups included, and observed associations for each of the 57 reviewed studies are provided in Table 1 and Figure 3A–F. Of note, the figures report non-mutually exclusive percentages because some studies include multiple categories (e.g., multiple age groups or outcomes of interest). By age group, 19 studies included < 18-year-olds (33%), 7 studies included 18–44-year-olds (12%), 20 studies included 45–64-year-olds (35%), and 38 studies included ≥65-year-olds (67%) (Figure 3A). The large majority (n = 46, 81%) of the studies were in developed countries, with the remaining conducted in Latin America/Caribbean (n = 1, 2%) and East Asia (n = 12, 21%) (Figure 3B). Among those studies explicitly identifying the racialized/ethnic group of participants, 10 included Black individuals (18%), 5 included Hispanic/Latinx individuals (9%), 5 included Asian individuals (9%), and 14 included White individuals (25%) (i.e., studies identified at least a subsample of these individuals) (Figure 3C). Altogether 12 studies (21%) specifically mentioned including individuals who were Black, Hispanic/Latinx, or Asian. Seventy-two percent of studies (n = 41) did not specify the participants’ racialized/ethnic groups, and as described in the methods, we did not automatically assign racialized/ethnic groups based on a presumptive racialized/ethnic group for a given study region. No individuals were identified as American Indian/Alaska Native or Native Hawaiian/Pacific Islander.

Table 1.
Summary of rapid literature review findings by geographic location and racialized/ethnic group.
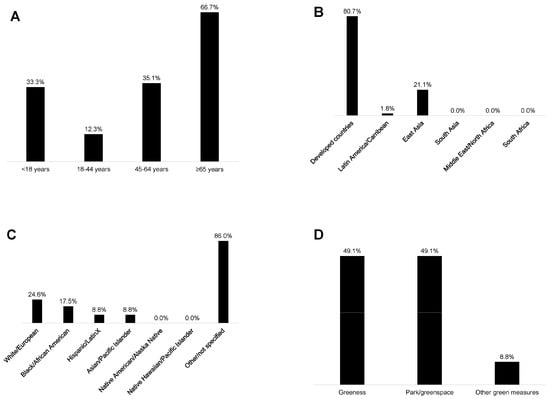
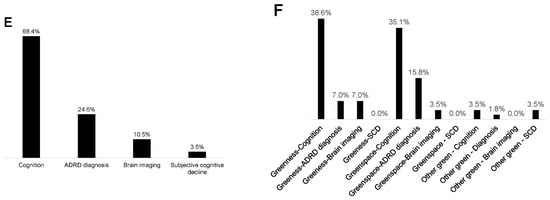
Figure 3.
Studies in the rapid literature review by (A) participants’ age group, (B) geographic region, (C) participants’ racialized/ethnic group, (D) green exposure studied, (E) brain health outcome studied, and (F) associations examined (not mutually exclusive categories).
Forty-nine percent of the studies used measures of greenness (e.g., normalized difference vegetation index), and 49% used measures of park/greenspace (Figure 3D). One study examined time spent in greenspace (2%) [58], eight included the distance to greenspace (14%) [27,64,66,67,74,80,91,93], and one used a measure of satisfaction in surrounding greenspaces (2%) [84]. Sixty-eight percent (n = 39) of the studies used cognitive test outcomes (e.g., global cognition, verbal intelligence, processing speed, etc.), 25% (n = 14) included ADRD diagnosis/incidence measures, 11% (n = 6) used neuroimaging measures (i.e., left hippocampal volume, white matter grade, etc.), and 4% (n = 2) used measures of subjective cognitive decline (Figure 3E). From the studies that evaluated greenness (e.g., normalized difference vegetation index), 39% of studies assessed associations with cognitive tests (n = 22), 7% with ADRD diagnosis (n = 4), and 7% with brain imaging (n = 4) (Figure 3F). From the studies that evaluated parks/greenspaces, 35% assessed associations with cognitive tests (n = 20), 16% with ADRD diagnosis (n = 9), and 4% with brain imaging measures (n = 2) (Figure 3F). Overall, 74% (n = 42) of the studies observed at least one positive association between greenspace and brain health, while 16% (n = 9) observed at least one negative association, and 81% (n = 46) observed at least one null association (Figure 4).
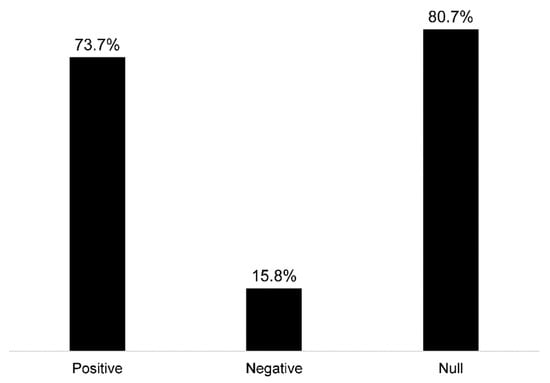
Figure 4.
Observed associations among reviewed studies between greenspace and ADRD brain health outcomes (not mutually exclusive categories).
Only 4 of the 57 studies (7%) investigated differences in greenspace—brain health associations by the racialized/ethnic group (Table 2) [27,54,65,94]. The first was a longitudinal study using data from the population-based multiethnic study of atherosclerosis, which enrolled participants from six US locations: Forsyth County, North Carolina; New York, New York; Baltimore, Maryland; St. Paul, Minnesota; Chicago, Illinois; Los Angeles, California [27]. While the study found an association between greater park access and maintained/improved global cognition over time in the overall sample (versus declining over time) (OR = 1.04; 95% CI = 1.00–1.08; p = 0.04), a statistically significant difference was not observed by the racialized group when testing an interaction term in the multivariable models (i.e., park access×race, p = 0.85). However, a borderline association was observed for Black (OR: 1.07, 95% CI: 1.00, 1.14; p = 0.07) but not White participants (OR = 1.04, 95% CI: 0.96–1.12; p = 0.35) when stratifying the model by race. The second study used data on >100,000 U.S. Medicare patients (federal health insurance for ≥65-year-old Americans). In stratified analyses, the authors found no significant difference between Black and White patients in the association of the proportion of neighborhood greenspace and Alzheimer’s disease (AD) risk [94]. Greater greenspace was associated with reduced AD risk for both Black and White individuals.

Table 2.
Four of fifty-seven reviewed studies examined differences in greenspace—brain health associations by the racialized/ethnic group.
The third study, which used the CARTaGENE cohort from six metropolitan areas in Quebec, Canada, found no association between neighborhood greenness and three measures of cognition (reasoning, visual memory, and reaction time) in either the entire sample or sample stratified by racialized/ethnic groups (i.e., White and non-White) [54]. The last study included US Medicare beneficiaries aged >65 years living in Miami-Dade County, Florida, from 2010 to 2011 [65]. The authors observed that higher greenness was associated with reduced risk of AD, ADRD, and non-AD dementia, after adjusting for individual and neighborhood sociodemographic characteristics. To better understand the role of demographic variables in the relationship between greenness and AD, they conducted separate tests for the interaction between greenness and each of the demographic variables but found no significant interactions with racialized/ethnic groups.
4. Discussion
In this rapid review of 57 published studies, we found that the majority (74%) observed at least one positive association suggesting a benefit between greenspace exposure and brain health outcomes. Twenty-one percent of studies explicitly indicated that they included Black, Hispanic/Latinx, and/or Asian participants. However, it must be noted that several reviewed studies were conducted in countries that are predominantly composed of one of these racialized/ethnic groups (e.g., most participants in Chinese studies were likely Asian), and we did not presume and assign a racial/ethnic identity for the participants in those studies. We found that only 7% of the studies investigated differences in greenspace—brain health associations by the racialized/ethnic group, although minoritized racialized/ethnic groups are present in countries represented in the reviewed studies (e.g., Zhuang and Hui in China). Most studies were conducted in developed/high-income countries (81%). Lastly, none of the studies were framed by health disparities, social/structural determinants of health, or related frameworks. Overall, our rapid review highlights the significant lack of studies conducted in developing countries, specifically studies that examined differences in associations by the racialized/ethnic group and that were framed according to fundamental mechanisms (e.g., structural racism) that are responsible for the unequal distribution of greenspaces in our communities and that are hypothesized to relate to disparities in ADRD risk.
An identified gap in the published greenspace and brain health literature is the lack of attention to how greenspaces differentially affect ADRD risk factors by the racialized/ethnic group, as well as how ADRD risk factors differentially impact ADRD outcomes by the racialized/ethnic group (Figure 1). For instance, the availability of neighborhood parks may be more important for social interaction depending on the racialized/ethnic group [99]. Similarly, social interaction may be more important for preserving cognitive function in older age depending on the racialized/ethnic group [100]. Thus, new studies should better incorporate this type of upstream and downstream effect modification of the causal pathway between greenspaces and brain health outcomes. Table 3 lists other major methodological gaps to address in the future. The list has been devised to help guide subsequent studies that aim to be more inclusive of minoritized, racialized/ethnic groups and individuals from historically disadvantaged communities and lower- to middle-income countries and to increase awareness of underlying issues that likely will accompany studies of greenspace and brain health associations among minoritized, racialized/ethnic groups.

Table 3.
Methodological concerns in examining differences in greenspace—brain health associations by the racialized/ethnic group.
Health studies historically have had difficulty in recruiting diverse participants, and ADRD research is no exception. Results on greenspace—brain health associations from majority White cohorts and higher-income countries cannot be automatically extrapolated to minoritized groups and developing countries.
To address the longstanding issue of diverse recruitment and retention, the US National Institute on Aging developed a guide that can be used to increase diversity in clinical studies of ADRD [102]. Recommended strategies that can be incorporated into brain health studies, such as those studying greenspace exposure/interventions, include developing partnerships and trust with underrepresented communities, promoting health and science literacy in the community, and increasing diversity in the research workforce to address bias. These strategies are particularly useful for new, prospective studies, whereas techniques for retention of underrepresented should be incorporated in preestablished cohort studies to minimize attrition (e.g., offering direct benefits whenever possible and researching topics directly pertinent to the community under study) [103].
Another methodological concern when examining greenspace—brain health associations by the racialized/ethnic group is the complex intertwining of neighborhood segregation, neighborhood SES, and racialized/ethnic group identity. These factors are difficult to disentangle given the historical roots of racism and residential segregation in the US and other countries [104,105]. Any studies of racialized/ethnic group differences in the neighborhood greenspace and brain health associations need to include sufficient samples of minoritized individuals from all neighborhood types (e.g., including lower segregation and higher SES neighborhoods) and/or should consider stratifying findings by these factors. Otherwise, any negative or null associations observed between greater access to greenspace and brain health outcomes among minoritized groups may be reflective of the underlying neighborhood conditions of segregated, low SES neighborhoods, which are often also more urban in character (e.g., with more crime, physical disorder, and fewer health-promoting resources and opportunities).
Inequities in S/SDOH, both proximal and distal, begin at birth and influence health outcomes across the life course [106]. Studies of dementia are often studies of survival. In the US, provisional estimates for 2021 show disparities in life expectancy by the racialized/ethnic group: 83.5 years for Asian individuals, 77.7 years for Hispanic/Latinx individuals, 76.4 years for White individuals, 70.8 years for Black individuals, and 65.2 years for Americans Indian/Alaskan Natives [107]. Age is the strongest risk factor for dementia, and the risk of ADRD is highest among those ages 65 and older [20]. Many minoritized individuals die before they can receive a diagnosis or are diagnosed at later stages of the disease [108]. This combined with a higher burden or comorbid disease and difficulty in continuously participating in research studies due to lack of time, transportation, or trust in the researchers can result in higher attrition of minoritized, racialized/ethnic groups in studies of brain health, including those focused on greenspace exposure. This review has identified greenspace as a potential protective factor for brain health, but more work is needed to measure how timing and duration of access to greenspace across the entire life impact survival (i.e., aging) and dementia risk and to ensure that minoritized groups remain enrolled in studies at similar rates as majoritized groups to avoid bias of findings.
A few studies have examined the relationship between park quality, access, and use by racialized/ethnic groups. Yet, perceptions of neighborhood quality, walkability, safety, and accessibility of green spaces, parks, and natural areas are known to affect physical activity among older adults [98,109]. Findings from previous studies suggest that in lower-income neighborhoods, proximity to greenspace and parks per se does not provide sufficient incentives for older adults to visit parks due to safety concerns and/or lack of amenities or adequate maintenance. Tinsley et al. (2002) found statistically significant differences among older adults from different racialized/ethnic groups in terms of intended park use, social and built environment characteristics, and perceived benefits [110]. Often parks in predominantly Black neighborhoods are perceived by the local residents as unattractive, unsafe, or exhibiting signs of disrepair [111]. Improvements in park quality in these neighborhoods as well as developing park programs and providing park fitness equipment for older adults are key interventions that can incentivize park usage by older adults [109,112]. Thus, future studies are needed to determine the importance of greenspace quality in promoting brain health throughout the life course, particularly for minoritized, racialized/ethnic communities.
Clinical evaluations for cognitive impairment and ADRD are based on healthcare systems and diagnostic tools that are often biased against minoritized, racialized groups. Black and other minoritized groups have been found to be at increased risk of underdiagnosis for dementia, which has been attributed to a multitude of factors, including but not limited to differences in dementia risk factors, disease etiology and presentation, cognitive test performance, and care-seeking behavior [113]. Undoubtedly, disparities in diagnosis are also related to inadequate access to healthcare, culturally/linguistically appropriate screening tools, and overarching structural racism, which suppresses opportunities for minoritized groups to receive appropriate evaluation and diagnosis [114]. Neuropsychological tests, which evaluate an individual’s cognitive functioning and decline over time and detect cognitive impairment and ADRD, are traditionally developed for majoritized populations, specifically White and English-speaking individuals. As such, numerous studies have demonstrated that non-White and non-English speaking individuals score consistently lower than majoritized groups on tests measuring various cognitive domains, necessitating different sets of norms for minoritized populations to accurately detect impairment [115]. Reasons for these disparities have been discussed in depth elsewhere, but they include structural factors such as differences in educational and occupational opportunity [115,116]. Disparities in cognitive testing and diagnosis need to be considered in any study of greenspace and ADRD outcomes that includes racialized/ethnic groups. Ideally, future studies will include cognitive tests that have been validated and/or back-translated for any racialized/ethnic group of interest, but at a minimum, care should be taken in the interpretation and potential bias of findings based on racialized/ethnic group disparities in testing and diagnosis.
Health studies on the built environment including greenspaces regularly note their limitations due to missing data on residential histories and participants’ reasons for moving to neighborhoods, which ultimately may bias study findings [117]. Accumulated exposure to greenspace environments over the life course may be a better predictor of late-life cognition than greenspace exposure in later life. In addition, controlling for early and midlife neighborhood conditions, such as neighborhood SES, is important when trying to understand the independent influence of neighborhood greenspace on late-life brain health. Exposure to environments with greater or fewer greenspaces may have accumulated impacts on individuals, and the differences may be particularly stark when comparing minoritized, racialized/ethnic groups with majoritized groups. The characterization of neighborhood environments throughout the life course is not possible without detailed residential history data (e.g., using life grid methods [26] or other questionnaires [118,119]), or at a minimum, self-reported measures of neighborhood environments over time. Yet few studies have incorporated such data collection to date. Similarly, most studies do not collect information on reasons for living in and moving to certain neighborhoods, particularly in late life. Self-selection into neighborhoods can result in reverse causation (e.g., harbingers of dementia prompt a move from an urban area to an assisted living facility in a suburban locale that is greener). However, unlike majoritized groups who have greater neighborhood choices, self-selection is a misnomer for minoritized groups, who have restricted choices in the neighborhoods in which they can live. Future studies will need to adequately consider these factors and their potential influence on study findings regarding differences by racialized/ethnic groups.
Lastly, researchers have called for measures of spatial polygamy, which is the “simultaneous belonging or exposure to multiple nested and non-nested, social and geographic, real, virtual and fictional, and past and present contexts” [101]. In this review, a single study assessed time spent in greenspace [58]; however, none captured multifaceted environmental exposures, including greenspace, as proposed by spatial polygamy. A motivation of spatial polygamy is to avoid exposure misclassification by singling out one element in our environments in a particular place (e.g., neighborhood) or time and confounding by factors closely related to the environment characteristic of interest (e.g., greenspaces and air pollution exposure). While studies would benefit from an eye toward spatial polygamy, tradeoffs must be considered (e.g., selection bias and generalizability to diverse populations) when asking for multiple detailed questionnaires/diaries over time or consent to use of mobility/exposure tracking devices or apps over multiple periods, which may deter minoritized, racialized/ethnic groups due to factors such as mistrust/privacy concerns and burden [120,121,122].
The primary limitation of this study is that it is a rapid review. Systematic reviews employ more rigorous methods to provide a comprehensive review of published literature, sometimes additionally including grey literature, risk of bias, and meta-analyses to estimate effect size. This study’s sample size of 57 papers may not be representative of the entire body of literature on the topic. Despite the benefits of a systematic review, a rapid review served our purpose to estimate the extent to which the published literature has included diverse individuals/regions and has examined associations by the racialized/ethnic group, and provided avenues for future research on greenspace and brain health among minoritized, historically disadvantaged, and developing country populations. Another limitation of this study is that many reviewed studies (72%) did not explicitly specify the racialized/ethnic groups included, particularly among studies conducted in international settings. For studies conducted in regions with at least moderate diversity in their racialized/ethnic groups (e.g., Ontario), this lack of attention to racialized/ethnic group identity of their participants highlights a lack of sensitivity to diversity, either through the absence of a description, lack of pertinent data, of or lack of inclusion of minoritized individuals. A lack of racial/ethnic and geographic diversity has been described in other medical research fields, particularly regarding participant inclusion in clinical trials [123]. In this rapid review, we observed a dearth of research on greenspace and brain health guided by health disparities, social/structural determinants of health, or related frameworks, despite the known differences in both greenspace availability/quality and dementia risk by the racialized/ethnic group and geography. Our findings imply that non-representativeness of participants from diverse racial and ethnic groups in study cohorts or geographic regions obstructs the external validity of the majority of current observational dementia research [124]. This has important implications for future researchers that seek to examine greenspace exposure as a potential population-level approach to improve cognitive health and suggests the need to more carefully consider underrepresented groups and regions and incorporate health disparities frameworks in future work.
5. Conclusions
Overall, this study demonstrated a paucity of greenspace—brain health research from developing countries, studies focused on differences in associations and differences in causal mechanisms between racialized/ethnic groups, and studies purposefully focused on associations within a specific minoritized, racialized/ethnic group. These scientific gaps should be addressed to ensure that greenspace interventions, policies, and plans to improve well-being and brain health will truly benefit historically disadvantaged communities. For instance, while in theory, new and improved parks in disadvantaged communities should improve park use and, thereby, the health of residents, it may have the opposite effect by gentrifying neighborhoods, which can decrease long-term residents’ sense of belonging and park use compared with incoming residents [125]. Despite the potential negative benefits, disadvantaged communities have the right to equal access to health-promoting greenspaces, which in several studies have been tied to health benefits for minoritized groups [126]. Ultimately, affordable housing and related policies may be required to be implemented in tandem with greenspace interventions to counteract any potentially negative impacts on historically disadvantaged communities [127].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph20095666/s1, Table S1: Characteristics of the 57 published studies of greenspace-ADRD brain health associations; Table S2: Greenspace and ADRD brain health measures in the 57 published studies of greenspace-ADRD brain health associations; Table S3: Greenspace and ADRD brain health measures in the 57 published studies of greenspace-ADRD brain health associations.
Author Contributions
Conceptualization, L.M.B. and M.P.J.; methodology, L.M.B.; data curation, L.M.B., M.P.J. and C.J.R.; writing—original draft preparation, L.M.B. and M.P.J.; writing—review and editing, L.M.B., M.P.J., C.J.R., O.L.M., D.M., K.M.G., P.B.A.-J. and J.E.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
As a review of published papers, this study has no accompanying data.
Acknowledgments
Besser is supported by NIH/NIA K01AG063895, NIH/NIA R21AG075291, and an Alzheimer’s Association Research Grant (AARG-21-850963). Meyer is supported by NIH/NIA P30AG072972. Pescador Jimenez is supported by NIH/NIA R00AG066949.
Conflicts of Interest
The authors declare no conflict of interest.
References
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Barton, B.; Grabli, D.; Bernard, B.; Czernecki, V.; Goldman, J.G.; Stebbins, G.; Dubois, B.; Goetz, C.G. Clinical validation of Movement Disorder Society-recommended diagnostic criteria for Parkinson’s disease with dementia. Mov. Disord. 2012, 27, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Plassman, B.L.; Langa, K.M.; Fisher, G.G.; Heeringa, S.G.; Weir, D.R.; Ofstedal, M.B.; Burke, J.R.; Hurd, M.D.; Potter, G.G.; Rodgers, W.L.; et al. Prevalence of dementia in the United States: The aging, demographics, and memory study. Neuroepidemiology 2007, 29, 125–132. [Google Scholar] [CrossRef]
- Hebert, L.E.; Weuve, J.; Scherr, P.A.; Evans, D.A. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013, 80, 1778–1783. [Google Scholar] [CrossRef]
- Frey, W. The US Will Become ‘Minority White’ in 2045, Census Projects. Available online: https://www.brookings.edu/blog/the-avenue/2018/03/14/the-us-will-become-minority-white-in-2045-census-projects/ (accessed on 18 October 2022).
- Hurd, M.D.; Martorell, P.; Langa, K.M. Monetary costs of dementia in the United States. N. Engl. J. Med. 2013, 369, 489–490. [Google Scholar] [CrossRef]
- Dilworth-Anderson, P.; Moon, H.; Aranda, M.P. Dementia Caregiving Research: Expanding and Reframing the Lens of Diversity, Inclusivity, and Intersectionality. Gerontologist 2020, 60, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.A.; Xu, W.; Gaglioti, A.H.; Holt, J.B.; Croft, J.B.; Mack, D.; McGuire, L.C. Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged ≥65 years. Alzheimer’s Dement. 2019, 15, 17–24. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2019 Alzheimer’s Disease Facts and Figures. 2019. Available online: https://www.alz.org/media/Documents/alzheimers-facts-and-figures-2019-r.pdf (accessed on 24 June 2019).
- Weuve, J.; Barnes, L.L.; Mendes de Leon, C.F.; Rajan, K.B.; Beck, T.; Aggarwal, N.T.; Hebert, L.E.; Bennett, D.A.; Wilson, R.S.; Evans, D.A. Cognitive Aging in Black and White Americans: Cognition, Cognitive Decline, and Incidence of Alzheimer Disease Dementia. Epidemiology 2018, 29, 151–159. [Google Scholar] [CrossRef]
- Froehlich, T.E.; Bogardus, S.T., Jr.; Inouye, S.K. Dementia and race: Are there differences between African Americans and Caucasians? J. Am. Geriatr. Soc. 2001, 49, 477–484. [Google Scholar] [CrossRef]
- Hachinski, V.; Einhäupl, K.; Ganten, D.; Alladi, S.; Brayne, C.; Stephan, B.C.M.; Sweeney, M.D.; Zlokovic, B.; Iturria-Medina, Y.; Iadecola, C.; et al. Preventing dementia by preventing stroke: The Berlin Manifesto. Alzheimer’s Dement. 2019, 15, 961–984. [Google Scholar] [CrossRef]
- GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. 2022 Alzheimer’s Disease Facts and Figures. Available online: https://www.alz.org/media/Documents/alzheimers-facts-and-figures.pdf (accessed on 17 January 2023).
- Galvin, J.E.; Chrisphonte, S.; Chang, L.C. Medical and Social Determinants of Brain Health and Dementia in a Multicultural Community Cohort of Older Adults. J. Alzheimer’s Dis. 2021, 84, 1563–1576. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. About Social Determinants of Health (SDOH). Available online: https://www.cdc.gov/socialdeterminants/about.html (accessed on 18 October 2022).
- Walker, R.J.; Williams, J.S.; Egede, L.E. Influence of Race, Ethnicity and Social Determinants of Health on Diabetes Outcomes. Am. J. Med. Sci. 2016, 351, 366–373. [Google Scholar] [CrossRef]
- Forde, A.T.; Lewis, T.T.; Kershaw, K.N.; Bellamy, S.L.; Diez Roux, A.V. Perceived Discrimination and Hypertension Risk Among Participants in the Multi-Ethnic Study of Atherosclerosis. J. Am. Heart Assoc. 2021, 10, e019541. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Brain Health. Available online: https://www.who.int/health-topics/brain-health#tab=tab_1 (accessed on 18 October 2022).
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef] [PubMed]
- Norton, S.; Matthews, F.E.; Barnes, D.E.; Yaffe, K.; Brayne, C. Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. Lancet Neurol. 2014, 13, 788–794. [Google Scholar] [CrossRef]
- Nianogo, R.A.; Rosenwohl-Mack, A.; Yaffe, K.; Carrasco, A.; Hoffmann, C.M.; Barnes, D.E. Risk Factors Associated With Alzheimer Disease and Related Dementias by Sex and Race and Ethnicity in the US. JAMA Neurol. 2022, 79, 584–591. [Google Scholar] [CrossRef]
- Krieger, N. Theories for social epidemiology in the 21st century: An ecosocial perspective. Int. J. Epidemiol. 2001, 30, 668–677. [Google Scholar] [CrossRef]
- Besser, L. Outdoor green space exposure and brain health measures related to Alzheimer’s disease: A rapid review. BMJ Open 2021, 11, e043456. [Google Scholar] [CrossRef]
- Zagnoli, F.; Filippini, T.; Jimenez, M.P.; Wise, L.A.; Hatch, E.E.; Vinceti, M. Is Greenness Associated with Dementia? A Systematic Review and Dose-Response Meta-analysis. Curr. Environ. Health Rep. 2022, 9, 574–590. [Google Scholar] [CrossRef]
- Cherrie, M.P.C.; Shortt, N.K.; Mitchell, R.J.; Taylor, A.M.; Redmond, P.; Thompson, C.W.; Starr, J.M.; Deary, I.J.; Pearce, J.R. Green space and cognitive ageing: A retrospective life course analysis in the Lothian Birth Cohort 1936. Soc. Sci. Med. 2018, 196, 56–65. [Google Scholar] [CrossRef]
- Besser, L.M.; Chang, L.C.; Evenson, K.R.; Hirsch, J.A.; Michael, Y.L.; Galvin, J.E.; Rapp, S.R.; Fitzpatrick, A.L.; Heckbert, S.R.; Kaufman, J.D.; et al. Associations between neighborhood park access and longitudinal change in cognition in older adults: The Multi-Ethnic Study of Atherosclerosis. J. Alzheimer’s Dis. 2021, 82, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, M.P.; Elliott, E.G.; DeVille, N.V.; Laden, F.; Hart, J.E.; Weuve, J.; Grodstein, F.; James, P. Residential Green Space and Cognitive Function in a Large Cohort of Middle-Aged Women. JAMA Netw. Open 2022, 5, e229306. [Google Scholar] [CrossRef] [PubMed]
- de Keijzer, C.; Tonne, C.; Basagaña, X.; Valentín, A.; Singh-Manoux, A.; Alonso, J.; Antó, J.M.; Nieuwenhuijsen, M.J.; Sunyer, J.; Dadvand, P. Residential Surrounding Greenness and Cognitive Decline: A 10-Year Follow-up of the Whitehall II Cohort. Environ. Health Perspect. 2018, 126, 077003. [Google Scholar] [CrossRef] [PubMed]
- Dadvand, P.; Pujol, J.; Macià, D.; Martínez-Vilavella, G.; Blanco-Hinojo, L.; Mortamais, M.; Alvarez-Pedrerol, M.; Fenoll, R.; Esnaola, M.; Dalmau-Bueno, A.; et al. The Association between Lifelong Greenspace Exposure and 3-Dimensional Brain Magnetic Resonance Imaging in Barcelona Schoolchildren. Environ. Health Perspect. 2018, 126, 027012. [Google Scholar] [CrossRef]
- Kühn, S.; Düzel, S.; Eibich, P.; Krekel, C.; Wüstemann, H.; Kolbe, J.; Martensson, J.; Goebel, J.; Gallinat, J.; Wagner, G.G.; et al. In search of features that constitute an “enriched environment” in humans: Associations between geographical properties and brain structure. Sci. Rep. 2017, 7, 11920. [Google Scholar] [CrossRef] [PubMed]
- De Keijzer, C.; Gascon, M.; Nieuwenhuijsen, M.J.; Dadvand, P. Long-Term Green Space Exposure and Cognition Across the Life Course: A Systematic Review. Curr. Environ. Health Rep. 2016, 3, 468–477. [Google Scholar] [CrossRef]
- Casey, J.A.; James, P.; Cushing, L.; Jesdale, B.M.; Morello-Frosch, R. Race, Ethnicity, Income Concentration and 10-Year Change in Urban Greenness in the United States. Int. J. Environ. Res. Public. Health 2017, 14, 1546. [Google Scholar] [CrossRef]
- Nardone, A.; Rudolph, K.E.; Morello-Frosch, R.; Casey, J.A. Redlines and Greenspace: The Relationship between Historical Redlining and 2010 Greenspace across the United States. Environ. Health Perspect. 2021, 129, 017006. [Google Scholar] [CrossRef]
- Locke, D.H.; Hall, B.; Grove, J.M.; Pickett, S.T.A.; Ogden, L.A.; Aoki, C.; Boone, C.G.; O’Neil-Dunne, J.P.M. Residential housing segregation and urban tree canopy in 37 US Cities. npj Urban Sustain. 2021, 1, 15. [Google Scholar] [CrossRef]
- Wen, M.; Zhang, X.; Harris, C.D.; Holt, J.B.; Croft, J.B. Spatial disparities in the distribution of parks and green spaces in the USA. Ann. Behav. Med. 2013, 45 (Suppl. S1), S18–S27. [Google Scholar] [CrossRef] [PubMed]
- Dai, D. Racial/ethnic and socioeconomic disparities in urban green space accessibility: Where to intervene? Landsc. Urban Plan. 2011, 102, 234–244. [Google Scholar] [CrossRef]
- Moore, L.V.; Diez Roux, A.V.; Evenson, K.R.; McGinn, A.P.; Brines, S.J. Availability of recreational resources in minority and low socioeconomic status areas. Am. J. Prev. Med. 2008, 34, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Duncan, D.T.; Kawachi, I.; White, K.; Williams, D.R. The geography of recreational open space: Influence of neighborhood racial composition and neighborhood poverty. J. Urban Health 2013, 90, 618–631. [Google Scholar] [CrossRef]
- Boone, C.G.; Buckley, G.L.; Grove, J.M.; Sister, C. Parks and People: An Environmental Justice Inquiry in Baltimore, Maryland. Ann. Assoc. Am. Geogr. 2009, 99, 767–787. [Google Scholar] [CrossRef]
- Chen, B.; Wu, S.B.A.; Song, Y.M.; Webster, C.; Xu, B.; Gong, P. Contrasting inequality in human exposure to greenspace between cities of Global North and Global South. Nat. Commun. 2022, 13, 4636. [Google Scholar] [CrossRef]
- Temple University. What is a Rapid Review? Systematic Reviews and Other Review Types. Available online: https://guides.temple.edu/c.php?g=78618&p=4156608#:~:text=%22Rapid%20reviews%20are%20a%20form,in%20less%20than%205%20weeks (accessed on 18 October 2022).
- Giedd, J.N.; Blumenthal, J.; Jeffries, N.O.; Castellanos, F.X.; Liu, H.; Zijdenbos, A.; Paus, T.; Evans, A.C.; Rapoport, J.L. Brain development during childhood and adolescence: A longitudinal MRI study. Nat. Neurosci. 1999, 2, 861–863. [Google Scholar] [CrossRef]
- Tierney, A.L.; Nelson, C.A., 3rd. Brain Development and the Role of Experience in the Early Years. Zero Three 2009, 30, 9–13. [Google Scholar]
- Irwin, K.; Sexton, C.; Daniel, T.; Lawlor, B.; Naci, L. Healthy Aging and Dementia: Two Roads Diverging in Midlife? Front. Aging Neurosci. 2018, 10, 275. [Google Scholar] [CrossRef]
- United Nations. Statistical Annex: Country Classifications. Available online: https://www.un.org/development/desa/dpad/wp-content/uploads/sites/45/WESP2022_ANNEX.pdf (accessed on 21 February 2023).
- Brown, S.C.; Perrino, T.; Lombard, J.; Wang, K.; Toro, M.; Rundek, T.; Gutierrez, C.M.; Dong, C.; Plater-Zyberk, E.; Nardi, M.I.; et al. Health Disparities in the Relationship of Neighborhood Greenness to Mental Health Outcomes in 249,405 U.S. Medicare Beneficiaries. Int. J. Environ. Res. Public. Health 2018, 15, 430. [Google Scholar] [CrossRef]
- Cherrie, M.P.C.; Shortt, N.K.; Ward Thompson, C.; Deary, I.J.; Pearce, J.R. Association Between the Activity Space Exposure to Parks in Childhood and Adolescence and Cognitive Aging in Later Life. Int. J. Environ. Res. Public. Health 2019, 16, 632. [Google Scholar] [CrossRef] [PubMed]
- Clarke, P.J.; Ailshire, J.A.; House, J.S.; Morenoff, J.D.; King, K.; Melendez, R.; Langa, K.M. Cognitive function in the community setting: The neighbourhood as a source of ‘cognitive reserve’? J. Epidemiol. Community Health 2012, 66, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Dadvand, P.; Nieuwenhuijsen, M.J.; Esnaola, M.; Forns, J.; Basagaña, X.; Alvarez-Pedrerol, M.; Rivas, I.; López-Vicente, M.; De Castro Pascual, M.; Su, J.; et al. Green spaces and cognitive development in primary schoolchildren. Proc. Natl. Acad. Sci. USA 2015, 112, 7937–7942. [Google Scholar] [CrossRef] [PubMed]
- Dadvand, P.; Tischer, C.; Estarlich, M.; Llop, S.; Dalmau-Bueno, A.; López-Vicente, M.; Valentín, A.; de Keijzer, C.; Fernández-Somoano, A.; Lertxundi, N.; et al. Lifelong Residential Exposure to Green Space and Attention: A Population-based Prospective Study. Environ. Health Perspect. 2017, 125, 097016. [Google Scholar] [CrossRef]
- Dzhambov, A.M.; Bahchevanov, K.M.; Chompalov, K.A.; Atanassova, P.A. A feasibility study on the association between residential greenness and neurocognitive function in middle-aged Bulgarians. Arh. Hig. Rada Toksikol. 2019, 70, 173–185. [Google Scholar] [CrossRef]
- Flouri, E.; Papachristou, E.; Midouhas, E. The role of neighbourhood greenspace in children’s spatial working memory. Brit. J. Educ. Psychol. 2019, 89, 359–373. [Google Scholar] [CrossRef]
- Hystad, P.; Payette, Y.; Noisel, N.; Boileau, C. Green space associations with mental health and cognitive function: Results from the Quebec CARTaGENE cohort. Environ. Epidemiol. 2019, 3, e040. [Google Scholar] [CrossRef]
- Liao, J.; Zhang, B.; Xia, W.; Cao, Z.; Zhang, Y.; Liang, S.; Hu, K.; Xu, S.; Li, Y. Residential exposure to green space and early childhood neurodevelopment. Environ. Int. 2019, 128, 70–76. [Google Scholar] [CrossRef]
- Reuben, A.; Arseneault, L.; Belsky, D.W.; Caspi, A.; Fisher, H.L.; Houts, R.M.; Moffitt, T.E.; Odgers, C. Residential neighborhood greenery and children’s cognitive development. Soc. Sci. Med. 2019, 230, 271–279. [Google Scholar] [CrossRef]
- Wang, D.; Lau, K.K.; Yu, R.; Wong, S.Y.S.; Kwok, T.T.Y.; Woo, J. Neighbouring green space and mortality in community-dwelling elderly Hong Kong Chinese: A cohort study. BMJ Open 2017, 7, e015794. [Google Scholar] [CrossRef]
- Ward, J.S.; Duncan, J.S.; Jarden, A.; Stewart, T. The impact of children’s exposure to greenspace on physical activity, cognitive development, emotional wellbeing, and ability to appraise risk. Health Place 2016, 40, 44–50. [Google Scholar] [CrossRef]
- Wu, Y.T.; Prina, A.M.; Jones, A.; Matthews, F.E.; Brayne, C.; Medical Research Council Cognitive Function and Ageing Study Collaboration. The Built Environment and Cognitive Disorders: Results From the Cognitive Function and Ageing Study II. Am. J. Prev. Med. 2017, 53, 25–32. [Google Scholar] [CrossRef]
- Wu, Y.T.; Prina, A.M.; Jones, A.P.; Barnes, L.E.; Matthews, F.E.; Brayne, C.; Medical Research Council Cognitive Function and Ageing Study. Community environment, cognitive impairment and dementia in later life: Results from the Cognitive Function and Ageing Study. Age Ageing 2015, 44, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Wang, D.; Leung, J.; Lau, K.; Kwok, T.; Woo, J. Is Neighborhood Green Space Associated With Less Frailty? Evidence From the Mr. and Ms. Os (Hong Kong) Study. J. Am. Med. Dir. Assoc. 2018, 19, 528–534. [Google Scholar] [CrossRef]
- Yuchi, W.; Sbihi, H.; Davies, H.; Tamburic, L.; Brauer, M. Road proximity, air pollution, noise, green space and neurologic disease incidence: A population-based cohort study. Environ. Health 2020, 19, 8. [Google Scholar] [CrossRef]
- Zhu, A.; Yan, L.; Shu, C.; Zeng, Y.; Ji, J.S. APOE epsilon4 Modifies Effect of Residential Greenness on Cognitive Function among Older Adults: A Longitudinal Analysis in China. Sci. Rep. 2020, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Zijlema, W.L.; Triguero-Mas, M.; Smith, G.; Cirach, M.; Martinez, D.; Dadvand, P.; Gascon, M.; Jones, M.; Gidlow, C.; Hurst, G.; et al. The relationship between natural outdoor environments and cognitive functioning and its mediators. Environ. Res. 2017, 155, 268–275. [Google Scholar] [CrossRef]
- Aitken, W.W.; Lombard, J.; Wang, K.; Toro, M.; Byrne, M.; Nardi, M.I.; Kardys, J.; Parrish, A.; Dong, C.; Szapocznik, J.; et al. Relationship of Neighborhood Greenness to Alzheimer’s Disease and Non-Alzheimer’s Dementia Among 249,405 U.S. Medicare Beneficiaries. J. Alzheimer’s Dis. 2021, 81, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.Q.; Barros, H.; Ribeiro, A.I. Residential and school green and blue spaces and intelligence in children: The Generation XXI birth cohort. Sci. Total. Environ. 2022, 813, 151859. [Google Scholar] [CrossRef]
- Anabitarte, A.; Ibarluzea, J.; García-Baquero, G.; Santa Marina, L.; Fernández-Somoano, A.; Tardón, A.; Nieuwenhuijsen, M.; de Castro, M.; Dadvand, P.; Lertxundi, A. Effects of residential greenness on attention in a longitudinal study at 8 and 11-13 years. Environ. Res. 2022, 210, 112994. [Google Scholar] [CrossRef]
- Asta, F.; Michelozzi, P.; Cesaroni, G.; De Sario, M.; Davoli, M.; Porta, D. Green spaces and cognitive development at age 7 years in a rome birth cohort: The mediating role of nitrogen dioxide. Environ. Res. 2021, 196, 110358. [Google Scholar] [CrossRef] [PubMed]
- Astell-Burt, T.; Navakatikyan, M.A.; Feng, X. Urban green space, tree canopy and 11-year risk of dementia in a cohort of 109,688 Australians. Environ. Int. 2020, 145, 106102. [Google Scholar] [CrossRef] [PubMed]
- Astell-Burt, T.; Feng, X. Greener neighbourhoods, better memory? A longitudinal study. Health Place 2020, 65, 102393. [Google Scholar] [CrossRef] [PubMed]
- Besser, L.M.; Hirsch, J.; Galvin, J.E.; Renne, J.; Park, J.; Evenson, K.R.; Kaufman, J.D.; Fitzpatrick, A.L. Associations between neighborhood park space and cognition in older adults vary by US location: The Multi-Ethnic Study of Atherosclerosis. Health Place 2020, 66, 102459. [Google Scholar] [CrossRef]
- Besser, L.M.; Lovasi, G.S.; Michael, Y.L.; Garg, P.; Hirsch, J.A.; Siscovick, D.; Hurvitz, P.; Biggs, M.L.; Galvin, J.E.; Bartz, T.M.; et al. Associations between neighborhood greenspace and brain imaging measures in non-demented older adults: The Cardiovascular Health Study. Soc. Psychiatry Psychiatr. Epidemiol. 2021, 56, 1575–1585. [Google Scholar] [CrossRef]
- EBijnens, M.; Derom, C.; Thiery, E.; Weyers, S.; Nawrot, T.S. Residential green space and child intelligence and behavior across urban, suburban, and rural areas in Belgium: A longitudinal birth cohort study of twins. PLoS Med. 2020, 17, e1003213. [Google Scholar] [CrossRef]
- Bijnens, E.M.; Vos, S.; Verheyen, V.V.; Bruckers, L.; Covaci, A.; De Henauw, S.; Den Hond, E.; Loots, I.; Nelen, V.; Plusquin, M.; et al. Higher surrounding green space is associated with better attention in Flemish adolescents. Environ. Int. 2022, 159, 107016. [Google Scholar] [CrossRef]
- Binter, A.C.; Bernard, J.Y.; Mon-Williams, M.; Andiarena, A.; González-Safont, L.; Vafeiadi, M.; Lepeule, J.; Soler-Blasco, R.; Alonso, L.; Kampouri, M.; et al. Urban environment and cognitive and motor function in children from four European birth cohorts. Environ. Int. 2022, 158, 106933. [Google Scholar] [CrossRef]
- Cerin, E.; Barnett, A.; Shaw, J.E.; Martino, E.; Knibbs, L.D.; Tham, R.; Wheeler, A.J.; Anstey, K.J. From urban neighbourhood environments to cognitive health: A cross-sectional analysis of the role of physical activity and sedentary behaviours. BMC Public Health 2021, 21, 2320. [Google Scholar] [CrossRef]
- Crous-Bou, M.; Gascon, M.; Gispert, J.D.; Cirach, M.; Sánchez-Benavides, G.; Falcon, C.; Arenaza-Urquijo, E.M.; Gotsens, X.; Fauria, K.; Sunyer, J.; et al. Impact of urban environmental exposures on cognitive performance and brain structure of healthy individuals at risk for Alzheimer’s dementia. Environ. Int. 2020, 138, 105546. [Google Scholar] [CrossRef]
- Dockx, Y.; Bijnens, E.M.; Luyten, L.; Peusens, M.; Provost, E.; Rasking, L.; Sleurs, H.; Hogervorst, J.; Plusquin, M.; Casas, L.; et al. Early life exposure to residential green space impacts cognitive functioning in children aged 4 to 6years. Environ. Int. 2022, 161, 107094. [Google Scholar] [CrossRef] [PubMed]
- Falcón, C.; Gascon, M.; Molinuevo, J.L.; Operto, G.; Cirach, M.; Gotsens, X.; Fauria, K.; Arenaza-Urquijo, E.M.; Pujol, J.; Sunyer, J.; et al. Brain correlates of urban environmental exposures in cognitively unimpaired individuals at increased risk for Alzheimer’s disease: A study on Barcelona’s population. Alzheimer’s Dement. 2021, 13, e12205. [Google Scholar] [CrossRef]
- Fangfang, H.; Xiao, H.; Shuai, Z.; Qiong, W.; Jingya, Z.; Guodong, S.; Yan, Z. Living Environment, Built Environment and Cognitive Function among Older Chinese Adults: Results from a Cross-Sectional Study. J. Prev. Alzheimer’s Dis. 2022, 9, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Finlay, J.; Esposito, M.; Li, M.; Colabianchi, N.; Zhou, H.; Judd, S.; Clarke, P. Neighborhood active aging infrastructure and cognitive function: A mixed-methods study of older Americans. Prev. Med. 2021, 150, 106669. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, M.P.; Shoaff, J.; Kioumourtzoglou, M.A.; Korrick, S.; Rifas-Shiman, S.L.; Hivert, M.F.; Oken, E.; James, P. Early-Life Exposure to Green Space and Mid-Childhood Cognition in the Project Viva Cohort, Massachusetts. Am. J. Epidemiol. 2022, 191, 115–125. [Google Scholar] [CrossRef]
- Jin, X.; Shu, C.; Zeng, Y.; Liang, L.; Ji, J.S. Interaction of greenness and polygenic risk score of Alzheimer’s disease on risk of cognitive impairment. Sci. Total Environ. 2021, 796, 148767. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.J.; Lee, J.E.; Lee, S.Y. Perceived environmental pollution and subjective cognitive decline (SCD) or SCD-related functional difficulties among the general population. Environ. Sci. Pollut. Res. Int. 2021, 28, 31289–31300. [Google Scholar] [CrossRef]
- Julvez, J.; López-Vicente, M.; Warembourg, C.; Maitre, L.; Philippat, C.; Gützkow, K.B.; Guxens, M.; Evandt, J.; Andrusaityte, S.; Burgaleta, M.; et al. Early life multiple exposures and child cognitive function: A multi-centric birth cohort study in six European countries. Environ. Pollut. 2021, 284, 117404. [Google Scholar] [CrossRef]
- Lee, K.S.; Kim, B.N.; Cho, J.; Jang, Y.Y.; Choi, Y.J.; Lee, W.S.; Han, C.; Bae, H.J.; Lim, Y.H.; Kim, J.I.; et al. Associations between surrounding residential greenness and intelligence quotient in 6-year-old children. Sci. Total Environ. 2021, 759, 143561. [Google Scholar] [CrossRef]
- Lega, C.; Gidlow, C.; Jones, M.; Ellis, N.; Hurst, G. The relationship between surrounding greenness, stress and memory. Urban For. Urban Green. 2021, 59, 126974. [Google Scholar] [CrossRef]
- Liu, C.C.; Li, C.Y.; Kung, S.F.; Kuo, H.W.; Huang, N.C.; Sun, Y.; Hu, S.C. Association of Environmental Features and the Risk of Alzheimer’s Dementia in Older Adults: A Nationwide Longitudinal Case-Control Study. Int. J. Environ. Res. Public. Health 2019, 16, 2828. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Sun, Y.; Kung, S.F.; Kuo, H.W.; Huang, N.C.; Li, C.Y.; Hu, S.C. Effects of physical and social environments on the risk of dementia among Taiwanese older adults: A population-based case-control study. BMC Geriatr. 2020, 20, 226. [Google Scholar] [CrossRef] [PubMed]
- Paul, L.A.; Hystad, P.; Burnett, R.T.; Kwong, J.C.; Crouse, D.L.; van Donkelaar, A.; Tu, K.; Lavigne, E.; Copes, R.; Martin, R.V.; et al. Urban green space and the risks of dementia and stroke. Environ. Res. 2020, 186, 109520. [Google Scholar] [CrossRef] [PubMed]
- Slawsky, E.D.; Hajat, A.; Rhew, I.C.; Russette, H.; Semmens, E.O.; Kaufman, J.D.; Leary, C.S.; Fitzpatrick, A.L. Neighborhood greenspace exposure as a protective factor in dementia risk among US adults 75 years or older: A cohort study. Environ. Health 2022, 21, 14. [Google Scholar] [CrossRef]
- Tani, Y.; Hanazato, M.; Fujiwara, T.; Suzuki, N.; Kondo, K. Neighborhood Sidewalk Environment and Incidence of Dementia in Older Japanese Adults. Am. J. Epidemiol. 2021, 190, 1270–1280. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.T.; Brayne, C.; Liu, Z.; Huang, Y.; Sosa, A.L.; Acosta, D.; Prina, M. Neighbourhood environment and dementia in older people from high-, middle- and low-income countries: Results from two population-based cohort studies. BMC Public Health 2020, 20, 1330. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jackson, L. Greenspace Inversely Associated with the Risk of Alzheimer’s Disease in the Mid-Atlantic United States. Earth 2021, 2, 140–150. [Google Scholar] [CrossRef]
- Zhu, A.; Wu, C.; Yan, L.L.; Wu, C.D.; Bai, C.; Shi, X.; Zeng, Y.; Ji, J.S. Association between residential greenness and cognitive function: Analysis of the Chinese Longitudinal Healthy Longevity Survey. BMJ Nutr. Prev. Health 2019, 2, 72–79. [Google Scholar] [CrossRef]
- Bagheri, N.; Mavoa, S.; Tabatabaei-Jafari, H.; Knibbs, L.D.; Coffee, N.T.; Salvador-Carulla, L.; Anstey, K.J. The Impact of Built and Social Environmental Characteristics on Diagnosed and Estimated Future Risk of Dementia. J. Alzheimer’s Dis. 2021, 84, 621–632. [Google Scholar] [CrossRef]
- Pirani, M.; Booth, E.R.; Shen, C.; Milligan, B.; Jones, E.K.; Toledano, M.B. Benefit of woodland and other natural environments for adolescents’ cognition and mental health. Nat. Sustain. 2021, 4, 851–858. [Google Scholar] [CrossRef]
- Sylvers, D.L.; Hicken, M.; Esposito, M.; Manly, J.; Judd, S.; Clarke, P. Walkable Neighborhoods and Cognition: Implications for the Design of Health Promoting Communities. J. Aging Health 2022, 34, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, C.A.; Cohen, D.A.; Han, B. How Do Racial/Ethnic Groups Differ in Their Use of Neighborhood Parks? Findings from the National Study of Neighborhood Parks. J. Urban Health 2018, 95, 739–749. [Google Scholar] [CrossRef]
- Hamlin, A.M.; Kraal, A.Z.; Sol, K.; Morris, E.P.; Martino, A.G.; Zaheed, A.B.; Zahodne, L.B. Social engagement and its links to cognition differ across non-Hispanic Black and White older adults. Neuropsychology 2022, 36, 640–650. [Google Scholar] [CrossRef]
- Matthews, S.A.; Yang, T.C. Spatial Polygamy and Contextual Exposures (SPACEs): Promoting Activity Space Approaches in Research on Place and Health. Am. Behav. Sci. 2013, 57, 1057–1081. [Google Scholar] [CrossRef] [PubMed]
- National Institute on Aging. National Strategy for Recruitment and Participation in Alzheimer’s and Related Dementias Clinical Research. Available online: https://www.nia.nih.gov/research/recruitment-strategy (accessed on 18 October 2022).
- Ejiogu, N.; Norbeck, J.H.; Mason, M.A.; Cromwell, B.C.; Zonderman, A.B.; Evans, M.K. Recruitment and retention strategies for minority or poor clinical research participants: Lessons from the Healthy Aging in Neighborhoods of Diversity across the Life Span study. Gerontologist 2011, 51 (Suppl. S1), S33–S45. [Google Scholar] [CrossRef] [PubMed]
- Kimble, J. Insuring inequality: The role of the federal housing administration in the urban ghettoization of African Americans. Law Soc. Inq. 2007, 32, 399–434. [Google Scholar] [CrossRef]
- Kushner, J.A. Apartheid in America—A Historical and Legal Analysis of Contemporary Racial Segregation in the United-States. Mich. Law Rev. 1981, 79, 856–858. [Google Scholar] [CrossRef]
- Galea, S. Moving Beyond the Social Determinants of Health. Int. J. Health Serv. 2022, 52, 423–427. [Google Scholar] [CrossRef]
- Arias, E.; Tejada-Vera, B.; Ahmad, F.; Kochanek, K.D. Provisional Life Expectancy Estimages for 2021; National Center for Health Statistics: Hyattsville, MA, USA, 2002; Volume 23.
- Lin, P.J.; Daly, A.T.; Olchanski, N.; Cohen, J.T.; Neumann, P.J.; Faul, J.D.; Fillit, H.M.; Freund, K.M. Dementia Diagnosis Disparities by Race and Ethnicity. Med. Care 2021, 59, 679–686. [Google Scholar] [CrossRef]
- Loukaitou-Sideris, A.; Levy-Storms, L.; Chen, L.; Brozen, M. Parks for an Aging Population: Needs and Preferences of Low-Income Seniors in Los Angeles. J. Am. Plan. Assoc. 2016, 82, 236–251. [Google Scholar] [CrossRef]
- Tinsley, H.E.A.; Tinsley, D.J.; Croskeys, C.E. Park usage, social milieu, and psychosocial benefits of park use reported by older urban park users from four ethnic groups. Leisure Sci. 2002, 24, 199–218. [Google Scholar] [CrossRef]
- Knapp, M.; Gustat, J.; Darensbourg, R.; Myers, L.; Johnson, C. The Relationships between Park Quality, Park Usage, and Levels of Physical Activity in Low-Income, African American Neighborhoods. Int. J. Environ. Res. Public. Health 2018, 16, 85. [Google Scholar] [CrossRef]
- Chu, Y.T.; Li, D.; Chang, P.J. Effects of Urban Park Quality, Environmental Perception, and Leisure Activity on Well-Being among the Older Population. Int. J. Environ. Res. Public. Health 2021, 18, 11402. [Google Scholar] [CrossRef] [PubMed]
- Gianattasio, K.Z.; Prather, C.; Glymour, M.M.; Ciarleglio, A.; Power, M.C. Racial disparities and temporal trends in dementia misdiagnosis risk in the United States. Alzheimer’s Dement. 2019, 5, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Bernstein Sideman, A.; Al-Rousan, T.; Tsoy, E.; Piña Escudero, S.D.; Pintado-Caipa, M.; Kanjanapong, S.; Mbakile-Mahlanza, L.; Okada de Oliveira, M.; De la Cruz-Puebla, M.; Zygouris, S.; et al. Facilitators and Barriers to Dementia Assessment and Diagnosis: Perspectives From Dementia Experts Within a Global Health Context. Front. Neurol. 2022, 13, 769360. [Google Scholar] [CrossRef]
- Zahodne, L.B.; Manly, J.J.; Azar, M.; Brickman, A.M.; Glymour, M.M. Racial Disparities in Cognitive Performance in Mid- and Late Adulthood: Analyses of Two Cohort Studies. J. Am. Geriatr. Soc. 2016, 64, 959–964. [Google Scholar] [CrossRef]
- Glymour, M.M.; Manly, J.J. Lifecourse Social Conditions and Racial and Ethnic Patterns of Cognitive Aging. Neuropsychol. Rev. 2008, 18, 223–254. [Google Scholar] [CrossRef]
- Besser, L.M.; Brenowitz, W.D.; Meyer, O.L.; Hoermann, S.; Renne, J. Methods to Address Self-Selection and Reverse Causation in Studies of Neighborhood Environments and Brain Health. Int. J. Environ. Res. Public. Health 2021, 18, 6484. [Google Scholar] [CrossRef] [PubMed]
- Lemelin, E.T.; Diez Roux, A.V.; Franklin, T.G.; Carnethon, M.; Lutsey, P.L.; Ni, H.; O’Meara, E.; Shrager, S. Life-course socioeconomic positions and subclinical atherosclerosis in the multi-ethnic study of atherosclerosis. Soc. Sci. Med. 2009, 68, 444–451. [Google Scholar] [CrossRef]
- Helbich, M.; O’Connor, R.C.; Nieuwenhuijsen, M.; Hagedoorn, P. Greenery exposure and suicide mortality later in life: A longitudinal register-based case-control study. Environ. Int. 2020, 143, 105982. [Google Scholar] [CrossRef]
- DeFrank, J.T.; Bowling, J.M.; Rimer, B.K.; Gierisch, J.M.; Skinner, C.S. Triangulating differential nonresponse by race in a telephone survey. Prev. Chronic Dis. 2007, 4, A60. [Google Scholar]
- Giuliano, A.R.; Mokuau, N.; Hughes, C.; Tortolero-Luna, G.; Risendal, B.; Ho, R.C.S.; Prewitt, T.E.; McCaskill-Stevens, W.J. Participation of minorities in cancer research: The influence of structural, cultural, and linguistic factors. Ann. Epidemiol. 2000, 10 (Suppl. S8), S22–S34. [Google Scholar] [CrossRef]
- Scharff, D.P.; Mathews, K.J.; Jackson, P.; Hoffsuemmer, J.; Martin, E.; Edwards, D. More than Tuskegee: Understanding mistrust about research participation. J. Health Care Poor Underserved. 2010, 21, 879–897. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.L.; Alsan, M.; Morris, A.A.; Halpern, S.D. Why Diverse Clinical Trial Participation Matters. N. Engl. J. Med. 2023, 388, 1252–1254. [Google Scholar] [CrossRef] [PubMed]
- Mooldijk, S.S.; Licher, S.; Wolters, F.J. Characterizing Demographic, Racial, and Geographic Diversity in Dementia Research: A Systematic Review. JAMA Neurol. 2021, 78, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Jelks, N.O.; Jennings, V.; Rigolon, A. Green Gentrification and Health: A Scoping Review. Int. J. Environ. Res. Public. Health 2021, 18, 907. [Google Scholar] [CrossRef]
- Besser, L.M.; Mitsova, D.P. Neighborhood Green Land Cover and Neighborhood-Based Walking in U.S. Older Adults. Am. J. Prev. Med. 2021, 61, e13–e20. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Healthy Places: Strategies. Available online: https://www.cdc.gov/healthyplaces/healthtopics/gentrification_strategies.htm (accessed on 18 October 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).