Abstract
We sought to estimate the prevalence of metabolic syndrome (MS) in the urban population of Mongolia and suggest a preferred definition. This cross-sectional study comprised 2076 representative samples, which were randomly selected to provide blood samples. MS was defined by the National Cholesterol Education Program’s Adults Treatment Panel III (NCEP ATP III), the International Diabetes Federation (IDF), and the Joint Interim Statement (JIS). The Cohen’s kappa coefficient (κ) was analyzed to determine the agreement between the individual MS components using the three definitions. The prevalence of MS in the 2076 samples was 19.4% by NCEP ATP III, 23.6% by IDF, and 25.4% by JIS criteria. For men, moderate agreement was found between the NCEP ATP III and waist circumference (WC) (κ = 0.42), and between the JIS and fasting blood glucose (FBG) (κ = 0.44) and triglycerides (TG) (κ = 0.46). For women, moderate agreement was found between the NCEP ATP III and high-density lipoprotein cholesterol (HDL-C) (κ = 0.43), and between the JIS and HDL-C (κ = 0.43). MS is highly prevalent in the Mongolian urban population. The JIS definition is recommended as the provisional definition.
1. Introduction
Metabolic syndrome (MS) is a combination of clustering risk factors, including central obesity, dyslipidemia, hyperglycemia, and hypertension, which eventually lead to cardiovascular diseases (CVDs) and diabetes [1,2,3,4]. The prevalence of MS is increasing worldwide, and many prior studies reported a varying prevalence of MS ranging from 20% to 30% in most countries, depending on the ethnicity, aging, sex, and race of the population [3,4,5,6]. In addition, the prevalence of MS has increased in Asia, including in Mongolia [7,8]. Mongolia has faced increased mortality from non-communicable diseases (NCDs) [9,10], such as CVDs, precipitated by MS. However, there is not yet an established diagnostic definition for MS in the Mongolian population. Therefore, in order to establish adequate criteria, this study compares three diagnostic definitions: the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (NCEP ATP III) [1,2]; the International Diabetes Federation (IDF) [3]; and the Joint Interim Statement (JIS) of the International Diabetes Federation Task Force on Epidemiology and Prevention: National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity [4], while adapting them to the Mongolian population.
Previous studies have reported the prevalence of MS in Mongolia. A previous Mongolian epidemiological study of MS reported the prevalence by IDF criteria (32.8%) in adults aged ≥ 40 years [11]. Another comparative study established the MS prevalence by the NCEP ATP III criteria (12–16%) in adults aged over 30 years, which was found to be higher than that in the Japanese and Korean populations [12]. A recent study on Mongolian national trends in MS reported that MS increased significantly (p for trend 0.023) when the JIS criteria were followed [13]. These studies have reported basic data on the prevalence of MS. To date, there is scarce information regarding the prevalence of MS in urban adults aged more than 20 years and the comparison of diagnostic definitions, increasing the evidence that early detection and prevention are targeted in the Mongolian urban population.
This seemingly different prevalence appears to be due to the use of the different thresholds and set of criteria established in different definitions, with varying cut-off values for waist circumference (WC), high-density lipoprotein cholesterol (HDL-C), or fasting blood glucose (FBG), and having different ways of combining and including them in blood pressure (BP), triglycerides (TG), and medications for hypertension, diabetes, and dyslipidemia to define MS. The NCEP ATP III definition does not require any specific risks, and it recognizes that MS is a complex disorder [1,2]. Therefore, IDF and JIS definitions use WC cut-off points based on ethnicity [3,4], and WC is now recognized as an important factor in the IDF definition [3]. However, the adaptability of different definitions to different populations is always arguable [3]. With the importance of early screening, determining, and diagnosing MS to prevent mortality from this condition, it is crucial to establish a personalized definition of MS in Mongolia, an Asian country where westernization is increasing.
Therefore, this study compared the differences in MS prevalence among Mongolian urban adults based on three currently used definitions of MS that have their own features to clarify the epidemiological situation of MS, and provide the necessary evidence for preparing diagnostic definitions. Furthermore, we determined the preferred provisional definition of MS for the Mongolians.
2. Materials and Methods
2.1. Study Design, Sampling, and Population
We conducted a cross-sectional survey on the prevalence of MS in an urban population in Mongolia. The survey followed the guidelines of the World Health Organization (WHO) STEPS Surveillance Manual which provides a complete overview, including guidelines and supporting materials, for countries wishing to undertake NCD risk factor surveys using the WHO STEPwise approach [14], and multistage cluster sampling was conducted for Mongolian residents. First, geopolitical units were sampled and then residents were sampled within these units. In Ulaanbaatar, 142 family healthcare centers (FHCCs) provide primary healthcare services to all citizens. According to the WHO STEPS Surveillance Manual, which recommends that at least 50 primary sampling units (PSUs) be selected from over 100, proportional probability sampling was used to select 52 FHCCs from the eight districts in the first stage of cluster sampling. In the next stage, 88 individuals aged ≥ 20 years were randomly selected from the registers of each of the 52 FHCCs. If the participants could not be reached by the research team, they were replaced by the next participant within the same age and sex category. In total, 4515 urban residents were included in this survey (response rate: 98.7%), and 2258 residents in Mongolia underwent biochemical measurements. A pilot study was conducted on five randomly selected FHCCs in November 2017. Data collection was conducted between December 2017 and January 2018, and the final study population included 2076 urban residents.
We obtained a de-identified dataset of Mongolians from the Onom Foundation, according to the Data Transfer Agreement. Written informed consent was obtained before conducting interviews and physical measurements. The study was approved by the Medical Ethics Committee of the Ministry of Health, Mongolia, and the Ethical Committee of Dokkyo Medical University (Protocol Number, 2021-014).
2.2. Measurements
The WHO STEPwise approach is comprised of three steps of risk factor assessment: questionnaire, physical measurements, and biochemical measurements. Before data collection, all field members, who were medical researchers, doctors, nurses, and laboratory technicians, successfully completed 5-day training programs regarding how to conduct interviews, measure anthropometry and BP, and take and collect blood samples. These training programs were organized by the ‘Technical Working Group’ in the Public Health Institute in collaboration with the WHO country office and experts from the relevant cooperating organizations. The pilot study was organized covering all steps of the actual survey. The entire data collection procedure was conducted using an electronic tablet (Fire HD 8, Amazon, Seattle, WA, USA). To avoid data loss, an Android application with an offline mode, QuickTapSurvey (TabbleDabble Inc., Toronto, ON, Canada), was used.
Interviews were conducted using the Mongolian version [15] of the WHO STEPS instrument for NCD risk factor surveillance. To ensure the adequacy of the Mongolian translation of the questionnaires, the Mongolian versions were separately back-translated and reviewed by two independent translators. The survey questionnaire was further adapted to country specifics with the help of local experts, the survey ‘Technical Working Group’, and with close collaboration and technical assistance from the WHO. Finally, it was reviewed and approved by international and national experts and consultants.
Blood pressure measurement: BP was measured using accuracy-validated BP A6 BTs (Microlife Corporation, Taipei, Taiwan) and digital automatic BP monitors. Participants were instructed to abstain from alcohol, cigarette smoking, caffeine consumption, and exercise for at least 30 min before BP measurement. Data collection teams ensured that participants were seated with their legs uncrossed and their back and arm supported, in accordance with the American Heart Association (AHA) guidelines, and that appropriate cuff sizes were used. After a 10 min rest, BP was measured three times, with 3 min intervals between measurements. The average value of the three measurements was then calculated. If one differed by ≥15 mmHg from the other two, it was discarded [16,17].
Physical measurements: Height was measured in centimeters (without shoes) and weight in kilograms (with heavy clothing removed) using a digital scale. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. We measured the WC of subjects while standing, using a soft tape midway between the lowest rib and iliac crest.
Biochemical measurements: All participants agreed that their blood samples would be collected using a clot activator. The serum samples were centrifuged at 3500 rpm for 10 min. Overnight fasting blood samples were obtained for the measurement of serum lipids and glucose. Concentrations of HDL-C, TG, and FBG following standard operating procedure (SOP)-Liver Center-005 protocols were assessed using a fully automated biochemical analyzer (ERBA-XL200, Mannheim, Germany).
2.3. Definition of the Metabolic Syndrome
The NCEP ATP III definition was chosen in this study because Mongolia, as an Asian country, was reported as having a higher BMI compared to Japan and China [18,19]. When using the IDF and JIS definitions, the other two widely accepted definitions of MS chosen in this study, we followed the Asian population thresholds for abdominal obesity [3,4]: WC ≥ 90 cm for men, and ≥80 cm for women. The NCEP ATP III, IDF, and JIS definitions are different from each other in the diagnostic process (Table 1).

Table 1.
Diagnosis criteria of metabolic syndrome used in the current study.
2.4. Socioeconomic Status (SES) Variables
Education variables were divided into two categories of high school and lower (≤12 years) and higher educational attainment (>12 years) according to the Mongolian education system. Occupational class was defined into three groups: non-manual, manual, and “others” referring to the Erikson–Goldthorpe–Portocarero scheme [20]. Individuals classified as students, retired, and individuals whose stated occupation could not be classified were placed in the “others” group. Monthly income was divided into three types: upper, middle, and lower, according to the average salary per month [21]. The average monthly salary in Mongolian is about MNT 1,500,000 (USD ~450) [22]. Housing was categorized into two types: apartment and Ger district. Ulaanbaatar, the capital city of Mongolia, consists of two different housing-type areas: apartment areas, which are located in the central part of the city; and “Ger areas”, which are a very common housing type among nomads located in the suburbs.
2.5. Statistical Analysis
Descriptive analyses were used to report the demographic characteristics of the study participants and the prevalence of MS. The sample was divided into three age groups: young adults (<40), middle adults (40–59), and old adults (60 and over) [23]. For validity between the individual MS components and different definitions, we measured the sensitivity and specificity. Cohen’s kappa coefficient (95% confidence interval (CI)) (poor, κ ≤ 0.20; fair, κ = 0.21–0.40; moderate, κ = 0.41–0.60; substantial, κ = 0.61–0.80; very good, κ > 0.80) was used to determine the level of agreement [24]. As a result of the greater agreement, we used the JIS definition to estimate the odds ratios (ORs) and 95% CIs of SES for MS prevalence using logistic regression. The analyses were stratified according to sex. All p values were two-sided, and the alpha level was set at 0.05. Data were analyzed using SPSS version 28.0 (SPSS Inc., Chicago, IL, USA). Mosaic plots were shown for the relationships between MS and individual MS components using JMP Statistical Discovery Software version 16 (SAS Institute Inc., Cary, NC, USA).
3. Results
Table 2 shows the characteristics of the study population and the clinical components of MS in Mongolians. Of the total study participants, the mean age was 39.9 ± 13.7 years, and 53.6% were female. The height, weight, WC, BP, and TG levels were all higher in men, whereas the HDL-C levels were lower. The differences in height, weight, WC, and BP between males and females were statistically significant (p < 0.05).

Table 2.
Anthropometric and clinical characteristics of the survey participants.
The overall prevalence rate of MS was 19.4% according to the NCEP ATP III, 23.6% according to the IDF, and 25.4% according to the JIS definitions. The prevalence of MS was higher in female participants and in the 40–59 and 60 and over age groups when stratified by sex and age (Figure 1).
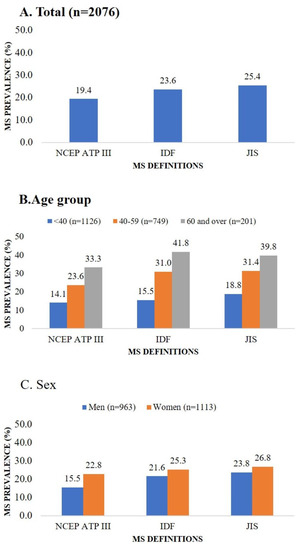
Figure 1.
Metabolic syndrome prevalence according to three definitions by total population, age group, and sex. Abbreviations: IDF, International Diabetes Federation; JIS, Joint Interim Statement; MS, metabolic syndrome; NCEP ATP III, National Cholesterol Education Program’s Adults Treatment Panel III.
The agreements between the NCEP ATP III, IDF, and JIS definitions, and the individual MS components are presented in Table 3. For men, a moderate agreement was found between the NCEP ATP III and WC (κ = 0.42), and between the JIS and FBG (κ = 0.44) and TG (κ = 0.46). For women, a moderate agreement was found between the NCEP ATP III and HDL-C (κ = 0.43), and between the JIS and HDL-C (κ = 0.43).

Table 3.
Validity and agreement between individual metabolic syndrome components and three definitions.
Table 4 shows the sex-divided ORs of SES factors for MS prevalence based on the JIS definition, which had moderate agreement between more MS components in men and showed a higher prevalence in both sexes. In men, the 40–59, and 60 and over age groups and married participants were significantly associated with MS, while for women, the 40–59, and 60 and over age groups as well as those with lower educational attainment, “others” group for occupation, and married participants were significantly associated with MS. In the multivariable logistic regression analysis, the 40–59 age group (aOR = 1.44, 95% CI 1.01 to 2.04 in men, aOR = 2.28, 95% CI 1.68 to 3.09 in women) and female 60 and over age group (aOR = 3.22, 95% CI 2.04 to 5.08) in addition to married female participants (aOR = 1.57, 95% CI 1.04 to 2.36) were significantly associated with MS.

Table 4.
Prevalence and odds ratios for the metabolic syndrome by JIS definition against SES factors.
Mosaic plots showed the MS distribution within the individual MS components by sex. Male participants with elevated MS components had a higher percentage of MS based on the JIS definition except for WC. For females, high glucose levels, and elevated cholesterol level participants had a higher percentage of MS according to the JIS definition. Based on the IDF definition, the elevated systolic blood pressure (SBP) participants had a higher percentage of MS, and the elevated diastolic blood pressure (DBP) participants had a higher percentage of MS based on both JIS and IDF definitions. However, according to the NCEP ATP III definition, the high WC participants had a higher percentage of MS (Figure S1).
4. Discussion
Using an urban population in Mongolia, we established the prevalence of MS, as 15.5%, 21.6%, and 23.8%, respectively, in men, and 22.8%, 25.3%, and 26.8%, respectively, in women as defined by the NCEP ATP III, IDF, and JIS definitions. Concerning the level of agreement, JIS definition had moderate agreements with more MS components in men, while both the NCEP ATP III and JIS definition had moderate agreements with HDL-C in women. According to our knowledge, this is the first study determining the preferred provisional definition of MS for the Mongolians.
Considering the greater emphasis of the JIS definition on FBG and TG for Mongolian men, and on HDL-C for Mongolian women, a moderate agreement between the JIS and these components is plausible. Furthermore, the agreement of the JIS definition on FBG and TG was slightly higher in women compared to the agreements of the other two definitions. However, the agreement of the JIS definition on WC was lower in both sexes than the agreements of the other two definitions because WC suffers from a higher measurement error [25,26]. Regarding the high prevalence of CVDs among the Mongolian population, applying these criteria to identify persons at risk might be helpful. In addition, the prevalence of MS measured using the JIS definition was higher in both sexes than that measured using other definitions. The Mongolian government has been building policies to prevent and control NCDs [27] and to set the Mongolia Sustainable Development Vision 2030 report, which aims to reduce the main NCDs, such as CVDs, health risk factors, and preventable deaths [28]. For policy responses, addressing MS is necessary. According to the national NCD STEP surveys, the prevalence of most NCD risk factors remained stable, while that of overweight and obesity increased [29,30]. Thus, the JIS definition could be used to identify more people at high risk and make early interventions for controlling MS in urban populations. A moderate agreement was more related to elevated glucose and cholesterol levels. In Mongolia, the incidence of diabetes and dyslipidemia has increased and is more common in the urban areas [29,31]. A cross-sectional national survey reported in 2019 that the prevalence of dyslipidemia was 58.6%, among which 6.2% were aware, 18.9% were treated, and 21.5% were controlled [31]. Another study noted that only a small proportion of the total hypertensive or diabetic population had adequately controlled blood pressure or blood sugar due to a large diagnosis gap, non-treatment of previously diagnosed populations, and inadequate control of the treated population [32].
Traditional and ecological aspects may play a role in the observed patterns of the results. A harsh climate, average atmospheric temperature, atmospheric pressure, precipitation, and mineralization of rivers can have an influence [33]. Traditionally, Mongolians have a unique nomadic lifestyle, and its population prefers a diet of meat, milk, and its derivatives [34,35,36]. Most rural families are physically active, involved in caring for livestock, transportation by horses and camels, milking, shearing, and combing. This unique lifestyle might be associated with a low BMI in Mongolians [30]. However, nearly 67% of the population is living in the capital city, seeking education and a professional job over traditional nomadic life, and migration from rural areas to Ulaanbaatar has been increasing in recent years [37]. For herders, there is a lack of access to education and healthcare services because of nomadic communities living and moving in remote areas [38]. Along with this rapid modernization of the whole country, diet and nutrition have shifted toward a diet with high fat, high energy, and low dietary fibers [39]. Dietary habits are associated with lifestyle-related diseases and early aging in Mongolia [40]. According to the NCD risk factor surveys, urban residents had higher risks, such as smoking, physical inactivity, obesity, and high cholesterol levels [29]. There was a significant association between MS and poor lifestyle habits and some SES factors such as education and marital status [11,13]. Additionally, Mongolian urban adults have a higher prevalence of MS [9,29]. Establishing health promotion policies based on the needs and convenience of urban residents is an urgent matter.
In addition, results of the logistic regression showed that the middle (40–59) and older adult (60 and over) groups had one to three times higher prevalence of MS, and married female participants had a higher prevalence of MS according to the JIS definition, which was suggested to be superior in defining MS in Mongolians than the other two definitions based on our main results. The major role of age in predicting MS was consistent with other previous studies [41]. The present study reported that married women had a higher prevalence of MS than those who were never married, which was consistent with the results of some studies [42]. In our study, unmarried participants were younger (93.1% of unmarried male young adults (<40) and 83.9% of unmarried female young adults (<40)). Nevertheless, due to their traditional social roles, Mongolian married women raise their children, do the housework, and cook the food. This may be related to the higher prevalence of MS components, such as physical inactivity, overweight, and obesity, in Mongolian women when compared to men [29]. Further research on how marital status, especially among women, is associated with MS is needed to clarify these findings.
Several limitations of this study should be noted. First, our cross-sectional study did not allow us to derive conclusions regarding causal mechanisms. Second, we did not evaluate visceral fat using computed tomography (CT) and magnetic resonance imaging (MRI) to measure central obesity. Third, our data were extracted only from the Mongolian urban population using multistage cluster sampling which included some sampling errors. However, our sampling was carried out following the WHO STEPS Surveillance Manual, and selection bias was smaller than non-random sampling. Furthermore, the current study provides the first report of a comparative study of MS prevalence in Mongolians according to three different definitions and suggests a provisional definition for Mongolians.
5. Conclusions
In conclusion, the current findings indicate that the prevalence of MS is high among the general population in the urban area of Mongolia. The JIS definition is recommended as the preferred provisional definition to identify more people at risk of MS. Hence, national preventive strategies and interventions should interfere as early as possible in detecting relevant risk factors. Therefore, further discussion is needed to establish the standardized measurement criteria and definitions of MS to allow Mongolians to define cut-offs as the best indicators of morbidity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph20064956/s1. Figure S1: Comparison of the distribution of metabolic syndrome in individual metabolic syndrome components by three definitions.
Author Contributions
Conceptualization, E.M.-O. and Y.H.; methodology, E.M.-O. and Y.H.; software, E.M.-O.; validation, E.M.-O. and Y.H.; formal analysis, E.M.-O. and Y.H.; investigation, N.D. and M.D.; resources, N.D.; data curation, N.D. and M.D.; writing—original draft preparation, E.M.-O.; writing—review and editing, N.T. and K.T.; visualization, E.M.-O. and Y.H.; supervision, G.K.; project administration, G.K.; funding acquisition, G.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Medical Ethics Committee of the Ministry of Health, Mongolia, and the Ethical Committee of Dokkyo Medical University (Protocol Number, 2021-014).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank Ken-Ichiro Yoshida and Yuichi Chigusa of the Dokkyo Medical University for their cooperation with the data transfer agreement, and all members of the Onom Foundation for their assistance with managing the survey and preparing the dataset. We would also like to thank William Hassett and Chris Smith, from the Research Planning and Analysis Support Office, Dokkyo Medical University, for English language editing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Expert Panel on Detection E. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.; Shaw, J.; IDF Epidemiology Task Force Consensus Group. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Metabolic syndrome pandemic. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 629–636. [Google Scholar] [CrossRef]
- Ervin, R.B. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. Natl. Health Stat. Rep. 2009, 13, 1–7. [Google Scholar]
- Pan, W.H.; Yeh, W.T.; Weng, L.C. Epidemiology of metabolic syndrome in Asia. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl. 1), 37–42. [Google Scholar] [PubMed]
- Ranasinghe, P.; Mathangasinghe, Y.; Jayawardena, R.; Hills, A.P.; Misra, A. Prevalence and trends of metabolic syndrome among adults in the asia-pacific region: A systematic review. BMC Public Health 2017, 17, 101. [Google Scholar] [CrossRef]
- Center for Health Development, Ministry of Health Mongolia, Health indicators-Mongolia; Munkhiin Useg: Ulaanbaatar, Mongolia, 2018.
- World Health Organization. Noncommunicable Diseases Country Profiles. Mongolia. 2018. Available online: https://www.who.int/publications/m/item/noncommunicable-diseases-mng-country-profile-2018 (accessed on 2 August 2022).
- Enkh-Oyun, T.; Kotani, K.; Davaalkham, D.; Davaa, G.; Ganchimeg, U.; Angarmurun, D.; Khuderchuluun, N.; Batzorig, B.; Tsuboi, S.; Ae, R.; et al. Epidemiologic features of metabolic syndrome in a general Mongolian population. Metab. Syndr. Relat. Disord. 2015, 13, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Shiwaku, K.; Nogi, A.; Kitajima, K.; Anuurad, E.; Enkhmaa, B.; Yamasaki, M.; Kim, J.M.; Kim, I.S.; Lee, S.K.; Oyunsuren, T.; et al. Prevalence of the metabolic syndrome using the modified ATP III definitions for workers in Japan, Korea and Mongolia. J. Occup. Health 2005, 47, 126–135. [Google Scholar] [CrossRef]
- Pengpid, S.; Peltzer, K. National trends in metabolic syndrome among adults in Mongolia from three cross-sectional surveys in 2009, 2013 and 2019. Diabetes Metab. Syndr. 2022, 16, 102375. [Google Scholar] [CrossRef]
- Riley, L.; Guthold, R.; Cowan, M.; Savin, S.; Bhatti, L.; Armstrong, T.; Bonita, R. The World Health Organization STEPwise Approach to Noncommunicable Disease Risk-Factor Surveillance: Methods, Challenges, and Opportunities. Am. J. Public Health 2016, 106, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Potts, H.; Baatarsuren, U.; Myanganbayar, M.; Purevdorj, B.; Lkhagvadorj, B.U.; Ganbat, N.; Dorjpalam, A.; Boldbaatar, D.; Tuvdendarjaa, K.; Sampilnorov, D.; et al. Hypertension prevalence and control in Ulaanbaatar, Mongolia. J. Clin. Hypertens. (Greenwich) 2020, 22, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Pickering, T.G.; Hall, J.E.; Appel, L.J.; Falkner, B.E.; Graves, J.; Hill, M.N.; Jones, D.W.; Kurtz, T.; Sheps, S.G.; Roccella, E.J. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005, 111, 697–716. [Google Scholar] [CrossRef] [PubMed]
- Kraus, W.E.; Bhapkar, M.; Huffman, K.M.; Pieper, C.F.; Krupa Das, S.; Redman, L.M.; Villareal, D.T.; Rochon, J.; Roberts, S.B.; Ravussin, E.; et al. 2 years of calorie restriction and cardiometabolic risk (CALERIE): Exploratory outcomes of a multicentre, phase 2, randomised controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zheng, L.; Xu, C.; Li, J.; Zhang, X.; Liu, S.; Hu, D.; Sun, Y. Prevalence of prehypertension, hypertension and, associated risk factors in Mongolian and Han Chinese populations in Northeast China. Int. J. Cardiol. 2008, 128, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Shiwaku, K.; Anuurad, E.; Enkhmaa, B.; Nogi, A.; Kitajima, K.; Shimono, K.; Yamane, Y.; Oyunsuren, T. Overweight Japanese with body mass indexes of 23.0-24.9 have higher risks for obesity-associated disorders: A comparison of Japanese and Mongolians. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 152–158. [Google Scholar] [CrossRef]
- Erikson, R.; Goldthorpe, J.H.; Portocarero, L. Intergenerational class mobility and the convergence thesis: England, France and Sweden. 1979. Br. J. Sociol. 2010, 61 (Suppl. 1), 185–219. [Google Scholar] [CrossRef]
- National Statistics Office of Mongolia. Mongolian Statistical Yearbook. 2018. Available online: https://www.1212.mn/en/statistic/file-library/view/47811309 (accessed on 23 September 2022).
- National Statistics Office of Mongolia. Mongolian Statistical Information Service. Available online: https://www2.1212.mn (accessed on 1 March 2023).
- Chovalopoulou, M.E.; Bertsatos, A.; Papageorgopoulou, C. Age-related changes in the craniofacial region in a modern Greek population sample of known age and sex. Int. J. Legal. Med. 2017, 131, 1103–1111. [Google Scholar] [CrossRef]
- Altman, D.G. Practical Statistics for Medical Research; Chapman & Hall/CRC: London, UK, 1991. [Google Scholar]
- Ulijaszek, S.J.; Kerr, D.A. Anthropometric measurement error and the assessment of nutritional status. Br. J. Nutr. 1999, 82, 165–177. [Google Scholar] [CrossRef]
- Verweij, L.M.; Terwee, C.B.; Proper, K.I.; Hulshof, C.T.; van Mechelen, W. Measurement error of waist circumference: Gaps in knowledge. Public Health Nutr. 2013, 16, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Chimeddamba, O.; Peeters, A.; Walls, H.L.; Joyce, C. Noncommunicable Disease Prevention and Control in Mongolia: A Policy Analysis. BMC Public Health 2015, 15, 660. [Google Scholar] [CrossRef]
- The Secretariat of the State Great Khural of Mongolia. Mongolia Sustainable Development Vision 2030. 2016. Available online: https://archive.un-page.org/files/public/20160205_mongolia_sdv_2030.pdf (accessed on 23 September 2022).
- National Center for Public Health. Fourth National STEPs Survey on Prevalence of Noncommunicable Disease and Injury Risk Factors-2019; Khukh Mongol Printing: Ulaanbaatar, Mongolia, 2019. [Google Scholar]
- Chimeddamba, O.; Gearon, E.; Stevenson, C.; Liviya Ng, W.; Baasai, B.; Peeters, A. Trends in adult overweight and obesity prevalence in Mongolia, 2005–2013. Obesity 2016, 24, 2194–2201. [Google Scholar] [CrossRef] [PubMed]
- Pengpid, S.; Peltzer, K. National high prevalence, and low awareness, treatment and control of dyslipidaemia among people aged 15–69 years in Mongolia in 2019. Sci. Rep. 2022, 12, 10478. [Google Scholar] [CrossRef]
- Otgontuya, D.; Oum, S.; Palam, E.; Rani, M.; Buckley, B.S. Individual-based primary prevention of cardiovascular disease in Cambodia and Mongolia: Early identification and management of hypertension and diabetes mellitus. BMC Public Health 2012, 12, 254. [Google Scholar] [CrossRef] [PubMed]
- Enkh-Oyun, T.; Kotani, K.; Davaalkham, D.; Uehara, R.; Sadakane, A.; Aoyama, Y.; Tsuboi, S.; Nakamura, Y. Hypertension in Mongolia: An overview. Ethn. Dis. 2013, 23, 363–368. [Google Scholar]
- Suvd, J.; Gerel, B.; Otgooloi, H.; Purevsuren, D.; Zolzaya, H.; Roglic, G.; King, H. Glucose intolerance and associated factors in Mongolia: Results of a national survey. Diabet. Med. 2002, 19, 502–508. [Google Scholar] [CrossRef]
- Manaseki, S. Mongolia: A health system in transition. BMJ 1993, 307, 1609–1611. [Google Scholar] [CrossRef]
- Bromage, S.; Daria, T.; Lander, R.L.; Tsolmon, S.; Houghton, L.A.; Tserennadmid, E.; Gombo, N.; Gibson, R.S.; Ganmaa, D. Diet and Nutrition Status of Mongolian Adults. Nutrients 2020, 12, 1514. [Google Scholar] [CrossRef]
- Mongolia Demographics 2020. Available online: https://www.worldometers.info/demographics/mongolia-demographics/#poplation-pyramid (accessed on 2 August 2022).
- National centre for Non Formal and Distance Education, Ministry of Education, Culture and Science. National Report on the Situation of Adult Learning and Education-Mongolia. 2008. Available online: https://uil.unesco.org/fileadmin/multimedia/uil/confintea/pdf/National_Reports/Asia%20-%20Pacific/Mongolia.pdf (accessed on 23 September 2022).
- Popkin, B.M. Global nutrition dynamics: The world is shifting rapidly toward a diet linked with noncommunicable diseases. Am. J. Clin. Nutr. 2006, 84, 289–298. [Google Scholar] [CrossRef]
- Komatsu, F.; Kagawa, Y.; Kawabata, T.; Kaneko, Y.; Purvee, B.; Otgon, J.; Chimedregzen, U. Dietary habits of Mongolian people, and their influence on lifestyle-related diseases and early aging. Curr. Aging Sci. 2008, 1, 84–100. [Google Scholar] [CrossRef] [PubMed]
- Bener, A.; Zirie, M.; Musallam, M.; Khader, Y.S.; Al-Hamaq, A.O. Prevalence of metabolic syndrome according to Adult Treatment Panel III and International Diabetes Federation criteria: A population-based study. Metab. Syndr. Relat. Disord. 2009, 7, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Bhanushali, C.J.; Kumar, K.; Wutoh, A.K.; Karavatas, S.; Habib, M.J.; Daniel, M.; Lee, E. Association between Lifestyle Factors and Metabolic Syndrome among African Americans in the United States. J. Nutr. Metab. 2013, 2013, 516475. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).