COVID-19: Protocol and Checklist for Nursing Care Management at Urgent Care Units
Abstract
1. Introduction
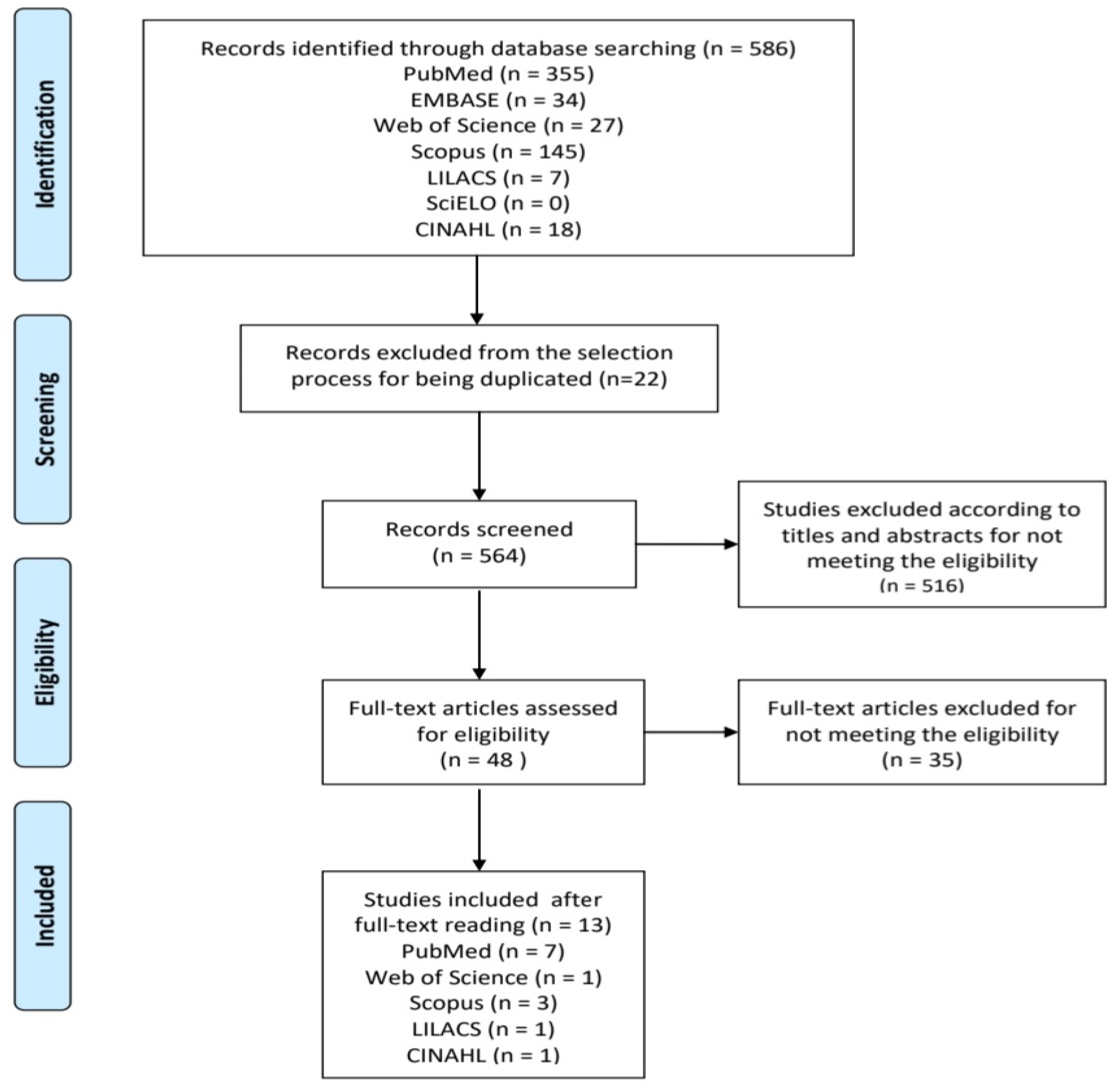
2. Methods
2.1. Study Design and Setting
2.2. Data Analysis
2.3. Ethical Aspects
3. Results
4. Discussion
5. Conclusions
6. Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brasil. Ministério da Saúde. Protocolo de Manejo Clínico da COVID-19 na Atenção Especializada [Recurso Eletrônico]. 2020. Available online: http://bvsms.saude.gov.br/bvs/publicacoes/manejo_clinico_covid-19_atencao_especializada.pdf (accessed on 10 October 2020).
- Brasil. Ministério da Saúde. Boletim Epidemiológico Especial n°80. Doença pelo Novo Coronavírus-COVID-19. 2021. Available online: https://www.gov.br/saude/pt-br/media/pdf/2021/setembro/17/boletim_epidemiologico_covid_80-final17set.pdf (accessed on 18 September 2021).
- Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Análise em Saúde e Doenças não Transmissíveis. Guia de Vigilância Epidemiológica: Emergência de Saúde Pública de Importância Nacional pela Doença pelo Coronavírus 2019—COVID-19. Versão 3–15 de Março de 2021; Ministério da Saúde: Brasilia (DF), Brazil, 2021. [Google Scholar]
- World Health Organization. COVID-19 Clinical Management: Living Guidance. 2021. Available online: https://app.magicapp.org/#/guideline/j1WBYn (accessed on 18 September 2021).
- Brasil. Ministério da Saúde. Portaria MS/GM no 342, de 4 de Março de 2013. Redefine as Diretrizes para Implantação do Componente Unidade de Pronto Atendimento (UPA 24h) em Conformidade com a Política Nacional de Atenção às Urgências, e Dispõe sobre Incentivo Financeiro de Investimento para Novas UPA 24h (UPA Nova) e UPA 24h Ampliadas (UPA Ampliada) e Respectivo Incentivo Financeiro de Custeio Mensal. 2013. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/gm/2013/prt0342_04_03_2013.html (accessed on 6 June 2020).
- Konder, M.T.; O’Dwyer, G. As Unidades de Pronto-Atendimento na Política Nacional de Atenção às Urgências. Physis Rev. Saúde Coletiva 2015, 25, 525–545. [Google Scholar] [CrossRef]
- Konder, M.; O’Dwyer, G. As Unidades de Pronto Atendimento como unidades de internação: Fenômenos do fluxo assistencial na rede de urgências. Physis Rev. Saúde Coletiva 2019, 29, e290203. [Google Scholar] [CrossRef]
- Brasil. Ministério da Saúde. Fluxo de Pacientes com Sintomas Respiratórios em Unidade de Urgência Hospitalar e Não-hospitalar. 2020. Available online: https://www.saude.ms.gov.br/wp-content/uploads/2020/03/2-Etapa-Fluxogramas-COVID-19-SAES-Z.pdf (accessed on 20 July 2020).
- Brasil. Ministério da Saúde. Fluxograma para Atendimento e Detecção Precoce de COVID-19 em Pronto Atendimento UPA 24 Horas e Unidade Hospitalar não Definida como Referência. 2020. Available online: https://docs.bvsalud.org/biblioref/2020/03/1052504/fluxogramas-covid-19-saes-1.pdf (accessed on 8 July 2020).
- ABRAMEDE—Associação Brasileira de Medicina de Emergência. Recomendações para o Atendimento de Pacientes Suspeitos ou Confirmados de Covid-19 pelas Equipes de Enfermagem de Serviços de Emergência (Pré-Hospitalar Fixo e Intra-Hospitalar). 2020. Available online: http://abramede.com.br/wp-content/uploads/2020/04/RECOMENDACOES-ENFERMAGEM-200420.pdf (accessed on 8 July 2020).
- Thomas, L.S.; Pietrowski, K.; da Silva Kinalski, S.; Bittencourt VL, L.; Sangoi KC, M. Atuação do enfermeiro emergencista na pandemia de covid-19: Revisão narrativa da literatura/The role of emergency nurses in the covid-19 pandemic: A narrative review of the literature. Braz. J. Health Rev. 2020, 3, 15959–15977. [Google Scholar] [CrossRef]
- de Sá Mororó, D.D.; Enders, B.C.; de Carvalho Lira, A.L.B.; da Silva, C.M.B.; de Menezes, R.M.P. Análise conceitual da gestão do cuidado em enfermagem no âmbito hospitalar. Acta Paul. Enferm. 2017, 30, 323–332. [Google Scholar] [CrossRef]
- Bardin, L. Análise de Conteúdo; Edições: Lisboa, Portugal, 2010; Volume 70. [Google Scholar]
- DeVellis, R.F. Scale Development: Theory and Applications, 3rd ed.; Applied Social Research Methods Series; SAGE: Thousand Oaks, CA, USA, 2012; 205 p. [Google Scholar]
- Terwee, C.B.; Prinsen, C.A.C.; Chiarotto, A.; Westerman, M.J.; Patrick, D.L.; Alonso, J.; Bouter, L.M.; de Vet, H.C.W.; Mokkink, L.B. COSMIN methodology for evaluating the content validity of patient-reported outcome measures: A Delphi study. Qual. Life Res. 2018, 27, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Melo, R.P.; Moreira, R.P. Critérios de seleção de experts para estudos de validação de fenômenos de enfermagem. Rev. Rene 2011, 12, 424–431. [Google Scholar]
- Alexandre, N.M.C.; Coluci, M.Z.O. Validade de conteúdo nos processos de construção e adaptação de instrumentos de medidas. Ciênc. Saúde Coletiva 2011, 16, 3061–3068. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, C.A.M.; Pastana, I.C.A.S.S.; Sichieri, K.; Solha, R.K.T. Guia para Construção de Protocolos Assistenciais de Enfermagem; COREN-SP: São Paulo, Brazil, 2015; 649p. [Google Scholar]
- Coluci, M.Z.O.; Alexandre, N.M.C.; Milani, D. Construção de instrumentos de medida na área da saúde. Ciênc. Saúde Coletiva 2015, 20, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Polit, D.F.; Beck, C.T. The content validity index: Are you sure you know what’s being reported? critique and recommendations. Res. Nurs. Health 2006, 29, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Yusoff, M.S.B. ABC of Content Validation and Content Validity Index Calculation. Educ. Med. J. 2019, 11, 49–54. [Google Scholar] [CrossRef]
- Medeiros SG, D.; Lima AV, D.; Saraiva CO PD, O.; Barbosa, M.L.; Santos VE, P. Avaliação da segurança no cuidado com vacinas: Construção e validação de protocolo. Acta Paul. Enferm. 2019, 32, 53–64. [Google Scholar] [CrossRef]
- Gomide, M.F.S.; Pinto, I.C.; de Figueiredo, L.A. Accessibility and demand at an Emergency Care Unit: The user’s perspective. Acta Paul. Enferm. 2012, 25, 19–25. [Google Scholar] [CrossRef]
- O’Dwyer, G.; Konder, M.T.; Reciputti, L.P.; Lopes, M.G.M.; Agostinho, D.F.; Alves, G.F. O processo de implantação das unidades de pronto atendimento no Brasil. Rev. Saúde Pública 2017, 51, 125. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Overview of Testing for SARS-CoV-2 (COVID-19). Updated 22 October 2021. CDC—Centers for Disease Control and Prevention. 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html (accessed on 29 November 2021).
- Morris, S.B.; Schwartz, N.G.; Patel, P.; Abbo, L.; Beauchamps, L.; Balan, S.; Lee, E.H.; Paneth-Pollak, R.; Geevarughese, A.; Lash, M.K.; et al. Case Series of Multisystem Inflammatory Syndrome in Adults Associated with SARS-CoV-2 Infection —United Kingdom and United States, March–August 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- ANVISA—Agência Nacional de Vigilância Sanitária. Nota Técnica GVIMS/GGTES/Anvisa No 04/2020. Orientações para Serviços de Saúde: Medidas de Prevenção e Controle que Devem ser Adotadas Durante a Assistência aos casos Suspeitos ou Confirmados de Infecção pelo Novo Coronavírus–Atualizada em 9 September 2021. 2021. Available online: https://www.gov.br/anvisa/pt-br/centraisdeconteudo/publicacoes/servicosdesaude/notas-tecnicas/nota-tecnica-gvims_ggtes_anvisa-04-2020-09-09-2021.pdf (accessed on 27 November 2021).
- World Health Organization. Rational Use of Personal Protective Equipment for Coronavirus Disease (COVID-19): Interim Guidance. World Health Organization. 2020. Available online: https://apps.who.int/iris/handle/10665/331215 (accessed on 27 November 2021).
- CDC—Centers for Disease Control and Prevention. Using Personal Protective Equipment (PPE). Updated 19 August 2020. CDC—Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/using-ppe.html (accessed on 27 November 2021).
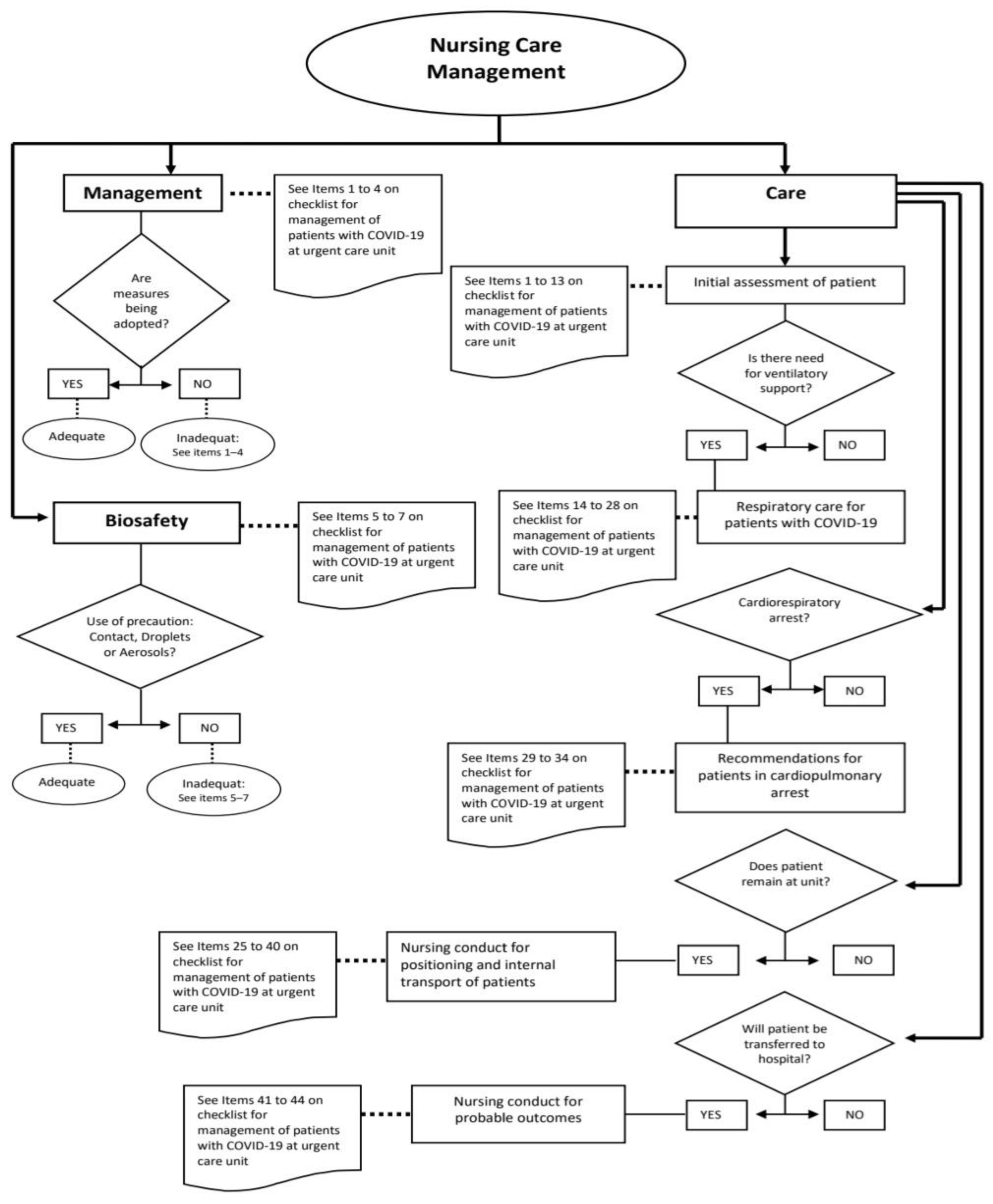
| Categories | Definition | Subcategories |
|---|---|---|
| Management | Involves actions related to the organization of the service for providing patient care and workflow strategies. | -Differentiated flow for respiratory cases. -Material resources and equipment necessary for providing care in severe cases. -Safety measures recommended. -Strategy developed at the service for the identification of respiratory cases and standardized classification of risk to facilitate recognition on the electronic patient records and prioritize care. |
| Biosafety | Actions for the prevention, control, reduction, or elimination of the risks inherent to activities that can compromise the health of professionals and patients. | -Measures of professional good practices (use of PPE, protocol for putting on and removing garments). |
| Care | Actions inherent to direct care by the nursing staff for patients with a suspicion or confirmation of COVID-19. | -Nursing conduct at initial care for patients with suspicion or confirmation of COVID-19. -Criteria for definition, reporting and conduct in cases of severe acute respiratory syndrome—SARS. -Recommendation for ventilatory care: invasive and non-invasive. -Recommendations for providing care in cases of cardiorespiratory arrest. -Nursing conduct regarding positioning of patient and internal transport of patients. -Conduct for transference of patient to reference hospital. -Nursing conduct after death of patient. -Other nursing care not exclusive to cases of COVID-19. |
| Dimension | Suggestion | Item after Change |
|---|---|---|
| Management | Item 1: Exclude the word “crossing”. Item 2: Include examples of common areas and exclude the word “performance” at the end of the sentence. | Item 1- Promoted distancing between patients and minimized circulation of people and patients with respiratory symptoms. Item 2- Prioritized radiological exam so that patient does not wait in common areas (reception, waiting room, corridor). |
| Care | Item 10: Include “SARS criteria in patients with influenza syndrome”. Item 12: Include field for annotating collected answers. Item 20: Write HEPA and HMEF out in full. Item 30: Explain reason for avoiding the procedure. Item 34: Specify side: right or left. Item 41: Specify whether transfers to tertiary sector require regulation via CROSS (service regulation center). | Item 10- Assessed criteria for severe acute respiratory syndrome (SARS) in patients with influenza syndrome:( ) Dyspnoea OR ( ) Respiratory distress OR ( ) Persistent pressure in chest OR ( ) SpO2< 95% in ambient air OR ( ) Cyanosis in lips or face Item 12- Investigated patient’s vaccinal situation for COVID-19: Laboratory/Manufacturer of vaccine; Number of doses received Item 20- HEPA (High Efficiency Particulate Arrestance) or HMEF (Heat and Moisture Exchanger) filter coupled to orotracheal tube and corrugated extension Item 30-Not used manual resuscitator due to high risk of generation of aerosols and contamination of the team. Item 34- For defibrillation of patient in PRONE position, sternal paddle was placed in dorsal region and apical paddle was placed on patient’s left side. Item 41- Made telephone contact with dispatcher of mobile urgent care service to request transport of patient to hospital reference units after confirmation of availability by CROSS (service regulation center); patient’s current clinical condition as well as the type of transport requested were informed. |
| Item/Dimension 1: Management | C | R | P | Cp |
|---|---|---|---|---|
| Item 1- Promoted distancing between patients and minimized circulation [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 2- Prioritized radiographic exam [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 3- Minimized patient’s contact with other patients during stay [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 4- Maintained small team, at most, three professionals during intubation [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item/Dimension2: Biosafety | ||||
| Item 5- Put on garments prior to onset of patient care [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 6- Removed garments after care with measures taken to avoid contamination [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 7- Discarded all waste from care provided in recipients [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item/Dimension3: Care | ||||
| Item 8- Assessed vital signs:() RR= ; () SpO2 AA. Annotate: ; () Temperature= ; () HR=[...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 9- Assessed breathing pattern: () Eupnea; () Dyspnoea [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 10- Assessed criteria of severe acute respiratory syndrome (SARS) [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 11- Collected sample for investigation of COVID-19 and sent it to [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 12- Investigated patient’s vaccinal situation for COVID-19: [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 13- Performed Notification of SARS and communicated with municipal[...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 14- Initiated oxygen supplementation, if patient had SpO2 ≤ 94% AA [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 15- Not placed water or saline solution in humidifier when O2 supplemented [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 16- Used surgical mask on patients using nasal tube. [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 17- Observed level of consciousness, drop inSpO2and/or signs of respiratory distress[...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 18- Verified whether patient presents criteria for indication for orotracheal intubation [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 19- Set up and tested functioning of mechanical ventilator [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 20- Coupled HEPA (High Efficiency Particulate Arrestance) filter to orotracheal tube [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 21- Sealed orotracheal tube with Reynalds forceps or plastic cap [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 22- Prepared suction device (aspirator) for closed system [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 23- Performed pre-oxygenation for 5 minutes using oxygen flow of [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 24- Assisted in intubation performed by physician and then confirmed [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 25- In the case of two failed orotracheal intubation attempts, assisted in [...] | 1.00 | 0.88 | 0.88 | 1.00 |
| Item 26- Performed clamping of orotracheal tube with Reynalds forceps [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 27- Performed aspiration of orotracheal tube and upper airways [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 28- Performed arterial gas exchange reading after 30 minutes of orotracheal intubation [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 29- Maintained pre-oxygenation non-re-inhalation face mask with O2 flow of [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 30- Not used manual resuscitator due to high risk of generation [...] | 0.88 | 1.00 | 1.00 | 1.00 |
| Item 31- In patients on mechanical ventilation, connection to closed circuit ventilator [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 32- Patient in prone position without advanced airway was quickly[...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 33- Patient in prone position with invasive ventilation, chest compressions were [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 34- For defibrillation of patient in prone position, sternal paddle was placed in [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 35- Placed patient in bed and maintained upper portion of bed elevated between 30° [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 36- Positioned patients with RR > 24 bpm and hypoxemia with no signs [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 37- Positioned and/or assisted in positioning patient in prone position, [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 38- Maintained self-pronated patients with spontaneous ventilation [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 39- Informed team of suspicion or confirmation of COVID-19 [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 40- Transported patient with surgical mask in wheelchair [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 41- Made telephone contact with dispatcher of mobile urgent care service [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 42- Prepared body at site of occurrence of death [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 43- Wrapped body in three layers: roll body in sheets; place [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Item 44- Labeled outer transport bag with information related to biological risk [...] | 1.00 | 1.00 | 1.00 | 1.00 |
| Nurse: | Patient: | ||||
|---|---|---|---|---|---|
| Date: | Bed/Sector: | Y | N | ||
| Dimension 1: Management | |||||
| 1.Promoted distancing between patients and minimized circulation of people and patients with respiratory symptoms. | |||||
| 2.Prioritized radiographic exam so that patient does not wait in common areas (reception, waiting room, corridor). | |||||
| 3.Minimized patient’s contact with other patients during their stay in emergency room or ward and observation room, closed curtains and increased distancing between beds when possible. | |||||
| 4.Maintained small team, at most, three professionals during orotracheal intubation. | |||||
| Dimension2: Biosafety | |||||
| 5.Put on garments prior to onset of patient care in anteroom of the emergency room or isolation room. Necessary equipment and way of performing procedure were in accordance with risk of exposure: | |||||
| Droplet and contact precaution, in the following sequence: 1- hand sanitization; 2- Long-sleeve apron or gown, knit or elastic wrist cuffs (minimum grammage: 30g/m2); 3- surgical mask; 4- protective eyewear; 5- cap; 6- gloves. | |||||
| Aerosol precaution, in following sequence: 1- hand sanitization; 2- Long-sleeve waterproof apron, knit or elastic wrist cuffs and open back (waterproof structure and minimum grammage of 50 g/m2); 3- N95 or PFF2 mask; 4- protective eyewear or face shield; 5- cap; 6- gloves. | |||||
| 6.Removed garments after care with measures taken to avoid contamination, using following removal sequence: In patient room or emergency room: 1- remove gloves; 2- remove apron; 3- sanitize hands; leave room or emergency room where patient is located: 4- sanitize hands; 5- remove cap; 6- remove protective eyewear or face shield; 7- sanitize hands; 8- remove N95 or PFF2 mask; 9- sanitize hands. | |||||
| 7.Discarded all waste from care provided in recipients for infectant waste, which will subsequently be labelled COVID-19. | |||||
| Dimension3: Care | |||||
| Initial patient care | 8.Assessed vital signs: ( ) RR = ___ ( ) SpO2 AA Annotate: ___( ) Temperature = ___ ( ) HR = ___ _________ | ||||
| 9.Assessed breathing pattern: ( )Eupnea ( ) Dyspnoea ( ) Tachypnoea ( ) Bradypnea ( )Signs of respiratory effort.( )Use of accessory muscles.( )Signs of cyanosis. | |||||
| Criteria for definition, reporting and conduct in SARS | 10.Assessed criteria of severe acute respiratory syndrome (SARS) in patients with influenza syndrome: ( ) Dyspnoea OR ( ) Respiratory distress OR ( ) Persistent pressure in chest OR ( ) SpO2< 95% in ambient air OR ( ) Cyanosis in lips or face | ||||
| 11.Collected sample for investigation of COVID-19 and sent it to reference laboratory (Adolfo Lutz Institute), in accordance with local flow: ( )Nasal oropharyngeal swab (rapid antigen test) – SARS: 1st to 7th day of symptom; ( ) Nasal oropharyngeal swab (RT-PCR) – SARS: 1st to 14th day of symptom; ( )Blood sample (rapid IgM/IgG test) – SARS: beginning with 8th day of symptom (NOTE: not recommended for diagnosis of vaccinated patients due to expected vaccinal response) | |||||
| 12.Investigated patient’s vaccinal situation for COVID-19: Laboratory/manufacturer of vaccine:_____; Number of doses received:_____. | |||||
| 13.Performed Notification of SARS and communicated with municipal epidemiological surveillance, in accordance with local flow. | |||||
| Respiratory care for patients with suspicion or confirmation of COVID-19 | |||||
Recommendations for ventilatory care: non-invasive | 14.Initiated oxygen supplementation, if patient had SpO2 ≤ 94% AA ORRR ≥ 24 bpm. -Offered oxygen through nasal tube 1 to 6 L/min. Annotate (L/min): __________ | ||||
| - Offered oxygen through mask with non-re-inhalation reservoir 6 to 15 L/min. Annotate (L/min): __________ | |||||
| 15.Not placed water or saline solution in humidifier when O2supplemented. | |||||
| 16.Used surgical mask on patients using nasal tube. | |||||
| 17.Observed level of consciousness, drop inSpO2and/or signs of respiratory distress in patient with high oxygen flow (>10 L/min) | |||||
| Recommendations for ventilatory care: invasive | 18.Verified whether patient presents criteria for indication for orotracheal intubation and communicated to medical team, in accordance with following criteria: -Respiratory failure and lowered level of consciousness and/or increase in ventilatory work/high ventilatory effort; need for O2supplementation > 5L/m into maintain SpO2>94% orRR ≤ 24 bpm; retention of CO2 (PaCO2 > 50mmHg and/or pH< 7.25). | ||||
| 19.Set up and tested functioning of mechanical ventilator, adjusting it with initial parameters of mechanical ventilation after orotracheal intubation in accordance with medical orientation, respecting the following recommendations: ❖ Modality: controlled volume; tidal volume: 6 ml/kg of predicted weight; FiO2 100%; initial PEEP of 10 cmH20; RR: 24/min (20 to 28/min); inspiratory flow: 60 L/min (40-80 L/min) or I:E ratio of 1:2 to 1:4. | |||||
| 20.Coupled HEPA (High Efficiency Particulate Arrestance) or HMEF (Heat and Moisture Exchanger)filter to orotracheal tube and corrugated extension. | |||||
| 21.Sealed orotracheal tube with Reynalds forceps or plastic cap (or rubber stopper) when used guide wire or Bougie for intubation. | |||||
| 22.Prepared suction device (aspirator) for closed system. | |||||
| 23.Performed pre-oxygenation for 5 minutes using oxygen flow of 15 L/min with care to avoid leakage or use non-re-inhalation mask. | |||||
| 24.Assisted in intubation performed by physician and then confirmed position of tube by capnography (preferably). | |||||
| 25.In the case of two failed orotracheal intubation attempts, assisted in passage of extra-glottal device (laryngeal mask) coupled to a HEPA or HME filter. | |||||
| 26.Performed clamping of orotracheal tube with Reynalds forceps when its disconnection or the exchange of the circuit of the mechanical ventilator is necessary. | |||||
| 27.Performed aspiration of orotracheal tube and upper airways using closed suction system, whenever necessary. | |||||
| 28.Performed arterial gas exchange reading after 30 minutes of orotracheal intubation and when requested by the medical team. | |||||
| Recommendations for cardiorespiratory arrest | |||||
| Care for cardiorespiratory arrest | 29.Maintained pre-oxygenation non-re-inhalation face mask with O2flow of 6-15 L/min until intubation to avoid generation of aerosols. | ||||
| 30.Not used manual resuscitator due to high risk of generation of aerosols and contamination of team. | |||||
| 31.In patients on mechanical ventilation, connection to closed circuit ventilator was maintained and set under medical orientation to “RCP/cardiorespiratory arrest” function of equipment; equipment without this function was adjusted to non-asynchronous ventilation with the following recommendations: ventilatory mode: asynchronous; 100%FiO2; RR: 10 to 12; PEEP = 0. | |||||
| 32.Patient in prone position without advanced airway was quickly positioned in supine position and cardiopulmonary resuscitation manoeuvres initiated. | |||||
| 33.Patient in prone position with invasive ventilation, chest compressions were performed with hands in normal position over vertebrae T7 to 10. | |||||
| 34.For defibrillation of patient in prone position, sternal paddle was placed in dorsal region and apical paddles was placed on patient’s left side. | |||||
| Nursing conduct in positioning and internal transport of patients | |||||
| Positioning of patient | 35.Placed patient in bed and maintained upper portion of bed elevated between 30° and 45° (semi-reclined or Fowler position) as tolerated by patient. | ||||
| 36.Positioned patients with RR >24 bpm and hypoxemia with no signs of respiratory failure in prone position or conscious pronation. | |||||
| 37.Positioned and/or assisted in positioning patient in prone position and repositioned devices, wires and catheters. | |||||
| 38.Maintained self-pronated patients with spontaneous ventilation under constant surveillance. | |||||
| Internal transport of patient | 39.Informed team of suspicion or confirmation of COVID-19. | ||||
| 40.Transported patient with surgical mask in wheelchair or stretcher using devices to maintain oxygen support during transport. | |||||
| Nursing conduct for probable outcomes | |||||
| Transfer to reference hospital | 41.Made telephone contact with dispatcher of mobile urgent care service to request transport of patient to reference hospital units after confirmation of availability by CROSS (service regulation center). | ||||
| After death | 42.Prepared body at site of occurrence of death. | ||||
| 43.Wrapped body in three layers: roll body in sheets; place body in waterproof bag; place body in second (outer) bag and disinfect with 70% alcohol or other sanitizing agent compatible with material of bag. | |||||
| 44.Labeled outer transport bag with information related to biological risk: COVID-19- biological agent – risk class 3; and then send to morgue. | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orestes, L.P.; Meneguin, S.; de Leo, A.; Patini, M.S.G.; Santos, B.P.; de Oliveira, C. COVID-19: Protocol and Checklist for Nursing Care Management at Urgent Care Units. Int. J. Environ. Res. Public Health 2023, 20, 2169. https://doi.org/10.3390/ijerph20032169
Orestes LP, Meneguin S, de Leo A, Patini MSG, Santos BP, de Oliveira C. COVID-19: Protocol and Checklist for Nursing Care Management at Urgent Care Units. International Journal of Environmental Research and Public Health. 2023; 20(3):2169. https://doi.org/10.3390/ijerph20032169
Chicago/Turabian StyleOrestes, Leticia Pereira, Silmara Meneguin, Aniele de Leo, Mayara Salles Gasparini Patini, Bruna Pegorer Santos, and Cesar de Oliveira. 2023. "COVID-19: Protocol and Checklist for Nursing Care Management at Urgent Care Units" International Journal of Environmental Research and Public Health 20, no. 3: 2169. https://doi.org/10.3390/ijerph20032169
APA StyleOrestes, L. P., Meneguin, S., de Leo, A., Patini, M. S. G., Santos, B. P., & de Oliveira, C. (2023). COVID-19: Protocol and Checklist for Nursing Care Management at Urgent Care Units. International Journal of Environmental Research and Public Health, 20(3), 2169. https://doi.org/10.3390/ijerph20032169






