Abstract
The spread of drug-resistant tuberculosis (DR TB) poses significant challenges to the control and successful eradication of TB globally. The current retrospective study was designed to evaluate the treatment outcomes and identify the risk factors associated with unsuccessful outcomes among DR TB patients. A total of 277/308 eligible DR TB patients were enrolled for treatment at the programmatic management unit of DR TB at the Pakistan Institute of Medical Sciences, Islamabad between January 2014 and July 2019. Treatment outcomes were defined according to the WHO recommendations. Death, treatment failure, and lost to follow-up (LTFU) were collectively grouped as unsuccessful treatment outcomes, whereas cured and treatment completed were summed up together as successful treatment outcomes. Out of the total 277 patients, 265 (95.67%) were multidrug/rifampicin-resistant TB (MDR/RR-TB) cases, 8 (2.89%) were isoniazid resistant cases, and 4 (1.44%) were extensively drug-resistant ones. In the current cohort, a total of 177 (63.9%) achieved successful treatment outcomes. Among them, 153 (55.2%) were declared cured and 24 (8.7%) completed their treatment. Of the remaining 100 (36.1%) patients with unsuccessful outcomes, 60 (21.7%) died, 32 (11.5%) were LTFU, and 8 (2.9%) had failed treatment. The proportion of male patients was relatively higher (55.2%), within the age group of 21–40 years (47.3%) and lived in rural areas (66.8%). The multivariate analysis revealed that unsuccessful outcomes had a statistically significant association with being male (adjusted odds ratio, AOR: 1.92, 95% confidence interval (CI): 1.10–3.36), being in an age group above 60 years (AOR: 3.34, 95% CI: 1.09–10.1), suffering from any comorbidity (AOR: 2.69, 95% CI: 1.35–5.38), and the history of use of second-line drugs (AOR; 3.51, 95% CI 1.35–9.12). In conclusion, treatment outcomes among DR TB patients at the study site were poor and did not achieve the treatment success target (≥75%) set by the World Health Organization.
1. Introduction
Irrespective of global efforts, tuberculosis (TB) continues to be a leading public health concern [1]. The spread of drug-resistant tuberculosis (DR TB) remains a threat to the TB control. Approximately half a million cases occurred worldwide in 2019 [2]. Treatment regimens used against DR TB are costly, prolonged, less effective, and are associated with more side effects as compared with drug-susceptible TB [3]. As a result, the global treatment success rate for DR TB remains less than 60%, and a large number of patients die each year [4,5]. The World Health Organization (WHO) states that approximately 9% of DR TB patients have a more likely chance of unsuccessful outcomes and their disease further develop into extensively drug-resistant TB (XDR TB) [6]. Currently, less developed countries are confronted with many DR TB cases that are alarmingly increasing every year [7]. To optimize the DR TB care and prevention requires a thorough understanding of the main factors that lead to poor treatment outcomes.
According to the WHO, Pakistan ranks fifth in the Eastern Mediterranean Region for DR TB [8,9]. The country has come a long way in enhancing DR TB management through several initiatives, such as the establishment of direct observation short course therapy (DOTs) and programmatic management of DR TB. Despite these efforts in the past decades, the country continues to face significant challenges in controlling and eradicating DR TB [1,10]. According to a study, the incidence of new drug-resistant TB cases was 4%, whereas 19.4% were previously TB treated patients [11]. Similarly, A. Javaid et al., in 2018, reported that mortality due to DR cases is increasing every year in Pakistan [5]. To improve the successful outcomes of DR TB, there must be a consistency with the DOTs rules related to the management [12]. To maintain a consistency in management, it is essential to identify socioeconomic factors at the population level that create hurdles in care and prevention [1]. Therefore, the WHO has directed that the treatment outcomes for DR TB patients must be regularly reviewed at national and district levels [13]. Regular monitoring of treatment outcomes will not only support to assess the performance of national TB program, but it will also help to identify treatment sites that require improvement in the future. Nevertheless, there have been no reports of DR TB patients’ treatment outcomes and socioeconomic factors at the population level from the study site. Therefore, the current study was designed to investigate treatment outcomes and risk factors associated with unsuccessful outcomes among drug-resistant TB patients in Pakistan.
2. Materials and Methods
2.1. Study Design
The current retrospective observational study was carried out at the Programmatic Management of Drug-resistant Tuberculosis (PMDT) unit at the Pakistan Institute of Medical Sciences, Islamabad, Pakistan (PIMS). The study center is well-equipped and has a staff that includes doctors, nurses, data operators, coordinators, pharmacists, psychologists, and other supporting staff. All culture-confirmed drug-resistant TB patients enrolled for treatment at the study site between January 2014 and July 2019 were included in the final analysis. It covers patients from different parts of the country, primarily registered from the capital (Islamabad), and two provinces of Pakistan, namely Punjab and Khyber Pakhtunkhwa, and a self-governing state, Azad Jammu and Kashmir.
2.2. Study Data Collection and Eligibility Criteria
A standardized data collection form based on WHO guidelines for the management of DR TB, previously published studies, and recommendations of the supervisory committee and healthcare professionals at the study site was used to abstract patients’ sociodemographic, microbiological, and clinical data from Electronic Nominal Record System records (ENRS) and patients’ medical record files. Enrolled patients were retrospectively followed until their end treatment outcomes were reported. Patients were carefully examined by specialist clinicians, data coordinators, and pharmacists to manage care.
Patients who were enrolled for the treatment before January 2014, transferred outpatients and those who were still under treatment on the final day of data collection, as well as resistance other than the most crucial drugs isoniazid and rifampicin were excluded from the study (Figure 1).
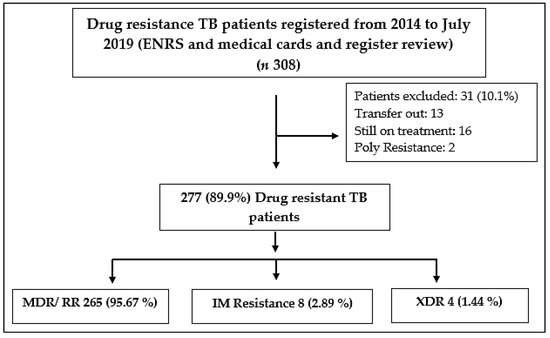
Figure 1.
Overall drug resistance patient enrollment in the study, MDR (Multidrug-resistant), RR (Rifampicin resistant, XDR (Extensively drug-resistant), I M (Isoniazid Mono resistance), ENRS (Electronic Nominal Record System).
2.3. Variables Outcomes and Definitions
Treatment outcomes of the study participants were categorized according to the WHO definition, death, treatment failure and lost to follow-up were collectively grouped as “unsuccessful treatment outcomes” while cure and treatment completion were grouped as “successful treatment outcomes”. Based on starting of TB treatment after the onset of the symptoms, patients were classified into “Delayed” and “Not-Delayed,” taking 30 days (4 weeks) as cut-off points. All definitions are presented in Table 1 [14,15].

Table 1.
Category of treatment outcome, type of TB resistance, and previous history of TB patients registered modified from WHO definitions, comorbidities.
2.4. Identification and Antimicrobial Susceptibility Testing
All DR TB samples were collected under the supervision of qualified professionals in the TB control center. A sample was subjected to equal division into two parts, i.e., one portion of samples for the Xpert MTB/RIF assay (Cephid, Sunnyvale, CA, USA) and smear microscopy, and similarly, the second portion of samples was assigned for DST (drug-susceptible test) and Lowenstein–Jensen culture medium. Sputum samples from patients with positive Xpert MTB/RIF and Ziehl–Neelsen stain results were sent to the National Institute of Health Islamabad (NIH) for culture and DST analysis at the NIH laboratory. Drug-susceptible tests against RIF, ethambutol (EMB), isoniazid (INH), ofloxacin (OFX), capreomycin (CM), streptomycin (SM) kanamycin (KM), amikacin (AM), and ethionamide were performed utilising the agar proportion methods in medium on Middle Brook 7H10 as reported previously [16]. The concentrations include rifampicin (1 µg/mL), EMB (5 µg/mL), INH (0.2 µg/mL), OFX (2 µg/mL), SM (2 µg/mL), KM (5 µg/mL), ethionamide (5 µg/mL), CM (4 µg/mL), and AMK (4 µg/mL). Similarly, DST was carried out for pyrazinamide (PZA) by means of BACTEC 7H12 radiometric medium (Becton Dickinson, New Jersey, USA) following the manufacturer’s instructions. Furthermore, DST was made available for all the DR TB patients at registration time and repeated when considered essential. A sputum smear and culture were carried out based on a scheduled visit.
2.5. Treatment Protocol
Patients found to be resistant in Xpert MTB/RIF diagnostic test were registered as DR TB cases and treated with treatment regimen protocol in compliance with WHO and Pakistan national MDR TB control guidelines [9,15]. Before initiation of the regimen, baseline laboratory diagnostic tests were performed to determine complete blood count, hepatitis, HIV, blood sugar level, kidney, and liver function tests. All patients’ adherence was ensured by the pharmacist, doctors, treatment coordinators, and trained supporters. Patients were prior informed for every scheduled follow-up visit, and free laboratory tests and medications were provided at each follow-up visit to patients.
2.6. Statistical Analysis
Statistical Package for the Social Sciences, version 23 (SPSS® IBM Corp., Chicago, IL, USA) was used for performing statistical analysis. Factors related to the treatment outcomes of drug-resistant tuberculosis were assessed using descriptive statistics (frequency, percentages) and logistic regression models. Multivariate binary logistic regression analysis was conducted to determine the final factors associated with unsuccessful outcomes statistically significant (p ≤ 0.05). Variables found significant with p-value (<0.15) in the univariate regression analysis were the criteria for addition in the final multivariate regression model [17,18]. In developing the multivariate binary logistic regression, we have checked the collinearity and tolerance value for all variables. If the variables had a high association with one another (Variance inflation factor = 10 and Tolerance value > 0.1), then one of them was removed from the concluding model [19]. Hosmere Lemeshow test was also applied for the adjustment of the final multivariate binary logistic regression model. Two different categories with binary variables were made for the treatment outcome, i.e., successful and unsuccessful. Odds ratios with 95% Confidence intervals with (p ≤ 0.05) were calculated to measure the level of association between variables and outcomes.
3. Results
During the study period, 308 DR TB patients were treated at the study site and 277 of them met the inclusion criteria and were analysed. The group of patients who were excluded from the study, included two poly-drug-resistant cases and 13 patients had left the study center. Similarly, the final treatment outcomes for 16 individuals were unknown because they were still on treatment. Out of the total 277, MDR/RR-TB cases were (265, 95.67%), isoniazid mono resistance cases were (8, 2.89), and XDR TB (4, 1.44%) (Figure 1). In most of the cases (47.3%), patients were between 18 and 40 years old. Patients from rural areas made up 66.8%, with males accounting for 55.2 percent of the total. About 52.7% of patients showed 30 days of delay before reporting to the MDR TB center and 9.4% of patients have already used second-line drugs and found resistance before being diagnosed with drug-resistant tuberculosis. Among the other patients, 17.7% had any comorbidity such as diabetes (26 patients), hypertension (11 patients), hepatitis (6 patients), and HIV (5 patients) (Table 2).

Table 2.
Sociodemographic and clinical characteristics of MDR TB patients (n = 277).
3.1. Drug Resistance
In this study, 84.5% of participants were previously treated for TB infection. Resistance to first-line drugs (FLD) was seen in almost all patients with at least one or more first-line drugs. Resistance to two major FLD was noted in 60 patients (21.6%), followed by resistance to four FLD drugs 18.4%, while 7.9% of patients were resistant to all five FLDs. Out of the total second-line resistant cases, i.e.,27.1%, 22.02% were resistant to at least any SLD fluoroquinolones (ofloxacin, levofloxacin, and moxifloxacin), followed by capreomycin, kanamycin, and amikacin (Table 3).

Table 3.
Patterns of drug resistance among drug-resistant tuberculosis patients (n = 277).
3.2. Predictors of Unsuccessful Treatment Outcomes
Treatment outcomes of the study participants (36.1%) were categorized according to the outcomes of death (21.7%), treatment failure (11.5%), and lost to follow-up (2.9%) and were collectively grouped as unsuccessful treatment outcomes. Cure (153, 55.2%), and treatment completed (24, 8.7%) were grouped as successful treatment outcomes (63.9%) (Table 4).

Table 4.
Trends of TB treatment outcome audit of 6 years among TB patients (n = 277).
The logistic regression univariate analysis has shown that the following variables were significantly associated with unsuccessful treatment outcomes: gender, age above 60 years, and delay in reporting to drug-resistant tuberculosis center, comorbidities, and history of second-line drug resistance before being diagnosed with drug-resistant tuberculosis. In the multivariate analysis, only four major significant predictors of unsuccessful treatment were identified, such as male gender (AOR; 1.92, 95% CI 1.10–3.36), age group above 60 years (AOR; 3.34, 95% CI 1.09–10.1), comorbidities (AOR; 3.51, 95% CI 1.35–9.12), and history of second-line drugs resistance before being diagnosed with drug-resistant tuberculosis (AOR; 3.51, 95% CI 1.35–9.12) Table 5.

Table 5.
Univariate and multivariable logistic regression for successful and unsuccessful predictor related treatment outcomes among patients (n = 277).
4. Discussion
This research showed the prevalence of predictors that impact DR TB treatment outcomes in Pakistan, a highly TB-endemic low-middle-income country. The treatment outcomes were briefly analyzed in accordance with the definitions put forward by the WHO [14,15]. In the current cohort, a total of 177 (63.9%) cases achieved successful treatment outcomes. Among these, 153 (55.2%) were declared cured, while 24 (8.7%) had completed their treatment. Of the remaining 100 (36.1%) patients with unsuccessful outcomes, 60 (21.7%) died, 32 (11.5%) were LTFU, and 8 (2.9%) were declared treatment failure. Thus, the site did not achieve the WHO recommended target of ≥75% for treatment success [18]. The treatment success rate (63.9%) observed in the current cohort was in line with the success rates reported by a meta-analysis (63.8%) [20], a study conducted in Sudan (63.5%) [21], and in China (63.4%) [22]. However, it was comparatively better than the treatment success rates reported in studies conducted in Morocco (53.5%) [23], Armenia (56.5%) [24], Ukraine (18.1%) [25], and India (38%) [26]. Differences in the study population in terms of age, gender, presence of comorbidities, disease severity, tobacco use, drug resistance pattern, social determinants of health, and socioeconomic characteristics could be some of the possible reasons for the discrepancy in treatment outcomes across these studies [13,27,28,29]. Another factor that may have contributed to the poor outcomes in the current study is the overburden of patients who were registered from different parts of the country in the TB care unit, which restricts the TB treatment coordinators access to the patients. The statement of the current study is supported by earlier studies that found overburdened healthcare staff in Pakistan TB centers [1,13]. Based on the results of this research, we recommend that early diagnosis, appropriate therapy, regular supportive care, and health advocates programmes should be implemented for patients who are at risk of poor outcomes. This might be possible by giving awareness about district and provincial drug-resistant tuberculosis centers to all communities and health care centers to register patients at the nearest DR TB control center.
In the present study, the prevalence of death rate was 21.7%; this figure was similar to reports from previous studies conducted in Colombia, India, and South Africa [30,31,32]. However, this was lower than the death rate reported in studies from Western India and Ukraine [25,26]. The higher death rate in the current study may be due to the delayed diagnosis and low education regarding DR TB, disease severity, comorbidities, and previously TB treated cases [24,25]. In the current study, the failure rate of 2.8% was lower than the failure rates reported in previous studies conducted in Ethiopia 12.8% [33] and Armenia 14.3% [24]. This might be due to a regular supply of drugs and counselling by the pharmacist and psychiatrist, regular checkups by the medical officer, and scheduled monthly visits by data coordinators. Lost to follow-up from TB treatment health centers is one of the main challenges for TB control programs. In the current study, overall, 11.5% of patients were LTFU. Previous studies conducted in Morocco 34.6% [23], Ukraine 31.9% [25], South Africa 20.9% [32], and Ethiopia 9.7% [33] showed more than 11.5% of patients were LTFU. The difference in the percentage of LTFU rates among studies may be due to regular home visits by the treatment coordinator, the presence of qualified doctors for follow-up checkups, proper social support by a psychiatrist, and the provision of free medicine with proper counselling by the pharmacist. Despite free therapy, psychologist and pharmacist counseling, and home coordinator visits, our study’s LTFU rate is also a point of concern for the management of DR TB. Perhaps it indicates a need for better access to more effective, less toxic, and easier to implement drug regimens, along with proper engagement of patients in the treatment plan. The current study LTFU rate may be associated with comorbidities, resistance to SLD, previous history of pulmonary TB treatment, gender, and deaths that were not reported to the TB center and access to the PMDT site [34,35].
The multivariate analysis showed numerous other factors that played an essential role in poor treatment outcomes including gender (male), age (above 60), history of past used SLD, and comorbidities. In this study, male participants have a more likely chance of poor treatment outcomes than females. The findings are consistent with previous studies [36,37], while other studies explained an opposite statement [38,39]. This difference between the reports might be due to financial requirements, illiteracy rate, exposure to environmental air-pollution, tobacco use, and fear of stigmatization which make it more difficult for patients in less developed countries to achieve therapeutic goals [40,41,42]. Therefore, community surveys and randomized controlled trials need to be conducted on gender discrepancies and socioeconomics parameters at the national level. The other significant predictor that we assessed was the age group of more than 50 years, which had 3.34 times the risk to develop a poor treatment outcome. Older age has previously been studied as a significant factor associated with poor treatment outcome [43,44]. The reason behind this may be the physical weakness, daily complex medications routine, follow-up visits, malnourishment, comorbidities, and weak immunity. All these factors together increase the possibility of older age patients being more towards poor outcomes [20].
In the present study, comorbidities were also found associated with an increased relative risk of poor treatment outcome in DR TB patients. This study result is in concordance with a meta-analysis [20] and study conducted in Brazil and Yemen [45,46]. This current finding will allow and help policymakers to develop new care strategies with more focus on early detection and patient-centered care during comorbid conditions. Patient-centered care has been described as an essential predicator for positive outcomes in DR TB [47].
The odds ratio of poor treatment outcomes was high among those patients who had already used any SLD and found resistance before proper DR TB treatment. The results of this study are similar in comparison to other studies [45,48]. An approximate 21.02% of cases were subject to any form of fluoroquinolone resistance. The fluoroquinolone resistance rate is higher in this study, while the observed rate of fluoroquinolone resistance is similar to other studies conducted in Pakistan [5,49]. Such a high proportion of fluoroquinolone resistance could be related to the non-prescription sale of antibiotics, delay in diagnosis, easy access of patients to antibiotics, non-formal health care practices, and irrational prescriptions [50].
The current study was conducted according to standardized WHO procedures, but it has several limitations. First of all, this study was carried out in a single center where patients were registered from most parts of the country. Secondly, because of the retrospective nature of this study, numerous significant clinical characteristics that may have influenced an unsuccessful treatment outcome were not observed and evaluated. Therefore, future prospective interventional studies should be based on patient-related, drug-related, and health system-related factors, to find out compact national decisions for successful treatment outcomes.
5. Conclusions
Our study showed a treatment success rate of 63.9% among DR TB patients, and we conclude that the successful treatment outcome was lower than the success rate set by WHO (≥75%). Gender (male), age above 60, history of past SLD use, and comorbidities were found to be significantly associated with poor treatment outcomes. All of these variables show a need for the development of unique and innovative strategies to monitor and evaluate DR tuberculosis patients. These findings demonstrate how serious DR TB is in this region and we recommend a strong systematic approach to decrease the number of deaths and default rates through proper management and long-term interventions.
Author Contributions
Conceptualization, methodology, F.U.K. (Farman Ullah Khan) and Y.F.; software Statistical Package for the Social Sciences; formal analysis, A.K. and F.U.K. (Faiz Ullah Khan); data curation, A.K. and U.R.M.; writing original draft preparation, F.U.K. (Farman Ullah Khan); writing review and editing, A.u.R., N.A., J.C. and K.H.; funding acquisition resources and supervision, Y.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the “Young Talent Support Plan”; by the “High Achiever Plan” of the Health Science Center, Xi’an Jiaotong University; and by the Central University Basic Research Fund (2015qngz05).
Institutional Review Board Statement
The study protocol was approved by the Ethics Committee of Pakistan Institute of Medical Sciences hospital, Islamabad and Shaheed Zulfiqar Ali Medical University, Islamabad (F.1-1/2015/ERB/SZABMU/359). The study was also approved by Xian Jiaotong University, Health Science Center Biology Scientific and Research Ethics Committee (12.31.2019-1258).
Informed Consent Statement
Not applicable (this retrospective study is based on recorded data).
Data Availability Statement
The data sets used and analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors would like to acknowledge the programmatic management of drug-resistant tuberculosis staff of Pakistan Institute of Medical Sciences, Asad Naimeti.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Atif, M.; Anwar, Z.; Fatima, R.K.; Malik, I.; Asghar, S.; Scahill, S. Analysis of tuberculosis treatment outcomes among pulmonary tuberculosis patients in Bahawalpur, Pakistan. BMC Res. Notes 2018, 11, 370. [Google Scholar] [CrossRef]
- Chakaya, J.; Khan, M.; Ntoumi, F.; Aklillu, E.; Fatima, R.; Mwaba, P.; Kapata, N.; Mfinanga, S.; Hasnain, S.E.; Katoto, P.D.; et al. Global Tuberculosis Report 2020–Reflections on the Global TB burden, treatment and prevention efforts. Int. J. Infect. Dis. 2021, 113, S7–S12. [Google Scholar] [CrossRef]
- Sloan, D.J.; Lewis, J.M. Management of multidrug-resistant TB: Novel treatments and their expansion to low resource settings. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Baluku, J.B.; Nakazibwe, B.; Naloka, J.; Nabwana, M.; Mwanja, S.; Mulwana, R.; Sempiira, M.; Nassozi, S.; Babirye, F.; Namugenyi, C.; et al. Treatment outcomes of drug resistant tuberculosis patients with multiple poor prognostic indicators in Uganda: A countrywide 5-year retrospective study. J. Clin. Tuberc. Other Mycobact. Dis. 2021, 23, 100221. [Google Scholar] [CrossRef] [PubMed]
- Javaid, A.; Ullah, I.; Masud, H.; Basit, A.; Ahmad, W.; Butt, Z.; Qasim, M. Predictors of poor treatment outcomes in multidrug-resistant tuberculosis patients: A retrospective cohort study. Clin. Microbiol. Infect. 2018, 24, 612–617. [Google Scholar] [CrossRef]
- Khalid, W.; Hamid, H.; Naveed, R.; Khan Baig, T.H.; Masood, R.A.; Tariq, H.; Pervaiz, A.; Mehmood, A. Potential Predictors of Unsuccessful Treatment Outcomes of Multiple Drug Resistance Tuberculosis in Program Management Drug Resistance Tuberculosis Setting. Lat. Am. J. Pharm. 2020, 39, 1977–1982. [Google Scholar]
- Khoshnood, S.; Goudarzi, M.; Taki, E.; Darbandi, A.; Kouhsari, E.; Heidary, M.; Motahar, M.; Moradi, M.; Bazyar, H. Bedaquiline: Current status and future perspectives. J. Glob. Antimicrob. Resist. 2021, 25, 48–59. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO EMRO Tuberculosis 2018. Available online: http://www.emro.who.int/pak/programmes/stop-tuberculosis.html (accessed on 2 May 2021).
- National TB Control Program. National Guidelines for Programmatic Management of Drug-Resistant Tuberculosis (PMDT); NTP: Islamabad, Pakistan, 2012. Available online: http://ntp.gov.pk/uploads/ntp1368669324 (accessed on 4 November 2020).
- Ullah, I.; Javaid, A.; Tahir, Z.; Ullah, O.; Shah, A.A.; Hasan, F.; Ayub, N. Pattern of Drug Resistance and Risk Factors Associated with Development of Drug Resistant Mycobacterium tuberculosis in Pakistan. PLoS ONE 2016, 11, e0147529. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, A.M.; Arif, M.A.; Kanwal, S.; Majeed, S. Prevalence and drug resistance pattern of MDR TB in retreatment cases of Punjab, Pakistan. J. Pak. Med. Assoc. 2016, 66, 989–993. [Google Scholar]
- Zong, K.; Luo, C.; Zhou, H.; Jiang, Y.; Li, S. Xpert MTB/RIF assay for the diagnosis of rifampicin resistance in different regions: A meta-analysis. BMC Microbiol. 2019, 19, 177. [Google Scholar] [CrossRef]
- Atif, M.; Ahmad, W.; Ahmad, N.; Malik, I.; Sarwar, S. Treatment outcomes among multidrug-resistant TB patients in Bahawal Victoria Hospital, Bahawalpur, Pakistan: A retrospective record review. Trans. R. Soc. Trop. Med. Hyg. 2020, 114, 733–741. [Google Scholar] [CrossRef]
- World Health Organization. Meeting report of the WHO expert consultation on the definition of extensively drug-resistant tuberculosis. In Proceedings of the WHO Expert Consultation on the Definition of Extensively Drug-Resistant Tuberculosis, Geneva, Switzerland, 27–29 October 2020. [Google Scholar]
- World Health Organization. WHO Consolidated Guidelines on Drug-Resistant Tuberculosis Treatment 2019. Available online: https://apps.who.int/iris/bitstream/handle/10665 (accessed on 2 March 2021).
- Mboowa, G.; Namaganda, C.; Ssengooba, W. Rifampicin resistance mutations in the 81 bp RRDR of rpoB gene in Mycobacterium tuberculosis clinical isolates using Xpert®MTB/RIF in Kampala, Uganda: A retrospective study. BMC Infect. Dis. 2014, 14, 481. [Google Scholar] [CrossRef]
- Steyerberg, E.W. Applications of prediction models. In Clinical Prediction Models; Springer: New York, NY, USA, 2009; pp. 11–31. [Google Scholar]
- Khan, F.U.; Khan, F.U.; Hayat, K.; Chang, J.; Kamran, M.; Khan, A.; Malik, U.R.; Khan, A.; Fang, Y. Impact of Protracted Displacement on Delay in the Diagnosis Associated with Treatment Outcomes: A Cross-Sectional Study in Internally Displaced Tuberculosis Patients of Pakistan. Int. J. Environ. Res. Public Health 2021, 18, 11984. [Google Scholar] [CrossRef]
- Pallant, J. SPSS Survival Manual: A Step by Step Guide to Data Analysis Using IBM SPSS; Routledge: London, UK, 2020. [Google Scholar]
- Alemu, A.; Bitew, Z.W.; Worku, T. Poor treatment outcome and its predictors among drug-resistant tuberculosis patients in Ethiopia: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 98, 420–439. [Google Scholar] [CrossRef]
- Ali, M.H.; Alrasheedy, A.A.; Kibuule, D.; Godman, B.; Hassali, M.A.; Ali, H.M.H. Assessment of multidrug-resistant tuberculosis (MDR-TB) treatment outcomes in Sudan; Findings and implications. Expert Rev. Anti-Infect. Ther. 2019, 17, 927–937. [Google Scholar] [CrossRef]
- Bartholomay, P.; Pinheiro, R.S.; Dockhorn, F.; Pelissari, D.M.; de Araújo, W.N. Brazilian cohort study of risk factors associated with unsuccessful outcomes of drug resistant tuberculosis. BMC Infect. Dis. 2021, 21, 1049. [Google Scholar] [CrossRef]
- El Hamdouni, M.; Bourkadi, J.E.; Benamor, J.; Hassar, M.; Cherrah, Y.; Ahid, S. Treatment outcomes of drug resistant tuberculosis patients in Morocco: Multi-centric prospective study. BMC Infect. Dis. 2019, 19, 316. [Google Scholar] [CrossRef]
- Bastard, M.; Sanchez-Padilla, E.; Hewison, C.; Hayrapetyan, A.; Khurkhumal, S.; Varaine, F.; Bonnet, M. Effects of Treatment Interruption Patterns on Treatment Success among Patients with Multidrug-Resistant Tuberculosis in Armenia and Abkhazia. J. Infect. Dis. 2015, 211, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Aibana, O.; Bachmaha, M.; Krasiuk, V.; Rybak, N.; Flanigan, T.P.; Petrenko, V.; Murray, M.B. Risk factors for poor multidrug-resistant tuberculosis treatment outcomes in Kyiv Oblast, Ukraine. BMC Infect. Dis. 2017, 17, 129. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.V.; Nimavat, K.B.; Alpesh, P.B.; Shukla, L.K.; Shringarpure, K.S.; Mehta, K.G.; Joshi, C.C. Treatment outcome among cases of multidrug-resistant tuberculosis (MDR TB) in Western India: A prospective study. J. Infect. Public Health 2015, 9, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Agyare, S.A.; Osei, F.A.; Odoom, S.F.; Mensah, N.K.; Amanor, E.; Martyn-Dickens, C.; Owusu-Ansah, M.; Mohammed, A.; Yeboah, E.O. Treatment Outcomes and Associated Factors in Tuberculosis Patients at Atwima Nwabiagya District, Ashanti Region, Ghana: A Ten-Year Retrospective Study. Tuberc. Res. Treat. 2021, 2021, 9952806. [Google Scholar] [CrossRef] [PubMed]
- Kebede, H.K.; Mwanri, L.; Ward, P.; Gesesew, H.A. Predictors of lost to follow up from antiretroviral therapy among adults in sub-Saharan Africa: A systematic review and meta-analysis. Infect. Dis. Poverty 2021, 10, 33. [Google Scholar] [CrossRef]
- Hargreaves, J.R.; Boccia, D.; Evans, C.A.; Adato, M.; Petticrew, M.; Porter, J.D.H. The Social Determinants of Tuberculosis: From Evidence to Action. Am. J. Public Health 2011, 101, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Chaves-Torres, N.M.; Fadul, S.; Patiño, J.; Netto, E. Factors associated with unfavorable treatment outcomes in patients with rifampicin-resistant tuberculosis in Colombia 2013–2015: A retrospective cohort study. PLoS ONE 2021, 16, e0249565. [Google Scholar] [CrossRef]
- Parmar, M.M.; Sachdeva, K.S.; Dewan, P.K.; Rade, K.; Nair, S.A.; Pant, R.; Khaparde, S.D. Unacceptable treatment outcomes and associated factors among India’s initial cohorts of multidrug-resistant tuberculosis (MDR-TB) patients under the revised national TB control programme (2007–2011): Evidence leading to policy enhancement. PLoS ONE 2018, 13, e0193903. [Google Scholar] [CrossRef] [PubMed]
- Farley, J.E.; Ram, M.; Pan, W.; Waldman, S.; Cassell, G.H.; Chaisson, R.E.; Weyer, K.; Lancaster, J.; van der Walt, M. Outcomes of Multi-Drug Resistant Tuberculosis (MDR-TB) among a Cohort of South African Patients with High HIV Prevalence. PLoS ONE 2011, 6, e20436. [Google Scholar] [CrossRef] [PubMed]
- Tola, H.; Holakouie-Naieni, K.; Mansournia, M.A.; Yaseri, M.; Gamtesa, D.F.; Tesfaye, E.; Mahamed, Z.; Sisay, M.M. National treatment outcome and predictors of death and treatment failure in multidrug-resistant tuberculosis in Ethiopia: A 10-year retrospective cohort study. BMJ Open 2021, 11, e040862. [Google Scholar] [CrossRef] [PubMed]
- Teferi, M.Y.; El-Khatib, Z.; Boltena, M.T.; Andualem, A.T.; Asamoah, B.O.; Biru, M.; Adane, H.T. Tuberculosis Treatment Outcome and Predictors in Africa: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Healh 2021, 18, 10678. [Google Scholar] [CrossRef]
- Xu, Z.; Xiao, T.; Li, Y.; Yang, K.; Tang, Y.; Bai, L. Reasons for Non-Enrollment in Treatment among Multi-Drug Resistant Tuberculosis Patients in Hunan Province, China. PLoS ONE 2017, 12, e0170718. [Google Scholar] [CrossRef]
- Melese, A.; Zeleke, B. Factors associated with poor treatment outcome of tuberculosis in Debre Tabor, northwest Ethiopia. BMC Res. Notes 2018, 11, 25. [Google Scholar] [CrossRef]
- Muluye, A.B.; Kebamo, S.; Teklie, T.; Alemkere, G. Poor treatment outcomes and its determinants among tuberculosis patients in selected health facilities in East Wollega, Western Ethiopia. PLoS ONE 2018, 13, e0206227. [Google Scholar] [CrossRef]
- Ahmad, N.; Javaid, A.; Basit, A.; Afridi, A.K.; Khan, M.A.; Ahmad, I.; Sulaiman, S.A.S.; Khan, A.H. Management and treatment outcomes of MDR-TB: Results from a setting with high rates of drug resistance. Int. J. Tuberc. Lung Dis. 2015, 19, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Torres, N.M.C.; Rodríguez, J.J.Q.; Andrade, P.S.P.; Arriaga, M.B.; Netto, E.M. Factors predictive of the success of tuberculosis treatment: A systematic review with meta-analysis. PLoS ONE 2019, 14, e0226507. [Google Scholar]
- Krishnan, L.; Akande, T.; Shankar, A.V.; McIntire, K.N.; Gounder, C.R.; Gupta, A.; Yang, W.-T. Gender-Related Barriers and Delays in Accessing Tuberculosis Diagnostic and Treatment Services: A Systematic Review of Qualitative Studies. Tuberc. Res. Treat. 2014, 2014, 215059. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, U.; Sahito, A.; Nafees, A.A.; Kazi, A.; Fatmi, Z. Pulmonary Tuberculosis Is Associated with Biomass Fuel Use Among Rural Women in Pakistan: An Age- and Residence-Matched Case-Control Study. Asia Pac. J. Public Healh 2017, 29, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-G.; Huang, W.-W.; Wang, Y.; Zhang, Y.-X.; Zhang, M.-M.; Wu, S.-Q.; Sandford, A.J.; He, J.-Q. Association between tobacco smoking and drug-resistant tuberculosis. Infect. Drug Resist. 2018, 11, 873–887. [Google Scholar] [CrossRef]
- Makhmudova, M.; Maxsumova, Z.; Rajabzoda, A.; Makhmadov, A.; van den Hof, S.; Mirtskhulava, V. Risk factors for unfavourable treatment outcomes among rifampicin-resistant tuberculosis patients in Tajikistan. Int. J. Tuberc. Lung Dis. 2019, 23, 331–336. [Google Scholar] [CrossRef]
- Zhang, L.; Meng, Q.; Chen, S.; Zhang, M.; Chen, B.; Wu, B.; Yan, G.; Wang, X.; Jia, Z. Treatment outcomes of multidrug-resistant tuberculosis patients in Zhejiang, China, 2009–2013. Clin. Microbiol. Infect. 2018, 24, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Bastos, M.L.; Cosme, L.B.; Fregona, G.; Prado, T.N.D.; Bertolde, A.I.; Zandonade, E.; Sanchez, M.N.; Dalcolmo, M.P.; Kritski, A.; Trajman, A.; et al. Treatment outcomes of MDR-tuberculosis patients in Brazil: A retrospective cohort analysis. BMC Infect. Dis. 2017, 17, 718. [Google Scholar] [CrossRef]
- Jaber, A.A.S.; Ibrahim, B. Evaluation of risk factors associated with drug-resistant tuberculosis in Yemen: Data from centres with high drug resistance. BMC Infect. Dis. 2019, 19, 464. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Javaid, A.; Sulaiman, S.A.S.; Afridi, A.K.; Zainab; Khan, A.H. Occurrence, Management, and Risk Factors for Adverse Drug Reactions in Multidrug Resistant Tuberculosis Patients. Am. J. Ther. 2018, 25, e533–e540. [Google Scholar] [CrossRef] [PubMed]
- Falzon, D.; Gandhi, N.; Migliori, G.B.; Sotgiu, G.; Cox, H.S.; Holtz, T.H.; Hollm-Delgado, M.-G.; Keshavjee, S.; DeRiemer, K.; Centis, R.; et al. Resistance to fluoroquinolones and second-line injectable drugs: Impact on multidrug-resistant TB outcomes. Eur. Respir. J. 2013, 42, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.M.A.; Haseeb, A.; Habib, S.S.; Malik, A.; Khowaja, S.; Saifullah, N.; Rizvi, N. Emergence of fluoroquinolone resistance among drug resistant tuberculosis patients at a tertiary care facility in Karachi, Pakistan. BMC Res. Notes 2017, 10, 313. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Malik, U.R.; Chang, J.; Hashmi, F.; Atif, N.; Basir, H.; Hayat, K.; Khan, F.U.; Kabba, J.A.; Lambojon, K.; Fang, Y. A Simulated Client Exploration of Nonprescription Dispensing of Antibiotics at Drugstores for Pediatric Acute Diarrhea and Upper Respiratory Infection in Lahore, Pakistan. Infect. Drug Resist. 2021, 14, 1129–1140. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).