The Effect of Iron Content on the Ammonia Selective Catalytic Reduction Reaction (NH3-SCR) Catalytic Performance of FeOx/SAPO-34
Abstract
1. Introduction
2. Experimental
2.1. Catalyst Preparation
2.2. Catalyst Characterization
2.3. NH3-SCR Activity Evaluation
3. Results and Discussion
3.1. Catalyst Characterization Results
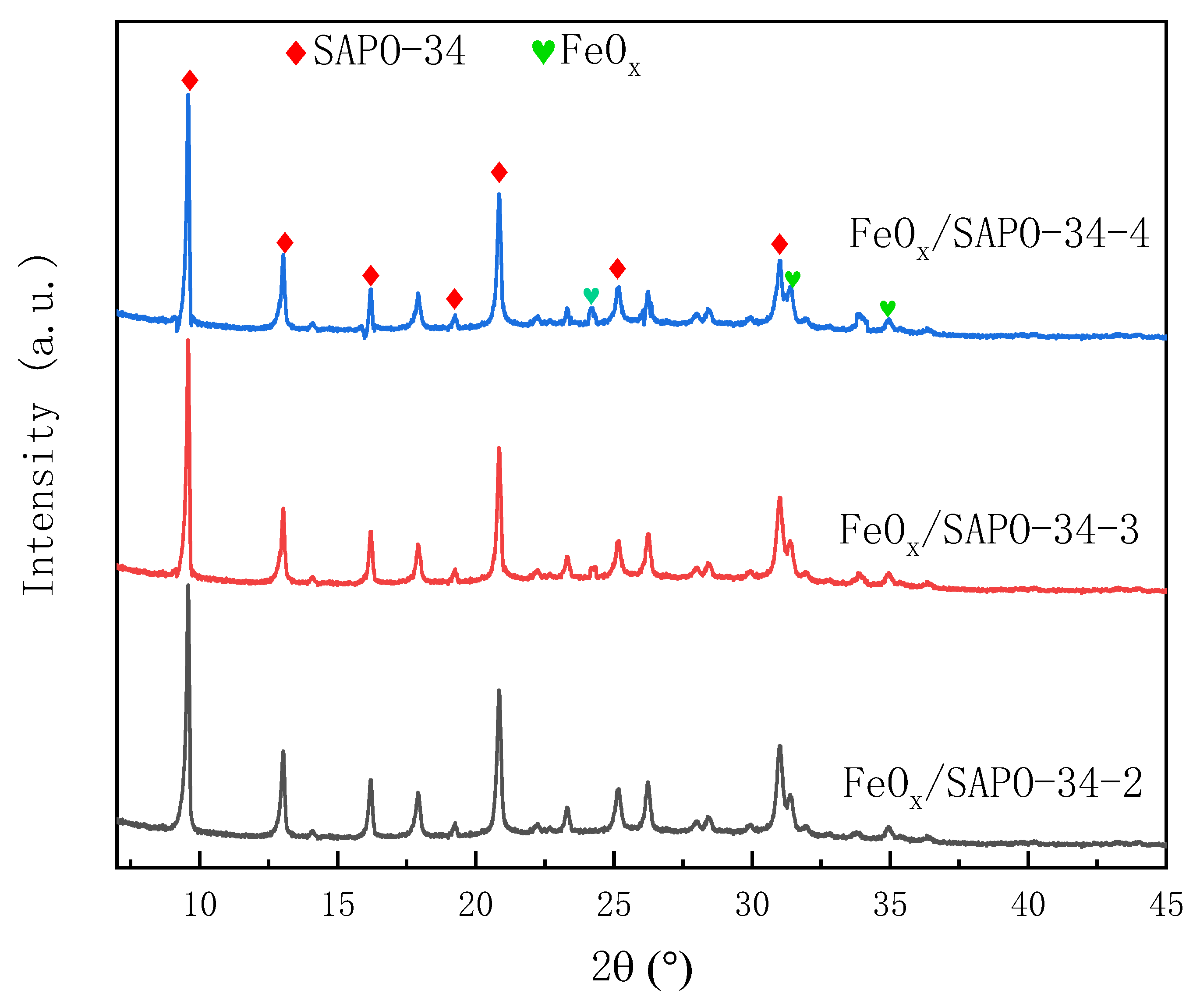
3.1.1. XRD
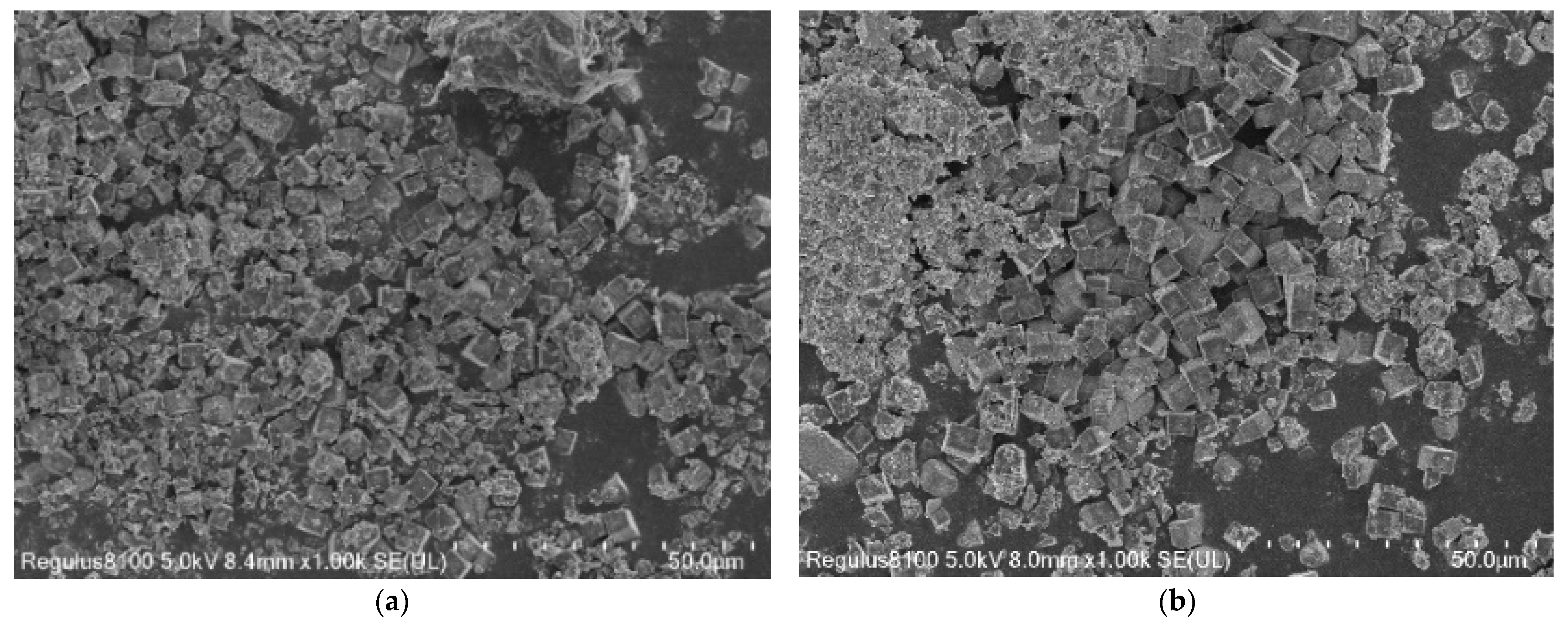
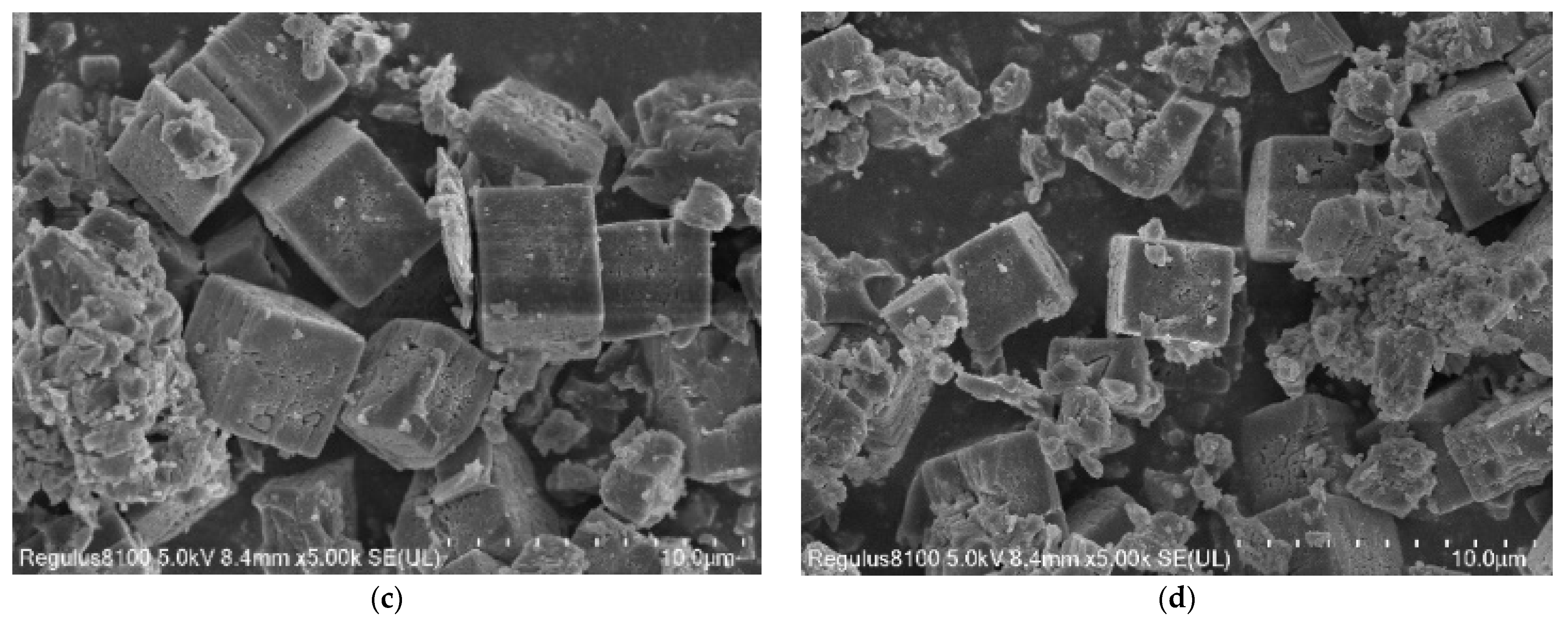
3.1.2. SEM
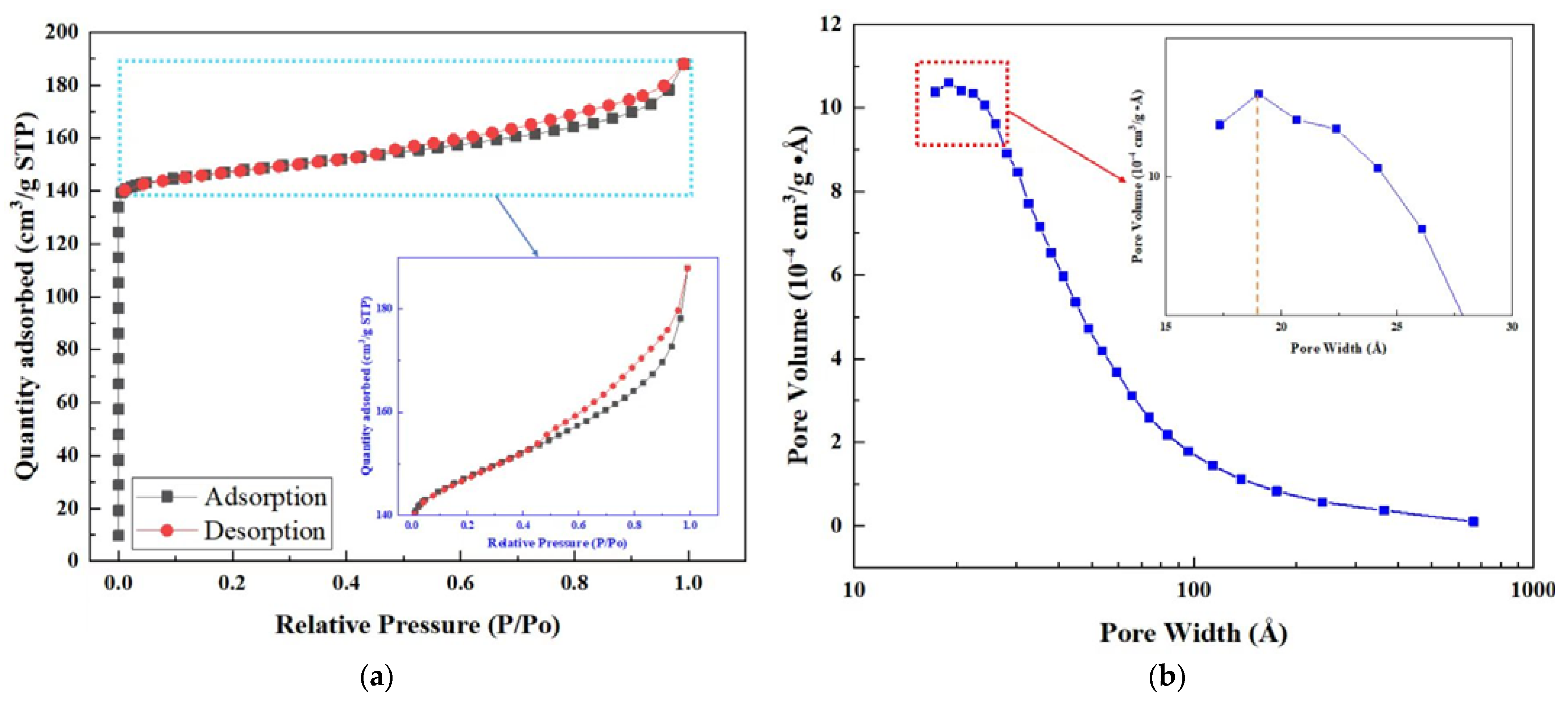
3.1.3. BET
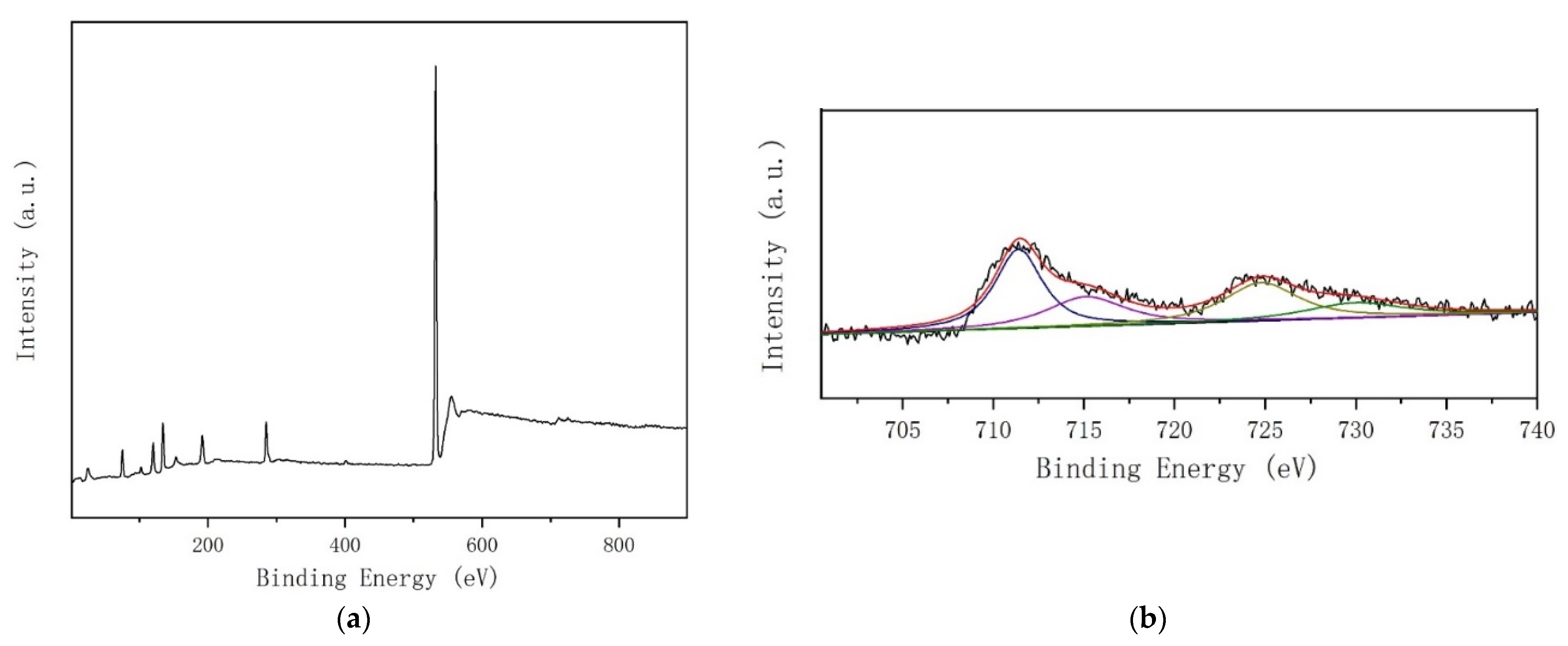
3.1.4. XPS
3.1.5. H2-TPR
3.1.6. NH3-TPD
3.2. De-NOx Performance of the SCR Catalysts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, Y.J.; Kwon, H.J.; Heo, I.; Nam, I.-S.; Cho, B.K.; Choung, J.W.; Cha, M.-S.; Yeo, G.K. Mn–Fe/ZSM5 as a low-temperature SCR catalyst to remove NOx from diesel engine exhaust. Appl. Catal. B 2012, 126, 9–21. [Google Scholar] [CrossRef]
- Chen, K.; Chen, R.; Cang, H.; Mao, A.; Tang, Z.; Xu, Q. Plasma-treated Ce/TiO2-SiO2 catalyst for the NH3-SCR of NOx. Environ. Technol. 2018, 39, 1753–1764. [Google Scholar] [CrossRef] [PubMed]
- Grossale, A.; Nova, I.; Tronconi, E. Study of a Fe–zeolite-based system as NH3-SCR catalyst for diesel exhaust aftertreatment. Catal. Today 2008, 136, 18–27. [Google Scholar] [CrossRef]
- Brandenberger, S.; Kröcher, O.; Tissler, A.; Althoff, R. The State of the Art in Selective Catalytic Reduction of NOx by Ammonia Using Metal-Exchanged Zeolite Catalysts. Catal. Rev. 2008, 50, 492–531. [Google Scholar] [CrossRef]
- Gao, F. Fe-Exchanged Small-Pore Zeolites as Ammonia Selective Catalytic Reduction (NH3-SCR) Catalysts. Catalysts 2020, 10, 1324. [Google Scholar] [CrossRef]
- Ellmers, I.; Pérez Vélez, R.; Bentrup, U.; Schwieger, W.; Brückner, A.; Grünert, W. SCR and NO oxidation over Fe-ZSM-5—The influence of the Fe content. Catal. Today 2015, 258, 337–346. [Google Scholar] [CrossRef]
- Andonova, S.; Tamm, S.; Montreuil, C.; Lambert, C.; Olsson, L. The effect of iron loading and hydrothermal aging on one-pot synthesized Fe/SAPO-34 for ammonia SCR. Appl. Catal. B 2016, 180, 775–787. [Google Scholar] [CrossRef]
- Niu, K.; Li, G.; Liu, J.; Wei, Y. One step synthesis of Fe-SSZ-13 zeolite by hydrothermal method. J. Solid State Chem. 2020, 287, 121330. [Google Scholar] [CrossRef]
- Brandenberger, S.; Kröcher, O.; Tissler, A.; Althoff, R. The determination of the activities of different iron species in Fe-ZSM-5 for SCR of NO by NH3. Appl. Catal. B 2010, 95, 348–357. [Google Scholar] [CrossRef]
- Gao, F.; Zheng, Y.; Kukkadapu, R.K.; Wang, Y.; Walter, E.D.; Schwenzer, B.; Szanyi, J.; Peden, C.H.F. Iron Loading Effects in Fe/SSZ-13 NH3-SCR Catalysts: Nature of the Fe Ions and Structure–Function Relationships. ACS Catal. 2016, 6, 2939–2954. [Google Scholar] [CrossRef]
- Lai, S.; She, Y.; Zhan, W.; Guo, Y.; Guo, Y.; Wang, L.; Lu, G. Performance of Fe-ZSM-5 for selective catalytic reduction of NOx with NH3: Effect of the atmosphere during the preparation of catalysts. J. Mol. Catal. A Chem. 2016, 424, 232–240. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, Y.; Fan, Z.; Chen, M.; Zhang, Z.; Shangguan, W. Widened Active Temperature Window of a Fe-ZSM-5 Catalyst by an Impregnation Solvent for NH3-SCR of NO. Ind. Eng. Chem. Res. 2018, 57, 13703–13712. [Google Scholar] [CrossRef]
- Khan, H.; Yerramilli, A.S.; D’Oliveira, A.; Alford, T.L.; Boffito, D.C.; Patience, G.S. Experimental methods in chemical engineering: X-ray diffraction spectroscopy—XRD. Can. J. Chem. Eng. 2020, 98, 1255–1266. [Google Scholar] [CrossRef]
- Liu, Q.; Fu, Z.; Ma, L.; Niu, H.; Liu, C.; Li, J.; Zhang, Z. MnO-CeO2 supported on Cu-SSZ-13: A novel SCR catalyst in a wide temperature range. Appl. Catal. A Gen. 2017, 547, 146–154. [Google Scholar] [CrossRef]
- Zhang, M.; Cao, H.; Chen, Y.; Jiang, H. Role of Mn: Promotion of Fast-SCR for Cu-SAPO-34 in Low-Temperature Selective Catalytic Reduction with Ammonia. Catal. Surv. Asia 2019, 23, 245–255. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Zhao, H.; Liu, M.; Ma, Y.; Yong, X.; Chen, H.; Li, Y. The Cu migration of Cu-SAPO-34 catalyst for ammonia selective catalytic reduction of NOx during high temperature hydrothermal aging treatment. Catal. Today 2019, 327, 126–133. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Gao, F.; Washton, N.M.; Wang, Y.; Kollár, M.; Szanyi, J.; Peden, C.H.F. Effects of Si/Al ratio on Cu/SSZ-13 NH3-SCR catalysts: Implications for the active Cu species and the roles of Brønsted acidity. J. Catal. 2015, 331, 25–38. [Google Scholar] [CrossRef]
- Bing, L.; Wang, G.; Yi, K.; Tian, A.; Wang, F.; Wu, C. One-pot synthesis of Cu-SAPO-34 catalyst using waste mother liquid and its application in the selective catalytic reduction of NO with NH3. Catal. Today 2018, 316, 37–42. [Google Scholar] [CrossRef]
- Cao, Y.; Feng, X.; Xu, H.; Lan, L.; Gong, M.; Chen, Y. Novel promotional effect of yttrium on Cu–SAPO-34 monolith catalyst for selective catalytic reduction of NOx by NH3 (NH3-SCR). Catal. Commun. 2016, 76, 33–36. [Google Scholar] [CrossRef]
- Pang, L.; Fan, C.; Shao, L.; Song, K.; Yi, J.; Cai, X.; Wang, J.; Kang, M.; Li, T. The Ce doping Cu/ZSM-5 as a new superior catalyst to remove NO from diesel engine exhaust. Chem. Eng. J. 2014, 253, 394–401. [Google Scholar] [CrossRef]
- Gao, F.; Kollár, M.; Kukkadapu, R.K.; Washton, N.M.; Wang, Y.; Szanyi, J.; Peden, C.H.F. Fe/SSZ-13 as an NH3-SCR catalyst: A reaction kinetics and FTIR/Mössbauer spectroscopic study. Appl. Catal. B 2015, 164, 407–419. [Google Scholar] [CrossRef]



| Sample | Micro Pore | Full Pore | |||
|---|---|---|---|---|---|
| Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) | |
| SAPO-34 | 534.4 | 0.29 | 541.8 | 0.30 | 2.0 |
| FeOx/SAPO-34-3 | 415.5 | 0.20 | 474.8 | 0.29 | 2.4 |
| Sample | Low-Temperature Peak Ammonia Desorption Volume (mmol/g) | High-Temperature Peak Ammonia Desorption Volume (mmol/g) | Total Ammonia Desorption (mmol/g) |
|---|---|---|---|
| FeOx/SAPO-34-1 | 0.79 | 0.52 | 1.31 |
| FeOx/SAPO-34-2 | 0.77 | 0.50 | 1.27 |
| FeOx/SAPO-34-3 | 0.72 | 0.46 | 1.18 |
| FeOx/SAPO-34-4 | 0.65 | 0.41 | 1.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Chen, G.; Shao, Z.; Zhang, H.; Guo, X. The Effect of Iron Content on the Ammonia Selective Catalytic Reduction Reaction (NH3-SCR) Catalytic Performance of FeOx/SAPO-34. Int. J. Environ. Res. Public Health 2022, 19, 14749. https://doi.org/10.3390/ijerph192214749
Li Z, Chen G, Shao Z, Zhang H, Guo X. The Effect of Iron Content on the Ammonia Selective Catalytic Reduction Reaction (NH3-SCR) Catalytic Performance of FeOx/SAPO-34. International Journal of Environmental Research and Public Health. 2022; 19(22):14749. https://doi.org/10.3390/ijerph192214749
Chicago/Turabian StyleLi, Zhaoyang, Geng Chen, Zhenghua Shao, Haonan Zhang, and Xiujuan Guo. 2022. "The Effect of Iron Content on the Ammonia Selective Catalytic Reduction Reaction (NH3-SCR) Catalytic Performance of FeOx/SAPO-34" International Journal of Environmental Research and Public Health 19, no. 22: 14749. https://doi.org/10.3390/ijerph192214749
APA StyleLi, Z., Chen, G., Shao, Z., Zhang, H., & Guo, X. (2022). The Effect of Iron Content on the Ammonia Selective Catalytic Reduction Reaction (NH3-SCR) Catalytic Performance of FeOx/SAPO-34. International Journal of Environmental Research and Public Health, 19(22), 14749. https://doi.org/10.3390/ijerph192214749





